Abstract
Background
There currently is no consensus on the optimal localization procedure and imaging protocol for parathyroid adenoma. Parathyroid four-dimensional (4D) CT has emerged as a promising method for preoperative localization.
Purpose
To evaluate the diagnostic performance of parathyroid 4D CT and technetium 99m–sestamibi (hereafter, referred to as sestamibi) SPECT/CT in preoperative localization in patients with primary hyperparathyroidism.
Materials and Methods
This was a single-institution retrospective study of patients with primary hyperparathyroidism who underwent a combined imaging protocol of sestamibi SPECT/CT and 4D CT (noncontrast, contrast agent–enhanced, arterial, and delayed venous phases) acquired in a single setting from February 2013 to May 2016, with subsequent parathyroidectomy within 6 months. Reference standard for correct localization was on the basis of location denoted on operative reports, with pathologic confirmation of parathyroid adenoma or hyperplasia. By using a four-quadrant analysis, sensitivity, specificity, and area under the curve (AUC) for localization of the hyperfunctioning parathyroid gland or glands at sestamibi SPECT/CT and 4D CT were compared, per modality and in combination.
Results
Four hundred patients (319 women, 81 men; mean age, 61 years ± 14 [standard deviation]) were evaluated. Similar diagnostic performance was found in both combined 4D CT with sestamibi SPECT/CT and 4D CT alone (area under the curve [AUC], 0.88 [95% CI: 0.86, 0.90] and 0.87 [95% CI: 0.85, 0.90], respectively; P = .82). Both modalities outperformed sestamibi SPECT/CT (AUC, 0.78; 95% CI: 0.76, 0.81; P < .001). Four-dimensional CT showed higher sensitivity than did sestamibi SPECT/CT (sensitivity, 79.3% [414 of 522] vs 58.0% [303 of 522], respectively; P < .001). In a subset analysis, 4D CT had higher sensitivity than sestamibi SPECT/CT in patients with single-gland disease (sensitivity, 92.5% [297 of 321] vs 75.1% [241 of 321], respectively; P < .001) and with multigland disease (sensitivity, 58.2% [117 of 201] vs 30.8% [62 of 201], respectively; P < .001).
Conclusion
Four-dimensional CT provided superior preoperative localization compared with sestamibi SPECT/CT in patients with single and multigland disease. The combination of the two modalities did not improve diagnostic performance compared with four-dimensional CT alone.
© RSNA, 2019
Online supplemental material is available for this article.
See also the editorial by Sinha and Oates in this issue.
Summary
Four-dimensional CT (noncontrast, contrast agent–enhanced, arterial, and delayed phases) provides superior preoperative localization in patients with primary hyperparathyroidism compared with sestamibi SPECT/CT in a direct head-to-head comparison. The combination of four-dimensional CT and sestamibi does not improve diagnostic performance compared with four-dimensional CT alone.
Key Points
■ Combined four-dimensional CT with sestamibi SPECT/CT and four-dimensional CT alone had similar diagnostic performance (area under the receiver operating characteristic curve [AUC], 0.88 and 0.87, respectively; P = .82).
■ Four-dimensional CT had higher diagnostic performance than sestamibi SPECT/CT (AUC, 0.88 vs 0.78, respectively; P < .001).
■ Four-dimensional CT had higher sensitivity than sestamibi SPECT/CT in all patients (79% vs 58%, respectively; P < .001) and in patients with single-gland disease (92% vs 75%, respectively; P < .001) and multigland disease (58% vs 31%, respectively; P < .001).
Introduction
Over the past 2 decades, minimally invasive parathyroidectomy has been widely adopted because of improved preoperative imaging and routine use of intraoperative parathyroid hormone monitoring (1). The rationale for this surgical approach is that most patients with primary hyperparathyroidism have a single, benign parathyroid adenoma (up to 88%) (2). Compared with bilateral neck exploration, minimally invasive parathyroidectomy is associated with lower complication rates, shorter operation times, reduced costs, and improved cosmetic results while it maintains similar cure rates (3). Accurate preoperative localization of a single parathyroid adenoma is critically important to the success of minimally invasive parathyroidectomy because it directs the surgeon to the adenoma, without which minimally invasive parathyroidectomy becomes a problematic surgical approach (4,5). Conversely, negative or equivocal preoperative imaging directs the surgeon to the less focused bilateral neck exploration.
With a variety of imaging-based localization modalities available and different acquisition protocols within a given modality, to our knowledge there is no current consensus regarding the optimal localization procedure and imaging protocol (6). The choice often depends on regional imaging capabilities, radiologist expertise, and surgeon preference. Many institutions use the traditional methods of technetium 99m (99 mTc) sestamibi (hereafter, referred to as sestamibi) scintigraphy, US, or a combination of both for preoperative localization (4,7). First reported by Rodgers et al (1) in 2006, parathyroid four-dimensional (4D) CT has emerged as a promising method for preoperative localization and consists of multiphase CT acquired at noncontrast, contrast agent–enhanced, arterial, and delayed phases. 4D CT has been shown to be useful in a variety of clinical scenarios including in patients with and without previous neck surgery, mild hyperparathyroidism, multigland disease, and in patients with sestamibi and US examinations that are negative for adenoma (4,7–9). Studies that compared 4D CT with sestamibi and US have generally demonstrated that 4D CT is superior (1,8–12). This experience led some to suggest that 4D CT should be used as a first-line imaging modality (9,10). However, rigorous comparison of 4D CT and sestamibi has been limited by small numbers of study participants, exclusion of patients with multigland disease, and variability in the imaging protocols.
In this study, we aimed to assess the diagnostic performance of a combined imaging protocol of 4D CT and 99 mTc sestamibi SPECT/CT for preoperative localization in patients with primary hyperparathyroidism, and we performed a direct head-to-head comparison of the success of either modality. We hypothesized that the synergistic combination of 4D CT and sestamibi SPECT/CT would outperform the individual modalities, and 4D CT would outperform sestamibi SPECT/CT.
Materials and Methods
Patients
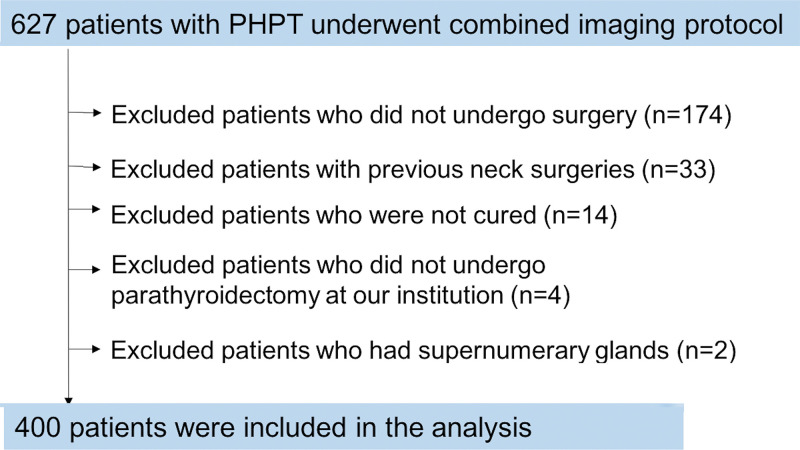
We conducted a retrospective review of consecutive patients with biochemical evidence of primary hyperparathyroidism who underwent a combined imaging protocol of 4D CT and sestamibi SPECT/CT at a single institution from February 2013 to May 2016. Figure 1 summarizes our study’s patient flow. A total of 627 patients with primary hyperparathyroidism underwent the imaging protocol. Exclusion criteria were patients who did not undergo surgery (n = 174), who underwent prior parathyroid or thyroid surgery (n = 33), who were not cured (n = 14), who did not undergo parathyroid surgery at our institution (n = 4), and who have supernumerary glands (n = 2). Thus, 400 patients were included in the analysis. Our study was Health Insurance Portability and Accountability Act compliant and approved by the institutional review board.
Figure 1:
Study flowchart with consecutive patients and inclusion and exclusion criteria. PHPT = primary hyperparathyroidism.
Combined 4D CT and Sestamibi SPECT/CT Imaging Protocol
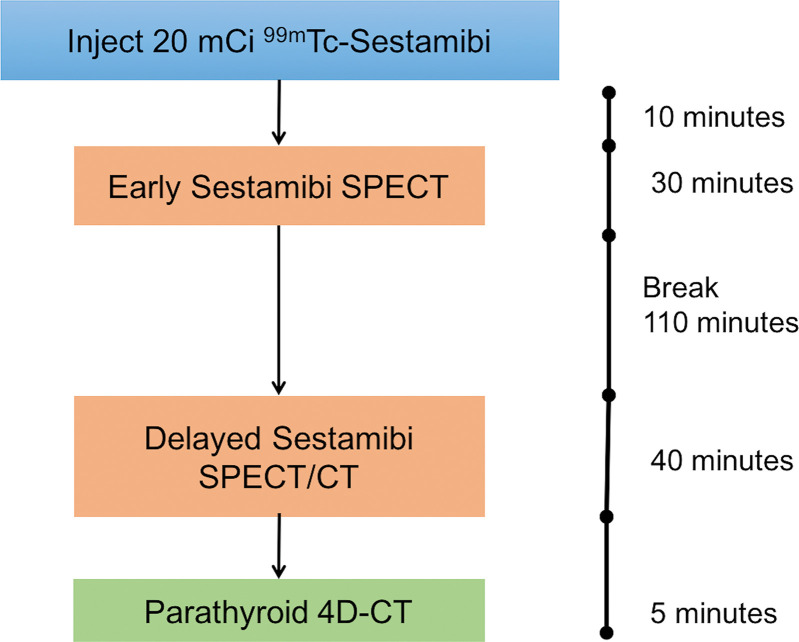
Imaging was performed with a 16-section SPECT/CT scanner (Symbia T; Siemens, Erlangen, Germany). The imaging protocol consisted of a dual-phase 99 mTc sestamibi SPECT followed by 4D CT performed immediately after acquisition of the delayed phase of the sestamibi SPECT/CT (Fig 2). The patients remained in the same position on the same SPECT/CT scanner for both components of the combined protocol.
Figure 2:
Sequence and time points of the combined imaging acquisition protocol performed on single SPECT/CT camera. 4D = four-dimensional, 99 mTc = technetium 99m.
The dual-phase sestamibi examination consisted of an early phase SPECT and a delayed-phase SPECT/CT. An 18-gauge catheter was placed in the antecubital vein. Patients were intravenously administered approximately 740 MBq (20 mCi) of 99 mTc sestamibi. Early phase SPECT images were obtained after 10 minutes and delayed-phase SPECT/CT images were acquired after 150 minutes. The x-axis field of view was from the level of the parotid glands to the heart. Patients were positioned supine, with the neck supported in an extended position and arms lowered alongside the body. SPECT images were acquired with a 128 × 128 matrix by using a magnification of 1.00×, with a 15% window centered around a 140-keV photopeak by using a low-energy, high-spatial-resolution parallel hole collimator. A step-and-shoot protocol was used and consisted of 20 seconds per frame with a total of 64 frames. Transverse, coronal, and sagittal SPECT images were generated by using a Gaussian 2.0 prefilter, and they were postprocessed by using fast low-angle shot three-dimensional iterative reconstruction (four iterations, eight subsets). An attenuation correction factor of 0.15/cm was applied with the Chang method. Attenuation correction of delayed SPECT was performed by using noncontrast CT images acquired at 4D CT.
Immediately after delayed-phase SPECT, patients were prepared for 4D CT imaging by connecting the CT contrast injector to the patient’s intravenous catheter. The 4D CT protocol consisted of helical scans performed at noncontrast, arterial, and delayed venous phases at predetermined times. After noncontrast CT was performed, iodinated contrast (Omnipaque 350, GE Healthcare, Princeton, NJ) was injected at 4 mL per second with a total dose of 75 mL. The arterial phase CT was performed 30 seconds after the beginning of contrast agent infusion and the delayed-phase CT was performed 30 seconds after arterial phase imaging. For all phases, scanning parameters were as follows: 130 kVp; 120–300 mA by automatic exposure control; rotation time, 0.6 seconds; pitch, 0.8; detector configuration, 1.0 mm; and beam width, 10 mm. Coronal and sagittal reformats were reconstructed with 1-mm section thickness. The field of view extended from the level of the mandibular angle to the carina. Noncontrast CT images were fused for the delayed SPECT/CT.
Image Interpretation
All images were reviewed and interpreted for preoperative localization and surgical planning by one of two radiologists (D.K.L., with 9 years of experience, and R.Y., with 4 years of experience) with dual board certification in radiology and nuclear medicine. Both 4D CT and sestamibi images were reviewed on a picture archiving and communication system (GE PACS; GE Healthcare, Barrington, Ill), with additional review of sestamibi images on an advanced workstation (AW Server; GE Healthcare) as deemed necessary by the interpreting radiologist.
A single, combined imaging report was rendered and entered into the electronic medical record. Abnormal parathyroid lesions were categorized on the basis of the anatomic position in reference to midline and thyroid gland into four quadrants: right upper, right lower, left upper, and left lower quadrants. The sestamibi SPECT/CT images were reviewed and a positive lesion was defined as an abnormal focus that localized to an expected location of a parathyroid gland on either early phase or delayed-phase images. A single focus of uptake was interpreted as single-gland disease and more than one focus was interpreted as multigland disease. Four-dimensional CT images were reviewed for abnormal parathyroid glands by using the pattern of contrast enhancement at serial phases. Additionally, the exact anatomic location of the suspected abnormal parathyroid gland was described regarding the thyroid gland, trachea, esophagus, carotid artery, and sternal notch. Relevant clinical history and laboratory values were reviewed at the time of image interpretation.
Surgical Procedures
All operations were performed by one of three board-certified endocrine surgeons (J.A.L., with 13 years of experience, J.H.K., with 5 years of experience, and a third surgeon, with 11 years of experience). The combined imaging report was used for surgical planning, with review of the images performed at the discretion of the surgeon. Intraoperative parathyroid hormone monitoring levels were drawn and measured by using a chemiluminescent immunometric assay (STAT intraoperative PTH assay; Future Diagnostics, Wijchen, the Netherlands). A successful operation was defined as a 50% or greater drop in intraoperative parathyroid hormone levels from the higher of the two pre-excision values (Miami criterion) (13). Pathologic examination of resected parathyroid glands was performed to confirm parathyroid adenoma or hyperplasia.
The reference standard for correct localization was the location of the gland or glands on the basis of surgical reports, with pathologic confirmation of resected glands as parathyroid adenoma or hyperplasia.
The original radiology, operative, and pathologic reports were retrieved from the electronic medical record and results were collated and tabulated in a spreadsheet (Excel version 15.23, 2016; Microsoft, Redmond, Wash). The imaging data were based on retrospective review of the radiology reports, and no reinterpretation of the images was performed.
Statistical Analysis
Data were presented as frequency and percentages for qualitative variables and as mean with standard deviation for continuous variables. Calculated data with P values less than .05 were considered to indicate statistical significance. For the combined 4D CT and sestamibi analysis, two analytic approaches were performed in the interpretation of the results, one by using a sensitive analysis and another by using a specific analysis. In the sensitive analysis, if either 4D CT or sestamibi was positive for a hyperfunctioning gland within a quadrant, this was considered to be a positive result. In the specific analysis, both 4D CT and sestamibi had to be positive to be considered a positive result. The diagnostic performance of 4D CT, sestamibi SPECT/CT, combined sensitive reading, and combined specific reading to detect a parathyroid adenoma was calculated by using a binary classifier system (healthy tissue vs parathyroid adenoma or hyperplasia) and nonparametric receiver operating characteristic curve. All statistical analyses were performed by using software (SPSS 24.0; IBM, Armonk, NY).
Results
Table 1 summarizes the patient demographics. The mean age of the patient cohort was 61 years ± 14 (standard deviation; age range, 19–91 years). Of the 400 patients, 319 patients (79.8%) were women and 81 (20.2%) were men. The women were older (mean age, 62 years ± 12; age range, 19–88 years) than the men (mean age, 57 years ± 17; age range, 19–91 years; P = .01). The majority of patients were white (68.8%; 275 of 400). The median calcium level was 10.7 mg/dL (interquartile range, 10.4–11.1 mg/dL) and parathyroid hormone level was 91.3 pg/mL (interquartile range, 67.9–121.0); normal range for serum calcium was 8.4–10.2 mg/dL and normal range for parathyroid hormone was 10–65 pg/mL.
Table 1:
Patient Demographics and Baseline Characteristics
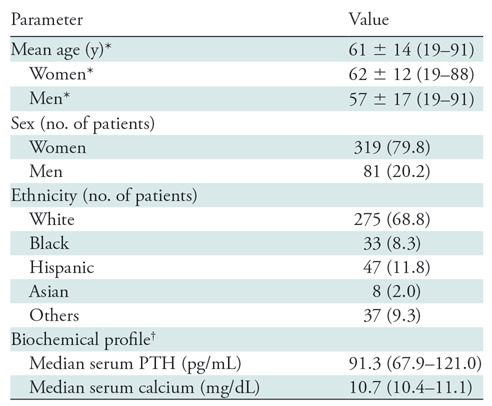
Note.—Unless otherwise indicated, data in parentheses are percent. Healthy ranges for parathyroid hormone and serum calcium are 10–65 pg/mL and 8.4–10.2 mg/dL, respectively. PTH = parathyroid hormone.
*Data are ± standard deviation; data in parentheses are range.
†Data in parentheses are interquartile range.
Parathyroid Disease Characteristics
Table 2 summarizes parathyroid disease characteristics analyzed by patient and per lesion. Of the 400 patients, 321 patients (80.2%) had single-gland disease and 79 patients (19.8%) had multigland disease. Of patients with multigland disease (n = 79), over half (n = 44; 56%) had double adenomas or hyperplasia, about a third of patients (n = 27; 34%) had three abnormal glands, and the remaining eight patients had four-gland disease (10%).
Table 2:
Parathyroid Disease Characteristics
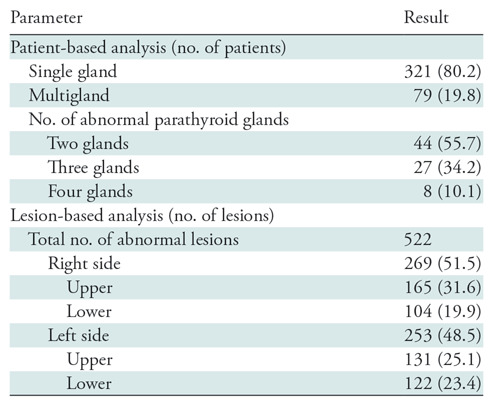
Note.—Data in parentheses are percent.
Lesion-based Four-Quadrant Analysis
Four hundred patients were assumed to have four parathyroid glands for the four-quadrant analysis (n = 1600). A total of 522 abnormal glands were found at surgery; 269 of 522 (51.5%) were from the right and 253 of 522 (48.5%) were from the left. The distribution of abnormal glands per quadrant is summarized in Table 2.
Diagnostic Performance
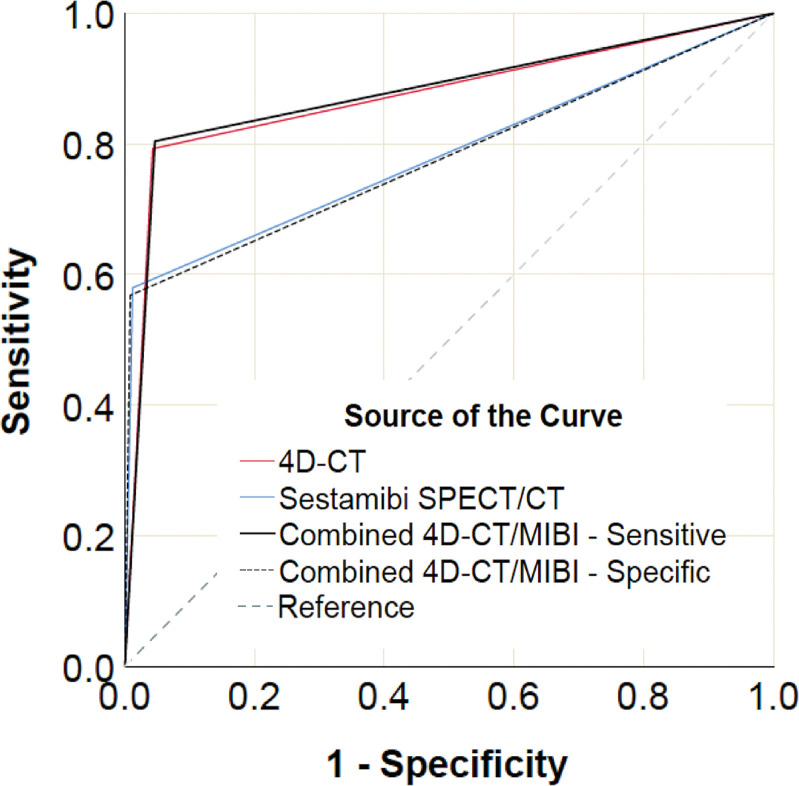
The diagnostic performance of each imaging modality for the classification of each parathyroid gland as normal or abnormal was calculated on a per-quadrant basis for 1600 lesions in 400 patients (Table E1 [online]). Figure 3 shows the receiver operating characteristic curves for all modalities and Table 3 summarizes their respective areas under the receiver operating characteristic curve (AUCs) and compares the AUCs to that of 4D CT and sestamibi. Four-dimensional CT and combined-modality sensitive reading had the highest AUCs and they were not statistically different (P = .82). Four-dimensional CT had a statistically significant (P < .001) AUC that was higher than the AUCs of sestamibi SPECT/CT and combined-modality specific reading. There was no statistical difference in AUCs for sestamibi SPECT/CT and combined-modality specific reading (P = .85). AUCs were also correct for within-patient correlation and summarized in Table E2 (online).
Figure 3:
Receiver operating characteristic curves for all modalities by using a per-lesion, four-quadrant analysis. MIBI = sestamibi SPECT/CT.
Table 3:
Comparison of Areas Under the Curve
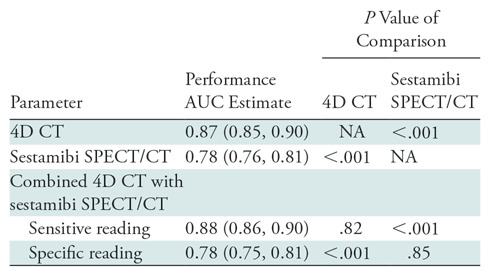
Note.—Data in parentheses are 95% confidence intervals. Area under the receiver operating characteristic curve comparisons for correlated receiver operating characteristic curves were performed by using Hanley and McNeil (14). Area under the curve analyses were performed by using a per-lesion, four-quadrant analysis in 1600 glands. 4D = four dimensional, AUC = area under the receiver operating characteristic curve, NA = not applicable.
Table 4 summarizes the sensitivity, specificity, positive predictive value, and negative predictive value of all modalities. Four-dimensional CT showed sensitivity superior to sestamibi SPECT/CT (sensitivity, 4D CT vs sestamibi SPECT/CT: 79.3% [95% confidence interval: 76%, 83%; 414 of 522] vs 58.0% [95% confidence interval: 54%, 62%; 303 of 522], respectively). Combined-modality sensitive reading demonstrated a sensitivity of 80.4% (95% confidence interval: 77%, 84%; 420 of 522), similar to 4D CT. Sestamibi SPECT/CT and combined-modality specific reading showed the highest specificity (98.8% [95% confidence interval: 98%, 99%; 1065 of 1078] and 99.2% [95% confidence interval: 99%, 100%; 1069 of 1078], respectively), however, both 4D CT and combined-modality sensitive reading demonstrated high specificity (95.6% [95% confidence interval: 94%, 97%, 1031 of 1078] and 95.3% [95% confidence interval: 94%, 97%; 1027 of 1078], respectively).
Table 4:
Diagnostic Performance in All Patients
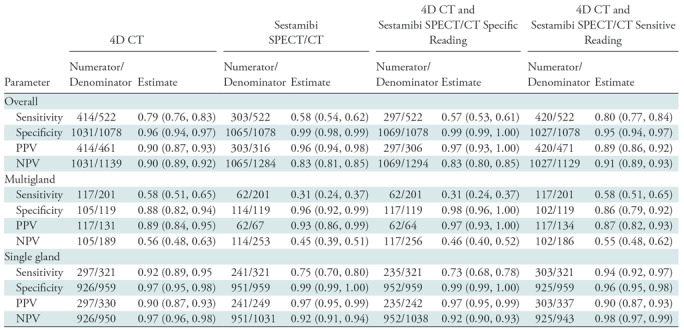
Note.—Data in parentheses are 95% confidence intervals. 4D = four dimensional, PPV = positive predictive value, NPV = negative predictive value.
Similar trends were observed in a subset analysis of patients with single-gland disease: the sensitivity of 4D CT was 92.5% (95% confidence interval: 89%, 95%; 297 of 321) compared with 75.1% (95% confidence interval: 70%, 80%; 241 of 321) for sestamibi SPECT/CT and 94.4% (95% confidence interval: 92%, 97%; 303 of 321) for combined-modality sensitive reading. Similar to the results for all patients, specificity for all modalities in patients with single-gland disease was high and ranged from 96% to 99%.
In patients with multigland disease, 4D CT also had a higher sensitivity compared with sestamibi SPECT/CT (58.2% [95% confidence interval: 51%, 65%; 117 of 201] vs 30.8% [95% confidence interval: 24%, 37%; 62 of 201], respectively), and similar to the combined-modality sensitive reading (58.2%; 95% confidence interval: 51%, 65%; 117 of 201). Higher specificity was observed for combined-modality specific reading and sestamibi SPECT/CT (98.3% [95% confidence interval: 96%, 100%; 117 of 119] vs 95.8% [95% confidence interval: 92%, 99%; 114 of 119], respectively) compared with 4D CT and combined-modality sensitive reading (88.2% [95% confidence interval: 82%, 94%; 105 of 119] vs 85.7% [95% confidence interval: 79%, 92%; 102 of 119], respectively). However, as demonstrated by the receiver operating characteristic curve and AUC analysis, the slightly higher specificity of combined-modality specific reading and sestamibi SPECT/CT also meant that they had lower sensitivities. Figures 4 and 5 show examples of the modalities in patients with single-gland and multigland disease, and the Movie (online) shows combination of anatomic imaging of 4D CT and functional imaging of sestamibi.
Figure 4a:
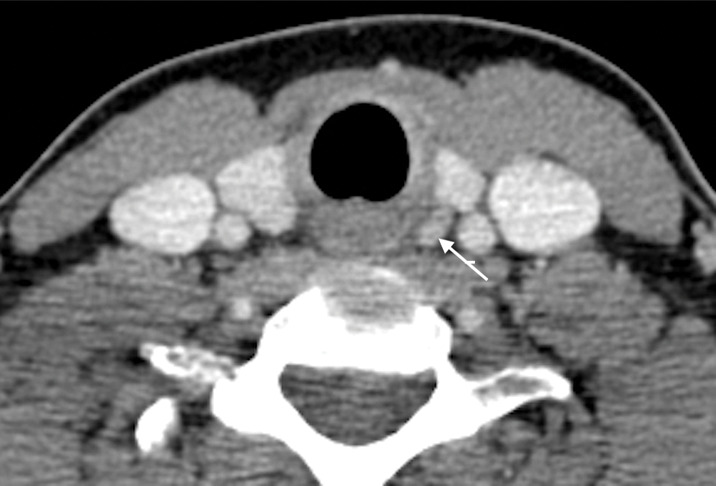
Images in a 63-year-old woman with primary hyperparathyroidism. Superior localization of four-dimensional (4D) CT compared with sestamibi SPECT/CT in a patient with single-gland disease. (a) Axial contrast-enhanced arterial phase of parathyroid 4D CT shows a single abnormal left upper parathyroid adenoma (arrow). (b) Axial delayed-phase SPECT/CT and (c) axial delayed-phase SPECT-only images show persistent mild diffuse sestamibi uptake in the thyroid gland, but no focal uptake corresponding with left upper parathyroid adenoma identified at 4D CT. The patient underwent minimally invasive parathyroidectomy for a single left upper parathyroid adenoma and experienced an appropriate drop in intraoperative parathyroid hormone.
Figure 5a:
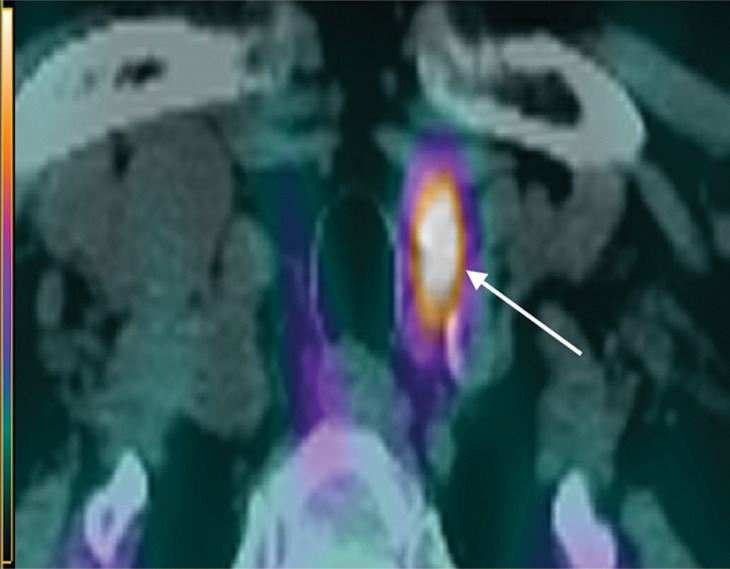
Images in a 69-year-old woman with primary hyperparathyroidism. Superior localization of four-dimensional (4D) CT compared with sestamibi SPECT/CT in a patient with multigland disease. (a) Axial delayed-phase sestamibi SPECT/CT shows focal sestamibi uptake localizing to a single abnormal left lower parathyroid gland (arrow). No additional abnormal focus is identified to suggest an additional abnormal parathyroid gland. (b) Axial contrast-enhanced arterial phase 4D CT shows enhanced left lower parathyroid gland (arrows) corresponding to same gland identified at sestamibi SPECT/CT. However, additional (c) enhanced right upper (arrow) and (d) left upper (arrows) parathyroid glands were identified at 4D CT, which were not observed at sestamibi SPECT/CT. The patient underwent bilateral four-gland exploration for multigland disease involving all three parathyroid glands.
Figure 4b:
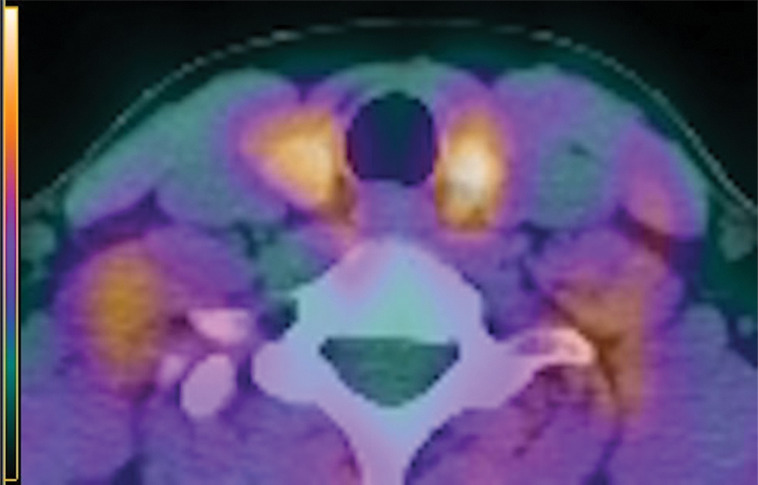
Images in a 63-year-old woman with primary hyperparathyroidism. Superior localization of four-dimensional (4D) CT compared with sestamibi SPECT/CT in a patient with single-gland disease. (a) Axial contrast-enhanced arterial phase of parathyroid 4D CT shows a single abnormal left upper parathyroid adenoma (arrow). (b) Axial delayed-phase SPECT/CT and (c) axial delayed-phase SPECT-only images show persistent mild diffuse sestamibi uptake in the thyroid gland, but no focal uptake corresponding with left upper parathyroid adenoma identified at 4D CT. The patient underwent minimally invasive parathyroidectomy for a single left upper parathyroid adenoma and experienced an appropriate drop in intraoperative parathyroid hormone.
Figure 4c:
Images in a 63-year-old woman with primary hyperparathyroidism. Superior localization of four-dimensional (4D) CT compared with sestamibi SPECT/CT in a patient with single-gland disease. (a) Axial contrast-enhanced arterial phase of parathyroid 4D CT shows a single abnormal left upper parathyroid adenoma (arrow). (b) Axial delayed-phase SPECT/CT and (c) axial delayed-phase SPECT-only images show persistent mild diffuse sestamibi uptake in the thyroid gland, but no focal uptake corresponding with left upper parathyroid adenoma identified at 4D CT. The patient underwent minimally invasive parathyroidectomy for a single left upper parathyroid adenoma and experienced an appropriate drop in intraoperative parathyroid hormone.
Figure 5b:
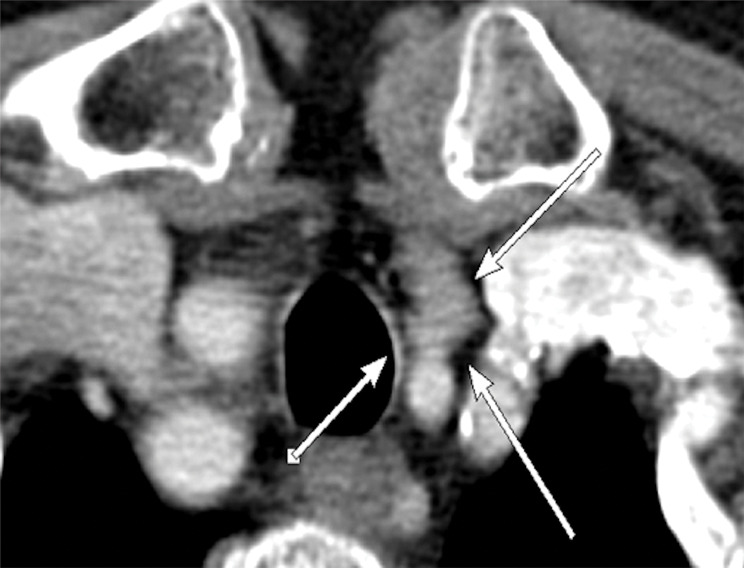
Images in a 69-year-old woman with primary hyperparathyroidism. Superior localization of four-dimensional (4D) CT compared with sestamibi SPECT/CT in a patient with multigland disease. (a) Axial delayed-phase sestamibi SPECT/CT shows focal sestamibi uptake localizing to a single abnormal left lower parathyroid gland (arrow). No additional abnormal focus is identified to suggest an additional abnormal parathyroid gland. (b) Axial contrast-enhanced arterial phase 4D CT shows enhanced left lower parathyroid gland (arrows) corresponding to same gland identified at sestamibi SPECT/CT. However, additional (c) enhanced right upper (arrow) and (d) left upper (arrows) parathyroid glands were identified at 4D CT, which were not observed at sestamibi SPECT/CT. The patient underwent bilateral four-gland exploration for multigland disease involving all three parathyroid glands.
Figure 5c:
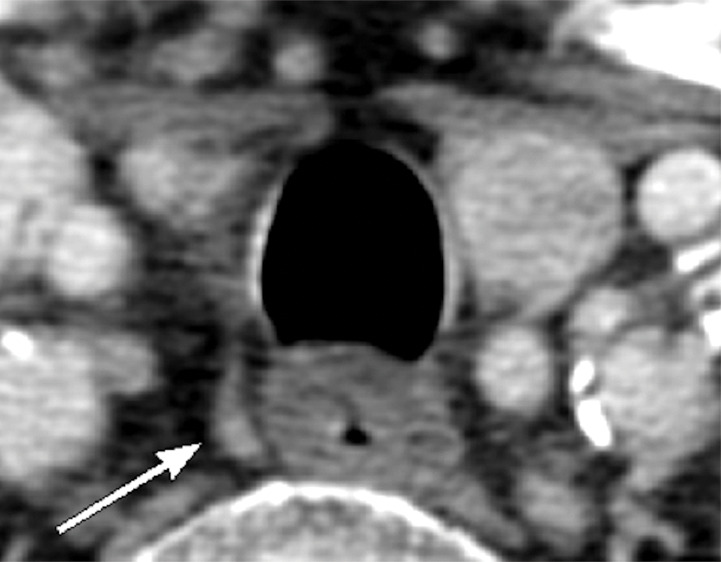
Images in a 69-year-old woman with primary hyperparathyroidism. Superior localization of four-dimensional (4D) CT compared with sestamibi SPECT/CT in a patient with multigland disease. (a) Axial delayed-phase sestamibi SPECT/CT shows focal sestamibi uptake localizing to a single abnormal left lower parathyroid gland (arrow). No additional abnormal focus is identified to suggest an additional abnormal parathyroid gland. (b) Axial contrast-enhanced arterial phase 4D CT shows enhanced left lower parathyroid gland (arrows) corresponding to same gland identified at sestamibi SPECT/CT. However, additional (c) enhanced right upper (arrow) and (d) left upper (arrows) parathyroid glands were identified at 4D CT, which were not observed at sestamibi SPECT/CT. The patient underwent bilateral four-gland exploration for multigland disease involving all three parathyroid glands.
Figure 5d:
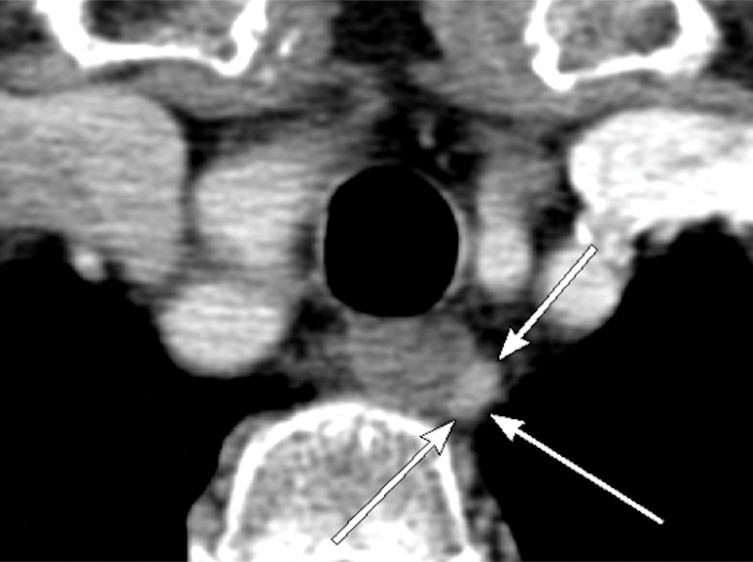
Images in a 69-year-old woman with primary hyperparathyroidism. Superior localization of four-dimensional (4D) CT compared with sestamibi SPECT/CT in a patient with multigland disease. (a) Axial delayed-phase sestamibi SPECT/CT shows focal sestamibi uptake localizing to a single abnormal left lower parathyroid gland (arrow). No additional abnormal focus is identified to suggest an additional abnormal parathyroid gland. (b) Axial contrast-enhanced arterial phase 4D CT shows enhanced left lower parathyroid gland (arrows) corresponding to same gland identified at sestamibi SPECT/CT. However, additional (c) enhanced right upper (arrow) and (d) left upper (arrows) parathyroid glands were identified at 4D CT, which were not observed at sestamibi SPECT/CT. The patient underwent bilateral four-gland exploration for multigland disease involving all three parathyroid glands.
Movie.
Video of volume-rendered 3D reconstruction derived from the combination of 4D-CT and sestamibi imaging. The case demonstrates a right lower parathyroid adenoma with the 4D-CT information displayed first (parathyroid adenoma: red, thyroid: blue) followed by the sestamibi information overlaid on the 4D-CT, with the adenoma in yellow. Physiologic sestamibi uptake in the salivary glands and heart are also displayed in yellow.
Discussion
We evaluated the diagnostic utility of combined 4D CT and sestamibi SPECT/CT by using a combined imaging protocol and performed a direct head-to-head comparison of the two individual modalities. In a large and varied cohort of patients, our results showed that the combination of two modalities did not improve localization performance compared with 4D CT; this was demonstrated by similar AUCs (AUC, 4D CT vs combined: 0.87 vs 0.88, respectively), but did improve performance versus sestamibi SPECT/CT (AUC, 0.78). As individual modalities, 4D CT outperformed sestamibi SPECT/CT in preoperative localization (AUC, 0.87 vs 0.78, respectively; P < .001), with incremental value of 4D CT because of superior sensitivity in all patients (79% vs 58%, respectively; P < .001) and in patients with single-gland disease (92% vs 75%, respectively; P < .001) and multigland disease (58% vs 31%, respectively; P < .001).
Several studies compared 4D CT and sestamibi for preoperative localization, and most analyzed sensitivity or so-called quadrant localization accuracy. In the seminal study regarding 4D CT in 75 patients by Rodgers et al (1), 4D CT had higher sensitivity compared with sestamibi (70% vs 33%, respectively). Our study similarly demonstrated that 4D CT had a higher sensitivity compared with sestamibi (79% vs 58%, respectively). The higher sensitivity of sestamibi in our study is likely because of the dual-time-point SPECT acquisition with delayed SPECT/CT, whereas Rodgers et al acquired dual-phase planar images with a single-time-point early SPECT/CT. Sestamibi protocols often vary among institutions, and it is important to compare the details of the sestamibi protocols between studies because differences can influence diagnostic performance. Kukar et al (10) also showed results similar to our study in 202 patients with 4D CT sensitivity of 94% compared with sestamibi sensitivity of 61% in single-gland disease. Less successful localization was also found in multigland disease, with 32% for 4D CT and 0% for sestamibi (10), compared with 58% versus 31%, respectively, in our study. Of note, most of the sestamibi scans in Kukar et al were performed outside of their institution with no reported standardized acquisition method. Starker et al (11) showed improved localization accuracy of 4D CT in 87 patients, with a high multigland disease detection rate at 4D CT of 86% versus 0% for sestamibi, however, only 38% of patients underwent 4D CT and 60% underwent sestamibi. Recent studies similarly showed the superiority of 4D CT versus sestamibi in patients with normocalcemic primary hyperparathyroidism (15) and low baseline intact parathyroid hormone (16), which are associated with higher rates of multigland disease.
To our knowledge, our study of 400 patients with a lesion-based analysis of 1600 glands is the largest to directly compare the two modalities and to evaluate 4D CT in preoperative localization. Our study differs from previous studies because we also investigated the utility of combining 4D CT and sestamibi SPECT/CT. Our hypothesis, that the combination of the two modalities would be superior to the individual modalities alone, was not supported by our results, which showed that the combined imaging protocol versus 4D CT alone did not provide added benefit in patients who had not undergone previous parathyroidectomy. The performance of combined SPECT and 4D CT imaging is not justified on the basis of our data. In addition, combined imaging results in greater radiation dose: effective radiation doses for sestamibi SPECT/CT, 4D CT, and combined imaging are 10.6 mSv, 22 mSv, and 28 mSv, respectively.
Our study had limitations. First, our study was retrospective; therefore, some patients who underwent imaging did not subsequently undergo an operation and were excluded from the analysis. Second, images from both 4D CT and sestamibi SPECT/CT were available to the radiologist at the time of interpretation and reporting. This may bias the individual diagnostic performances of each modality because findings at one modality may have influenced the interpretation of the other modality, and therefore would affect the direct head-to-head comparison. We attempted to limit this bias by using a step-wise interpretation and standardized reporting of 4D CT and SPECT/CT findings. Finally, 4D CT is not yet widely performed. Ultimately, the choice of imaging method depends on several factors such as surgeon preference, availability, radiologist expertise, and patient’s renal function and history of allergy to CT contrast agents.
Four-dimensional CT provided superior preoperative localization compared with sestamibi SPECT/CT in patients with primary hyperparathyroidism. The combination of 4D CT and sestamibi did not improve diagnostic performance compared with 4D CT alone. However, dual-modality imaging could prove to be more beneficial than single-modality imaging in other clinical settings, such as persistent or recurrent primary hyperparathyroidism after surgery, and the use of dual-modality imaging in these settings is a potential future research direction.
SUPPLEMENTAL TABLES
Acknowledgments
Acknowledgment
The authors thank Elias Kazam, MD, for his guidance during development of the combined 4D CT and sestamibi imaging protocol.
Disclosures of Conflicts of Interest: R.Y. disclosed no relevant relationships. Y.K.D.T. disclosed no relevant relationships. G.T. disclosed no relevant relationships. L.D. disclosed no relevant relationships. J.H.K. disclosed no relevant relationships. L.B. disclosed no relevant relationships. C.M. disclosed no relevant relationships. D.K.L. disclosed no relevant relationships. J.A.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to author for board membership from Medscape; disclosed money paid to author for stock/stock options from Collectedmed. Other relationships: disclosed no relevant relationships. J.P.B. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed grant from the National Institutes of Health (DK32333). Other relationships: disclosed no relevant relationships.
Abbreviations:
- 4D
- four dimensional
- AUC
- area under the receiver operating characteristic curve
References
- 1.Rodgers SE, Hunter GJ, Hamberg LM, et al. Improved preoperative planning for directed parathyroidectomy with 4-dimensional computed tomography. Surgery 2006;140(6):932–940; discussion 940–941. [DOI] [PubMed] [Google Scholar]
- 2.Ruda JM, Hollenbeak CS, Stack BC, Jr. A systematic review of the diagnosis and treatment of primary hyperparathyroidism from 1995 to 2003. Otolaryngol Head Neck Surg 2005;132(3):359–372. [DOI] [PubMed] [Google Scholar]
- 3.Hoang JK, Sung WK, Bahl M, Phillips CD. How to perform parathyroid 4D CT: tips and traps for technique and interpretation. Radiology 2014;270(1):15–24. [DOI] [PubMed] [Google Scholar]
- 4.Hunter GJ, Schellingerhout D, Vu TH, Perrier ND, Hamberg LM. Accuracy of four-dimensional CT for the localization of abnormal parathyroid glands in patients with primary hyperparathyroidism. Radiology 2012;264(3):789–795. [DOI] [PubMed] [Google Scholar]
- 5.Hoang JK, Williams K, Gaillard F, Dixon A, Sosa JA. Parathyroid 4D-CT: Multi-institutional International Survey of Use and Trends. Otolaryngol Head Neck Surg 2016;155(6):956–960. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm SM, Wang TS, Ruan DT, et al. The American Association of Endocrine Surgeons guidelines for definitive management of primary hyperparathyroidism. JAMA Surg 2016;151(10):959–968. [DOI] [PubMed] [Google Scholar]
- 7.Kelly HR, Hamberg LM, Hunter GJ. 4D-CT for preoperative localization of abnormal parathyroid glands in patients with hyperparathyroidism: accuracy and ability to stratify patients by unilateral versus bilateral disease in surgery-naive and re-exploration patients. AJNR Am J Neuroradiol 2014;35(1):176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Day KM, Elsayed M, Beland MD, Monchik JM. The utility of 4-dimensional computed tomography for preoperative localization of primary hyperparathyroidism in patients not localized by sestamibi or ultrasonography. Surgery 2015;157(3):534–539. [DOI] [PubMed] [Google Scholar]
- 9.Eichhorn-Wharry LI, Carlin AM, Talpos GB. Mild hypercalcemia: an indication to select 4-dimensional computed tomography scan for preoperative localization of parathyroid adenomas. Am J Surg 2011;201(3):334–338; discussion 338. [DOI] [PubMed] [Google Scholar]
- 10.Kukar M, Platz TA, Schaffner TJ, et al. The use of modified four-dimensional computed tomography in patients with primary hyperparathyroidism: an argument for the abandonment of routine sestamibi single-positron emission computed tomography (SPECT). Ann Surg Oncol 2015;22(1):139–145. [DOI] [PubMed] [Google Scholar]
- 11.Starker LF, Mahajan A, Björklund P, Sze G, Udelsman R, Carling T. 4D parathyroid CT as the initial localization study for patients with de novo primary hyperparathyroidism. Ann Surg Oncol 2011;18(6):1723–1728. [DOI] [PubMed] [Google Scholar]
- 12.Suh YJ, Choi JY, Kim SJ, et al. Comparison of 4D CT, ultrasonography, and 99mTc sestamibi SPECT/CT in localizing single-gland primary hyperparathyroidism. Otolaryngol Head Neck Surg 2015;152(3):438–443. [DOI] [PubMed] [Google Scholar]
- 13.Carneiro DM, Solorzano CC, Nader MC, Ramirez M, Irvin GL, 3rd. Comparison of intraoperative iPTH assay (QPTH) criteria in guiding parathyroidectomy: which criterion is the most accurate? Surgery 2003;134(6):973–979; discussion 979–981. [DOI] [PubMed] [Google Scholar]
- 14.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983;148(3):839–843. [DOI] [PubMed] [Google Scholar]
- 15.Cunha-Bezerra P, Vieira R, Amaral F, et al. Better performance of four-dimension computed tomography as a localization procedure in normocalcemic primary hyperparathyroidism. J Med Imaging Radiat Oncol 2018;62(4):493–498. [DOI] [PubMed] [Google Scholar]
- 16.Rameau A, Eng S, Vu J, Saket R, Jun P, Friduss M. Four-dimensional computed tomography scan utility in parathyroidectomy for primary hyperparathyroidism with low baseline intact parathyroid hormone. Laryngoscope 2017;127(6):1476–1482. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.