Abstract
Several emerging small molecules have been established for the repair or regeneration of musculoskeletal tissues. Cyclic AMP (cAMP) and its analogs have been the purview of many researchers in that they have been implicated in the repair or regeneration of several musculoskeletal tissues, such as bone, muscle, ligament, cartilage, blood vessels, skin, and nerve. Owing to the varied signal transduction pathways associated with cAMP, it elicits several processes and is thought to either activate or enhance native growth factors, thereby circumventing the need for supraphysiological doses of therapeutic growth factors in musculoskeletal tissue regeneration. In this review, we highlight the developments in using cAMP and its analogs for musculoskeletal regenerative engineering.
Keywords: small molecules, regenerative engineering, musculoskeletal regeneration, cAMP signaling, drug delivery
Introduction
With the aging of the US population, the burden of musculoskeletal diseases is fast increasing with each passing year, with significant impacts on the US economy. In the quest for a promising strategy that can repair, regrow, or regenerate complex musculoskeletal tissues, ‘regenerative engineering’ was put forward by Laurencin et al. to address this need. Regenerative engineering is the convergence of multidisciplinary approaches in advanced material science, stem cell technologies, developmental biology, physics, and clinical translation toward the goal of regenerating complex musculoskeletal tissues or an entire organ system [1].
Increased knowledge in cell and developmental biology has advanced our understanding of the molecular processes of the myriad growth factors, pro-osteogenic chemokines, cytokines, inducerons (i.e., a variety of inductive simple signaling molecules, such as gases, ions, and redox reagents, that are able to induce other stem cell differentiation through endogenous growth factor production), and angiogenic factors that are actively involved in complex tissue regeneration [2–4]. Therefore, it is a well-established fact that bioactive factors are an essential part of musculoskeletal regenerative engineering in that they guide cellular behavior by interacting with cell surface receptors, which eventually triggers cell signaling cascades, eliciting cell proliferation and differentiation in such a manner that is similar to native tissue development and healing mechanisms [5,6]. Several preclinical and clinical successes have been recorded with growth factors, such as bone morphogenetic proteins (BMPs), and some therapeutic proteins (i.e., BMP2 and BMP7) have now been approved by the US Food and Drug Administration (FDA) for clinical use for long bone non-unions, spinal fusions, fracture healing, among others [7–10]. Nevertheless, the use of these therapeutic proteins for expediting musculoskeletal regeneration process has been encumbered with high costs, immunogenicity, supraphysiological dosages, and instability, among others [11]. As a result, short peptides that are derived from therapeutic proteins have been extensively studied for regeneration purposes. Although they are more stable and smaller in size, with less anticipated immunogenicity compared with growth factors, they are still saddled with high costs and short half-lives, and could provoke immune responses in vivo. Despite their ease of synthesis from proteins and self-assembly properties, small peptides are associated with several controversial results that have impeded their clinical translation. Thus, there are limited preclinical and clinical data reported on their osteogenic and other musculoskeletal capacities [5,6,12]. This has necessitated the search for stable, inexpensive, safe, and promising alternatives that can stimulate cell differentiation by activating intracellular signaling cascades, promote tissue healing with high efficiency, and reduce adverse effects.
The small size, hydrophobicity, and (usually) uncharged nature of small molecules [5,6] accord them the ability to penetrate the phospholipid bilayer of cellular membranes to trigger cell transduction, which results in gene transcription and phenotype expression [5]. Recently, the musculoskeletal therapeutic potential of over 100 small molecules, including natural small molecules, synthetic novel small molecules, and other FDA-approved small molecules, was revealed via phenotype or target-based approaches [13–22]. Of the several small molecules unraveled, secondary messengers have gained significant interest among researchers in the area of tissue regeneration. Secondary messengers are responsible for transmitting signals received from the surface of the cell throughout its remaining inner compartments. They comprise four main categories: cyclic nucleotides; lipid and lipid-derived messengers; ions; and gases or free radicals [23,24]. Examples of secondary messengers used in regenerative engineering include calcium ions [25], sphingolipids [26], and nitric oxide (NO) [27].
cAMP nucleotide analogs have been the purview of many excellent reviews in musculoskeletal tissue repair and regeneration. cAMP, a derivative of ATP (Figure 1) is a physiologically important secondary messenger used for intracellular signal transduction in several different organisms, often conveying the cAMP-dependent pathway. cAMPs are key regulators of biological processes via cyclic nucleotide-gated ion channels, protein kinase A (PKA), guanine-nucleotide-exchange factor (GEF), exchange proteins activated by cAMP (EPAC; e.g., RAPGEF3), hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, popeye domain-containing proteins (Popdc), Wnt/beta catenin, and Hedgehog-dependent pathways, among others [5,28]. One of the most important signaling pathways is the cAMP-PKA pathway, which has been extensively studied and well correlated with osteogenesis. Once activated, PKA phosphorylates metabolic enzymes to regulate glucose metabolism and the transcription factor cAMP response element-binding protein (CREB) (Figure 2) to enhance gene transcription and phenotype expression by stimulating the interaction of CREB with its co-activator, CREB-binding protein [28,29].
Figure 1.

The conversion of ATP into cyclic (c)AMP.
Figure 2.

An updated cyclic (c)AMP signaling pathway. Reproduced, with permission, from [30]. For definitions of abbreviations, please see the main text.
Furthermore, the intracellular level of cAMP is mediated by leveling the activities of adenyl cyclase (AC) and cyclic nucleotide phosphodiesterase (PDE), although PDE tends to contribute more because the rate of cAMP hydrolysis in all human tissues surpasses the rate of synthesis [30,31]. Given that the abnormal regulation of the cAMP signaling cascade occurs in several musculoskeletal disorders, cancers, and Carney complex diseases [32], targeting cAMP signaling pathways could serve as a model for studying several diseases [33]. Nevertheless, limited understanding of the cellular and molecular mechanisms of the proapoptotic or antiapoptotic effects of cAMP [33,34] has necessitated the need to synthesize functionally and structurally similar compounds that can mimic its bioactivities. cAMP analogs are synthetic compounds that stimulate endogenous cAMP production or increase its systemic concentration. Owing to their structure–activity relationships, hundreds of cAMP analogs (Table 1) have been developed to study the underlying mechanisms behind the cAMP signal transduction cascades and their therapeutic properties and/or potentials. Thus, these natural and/or synthetic analogs have served as instrumental biological tools over the decades. Following the administration of therapeutic cAMP analogs or cAMP inducers, cells usually sense the extracellular stimuli through membranous or intracellular receptors, transduce the signals via intracellular molecules, and, thus, regulate the biological function of the cells, such as phenotypic expression [5,7]. It is thought that these cAMPs either activate native growth factors, enhance intracellular growth factors, or to a lesser extent, replace the use of supraphysiological therapeutic growth factors [5]. As a result of the many signal transduction pathways that have been identified with cAMP, it has been implicated in the repair or regeneration of several musculoskeletal tissues, such as bone, muscle, ligament, cartilage, blood vessels, skin, and nerves. Therefore, here, we highlight the recent applications of cAMP in musculoskeletal regenerative engineering. Based on the feasibility of adenosine compounds to concomitantly elicit several processes, a combination of numerous cAMP molecules (Table 1), which act following different known pathways, could be used in a sequential manner such that they would imitate the developmental pathways of resident tissues during musculoskeletal tissue repair or regeneration [5].
Table 1.
Classification of common cAMP analogs
| Classifications of cAMPs | Representative cAMPs | Molecular formula | Description/mechanisms of action |
|---|---|---|---|
| PDE-resistant PKA activators | Adenosine-3’,5’-cyclic monophosphorothioate, Sp-isomer (Sp-cAMPS), sodium salt; C10H11N5O5PS · Na |  |
PDE-resistant PKA agonist; considerably higher resistance against PDE compared with dibutyryl-or 8-Br-cAMP; membrane permeability comparable with that of 8-Br-cAMP |
| 2-Chloroadenosine-3’,5’-cyclic monophosphorothioate, Sp-isomer (Sp-2-Cl-cAMPS), sodium salt; C10H10ClN5O5PS · Na |  |
Analog of the PKA activator Sp-cAMPS with increased lipophilicity and PDE stability; halogen in position 2 of the adenine nucleobase can be used for various substitutions, including spacers and labels | |
| N6-Benzoyladenosine-3’,5’-cyclic monophosphorothioate, Sp-isomer (Sp-6-Bnz-cAMPS), sodium salt; C17H15N5O6PS · Na |  |
PDE-resistant form of 6-Bnz-cAMP; membrane permeant and does not activate EPAC; thus, can be used as an EPAC-negative control | |
| N6-Phenyladenosine-3’,5’-cyclic monophosphorothioate, Sp-isomer (Sp-6-Phe-cAMPS), sodium salt; C16H15N5O5PS · Na |  |
PDE-resistant form of N6-phenyl-cAMP; potent site-selective, highly membrane-permeant, and PDE-resistant activator of PKA; Sp-6-Phe-cAMPS does not activate EPAC; thus, can be used as an EPAC-negative control | |
| 8-Bromoadenosine-3’,5’-cyclic monophosphorothioate, Sp-isomer (Sp-8-Br-cAMPS), sodium salt; C10H10BrN5O5PS · Na |  |
Metabolically resistant, membrane-permeant, and PDE-resistant PKA agonist; no metabolic adverse effects as observed with dibutyryl-cAMP or 8-Br-cAMP; significantly more lipophilic and membrane-permeant compared with Sp-AMPS | |
| 8-(4-Chlorophenylthio)adenosine-3’,5’-cyclic monophosphorothioate, Sp-isomer (Sp-8-CPT-cAMPS), sodium salt; C16H14ClN5O5PS2 · Na |  |
Lipophilic, site-selective, and PDE-resistant activator of PKA | |
| 8-Piperidinoadenosine-3’,5’-cyclic monophosphorothioate, Sp-isomer (Sp-8-PIP-cAMPS), sodium salt; C15H20N6O5PS · Na |  |
Lipophilic site-selective cAMP analog with extremely high selectivity for site B of PKA type II; acts synergistically with site A-activating analogs for selective activation of type II; metabolically stable and good membrane permeability | |
| 8-Hydroxyadenosine-3’,5’-cyclic monophosphorothioate, Sp-isomer (Sp-8-OH-cAMPS), sodium salt; C10H11N5O6PS · Na |  |
Polar, membrane-impermeant, PKA agonist Sp-cAMPS; e.g., for the study of extracellular cAMP receptors. If applied by patch clamp techniques, Sp-8-OH-cAMPS should remain trapped inside the cell | |
| PDE-resistant PKA inhibitors | Adenosine-3’,5’-cyclic monophosphorothioate, Rp-isomer (Rp-cAMPS), sodium salt; C10H11N5O5PS · Na |  |
Competitive inhibitor of PKA type I and II (cAMP antagonist); prefers type II PKA; membrane permeant and PDE resident; significantly more lipophilic than cAMP |
| 8-Bromoadenosine-3’,5’-cyclic monophosphorothioate, Rp-isomer (Rp-8-Br-cAMPS), sodium salt; C10H10BrN5O5PS · Na |  |
Lipophilic analog of cAMP antagonist Rp-cAMPS. PDE resistant; prefers type I PKA; significantly more lipophilic and membrane permeant compared with Rp-cAMPS or 8-Br-cAMP | |
| EPAC agonists | 2’-O-Methyladenosine-3’,5’-cyclic monophosphate (2’-O-Me-cAMP), sodium salt; C11H13N5O6P · Na |  |
Relatively polar EPAC agonist that does not activate PKA. Suitable for EPAC activation by patch clamp application techniques and for receptor mapping studies |
| 8-Bromo-2’-O-methyladenosine-3’,5’-cyclic monophosphate (8-Br-2’-O-Me-cAMP), sodium salt; C11H12BrN5O6P · Na |  |
Specific EPAC activator, while PKA is not affected; suitable for direct comparison with common 8-Br-cAMP, which activates both PKA and EPAC | |
| 8-(4-Chlorophenylthio) adenosine-3’,5’-cyclic monophosphate (8-CPT-cAMP), sodium salt; C16H14ClN5O6PS · Na |  |
Lipophilic activator of both cAMP-and cGMP-dependent protein kinase and of EPAC; excellent cell membrane permeability, improved PDE stability, and high site selectivity preferring site B of cAMP-dependent protein kinase type II | |
| 8-(4-Chlorophenylthio)-2’-O-methyladenosine-3’,5’-cyclic monophosphate (8-pCPT-2’-O-Me-cAMP), sodium salt; C17H16ClN5O6PS · Na |  |
Specific, membrane-permeant EPAC activator with increased PDE stability, which does not activate PKA | |
| 8-Hydroxy-2’-O-methyladenosine-3’,5’-cyclic monophosphate (8-OH-2’-O-Me-cAMP), sodium salt; C11H13N5O7P · Na |  |
Polar EPAC agonist that does not activate PKA; due to its low lipophilicity and membrane permeability, it can be used in patch clamp applications | |
| EPAC-negative controls | N6-Benzyladenosine-3’,5’-cyclic monophosphate (6-Bn-cAMP), sodium salt; C17H17N5O6P · Na | 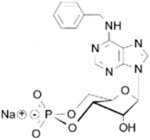 |
Site-selective activator of cAMP-dependent protein kinase that does not activate EPAC; increased hydrolytic stability against PDE, esterases, and amidases, and considerably higher membrane permeability compared with cAMP |
| Other PKA Activators | N⁶-Benzoyladenosine-3’,5’-cyclic monophosphate (6-Bnz-cAMP), sodium salt; C17H15N5O7P · Na | 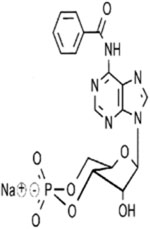 |
Site-selective and membrane-permeant activator of cAMP-dependent protein kinase that does not activate EPAC; thus, can be used as an EPAC-negative control. High PDE resistance compared with cAMP; selects site A of both cAMP-dependent protein kinase isozymes; thus, can be used for synergistic activation of type II when combined with site B II-selective analogs, such as Sp-8-CPT-cAMPS or 8-PIP-cAMP. For preferential stimulation of cAMP-dependent protein kinase type I, a combination with the site B I-selective analog 2-Cl-8-MA-cAMP or 8-HA-cAMP can be used |
| N6-Phenyladenosine-3’,5’-cyclic monophosphate (6-Phe-cAMP), sodium salt; C16H15N5O6P · Na |  |
Potent site-selective and highly membrane-permeant activator of PKA showing strong preference for site A of both isozymes. Together with, e.g., SP-5,6-DCl-cBIMPS, selective stimulation of type II can be achieved | |
| N6-Mono-t. butylcarbamoyladenosine-3’,5’-cyclic monophosphate, sodium salt (6-MBC-cAMP); C15H20N6O7P · Na |  |
Site selective activator of PKA (cAMP agonist) strongly preferring the A site of cAMP-dependent protein kinase II. Together with, e.g., Sp-5,6-DCl-cBIMPS, selective stimulation of type II can be achieved; not considered to activate EPAC | |
| N6, 2’-O-Dibutyryladenosine-3’,5’-cyclic monophosphate (db-cAMP), sodium salt |  |
Membrane-permeant activator of PKA (cAMP agonist). Caution: it releases butyrate because of intracellular and extracellular esterase action. Butyrate was shown to have distinct biological effects | |
| cAMP ligands for immobilization | 2-(6-Aminohexylamino)adenosine-3’,5’-cyclic monophosphate (2-AHA-cAMP); C16H26N7O6P |  |
cAMP functionalized for conjugation with markers and dyes or for immobilization; suitable as a ligand in affinity chromatography (2-AHA-cAMP-Agarose) and for modification with fluorophores and other markers |
| 8-(4-Mercaptobutylthio)adenosine-3’,5’ cyclic monophosphate (8-MBT-cAMP), triethyl ammonium salt; C14H20N5O6PS2 |  |
Functionalized cAMP analog, suitable for immobilization as affinity ligand (e.g., for purification of PDEs) or for coupling of various labeling structures, including fluorophores | |
| Special task cyclic nucleotides | 2’-Deoxyadenosine-3’,5’-cyclic monophosphate (2’-dcAMP), sodium salt |  |
PKA-inactive analog of cAMP with modified 2’-moiety for cAMP receptor mapping studies |
| 8-Azidoadenosine-3’,5’-cyclic monophosphate, sodium salt (8-N₃-cAMP); C10H10N8O6P · Na |  |
Analog for photoaffinity labeling of cAMP-binding proteins | |
| N6-Phenyladenosine-3’,5’-cyclic monophosphorothioate, Rp-isomer; C16H15N5O5PS · Na |  |
Ideal for obtaining only small amounts of structures otherwise accessible only by custom syntheses (e.g., references for chromatography) |
cAMPs in bone regeneration
The human bone is one tissue that can regenerate or repair as nicely as it develops (skeletogenesis), whereby all the pre-existing properties are largely restored without any scar formation [35]. During the bone fracture healing process, intramembranous and endochondral ossification, and other fetal skeletogenesis pathways are often recapitulated, thereby forming new bones that are identical to the existing bone [35–37]. Although bone regeneration is actively involved in bone remodeling throughout adult life, its regenerative process is truncated by cases of avascular necrosis, osteoporosis, atrophic non-unions, and critical size bone defects caused by trauma, tumor resection, infection, or other skeletal abnormalities [37]. Given the many patients found to have bone disorders and/or diseases annually in the USA, it is vital to establish new, safer, bone treatment strategies that will replace or enhance conventional treatment methods, such as autograft,, allograft,, xenograft,, use of growth factors, osteoconductive scaffolds, osteoprogenitor cells, distraction osteogenesis, and so on. The growth factor therapeutic BMPs (2 or 7) tend to be excellent candidates for bone regeneration but with limitations, such as the formation of unwanted ectopic bone [38,39] and increased rate of benign neoplasia [40]. Thus, many researchers have begun to investigate the osteogenic properties of small osteoinductive molecules, particularly the cyclic adenosine nucleotides.
cAMP analogs are promising candidates for bone regeneration because their signaling pathways regulate osteoclast differentiation and promote bone formation by inhibiting PDEs [41]. cAMP is also known to promote osteoblastogenesis directly or by enhancing other biological activators, such as BMPs, parathyroid hormone, and prostaglandin effectors [41]. Several researchers have reported that the cAMP signaling channel is responsible for regulating osteoblast cell differentiation. For example, Tintut and co-workers demonstrated that calcifying vascular cells (CVC) for 3 days with either N6,2′-O-dibutyryladenosine-3′,5′-cyclic monophosphate (db-cAMP) (a synthetic analog of cAMP) or forskolin (intracellular cAMP activator) induced osteoblast-like ‘cuboidal’ morphology (i.e., osteoblastic differentiation with elevated alkaline phosphatase activity but inhibited CVC proliferation) [42]. They further showed that the cAMP pathway enhanced vascular calcification in vitro and in vivo as evident by elevated type I procollagen production and expression of matrix γ-carboxyglutamic acid protein [42]. When the potential application of db-cAMP-treated human mesenchymal stem cells (hMSCs) in bone tissue engineering was evaluated by de Boer’s research group, the authors reported remarkable osteogenic differentiation in vitro with hMSC proliferation inhibition [43], although the authors postulated that cAMP can either promote or inhibit osteogenesis in hMSCs based on the duration, rather than on the drug concentration or strength of the signal provided [44]. The authors also implanted various tissue-engineered constructs with or without db-cAMP into the subcutaneous pockets of immune-deficient mice and showed robust bone formation with db-cAMP after 6 weeks of implantation [43]. Their results imply that articulated manipulation of hMSCs with a suitable cAMP analog will induce osteogenic differentiation in vitro and bone formation in vivo, thus supporting the osteogenic effect of PKA activation. By contrast, when the researchers treated mouse calvarial osteoblasts (MC3T3-E1 cells), mouse MSCs, and rat MSCs with db-cAMP and 8-bromo cAMP (8Br-cAMP), both cAMP analogs inhibited osteogenesis in these rodent cells in vitro [45]. To support their findings further, the authors exposed ex vivo-cultured mouse calvaria to db-cAMP, which resulted in a reduction in bone volume [45]. This indicates the essence of evaluating the variations in species morphology in relation to various osteogenic signals when considering clinical translation from clinically relevant models [45].
In 2012, researchers from de Boer’s group addresses some of the limitations present in their earlier studies [46]. For instance, they had previously shown that db-cAMP induced osteogenic differentiation of hMSCs in vitro and bone formation in vivo, but inhibited hMSC proliferation [43]. In an attempt to overcome this limitation, they investigated the effect of timing of application, rather than the concentration, of db-cAMP on the osteogenic differentiation of hMSCs, and revealed that intermittent exposure of hMSCs to dibutyryl-cAMP inhibited ALP expression, but with no inhibitory effect at low db-cAMP concentrations [44]. To further investigate whether slightly adjusting the dose and timing of PKA activation promoted both hMSC proliferation and better bone formation in vivo, the researchers compared the proliferative and osteogenic differentiation effects of two different PKA activators (8Br-cAMP and forskolin) with db-cAMP [46]. According to their results, all three compounds induced alkaline phosphatase levels, bone-specific target genes, and secretion of insulin-like growth factor-1, with 8-br-cAMP inducing adipogenic differentiation in long-term cultures. The three cAMP analogs inhibited hMSC proliferation in a dose-dependent manner, with forskolin preventing proliferation the most. Nonetheless, the investigators noticed larger amounts of in vivo bone formation using forskolin compared with db-cAMP [46].
It has also been demonstrated that the small molecule 6-Bnz-cAMP promotes osteoblastic differentiation and matrix mineralization of osteoblast-like MC3T3-E1 cells by activating the PKA signaling pathway [47]. The expression of osteoblast-specific markers, such as alkaline phosphatase (ALP) activity, runt transcription factor 2 (Runx2), osteopontin (OPN), and osteocalcin (OCN), was upregulated. Cell proliferation studies showed no cytotoxicity, indicating that 6-Bnz-cAMP could serve as a novel bone-inducing growth factor for bone repair and regeneration [47]. Several cAMP analogs with various signaling pathways are currently being investigated for their osteogenic potential in an attempt to introduce newer promising osteoinductive small molecules for bone regenerative engineering (Ifegwu et al., unpublished data, 2017).
Coupled with the fact that the cAMP signaling pathway modulates osteoblast cell differentiation, it is also known to be essential for regulating the activity of integrin-mediated cell adhesion in several cell types [48–50]. Other than the contribution of the PKA signaling pathway to osteogenesis, the discovery of an exchange protein activated by cAMP [51] serves as a Rap-1-specific cAMP guanine nucleotide exchange factor and stimulates active GTP-Rap1 accumulation [51,52]. The Epac-Rap1 signal transduction pathway has been reported to be actively involved in cAMP-induced integrin-mediated cell adhesion in many cell types [52,53]. Given that the conventional graft system (autografts, allografts, xenografts, etc.) in bone tissue engineering is being replaced with synthetic bioactive and biodegradable biomaterials, osteoblast cell adhesion to these materials is an indispensable requirement for the successful integration of the implants in vivo. Cell adhesion is a complex biological process that influences cell proliferation, differentiation, and bone formation, among others [48–50]. Following the report that 8Br-cAMP, a nonspecific target cAMP analog, induced initial cell adhesion of ovarian carcinoma cells to a fibronectin-coated plate [51], the effects of 8Br-cAMP and forskolin on cell adhesion were investigated using osteoblast-like cells (MC3T3-E1) and the FDA-approved biomaterial poly lactic-co-glycolic acid (PLGA) [50]. Both molecules increased initial adhesion (after 1 h) of osteoblast-like MC3T3 cells on PLGA thin films in a concentration-dependent manner, although 8Br-cAMP was slightly quicker than forskolin [50]. Owing to the fact that 8Br-cAMP is a dual activator of both the PKA/cAMP and Epac/cAMP pathways, it was demonstrated that 8-BrcAMP can induce initial cell adhesion to PLGA via the PKA and/or Epac signaling mechanisms. In an attempt to study the individual contribution of Epac or PKA to osteoblast cell adhesion, it was shown using two different target-specific cAMP analogs (the Epac activator, 8-CPT-2Me-cAMP, and the PKA activator, 6-Bnz-cAMP) that the PKA signaling pathway has an important role in the adhesion process. From the data obtained, 6-Bnz-cAMP imitated 8-Br-cAMP-enhanced cell adhesion, whereas 8-CPT-2Me-cAMP was incapable of significantly increasing cell adhesion compared with untreated control cells. This showed that PKA signaling pathway activation alone is enough to improve cell adhesion, while Epac has minimum or no effect on cAMP-induced cell adhesion [50]. It is important to point out that the failure of anchorage-dependent cells, such as osteoblasts, to adhere will often result in cell apoptosis [51], suggesting that the ability to control osteoblast cell adhesion to biomaterial surfaces is crucial for osseointegration, cell specialization, and, ultimately, bone regenerative engineering [50,54].
In another study, Caroll and colleagues demonstrated that the A2B adenosine receptor (A2BAR), a Gαs/αq-protein-coupled receptor that signals via cAMP, was capable of promoting MSC differentiation to osteoblasts and bone development in vivo. First, by studying the role of A2BAR in the differentiation of MSCs to osteoblasts, the authors found that in vitro differentiation of bone marrow-derived MSCs from A2BAR-knockout (KO) mice resulted in decreased expression of osteoblastic differentiation transcription factors and the formation of fewer mineralized nodules compared with wild-type (WT) mice. Their next experiment confirmed that A2BAR regulation of bone homeostasis involved cAMP signaling. Using micro-CT analysis of adult femurs, the researchers revealed decreased bone density in A2BAR-KO mice compared with WT. According to their results, ABAR-KO mice equally displayed a delay in normal fracture physiology, with lower expression of osteoblast differentiation genes, which implied that baseline bone density and bone fracture healing are compromised in A2BAR-KO mice. Taken together, the study identified A2BAR via cAMP signaling as a new regulator of osteoblast differentiation, bone formation, and fracture repair in vitro and in vivo [55,50].
cAMPs in the development and repair of skeletal muscle
Skeletal muscle is arguably the most structurally specialized and abundant tissue of the human body and stimulates well-articulated body movements via their attachment to the skeleton [56,57]. Its striking subcellular architecture and intrinsic signaling mechanisms enable smooth movement with instructions from motor neurons [57]. Thus, muscles are extensively vascularized and well innervated, which afford them the ability to self-regenerate when damaged. The actin (which forms the thin filaments) and the myosin (which forms the thick filaments) of the myofibril repeating sarcomeres are crucial to the contractile properties of skeletal muscle. During skeletal contraction, nerve activation usually elicits myofiber (formed by the fusion of myoblasts) depolarization, which results in the elevation of intracellular calcium released from the sarcoplasmic reticulum [56]. Consequently, the myosin is bound to the actin by calcium, which eventually leads to the contraction of the myofibers and, thus, the entire skeletal muscle [56]. The robust regenerative ability of skeletal muscles is compromised under severe trauma, therefore resulting in the loss of muscle functionality. Despite the fact that, similar to stem cells, satellite cells can restore damaged muscle fibers or form completely new muscle fibers, muscle tissues are saddled with the formation of dysfunctional scar tissue (fibrosis), leading to incomplete functional recovery with esthetic problems [56,57]. Hence, the identification of factors that reduce scar formation and enhance the muscle-healing process has resulted in huge scientific interest, because this knowledge would be useful in developing treatment modalities for major muscle injuries, diseases, or dysfunction [56].
Skeletal muscle development and regeneration studies have illuminated intrinsic skeletal muscle growth and repair mechanisms, and attempted to identify a variety of bioactive factors that influence regeneration. Increased understanding of biological factors, mechanisms, signaling cascades, and pathways associated with skeletal muscle development and regeneration will have a central role in developing promising therapeutics as treatment modalities for major muscle injuries or muscle diseases. During myogenesis, the mammalian trunk skeletal muscle is derived from the somites, a group of cell clusters formed by the paraxial mesoderm, starting at the head region and sequentially added caudally [58,59]. The somites are one of the mesodermal segments (axial, intermediate, paraxial, and lateral plate) on either side of the neural tube. Upon receiving a signal from the neural tube and ectoderm, cells of the ventral somites transit from the epithelium to mesenchyme, resulting in the sclerotome, which ultimately forms the vertebrae and ribs, whereas cells of the dorsal somites (dermomyotome) delaminate from the dermomyotome to form the myotome, which precipitates the deep back muscles (medial myotome), the limb and body wall muscles (lateral myotome) [58,60]. Pax 3, Pax 7, c-Met, Myf5, Mrf4, MyoD, myogenin, BMPs, cAMP, Hedgehog (hh), and Wnt proteins are all essential for the formation of these skeletal muscles [56–62]. Most of the inducerons, growth factors, and signaling molecules implicated in muscle embryogenesis are equally involved in satellite cell activation and postnatal muscle regeneration, particularly cAMP [56–62].
Most importantly, cAMP has been shown to contribute immensely to muscle development and regeneration regulated by satellite cells, implying that its signal transduction can be manipulated to enhance muscle regeneration in patients with acute damage or muscle disorder [57,58,62–64]. More so, cAMP signaling is actively involved in almost all of the essential cellular activities required to effectively regenerate adult skeletal muscle, such as myoblast differentiation [58], migration [65], and fusion [57,58,63,66]. Hence, muscular dystrophy, disuse atrophy, denervation injury, age-related atrophy, and similar animal model muscle disorders can be combated via sustained activation of the cAMP signaling cascade, which results in hypertrophic responses in skeletal myofibers [57,67,68]. These responses also promote myofiber maturation and myofiber metabolic phenotype, and stimulate acute changes in myofibers during exercise. Acute cAMP signaling mediates glycogenolysis, exerts elevated contractile forces in skeletal muscle, and encourages quick ion balance recovery from sustained muscle contraction, especially during exercise (energy utilization), where epinephrine is discharged into the circulation, whereas cAMP accumulates in muscle [69–71]. Intriguingly, identifying ligands or signal-activating small molecules that can elicit relevant cAMP signaling and downstream effectors may represent a new generation of therapies for muscle repair and regeneration [57]. In addition, several cAMP-inducing agents, cAMP-associated proteins, and cAMP-activated transcription factors have been demonstrated to enhance muscle regeneration and functional repair after injury by increasing myofiber size and promoting fiber-type transitions to glycolytic fibers [67,68].
cAMP signaling is dynamically regulated during embryonic muscle development, ex vivo myogenesis, and muscle regeneration [58,62–64]. In 2008, by incubating cultured rat skeletal muscle with exogenous cAMP stimulant, forskolin or isoprenaline, while quantifying the expressed cAMP and its metabolites as a representative of cAMP efflux and its extracellular degradation using radioassays and high-performance liquid chromatography (HPLC), respectively, Chiavegatti et al. demonstrated that skeletal muscle expresses the extracellular cAMP-adenosine pathway [72]. The researchers also showed that the activation of AC in cultured cells with forskolin or isoprenaline resulted in higher cAMP efflux and extracellular generation of 5’-AMP and adenosine [72]. They equally observed an extracellular cAMP-adenosine pathway after direct and receptor-dependent stimulation of AC in rat extensor muscle ex vivo [72]. As mentioned above, forskolin is an activator of AC, and isoprenaline (isoproterenol) is a β1 and β2 adrenoceptor agonist, both of which are known to increase intracellular levels of cAMP. In contrast to β-blockers, the β-adrenergic agonists or β-agonists are sympathomimetic agents that act upon β-adrenoceptors by mimicking the action of epinephrine and norepinephrine (noradrenaline) signaling, which stimulates AC and ultimately activates intracellular cAMP. Although the underlying molecular mechanisms are still being elucidated, β-adrenergic receptor agonists have shown significant potential in promoting muscle function in patients with muscular dystrophy [73–75]. Other β2-AR agonists, such as clenbuterol, have also been reported to induce skeletal muscle hypertrophy in rodents, large mammals, and humans [76]. Muscle growth is thought to be a function of the regulation of protein synthesis and proteolysis. For example, clenbuterol has been shown to elicit total protein synthesis and translational efficiency as well as to reduce proteolysis in rabbit, rat, and chick skeletal muscles [57]. A cell-permeable cAMP analog, dibutyryl cAMP (db-cAMP), has also been shown to reduce proteolysis in muscle ex vivo [77,78], suggesting that these effects are brought about by cAMP because PDE inhibitors reduce atrophy in skeletal muscle [79]. Owing to the activation of β-adrenergic receptor signaling during exercise, β-adrenergic receptors and catecholamines are the most widely studied cAMP inducers in skeletal muscle among the G-protein-coupled receptor (GPCR) ligand pairs. Minetti and co-workers also demonstrated that Galphai2 (Gαi2) signaling enhances skeletal muscle hypertrophy, myoblast differentiation, and skeletal muscle regeneration in mice via protein kinase C (PKC)-mediated Gαi2-induced satellite cell activation, differentiation, hypertrophy, and myoblast fusion [80]. Despite the paucity of data regarding Epac action in skeletal muscle, the EPAC1-selective agonist 8-pCPT-2′-O-Me-cAMP has been used to show the physiological effects of Epac1 in skeletal muscle enhancement [81,82]. While Brennesvik et al. showed that epinephrine enhanced insulin-stimulated PKB activation via cAMP and Epac, Baviera et al. reported the involvement of the cAMP/Epac/PI3K-dependent pathway in the antiproteolytic effect of epinephrine on rat skeletal muscle. Results from these two studies suggest that EPAC mediates crosstalk between β-adrenoceptors and the PI 3-kinase-Akt pathway [81,82]. In 2009, Griffin et al. demonstrated that the GPCR MOR23 elicited cAMP signaling and promoted skeletal muscle regeneration while regulating myoblast cell migration, adhesion, and fusion [65]. The researchers observed the inhibition of muscle regeneration and unfused myofibers when MOR23 was silenced in vivo [65]. To further corroborate the fact that dynamic cAMP signaling is needed during regeneration, intramuscular injection of forskolin or a PDE inhibitor after myotoxic injury reduced the expression of a MEF2 reporter during regeneration, which suggests the direct inhibition of MEF2D by PKA [83]. CREB has also been implicated in muscle development, muscle survival, and muscle regeneration in mouse skeletal muscle [57,84]. In 2008, Berdeaux et al. reported muscle degeneration in mice expressing dominant-negative CREB in skeletal myofibers [84]. The authors suggested that the lowered expression of salt-inducible kinase-1 was responsible [84]. Collectively, cAMP, its analogs, activating, and signaling molecules are essential for skeletal muscle development and regeneration.
cAMP-mediated angiogenesis and vasculogenesis
Impaired wound healing remains a major clinical challenge for patients with musculoskeletal disorders as well as diabetes, and results from reduced angiogenesis, delayed cell infiltration, and the reduced formation and alignment of collagen fibers. Beyond wound healing, angiogenesis and vasculogenesis remain crucial to the clinical application of engineered tissues. Angiogenesis is the proliferation and migration of pre-existing, fully differentiated endothelial cells native to existing vessels, whereas vasculogenesis is the process of isolating endothelial progenitor cells (EPC) from peripheral blood cells and their incorporation into new vessels in ischemic regions [85–87]. Both processes are pivotal to neovascularization during adult wound healing because vasculogenesis is a crucial mechanism in the development of primordial vascular networks in the embryo [88,89]. Owing to reports of the involvement of cAMP-activating and/or -elevating molecules in angiogenesis as well as the ability of cAMP to elicit several biological processes, it is possible that one of the major signaling pathways for enabling angiogenesis is a cAMP-dependent one. After reporting the elevation of cAMP-facilitated angiogenesis in rat sponge implants by endogenous prostaglandins (PGs) via vascular endothelial growth factor (VEGF) [90], Majima’s group demonstrated that 8-bromo-cAMP, forskolin-, and amrinone-induced VEGF enhanced angiogenesis in Sprague-Dawley (SD) rats. With immunohistochemistry, the authors confirmed that the VEGF-expressed cells in their observed sponge granulated tissues were fibroblasts and the intensities of the positive reactions were increased by these cAMP-elevating molecules. 8-br-cAMP is a cAMP analog, forskolin is a cAMP activator, whereas amrinone is a PDE (cAMP-degrading enzyme) inhibitor; all three stimulate AC. Hence, these findings suggested that AC/PKA-dependent VEGF induction promotes angiogenesis in vivo [91]. Despite several in vitro reports, this study was the first in vivo report to show that an AC/PKA-dependent increase in the VEGF in fibroblasts is relevant to angiogenesis [91].
By applying DB-cAMP to a full-thickness dorsal skin wound of C57BL/KsJ-db/db (db/db) mice every second day, Asai et al. showed that DB-cAMP treatment led to the increased recruitment of EPCs and significant upregulation of angiogenic growth factors, thus significantly accelerating wound healing via angiogenesis-and vasculogenesis-related processes [86]. According to their data, DB-cAMP accelerated healing and neovascularization of diabetic wounds, increased the recruitment of EPCs into the wounds, upregulated VEGF and stromal cell-derived factor-1 (SDF-1) α production, and indirectly promoted EPC migration, granulation tissue formation and re-epithelialization. These findings experimentally support the stimulatory effects of DB-cAMP on diabetic-related impaired wound healing [86]. In 2009, cAMP-mediated signaling pathways were again implicated in angiogenesis regulation through increased VEGF expression by Namkoong and co-workers [92]. Forskolin elicited the angiogenesis of human endothelial cells (HUVEC) and in vivo neovascularization (in mice), which was accompanied by phosphorylation of CREB, ERK, Akt, and endothelial NO synthase (eNOS) as well as NO production and VEGF expression [92]. The authors further showed that 8CPT-2Me-cAMP, an EPAC activator, promoted the Akt/eNOS/NO pathway and ERK phosphorylation, but did not stimulate CREB phosphorylation and VEGF expression [92]. Hence, the investigators concluded that forskolin promotes angiogenesis via the coordinated crosstalk of PKA-dependent VEGF expression and EPAC-mediated PI3K/Akt/eNOS signaling.
Given that appropriate vascularization is essential to the clinical application of engineered tissues, the Tian group from Boston University recently investigated how physical and chemical signals affect vascularization of directly seeded capillary-scale channels to apply those signals to create microvessels that mimic human capillaries in size and function [93]. The group had earlier demonstrated that the cell-permeant cAMP analog, DB-cAMP, improved the stability of large (~120-Mm diameter) engineered microvessels [94,95]. Along this line of thought, the same group used DB-cAMP to promote the endothelial stability of newly formed endothelium in narrow (<30-Mm diameter) channels [93]. Owing to the fact that stiffness is a strong controller of endothelial cell function, the authors were able to successfully replicate the vascular stability in smaller, capillary-scale, channels using DB-cAMP [93]. Their results suggested how to optimize the physical and chemical environment to enhance the rapid generation and vascularization of perfusable capillary-scale channels with diameters as small as 20 Mm in type I collagen gels [93].
Coupled with the in vivo enhancement of angiogenesis and vasculogenesis, several in vitro studies have implicated cAMP-mediated angiogenesis in retinal pericytes [96], adipocytes [97,98], lung cancer cells [99], rat cultured Muller cells [100], rat aorta fibroblasts [101], synovial fibroblasts from patients with rheumatoid arthritis [102], perfused rat lungs, and human fetal lung explants [103,104]. Recently, the effects of various cAMP analogs (8-Br-cAMP, 6-Bnz-cAMP, and 8-CPT-2Me-cAMP) on VEGF production were investigated using MC3T3-E1 osteoblast-like cells [104]. 8-Br-cAMP is an activator of both PKA and EPAC, whereas 6-Bnz-cAMP and 8-CPT-2Me-cAMP exclusively target PKA and EPAC, respectively. The resulting data indicated that 1-day treatment with 8Br-cAMP was capable of inducing in vitro cell-based VEGF production for promoting angiogenesis [105]. When osteoblast-like MC3T3-E1 cells were first treated with 8Br-cAMP for 1 day, VEGF production and secretion were significantly increased [105]. Thereafter, it was shown that 8Br-cAMP induced cell-secreted VEGF was biologically active and might have promoted angiogenesis, as evidenced by increased HUVEC migration and tubule formation [105].
cAMP-expedited chondrogenesis
Cartilage mainly comprises collagens and proteoglycans, although it is a connective tissue that holds relatively sparse populations of chondrocytes [106]. These chondrocytes help in matrix generation and the maintenance of the various types of cartilage, such as hyaline, fibrous, and elastic cartilage [106,107]. The inability of resident chondrocytes to lay down new matrix with similar properties that it had during its development has been a huge challenge in efforts to successfully repair damaged cartilage [106]. To attempt to develop effective strategies for preventing cartilage damage as well as promoting cartilage repair, it is important to understand the underlying mechanisms of cartilage remodeling during its development, diseased state (e.g., osteoarthritis), repair, and aging [106]. Chondrogenesis (chondrification) involves the formation of cartilage from condensed mesenchyme tissue, which differentiates into chondroblasts and starts secreting the molecules that make the extracellular matrix. Chondrogenesis and endochondral ossification are cartilage differentiation processes that result in skeletal formation, development, growth (in embryos), and skeletal muscle repair (in adults) [107]. During skeletal development, chondrocytes develop from mesenchymal progenitors to synthesize the templates, or cartilage anlagen, for the developing bone [106,107]. After chondrogenesis, the chondrocytes remain as resting cells to either become cartilage or undergo proliferation, terminal differentiation to chondrocyte hypertrophy, or apoptosis by endochondral ossification, such that the hypertrophic cartilage is replaced by bone [106]. There have been several reports of cAMP-enhanced chondrogenesis and regeneration. Ephemeral elevation of intracellular cAMP during the onset of chondrogenesis in mouse and chick limb buds,was reported by Ho et al. [107] and Solursh et al. [108], respectively. It has long been established that cAMP stimulates a remarkable elevation in the steady-state cytoplasmic levels of mRNAs for cartilage-characteristic type II collagen as well as in the core protein of cartilage-specific proteoglycan in limb mesenchymal cells in vitro [110]. While investigating the role of cAMP in the regulation of synthesis of macromolecules (RNA, total protein, and proteoglycan) in embryonic chick cartilage, Drezner et al. reported the stimulation of macromolecule synthesis by db-cAMP and matrix sulfated proteoglycan deposition by chondrocytes (anabolic processes), mediated by intracellular cAMP levels and occurring during the G1 phase of the cell cycle [111]. Consistent with Drezner et al.’s results, Miller and colleagues showed that db-cAMP and 8Br-cAMP treatment enhanced sulfated proteoglycan deposition, which in turn resulted in increased chondrocyte cell volume [112]. These are all indications that cAMP is essential in mediating chondrogenesis and cartilage formation. In the same vein, db-cAMP was again reported to promote cartilage differentiation in the limb-bud mesoderm in both cell and organ cultures [113], suggesting the necessity of cAMP in complex tissue regeneration.
Many of the above data indicate that the effect of cAMP on cartilage tissue is mostly to elicit anabolic processes. PGs, such as PGE2, are thought to be mediating the elevation of cAMP at the onset of chondrogenesis in vitro because the remarkable increase observed in PGs (especially PGE2) synthesis corresponds temporally with cAMP elevation at the onset of chondrogenesis [114–117]. By implication, PGE induces significant increase in cAMP content in limb mesenchymal cells [118], while exogenous PGE elicits ‘PDE-inhibitor potentiated’ chondrogenesis in vitro [116]. Endogenous inhibitors of PG synthesis were also shown to suppress in vitro chondrogenesis [116]. Some researchers suggested that cAMP promotes chondrogenesis by the activation of a nuclear cAMP-dependent protein kinase, which phosphorylates a nonhistone chromatin protein that is potentially involved in mediating cartilage-specific gene expression [119]. Other investigators were able to show that it is the close cellular juxtaposition and interaction of limb mesenchymal cells cultured at very high densities (confluent) that lead to spontaneous chondrogenic differentiation in vitro, whereas chondrogenesis fails at low densities, except that the cells remained in a rounded configuration in collagen gels or cytochalasin D [120,121]. This was corroborated by a study by Rodgers et al. in which they observed that db-cAMP exerts its regulatory effect in part by facilitating cell to cell communication during the crucial condensation phase of chondrogenesis [114]. Given that cartilage mainly comprises type II collagen, matrix metalloproteinase 13 (MMP-13) is the main type II collagen-degrading collagenase that marks osteoarthritis progression, and is mostly controlled by stress and inflammatory signals [106]. Hence, any molecule or agent that can inhibit MMP is anticipated to suppress cartilage degradation but to promote cartilage development and repair. In 2007, Karsdal and co-workers induced cartilage degradation in bovine articular cartilage explants using 10 ng/ml oncostatin M and 20 ng/ml tumor necrosis factor (TNF) [122]. They used forskolin (4, 16, or 64 M) or 3-isobutyl-1-methyl xanthine (IBMX; 4, 16, or 64 M) to augment cAMP levels in the cultures and showed that elevated cAMP levels in chondrocytes prevented MMP expression and activity, which in turn inhibited cartilage degradation [122]. Overall, specific cAMP activators or modulators in chondrocytes could serve as potential clinically relevant therapeutics for cartilage degenerative diseases.
Furthermore, a study by Kim et al. demonstrated that PKA activation is required for chondrogenic differentiation [123]. The authors determined this by treating mesenchymal cells with the PKA-specific inhibitors H89 and KT5720 for 4 days. When compared with the control, the accumulation of proteoglycan, as demonstrated by Alcian blue staining, was significantly inhibited by treatment with 1 μM KT5720 and entirely blocked by 20 mM H89 [123]. The data obtained indicated that the inhibition of PKA led to suppression of the chondrogenesis of mesenchymal cells in vitro [123]. The authors also investigated the role of PKA in the expression of the Sox9 gene, which encodes a transcription factor required for chondrocyte differentiation and cartilage formation [123,124]. Based on their RT-PCR data, pre-chondrogenic mesenchymal cells treated with forskolin significantly increased Sox9 mRNA levels compared with cells treated with the PKA inhibitor H89 [123]. Furthemore, Kim et al. examined the downstream signaling pathway of PKA, which involved the regulation of CREB activity via phosphorylation along with other nuclear transcription factors that regulated the expression of cAMP-inducible gene expression during the early stages of chondrogenesis [123]. These results indicated that PKA has an important role in chondrocyte differentiation by downregulating the activities of the Sox9 and CREB transcriptional factors.
cAMP as a modulator of axonal regeneration
The lack of the ability of axons to regenerate after spinal cord injury constitutes a serious health concern for both neuroscientists and clinicians. The inability of spinal axons to regenerate after injury is usually attributed to signaling of F-actin depolymerization and growth cone collapse by molecules such as neurite outgrowth inhibitor (Nogo) and other myelin-associated growth (MAG) inhibitors [125]. The ability of cAMP to neutralize this effect directly by acting on the growth cone or indirectly by transcriptional mechanism renders it a potential therapy [125]. It has been theorized that regeneration of an injured axon mimics the axon development mechanism, where the polymerization of F-actin stimulates the elongation of growth cone filopodia or lamellipodia at their distal ends, adhering to the adhesive extracellular surface while stretching the axon forward via forces generated by an actin–myosin molecular motor [125–127]. By contrast, fixed specimens obtained during the regeneration of a spinal cord transection in the highly regenerating sea lamprey showed that the axon tips lack filopodia and lamellipodia, but are instead filled with neurofilaments (NFs) and have little F-actin [128]. This suggests that, unlike the embryonic development of axons, actin and myosin-based growth cone-pulling mechanisms do not underlie the regeneration of more mature axons in the central nervous system; it might be that this is the result of intracellular protrusive forces, conceivably the synthesis, transport, and aggregation of NFs [129] or polymerization of microtubules (MTs) [130].
Several promising therapeutics have resulted from the identification of cAMP as a modulator of axonal regeneration, such as cAMP analogs [e.g., db-cAMP, 8Br-cAMP, and 8-(4-chlorophenylthio)-adenosine-3,5-cyclic monophosphate (CPT-cAMP)], cAMP activators (e.g., forskolin), PDE inhibitors [e.g., 3-isobutyl-1-methylxanthine (IBMX) or Rolipram], and partial peripheral conditioning lesion. Conditioning lesion is the acquisition of enhanced regeneration potential by the central branch of a dorsal root ganglion neuron via the activation of specific intrinsic growth signaling, after its peripheral branch has been injured [131]. Hence, the central branch can circumvent the inhibitory environment of the spinal cord to regenerate into and beyond a lesion if created in the central nervous system [132,133]. Interestingly, the observed elevation of cAMP in neuron somata enables the axon to outclass MAG inhibitors of elongation by eliciting a transcription-dependent increase in arginase 1 and other regeneration-associated genes [134,135]. Nevertheless, it is sometimes problematic to differentiate the regeneration of injured axons from collateral sprouting by uninjured axons in the conditioning lesion [125]. To differentiate actively growing axons from static or retracting ones and confirm the exclusion of a growth cone mechanism in axon regeneration, fluorescently labeled large reticulospinal axons were visualized in the living, transected lamprey cord with and without the application of cAMP analogs [8-hydroxy adenosine-3,5-cyclic monophosphate (8-OH-cAMP) and db-cAMP], and then studied with 2-photon microscopy [125]. According to Jin and colleagues, the results revealed that regeneration was intermittent; cAMP inhibited initial axon retraction and elevated regeneration up to 11-fold; the increase in regeneration was a result of an increase in the mean velocity of axon growth, but not in the time spent in forward movement; tips of actively regenerating axons had a sharp contour, whereas the static tips were more rounded; no filopodia or lamellipodia were reported, even in db-cAMP-treated samples; axon tips contained vesicle-like inclusions and were highly immunoreactive for neurofilaments during active growth; and F-actin and microtubule staining revealed that F-actin was not concentrated at the leading edge [125]. Taking these results together, the authors concluded that cAMP accelerates the regeneration of lamprey spinal axons without stimulating the formation of growth cones.
Having determined cAMP signaling to be a key player in the conditioning lesion, it was reported by several researchers that intraganglionic injection of cAMP synthetic analogs, such as db-cAMP, could recapitulate the effect of conditioning lesion through the activation of PKA [136–139]. Researchers have reported the transcription-dependent effects of cAMP on regeneration progresses via both PKA-dependent [140] and PKA-independent signaling, through activation of the gene encoding the cytokine interleukin 6 (IL-6) [141] by cAMP. Kilmer and Carlsen had previously shown that, after freeze-lesioning of the sciatic nerve, daily administration of forskolin injection via an implanted osmotic pump resulted in a 40% increase in the rate of sensory nerve regeneration and an almost 40-fold greater elevation in neuronal cAMP compared with an equimolar concentration of the control, isoprenaline [142]. To further ascertain that cAMP modulates nerve regeneration in mammals, the same research group demonstrated that chronically infused forskolin with that of infused db-cAMP, 8Br-cAMP, or theophylline (a known PDE inhibitor) in hamsters resulted in enhanced regeneration, although forskolin and 8-bromo cAMP had the most beneficial effect on axonal elongation whereas theophylline exhibited the largest decrease in the initiation time needed for neurite sprouting [142]
A major set-back reported for forskolin is that, although forskolin alone can increase cAMP levels in normal nerves as well nerves crushed during the axon regeneration period, following nerve transection, cAMP could only be increased by the combination of forskolin (to activate AC) and IBMX, which hydrolyzes cAMP. Therefore, it is possible that PDE inhibition is necessary to increase cAMP levels in nonmyelinating nerves, which exhibited a robust induction of PDE activity [143]. More intriguing was the PDE4 inhibitor rolipram, which exhibited excellent enhancement of peripheral nerve (PN) regeneration after injury and also expedited the reinnervation of denervated skeletal muscles [144,145]. Rolipram, originally developed as an antidepressant, has now been shown to promote neurite outgrowth and axonal regeneration in the presence of myelin inhibitors [139]. The ability to cross the blood–brain barrier makes oral and subcutaneous administration of this drug feasible. In an in vitro and in vivo study by Nikulina and colleagues, in which they performed spinal cord hemisection in adult rats and transplanted embryonic spinal cord tissue into the lesion site, followed by subcutaneous delivery of rolipram, significantly more growth of serotonergic fibers and greater functional recovery were reported in the rolipram-treated transplants [146].
Crosstalk between cAMP and other signal transductions, such as the Rho-A/ROCK pathway as well as intermediates from its downstream effector cascades and transcription factors [i.e., PKA, EPAC, CREB, activation transcription factor III (ATF-3), and STAT-3] have all been suggested to be some of the active roles of cAMP in the successful regeneration of PNs [131]. Although the proregenerative effects of ATF-3 are well reported, they are not strong enough to override MAG inhibitors or to enhance in vivo central axonal regeneration in spinal cord [147,148]. Alternatively, CREB alone is able to overcome MAG inhibitors [149]. This implies that several cAMP activation events occur at the transcriptional level to enhance axon elongation. Given that changes directly elicited by PKA in cytoskeleton effectors at the axon can induce axon growth via disinhibition, MAG activates GTPase, Rho-A, in a p75NTR-dependent manner and Rho-A GTPase transduces via stimulation of Rho-associated kinase (ROCK) to impede axon cytoskeletal assembly [149–150]. It is believed that the inhibition of Rho-A enhances axon growth through disinhibition of cytoskeleton assembly regulated by ROCK [131], as evident by the results of a study of Hiraga et al. [150]. To investigate the effects of db-cAMP on axon regeneration, motor function recovery, and RhoA signal pathway in cerebral ischemia-reperfusion rats, Niu et al. established a middle cerebral artery ischemia-reperfusion model using the nylon 6-occlusion method in 105 SD rats [152]. Staircase test score and semi-quantitative western blot results revealed that db-cAMP enhanced axon regeneration and the recovery of motor function by inhibiting the RhoA signal pathway [152]. Murray and Shewan demonstrated that EPAC expression was developmentally regulated in the rat nervous system, showing that, similar to a gradient of a cAMP agonist, developing axons were attracted to a gradient of a EPAC agonist, and the activation of EPAC promoted dorsal root ganglion (DRG) neurite outgrowth, especially axon regeneration in central nervous system tissue [153]. The authors further showed that EPAC mediates cAMP-regulated axon growth and guidance, and could equally serve as a good target for enhancing axon regeneration after injury while leaving other PKA-dependent cAMP-mediated functions unperturbed [153]. In a recent study, Hellstrom and Harvey extensively reported how the intravitreal injection of CPT-cAMP significantly promoted the regeneration of adult rat ganglion cell (RGC) axons into PNs grafted onto transected optical nerves [154]. To evaluate the neurotrophic influence of db-cAMP on the facial nerve regeneration of Wistar rats, topical cAMP was applied to 32 animals all with transected and sutured right facial nerves [155]. According to Borin et al., behavioral and histometric analyses of the sutured rats showed a possible neurotrophic effect of cAMP on facial nerve regeneration in rats [155]. Kim and co-workers demonstrated that the combination strategy of db-cAMP-treated neural stem/progenitor cell (NSPC)-loaded chitosan channels implanted in a fully transected spinal cord resulted in excellent axonal regeneration into an injury site, as well as functional recovery after 6 weeks of implantation [156].
Intriguingly, studies have shown that low-frequency electrical stimulation of injured PNs and lacerated rat femoral nerves restored function in re-innervated leg muscles [157,158] and accelerated axonal growth [159], respectively. In a separate experiment, the authors revealed that the observed accelerated axonal growth required cAMP and PKA activation [160], which suggests that the low-frequency electrical stimulation was activating upstream or downstream cAMP effectors.
cAMP-mediated ligament growth and repair
Owing to the significant roles of PGE and cAMPs in physiological processes, Saito et al. showed that human periodontal ligament fibroblasts respond to cytokines with the production of cAMP, which was preceded by elevated PGE synthesis [161], suggesting that the inflammatory reactions and roles of fibroblasts in periodontium physiological remodeling are mediated by the aforesaid cytokines [161]. Similarly, Kanaya and colleagues demonstrated that higher extracellular calcium increased fibroblast growth factor 2 (Fgf-2) gene and protein expression levels via a cAMP/PKA-dependent pathway in cementoblasts [162]. First, they showed that increased concentrations of extracellular Ca2+ resulted in higher expression level of Fgf2 mRNA in transgenic mice, but that the CaCl2-stimulated Fgf2 expression required the intracellular cAMP and PKA pathways. They further demonstrated an increase in FGF-2 protein by CaCl2 and its subsequent enhancement by cAMP treatment (8-Br-cAMP and forskolin). Interestingly, the researchers revealed that extracellular Ca2+ influx via Ca2+ channels was not involved in CaCl2-mediated Fgf2 regulation [162]. In an attempt to unravel the therapeutic mechanism of low-power laser irradiation (LPLI) in the proliferation and differentiation of periodontal ligament cells and ligament gene marker expression, Wu et al. reported that LPLI promoted the osteogenic differentiation of human periodontal ligament (hPDL) cells via cAMP. They revealed that the observed LPLI-mediated proliferation and osteogenic differentiation of hPDL cells were regulated by cAMP [163].
Concluding remarks and future directions
Owing to the implication of cAMPs in several physiological processes, many promising treatments have resulted from the identification of cAMP as a modulator of musculoskeletal regeneration because of reported advantages in multi-functionality, commercial availability, target specificity, and demonstrated usage in clinical studies. However, the activation of cAMP elicits several signaling cascades, some wanted while others might be unwanted for specific purposes. Further identification of site-selective cAMP analogs could yield additional therapeutic agents for musculoskeletal regenerative engineering. The therapeutic strategy of targeting a specific effector of cAMP will be more specific and effective, and produce less detrimental adverse effects, facilitating the design and development of new safer clinically relevant therapies for musculoskeletal regenerative engineering. More so, the efficient use of pharmacological and nonpharmacological strategies to target specific upstream or downstream effectors of cAMPs will complement the currently utilized surgical approaches, resulting in more comprehensive and potentially efficacious musculoskeletal therapies.
The emergence of high-throughput screenings (HTS) has helped the discovery of several novel small molecules, such as cAMP analogs, for musculoskeletal regenerative engineering. Although HTS techniques have long been in existence, they often yield good and reproducible results [5]. In a typical HTS, chemical libraries are often selected based on a desired biological function (phenotype based) or a defined molecular structure (target based) [5,17,18]. These molecules are assayed to identify those that are capable of eliciting desired enzymatic activity or signal cascades as hits. Complementary secondary assays are further used to confirm the biological activities of the hits and to make a final selection of the lead molecule. Unknown molecular targets and pathways of action are finally identified before preclinical studies. New drug screening tools, such as zebrafish, are cheap, quick, and effective in vivo methods to reveal novel cAMP analogs with tissue-regenerative properties. As an embryologically and genetically tractable disease model, zebrafish has attracted overwhelming scientific and preclinical attention because of its genetic similarity to humans, ease of care compared with rodents, transparency that clearly reveals the impact of any genetic mutation or drug treatment, ease of introducing genetic changes, and its ability to produce large numbers of offspring. Zebrafish biology allows ready access to all developmental stages, and the optical clarity of embryos and larvae allows for real-time imaging of developing pathologies [164]. Advanced mutagenesis and screening strategies on a large scale, a phenomenon that is scarce in other vertebrate systems, have created zebrafish models of a variety of human diseases [164]. Hence, zebrafish has been extensively used for modeling human diseases and for drug discovery and development. Coupled to these advances, the development of state-of-the-art analytical techniques that are ultrasensitive and highly specific have expanded the field of drug discovery, drug design, and drug development. Interestingly, several researchers have reported a large number of novel small osteoinductive molecules that were discovered through chemical screens [10,13–22].
A major concern for small-molecule drugs such as cAMP analogs, is their non-target adverse effects. They can easily penetrate the cell membrane to stimulate unwanted physiological response as a result of their small size [6,15,16,47,50,105,165]. In an attempt to circumvent this drawback, researchers have either reduced the frequency of drug administration [16,105] or fabricated drug delivery devices that can deliver these cAMP molecules locally to their target sites with suitable controlled release kinetics [15]. For example, it was previously demonstrated that, even though short administration (12 h) and continuous administration of phenamil both have beneficial effects on osteoblastic differentiation and mineralization at the same time points, short-term treatment of phenamil reduced the risk of nonspecific cytotoxicity compared with continuous phenamil treatment [16]. More so, the increased knowledge in advanced biomaterial science has opened several drug delivery systems as well as strategies to incorporate cAMPs and other small molecules into/onto fabricated scaffolds. These regenerative small molecules have been successfully incorporated into polymeric particulate systems via emulsion techniques [15]; immersion of scaffolds into a saturated drug solution [166]; direct mixing of polymeric solutions and small molecules before scaffold fabrication; and the immobilization of the bioactive molecules onto biomaterials either by physical adsorption, entrapment, covalent bonding, or crosslinking [5,167]. The choice of biomaterials for controlled and/or sustained drug release is dependent on the particular musculoskeletal tissue and specific application. In bone regeneration, for instance, ceramics [e.g., hydroxyapatite (HA), calcium phosphate (CaP), and calcium sulfate (CaS)] have been extensively used for the delivery of osteoinductive molecules because they largely mimic native bone. They are equally characterized by high porosity, large pores for loading a large concentration of drug, as well as a structured pore network that influences drug loading and release kinetics [168]. Simvastatins [169], purmorphamine [170], and bisphosphonates [171] have been successfully delivered using CaP matrices and HA granules (bisphosphonates) [172]. Ceramics have also been surface functionalized with silanes in an attempt to improve their absorption capabilities [173]. Natural and synthetic polymers, such as PLGA, polyurethanes, collagen, gelatin, and methylcellulose, are fast replacing ceramics in the delivery of osteoinductive molecules because of their biodegradability and wider options for surface immobilization. Pradeep et al. demonstrated in clinical trials that statin-loaded methylcellulose gels significantly promoted intrabony defect fill for all statin drug delivery systems evaluated in treating patients with chronic periodontitis [174–177]. Another group of researchers combined the osteoinductive properties of ceramics with the biodegradable and tunable properties of polymers to design efficient drug delivery systems [5,178]. In the recent past, Moore and co-workers developed and patented a novel composite bone-enhancing implant comprising β-TCP mixed with poly(lactide-co-caprolactone) or collagen [178]. They loaded these scaffolds with cAMP analogs and other osteoinductive small molecules and demonstrated controlled release of the small molecules with high osteogenic properties in vitro and in vivo [178].
Finally, the integration of cutting-edge engineering technologies, advanced biomaterials science, stem cell science, bioactive molecules, cellular and developmental biology, physics, and clinical translation will help address the challenges of regenerating complex tissues. Intriguingly, 3D bioprinting is central to all the aforementioned fields in that it is a tissue regeneration technique that uses layer-by-layer precise positioning of biological materials, biochemicals, and living cells, with spatial control of the placement of functional components [179]. Hence, with 3D printing, it is feasible to incorporate different cAMP analogs for complex tissue regeneration and perhaps limb regeneration. The timely introduction of 3D bioprinting is anticipated to revolutionize the field of musculoskeletal regenerative engineering. Combinations of biomimicry, autonomous self-assembly, and mini tissue-building blocks are essential to fabricate a complex 3D biological structure with multiple functional, structural, and mechanical properties for clinical restoration [179]. High-throughput 3D-bioprinted tissue models for research, drug discovery, and toxicology are other fascinating applications that can be harnessed from additive manufacturing [179].
Highlights.
Regenerative engineering is a new concept for regenerating injured tissues
Regenerative small molecules have been proposed to regenerate musculoskeletal tissues
Cyclic AMP and its analogues have gained its importance in tissue regeneration
Acknowledgments
The authors gratefully acknowledge funding from the Raymond and Beverly Sackler Center for Biomedical, Biological, Physical and Engineering Sciences and NIH DP1 AR068147.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Laurencin CT and Khan Y (2012) Regenerative engineering. Sci. Transl. Med 4, 160ed9. [DOI] [PubMed] [Google Scholar]
- 2.Phan AQ et al. (2015) Positional information in axolotl and mouse limb extracellular matrix is mediated via heparan sulfate and fibroblast growth factor during limb regeneration in the axolotl (Ambystoma mexicanum). Regeneration 2, 182–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cushnie EK et al. (2014) Simple signaling molecules for inductive bone regenerative engineering. PLoS ONE 9, e101627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laurencin CT and Nair LS (2016) The quest toward limb regeneration: a regenerative engineering approach. Regenerative Biomaterials 3, 123–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elizabeth RB (2015) Targeted delivery as key for the success of small osteoinductive molecules. Adv. Drug Deliv. Rev 94, 13–27 [DOI] [PubMed] [Google Scholar]
- 6.Lo K-W. (2011) Current patents on osteoinductive molecules for bone tissue engineering. Recent Pat. Biomed. Eng 4, 153–167 [Google Scholar]
- 7.Cipitria A et al. (2013) Polycaprolactone scaffold and reduced rhBMP-7 dose for the regeneration of critical-sized defects in sheep tibiae. Biomaterials 34, 9960–9968 [DOI] [PubMed] [Google Scholar]
- 8.Govender S et al. (2002) Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Joint Surg. Am 84A, 2123–2134 [DOI] [PubMed] [Google Scholar]
- 9.White AP et al. (2007) Clinical applications of BMP-7/OP-1 in fractures, nonunions and spinal fusion. Int. Orthop 31, 735–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lo K-W. et al. (2012) The role of small molecules in musculoskeletal regeneration. Regen. Med 7, 535–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awale G et al. (2017) Engineered bone tissue with naturally-derived small molecules. Curr. Pharm. Des 7, 535–549 [DOI] [PubMed] [Google Scholar]
- 12.Moore NM et al. (2010) The use of immobilized osteogenic growth peptide on gradient substrates synthesized via click chemistry to enhance MC3T3-E1 osteoblast proliferation. Biomaterials 31, 1604–1611 [DOI] [PubMed] [Google Scholar]
- 13.Lo KW et al. (2014) Small-molecule based musculoskeletal regenerative engineering. Trends Biotechnol. 32, 74–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laurencin CT et al. (2014) Delivery of small molecules for bone regenerative engineering: preclinical studies and potential clinical applications. Drug Discov. Today 19, 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo WH et al. (2014) Evaluating the feasibility of utilizing the small molecule phenamil as a novel biofactor for bone regenerative engineering. J. Tissue Eng. Regen. Med 8, 728–736 [DOI] [PubMed] [Google Scholar]
- 16.Lo WH et al. (2016) Short-term administration of small molecule phenamil induced a protracted osteogenic effect on osteoblast-like MC3T3-E1 cells. J. Tissue Eng. Regen. Med 10, 518–526 [DOI] [PubMed] [Google Scholar]
- 17.Wu TY and Ding S (2006) Applying chemical tools to the discovery of novel regenerative medicine. Drug Discov. Today Technol 3, 255–260 [DOI] [PubMed] [Google Scholar]
- 18.Brey DM et al. (2011) High-throughput screening of a small molecule library for promoters and inhibitors of mesenchymal stem cell osteogenic differentiation. Biotechnol. Bioeng 108, 163–174 [DOI] [PubMed] [Google Scholar]
- 19.Xu Y et al. (2008) A chemical approach to stem-cell biology and regenerative medicine. Nature 453, 338–344 [DOI] [PubMed] [Google Scholar]
- 20.Alves H et al. (2011) High-throughput assay for the identification of compounds regulating osteogenic differentiation of human mesenchymal stromal cells. PLoS One 6, e26678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu X et al. (2002) A small molecule with osteogenesis inducing activity in multipotent mesenchymal progenitor cells. J. Am. Chem. Soc 124, 14520–14521 [DOI] [PubMed] [Google Scholar]
- 22.North TE et al. (2007) Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans DH and Abrahamse H (2009) A review of laboratory-based methods to investigate second messengers in low-level laser therapy (LLLT). Medical Laser Application 24, 201–215 [Google Scholar]
- 24.Newton AC et al. (2016) Second messengers. Cold Spring Harb. Perspect. Biol 8, a005926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mouriño V et al. (2012) Metallic ions as therapeutic agents in tissue engineering scaffolds: an overview of their biological applications and strategies for new developments. J. R. Soc. Interface 9, 401–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nojima H et al. (2015) Sphingolipids in liver injury, repair and regeneration. Biol. Chem 396, 633–643 [DOI] [PubMed] [Google Scholar]
- 27.Buono R et al. (2012) Nitric oxide sustains long-term skeletal muscle regeneration by regulating fate of satellite cells via signaling pathways requiring Vangl2 and cyclic GMP. Stem Cells 30, 197–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doorn J et al. (2012). Diverse effects of cyclic amp variants on osteogenic and adipogenic differentiation of human mesenchymal stromal cells. Tissue Eng. Part A 18, 1431–1442 [DOI] [PubMed] [Google Scholar]
- 29.Alexandra M et al. (2014) The role of cyclic nucleotide signaling pathways in cancer: targets for prevention and treatment. Cancers 6, 436–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sassone-Corsi P (2012) The cyclic AMP pathway. Cold Spring Harb. Perspect. Biol 4, a011148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rehmann H et al. (2007) Capturing cyclic nucleotides in action: snapshots from crystallographic studies. Nat. Rev. Mol. Cell Biol 8, 63–73 [DOI] [PubMed] [Google Scholar]
- 32.Gold MG et al. (2013) Local cAMP signaling in disease at a glance. J. Cell Sci 126, 4537–4543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keshwani MM et al. (2015) Mechanisms of cyclic AMP/protein kinase A-and glucocorticoid-mediated apoptosis using S49 lymphoma cells as a model system. Proc. Natl. Acad. Sci. U S A 112, 12681–12686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Insel PA et al. (2012) Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol. 204, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin. Orthop. Relat. Res 355, S7–S21 [DOI] [PubMed] [Google Scholar]
- 36.Ferguson C et al. (1999) Does adult fracture repair recapitulate embryonic skeletal formation. Mech. Dev 87, 57–66 [DOI] [PubMed] [Google Scholar]
- 37.Dimitriou R et al. (2011) Bone regeneration: current concepts and future directions. BMC Medicine 9, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taghavi CE et al. (2010) Bone morphogenetic protein binding peptide mechanism and enhancement of osteogenic protein-1 induced bone healing. Spine 35, 2049–2056 [DOI] [PubMed] [Google Scholar]
- 39.Wong E et al. (2013) A novel low-molecular-weight compound enhances ectopic bone formation and fracture repair. J Bone Joint Surg. Am 95, 454–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lad SP et al. (2013) Cancer after spinal fusion: the role of bone morphogenetic protein. Neurosurgery 73, 440–449 [DOI] [PubMed] [Google Scholar]
- 41.Epstein PM (2012). Bone and the cAMP signaling pathway: emerging therapeutics In Bone-Metabolic Functions and Modulators (eds), pp. 271–287, Springer [Google Scholar]
- 42.Yin T et al. (1998) cAMP stimulates osteoblast-like differentiation of calcifying vascular cells. Potential signaling pathway for vascular calcification. J. Biol. Chem 273, 7547–7553 [DOI] [PubMed] [Google Scholar]
- 43.Siddappa R et al. (2008) cAMP/PKA pathway activation in human mesenchymal stem cells in vitro results in robust bone formation in vivo. Proc. Natl. Acad. Sci. U S A 105, 7281–7286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siddappa R et al. (2010) Timing, rather than the concentration of cyclic AMP, correlates to osteogenic differentiation of human mesenchymal stem cells. J. Tissue Eng. Regen. Med 4, 356–365 [DOI] [PubMed] [Google Scholar]
- 45.Siddappa R et al. (2009) cAMP/PKA signaling inhibits osteogenic differentiation and bone formation in rodent models. Tissue Eng. Part A 15, 2135–2143 [DOI] [PubMed] [Google Scholar]
- 46.Doorn J et al. (2012) Forskolin enhances in vivo bone formation by human mesenchymal stromal cells. Tissue Eng. Part A 18, 558–567 [DOI] [PubMed] [Google Scholar]
- 47.Lo KW et al. (2012) The small molecule PKA-specific cyclic AMP analogue as an inducer of osteoblast-like cells differentiation and mineralization. J. Tissue Eng. Regen. Med 6, 40–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rangarajan S et al. (2003) Cyclic AMP induces integrin-mediated cell adhesion through Epac and Rap1 upon stimulation of the beta 2–adrenergic receptor. J. Cell Biol 160, 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Enserink JM et al. (2004) The cAMP Epac-Rap1 pathway regulates cell spreading and cell adhesion to laminin-5 through the alpha3beta1 integrin but not the alpha6beta4 integrin. J. Biol. Chem 279, 44889–44896 [DOI] [PubMed] [Google Scholar]
- 50.Lo KW et al. (2011) Activation of cyclic amp/protein kinase: a signaling pathway enhances osteoblast cell adhesion on biomaterials for regenerative engineering. J. Orthop. Res 29, 602–608 [DOI] [PubMed] [Google Scholar]
- 51.de Rooij J et al. (1998) Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477 [DOI] [PubMed] [Google Scholar]
- 52.Carmona G et al. (2008) Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood 111, 2640–2646 [DOI] [PubMed] [Google Scholar]
- 53.Rosales C et al. (1995) Signal transduction by cell adhesion receptors. Biochim. Biophys. Acta 1242, 77–98 [DOI] [PubMed] [Google Scholar]
- 54.Marquis ME et al. (2009) Bone cells biomaterials interactions. Front. Biosci 14, 1023–1067 [DOI] [PubMed] [Google Scholar]
- 55.Carroll SH et al. (2012) A2B adenosine receptor promotes mesenchymal stem cell differentiation to osteoblasts and bone formation in vivo. J. Biol. Chem 287, 15718–15727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grefte S et al. (2007) Concise review: skeletal muscle development and regeneration. Stem Cells Dev. 16, 857–868 [DOI] [PubMed] [Google Scholar]
- 57.Berdeaux R and Stewart R (2012) Intracellular signal for skeletal muscle adaptation: cAMP signaling in skeletal muscle adaptation: hypertrophy, metabolism, and regeneration. Am. J. Physiol. Endocrinol. Metab 303, E1–E17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Alice E et al. (2005) Protein kinase A signalling via CREB controls myogenesis induced by Wnt proteins. Nature 433, 317–322 [DOI] [PubMed] [Google Scholar]
- 59.Christ B and Ordahl CP (1995) Early stages of chick somite development. Anat. Embryol 191, 381–396 [DOI] [PubMed] [Google Scholar]
- 60.Ordahl C (2000) Somitogenesis. 2, 129–268 [Google Scholar]
- 61.Wagers AJ and Conboy IM (2005) Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell 122, 659–667 [DOI] [PubMed] [Google Scholar]
- 62.Carlsen RC (1975) The possible role of cyclic AMP in the neurotrophic control of skeletal muscle. J. Physiol 247, 343–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zalin RJ and Montague W (1975) Changes in cyclic AMP, adenylate cyclase and protein kinase levels during the development of embryonic chick skeletal muscle. Exp. Cell Res 93, 55–62 [DOI] [PubMed] [Google Scholar]
- 64.Keren A et al. (2008) A p38 MAPK–CREB pathway functions to pattern mesoderm in Xenopus. Dev. Biol 322, 86–94 [DOI] [PubMed] [Google Scholar]
- 65.Griffin CA et al. (2009) MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev. Cell 17, 649–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hawke TJ and Garry DJ (2001) Myogenic satellite cells: physiology to molecular biology. J Appl. Physiol 91,534–551 [DOI] [PubMed] [Google Scholar]
- 67.Stewart R et al. (2011) CREB is activated by muscle injury and promotes muscle regeneration. PLoS ONE 6, e24714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryall JG et al. (2008) Intramuscular beta2-agonist administration enhances early regeneration and functional repair in rat skeletal muscle after myotoxic injury. J. Appl. Physiol 105, 165–172 [DOI] [PubMed] [Google Scholar]
- 69.Goldfarb AH et al. (1989) Intensity and duration of exercise effects on skeletal muscle cAMP, phosphorylase, and glycogen. J. Appl. Physiol 66, 190–194 [DOI] [PubMed] [Google Scholar]
- 70.Soderling TR et al. (1970) Inactivation of glycogen synthetase and activation of phosphorylase kinase by muscle adenosine 3′,5′-monophosphate-dependent protein kinases. J. Biol. Chem 245, 6317–6328 [PubMed] [Google Scholar]
- 71.Hartley LH et al. (1972) Multiple hormonal responses to graded exercise in relation to physical training. J. Appl. Physiol 33, 602–606 [DOI] [PubMed] [Google Scholar]
- 72.Chiavegatti T et al. (2008) Skeletal muscle expresses the extracellular cyclic. AMP-adenosine pathway. Br. J. Pharmacol 153, 1331–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Franko A et al. (2008) CREB-1alpha is recruited to and mediates upregulation of the cytochrome c promoter during enhanced mitochondrial biogenesis accompanying skeletal muscle differentiation. Mol. Cell Biol 28, 2446–2459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Skura CL et al. (2008) Albuterol increases lean body mass in ambulatory boys with Duchenne or Becker muscular dystrophy. Neurology 70, 137–143 [DOI] [PubMed] [Google Scholar]
- 75.Fowler EG et al. (2004) Pilot trial of albuterol in Duchenne and Becker muscular dystrophy. Neurology 62, 1006–1008 [DOI] [PubMed] [Google Scholar]
- 76.Lynch GS and Ryall JG (2008) Role of beta-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev 88, 729–767 [DOI] [PubMed] [Google Scholar]
- 77.Baviera AM et al. (2007) Pentoxifylline inhibits Ca2+-dependent and ATP proteasome-dependent proteolysis in skeletal muscle from acutely diabetic rats. Am. J. Physiol. Endocrinol. Metab 292, E702–E708 [DOI] [PubMed] [Google Scholar]
- 78.Navegantes LC et al. (2000) Role of adrenoceptors and cAMP on the catecholamine-induced inhibition of proteolysis in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab 279, E663–E668 [DOI] [PubMed] [Google Scholar]
- 79.Hinkle RT et al. (2005) Phosphodiesterase 4 inhibition reduces skeletal muscle atrophy. Muscle Nerve 32, 775–781 [DOI] [PubMed] [Google Scholar]
- 80.Minetti GC et al. (2011) Galphai2 signaling promotes skeletal muscle hypertrophy, myoblast differentiation, and muscle regeneration. Sci. Signal 4, ra80. [DOI] [PubMed] [Google Scholar]
- 81.Baviera AM et al. (2010) Involvement of cAMP/Epac/PI3K-dependent pathway in the antiproteolytic effect of epinephrine on rat skeletal muscle. Mol. Cell Endocrinol 315, 104–112 [DOI] [PubMed] [Google Scholar]
- 82.Brennesvik EO et al. (2005) Adrenaline potentiates insulin-stimulated PKB activation via cAMP and Epac: implications for cross talk between insulin and adrenaline. Cell Signal 17, 1551–1559 [DOI] [PubMed] [Google Scholar]
- 83.Du M et al. (2008) Protein kinase A represses skeletal myogenesis by targeting myocyte enhancer factor 2D. Mol. Cell Biol 28, 2952–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Berdeaux R et al. (2007) SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat. Med 13, 597–603 [DOI] [PubMed] [Google Scholar]
- 85.Risau W (1995) Differentiation of endothelium. FASEB J. 9, 926–933 [PubMed] [Google Scholar]
- 86.Asai J et al. (2006) Dibutyryl cAMP influences endothelial progenitor cell recruitment during wound neovascularization. J. Invest. Dermatol 126, 1159–1167 [DOI] [PubMed] [Google Scholar]
- 87.Asahara T et al. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 [DOI] [PubMed] [Google Scholar]
- 88.Galiano RD et al. (2004) Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol 164, 1935–1947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kalka C et al. (2000) Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc. Natl. Acad. Sci. USA 97, 3422–3427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Majima M et al. (2000) Cyclo-oxygenase-2 enhances basic fibroblast growth factor-induced angiogenesis through induction of vascular endothelial growth factor in rat sponge implants. Br. J. Pharmacol 130, 641–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amano H et al. (2001) Adenylate cyclase / protein kinase A signaling pathway enhances angiogenesis through induction of vascular endothelial growth factor in vivo. Jpn. J. Pharmacol 87, 181–188 [DOI] [PubMed] [Google Scholar]
- 92.Namkoong S et al. (2009) Forskolin increases angiogenesis through the coordinated cross-talk of PKA-dependent VEGF expression and Epac-mediated PI3K/Akt/eNOS signaling. Cell Signal 21, 906–915 [DOI] [PubMed] [Google Scholar]
- 93.RALEIGH ML et al. (2016) Physical and chemical signals that promote vascularization of capillary-scale channels. Cell Mol. Bioeng 9, 73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Price GM (2008) Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc. Res 76, 46–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong KHK et al. (2010) The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 31, 4706–4714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Takagi H et al. (1996) Adenosine mediates hypoxic induction of vascular endothelial growth factor in retinal pericytes and endothelial cells. Invest. Ophthalmol. Vis. Sci 37, 2165–2176 [PubMed] [Google Scholar]
- 97.Claffey KP et al. (1992) Vascular endothelial growth factor. Regulation by cell differentiation and activated second messenger pathways. J. Biol. Chem 267, 16317–16322 [PubMed] [Google Scholar]
- 98.Fredriksson JM et al. (2000) Norepinephrine induces vascular endothelial growth factor gene expression in brown adipocytes through a beta-adrenoreceptor/cAMP/protein kinase A pathway involving Src but independently of Erk1/2. J. Biol. Chem 275, 13802–13811 [DOI] [PubMed] [Google Scholar]
- 99.Casibang M et al. (2001) Prostaglandin E2 and vasoactive intestinal peptide increase vascular endothelial cell growth factor mRNAs in lung cancer cells. Lung Cancer 31, 203–212 [DOI] [PubMed] [Google Scholar]
- 100.Cheng T et al. (1998) Prostaglandin E2 induces vascular endothelial growth factor and basic fibroblast growth factor mRNA expression in cultured rat Muller cells. Invest. Ophthalmol. Vis. Sci 39, 581–591 [PubMed] [Google Scholar]
- 101.Pueyo ME et al. (1998) Regulation of vascular endothelial growth factor expression by cAMP in rat aortic smooth muscle cells. Exp. Cell Res 238, 354–358 [DOI] [PubMed] [Google Scholar]
- 102.Harada S et al. (1994) Induction of vascular endothelial growth factor expression by prostaglandin E2 and E1 in osteoblast. J. Clin. Invest 93, 2490–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hoper MM et al. (1997) Prostaglandins induce vascular endothelial growth factor in a human monocytic cell line and rat lungs via cAMP. Am. J. Respir. Cell Mol. Biol 17, 748–756 [DOI] [PubMed] [Google Scholar]
- 104.Acarregui MJ et al. (1999) Vascular endothelial growth factor gene expression in human fetal lung in vitro. Am. J. Respir. Cell Mol. Biol 20, 14–23 [DOI] [PubMed] [Google Scholar]
- 105.Lo KW et al. (2013) One-day treatment of small molecule 8-bromo-cyclic AMP analogue induces cell–based VEGF production for in vitro angiogenesis and osteoblastic differentiation. J. Tissue Eng. Regen. Med 10.1002/term.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goldring MB (2012) Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. The Adv Musculoskelet Dis. 4, 269–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zuscik MJ et al. (2008) Regulation of chondrogenesis and chondrocyte differentiation by stress. J. Clin. Invest 118, 429–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ho WC et al. (1982) Cyclic nucleotides during chondrogenesis: concentration and distribution in vivo and in vitro. J. Exp. Zool 224, 321–330 [DOI] [PubMed] [Google Scholar]
- 109.Solursh M et al. (1979) Increase in levels of cyclic AMP during avian limb chondrogenesis in vitro. Differentiation 15, 183–186 [DOI] [PubMed] [Google Scholar]
- 110.Kosher RA et al. (1986) Cartilage proteoglycan core protein gene expression during limb cartilage differentiation. Dev. Biol 118, 112–117 [DOI] [PubMed] [Google Scholar]
- 111.Drezner MK et al. (1976) Stimulation of cartilage macromolecule synthesis by adenosine 3,5’-monophosphate. Biochim. Biophys. Acta 425,521–531 [DOI] [PubMed] [Google Scholar]
- 112.Miller RP et al. (1979) Long acting CAMP analogues enhance sulfate incorporation into matrix proteoglycans and suppress cell division of fetal rat chondrocytes in monolayer culture. J. Cell. Physiol 100, 63–76 [DOI] [PubMed] [Google Scholar]
- 113.Kosher RA and Savage MP (1980) Studies on the possible role of cyclic AMP in limb morphogenesis and differentiation. J. Embryol. Exp. Morphol 56, 91–105 [PubMed] [Google Scholar]
- 114.Barbara J et al. (1989) Stimulation of limb cartilage differentiation is dependent on cell density by cyclic AMP. Cell Differ Dev. 28, 179–188 [DOI] [PubMed] [Google Scholar]
- 115.Biddulph DM et al. (1988) Chondrogenesis in chick limb mesenchyme in vitro derived from distal limb bud tips: changes in cyclic AMP and in prostaglandin responsiveness. J. Cell Physiol 136, 81–87 [DOI] [PubMed] [Google Scholar]
- 116.Chepenik DP et al. (1984) Arachidonate metabolism during chondrogenesis in vitro. Calcified Tiss. Int 36, 175–181 [DOI] [PubMed] [Google Scholar]
- 117.Gay SW and Kosher RA (1985) Prostaglandin synthesis during the course of limb cartilage differentiation in vitro. J. Embryol. Exp. Morphol 89, 367–382 [PubMed] [Google Scholar]
- 118.Kosher RA and Gay SW (1985) The effect of prostaglandins on the cyclic AMP content of limb mesenchymal cells. Cell Differ. 17, 159–167 [DOI] [PubMed] [Google Scholar]
- 119.Leonard CM and Newman SA (1987) Nuclear events during early chondrogenesis: phosphorylation of the precartilage 35.5kDa domain-specific chromatin protein and its regulation by cyclic AMP. Dev. Biol 120, 92–100 [DOI] [PubMed] [Google Scholar]
- 120.Zanetti NC and Solursh M (1984) Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J. Cell Biol 99, 115–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Caplan AI (1970) Effects of the nicotinamide-sensitive teratogen 3-acetylpyridine on chick limb cells in culture. Exp. Cell Res 62, 341–355 [DOI] [PubMed] [Google Scholar]
- 122.Karsdal MA et al. (2007) Induction of increased cAMP levels in articular chondrocytes blocks matrix metalloproteinase-mediated cartilage degradation, but not aggrecanase-mediated cartilage degradation. Arthritis Rheum. 56, 1549–1558. [DOI] [PubMed] [Google Scholar]
- 123.Kim K-H. and Lee Y-S. (2009) Activation of CREB by PKA promotes the chondrogeneic differentiation of chick limb bud mesenchymal cells. Anim. Cells Syst 13, 289–295 [Google Scholar]
- 124.Huang W et al. (2000) Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9’s ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol. Cell. Biol 20, 4149–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nishiyama M et al. (2003) Cyclic AMP/GMP-dependent modulation of Ca2+ channels sets the polarity of nerve growth-cone turning. Nature 423, 990–995 [DOI] [PubMed] [Google Scholar]
- 126.He Z and Koprivica V (2004) The Nogo signaling pathway for regeneration block. Annu Rev. Neurosci 27, 341–368 [DOI] [PubMed] [Google Scholar]
- 127.Brown J and Bridgman PC (2003) Role of myosin II in axon outgrowth. J. Histochem. Cytochem 51, 421–428 [DOI] [PubMed] [Google Scholar]
- 128.Zhang G et al. (2005) Live imaging of regenerating lamprey spinal axons. Neurorehabil. Neural Repair 19, 46–57 [DOI] [PubMed] [Google Scholar]
- 129.Jacobs AJ et al. (1997) Recovery of neurofilament expression selectively in regenerating reticulospinal neurons. J. Neurosci 17, 5206–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jones SL et al. (2006) Developmental regulation of sensory axon regeneration in the absence of growth cones. J. Neurobiol 66, 1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Knott EP et al. (2014) Cyclic AMP signaling: a molecular determinant of peripheral nerve regeneration. BioMed. Res. Int 2014, 651625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Neumann S and Woolf CJ (1999) Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron 23, 83–91 [DOI] [PubMed] [Google Scholar]
- 133.Richardson PM and Issa VMK (1984) Peripheral injury enhances central regeneration of primary sensory neurons. Nature 309, 791–793 [DOI] [PubMed] [Google Scholar]
- 134.Cai D et al. (2002) Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth, MAG and myelin in vitro. Neuron 35, 711–716 [DOI] [PubMed] [Google Scholar]
- 135.Boeshore KL et al. (2004) Novel changes in gene expression following axotomy of a sympathetic ganglion: a microarray analysis. J. Neurobiol 59, 216–235 [DOI] [PubMed] [Google Scholar]
- 136.Neumann S et al. (2002) Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron 34, 885–893 [DOI] [PubMed] [Google Scholar]
- 137.Qiu J et al. (2002) Spinal axon regeneration induced by elevation of cyclic AMP. Neuron 34, 895–903 [DOI] [PubMed] [Google Scholar]
- 138.Qiu J et al. (2005) Conditioning injury-induced spinal axon regeneration requires signal transducer and activator of transcription 3 activation. J. Neurosci 25, 1645–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sari S et al. (2008) The role of cyclic AMP signaling in promoting axonal regeneration after spinal cord injury. Exp. Neurol 209, 321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gao Y et al. (2004) Activated CREB is sufficient to overcome inhibitors in myelin and promote spinal axon regeneration in vivo. Neuron 44, 609–621 [DOI] [PubMed] [Google Scholar]
- 141.Cao Z et al. (2006) The cytokine interleukin-6 is sufficient but not necessary to mimic the peripheral conditioning lesion effect on axonal growth. J. Neurosci 26, 5565–5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kilmer SL and Carlsen RC (1987) Chronic infusion of agents that increase cyclic AMP concentration enhances the regeneration of mammalian peripheral nerves in vivo. Exp. Neurol 95, 357–367 [DOI] [PubMed] [Google Scholar]
- 143.Walikonis RS and Poduslo JF (1998) Activity of cyclic AMP phosphodiesterases and adenylyl cyclase in peripheral nerve after crush nd permanent transection injuries. J. Biol. Chem 273, 9070–9077 [DOI] [PubMed] [Google Scholar]
- 144.Gordon T et al. (2009) Accelerating axon growth to overcome limitations in functional recovery after peripheral nerve injury. Neurosurgery 65, A132–A144 [DOI] [PubMed] [Google Scholar]
- 145.Udina E et al. (2010) Rolipram-induced elevation of cAMP or chondroitinase ABC breakdown of inhibitory proteoglycans in the extracellular matrix promotes peripheral nerve regeneration. Exp. Neurol 223, 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nikulina E et al. (2004) The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc. Natl. Acad. Sci. U. S. A 101, 8786–8790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Seijffers R et al. (2007) ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J. Neurosci 27, 7911–7920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bareyre FM et al. (2011) In vivo imaging reveals a phase-specific role of stat3 during central and peripheral nervous system axon regeneration. Proc. Natl. Acad. Sci. U. S. A 108, 6282–6287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yamashita T et al. (2002) The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol 157, 565–570, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cheng C et al. (2008) Activated RHOA and peripheral axon regeneration. Exp. Neurol 212, 358–369 [DOI] [PubMed] [Google Scholar]
- 151.Hiraga A et al. (2006) Rho-kinase inhibition enhances axonal regeneration after peripheral nerve injury. J. Peripher. Nerv. Syst 11, 217–224 [DOI] [PubMed] [Google Scholar]
- 152.Niu L et al. (2010) db-Cyclic adenosine monophosphate promotes axon regeneration and motor function recovery in cerebral ischemia-reperfusion rats. Neurology India 58, 195. [DOI] [PubMed] [Google Scholar]
- 153.Murray AJ and Shewan DA (2008). Epac mediates cyclic AMP-dependent axon growth, guidance and regeneration. Mol. Cell. Neurosci 38 578–588 [DOI] [PubMed] [Google Scholar]
- 154.Hellström M and Harvey AR (2014) Cyclic {AMP} and the regeneration of retinal ganglion cell axons. Int. J. Biochem. Cell Biol 56, 66–73 [DOI] [PubMed] [Google Scholar]
- 155.Borin A et al. (2008) Influence of cyclic AMP on facial nerve regeneration in rats. Rev. Brasil. Otorrinolaringol 74, 675–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim H et al. (2011) Effects of dibutyryl cyclic-AMP on survival and neuronal differentiation of neural stem/progenitor cells transplanted into spinal cord injured rats. PLoS ONE 6, e21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nix WA and Hopf HC (1983) Electrical stimulation of regenerating nerve and its effect on motor recovery. Brain Res. 272, 21–25 [DOI] [PubMed] [Google Scholar]
- 158.Pockett S and Gavin RM (1985) Acceleration of peripheral nerve regeneration after crush injury in rat. Neurosci. Lett 59, 221–224 [DOI] [PubMed] [Google Scholar]
- 159.Al-Majed AA et al. (2000) Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. J. Neurosci, 20, 2602–2608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gordon E et al. (2009) Brief electrical stimulation accelerates axon regeneration in the peripheral nervous system and promotes sensory axon regeneration in the central nervous system. Motor Control 13, 412–441 [DOI] [PubMed] [Google Scholar]
- 161.Saito S et al. (1990) Effects of cytokines on prostaglandin E and cAMP levels in human periodontal ligament fibroblasts in vitro. Arch. Oral Biol 35, 387–395 [DOI] [PubMed] [Google Scholar]
- 162.Kanaya S et al. (2010) Elevated extracellular calcium increases fibroblast growth factor-2 gene and protein expression levels via a cAMP/PKA dependent pathway in cementoblasts. Bone 47, 564–572 [DOI] [PubMed] [Google Scholar]
- 163.Wu JY et al. (2013) Low-power laser irradiation promotes the proliferation and osteogenic differentiation of human periodontal ligament cells via cyclic adenosine monophosphate. Int. J. Oral Sci 5, 85–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Graham J et al. (2007) Animal models of human disease: zebrafish swim into view. Nat. Rev. Genet 8, 353–367 [DOI] [PubMed] [Google Scholar]
- 165.Brouwers L et al. (2011) Network neighbors of drug targets contribute to drug side-effect similarity. PLoS ONE 6, e22187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Monjoa M et al. (2010) In vivo performance of absorbable collagen sponges with rosuvastatin in critical-size cortical bone defects. Acta Biomater. 6, 1405–1412 [DOI] [PubMed] [Google Scholar]
- 167.Nguyen LTH et al. (2012) Electrospun poly(L-lactic acid) nanofibres loaded with dexamethasone to induce osteogenic differentiation of human mesenchymal stem cells. J. Biomater. Sci. Polym. Ed 23, 1771–1791 [DOI] [PubMed] [Google Scholar]
- 168.Colilla M et al. (2008) Recent advances in ceramic implants as drug delivery systems for biomedical applications. Int. J. Nanomedicine 3, 403–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ito T et al. (2013) Preparation of calcium phosphate nanocapsules including simvastatin/deoxycholic acid assembly, and their therapeutic effect in osteoporosis model mice. J. Pharm. Pharmacol 65, 494–502 [DOI] [PubMed] [Google Scholar]
- 170.Gellynck K et al. (2011) Cell attachment and response to photocured, degradable bone adhesives containing tricalcium phosphate and purmorphamine. Acta Biomater. 7, 2672–2677 [DOI] [PubMed] [Google Scholar]
- 171.Pascaud P et al. (2014) Adsorption on apatitic calcium phosphates for drug delivery: interaction with bisphosphonate molecules. J. Mater. Sci. Mater. Med 25, 2373–2381 [DOI] [PubMed] [Google Scholar]
- 172.Seshima H et al. (2006) Control of bisphosphonate release using hydroxyapatite granules, J. Biomed. Mater. Res. B Appl. Biomater 78, 215–221 [DOI] [PubMed] [Google Scholar]
- 173.Treccani L et al. (2013) Yvonne Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 9, 7115–7150 [DOI] [PubMed] [Google Scholar]
- 174.Pradeep AR et al. (2013) Clinical efficacy of subgingivally delivered 1.2% atorvastatin in chronic periodontitis: a randomized controlled clinical trial. J. Periodontol 84, 871–879 [DOI] [PubMed] [Google Scholar]
- 175.Pradeep AR et al. (2012) Clinical efficacy of subgingivally delivered 1.2–mg simvastatin in the treatment of individuals with Class II furcation defects: a randomized controlled clinical trial, J. Periodontol 83, 1472–1479 [DOI] [PubMed] [Google Scholar]
- 176.Pradeep AR et al. (2013) Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. J. Periodontol 84, 24–31 [DOI] [PubMed] [Google Scholar]
- 177.Pradeep AR and Thorat MS (2010) Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J. Periodontol 81, 214–222 [DOI] [PubMed] [Google Scholar]
- 178.Moore MH et al. DePuy Synthes Products. Osteogenic promoting implants and methods of inducing bone growth. European Patent EP2678051 A1
- 179.Murphy SV and Atala A (2014) 3D bioprinting of tissues and organs. Nature Biotechnol. 32, 773–785 [DOI] [PubMed] [Google Scholar]


