Abstract
Background
Coronary artery calcium (CAC) predicts atherosclerotic cardiovascular disease (ASCVD) events, inclusive of coronary heart disease (CHD) and stroke, and is a decision-making aid for primary prevention. The predictive value of CAC categories for CHD and stroke separately, and across sex and race groups of an asymptomatic population is unclear.
Methods
White, Black, and Hispanic participants of Multi-Ethnic Study of Atherosclerosis and Dallas Heart Study underwent CAC measurement at enrollment and were followed for incident ASCVD events. Ten-year CHD-to-stroke incidence ratios across CAC score categories 0, 1 to 99, and ≥100 were assessed. Associations of CAC with incident CHD and stroke events were evaluated using multivariable-adjusted Cox models and multiplicative interactions of CAC with sex/race were tested.
Results
Among 7,042 participants (mean age 57 years, 54% women, 36% Black, 23% Hispanic, 49% CAC=0, 19% CAC ≥100), 574 incident ASCVD events (333 CHD and 241 stroke) were observed over 12.3-year follow-up. 10-year CHD-to-stroke incidence ratio increased significantly across CAC categories in men, women, Whites, Blacks, and Hispanics (all p<0.001). High CAC burden (score ≥100) was independently associated with ASCVD and CHD risk in all groups, and with stroke risk in the overall cohort and Blacks. No sex- or race-based CAC interactions for ASCVD, CHD, and stroke events were observed. Adding CAC to a traditional risk factor model improved risk discrimination and reclassification for CHD, but not for stroke events.
Conclusions
In two population-based cohorts of asymptomatic individuals, 10-year CHD-to-stroke incidence ratio was higher with increasing CAC score categories across sex and race groups, and CAC was consistently a better predictor of CHD than stroke. High CAC burden comparably associated with ASCVD risk across sex and race groups.
Keywords: Calcium score, atherosclerotic cardiovascular disease, risk prediction, primary prevention
Journal Subject Terms: Computerized Tomography (CT), Prognosis, Epidemiology
INTRODUCTION
The 2013 American College of Cardiology (ACC)/American Heart Association (AHA) guidelines for cardiovascular risk assessment recommended using the pooled cohort equations (PCE) for estimating the 10-year risk of developing a first atherosclerotic cardiovascular disease (ASCVD) event, defined as coronary death, nonfatal myocardial infarction, or fatal/nonfatal stroke.(1) These guidelines changed the landscape of cardiovascular risk assessment by using racially diverse cohorts that allowed creation of separate risk estimation equations for Non-Hispanic White and Non-Hispanic Black men and women.(1) Furthermore, the cardiovascular end-point of interest was expanded beyond coronary heart disease (CHD) to include strokes in order to better identify modifiable ASCVD risk in women and in Blacks.(2) However, frequently there is uncertainty in clinical practice over the use of preventive therapies, even after utilizing the PCE, particularly for patients at borderline or intermediate risk.(2) In such a situation, testing for subclinical coronary atherosclerosis is thought to be reasonable.(3)
Coronary artery calcium (CAC) score, a marker of subclinical coronary atherosclerosis, reflects the cumulative exposure to cardiovascular risk factors over the lifetime and can inform shared decision-making regarding the use of preventive therapies.(4, 5) CAC burden independently predicts CHD events, improves CHD risk-discrimination, and correctly reclassifies individuals to appropriate risk categories.(6-9) However, fewer reports have examined the association of CAC with stroke,(10, 11) and the strength of this association is weaker than observed with CHD.(12) The collective evidence regarding the clinical utility of CAC is reflected in the 2018 AHA/ACC Multi-society Cholesterol management guidelines that recommend using CAC score categories 0, 1 to 99, and ≥100 if the decision about statin therapy is uncertain.(3)
The proportion of the two ASCVD subtypes, CHD and stroke, differs across sex and race/ethnic groups.(13) However, the relative predictive value of CAC categories for CHD vs. stroke has not been explored in detail across different demographic groups. Given the importance placed on stroke as an outcome along with the sex and racial variation in ASCVD risk, we sought to understand the performance of guideline-recommended CAC score thresholds to predict CHD and stroke risk in sex and race groups of an asymptomatic population. In this framework, two population-based, multi-ethnic American cohorts – the Multi-Ethnic Study of Atherosclerosis (MESA) and Dallas Heart Study (DHS) offer a unique opportunity to fill this knowledge gap.
METHODS
We performed individual-level data pooling of participants from MESA examination-1 and DHS phase-1 for the current analysis. The data that support the findings of this study are available from the corresponding author upon reasonable request. The MESA and DHS were selected because a pooled sample of these cohorts would be racially diverse and have a large number of participants with CAC scanning performed at enrollment. Additionally, these cohorts were recruited over similar time periods, and CHD and stroke events in both studies were adjudicated over long-term follow-up.
Study cohorts
The study designs for MESA and DHS have been previously published.(14, 15) Briefly, MESA is a population-based cohort study of White, Black, Hispanic, and Chinese individuals aged 45–84 years.(14) Examination-1 was performed between 2000 and 2002, and participants were recruited from six field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota).(14) The DHS is a probability-based, population cohort study of Dallas County (Texas) adults, with deliberate over-sampling of Blacks.(15) DHS phase-1 was conducted between 2000 and 2002 with participants aged 30–65 years completing a detailed in-home survey, laboratory testing, and multiple imaging studies.(15)
Both MESA and DHS were approved by Institutional Review Boards at each site and all participants provided written informed consent at enrollment. For the present analysis, we included White, Black, and Hispanic MESA (N=4838) and DHS (N=2971) participants who were free of prevalent clinical cardiovascular disease, had CAC score measured at time of study enrollment, and were followed for adjudicated ASCVD events. We excluded Chinese MESA participants in this analysis because DHS did not enroll Chinese participants. Cardiovascular risk factor measurement and CAC scanning procedures for both cohorts have been described previously and are discussed in detail in the Supplement.
Atherosclerotic cardiovascular disease events
An ASCVD event was defined as coronary death, nonfatal myocardial infarction, or fatal/nonfatal stroke. In MESA, the primary means of identifying ASCVD events was participant self-report during telephone follow-up calls conducted at 9 to 12-month intervals.(16) A trained interviewer administered a standardized interview during the telephone call to determine any new cardiovascular diagnoses, hospital admissions, and deaths. This information was supplemented by cardiovascular events identified by participant notification, during MESA clinic visits, investigations of other possible events, national death index search, and obituaries or public notices.(16) Medical records and death certificates were requested for all cases; and for participants who died of cardiovascular causes outside the hospital, interviewers contacted the next of kin and requested copies of death certificates. Trained personnel abstracted data from medical records with reports of cardiovascular events. Two blinded physician members of the MESA mortality and morbidity review committee independently classified all cardiovascular events and assigned incidence dates. In case of disagreement, the full mortality and morbidity review committee made the final decision.(16)
In DHS, multiple overlapping sources were utilized for identifying ASCVD events. The main source was the data coordinating center health survey, which is an annual formal survey administered by telephone to all participants to determine any new cardiovascular diagnoses, hospital admissions, and deaths.(9) This source was supplemented by the national death index and the Dallas Fort Worth (DFW) hospital council data initiative, which is a database comprising of 70 of 72 DFW area hospitals. The database consists of 100% of the discharge data from these institutions and is updated on a quarterly basis. The data retrieved from this database includes demographic data, hospitalization dates, discharge status (alive or deceased), primary and 10 secondary diagnoses by ICD-9 code, and in-hospital procedures. All potential cardiovascular events were reviewed by members of the clinical endpoints committee, which consists of DHS investigators. Each event was reviewed for adjudication by two blinded cardiologist reviewers. In the case of disagreement, a third review was required, and this review was considered final or could subsequently be referred to the entire clinical endpoints committee.(9) The detailed criteria for defining coronary death, nonfatal myocardial infarction, or fatal/nonfatal stroke events utilized during ASCVD event adjudication in MESA and DHS cohorts are provided in the Supplement.
Statistical analysis
Baseline characteristics of participants in the overall cohort, and among sex and race groups were described across three CAC score categories: 0, 1 to 99, and ≥100 Agatston Units (AU).(3) Categorical variables were presented as counts (proportions), continuous variables were presented as means (standard deviation) or medians [25th-75th percentile] depending on distribution. Categorical variables were compared using the Chi-square test and continuous variables were compared for a significant trend across CAC categories using the Kruskal Wallis test.
The unadjusted 10-year cumulative incidence of ASCVD and its sub-components, CHD and stroke events, were computed. Only the first ASCVD event for each participant was analyzed, and the 10-year CHD-to-stroke incidence ratio was additionally calculated. A similar analysis for participants stratified by CAC score categories was performed. The 10-year CHD-to-stroke incidence ratio was also compared across CAC score categories within the same sex and racial group participants. Furthermore, the CHD-to-stroke incidence ratio across CAC score categories and predicted 10-year ASCVD risk categories (<7.5%, 7.5–20%, and ≥20%) in the overall cohort was also calculated. The risk prediction model used to estimate 10-year ASCVD risk is described later.
The independent associations of ‘high’ CAC burden (score ≥100 versus 0), ‘moderate’ CAC burden (score 1 to 99 versus 0), and CAC ‘presence’ (score >0 versus 0) with time-to-first cardiovascular event (ASCVD, CHD, or stroke) were assessed using Cox proportional hazards regression models. We stratified baseline hazards by study site, and participants from each site (6 in MESA and 1 in DHS) were given their own baseline hazard function in regression models. Cox models were adjusted for PCE risk factors (age, sex, race, diabetes, smoking, systolic blood pressure, antihypertensive use, total cholesterol, and HDL-C level), FHx, and statin use at baseline. This analysis was first performed in the overall cohort and the multiplicative interactions of CAC score with sex and race were tested. Cox models were further stratified into sex and race groups. Lastly, Cox models were stratified by cohort and were adjusted for educational attainment among MESA participants (education data not available in DHS).
We conducted two sensitivity analyses. First, the association of CAC with time-to-first ASCVD, CHD, or stroke event was determined using Fine and Gray competing risk regression models. Second, Cox regression analyses were performed in a subgroup of participants that met the 2018 Cholesterol Guideline criteria for CAC scanning. We sub-selected participants with predicted 10-year ASCVD risk between 7.5% and 20%, and no statin use at baseline for this analysis.
Finally, the impact of CAC on improving ASCVD, CHD, and stroke risk discrimination and reclassification in the overall cohort, sex and race groups was assessed by computing the change in model calibration (C)-statistic and the net reclassification index (NRI), respectively. A risk prediction model comprising of risk factors used during multivariable adjustment in Cox models was constructed, and change in C-statistic and NRI after CAC score categories were added to the model were studied. We used the 10-year predicted risk cut-off of 7.5% to create low- and high-risk categories for NRI analyses. Also, this risk prediction modeling approach was used for creating the three 10-year ASCVD risk categories (<7.5%, 7.5–20%, and ≥20%) used in the CHD-to-stroke incidence ratio analysis described previously. All statistical analyses were performed using SAS 9.4 (SAS Inc., Cary, North Carolina) and a two-sided p-value less than 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
The study sample consisted of 7,042 participants (4,838 from MESA and 2,204 from DHS) and the mean age was 56.8 (12.7) years, 54.3% were women, 40.2% were White, 36.4% were Black, and 23.4% were Hispanic. No subclinical atherosclerosis was observed in 48.9% (N=3,443), while 31.7% (N=2,232) and 19.4% (N=1,367) had moderate (1 to 99 AU) and high (≥100 AU) CAC burden, respectively (Table 1). Traditional cardiovascular risk factor burden, apart from smoking history, increased across CAC categories and a high CAC burden was observed less frequently in DHS as compared with MESA participants (Table 1). The prevalence of antihypertensive medication use, FHx, and absent CAC was higher, while that of smoking and statin use was lower among women as compared with men (Online Table 1A). White participants were older, more frequently men, had higher CAC scores, higher prevalence of FHx, statin use, and lower prevalence of smoking and diabetes as compared with the other two race groups (Online Table 1B). Women and Black participants in the overall cohort were more frequently DHS participants.
Table 1.
Baseline characteristics of study participants stratified by coronary artery calcium score category
| Participant Characteristics | CAC score zero (n=3,443) |
CAC score 1 to 99 (n=2,232) |
CAC score ≥100 (n=1,367) |
p-value |
|---|---|---|---|---|
| Age (years) | 53.1 (11.5) | 56.2 (12.7) | 66.9 (6.7) | <0.001 |
| Women | 2204 (64.0) | 1110 (49.7) | 512 (37.5) | <0.001 |
| Blacks | 1284 (37.3) | 906 (40.6) | 376 (27.5) | <0.001 |
| Hispanics | 898 (26.1) | 491 (22.0) | 259 (18.9) | <0.001 |
| Smoking | 619 (18.0) | 420 (18.8) | 230 (16.8) | 0.319 |
| Diabetes | 248 (7.2) | 289 (12.9) | 229 (16.8) | <0.001 |
| Systolic blood pressure (mm Hg) | 122.4 (18.9) | 129.1 (18.8) | 133.7 (21.8) | <0.001 |
| Diastolic blood pressure (mm Hg) | 72.8 (10.1) | 75.7 (10.4) | 73.9 (10.7) | <0.001 |
| Antihypertensive use | 861 (25.0) | 753 (33.7) | 682 (49.9) | <0.001 |
| Total cholesterol (mg/dL) | 189.0 (36.2) | 191.0 (37.6) | 194.0 (37.6) | 0.002 |
| High density lipoprotein-cholesterol (mg/dL) | 52.5 (15.1) | 49.2 (13.8) | 49.8 (15.1) | <0.001 |
| Low density lipoprotein-cholesterol (mg/dL) | 113.0 (32.9) | 116.1 (33.4) | 118.0 (33.6) | <0.001 |
| Family history of myocardial infarction | 1392 (40.4) | 1024 (45.9) | 817 (59.8) | <0.001 |
| Statin use | 277 (8.1) | 307 (13.8) | 296 (21.7) | <0.001 |
| CAC score (Agatston units) | 0.0 [0.0–0.0] | 10.5 [2.3–36.5] | 329.5 [179.1–682.7] | <0.001 |
| Dallas Heart Study cohort | 1039 (30.2) | 988 (44.3) | 177 (12.9) | <0.001 |
| ASCVD event | 130 (3.8) | 182 (8.2) | 262 (19.2) | <0.001 |
| CHD event | 57 (1.7) | 97 (4.4) | 179 (13.1) | <0.001 |
| Stroke event | 73 (2.1) | 85 (3.8) | 83 (6.1) | <0.001 |
Values shown are mean (standard deviation) or median [25th-75th percentile] and number (proportion) depending on variable type. Abbreviations – CAC = coronary artery calcium, ASCVD = atherosclerotic cardiovascular disease, CHD = coronary heart disease.
Atherosclerotic cardiovascular disease incidence
Over a median follow-up of 12.3 [10.9–13.3] years, there were 574 adjudicated ASCVD events (130 in no CAC, 182 in moderate CAC, and 262 in high CAC category) in the study cohort. These comprised of 333 CHD (57 in no CAC [28 fatal], 97 in moderate CAC [46 fatal], and 179 in high CAC [100 fatal] category) and 241 stroke events (73 in no CAC [22 fatal], 85 in moderate CAC [34 fatal], and 83 in high CAC [40 fatal] category). The 10-year cumulative incidence of ASCVD, CHD, and stroke events along with CHD-to-stroke incidence ratio in the overall cohort, sex, and race groups is described in Figure 1 and Online Table 2. Overall, the 10-year CHD incidence was significantly higher than the stroke incidence, which was driven by a high CHD-to-stroke incidence ratio among men and White participants (Figure 1). Women on the other hand had a nominally higher incidence of stroke as compared with CHD and the ratio estimate was <1 (Figure 1).
Figure 1. 10-year cumulative incidence of atherosclerotic cardiovascular disease, coronary heart disease, and stroke events.
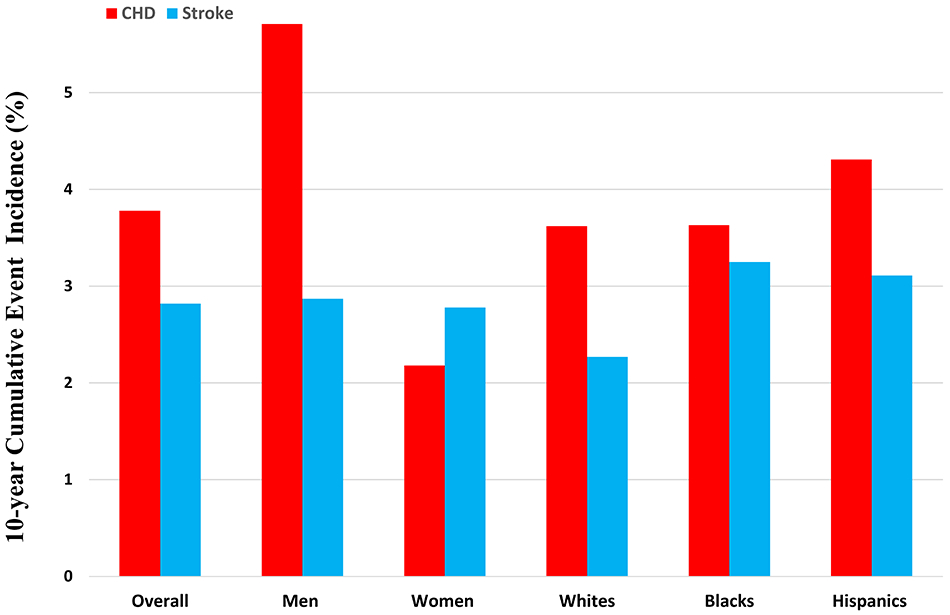
CHD-to-stroke incidence ratio (95% confidence interval) in the overall study cohort was 1.35 (1.15–1.57). This was primarily driven by men (ratio 2.01, 95% CI 1.63–2.49) and Whites (ratio 1.63, 95% CI 1.21–2.15). The ratios for Women, Blacks, and Hispanics were 0.79 (95% CI 0.59–1.01), 1.14 (95% CI 0.87–1.47), and 1.43 (95% CI 0.99–1.95), respectively.
Atherosclerotic cardiovascular disease incidence across CAC score categories
The 10-year cumulative incidence of ASCVD, CHD, and stroke events in the overall cohort, sex, and race groups across CAC score categories is described in Figure 2 and Online Table 2. The cumulative ASCVD incidence increased across CAC categories of none, moderate, and high, such that the estimates were <5%, 5–9%, and >13%, respectively, in all participant groups (Online Table 2). While the CHD and stroke incidence also increased across CAC score categories (Figure 2), there was a relatively greater increase in CHD incidence which is captured by the increasing CHD-to-stroke incidence ratio across CAC score categories (Figure 3) for the overall cohort as well as sex/race groups (p-trend for all groups <0.001). This observation remained consistent after the overall cohort was divided into predicted 10-year ASCVD risk groups (Online Table 3). Notably, in the moderate CAC burden category the ratio was significantly higher in men (1.73, 95% CI 1.19–2.48) as compared with women (0.55, 95% CI 0.31–0.84) (p<0.001). In the high CAC burden category, the CHD incidence was significantly higher than stroke in all groups except women where a nominal trend was observed (Figure 3).
Figure 2. 10-year cumulative incidence of atherosclerotic cardiovascular disease, coronary heart disease, and stroke events across coronary artery calcium score categories.
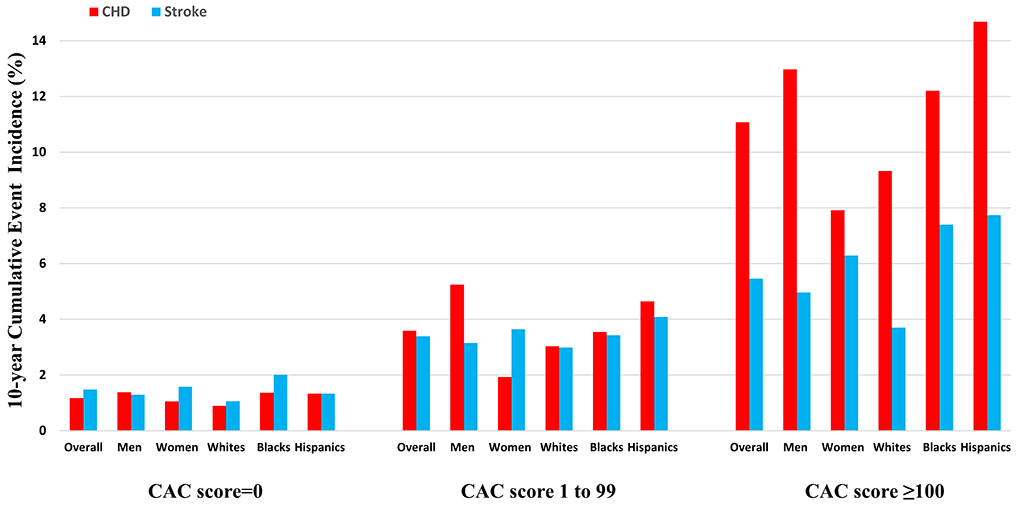
The 10-year cumulative incidence of ASCVD, CHD, and stroke events increased across CAC score categories in the overall cohort and all sex/race groups. The increase in CHD incidence across CAC categories was relatively higher compared with stroke incidence for all participant groups.
Figure 3. CHD-to-stroke cumulative incidence ratio across CAC score categories.
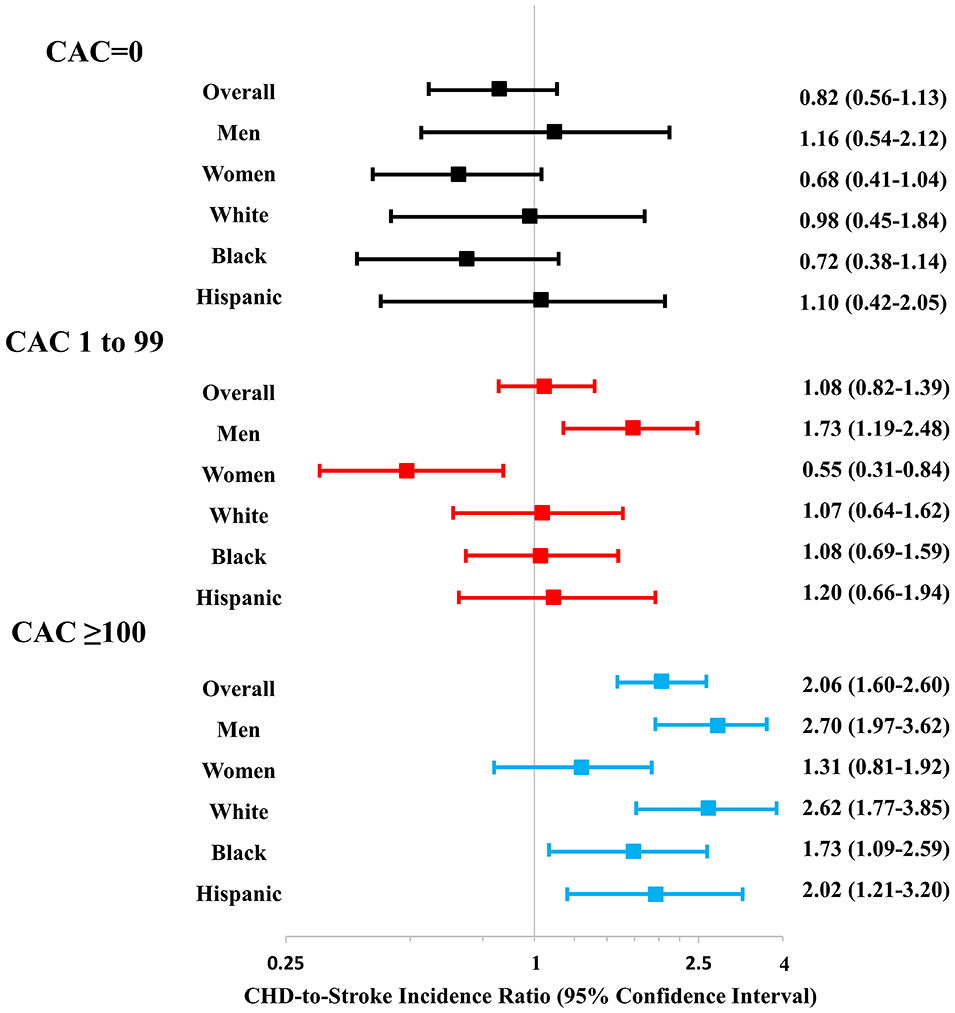
The CHD-to-stroke cumulative incidence ratio increased across three CAC score categories for the overall cohort and the all sex/race groups (all p-trend <0.001)
Independent association of CAC with ASCVD events
Compared with no CAC, a high CAC burden was independently associated with a 2.3- to 3.4-fold increased risk of ASCVD, and a 3.3- to 5.6-fold increased risk of CHD events in the overall cohort and sex/race groups in Cox regression models (Figure 4). This relationship was similar in MESA and DHS separately (Online Table 4), and was only slightly attenuated after adjustment for educational attainment among MESA participants (Online Table 5). High CAC burden was independently associated with stroke risk in the overall cohort and Blacks, but this association was not statistically significant in rest of the demographic groups (Figure 4). Importantly, the strength of this association was consistently lower than what was observed with CHD events in all groups (Figure 4). There was no high CAC-sex or -race interaction for ASCVD, CHD, or stroke events (all p-interaction>0.10).
Figure 4. Association of high coronary artery calcium burden (score ≥100 AU) with incident atherosclerotic cardiovascular disease, coronary heart disease, and stroke events.
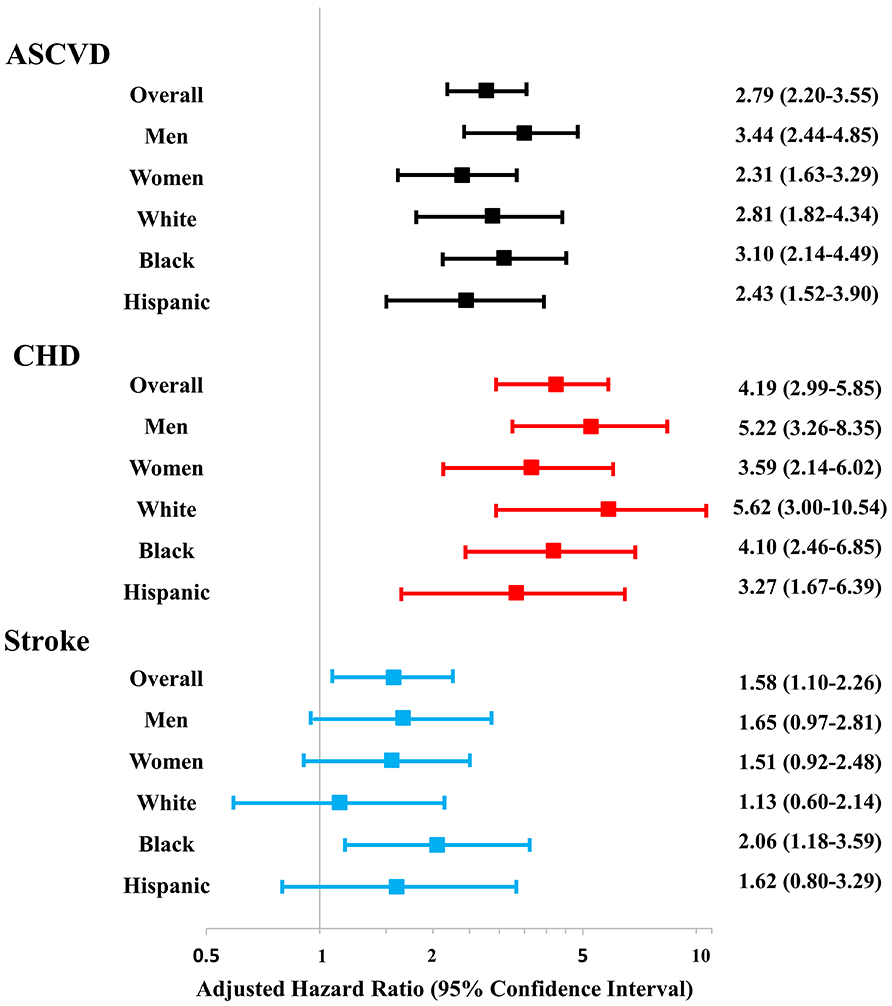
Results presented as adjusted hazard ratio (95% confidence interval) and referent group is participants with a zero CAC score. High CAC burden is independently associated with ASCVD, CHD, and stroke risk in the overall cohort; with ASCVD and CHD risk in all groups; and with stroke risk among Blacks. No significant sex- or race-based interactions for ASCVD, CHD, and stroke risk exist. Cox proportional hazards regression models adjusted for age, sex, race, smoking, diabetes, systolic blood pressure, antihypertensive use, total cholesterol, high density lipoprotein-cholesterol level, family history of myocardial infarction, and statin use.
Similar to high CAC burden, CAC presence was independently associated with ASCVD, CHD, and stroke risk in the overall cohort; and with ASCVD and CHD risk in all study groups when compared with no CAC (Table 2). A significant multiplicative interaction between CAC presence and sex for ASCVD and CHD risk was observed (p-interaction 0.048 and 0.043, respectively), such that the association of CAC presence with ASCVD and CHD risk was stronger among men as compared with women. There was no CAC presence-sex interaction for stroke events, and no CAC presence-race interaction for the three outcomes was observed.
Table 2.
Association of coronary artery calcium score with incident atherosclerotic cardiovascular disease, coronary heart disease, and stroke events
| ASCVD events | CHD events | Stroke events | |||||
|---|---|---|---|---|---|---|---|
| Group | HR (95%CI) | p-value | HR (95%CI) | p-value | HR (95%CI) | p-value | |
| CAC score >0 | Overall | 2.06 (1.67–2.55) | <0.001 | 2.74 (2.02–3.72) | <0.001 | 1.48 (1.09–2.01) | 0.012 |
| Men | 2.59 (1.89–3.56)* | <0.001 | 3.66 (2.35–5.69) † | <0.001 | 1.51 (0.95–2.42) | 0.084 | |
| Women | 1.68 (1.25–2.27)* | <0.001 | 1.99 (1.26–3.12) † | 0.003 | 1.47 (0.98–2.20) | 0.061 | |
| Whites | 2.28 (1.53–3.39) | <0.001 | 3.55 (1.98–6.37) | <0.001 | 1.38 (0.79–2.42) | 0.259 | |
| Blacks | 1.84 (1.33–2.55) | <0.001 | 2.32 (1.47–3.68) | <0.001 | 1.39 (0.87–2.21) | 0.171 | |
| Hispanics | 2.11 (1.40–3.19) | <0.001 | 2.56 (1.39–4.69) | 0.002 | 1.69 (0.95–3.03) | 0.076 | |
| CAC score 1 to 99 | Overall | 1.64 (1.30–2.08) | <0.001 | 1.92 (1.37–2.70) | <0.001 | 1.42 (1.02–1.98) | 0.039 |
| Men | 2.05 (1.45–2.89) ‡ | <0.001 | 2.69 (1.97–4.32)§ | <0.001 | 1.42 (0.85–2.37) | 0.183 | |
| Women | 1.35 (0.96–1.90) ‡ | 0.080 | 1.24 (0.72–2.12) § | 0.436 | 1.44 (0.93–2.24) | 0.100 | |
| Whites | 1.90 (1.23–2.93) | 0.004 | 2.30 (1.20–2.12) | 0.012 | 1.62 (0.89–2.93) | 0.112 | |
| Blacks | 1.30 (0.90–1.87) | 0.161 | 1.54 (0.92–2.58) | 0.101 | 1.09 (0.64–1.84) | 0.753 | |
| Hispanics | 1.92 (1.23–3.01) | 0.004 | 2.14 (1.11–4.13) | 0.023 | 1.74 (0.93–3.24) | 0.084 | |
Referent group is participants with a zero CAC score. Cox proportional hazards regression models adjusted for age, sex, race, smoking, diabetes, systolic blood pressure, antihypertensive use, total cholesterol, high density lipoprotein-cholesterol level, family history of myocardial infarction, and statin use. Abbreviations: HR = hazard ratio, CI = confidence interval, ASCVD = atherosclerotic cardiovascular disease, CHD = coronary heart disease. Bold values indicate statistically significant association (two-sided p-value <0.05).
p-interaction=0.048
p-interaction=0.043
p-interaction=0.065
p-interaction=0.022
Finally, compared with no CAC, moderate CAC burden was independently associated with ASCVD and CHD risk in the overall cohort, men, Whites, and Hispanics; while a nominal association was observed in women and Blacks (Table 2). Similar to CAC presence, there was a significant moderate CAC burden-sex interaction for CHD risk (p-interaction 0.022) and a nominal interaction for ASCVD risk (p=0.065), in that the association of moderate CAC burden with CHD and ASCVD was stronger among men than in women. Moderate CAC burden was independently associated with stroke in the overall cohort and a nominal association was observed in sex/race groups (Table 2). In this CAC category, there was no CAC-sex interaction for stroke and no race-based interactions were observed for the three outcomes.
During Fine and Gray competing risk regression analyses, the association of CAC presence, moderate burden, and high burden with cardiovascular events was qualitatively similar to Cox regression analyses as shown in Online Table 6. Among participants that met the 2018 cholesterol guideline criteria for CAC scanning (N=1,252), we observed that high CAC burden was independently associated with an increased risk of ASCVD events among all participant groups apart from Hispanics (Online Table 7). Additionally, the association of high CAC burden with CHD events was stronger than that for strokes.
Improvement in risk discrimination and reclassification with CAC
Addition of CAC score categories to a traditional risk factor model comprising of PCE risk factors, FHx, and statin use at baseline resulted in a significant improvement in ASCVD and CHD risk discrimination, but not stroke risk, as measured using the change in model c-statistic (Table 3). This observation was consistent across all study groups apart from CHD in Hispanics where a nominal improvement was seen likely due to small number of events. We also observed significant improvement in NRI for CHD events in the overall cohort, men, women, Whites, and Blacks (Online Tables 8A-F), but a similar improvement for stroke events was not observed in any study group.
Table 3.
Change in risk discrimination capability (C-statistic) of a traditional risk factor model* for predicting incident cardiovascular events after addition of coronary artery calcium score categories
| ASCVD events | CHD events | Stroke events | ||||
|---|---|---|---|---|---|---|
| Group | Baseline | Change (95%CI) | Baseline | Change (95%CI) | Baseline | Change (95%CI) |
| Overall | 0.749 | 0.021 (0.011,0.031) | 0.754 | 0.035 (0.020,0.051) | 0.737 | 0.003 (−0.004,0.012) |
| Men | 0.730 | 0.026 (0.010,0.043) | 0.717 | 0.047 (0.023,0.071) | 0.735 | -0.002 (−0.015,0.011) |
| Women | 0.757 | 0.015 (0.003,0.026) | 0.749 | 0.032 (0.007,0.057) | 0.761 | 0.003 (−0.006,0.012) |
| Whites | 0.757 | 0.015 (0.001,0.028) | 0.754 | 0.037 (0.013,0.062) | 0.754 | 0.002 (−0.009,0.014) |
| Blacks | 0.734 | 0.031 (0.012,0.051) | 0.740 | 0.040 (0.013,0.068) | 0.716 | 0.017 (−0.007,0.040) |
| Hispanics | 0.776 | 0.017 (0.002,0.033) | 0.790 | 0.022 (−0.002,0.046) | 0.775 | 0.003 (−0.009,0.015) |
Traditional risk factor model contains age, sex, race, smoking, diabetes, systolic blood pressure, antihypertensive use, total cholesterol, high density lipoprotein-cholesterol level, family history of myocardial infarction, and statin use. Abbreviations: CI = confidence interval, ASCVD = atherosclerotic cardiovascular disease, CHD = coronary heart disease. Bold values indicate statistically significant association (two-sided p-value <0.05).
DISCUSSION
We report three important findings in this study of the impact of sex and race on the predictive value of guideline recommended CAC score categories for CHD and stroke. First, CAC has a stronger association with CHD risk as compared with stroke risk. Second, the guideline-recommended CAC score threshold of ≥100 AU is independently associated with a significant increase in ASCVD risk and its predictive value is comparable across sex and race groups. Third, the hazard associated with moderate CAC burden (CAC 1–99) for future ASCVD events appears to be higher among men as compared with women, primarily driven by lower CHD events among women.
CAC score and ASCVD events
In the overall study population, the 10-year cumulative ASCVD incidence was 6.7%. After stratifying the cohort into guideline recommended CAC score categories, an overwhelming majority of ASCVD events occurred in the high CAC burden group. The observed 10-year ASCVD incidence increased from 2.7% to 7.0% to 16.5% across CAC score categories, supporting the 2018 Cholesterol Guideline’s recommendation of considering CAC score thresholds of 0 AU, 1 to 99 AU, and ≥100 AU to guide statin use in primary prevention settings.(3)
CAC score for predicting Coronary risk versus Stroke risk
Several studies have assessed the predictive performance of CAC for CHD and strokes separately among asymptomatic individuals.(6-11) Our findings are consistent with prior work in terms of CAC score’s strength of association with incident CHD and strokes, but we are the first to systematically study the differential impact of the guideline-recommended CAC score categories on CHD and stroke risk in a combined sample of two large, multi-ethnic, population-based cohorts. This is important in the context of current risk assessment and cholesterol management guidelines where the focus has shifted to ASCVD events.
There are several findings in our study highlighting the superior predictive ability of CAC for CHD as compared with strokes. The incidence of CHD events in the study cohort was 35% higher than strokes, and there was a significant increase in the CHD-to-stroke incidence ratio across CAC categories with a 106% relatively higher CHD incidence observed in the CAC ≥100 AU group. In multivariable adjusted analyses, the strength of the independent association of CAC presence, moderate or high CAC burden with CHD risk was higher than stroke risk. Furthermore, addition of CAC to a traditional risk factor model resulted in a significant improvement in risk discrimination and reclassification for CHD but not for stroke events.
However, despite the greater CHD predictive ability it is important to note that CAC presence and burden was an independent predictor of stroke risk in the overall cohort and high CAC burden independently predicted stroke risk in Blacks. The lack of a significant association in the remaining groups is possibly reflective of low statistical power in these subgroups.
Impact of sex and race on the predictive value of CAC
We explored the impact of sex and race on the predictive value of CAC because of variations in the relative contribution of CHD and stroke events to ASCVD incidence across sex and race/ethnicity.(13) Stroke incidence is known to be higher among women and Blacks. Given the relatively stronger association of CAC with CHD than stroke, it follows that the predictive value of CAC for ASCVD risk would be higher in demographic groups where the relative contribution of CHD to ASCVD incidence is higher than stroke.
In the current analysis men had a similar mean age but a higher CAC score than in women, and White participants had a higher CAC score as compared with the other two race groups, similar to previous reports.(17) However, the unadjusted ASCVD incidence was higher among men and similar across race groups. The difference between men and women was primarily driven by a relatively higher CHD incidence in men such that the CHD-to-stroke incidence ratio estimate among men was 2.0 and among women was 0.8. Among race groups on the other hand, CHD incidence was significantly higher than stroke incidence among White participants with a nominal trend observed among Hispanics. The unadjusted CHD-to-stroke incidence ratio estimate was close to even (~1) for all five sex/race groups in the CAC zero category but changed differentially for men and women with increasing CAC score. While the ratio increased for both men and women, the ratio estimate among men was higher than women in the moderate and high CAC burden categories.
CAC presence and high burden were independently associated with ASCVD and CHD risk among both men and women in multivariable-adjusted Cox models. Moderate CAC burden had a significant association with ASCVD risk in men and a nominal association (p=0.08) was observed in women. Similarly, a significant sex-based interaction for CAC presence and moderate burden, but not high burden, for predicting ASCVD and CHD risk was observed. This is likely related to the relatively lower CHD incidence among women in the moderate CAC burden category.
Lastly, there were no interactions between CAC presence or burden and race for ASCVD, CHD, or stroke risk such that the predictive value of CAC score was similar for White, Black and Hispanic participants of the study cohort. These findings differ from a recent MESA study where CAC was analyzed as a continuous variable and no sex- or race-based interaction with CAC was observed for ASCVD events.(18) Indeed, we did not observe any significant interactions when we assessed continuous CAC scores in our combined cohort (data not shown). Nonetheless, our findings with CAC categories are perhaps more impactful as they align better with recommended use of CAC testing per recent guidelines. Furthermore, recent studies from the CAC consortium, wherein participants underwent clinically indicated CAC scanning, have shown that women as well as Blacks and Hispanics with elevated CAC have a higher risk of cardiovascular mortality as compared with men and Whites, respectively.(19, 20) Our findings among the moderate CAC group are different from the CAC consortium because of possible referral biases due to differences in the two study populations.
Study findings in context of current guidelines
Our findings are particularly relevant in light of the 2018 cholesterol guideline recommending consideration of CAC scanning as a decision-making aid when the decision about initiating statin therapy for primary ASCVD prevention is uncertain. Our findings support the paradigm that CAC=0 is associated with low ASCVD risk, while scores ≥100 AU are associated with a dramatically increased risk across sex and race groups. Furthermore, we also found that CAC ≥100 AU is independently predictive of stroke risk among Blacks.
Strengths
This is the first study analyzing the impact of sex and race on the predictive value of guideline-recommended CAC score thresholds for CHD versus stroke events in middle-aged, asymptomatic American individuals. Our study cohort was large, multi-ethnic and participants had long-term follow-up for adjudicated ASCVD events.
Limitations
The results of our study should be interpreted in the context of its limitations. First, we report findings from observational, population-based cohorts of a pooled sample of adults aged 30 to 84 years (mean age 57 years) that were free of ASCVD at baseline; as such our results may not be generalizable to populations outside the United States and among patients undergoing clinically indicated CAC scanning. Second, we have not considered specific stroke types (ischemic, hemorrhagic, or embolic) and ‘soft’ ASCVD events like transient ischemic attacks and coronary revascularization via percutaneous intervention or coronary artery bypass grafting in our analysis. Third, MESA participants received information regarding their CAC score after scanning which could have altered participant health behavior and we have not accounted for incident use of cardioprotective medications like statins or more aggressive blood pressure management which might have biased our results to null.
CONCLUSIONS
In this large study of two contemporary, multi-ethnic, population-based cohorts we have shown that the predictive value of CAC categories for CHD is better than that for stroke. The CAC score threshold of ≥100 AU is highly predictive of ASCVD risk across demographic groups. CAC presence with a score <100 AU has a stronger association with ASCVD risk among men as compared with women, which is likely related to the relatively higher incidence of CHD events among men, for which CAC performs better. These nuances are important for guiding the patient-clinician shared decision-making process for deciding when to obtain a CAC scan and how to utilize CAC results to mitigate the risk of preventable CHD and stroke events.
Supplementary Material
CLINICAL PERSPECTIVE.
Coronary artery calcium (CAC) is a measure of subclinical coronary atherosclerosis and the 2018 American cholesterol management guidelines recommend that selective use of a CAC score can be helpful for guiding shared decision-making for primary atherosclerotic cardiovascular disease (ASCVD) prevention therapy. ASCVD is a composite of coronary and stroke events, and the incidence of ASCVD and its subtypes varies by sex and race. In this study, we have explored the predictive value of guideline-recommended CAC score categories (0, 1 to 99, and ≥100 Agatston Units) for incident ASCVD, CHD, and stroke events along with the impact of sex and race on this predictive value in a pooled sample of White, Black, and Hispanic participants from Multi-Ethnic Study of Atherosclerosis (MESA) and Dallas Heart Study (DHS). Across CAC categories, we observed an increase in 10-year cumulative ASCVD incidence and a greater increase in CHD incidence as compared with stroke, as captured by the CHD-to-stroke incidence ratio. Furthermore, high CAC burden (score ≥100) was independently associated ASCVD events and its subtypes, but the strength of this association was higher for CHD than for stroke. Lastly, CAC score categories resulted in significant improvement in CHD risk discrimination and reclassification, while such improvements were not seen for stroke risk. These observations were consistent across sex and race groups. Overall, our findings suggest that guideline-recommended CAC score categories are a better predictor of CHD than stroke risk and this predictive value is similar across sex and race groups.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of the MESA and DHS for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Source of Funding
The MESA study is supported by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168 and N01-HC-95169 from the NHLBI, and by grants UL1-TR-000040,UL1-TR-001079, and UL1-TR-001420 from National Center for Advancing Translational Sciences. The DHS was funded by the Donald W. Reynolds Foundation, Las Vegas, Nevada, and partially supported by the National Center for Advancing Translational Sciences of the NIH under award Number UL1TR001105.
Disclosures
A.M. is supported by American Heart Association postdoctoral fellowship award 19POST34400057 and by Abraham J. & Phyllis Katz foundation. P.H.J. has received grant support from the American Heart Association, Novo Nordisk, Sanofi/Regeneron, GlaxoSmithKline, AstraZeneca, and Pfizer and personal fees from Bayer and Regeneron; equity interest in Global Genomics Group. Rest of the authors have no disclosures.
ABBREVIATIONS
- ASCVD
Atherosclerotic Cardiovascular Disease
- CAC
Coronary artery calcium
- CI
Confidence Interval
- DHS
Dallas Heart Study
- EBCT
Electron Beam Computed Tomography
- FHx
Family History of Myocardial Infarction
- HR
Hazard Ratio
- MDCT
Multi-Detector Computed Tomography
- MESA
Multi-Ethnic Study of Atherosclerosis
- NRI
net reclassification index
- PCE
Pooled Cohort Equation
- sHR
sub-distribution Hazard Ratio
REFERENCES
- 1.Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–S73. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Braun LT, Ndumele CE, Smith SC Jr., Sperling LS, Virani SS, Blumenthal RS. Use of Risk Assessment Tools to Guide Decision-Making in the Primary Prevention of Atherosclerotic Cardiovascular Disease: A Special Report From the American Heart Association and American College of Cardiology. Circulation. 2019;139:e1162–e77. [DOI] [PubMed] [Google Scholar]
- 3.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toth PP. Subclinical atherosclerosis: what it is, what it means and what we can do about it. Int J Clin Pract. 2008;62:1246–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greenland P, Blaha MJ, Budoff MJ, Erbel R, Watson KE. Coronary Calcium Score and Cardiovascular Risk. J Am Coll Cardiol. 2018;72:434–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, Greenland P. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA. 2010;303:1610–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erbel R, Mohlenkamp S, Moebus S, Schmermund A, Lehmann N, Stang A, Dragano N, Gronemeyer D, Seibel R, Kalsch H, et al. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: the Heinz Nixdorf Recall study. J Am Coll Cardiol. 2010;56:1397–406. [DOI] [PubMed] [Google Scholar]
- 8.Elias-Smale SE, Proenca RV, Koller MT, Kavousi M, van Rooij FJ, Hunink MG, Steyerberg EW, Hofman A, Oudkerk M, Witteman JC. Coronary calcium score improves classification of coronary heart disease risk in the elderly: the Rotterdam study. J Am Coll Cardiol. 2010;56:1407–14. [DOI] [PubMed] [Google Scholar]
- 9.Paixao AR, Ayers CR, El Sabbagh A, Sanghavi M, Berry JD, Rohatgi A, Kumbhani DJ, McGuire DK, Das SR, de Lemos JA, et al. Coronary Artery Calcium Improves Risk Classification in Younger Populations. JACC Cardiovasc Imaging. 2015;8:1285–93. [DOI] [PubMed] [Google Scholar]
- 10.Hermann DM, Gronewold J, Lehmann N, Moebus S, Jockel KH, Bauer M, Erbel R, Heinz Nixdorf Recall Study Investigative G. Coronary artery calcification is an independent stroke predictor in the general population. Stroke. 2013;44:1008–13. [DOI] [PubMed] [Google Scholar]
- 11.Gibson AO, Blaha MJ, Arnan MK, Sacco RL, Szklo M, Herrington DM, Yeboah J. Coronary artery calcium and incident cerebrovascular events in an asymptomatic cohort. The MESA Study. JACC Cardiovasc Imaging. 2014;7:1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folsom AR, Kronmal RA, Detrano RC, O’Leary DH, Bild DE, Bluemke DA, Budoff MJ, Liu K, Shea S, Szklo M, et al. Coronary artery calcification compared with carotid intima-media thickness in the prediction of cardiovascular disease incidence: the Multi-Ethnic Study of Atherosclerosis (MESA). Arch Intern Med. 2008;168:1333–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 14.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr., Kronmal R, Liu K, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–81. [DOI] [PubMed] [Google Scholar]
- 15.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, et al. The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol. 2004;93:1473–80. [DOI] [PubMed] [Google Scholar]
- 16.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, Liu K, Shea S, Szklo M, Bluemke DA, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–45. [DOI] [PubMed] [Google Scholar]
- 17.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, sex, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–7. [DOI] [PubMed] [Google Scholar]
- 18.Budoff MJ, Young R, Burke G, Jeffrey Carr J, Detrano RC, Folsom AR, Kronmal R, Lima JAC, Liu KJ, McClelland RL, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the multi-ethnic study of atherosclerosis (MESA). Eur Heart J. 2018;39:2401–08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw LJ, Min JK, Nasir K, Xie JX, Berman DS, Miedema MD, Whelton SP, Dardari ZA, Rozanski A, Rumberger J, et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39:3727–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orimoloye OA, Budoff MJ, Dardari ZA, Mirbolouk M, Uddin SMI, Berman DS, Rozanski A, Shaw LJ, Rumberger JA, Nasir K, et al. Race/Ethnicity and the Prognostic Implications of Coronary Artery Calcium for All-Cause and Cardiovascular Disease Mortality: The Coronary Artery Calcium Consortium. J Am Heart Assoc. 2018;7:e010471. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


