Symptomatic and asymptomatic infection with the diarrheal pathogen enteroaggregative Escherichia coli (EAEC) is associated with growth faltering in children in developing settings. The mechanism of this association is unknown, emphasizing a need for better understanding of the interactions between EAEC and the human gastrointestinal mucosa. In this study, we investigated the role of the aggregative adherence fimbriae II (AAF/II) in EAEC adherence and pathogenesis using human colonoids and duodenal enteroids.
KEYWORDS: Escherichia coli, adherence, aggregative, barrier disruption, colonoid, fimbriae, organoid
ABSTRACT
Symptomatic and asymptomatic infection with the diarrheal pathogen enteroaggregative Escherichia coli (EAEC) is associated with growth faltering in children in developing settings. The mechanism of this association is unknown, emphasizing a need for better understanding of the interactions between EAEC and the human gastrointestinal mucosa. In this study, we investigated the role of the aggregative adherence fimbriae II (AAF/II) in EAEC adherence and pathogenesis using human colonoids and duodenal enteroids. We found that a null mutant in aafA, the major subunit of AAF/II, adhered significantly less than wild-type (WT) EAEC strain 042, and adherence was restored in a complemented strain. Immunofluorescence confocal microscopy of differentiated colonoids, which produce an intact mucus layer comprised of the secreted mucin MUC2, revealed bacteria at the epithelial surface and within the MUC2 layer. The WT strain adhered to the epithelial surface, whereas the aafA deletion strain remained within the MUC2 layer, suggesting that the presence or absence of AAF/II determines both the abundance and location of EAEC adherence. In order to determine the consequences of EAEC adherence on epithelial barrier integrity, colonoid monolayers were exposed to EAEC constructs expressing or lacking aafA. Colonoids infected with WT EAEC had significantly decreased epithelial resistance, an effect that required AAF/II, suggesting that binding of EAEC to the epithelium is necessary to impair barrier function. In summary, we show that production of AAF/II is critical for adherence and barrier disruption in human colonoids, suggesting a role for this virulence factor in EAEC colonization of the gastrointestinal mucosa.
INTRODUCTION
Linear growth faltering in children in developing countries is a significant public health concern, contributing to lifelong deficits in growth, cognitive abilities, and socioeconomic potential (1, 2). Infection with the diarrheal pathogen enteroaggregative Escherichia coli (EAEC), whether symptomatic or asymptomatic, is associated with reduced weight for age z-scores (3–5). The mechanism of this association is not known. Better elucidation of the interactions between EAEC and the human gastrointestinal mucosa is needed to understand the role of EAEC infection in the etiology of growth faltering.
Mucosal pathogens, including EAEC, produce filamentous polymeric structures called fimbriae that mediate adherence to target host tissues and promote colonization (6). In addition to their roles in adherence, interactions between fimbriae and their receptors on the host cell surface can initiate signaling events, further impacting the outcome of infection (7–9). The aggregative adherence fimbriae (AAF) are defining virulence factors of EAEC, and five different variants have been identified (10–14). The genes required for the production of AAFs are encoded on the pAA virulence plasmid along with the AggR regulator that promotes their expression (15). AAFs not only contribute to bacterial adherence to the epithelium, as demonstrated in human tissue culture cells and human tissue explant models, but also alter host cell physiology, leading to increased IL-8 secretion and opening of tight junctions (11, 16–18). Despite clear roles for AAF in human cell culture and explant model systems, a role for AAF in infection of animal models is not proven (19, 20), which could be attributed to host- and tissue-specificity of fimbrial binding. Importantly, animal models do not fully recapitulate EAEC disease (20, 21), and the lack of suitable animal models emphasizes the need for human-derived model systems to fully understand the role of AAF in EAEC infection.
Colonoids and enteroids are derived from human intestinal biopsy specimens of healthy donors, can express all major intestinal epithelial cell types, and recapitulate many aspects of normal intestinal physiology, including expression of multiple mucins (22). EAEC adherence has been demonstrated in multiple human intestinal segments (23, 24), but the site of colonization during infection has not been definitively determined. Recovery of EAEC from the duodenum of human volunteers infected with EAEC has been reported (25), but the presence of EAEC colonization at other sites was not investigated. Using pediatric intestinal samples from the small and large intestine, EAEC adhered to specimens derived from all sites, although the highest levels of adherence and cytotoxic effects were detected in colon samples (23). In this study, we explore the interaction between EAEC and human gastrointestinal mucosa using human colonoids and enteroids, derived from colon and duodenum biopsy specimens, respectively. We report that the presence of AAF/II determines the abundance and location of EAEC adherence and that these fimbriae are required for barrier disruption in human colonoids.
RESULTS
Prewashing colonoid monolayers increases EAEC adherence.
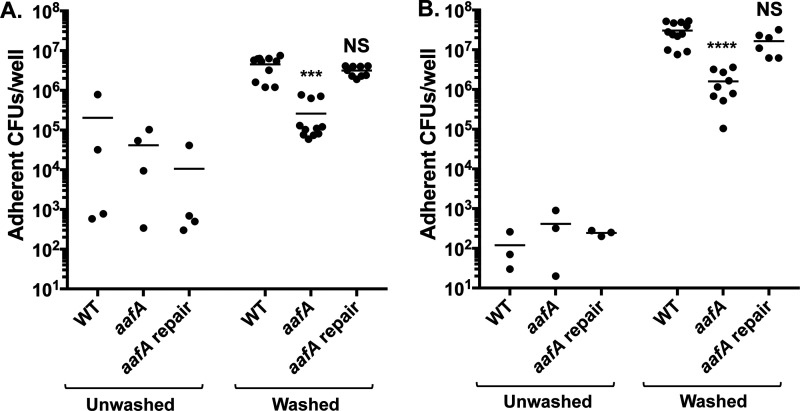
We have shown previously that AAFs mediate adherence to human intestine and T84 monolayers (11, 16, 17). A previous study found that AAF/II determines donor- and segment-specific patterns of adherence in WT EAEC strain 042 but does not affect the abundance of EAEC adherence (24). We were interested in further exploring the role of AAF/II in adherence to human colonoids to understand the discrepancies between these model systems. In order to retain the loose MUC2 layer present after differentiating human colonoids, we did not remove the media or wash colonoid monolayers before infection with EAEC strains in our first experiments. We infected the apical surface of human colonoid monolayers seeded on Transwell supports with EAEC strains for 3 or 6 h. The monolayers were then washed to remove nonadherent bacteria, and the colonic cells were lysed; adherent bacteria were quantified by enumerating CFU. After 3 h of incubation, the number of adherent bacteria was extremely variable and no difference between bacterial strains was observed (Fig. 1A). Even after a long incubation of 6 h, we detected very little adherence to unwashed colonoids and no effect of deletion of the AAF major subunit, aafA, on the amount of adherence (Fig. 1B). This low level of EAEC adherence to colonoids is consistent with previous published work (24).
FIG 1.
Prewashing cells before EAEC infection increases adherence. Adherence to unwashed colonoids or washed colonoids after 3 h (A) or 6 h (B) of incubation. One-way ANOVA was performed on log-transformed data followed by Bonferroni’s test for multiple comparisons: ***, P ≤ 0.001; ****, P ≤ 0.0001 compared to WT infection after washing. ****, P ≤ 0.0001 for comparisons of each bacterial strain in unwashed versus washed colonoids at each time point, except for the aafA strain at 3 h (not significant). No statistically significant differences were observed between strains using unwashed colonoid monolayers.
The MUC2 layer is absent in other human tissue culture lines that we have previously used to probe adherence (11, 17), and we hypothesized that perhaps its presence may contribute to the low levels of adherence detected on the colonoids. To understand if the thickness of the MUC2 layer restricted bacterial adherence, we washed the colonoid monolayers before EAEC infection. The washing procedure reduced the height of the MUC2 layer but did not remove all MUC2; the mucus layer was measured to be 34.44 ± 1.334 μm in unwashed colonoids versus 29.72 ± 1.837 μm (P = 0.0227) (Fig. S1 in the supplemental material). The presence of a highly soluble mucus layer and a firmer, adherent mucus layer is well-documented in the natural human intestine (26). Notably, adherence of EAEC wild-type (WT) strain 042 to washed colonoids after 3 or 6 h of incubation was significantly increased compared to adherence to unwashed colonoids (Fig. 1A and B). To determine whether medium toxicity also contributed to the low level of EAEC adherence to unwashed colonoids, we collected culture medium from naive, differentiated colonoids derived from two different donors. We cultured EAEC in these preconditioned media samples as well as in fresh medium and quantified bacterial growth at 37°C. We found that this preconditioned medium was toxic for the bacterium, as demonstrated by reduced bacterial number after incubation, in comparison to normal growth in fresh cell culture medium (Fig. S2). Thus, we hypothesize that both the presence of a thicker MUC2 layer and toxicity of the preconditioned media may have contributed to the very low levels of adherence to colonoids and high stochasticity detected in our preliminary studies (Fig. 1), as well as in previous reports (24).
AAF/II is required for abundant adherence to human colonoids.
With the refinements to our experimental system, we decided to reinvestigate the role of AAF/II in EAEC adherence to human colonoids. We infected washed monolayers of human colonoids seeded on Transwell supports with WT EAEC, an aafA mutant, and an aafA mutant strain where aafA has been restored to the pAA2 plasmid. After both 3 and 6 h of incubation, we observed that adherence of the aafA mutant was significantly reduced compared to WT EAEC, and adherence was restored in a strain where the aafA mutation was repaired (Fig. 1A and B). Thus, prewashing the monolayers revealed a role for AAF/II in EAEC adherence to human colonoids. Because we detected abundant aafA-mediated adherence by 3 h, we chose to perform all further experiments investigating adherence at the 3-h time point, as the role of AAF/II has been established in other tissue culture systems at this time point (27).
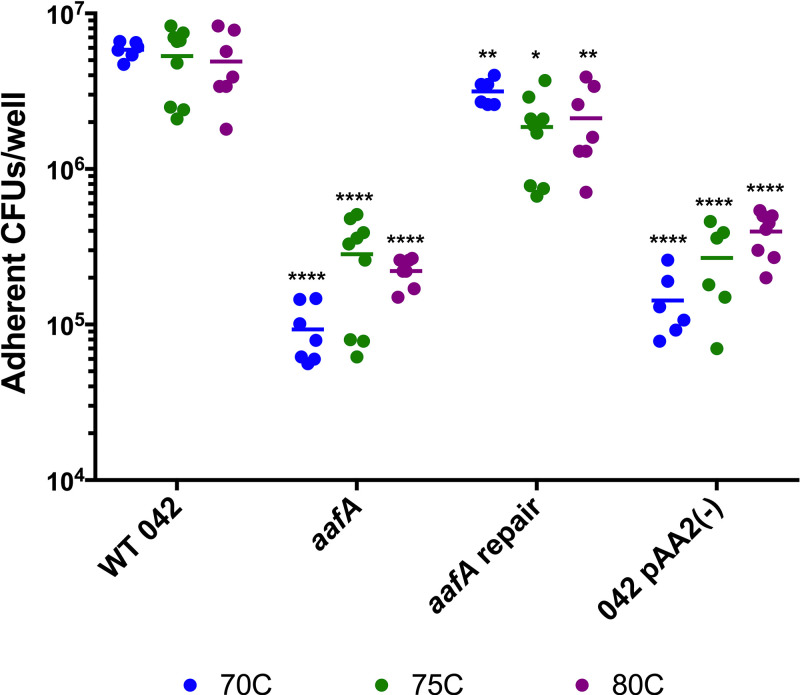
In addition to quantifying adherence to colonoid monolayers seeded on Transwell supports, we also assessed adherence to colonoid monolayers seeded into collagen-coated wells of 96-well plates, as previously described (24). We used this format for high-throughput testing of colonoid lines derived from three donors (designated 70C, 75C, and 80C). We observed abundant adherence in AAF/II-producing strains (WT EAEC and the aafA-repaired strain), and the number of adherent bacteria was significantly reduced in an aafA mutant and the strain lacking the pAA2 virulence plasmid (Fig. 2). This trend was observed using colonoid lines derived from three different donors (Fig. 2), suggesting that the role for aafA is not donor-specific.
FIG 2.
Role of AAF/II in adherence to human colonoids is not donor specific. Adherence to colonoid monolayers seeded in collagen-coated wells in a 96-well plate after 3 h of incubation. Lines (70C, 75C, and 80C) derived from three different donors are shown in blue, green, and purple, respectively. One-way ANOVA was performed on log-transformed data followed by Bonferroni’s test for multiple comparisons: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, as compared to WT infection of each colonoid line.
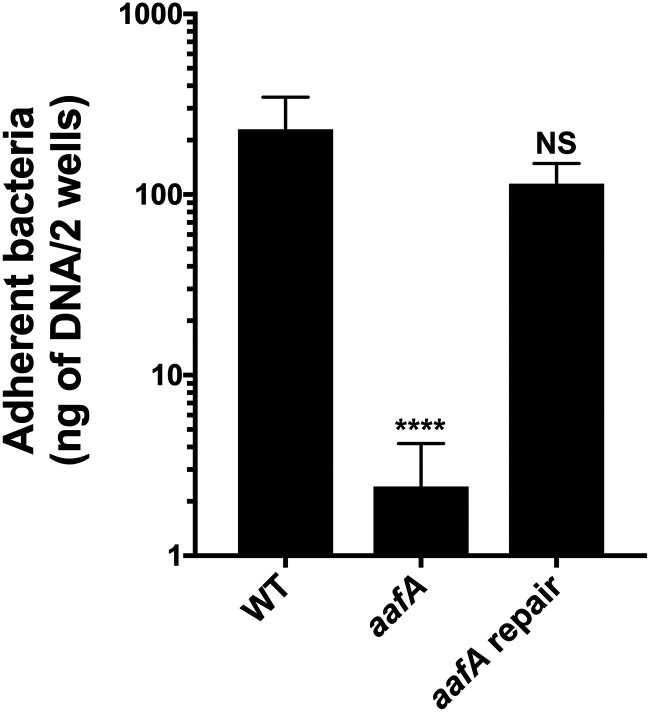
Bacterial aggregation can affect enumeration by CFU, and AAF/II-deficient strains are less aggregative than AAF/II-positive strains. To confirm that differences in bacterial aggregation did not affect our results, we also employed quantitative PCR (qPCR) to quantify adherent bacteria using the chromosomal gene aaiC after 3 h of infection (Fig. 3). Using this method, we again observed that AAF/II was required for abundant adherence to human colonoids.
FIG 3.
qPCR quantification of EAEC adherence to human colonoids. qPCR for aaiC for quantification of adherent bacteria after 3 h of incubation with colonoids from the 75C line. Six replicates were tested in two independent experiments. One-way ANOVA was performed on log-transformed data followed by Bonferroni’s test for multiple comparisons: ****, P ≤ 0.0001, compared to WT.
AAF/II also promotes adherence to duodenal enteroids.
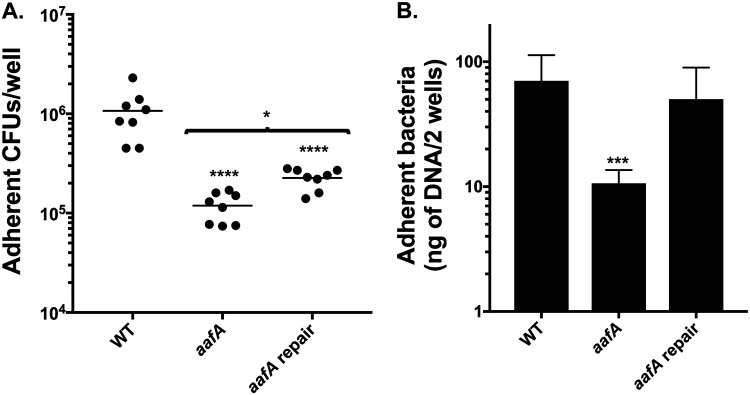
Previous work has suggested that EAEC adherence is greater to enteroids derived from the duodenal segment than to the colon segment (24). To understand the contribution of AAF/II to adherence to the human duodenum, we utilized a line of enteroids derived from the human duodenum. We found that adherence of the aafA mutant was lower than that of the parental strain (Fig. 4A). These results were confirmed by qPCR (Fig. 4B). Restoration of adherence of the aafA-repaired strain to wild-type levels could only be determined by quantitation by qPCR and not by enumeration by CFU. These data suggest that the ability to aggregate is increased in the repaired strain in comparison to the wild-type strain, and this effect may be more pronounced during adherence to duodenal cells in comparison to colonic cells.
FIG 4.
AAF/II mediates adherence to duodenal enteroids. Adherence to duodenal enteroid line DP30 after a 3 h of incubation. (A) Adherence to enteroid monolayers seeded into a collagen-coated wells in a 96-well plate after 3 h of incubation. (B) qPCR for aaiC for quantification of adherent bacteria after 3 h of incubation with colonoids from the 75C line. Six replicates were tested in two independent experiments. One-way ANOVA was performed on log-transformed data followed by Bonferroni’s test for multiple comparisons: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, compared to WT.
Strains expressing aafA remain adherent when the MUC2 layer is lost during fixation.
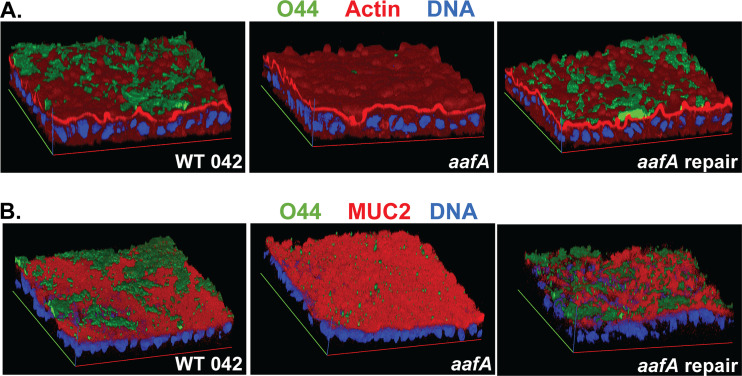
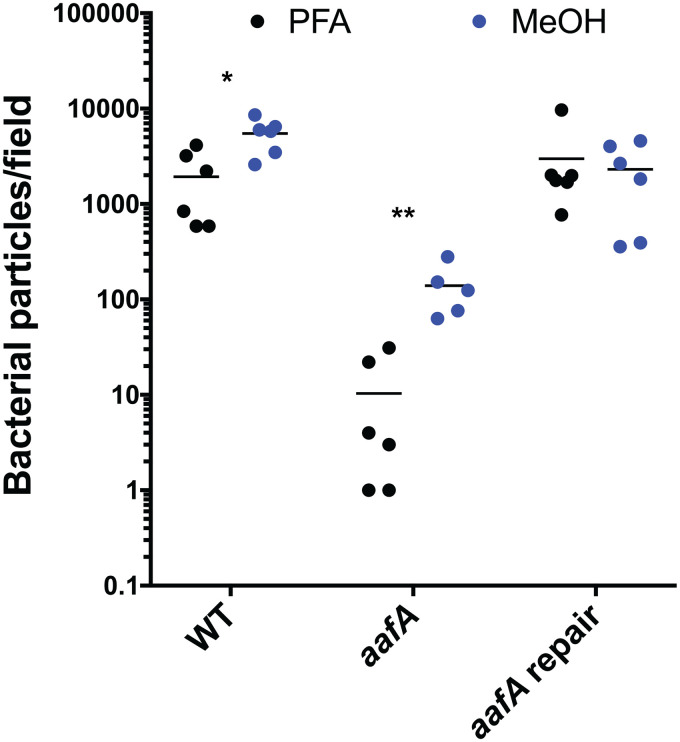
In addition to effects on the amount of adherence, we hypothesized that the location of adherence would also be different in EAEC strains expressing or lacking aafA. After bacterial binding had occurred, we utilized two different fixation methods that either preserve (methanol:acetic acid [9:1]) or dissolve (paraformaldehyde) the MUC2 layer to identify bacterial cells adhering to the epithelial surface versus within the MUC2 layer. MUC2 staining was not detected in samples fixed with paraformaldehyde (Fig. S3), which is consistent with previous reports (28, 29). Regardless of the fixative used, AAF/II-producing strains showed abundant adherence (Fig. 5). However, adherence of the aafA mutant was deficient when the MUC2 layer was retained with methanol:acetic acid fixation (primarily as single cells), and adherence was absent or even more reduced when the MUC2 layer was lost after fixation with paraformaldehyde. Quantification of the number of bacteria per field after paraformaldehyde fixation compared to methanol:acetic acid fixation was significantly different for the aafA mutant strain (Fig. 6). These data suggest that AAF/II-mediated adherence is independent of MUC2 and likely involves a receptor on the apical surface of the monolayer.
FIG 5.
EAEC strains expressing AAF/II adhere independently of MUC2. EAEC strains were added to prewashed 75C colonoids seeded on Transwells and incubated for 3 h. Samples were fixed with either 4% paraformaldehyde (stained for actin) (A) or methanol:acetic acid (stained for MUC2) (B) as indicated. EAEC is shown in green. Representative images are shown. Data are quantified in Fig. 6.
FIG 6.
Quantification of EAEC adherence with different fixative methods that affect retention of MUC2. The relative number of bacteria in every image was measured with ImageJ using the particle enumeration algorithm. The numbers of particles (representing a bacterial cell) per image were summed to generate the total number of bacteria per field. The effect of the two different fixative treatments on each strain was evaluated using an unpaired t test on log-transformed data: *, P ≤ 0.05; **, P ≤ 0.01.
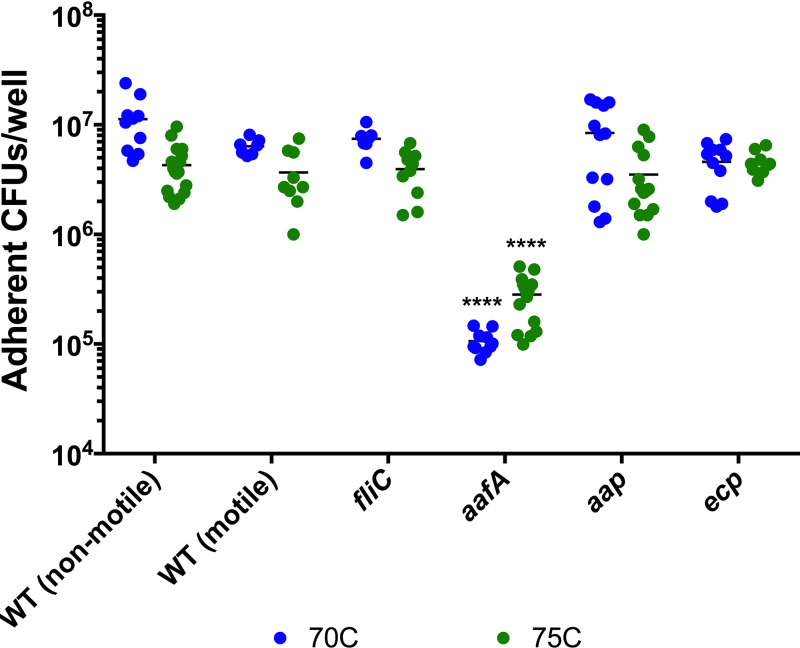
Investigation of other adhesins emphasizes the role of aafA as a key factor in EAEC adherence.
Although AAFs are a defining feature of EAEC strains, other adhesins have been described, and the interplay between these factors is not yet understood. We investigated whether other EAEC adhesins contribute to adherence to human colonoids. Dispersin, encoded by aap, is a hypothetical coat protein (30), and its role in EAEC pathogenesis is unclear. Deletion of aap causes a hyperaggregative and hyperadherent phenotype (30), prompting us to investigate whether deletion of aap would affect adherence to human colonoids. We did not observe a significant difference in adherence to either colonoid line between the aap mutant strain and WT EAEC (Fig. 7).
FIG 7.
Role of EAEC adhesins in adherence to human colonoids. Adherence to colonoid monolayers seeded in collagen-coated wells in a 96-well plate after 3 h of incubation. Lines 70C (blue) and 75C (green) were derived from two different donors. One-way ANOVA was performed on log-transformed data followed by Bonferroni’s test for multiple comparisons: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, compared to nonmotile WT infection of each colonoid line.
In addition to a role in bacterial motility, the flagellum also has a documented role in adherence in E. coli pathovars (summarized in reference 31). Motility is variable in EAEC isolates and within strains used in this study, introducing an additional variable to our experiments. We investigated whether expression of flagella or motility altered adhesion within our model system. We utilized motile and nonmotile variants of WT EAEC strain 042 as well as a strain lacking fliC (32), the major subunit that is polymerized to form the flagellar structure. We observed equivalent adherence among all three of these strains (Fig. 7), suggesting that the production of flagella and motility are not required for, nor do they contribute to, adherence to human colonoids.
The E. coli common pilus (ECP) is expressed by the majority of EAEC strains in the presence of epithelial cells, but its role in adherence in wild-type EAEC strains is unclear (33). We tested whether the deletion of ecpA, the major subunit of ECP, affected EAEC adherence to colonoids. The ecpA mutant strain adhered at levels comparable to WT EAEC (Fig. 7), indicating that ECP is not required for adherence to human colonoids.
In summary, neither flagella, dispersin, nor ECP contributed to adherence to the two human colonoid lines (70C and 75C) tested, highlighting the importance of AAF/II in the interaction of EAEC with the human gut mucosa.
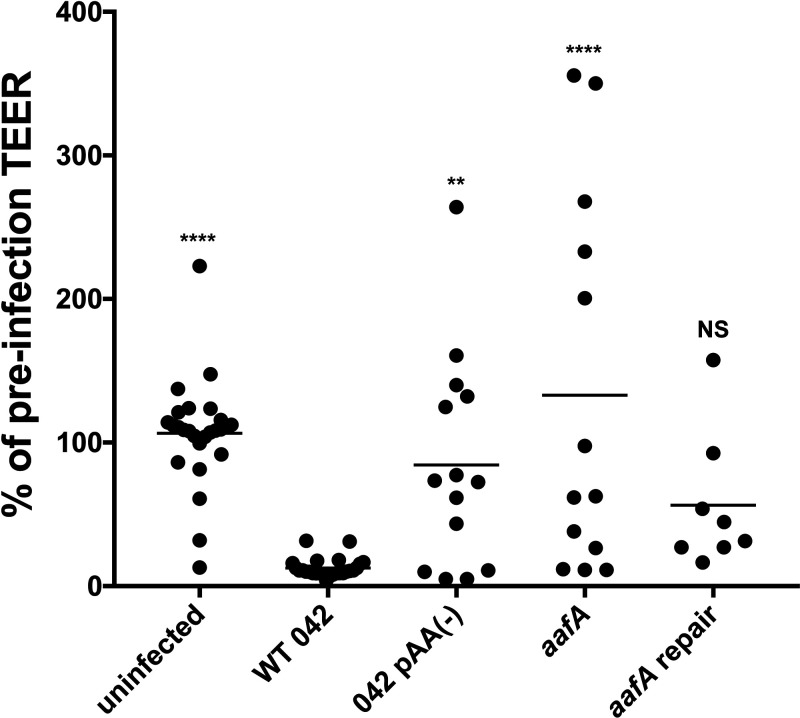
Effect of WT 042 and aafA on TEER.
We have previously shown that infection of T84 cells by WT EAEC induced barrier disruption, characterized by a sustained decrease in transepithelial electrical resistance (TEER), and that this effect required AAF/II (18). Because colonoids provide a more complete representation of the human colon than T84 cells, we investigated whether WT EAEC and AAF/II would alter barrier function in the colonoid model system. We employed the published protocol describing barrier disruption of T84 monolayers (18). Colonoid monolayers seeded on Transwell supports were infected with EAEC strains for 6 h. After infection, the monolayers were washed and fresh medium containing antibiotics was added for an additional 18 h to allow the cells to recover. Notably, TEER was significantly reduced after infection with WT EAEC compared to monolayers that were uninfected or infected with the commensal E. coli strain HS (Fig. 8). Additionally, AAF/II-lacking strains (an aafA mutant and a strain lacking the pAA2 virulence plasmid) were associated with less TEER disruption than WT EAEC (Fig. 8). The aafA-repaired strain did not show significant differences compared to WT EAEC (Fig. 8). These data are the first evidence supporting a role for AAF/II-mediated binding in alteration of barrier function in the human colonoid model system.
FIG 8.
Infection with AAF/II-positive EAEC strains decreases TEER. EAEC strains were added to prewashed colonoid monolayers (line 70C) seeded on Transwell supports and incubated for 6 h. Monolayers were then washed with PBS and medium containing antibiotics was added to both apical and basolateral chambers. Cells recovered for 18 h and were then assessed for TEER. A Kruskal-Wallis test was performed followed by a Dunn’s test for multiple comparisons: *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001, compared to WT strain 042.
DISCUSSION
Previous work using animal models of infection has failed to identify a role for AAF/II in colonization or disease (19, 20). This contrasts with in vitro experiments utilizing human cell lines, where AAF/II is critical for adherence (11, 17). These data suggest that the receptor for AAF/II is host specific, emphasizing the need for human-derived models to study the mechanisms of EAEC adherence in the human gut. In this study, we utilized human intestinal organoids derived from the duodenum or colon, also referred to as enteroids and colonoids, respectively. Human enteroids and colonoids have been shown to recapitulate pathogenesis of other enteric pathogens (34–37), which highlights the usefulness of this model system in studying the interactions between pathogens and the human intestinal epithelium. Also, enteroid and colonoid cell lines express secreted and transmembrane mucins (22, 34, 38), which are thought to play a significant role in EAEC colonization and disease.
A previous study has investigated EAEC adherence to human enteroids and colonoids (24). These authors reported differences in the level of EAEC adherence based on the segment or donor that the line was derived from. They found that the presence of AAF/II promoted aggregative adherence as opposed to diffuse adherence in the absence of AAF/II, but AAF/II did not affect the overall level of adherence (24). In our initial experiments, we also did not find a role for AAF/II in the level of EAEC adherence to human colonoids (Fig. 1) after either 3 or 6 h exposure. These results were inconsistent with previous studies that suggested a critical role for AAF/II in adherence to human tissue culture cells and human tissue explants (11, 16, 17). Major differences between human colonoids and human intestinal cell lines are the heterogeneity of cell types after differentiation, presence of transmembrane mucins and a soluble mucin layer, and lack of genetic and phenotypic changes required for immortalization (22, 34, 38). In our experiments, we also noted a lack of bacterial proliferation during the incubation with human colonoids. Incubation of EAEC in the colonoid differentiation medium (in the absence of cells) promoted growth (Fig. S2), suggesting that the medium itself was not inhibiting bacterial proliferation. However, incubation of EAEC in colonoid differentiation medium that had previously been incubated with colonoid cells resulted in reduced recovery of viable EAEC. This reduced recovery of viable CFU suggested that the depleted medium used in our initial adherence experiments contributed to the low recovery of viable adherent bacteria, either due to toxicity or nutrient depletion of the medium. When cells were prewashed and fresh medium replaced, EAEC adherence increased, stochasticity decreased, and a role for AAF/II emerged. We also noted that incorporation of prewashing did not remove the MUC2 layer but did reduce its thickness (Fig. S1). The human gastrointestinal mucosal surface is subject to the constant flow of intestinal contents, and it is possible that incorporation of this wash step better models normal turnover of the mucus layer.
Because of the inconsistencies between our study and a previously published report (24), we employed multiple methods of assessing the level of adherence to increase confidence that our observations were accurate. We enumerated the adherent bacteria by counting CFU after infection of monolayers seeded on Transwell supports (Fig. 1) as well as monolayers seeded in 96-well plates (Fig. 2). Use of 96-well plates allowed for higher throughput analyses that included multiple lines derived from different donors, as well as the addition of a plasmid-less strain (pAA2−) that lacks AAF/II. Because aggregation can affect enumeration by CFU, we also quantified adherent bacteria by qPCR (Fig. 3). Lastly, we also visually assessed the level of adherence by immunofluorescence and confocal microscopy (Fig. 5) and quantified the number of adherent bacteria in these images with a particle-counting algorithm (Fig. 6). By utilizing multiple methods, we provide strong support for a critical role for AAF/II in adherence to human colonoids.
The requirement for AAF/II for abundant adherence was not donor- or segment-specific. AAF/II promoted adherence in three different colonoid lines derived from different donors (Fig. 2). Previous reports have suggested that EAEC may colonize the duodenum (25) in addition to the colon. We also observed AAF/II-dependent adherence using an enteroid line derived from the duodenum (Fig. 4). Although these experiments do not indicate whether one site is preferred over the other for human colonization, they do suggest that AAF/II plays a critical role at either site.
In addition to effects on the amount of adherence, we were also interested in determining whether AAF/II affects the location of adherence. During infection, interaction with the secreted mucins and movement through the mucin layer is critical for pathogens to colonize the gastrointestinal epithelium. Colonoids produce a mucus layer that coats the surface of the monolayer, primarily composed of the secreted mucin MUC2. This important component of the mucosal surface is absent in many tissue culture systems that have previously been used to evaluate the role of AAF/II in adherence. To determine the location of AAF/II-positive and a derivative AAF/II-negative strain within the MUC2 layer, we utilized two different fixation protocols that either lose or retain MUC2. Aqueous fixations, like paraformaldehyde, dehydrate the mucus and result in loss of the mucus layer (28, 29). Water-free fixatives, like methanol:acetic acid (9:1) better retain the mucus layer (28, 29). We hypothesized that bacterial cells that are adhering only to the MUC2 layer would be visualized after methanol:acetic acid fixation but absent after paraformaldehyde fixation, allowing us to determine whether aafA-positive and aafA-negative strains associate differently with the epithelial surface and MUC2 layer. We observed abundant adherence with AAF/II-positive strains (wild-type 042 and the aafA-repaired strains) regardless of which fixative was used (Fig. 5 and 6), suggesting that the increased AAF/II-dependent adherence observed was independent of the MUC2 layer, but instead likely associated with one or more receptors present at the apical surface of the monolayer. Fewer aafA-negative bacteria were visualized, consistent with data shown in Fig. 1 and 3, and significantly more bacteria were present after methanol:acetic acid fixation versus paraformaldehyde fixation (Fig. 5 and 6), suggesting that aafA-negative stains may adhere within the mucus layer through an alternate adhesin.
EAEC encodes multiple putative adhesins, and their role in EAEC infection is not understood. We tested the contribution of multiple EAEC factors to adherence to human colonoids. Strains containing single mutations in other potential adhesins, including fliC, aap, and ecpA, displayed wild-type levels of adherence (Fig. 7). These data are consistent with previous reports using other tissue culture model systems, showing that ECP does not promote adherence in AAF/II-positive strains (33, 39). Deletion of the dispersin-encoding gene has previously been reported to increase adherence to HEp-2 cells and colonic biopsy specimens but to decrease movement through mucus (30). Since the colonoid model system includes a mucus layer, reduced movement through mucus may counteract the hyperaggregation and hyperadherence phenotypes reported using model systems that lack this mucin layer. It is well established that motility may influence bacterial colonization of host tissues. We found that motile and nonmotile 042 strains adhered equivalently, suggesting that expression of flagella and motility do not affect adherence in the colonoid model system tested here. The flagella and motility could play a more significant role in adherence to host surfaces with thicker mucus layers. It is possible that these factors mediate adherence in the absence of AAF/II or affect other aspects of EAEC infection, but our data suggest that AafA is the dominant adhesin when present. In WT EAEC, all the adhesins are present together (although their expression may be induced at different times during the pathogenetic sequence), and therefore the dominant role of AAF/II is germane. Further study in different model systems, or even slight modifications to this model system, might reveal roles for the other adhesins in EAEC adherence.
We have previously shown that EAEC infection of polarized T84 monolayers results in AAF/II-dependent disruption of tight junctions, characterized by a decrease in TEER and aberrant expression of tight junction proteins (18). We investigated whether EAEC infection affected barrier function by measuring TEER before and after EAEC treatment. We observed that infection of colonoids with EAEC but not with the nonpathogenic E. coli HS strain resulted in a decrease in TEER, which was AAF/II-dependent (Fig. 8). These data replicate the previous observations in the more complex human colonoid model system, providing evidence that AAF binding to the mucosal surface is required for effects on barrier function either through promoting adherence and/or directly modulating host processes. In addition to their roles in adherence, interactions between fimbriae and their ligands on the host cell surface can initiate signaling events, further impacting the outcome of infection. We speculate that AAF/II binding to target receptor(s) on human colonoids both promotes bacterial adherence and alters barrier function, either directly by affecting host cell signaling or indirectly by promoting delivery of other bacterial factors through close contact. Current work in our laboratory is focused on distinguishing between these two possibilities, as well as identifying receptors for AAF/II. A candidate receptor for AAF/II is MUC1 (9) and this interaction is currently being explored. Together, our results suggest a multifactorial role for AAF/II in EAEC infection, affecting the amount and location of EAEC adherence as well as disruption of barrier function in human colonoids. This work highlights the relevance of the colonoid model system in studying interactions of intestinal pathogens with the human gastrointestinal mucosa.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Bacterial strains used in this study are described in Table 1. Before addition to colonoid cultures, bacterial strains were incubated for 16 to 18 h at 37°C in Dulbecco’s modified Eagle’s medium with 0.4% glucose (Gibco) under static conditions. Cultures were then diluted to prepare an inoculum of approximately 2 × 106 CFU/10 μl.
TABLE 1.
Strains used in this study
| Strain name | Description | Reference |
|---|---|---|
| 042 | Prototype EAEC strain isolated from a child with diarrhea in Lima, Peru | 25 |
| aafA | Deletion mutant of aafA created using lambda red linear recombination method (40), also referred to as EAECaafA::km-sacB | 44 |
| aafA-repaired | Strain with aafA restored by pAA2 plasmid | This study |
| pAA(−) | 042 that has been cured of the pAA2 plasmid | 45 |
| HS | Human commensal E. coli strain | 46 |
| Motile 042 | Motile variant of 042 isolated after growth on 0.3% agar | This study |
| fliC | Aflagellar mutant that harbors the suicide plasmid pJP5603 inserted into the fliC gene | 32 |
| aap | Mutant that harbors the suicide plasmid pJP5603 inserted into the aap gene | 30 |
| ecp | Deletion mutant of ecpA created using lambda red linear recombination method (40) | 33 |
Construction of the aafA-repaired strain.
The aafA gene in the EAECaafA::km-sacB strain was reconstructed using the λ red recombination approach (40). Briefly, a linear DNA fragment encoding the aafA gene flanked by 60-bp upstream and downstream regions of the aafA locus was prepared by PCR using the pAA2 virulence plasmid as a DNA template. The DNA fragment was electroporated into the EAECaafA::km-sacB strain containing the pKD46 plasmid, which encodes the genes required for λ red recombination (40). The strain was grown in LB medium containing 5% sucrose. Sucrose-resistant electroporants were screened for sensitivity to kanamycin and by the insertion of aafA by PCR. In-frame insertion of aafA was verified by DNA sequencing.
Intestinal organoid (enteroid and colonoid) medium composition.
All cell culture media were prepared as reported previously (38). Advanced Dulbecco’s modified Eagle’s medium (DMEM)-F-12 medium supplemented with 1× GlutaMAX (Gibco), 10 mM HEPES (Sigma-Aldrich), and 100 units/ml penicillin-streptomycin (Sigma-Aldrich) was used as the basal medium. Complete medium with growth factor (CMGF+) is basal medium supplemented with 50% (vol/vol) Wnt3a-conditioned medium, 20% (vol/vol) R-spondin-1-conditioned medium, 10% (vol/vol) Noggin-conditioned medium, 1× B27 supplement (Gibco), 1 mM N-acetylcysteine (Sigma), 1× Primocin (InvivoGen), 50 ng/ml human epidermal growth factor (R&D Systems), 10 nM [Leu-15]-gastrin (AnaSpec), 500 nM A83-01 (Tocris), and 10 μM SB202190 (Tocris). Differentiation medium is comprised of basal medium (no penicillin-streptomycin added), 10% (vol/vol) Noggin-conditioned medium, 1 mM N-acetylcysteine (Sigma), 50 ng/ml human epidermal growth factor (R&D Systems), 10 nM [Leu-15]-gastrin (AnaSpec), 500 nM A83-01 (Tocris), and 10% fetal bovine serum (Sigma-Aldrich).
Human intestinal organoid cultures.
Human enteroid and colonoid cultures were established from deidentified biopsy specimens from healthy subjects obtained after endoscopic or surgical procedures using previously described methods (41). Subjects provided informed consent at Johns Hopkins University and all methods were carried out in accordance with approved guidelines and regulations. All experimental protocols were approved by the Johns Hopkins University Institutional Review Board (IRB) (protocol NA_00038329).
Organoids were cultured as 3D cysts embedded in Matrigel (Corning) and passaged approximately every 7 to 10 days. 3D organoids were harvested by gentle scraping in TrypLE express (Gibco), incubated at 37°C for 4 min, triturated 25 to 30 times, washed using an equal volume of basal medium, and collected by centrifugation at 500 × g for 5 min. For passaging, the pellet was resuspended in Matrigel and seeded such that each well contained at least 50 organoids. The plate was incubated at 37°C for 10 min to allow the Matrigel to polymerize. A total of 0.5 ml of CMGF+ containing 10 μM each Y-27632 (Tocris) and CHIR99021 (Tocris) was added to each well. The medium was replaced with CMGF+ without Y-27632 and CHIR99021 after 48 to 72 h. Fresh CMGF+ was added to the wells every other day.
To form monolayers, the triturated organoids were resuspended in CMGF+ containing Y-27632 and CHIR99021, as described previously (34, 35). Transparent polyester membrane 24-well cell culture inserts with 0.4-μm pore size (Transwell supports; Corning) or polystyrene 96-well plates (Corning) were precoated with 100 μl of a 34-μg/ml human collagen IV solution (Sigma) and incubated at 4°C overnight. An aliquot of 100 μl of resuspended organoid fragments was added to each well, and 600 μl of CMGF+ with Y-27632 and CHIR99021 was added to the receiver well for Transwell supports. Cultures were incubated at 37°C with 5% CO2. Typically, monolayer confluence was achieved in 7 to 14 days. Monolayer confluence was assessed visually and by increased transepithelial electrical resistance (TEER) measured using an epithelial volt/ohm meter (EVOM2; World Precision Instruments). Confluent monolayers were differentiated by incubation with Wnt3A-free and R-spondin-1-free medium (differentiation medium) for 3 to 5 days.
Adherence assays.
Prior to infection, monolayers prepared on Transwell supports were washed three times (unless otherwise indicated) with sterile phosphate-buffered saline (PBS), fresh differentiation medium was replaced, and cells rested for 1 h before infection. For monolayers seeded in 96-well plates, the medium was changed but the monolayers were not washed. Bacterial strains were added as indicated and incubated 3 or 6 h. The monolayers were then washed three times with PBS, lysed with 1% Triton X-100/PBS, and adherent bacteria were enumerated by dilution and plating on Luria agar.
Quantitative PCR.
Monolayers were infected as described for the adherence assay in 96-well plates. After 3 h of incubation, monolayers were washed 3 times with PBS, and resuspended in ATL buffer from the QIAamp DNA minikit (Qiagen). Two wells were pooled per sample. Genomic DNA was extracted following the manufacturer’s instructions.
The aaiC primers were previously validated and published (aaiC Fd 5′-CATTGTCCTCAGGCATTTCA-3′ and aaiC Rv 5′-TGCATACGACACCCCTGATA-3′) (42). Reaction mixtures were prepared containing 1× SYBR green master mix (Applied Biosystems) and 50 nM of each primer. Quantitative PCR (qPCR) was performed using an ABI 7500-FAST sequence detection system (Applied Biosystems). A standard curve with known concentrations of purified EAEC strain 042 genomic DNA was used to determine the concentration of EAEC DNA per sample.
Confocal microscopy.
Human colonoid monolayers were washed three times with PBS, fixed with 90% (vol/vol) methanol/10% (vol/vol) glacial acetic acid or 4% (vol/vol) paraformaldehyde (Electron Microscopy Sciences), permeabilized with 0.1% saponin, and blocked with 2% bovine serum albumin/fetal bovine serum for 30 min (all Sigma-Aldrich, USA). Cells were rinsed with PBS and incubated overnight at 4°C with primary antibodies diluted 1:100 in PBS. Primary antibodies included rabbit anti-Muc2 conjugated to Alexa-fluor 647 (Santa Cruz Biotechnology, USA), and rabbit sera anti-044 (Denka Seiken Co., Ltd., Tokyo, Japan) to detect EAEC. Stained cells were then washed 3 times for 5 min each with PBS followed by incubation with an Alexa-fluor 488-congugated goat anti-rabbit antibody (Molecular Probes/Invitrogen, USA) diluted 1:100 in PBS or an Alexa-fluor 594-conjugated phalloidin for actin staining diluted 1:40 in PBS (Thermo Fisher Scientific). Hoechst (Vector Laboratories, USA) was used at a 1:1,000 dilution in PBS for DNA labeling. After incubation, cells were washed 3 times for 5 min each and mounted in ProLong Diamond (Life Technologies). Samples were imaged at the Imaging Core Facility at the University of Virginia using an LSM-710 Multiphoton laser-scanning confocal microscope (Zeiss, Germany) running ZEN 2012 imaging software (Zeiss, Germany) using a 64× oil objective.
Z-stack images of 2-μm intervals were converted to single channel images showing only bacterial cells (green channel only). The relative number of bacteria in every image was measured with ImageJ using the particle enumeration algorithm as follows. Images were opened as 16-bit type images with ImageJ (43). Threshold values were adjusted to eliminate the background. For particle enumeration, images were processed as Binary>Watershed images. This algorithm separates particles that are close together (e.g., aggregated bacteria). Lastly, images were analyzed as particles set as: size (pixel̂2) = 20-infinite, which is close to the size of E. coli in 64× confocal images. Particle (bacteria) counts in each image were summed to enumerate the total number of bacterial cells per field.
TEER measurements.
Before infection, monolayers on Transwell supports were washed three times (unless otherwise indicated) with sterile PBS, fresh differentiation medium was replaced, and cells recovered for 1 h. Monolayers were incubated with bacterial strains for 6 h, and then washed three times with PBS. Fresh medium containing 1× primocin was replaced and the cells incubated for 18 h before measurement of TEER with an epithelial volt/ohm meter (EVOM2; World Precision Instruments). This protocol was adapted from that used in previous studies with T84 monolayers (18).
Statistics.
Statistical analyses were performed using GraphPad Prism version 7 for Mac OS X, (GraphPad Software, La Jolla, California). For data generated from adherence assays, data were log transformed and then analyzed by one-way ANOVA followed by Bonferroni’s test for multiple comparisons. For quantification of bacterial number per field by particle counting, the effect of the two different fixative treatments was evaluated using an unpaired t test on log-transformed data. The effect of EAEC treatment on TEER was assessed by a Kruskal-Wallis test followed by a Dunn’s test for multiple comparisons.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (P01-AI125181) to J.P.N. and by the Hartwell Foundation through a postdoctoral research fellowship to L.A.G.
We acknowledge the Integrated Physiology Core of the Hopkins Conte Digestive Disease Basic and Translational Research Core Center (NIH P30 DK-089502).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Victora CG, Adair L, Fall C, Hallal PC, Martorell R, Richter L, Sachdev HS, Maternal and Child Undernutrition Study Group. 2008. Maternal and child undernutrition: consequences for adult health and human capital. Lancet 371:340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima A. 2013. The impoverished gut—a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol 10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lima AAM, Soares AM, Filho JQS, Havt A, Lima IFN, Lima NL, Abreu CB, Junior FS, Mota RMS, Pan W-Y, Troeger C, Medeiros P, Vera HN, Prata MMG, McCormick B, McGrath M, Rogawski E, Houpt E, Platts-Mills J, Gratz J, Samie A, Bessong P, Babji S, Kang G, Shahida Q, Shakoor S, Bhutta Z, Haque R, Ahmed T, Mduma E, Svensen E, Kosek M, Penataro Yori P, Bodhidatta L, Jasmin S, Mason C, Lang D, Gottlieb M, Guerrant RL. 2017. Enteroaggregative E. coli subclinical infection and co-infections and impaired child growth in the MAL-ED cohort study. J Pediatr Gastroenterol Nutr 66:325–333. doi: 10.1097/MPG.0000000000001717. [DOI] [PubMed] [Google Scholar]
- 4.Rogawski ET, Guerrant RL, Havt A, Lima IFN, Medeiros P, Seidman JC, McCormick BJJ, Babji S, Hariraju D, Bodhidatta L, Shrestha J, Anania J, Maro A, Samie A, Yori PP, Qureshi S, Mahfuz M, Bessong PO, Kosek MN, Ahmed T, Bhutta ZA, Lang DR, Gottlieb M, Houpt ER, Lima A, the MAL-ED Network Investigators. 2017. Epidemiology of enteroaggregative Escherichia coli infections and associated outcomes in the MAL-ED birth cohort. PLoS Negl Trop Dis 11:e0005798. doi: 10.1371/journal.pntd.0005798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, Khan SS, Praharaj I, Murei A, Nshama R, Mujaga B, Havt A, Maciel IA, Operario DJ, Taniuchi M, Gratz J, Stroup SE, Roberts JH, Kalam A, Aziz F, Qureshi S, Islam MO, Sakpaisal P, Silapong S, Yori PP, Rajendiran R, Benny B, McGrath M, Seidman JC, Lang D, Gottlieb M, Guerrant RL, Lima AAM, Leite JP, Samie A, Bessong PO, Page N, Bodhidatta L, Mason C, Shrestha S, Kiwelu I, Mduma ER, Iqbal NT, Bhutta ZA, Ahmed T, Haque R, Kang G, Kosek MN, Houpt ER, MAL-ED Network Investigators. 2018. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health 6:e1319–e1328. doi: 10.1016/S2214-109X(18)30351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beachey EH. 1981. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis 143:325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- 7.Ambite I, Butler DSC, Stork C, Grönberg-Hernández J, Köves B, Zdziarski J, Pinkner J, Hultgren SJ, Dobrindt U, Wullt B, Svanborg C. 2019. Fimbriae reprogram host gene expression—divergent effects of P and type 1 fimbriae. PLoS Pathog 15:e1007671. doi: 10.1371/journal.ppat.1007671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedlund M, Duan RD, Nilsson A, Svensson M, Karpman D, Svanborg C. 2001. Fimbriae, transmembrane signaling, and cell activation. J Infect Dis 183 Suppl 1:S47–50. doi: 10.1086/318851. [DOI] [PubMed] [Google Scholar]
- 9.Boll EJ, Ayala-Lujan J, Szabady RL, Louissaint C, Smith RZ, Krogfelt KA, Nataro JP, Ruiz-Perez F, McCormick BA. 2017. Enteroaggregative Escherichia coli adherence fimbriae drive inflammatory cell recruitment via interactions with epithelial MUC1. mBio 8:e00717-17. doi: 10.1128/mBio.00717-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nataro JP, Yikang D, Giron JA, Savarino SJ, Kothary MH, Hall R. 1993. Aggregative adherence fimbria I expression in enteroaggregative Escherichia coli requires two unlinked plasmid regions. Infect Immun 61:1126–1131. doi: 10.1128/IAI.61.3.1126-1131.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czeczulin JR, Balepur S, Hicks S, Phillips A, Hall R, Kothary MH, Navarro-Garcia F, Nataro JP. 1997. Aggregative adherence fimbria II, a second fimbrial antigen mediating aggregative adherence in enteroaggregative Escherichia coli. Infect Immun 65:4135–4145. doi: 10.1128/IAI.65.10.4135-4145.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernier C, Gounon P, Le Bouguénec C. 2002. Identification of an aggregative adhesion fimbria (AAF) type III-encoding operon in enteroaggregative Escherichia coli as a sensitive probe for detecting the AAF-encoding operon family. Infect Immun 70:4302–4311. doi: 10.1128/iai.70.8.4302-4311.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boisen N, Struve C, Scheutz F, Krogfelt KA, Nataro JP. 2008. New adhesin of enteroaggregative Escherichia coli related to the Afa/Dr/AAF family. Infect Immun 76:3281–3292. doi: 10.1128/IAI.01646-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jønsson R, Struve C, Boisen N, Mateiu RV, Santiago AE, Jenssen H, Nataro JP, Krogfelt KA. 2015. Novel aggregative adherence fimbria variant of enteroaggregative Escherichia coli. Infect Immun 83:1396–1405. doi: 10.1128/IAI.02820-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nataro JP. 2005. Enteroaggregative Escherichia coli pathogenesis. Curr Opin Gastroenterol 21:4–8. [PubMed] [Google Scholar]
- 16.Boll EJ, Struve C, Sander A, Zachary D, Nataro JP, McCormick BA, Krogfelt KA. 2012. The fimbriae of enteroaggregative Escherichia coli induce epithelial inflammation in vitro and in a human intestinal xenograft model. J Infect Dis 206:714–722. doi: 10.1093/infdis/jis417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harrington SM, Strauman MC, Abe CM, Nataro JP. 2005. Aggregative adherence fimbriae contribute to the inflammatory response of epithelial cells infected with enteroaggregative Escherichia coli. Cell Microbiol 7:1565–1578. doi: 10.1111/j.1462-5822.2005.00588.x. [DOI] [PubMed] [Google Scholar]
- 18.Strauman MC, Harper JM, Harrington SM, Boll EJ, Nataro JP. 2010. Enteroaggregative Escherichia coli disrupts epithelial cell tight junctions. Infect Immun 78:4958–4964. doi: 10.1128/IAI.00580-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munera D, Ritchie JM, Hatzios SK, Bronson R, Fang G, Schadt EE, Davis BM, Waldor MK. 2014. Autotransporters but not pAA are critical for rabbit colonization by Shiga toxin-producing Escherichia coli O104:H4. Nat Commun 5:3080. doi: 10.1038/ncomms4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrington SM, Sheikh J, Henderson IR, Ruiz-Perez F, Cohen PS, Nataro JP. 2009. The Pic protease of enteroaggregative Escherichia coli promotes intestinal colonization and growth in the presence of mucin. Infect Immun 77:2465–2473. doi: 10.1128/IAI.01494-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kang G, Pulimood AB, Mathan MM, Mathan VI. 2001. Enteroaggregative Escherichia coli infection in a rabbit model. Pathology 33:341–346. doi: 10.1080/00313020126303. [DOI] [PubMed] [Google Scholar]
- 22.Zachos NC, Kovbasnjuk O, Foulke-Abel J, In J, Blutt SE, de Jonge HR, Estes MK, Donowitz M. 2016. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J Biol Chem 291:3759–3766. doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks S, Candy DC, Phillips AD. 1996. Adhesion of enteroaggregative Escherichia coli to pediatric intestinal mucosa in vitro. Infect Immun 64:4751–4760. doi: 10.1128/IAI.64.11.4751-4760.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rajan A, Vela L, Zeng X-L, Yu X, Shroyer N, Blutt SE, Poole NM, Carlin LG, Nataro JP, Estes MK, Okhuysen PC, Maresso AW. 2018. Novel segment- and host-specific patterns of enteroaggregative Escherichia coli adherence to human intestinal enteroids. mBio 9:e02419-17. doi: 10.1128/mBio.02419-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nataro JP, Deng Y, Cookson S, Cravioto A, Savarino SJ, Guers LD, Levine MM, Tacket CO. 1995. Heterogeneity of enteroaggregative Escherichia coli virulence demonstrated in volunteers. J Infect Dis 171:465–468. doi: 10.1093/infdis/171.2.465. [DOI] [PubMed] [Google Scholar]
- 26.Johansson MEV, Phillipson M, Petersson J, Velcich A, Holm L, Hansson GC. 2008. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc Natl Acad Sci U S A 105:15064–15069. doi: 10.1073/pnas.0803124105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nataro JP, Kaper JB, Robins-Browne R, Prado V, Vial P, Levine MM. 1987. Patterns of adherence of diarrheagenic Escherichia coli to HEp-2 cells. Pediatr Infect Dis J 6:829–831. doi: 10.1097/00006454-198709000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Swidsinski A, Weber J, Loening-Baucke V, Hale LP, Lochs H. 2005. Spatial organization and composition of the mucosal flora in patients with inflammatory bowel disease. J Clin Microbiol 43:3380–3389. doi: 10.1128/JCM.43.7.3380-3389.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johansson MEV, Hansson GC. 2012. Preservation of mucus in histological sections, immunostaining of mucins in fixed tissue, and localization of bacteria with FISH. Methods Mol Biol 842:229–235. doi: 10.1007/978-1-61779-513-8_13. [DOI] [PubMed] [Google Scholar]
- 30.Sheikh J, Czeczulin JR, Harrington S, Hicks S, Henderson IR, Le Bouguénec C, Gounon P, Phillips A, Nataro JP. 2002. A novel dispersin protein in enteroaggregative Escherichia coli. J Clin Invest 110:1329–1337. doi: 10.1172/JCI16172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haiko J, Westerlund-Wikström B. 2013. The role of the bacterial flagellum in adhesion and virulence. Biology (Basel) 2:1242–1267. doi: 10.3390/biology2041242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steiner TS, Nataro JP, Poteet-Smith CE, Smith JA, Guerrant RL. 2000. Enteroaggregative Escherichia coli expresses a novel flagellin that causes IL-8 release from intestinal epithelial cells. J Clin Invest 105:1769–1777. doi: 10.1172/JCI8892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avelino F, Saldaña Z, Islam S, Monteiro-Neto V, Dall'Agnol M, Eslava CA, Girón JA. 2010. The majority of enteroaggregative Escherichia coli strains produce the E. coli common pilus when adhering to cultured epithelial cells. Int J Med Microbiol 300:440–448. doi: 10.1016/j.ijmm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 34.In J, Foulke-Abel J, Zachos NC, Hansen A-M, Kaper JB, Bernstein HD, Halushka M, Blutt S, Estes MK, Donowitz M, Kovbasnjuk O. 2016. Enterohemorrhagic Escherichia coli reduce mucus and intermicrovillar bridges in human stem cell-derived colonoids. Cell Mol Gastroenterol Hepatol 2:48–62.e3. doi: 10.1016/j.jcmgh.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Noel G, Baetz NW, Staab JF, Donowitz M, Kovbasnjuk O, Pasetti MF, Zachos NC. 2017. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci Rep 7:45270. doi: 10.1038/srep45270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ranganathan S, Doucet M, Grassel CL, Delaine-Elias B, Zachos NC, Barry EM. 2019. Evaluating Shigella flexneri pathogenesis in the human enteroid model. Infect Immun 87:e00740-18. doi: 10.1128/IAI.00740-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zou WY, Blutt SE, Crawford SE, Ettayebi K, Zeng X-L, Saxena K, Ramani S, Karandikar UC, Zachos NC, Estes MK. 2019. Human intestinal enteroids: new models to study gastrointestinal virus infections. Methods Mol Biol 1576:229–247. doi: 10.1007/7651_2017_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. 2016. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology 150:638–649.e8. doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elias WP, Czeczulin JR, Henderson IR, Trabulsi LR, Nataro JP. 1999. Organization of biogenesis genes for aggregative adherence fimbria II defines a virulence gene cluster in enteroaggregative Escherichia coli. J Bacteriol 181:1779–1785. doi: 10.1128/JB.181.6.1779-1785.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Datsenko KA, Wanner BL. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A 97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato T, Stange DE, Ferrante M, Vries RGJ, Van Es JH, Van den Brink S, Van Houdt WJ, Pronk A, Van Gorp J, Siersema PD, Clevers H. 2011. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology 141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 42.Morin N, Santiago AE, Ernst RK, Guillot SJ, Nataro JP. 2013. Characterization of the AggR regulon in enteroaggregative Escherichia coli. Infect Immun 81:122–132. doi: 10.1128/IAI.00676-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez J-Y, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A. 2012. Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izquierdo M, Navarro-Garcia F, Nava-Acosta R, Nataro JP, Ruiz-Perez F, Farfan MJ. 2014. Identification of cell surface-exposed proteins involved in the fimbria-mediated adherence of enteroaggregative Escherichia coli to intestinal cells. Infect Immun 82:1719–1724. doi: 10.1128/IAI.01651-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nataro JP, Hicks S, Phillips AD, Vial PA, Sears CL. 1996. T84 cells in culture as a model for enteroaggregative Escherichia coli pathogenesis. Infect Immun 64:4761–4768. doi: 10.1128/IAI.64.11.4761-4768.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Levine MM, Bergquist EJ, Nalin DR, Waterman DH, Hornick RB, Young CR, Sotman S. 1978. Escherichia coli strains that cause diarrhoea but do not produce heat-labile or heat-stable enterotoxins and are non-invasive. Lancet 1:1119–1122. doi: 10.1016/s0140-6736(78)90299-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.