Insect-pathogenic Photorhabdus luminescens bacteria are widely used in biocontrol strategies against pests. Very little is known about the life of these bacteria in the rhizosphere. Here, we show that P. luminescens can specifically react to and interact with plant roots. Understanding the adaptation of P. luminescens in the rhizosphere is highly important for the biotechnological application of entomopathogenic bacteria and could improve future sustainable pest management in agriculture.
KEYWORDS: entomopathogenic bacteria, bacteria-plant interaction, entomopathogenic nematodes, phenotypic heterogeneity
ABSTRACT
The number of sustainable agriculture techniques to improve pest management and environmental safety is rising, as biological control agents are used to enhance disease resistance and abiotic stress tolerance in crops. Here, we investigated the capacity of the Photorhabdus luminescens secondary variant to react to plant root exudates and their behavior toward microorganisms in the rhizosphere. P. luminescens is known to live in symbiosis with entomopathogenic nematodes (EPNs) and to be highly pathogenic toward insects. The P. luminescens-EPN relationship has been widely studied, and this combination has been used as a biological control agent; however, not much attention has been paid to the putative lifestyle of P. luminescens in the rhizosphere. We performed transcriptome analysis to show how P. luminescens responds to plant root exudates. The analysis highlighted genes involved in chitin degradation, biofilm regulation, formation of flagella, and type VI secretion system. Furthermore, we provide evidence that P. luminescens can inhibit growth of phytopathogenic fungi. Finally, we demonstrated a specific interaction of P. luminescens with plant roots. Understanding the role and the function of this bacterium in the rhizosphere might accelerate the progress in biocontrol manipulation and elucidate the peculiar mechanisms adopted by plant growth-promoting rhizobacteria in plant root interactions.
IMPORTANCE Insect-pathogenic Photorhabdus luminescens bacteria are widely used in biocontrol strategies against pests. Very little is known about the life of these bacteria in the rhizosphere. Here, we show that P. luminescens can specifically react to and interact with plant roots. Understanding the adaptation of P. luminescens in the rhizosphere is highly important for the biotechnological application of entomopathogenic bacteria and could improve future sustainable pest management in agriculture.
INTRODUCTION
Pests and diseases considerably reduce crop yields. Without prevention programs using chemical pesticides, 70% of agricultural production would be lost (1). The use of agrochemicals ensures adequate crop yields that allow us to feed an increasingly growing population (2). While the use of pesticides has profited agricultural production and management, promiscuous use has led to environmental damage and toxicity toward nontarget organisms (i.e., bees and other wildlife) and human beings (3). Indeed, agricultural workers and people exposed to agrochemicals through occupational use (eating food, drinking liquids containing agrochemical residues, or inhalation or contact with pesticide-contaminated air) are at increasing risks of leukemia and myeloma (4). Furthermore, pesticides in the soil can interact with the rhizosphere microbiome, negatively impacting its composition, metabolism, and growth (5).
During the last decade, new sustainable agriculture techniques, e.g., use of beneficial microorganisms (plant growth-promoting rhizobacteria [PGPR]) and entomopathogenic nematodes (EPNs), have arisen to improve pest management, low energy consumption, and environmental and human safety (6). Beneficial microorganisms can protect plants from pests, enhancing disease resistance (i.e., induced systemic resistance [ISR]) and abiotic stress tolerance. In fact, plants can recognize the presence and activities of PGPR in the roots and respond with hormonal and metabolic changes to a wide range of pathogens, without impairing their fitness (7). EPNs from Steinernematidae and Heterorhabditidae became effective and popular biological control agents during the last 3 decades. They have direct effects on plant pathogens, plant parasitic nematodes, and pest insect populations, and they can indirectly improve the soil quality (8). A unique characteristic of EPNs is their symbiotic relationship with bacteria of the Xenorhabdus and Photorhabdus genera.
Photorhabdus luminescens is a Gram-negative entomopathogenic enterobacterium living in mutualistic symbiosis with EPNs. P. luminescens is characterized by a complex dualistic life cycle, i.e., (i) it is able to symbiotically interact with nematodes of the Heterorhabditidae family, and (ii) it is highly pathogenic toward a wide range of insect species since it produces a wide range of high-molecular-weight toxins and secondary metabolites that effectively kill insect larvae within 48 h (9, 10).
P. luminescens exists in two phenotypically different cell forms: the symbiosis phenotypic variant (primary [1°] cells) and the symbiosis “deficient” phenotypic variant (secondary [2°] cells) (11). The 1° and 2° cells are genetically identical (12) (R. Heermann, unpublished data) and equally pathogenic toward insect larvae. However, they differ in diverse phenotypic traits and in the success of their relationship with nematodes since 2° cells can neither support their development nor reassociate with them (13, 14). Furthermore, 1° cells display different distinct phenotypic characteristics as follows: (i) toxins, extracellular enzymes, and pigment production; (ii) secondary metabolites like antibiotics; (iii) bioluminescence; (iv) cell clumping factor; and (v) crystalline inclusion proteins (the majority of which are missing or have a reduced level in 2° cells) (12, 15). Since 2° cells are unable to live in symbiosis with EPNs, we have suggested earlier that they could adapt to a free life in soil and hence better respond to different environmental stress conditions, nutrient poverty, and plant-derived molecules (16, 17). Indeed, it has been reported that 2° cells had a more active cellular metabolism and accumulation of stock proteins to be responsive to new environments (18), such as those represented by the rhizosphere and plant roots.
The rhizosphere is characterized by plant root exudates that can act as a signal(s), influencing specific bacterial gene expression patterns and, thus, impacting the microbial ability to colonize roots and to survive in the rhizosphere (19). Despite the application of P. luminescens EPNs as a biopesticide, very little is known about the role of P. luminescens 2° cells in the rhizosphere.
For that reason, here we investigate the capacity of the P. luminescens strain DJC 2° variant (P. luminescens 2°) to interact with plant roots, their chemotactic response to plant-derived compounds, and their effect toward phytopathogenic microorganisms (e.g., pathogenic fungi). First, we examined the response of P. luminescens 2° cells to plant root exudates using RNA-seq transcriptome sequencing analysis, allowing the identification of putative genes involved in 2° P. luminescens-plant root interaction, adaptation, and colonization. Understanding the role and the function of this bacterium in the rhizosphere will contribute to the understanding of phenotypic heterogeneity in P. luminescens cell populations and will have profound implications on bioagriculture and pest management using EPNs.
RESULTS AND DISCUSSION
Transcriptome profile of P. luminescens 2° cells in response to plant root exudates.
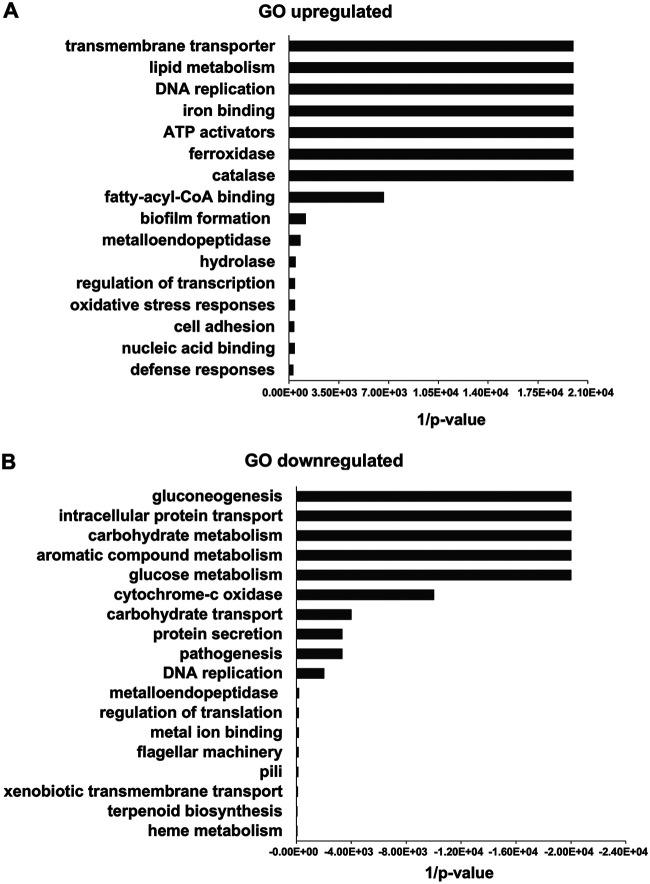
In order to identify genes in P. luminescens 2° cells that are important for the interaction of the bacteria with plant roots, we performed a comparative transcriptome analysis of P. luminescens 2° cells in the presence and absence of pea root exudates with cells collected at the logarithmic (6 h) as well as at the late stationary growth phase (24 h). Since we intended to gather all bacterial genes affected by the root exudates, we pooled the differentially expressed genes (DEGs) of both time points. Analysis of the transcriptome profile showed that the expression of 741 genes (see Fig. S1 and Table S1 in the supplemental material), representing ∼6% of the transcriptome, were significantly altered (−1 ≤ log2 fold change ≥ 1; P ≤ 0.05) in response to the root exudates; specifically, 233 DEGs were upregulated and 508 showed downregulation. The DEGs were analyzed to identify their function and the respective gene ontology (GO) terms. The GO terms highlighted as the most important functional classes of the DEGs upregulated in response to the root exudates are putative transmembrane transporters, lipid metabolic enzymes, transcriptional regulators, iron-binding proteins, ATP activators, ferroxidase, and catalase (Fig. 1a), whereas many of the significantly downregulated genes showed GO functional classes involved in gluconeogenesis, carbohydrate metabolism, protein and carbohydrate transport, and aromatic compound metabolism (Fig. 1b).
FIG 1.
Overview of DEG functional analysis of P. luminescens 2° cells in response to pea root exudates. Most significant gene ontology (GO) categories of DEGs upregulated (A) and downregulated (B) in P. luminescens 2° cells in the presence of pea root exudates.
The putative functions of the identified DEGs indicate a profound switch in the lifestyle of the bacteria, especially in metabolism. Particularly, the downregulation of gluconeogenesis and changes in carbohydrate metabolism support the idea of a switch in sugar metabolism when the cells are faced with the plant roots after an insect infection cycle where preferentially other carbohydrates are used. This is in accordance with the different sugars that we identified in the pea root exudates (see Table S2 in the supplemental material). In the presence of the exudates, we found that the gene PluDJC_05975, which is homologous to csrA, is upregulated. CsrA is a glycolysis activator and a gluconeogenesis repressor in Escherichia coli, and the corresponding gene was also found to be upregulated in the presence of spinach root exudates (20). Therefore, it is likely that PluDJC_05975 has a similar function to regulate sugar metabolism in P. luminescens 2° cells.
Moreover, the transcriptome analysis presented here spotlights a drastic transcriptional reprogramming of P. luminescens 2° cells, probably due to root signaling molecules contained in the medium. Indeed, the modulation of a large set of genes encoding transcriptional regulators, which represents ∼5.5% of all DEGs, was influenced by root exudates as, e.g., observed for XRE- and LuxR-like transcriptional regulator proteins (of which the majority showed positive regulation) (Fig. 2a; see also Table S1). The relationship between plant-derived molecules and LuxR- and XRE-like regulators has been demonstrated for other bacteria before. A plant compound from leaf macerate, an ethanolamine-derived small molecule, activates the LuxR-like receptor PipR and, therefore, its regulated genes in Pseudomonas GM79 (21, 22). Additionally, the LuxR-like protein OryR of Xanthomonas oryzae possesses an acyl homoserine lactone (AHL)-binding domain that specifically responds to a plant-derived molecule (23). XRE regulators can be associated with carbon metabolism (24), and thus, these regulators might also be involved in the regulation of carbohydrate metabolism and transport, processes found negatively modulated in the transcriptome analysis presented here. In addition, downregulation of protein transport and changes in carbohydrate metabolism as well as differential expression of several regulatory genes were also observed for Bacillus mycoides in response to potato root exudates (25).
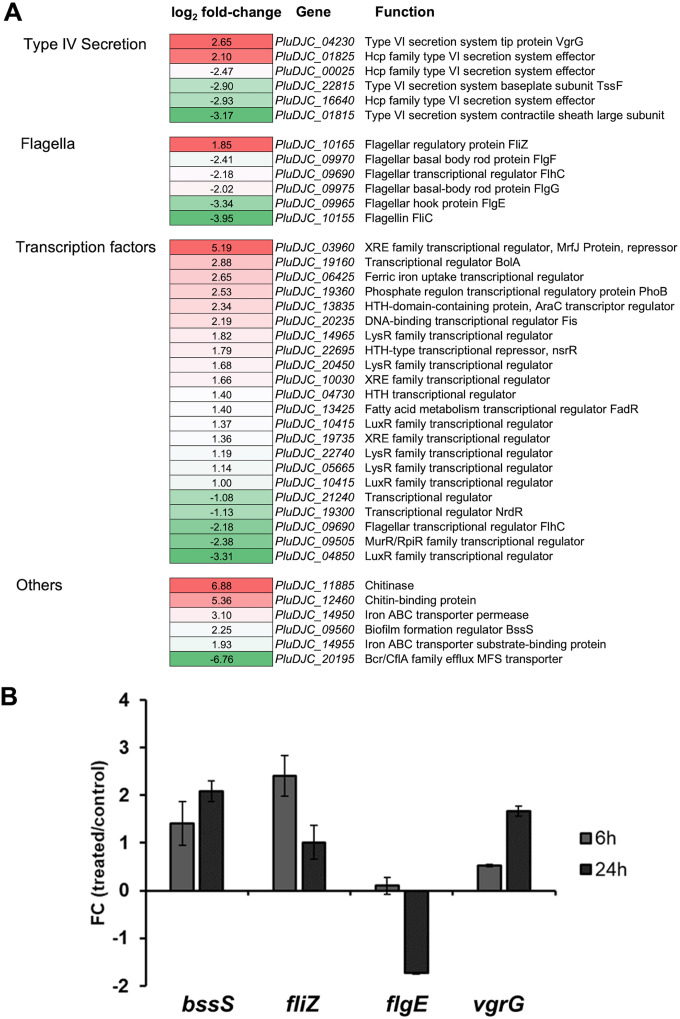
FIG 2.
(A) DEGs of P. luminescens 2° cells in response to pea root exudates. Subset of P. luminescens 2° DEGs that showed modulated expression in response to root exudates from Pisum sativum variant Arvica. The first column represents the different gene classes. The second column shows the relative gene expression level (−1 ≤ log2 fold change ≥ 1; P ≤ 0.05) of P. luminescens 2° cells cultivated with root exudates in comparison to that of those cultivated in the absence of the exudates. The third column describes the gene names and their putative function. Red represents upregulation, whereas green denotes downregulation of gene expression. (B) Real-time qPCR considering selected P. luminescens 2° DEGs to confirm the RNA-seq data analysis. The plot shows the fold change (FC) (P. luminescens 2° cells in LB with pea plant root exudates [“treated”]/P. luminescens 2° cells in LB [“control”]) expression level of the following selected genes of interest: PluDJC_09560 (bssS), PluDJC_10165 (fliZ), PluDJC_09965 (flgE), and PluDJC_04230 (vgrG). The analysis was performed at 6 h (gray) and 24 h (dark gray) postinoculation of the cells. Error bars represent standard deviation of at least three independently performed biological experiments.
Genes involved in flagellar motility and chemotaxis, i.e., flgG, flgE, and fliC, were downregulated (Fig. 2a), showing that root exudate attractants and their concentration could play a role in motility and chemotaxis for a successful colonization of the rhizosphere by P. luminescens 2° cells. In a transcriptional profiling of Pseudomonas aeruginosa PAO1, genes encoding FlgE and FliC were found to be downregulated in response to sugar beet root exudates (26). Moreover, for Pseudomonas putida KT2440, an enhanced chemotaxis at a certain distance to the roots could be demonstrated. Indeed, low root exudate concentration increased chemoreceptor transcription levels, thus positively modulating the motility and chemotaxis related genes. This process was reversed at root proximity, where the concentration of root exudates is higher (27). This observation could reflect the capacity of P. luminescens 2° cells to detect concentration differences of root exudates in the rhizosphere. Another interesting gene found positively modulated by the root exudates was fliZ. FliZ contains a DNA-binding domain that could play a direct role in type II flagellar gene transcriptional regulation by direct binding to the flhD promoter as reported for Xenorhabdus nematophila (28). Additionally, FliZ of Xenorhabdus could also be involved in the regulation of motility and mutualism. Moreover, FliZ together with RpoS promotes the adhesion of Xenorhabdus in the intestinal region of the soil-dwelling nematodes (29). Therefore, FliZ could also trigger in cooperation with the RpoS-encoding gene PluDJC_03680, which was upregulated in response to the root exudates, the adhesion of P. luminescens 2° cells onto plant roots.
Bacterial type VI secretion systems (T6SSs) play a key role in interbacterial competition. They are molecular weapons projected to deliver toxic effectors into prey cells, thus providing advantages for T6SS active strains in polymicrobial environments (30). For P. putida, it has been reported that the T6SS is important for the fight against competitors like Xanthomonas campestris, thereby reducing leaf necrosis of the plant Nicotiana benthamiana induced by this phytopathogen (31). In Pseudomonas fluorescens Pf29Arp, T6SS genes were expressed when the bacterium was located on healthy roots, which further increased on fungus-infected roots (32), suggesting not only an interbacterial competition role in P. fluorescens but also a possible root signal involved in the modulation of the T6SS in P. luminescens. Indeed, P. luminescens 2° T6SS genes showed a complex modulation pattern in response to the root exudates, which is not unusual since root exudates include a complex mixture of metabolites, small molecular signals, and inhibitory compounds (33, 34) (Fig. 2a; see also Table S2 for the root exudate composition).
The comparative transcriptome analysis further highlighted genes that are supposed to be implicated in microbe-plant interaction and colonization, such as the biofilm formation regulator BssS (PluDJC_09560), a putative chitinase (PluDJC_11885), an iron ABC transporter permease (PluDJC_14950) that was upregulated, and the xenobiotic transporter (PluDJC_20195), which was downregulated (Fig. 2a; see also Table S1). PluDJC_09560 is homologous to yceP (bssS) in E. coli K-12, a biofilm formation regulator, which regulates several genes involved in catabolite repression, stress responses, regulation of quorum sensing (QS), and putative stationary-phase signal(s). Moreover, it has been reported that YceP is implicated in the regulation of indole synthesis as well as its uptake and secretion together with YliH (35). Indole is involved in interkingdom signaling between bacteria and plants, and it acts as a potent plant growth modulator as reported for Arabidopsis thaliana (36). This suggests a possible similar function of BssS (PluDJC_09560) in P. luminescens 2° cells, besides regulation of stress response and QS, by regulating secretion of indole, which is used as a remote messenger to manipulate plant growth and development.
Chitinases are very useful in agriculture as biocontrol agents against phytopathogenic fungi due to their ability to hydrolyze the chitinous fungal cell wall (37). The transcriptome analysis presented here shows a chitinase-encoding gene (PluDJC_11885) upregulated in the presence of plant root exudates, hypothesizing that P. luminescens 2° cells secrete a chitinolytic enzyme in the rhizosphere environment, a characteristic behavior observed for PGPR such as Pseudomonas and Bacillus spp. (38, 39).
Iron ABC transporters are involved in siderophore-dependent iron uptake pathways, and they were highlighted as important plant root colonization genes in Pseudomonas simiae and P. putida (40). Iron is a highly insoluble important micronutrient required by microbes and plants in the rhizosphere. The production of iron-binding ligands and transporters ensures advantages over other microorganisms, e.g., phytopathogens (41). In fact, microorganisms producing siderophores restrict the growth of deleterious microorganisms by limiting iron availability and at the same time promoting plant growth (42). For instance, the expression of genes encoding iron binding and transporter activity was modulated in the presence of the root exudates in P. luminescens 2° cells. Particularly, PluDJC_14950, encoding a putative iron ABC transporter permease, was positively regulated. PluDJC_14950 is homologous to the cation ABC transporter ATP-binding protein PP_3802 of P. putida, which was found to be important for root colonization (40). This suggests that plant root exudates might influence the siderophore activity in P. luminescens 2° cells, which could be a survival strategy of the bacteria in plant root environments.
In the rhizosphere, rhizodeposits (exudates released from the root cap cells) and root exudates shape the microbial population, a process important for the defense against plant-pathogenic fungi, bacteria, nematodes, and viruses (43). Some microorganisms have developed strategies to increase their capacity to resist antimicrobial rhizodeposits and xenobiotic compounds released by the roots and heavy metals in the soil. For instance, Sinorhizobium meliloti can degrade rhizopine, a compound toxic for microorganisms and found in nitrogen-fixing nodules (44). Bcr/CflA xenobiotic antiporters are also involved in heavy metal resistance and copper homeostasis (45). We found the Bcr/CflA major facilitator superfamily members to be downregulated in P. luminescens 2° cells in the presence of root exudates. This indicates a putative capacity of P. luminescens 2° cells to modulate mechanisms to cope with xenobiotic compounds released by the roots or with heavy metals present in the soil, thus providing a selective advantage over other bacteria to survive in the rhizosphere, especially in the presence of heavy metals.
The comparative transcriptome analysis successfully identified candidate genes that are involved in the interaction of P. luminescens 2° cells with plant roots. We could also validate the results of the RNA-seq analysis via real-time quantitative PCR (RT-qPCR) using selected DEGs (Fig. 2b). Nevertheless, it is important to consider not only the advantages but also the limitations of our approach. One limitation is that the transcriptome profiling was performed from cultures grown in exudate-supplemented complex medium. Therefore, it is possible that exudate effects might be inhibited or overrun by medium components and, consequently, not affecting gene expression anymore. Furthermore, a putative dilution of several exudate molecules in the growth medium could lead to a loss of induction or repression of the bacterial gene expression. In the future, we will therefore consider different root exudate fractions to identify the signal molecule(s) that is important for the P. luminescens-plant root interaction. Finally, it will be necessary to analyze the molecular mechanism(s) behind the interaction between this insect pathogen and plants.
Evaluation of phenotypic traits important for the P. luminescens-rhizosphere interaction. (i) Chitin degradation and fungal growth inhibition activities.
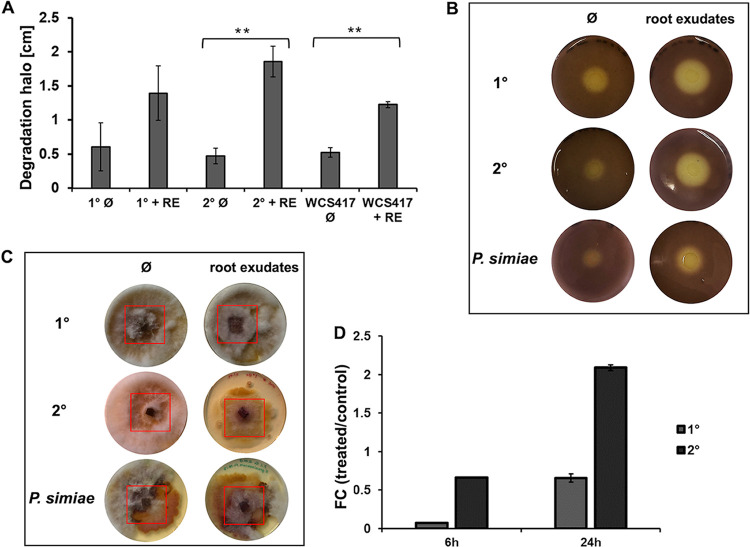
The comparative transcriptome analysis highlighted PluDJC_11885 encoding a putative chitinase as the most upregulated gene in response to the plant root exudates. This result suggested that P. luminescens 2° cells could have chitin degradation activity in the presence of plant root exudates. For that reason, we further investigated the ability of P. luminescens to degrade chitin and inoculated the two phenotypic forms of P. luminescens and P. simiae WCS417, a PGPR that is already characterized (46), on chitin agar plates. The chitin degradation activity of P. luminescens 2° cells cultivated in the presence of root exudates was significantly higher (P ≤ 0.05) than that in their absence (Fig. 3a and b). Although P. luminescens 1° cells also exhibited chitinase activity, the activity was not significatively influenced by the root exudates. The increasing chitin degradation activity of P. luminescens 2° cells in the presence of root exudates was in line with what was observed for P. simiae (Fig. 3a and b). We then tested the capacity of P. luminescens to inhibit fungal growth, considering P. luminescens 1° and 2° cells as well as P. simiae cells cultivated with or without root exudates against Fusarium graminearum strain HM6PIS. P. luminescens 2° cells were able to inhibit the growth of F. graminearum HM6PIS in the presence of the root exudates, a behavior also observed for P. simiae (Fig. 3c). In contrast, P. luminescens 1° cells did not show any fungal growth-inhibitory effect. This result is in line with our initial hypothesis that P. luminescens 2° cells could be more adapted to a free-soil lifestyle, subsequently interact with plant roots, and protect them from pathogenic fungi. However, this fungal growth-inhibitory effect observed for P. luminescens 2° cells is not only due to the chitinase-related gene PLUDJC_11885, since this inhibitory effect could also be observed for its ΔPLUDJC_11885 mutant (see Fig. S2 in the supplemental material). Therefore, chitinase activity could be involved in a more complex fungal growth-inhibitory pathway which is not yet clear. Finally, RT-qPCR was performed to confirm the chitinase expression pattern observed during RNA-seq analysis. In this analysis, we also considered P. luminescens 1° cells to test whether the corresponding gene PluDJC_11885 is also influenced by the presence of root exudates in this phenotypic variant. Gene expression analysis showed a positive effect of the root exudates on P. luminescens 2° cells at 24 h postinoculation (4-fold upregulation), whereas in 1° cells, expression of this gene was only slightly influenced by the root exudates (Fig. 3d).
FIG 3.
Plant root exudates influence the chitin degradation capacity and fungal growth inhibition of P. luminescens 2° cells. (A) Chitin degradation halos (shown in panel B) in centimeters obtained during the chitin degradation assay. The plot shows the degradation halo (Ø) measured with ImageJ represented by the average and the standard deviation of three biological replicates (**, P ≤ 0.05). RE, root exudates. (B) Chitin degradation halo of P. luminescens 1° cells (1°), P. luminescens 2° cells (2°), and P. simiae WCS417 cultivated with or without root exudates. (C) Fungal growth inhibition assay using phytopathogenic Fusarium graminearum HM6PIS performed on YMG agar plates. P. luminescens 1° cells, P. luminescens 2° cells, and P. simiae WCS417, cultivated with and without plant root exudates, were placed around HM6PIS (red square) and incubated for 14 days at 26°C. (D) Expression level (fold change) of the chitinase-encoding gene (PluDJC_11885) in P. luminescens 1° and 2° cells using real-time qPCR analysis. P. luminescens 1° and 2° cells were cultivated in LB medium with root exudates (treated) or without (control) and collected at 6 h and 24 h postinoculation. Error bars represent standard deviation of at least three independently performed biological experiments. All pictures represent one characteristic of at least three independently performed experiments with similar outcomes.
(ii) Chemotaxis and swimming assay.
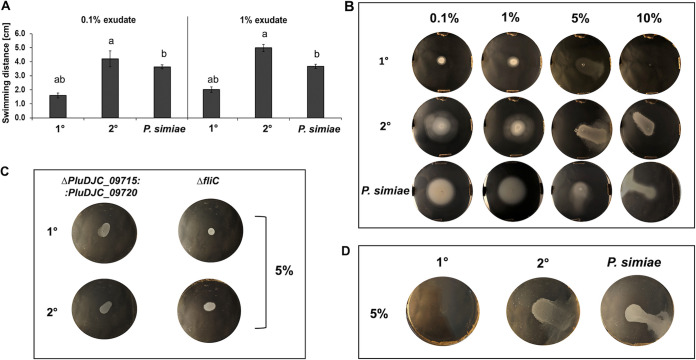
Chemotaxis is an important feature in PGPR. It allows bacterial movement toward the root surface, and it has been identified to be one of the first colonization steps (47). For that reason, we tested chemotaxis through swimming activity of P. luminescens, considering both cell variants in the presence of root exudates, using tryptone LB (without yeast extract) or M9 (to exclude any effect of LB compounds) soft agar plates. We could show that P. luminescens 2° cells chemotactically responded to the portion of the root exudates extracted with MetOH (Fig. 4a and b). A similar behavior was observed for P. simiae (Fig. 4a and b). Moreover, for a higher concentration of root exudates extracted with MetOH (5% to 10%), both P. luminescens 2° and P. simiae cells showed a similar swarming pattern in LB and M9 (Fig. 4b and d). Neither P. luminescens 1° cells nor P. luminescens mutants (with inactivation of the two chemotaxis receptor genes PluDJC_09715 and PluDJC_09720 or flagellin [ΔfliC used as negative control] were inactivated) (Fig. 4c) showed any chemotaxis activity. In summary, these results highlight the capacity of P. luminescens 2° cells to chemotactically respond to attractants or repellents in plant root exudates and focus the attention on chemotaxis receptors PluDJC_09715 and/or PluDJC_09720. These receptors are homologous to Tar (type II methyl-accepting chemotaxis protein) and Tsr (MCP-I) of E. coli, respectively. Tsr and Tar are involved in chemotaxis activity toward serine, maltose, and aspartate (48), compounds released by plant roots in the rhizosphere (49) and that are also present in the pea root exudates used here (see Table S2).
FIG 4.
Chemotaxis, swimming, and swarming. The chemotaxis assays were performed in LB swimming agar plates using the MetOH-extracted fraction of pea root exudates. (A) Quantification of the swimming assays shown in panel B using 0.1% and 1% pea plant root exudates. The plots show the swimming halo measured with ImageJ represented by the average and the standard deviation of four biological replicates (different lowercase letters between the bars indicate a P value of ≤0.05). (B) Chemotaxis assays of P. luminescens 1° and 2° cells as well as P. simiae WCS417 using 0.1%, 1%, 5%, and 10% of plant root exudates, respectively. P. luminescens 2° cells and P. simiae show swimming behavior at a concentration of 0.1% and 1% of plant root exudates, while at ≥5%, they showed swarming behavior. (C) Chemotaxis assays using P. luminescens 1° and 2° chemotaxis receptor ΔPluDJC_09715 ΔPluDJC_09720 (double deletion) and ΔfliC (negative control) mutants. (D) Swarming assays on M9 minimal medium with 5% of plant root exudates to exclude LB compounds to be responsible for swarming. All images represent one characteristic of four independently performed experiments with similar outcomes.
(iii) Lateral root formation induction and root colonization.
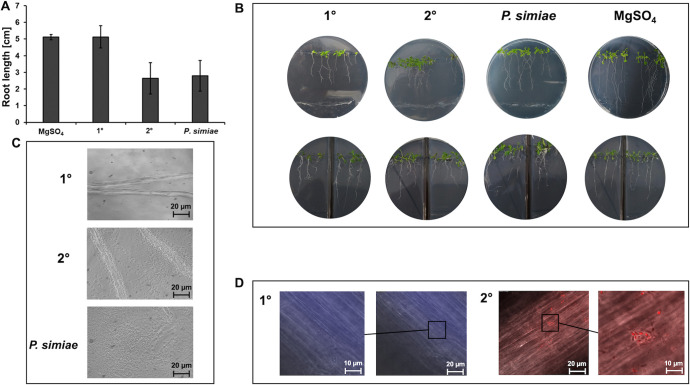
Root hairs (RH) and later roots (LR) are important root traits that facilitate plant anchorage and water and mineral scavenging. Beneficial microorganisms can induce alteration in root morphology, enhancing LR and RH formation as demonstrated for Pseudomonas species rhizobacteria (46). To get insights on the root development effect caused by P. luminescens, we analyzed the developmental responses of A. thaliana Col-0 to P. luminescens 1° and 2° cells considering also P. simiae WCS417 for comparison, since the effect of this microorganism on root development was already established. After 8 days of cocultivation, we observed a reduction of ∼20% of the primary roots exposed to P. luminescens 2° cells compared to that of the negative control. This result was similar for the seedlings exposed to P. simiae. In contrast, primary roots exposed to P. luminescens 1° cells showed only a small reduction (Fig. 5a and b). A similar root development was observed considering the same experiment using a split plate (Fig. 5b). In this experiment, the bacteria were placed only in one side of the plate, and the root development was analyzed. In cases of whether similar plant root development can be observed in both sides of the split plate, involvement of bacterial volatile organic compounds (VOCs) in this mechanism can be concluded, which was the case for P. luminescens 2° cells as well as for P. simiae.
FIG 5.
Bacterium-plant cocultivation assays and VOC tests on Arabidopsis thaliana Col-0 seedlings. (A) P. luminescens 1° and 2° cells as well as P. simiae WCS417 were spotted at a 5-cm distance from 4-day-old A. thaliana Col-0 seedlings on MS agar plates and cultivated for 8 days at 24°C (plates are shown in panel B, top). The root lengths were measured using ImageJ. Error bars represent standard deviation of at least three independently performed experiments. For the split agar assays (B, bottom), the left side of the MS agar plates contained only the seedlings, while on the right side, the respective bacteria were spotted at the bottom of the plate to test whether VOCs produced by bacteria have an influence on plant root length and development. (C) Phase contrast microscopy of A. thaliana roots colonized with P. luminescens 1° and 2° cells as well as P. simiae WCS417 (positive control). (D) Fluorescence microscopy of A. thaliana roots with attached P. luminescens 1° cells tagged with mTFP and P. luminescens 2° cells tagged with mCherry. All pictures show one representative of at least three independently performed experiments.
In summary, these results indicate that plant roots reduced primary root elongation in response to P. luminescens 2° cells, an effect that might be due to the inhibition of cell expansion as has also been reported for P. simiae (46). Finally, we investigated the capacity of P. luminescens 2° cells to colonize plant roots. For that purpose, Arabidopsis roots were colonized with P. luminescens 1° and 2° cells as well as P. simiae as a positive control. This analysis showed a similar colonization pattern for P. luminescens 2° cells and P. simiae, highlighting the capacity of P. luminescens 2° cells to specifically colonize the plant roots, features that were not observed for P. luminescens 1° cells (Fig. 5c). Following this observation, we then investigated the capacity of P. luminescens 1° and 2° cells to attach to the Arabidopsis roots. For that purpose, P. luminescens 1° cells tagged with monomeric teal fluorescent protein (mTFP) and P. luminescens 2° cells tagged with mCherry were exposed to Arabidopsis roots. Then, the roots were washed and analyzed by fluorescence microscopy using the appropriate fluorescence channels (Fig. 5d). P. luminescens 2° cells were found attached to the Arabidopsis root surface, whereas for 1° cells, we could not detect bacterial cells attached to the roots. The exact mechanisms of P. luminescens 2° cells that influence root development, alteration, and attachment remain to be clarified. Further analyses must be performed to understand how P. luminescens 2° cells and their volatile compounds can influence plant root development and their role in triggering plant ISR. For instance, some PGPR can influence auxin transport and signaling by influencing the ethylene and jasmonic acid pathways (50), thus suggesting a possible signaling mechanism of P. luminescens 2° cells to interact with plant roots and trigger their ISR.
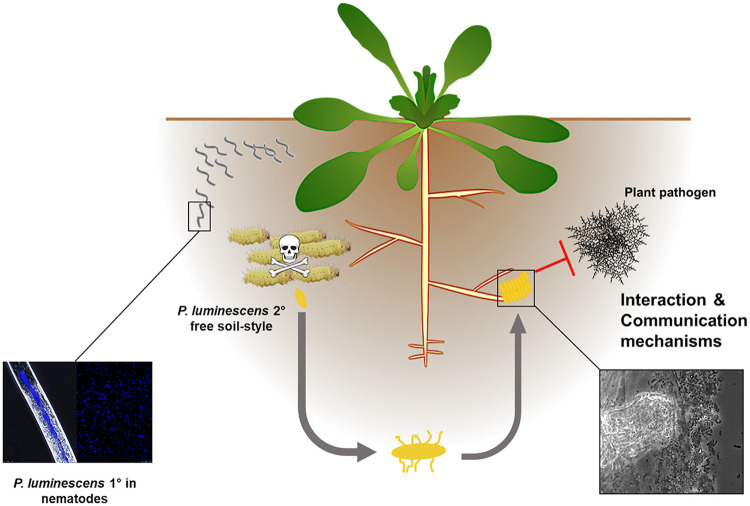
In conclusion, in this study, we could show that P. luminescens 2° cells have an alternative lifestyle in the soil in the absence of their nematode partners and away from infecting insects (Fig. 6). The bacteria can specifically respond to and interact with plant roots after undergoing the phenotypic switch from 1° to 2° cells and being left by the nematode partner in soil after the insect infection cycle. In this context, it seems that the plant can benefit from this interaction since the bacteria promote root development and can defend the plant from phytopathogens. Since 2° cells are still pathogenic toward insects, it can be assumed that they also protect the plant from insect predators. Whether and when the bacteria can reenter their pathogenic life cycle through a possible reswitch from 2° to 1° remain unclear. Overall, this work broadens our understanding of both beneficial and insect-pathogenic bacterial responses to a host plant and might help to improve sustainable agricultural techniques using EPNs in the future.
FIG 6.
Model of the P. luminescens 2° cell alternative life cycle in the rhizosphere. During the insect infection cycle, single P. luminescens cells undergo phenotypic switching from the 1° to the 2° phenotype. The 2° cells cannot reassociate with the nematode symbiosis partner and are left behind in the soil when the nematodes leave the depleted insect cadaver. Then, the 2° cells chemotactically respond to plant root exudates and specifically colonize and attach to the roots. The metabolism of the bacteria adapts to plant-derived nutrients, and the cells protect the plants from phytopathogens. Since 2° cells are still pathogenic against insects, it can be assumed that they also protect the plant roots from insect predators.
MATERIALS AND METHODS
Bacterial and fungal strains.
For this study, 1° and 2° cells of P. luminescens strain DJC were used (51). Pseudomonas simiae WCS417 (Utrecht University, The Netherlands) was used as a positive control strain for plant interactions. Fusarium graminearum HM6PIS (Institute of Biotechnology and Drug Research [IBWF], Kaiserslautern, Germany) was used for fungal growth inhibition activity assays. P. luminescens 1° and 2° ΔPluDJC_09715 ΔPluDJC_09720 (double mutant), ΔPluDJC_fliC, and ΔPluDJC_11885 in-frame deletion mutants were obtained through conjugation and homologous recombination. For that purpose, the upstream and downstream fragments (500 bp) of the desired regions were cloned into the pNPTS138-R6KT suicide vector using appropriate primers (listed in Table 1), and conjugation was performed as previously described (52). P. luminescens 1° and 2° cells tagged with mTFP and mCherry under the control from an exogenous Ptac promoter were obtained using the method described earlier (53).
TABLE 1.
List of primers used in this study
| Primer name | Sequence | Characteristic |
|---|---|---|
| Up09715_fw_BamHI | CCTAGGATCCTATCGAAATACTGAAAGTACAGGAG | PluDJC_09715 PluDJC_09720 deletion double mutant |
| Up09715_rv_ovl | CGTCAGTAGATCTTAAACATGTTTTCCCTTTTTACAATAG | PluDJC_09715 PluDJC_09720 deletion double mutant |
| Down09720_fw_ovl | GATCTACTGACGTCAGACTCACTGAGGCCAGATG | PluDJC_09715 PluDJC_09720 deletion double mutant |
| Down09720_rv_EagI | CGTTCGGCCGCATCCAGTCGATAAACCCCTTTG | PluDJC_09715 PluDJC_09720 deletion double mutant |
| UpfliC_fw_BamHI | ACGGGATCCGGCAACGAATGCATCATG | fliC deletion mutant |
| UpfliC_rv_ovl | CCCTAGCTGAGCGATTAACGTGCCATAGTTAGAGTTCC | fliC deletion mutant |
| DownfliC_fw_ovl | GGAACTCTAACTATGGCACGTTAATCGCTCAGCTAGGG | fliC deletion mutant |
| DownfliC_rv_EagI | ACTCGGCCGCAATCACGGCTCCTTAAC | fliC deletion mutant |
| Up11885_fw_BamHI | GAGGGATCCCCATATATAACCTCTCCTGA | PluDJC_11885 deletion mutant |
| Up11885_rv_ovl | CCTGAGCTTGACATAAATCACCTCGACTAG | PluDJC_11885 deletion mutant |
| Down11885_fw_ovl | AAGCTCAGGCATAATTAATTAAGCCAAGCCAC | PluDJC_11885 deletion mutant |
| Down11885_rv_EagI | TGACGGCCGGTTGGAATTTCACTGCGCAG | PluDJC_11885 deletion mutant |
| rpoDqPCR_fwDJC | CGGAAGATATCGTCGATTCCGA | Housekeeping, PluDJC_19710 |
| rpoDqPCR_rvDJC | TGTCGTTAGCGGTTTCTGCT | Housekeeping, PluDJC_19710 |
| chitinqPCR_fwDJC | GGTCGCAATATGACGGTCG | Chitinase for qPCR, PluDJC_11885 |
| chitinqPCR_revDJC | GGCAAATAATGGCGCTTGCT | Chitinase for qPCR, PluDJC_11885 |
| vgrGqPCR_fwDJC | ACAGCTTTATCGCCTGACGTT | vgrG for qPCR, PluDJC_04230 |
| vgrGqPCR_rvDJC | GTCCGTTCGGTGATGCCATT | vgrG for qPCR, PluDJC_04230 |
| flgEqPCR_fwDJC | AGGTGGGACTGGGGGTAAAA | flgE for qPCR, PluDJC_09965 |
| flgEqPCR_rvDJC | ACCGCCTTGCATACGGAAAA | flgE for qPCR, PluDJC_09965 |
| bssSregqPCR_fw | TTTGCAATGTCAGTTGTCAACCA | bssS for qPCR, PluDJC_09560 |
| bssSregqPCR_rv | AACGCATCCTGTTGTAGGCT | bssS for qPCR, PluDJC_09560 |
| fliZqPCR_fw | TTGTCACAAAGCTCTTGACCGT | fliZ for qPCR, PluDJC_10165 |
| fliZqPCR_rv | TGCAAAAACGACATAACGCGA | fliZ for qPCR, PluDJC_10165 |
The bacteria were aerobically cultivated at 30°C in LB medium (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, 0.5% [wt/vol] NaCl), M9 minimal medium (33.7 mM Na2HPO4, 22 mM KH2PO4, 8.55 mM NaCl, 9.35 mM NH4Cl, 1 mM MgSO4, 100 μM CaCl2, 0.2% [wt/vol] glucose), or YMG medium (1% [wt/vol] malt extract, 0.4% [wt/vol] yeast extract, 1% [wt/vol] glucose, pH 5.5), respectively.
Root exudate collection.
Root exudates were collected from Pisum sativum variant Arvica (Bayerische Futtersaatbau, Ismaning, Germany) grown in controlled conditions (2 weeks incubation at 24°C; 16 h light/8 h dark regime). Then, 75 plants were collected and washed of vermiculite residues, and roots were put into vessels containing 250 ml of sterile distilled H2O or methanol (MetOH) with continuous shaking for 12 to 14 h to ensure the extraction of most root exudate substances and signaling molecules. The root exudate solutions were filter sterilized, lyophilized (H2O portion), and stored at −20°C in the dark until use.
Transcriptome profiling and RNA-seq analysis.
The influence of root exudates collected from Pisum sativum variant Arvica on the transcriptome of P. luminescens 2° cells was investigated by using RNA-seq. P. luminescens 2° cultures were cultivated in 50 ml LB medium supplemented with 3% (vol/vol) root exudates (treated) or in LB medium without root exudates (control). The pea root exudates used were collected from the same batch. The cultures were aerobically grown under shaking at 30°C, and the cells were harvested after 6 h (exponential growth phase), when the culture reached an optical density at 600 nm (OD600) of 0.8 to 1, and 24 h (stationary growth phase; OD600 of 8 to 10). In total, three independent biological replicates were sampled for every condition considered, and the total RNA was isolated using AquaPhenol-chloroform-isoamyl alcohol as described previously (16). Successively, 5 μg of treated RNA was subjected to rRNA depletion using the RiboMinus kit (Invitrogen), and 150 ng of depleted RNA was processed using NEBNext Ultra II directional RNA library prep kit for Illumina (New England BioLabs [NEB]) according to the protocol of the distributor. Finally, a total concentration of 4 mM from the obtained library was sequenced on a MiSeq sequencer (Illumina; 2 × 75 bp paired-end sequencing, v3 chemistry) (Genomics Core Facility, LMU München). Raw reads were trimmed, mapped to the reference genome (P. luminescens DJC; GenBank accession number NZ_CP024900.1), and differentially expressed genes (DEGs = −1 ≤ log2 fold change ≥ 1; P ≤ 0.05) were identified. The function of the DEGs and gene ontology (GO) were extracted from UniProt (https://www.uniprot.org) and NCBI (https://www.ncbi.nlm.nih.gov).
Validation of the transcriptome profiling experiment was carried out by RT-qPCR on selected candidate genes identified from the RNA-seq experiment. The cDNA was synthesized using SuperScript III reverse transcriptase (Invitrogen) followed by qPCR using specifically designed primers (Table 1) and GoTaq qPCR master mix (Promega). The gene designated as rpoD (PluDJC_19710) was used as a reference. The relative expression values of the target genes and the standard error (SE) were calculated using the Pfaffl and Simon equations, respectively (54, 55). The primer efficiencies were calculated with the LingRegPCR program (http://LinRegPCR.nl).
HPLC-DAD analysis.
High-performance liquid chromatography with diode array detector (HPLC-DAD) analysis of P. sativum plant root exudates was performed on a Shimadzu LC 20A Prominence system (Shimadzu, Griesheim, Germany) equipped with two LC-20AD pumps, a DGU-20A degassing unit, a SIL 20AC autosampler, a CBM-20A controller, and a CTO-20AC column oven. Separations were performed using an analysis reversed-phase C18 column (Waters SunFire C18; particle size, 5 μm; 4.6 by 250 mm) at 20°C. A linear gradient starting from 99% 0.1% (vol/vol) trifluoroacetic acid and 1% (vol/vol) acetonitrile to 100% (vol/vol) acetonitrile in 20 min and then maintaining 100% (vol/vol) acetonitrile for 3 min and an additional equilibration time of 7 min was used at a flow rate of 1 ml/min. Injection volume of sample solution was 20 μl. A Shimadzu SPD-M20A diode array detector was used from 200 to 800 nm to record the spectra and detect separated metabolites at 210 nm, 250 nm, 300 nm, 350 nm, and 400 nm. Data and spectra were analyzed using the LabSolutions 5.54 software (Shimadzu, Griesheim, Germany).
Chitin degradation activity assays.
The chitin degradation activity assay was performed in chitin agar plates (0.01% [wt/vol] peptone, 0.025% [wt/vol] KCl, 0.2% [wt/vol] K2HPO4, 0.025% [wt/vol] MgSO4, 1% [wt/vol] colloidal chitin) (56). Overnight cultures of P. luminescens 1° and 2° cells and P. simiae WCS417 cultivated in LB medium with or without 3% (vol/vol) root exudates were adjusted to an OD600 of 0.1 (107 CFU/ml), and 50 μl was spotted in the center of the chitin agar plate, which was then incubated for 5 days at 30°C. The resulting halo diameter was measured using ImageJ (https://imagej.nih.gov/ij/), and statistical significance was evaluated through t test. Three biological independent replicates were performed.
Fungal growth inhibition assays.
For fungal growth inhibition assays, agar plugs harboring actively growing F. graminearum HM6PIS were placed into the middle of YMG agar plates. Then, P. luminescens 1° and 2° cells and P. simiae WCS417 were cultivated overnight in LB medium with or without 3% (vol/vol) root exudates at 30°C. Cultures were adjusted to an OD600 of 2. Then, four 50-μl aliquots were spotted and square connected around the fungal plug. The plates were further incubated at 26°C, and fungal growth was observed over 14 days. The experiment was repeated three times.
Chemotaxis and swimming assays.
Chemotaxis and swimming assays were performed using soft agar plates containing 0.3% (wt/vol) agar, 1% (wt/vol) tryptone, and 0.3% (wt/vol) NaCl or M9 soft agar plates (M9 medium supplemented with 0.3% [wt/vol] agar) with different concentrations of root exudates or without (control). Overnight cultures of P. luminescens 1° and 2° cells and the respective ΔPluDJC_09715 ΔPluDJC_09720 double mutant aerobically grown at 30°C were washed with 10 mM MgSO4 and adjusted to an OD600 of 0.1 (108 CFU/ml). Then, 10 μl of the cell suspensions were spotted in the center of the agar plates and incubated for 24 h at 30°C. The resulting swimming halo diameter was measured using ImageJ (https://imagej.nih.gov/ij/), and statistical significance was evaluated through t test. Four independent biological replicates for each considered condition were performed.
Bacterium-plant cocultivation assays and microscopy.
Bacterium-plant cocultivation and VOC assays on 4-day-old Arabidopsis thaliana Col-0 seedlings cultivated in MS agar (0.4% [wt/vol] MS basal salt mixture, 3% [wt/vol] sucrose, 0.8% [wt/vol] agar) at 24°C with a 16 h light/8 h dark regime were performed as reported previously (46). Briefly, P. luminescens 1° and 2° cells and P. simiae WCS417 were grown in LB medium at 30°C overnight. Cells were collected by centrifugation (5 min at 5,000 rpm), washed with 10 mM MgSO4, and adjusted to an OD600 of 0.002 (105 CFU/ml). Then, 240 μl of the bacterial suspension (or 10 mM MgSO4 as control) was spotted at a 5-cm distance of the seedlings. For experiments involving bacterial VOCs, 120 μl of the culture was spotted in one side of the split plate. For experiments involving root colonization, 120 μl (OD600 = 0.02) of the previously considered bacteria or P. luminescens 1° and 2° cell culture tagged with mTFP and mCherry, respectively, was spotted onto the root tip. For established colonization capacity, after 2 days, the roots were observed by phase-contrast microscopy (Leica; magnification, ×40). For root attachment assays, after 2 days, Col-0 roots were thoroughly washed and then analyzed by fluorescence microscopy (Leica DMi8 fluorescence imaging system) using 4′,6-diamidino-2-phenylindole (DAPI) and Texas Red fluorescence filter to verify the presence of P. luminescens cells attached on the roots. The experiments were performed three times.
Supplementary Material
ACKNOWLEDGMENTS
We thank Roeland Berendsen (Utrecht University, Netherlands) for providing P. simiae WCS417 and Luis Antelo (IBWF Kaiserslautern, Germany) for providing Fusarium graminearum HM6PIS. We thank Christiane Grünewald (JGU Mainz, Germany) for performing HPLC analysis. We are grateful to Bettina Bölter and Serena Schwenkert (LMU München, Germany) for providing Pisum sativum variant Arvica and Cordelia Bolle (LMU München, Germany) for providing Arabidopsis thaliana Col-0. We thank Andreas Brachmann for sequencing support. RNA-seq library sequencing was performed in the Genomics Core Facility of the LMU Biocenter.
Research was funded from the Deutsche Forschungsgemeinschaft, priority program SPP 1617 (HE 5247/5-2).
We declare no conflict of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Gerland P, Raftery AE, Sevčíková H, Li N, Gu D, Spoorenberg T, Alkema L, Fosdick BK, Chunn J, Lalic N, Bay G, Buettner T, Heilig GK, Wilmoth J. 2014. World population stabilization unlikely this century. Science 346:234–237. doi: 10.1126/science.1257469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fountain ED, Wratten SD. 2013. Conservation biological control and biopesticides in agricultural, p 377–381. In Fath B. (ed), Encyclopedia of ecology, 2nd ed Elsevier, Oxford, United Kingdom. [Google Scholar]
- 3.Clark M, Tilman D. 2017. Comparative analysis of environmental impacts of agricultural production systems, agricultural input efficiency, and food choice. Environ Res Lett 12:e064016. doi: 10.1088/1748-9326/aa6cd5. [DOI] [Google Scholar]
- 4.Rusiecki JA, Beane Freeman LE, Bonner MR, Alexander M, Chen L, Andreotti G, Barry KH, Moore LE, Byun HM, Kamel F, Alavanja M, Hoppin JA, Baccarelli A. 2017. High pesticide exposure events and DNA methylation among pesticide applicators in the agricultural health study. Environ Mol Mutagen 58:19–29. doi: 10.1002/em.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaplain V, Mamy L, Vieublé-Gonod L, Mougin C, Benoit P, Barriuso E, Nélieu S. 2011. Fate of pesticides in soils: toward an integrated approach of influential factors, p 535–560. In Stoytcheva M. (ed), Pesticides in the modern world—risks and benefits. IntechOpen, London, United Kingdom: http://www.intechopen.com/books/pesticides-in-the-modern-world-risks-and-benefits/fate-of-pesticides-in-soils-toward-an-integrated-approach-of-influential-factors. [Google Scholar]
- 6.Zhang D, Yan M, Niu Y, Liu X, van Zwieten L, Chen D, Bian R, Cheng K, Li L, Joseph S, Zheng J, Zhang X, Zheng J, Crowley D, Filley TR, Pan G. 2016. Is current biochar research addressing global soil constraints for sustainable agriculture? Agricult Ecosyst Environm 226:25–32. doi: 10.1016/j.agee.2016.04.010. [DOI] [Google Scholar]
- 7.Pieterse CMJ, Zamioudis C, Berendsen RL, Weller DM, Van Wees SCM, Bakker P. 2014. Induced systemic resistance by beneficial microbes. Annu Rev Phytopathol 52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 8.Lacey LA, Georgis R. 2012. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. J Nematol 44:218–225. [PMC free article] [PubMed] [Google Scholar]
- 9.Blackburn M, Golubeva E, Bowen D, Ffrench-Constant R. 1998. A novel insecticidal toxin from Photorhabdus luminescens, toxin complex a (Tca), and its histopathological effects on the midgut of Manduca sexta. Appl Environ Microbiol 64:3036–3041. doi: 10.1128/AEM.64.8.3036-3041.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajagopal R, Bhatnagar RK. 2002. Insecticidal toxic proteins produced by Photorhabdus luminescens Akhurstii, a symbiont of Heterorhabditis indica. J Nematol 34:23–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Boemare NE, Akhurst RJ. 1988. Biochemical and physiological characterization of colony form variants in Xenorhabdus spp. (Enterobacteriacea). J Gen Microbiol 134:751–761. doi: 10.1099/00221287-134-3-751. [DOI] [PubMed] [Google Scholar]
- 12.Forst S, Dowds B, Boemare N, Stackebrandt E. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu Rev Microbiol 51:47–72. doi: 10.1146/annurev.micro.51.1.47. [DOI] [PubMed] [Google Scholar]
- 13.Han RR, Ehlers RU. 2001. Effect of Photorhabdus luminescens phase variants on the in vivo and in vitro development and reproduction of the entomopathogenic nematodes Heterorhabditis bacteriophora and Steinernema carpocapsae. FEMS Microbiol Ecol 35:239–247. doi: 10.1111/j.1574-6941.2001.tb00809.x. [DOI] [PubMed] [Google Scholar]
- 14.Han RR, Ehlers RU. 2000. Pathogenicity, development, and reproduction of Heterorhabditis bacteriophora and Steinernema carpocapsae under axenic in vivo conditions. J Invertebr Pathol 75:55–58. doi: 10.1006/jipa.1999.4900. [DOI] [PubMed] [Google Scholar]
- 15.Joyce SA, Clarke DJ. 2003. A hexA homologue from Photorhabdus regulates pathogenicity, symbiosis and phenotypic variation. Mol Microbiol 47:1445–1457. doi: 10.1046/j.1365-2958.2003.03389.x. [DOI] [PubMed] [Google Scholar]
- 16.Eckstein S, Dominelli N, Brachmann A, Heermann R. 2019. Phenotypic heterogeneity of the insect pathogen Photorhabdus luminescens: insights into the fate of secondary cells. App Env Microbiol 85:e01910-19. doi: 10.1128/AEM.01910-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckstein S, Heermann R. 2019. Regulation of phenotypic switching and heterogeneity in Photorhabdus luminescens cell populations. J Mol Biol 431:4559–4568. doi: 10.1016/j.jmb.2019.04.015. [DOI] [PubMed] [Google Scholar]
- 18.Turlin E, Pascal G, Rousselle J-C, Lenormand P, Ngo S, Danchin A, Derzelle S. 2006. Proteome analysis of the phenotypic variation process in Photorhabdus luminescens. Proteomics 6:2705–2725. doi: 10.1002/pmic.200500646. [DOI] [PubMed] [Google Scholar]
- 19.Morrissey JP, Dow JM, Mark GL, O'Gara F. 2004. Are microbes at the root of a solution to world food production? Rational exploitation of interactions between microbes and plants can help to transform agriculture. EMBO Rep 5:922–926. doi: 10.1038/sj.embor.7400263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Crozier L, Hedley PE, Morris J, Wagstaff C, Andrews SC, Toth I, Jackson RW, Holden NJ. 2016. Whole-transcriptome analysis of verocytotoxigenic Escherichia coli O157:H7 (Sakai) suggests plant-species-specific metabolic responses on exposure to spinach and lettuce extracts. Front Microbiol 7:1088. doi: 10.3389/fmicb.2016.01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schaefer AL, Oda Y, Coutinho BG, Pelletier DA, Weiburg J, Venturi V, Greenberg EP, Harwood CS. 2016. A LuxR homolog in a cottonwood tree endophyte that activates gene expression in response to a plant signal or specific peptides. mBio 7:e01101-16. doi: 10.1128/mBio.01101-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Coutinho BG, Mevers E, Schaefer AL, Pelletier DA, Harwood CS, Clardy J, Greenberg EP. 2018. A plant-responsive bacterial-signaling system senses an ethanolamine derivative. Proc Natl Acad Sci U S A 115:9785–9790. doi: 10.1073/pnas.1809611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferluga S, Venturi V. 2009. OryR is a LuxR-family protein involved in interkingdom signaling between pathogenic Xanthomonas oryzae pv. oryzae and rice. J Bacteriol 191:890–897. doi: 10.1128/JB.01507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos CL, Tavares F, Thioulouse J, Normand P. 2009. A phylogenomic analysis of bacterial helix-turn-helix transcription factors. FEMS Microbiol Rev 33:411–429. doi: 10.1111/j.1574-6976.2008.00154.x. [DOI] [PubMed] [Google Scholar]
- 25.Yi Y, de Jong A, Frenzel E, Kuipers OP. 2017. Comparative transcriptomics of Bacillus mycoides strains in response to potato-root exudates reveals different genetic adaptation of endophytic and soil isolates. Front Microbiol 8:1487. doi: 10.3389/fmicb.2017.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mark GL, Dow JM, Kiely PD, Higgins H, Haynes J, Baysse C, Abbas A, Foley T, Franks A, Morrissey J, O'Gara F. 2005. Transcriptome profiling of bacterial responses to root exudates identifies genes involved in microbe-plant interactions. Proc Natl Acad Sci U S A 102:17454–17459. doi: 10.1073/pnas.0506407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.López-Farfán D, Reyes-Darias JA, Matilla MA, Krell T. 2019. Concentration dependent effect of plant root exudates on the chemosensory systems of Pseudomonas putida KT2440. Front Microbiol 10:78. doi: 10.3389/fmicb.2019.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lanois A, Jubelin G, Givaudan A. 2008. FliZ, a flagellar regulator, is at the crossroads between motility, haemolysin expression and virulence in the insect pathogenic bacterium Xenorhabdus. Mol Microbiol 68:516–533. doi: 10.1111/j.1365-2958.2008.06168.x. [DOI] [PubMed] [Google Scholar]
- 29.Jubelin G, Lanois A, Severac D, Rialle S, Longin C, Gaudriault S, Givaudan A. 2013. FliZ is a global regulatory protein affecting the expression of flagellar and virulence genes in individual Xenorhabdus nematophila bacterial cells. PLoS Genet 9:e1003915. doi: 10.1371/journal.pgen.1003915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho BT, Dong TG, Mekalanos JJ. 2014. A view to a kill: the bacterial type VI secretion system. Cell Host Microbe 15:9–21. doi: 10.1016/j.chom.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernal P, Allsopp LP, Filloux A, Llamas MA. 2017. The Pseudomonas putida T6SS is a plant warden against phytopathogens. ISME J 11:972–987. doi: 10.1038/ismej.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marchi M, Boutin M, Gazengel K, Rispe C, Gauthier J-P, Guillerm-Erckelboudt A-Y, Lebreton L, Barret M, Daval S, Sarniguet A. 2013. Genomic analysis of the biocontrol strain Pseudomonas fluorescens Pf29Arp with evidence of T3SS and T6SS gene expression on plant roots. Environ Microbiol Rep 5:393–403. doi: 10.1111/1758-2229.12048. [DOI] [PubMed] [Google Scholar]
- 33.Shank EA, Kolter R. 2009. New developments in microbial interspecies signaling. Curr Opin Microbiol 12:205–214. doi: 10.1016/j.mib.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu L, Robert CAM, Cadot S, Zhang X, Ye M, Li B, Manzo D, Chervet N, Steinger T, van der Heijden MGA, Schlaeppi K, Erb M. 2018. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nat Commun 9:2738. doi: 10.1038/s41467-018-05122-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domka J, Lee J, Wood TK. 2006. YliH (BssR) and YceP (BssS) regulate Escherichia coli K-12 biofilm formation by influencing cell signaling. Appl Environ Microbiol 72:2449–2459. doi: 10.1128/AEM.72.4.2449-2459.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bailly A, Groenhagen U, Schulz S, Geisler M, Eberl L, Weisskopf L. 2014. The inter-kingdom volatile signal indole promotes root development by interfering with auxin signalling. Plant J 80:758–771. doi: 10.1111/tpj.12666. [DOI] [PubMed] [Google Scholar]
- 37.Majeed A, Abbasi MK, Hameed S, Imran A, Rahim N. 2015. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol 6:198. doi: 10.3389/fmicb.2015.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ahemad M, Saghir Kha M. 2011. Assessment of plant growth promoting activities of rhizobacterium Pseudomonas putida under insecticide-stress. Microbiology J 1:54–64. doi: 10.3923/mj.2011.54.64. [DOI] [Google Scholar]
- 39.Khan N, Martínez-Hidalgo P, Ice TA, Maymon M, Humm EA, Nejat N, Sanders ER, Kaplan D, Hirsch AM. 2018. Antifungal activity of Bacillus species against Fusarium and analysis of the potential mechanisms used in biocontrol. Front Microbiol 9:2363. doi: 10.3389/fmicb.2018.02363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cole BJ, Feltcher ME, Waters RJ, Wetmore KM, Mucyn TS, Ryan EM, Wang G, Ul-Hasan S, McDonald M, Yoshikuni Y, Malmstrom RR, Deutschbauer AM, Dangl JL, Visel A. 2017. Genome-wide identification of bacterial plant colonization genes. PLoS Biol 15:e2002860. doi: 10.1371/journal.pbio.2002860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lambrese Y, Guiñez M, Calvente V, Sansone G, Cerutti S, Raba J, Sanz MI. 2018. Production of siderophores by the bacterium Kosakonia radicincitans and its application to control of phytopathogenic fungi. Biores Technol Rep 3:82–87. doi: 10.1016/j.biteb.2018.06.003. [DOI] [Google Scholar]
- 42.Scavino AF, Pedraza RO. 2013. The role of siderophores in plant growth-promoting bacteria, p 265–285. In Bacteria in agrobiology: crop productivity. Springer, Berlin, Germany. [Google Scholar]
- 43.Haichar FEZ, Santaella C, Heulin T, Achouak W. 2014. Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–80. doi: 10.1016/j.soilbio.2014.06.017. [DOI] [Google Scholar]
- 44.Saint CP, Wexler M, Murphy PJ, Tempé J, Tate ME, Murphy PJ. 1993. Characterization of genes for synthesis and catabolism of a new rhizopine induced in nodules by Rhizobium meliloti Rm220-3: extension of the rhizopine concept. J Bacteriol 175:5205–5215. doi: 10.1128/jb.175.16.5205-5215.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furlan JPR, de Almeida OGG, De Martinis ECP, Stehling EG. 2019. Characterization of an environmental multidrug-resistant Acinetobacter seifertii and comparative genomic analysis reveals co-occurrence of antimicrobial resistance and metal tolerance determinants. Front Microbiol 10:2151. doi: 10.3389/fmicb.2019.02151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zamioudis C, Mastranesti P, Dhonukshe P, Blilou I, Pieterse C. 2013. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol 162:304–318. doi: 10.1104/pp.112.212597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gupta Sood S. 2003. Chemotactic response of plant-growth-promoting bacteria towards roots of vesicular-arbuscular mycorrhizal tomato plants. FEMS Microbiol Ecol 45:219–227. doi: 10.1016/S0168-6496(03)00155-7. [DOI] [PubMed] [Google Scholar]
- 48.Boyd A, Kendall K, Simon MI. 1983. Structure of the serine chemoreceptor in Escherichia coli. Nature 301:623–626. doi: 10.1038/301623a0. [DOI] [PubMed] [Google Scholar]
- 49.Koo BJ, Adriano DC, Bolan NS, Barton CD. 2005. Root exudates and microorganisms, p 421–428. In Hillel D. (ed), Encyclopedia of soils in the environment. Elsevier, Oxford, United Kingdom. [Google Scholar]
- 50.Berendsen RL, Pieterse CMJ, Bakker P. 2012. The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486. doi: 10.1016/j.tplants.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Zamora-Lagos M-A, Eckstein S, Langer A, Gazanis A, Pfeiffer F, Habermann B, Heermann R. 2018. Phenotypic and genomic comparison of Photorhabdus luminescens subsp. laumondii TT01 and a widely used rifampicin-resistant Photorhabdus luminescens laboratory strain. BMC Genomics 19:854. doi: 10.1186/s12864-018-5121-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lassak J, Henche A-L, Binnenkade L, Thormann KM. 2010. ArcS, the cognate sensor kinase in an atypical Arc system of Shewanella oneidensis MR-1. Appl Environ Microbiol 76:3263–3274. doi: 10.1128/AEM.00512-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Glaeser A, Heermann R. 2015. A novel tool for stable genomic reporter gene integration to analyze heterogeneity in Photorhabdus luminescens at the single-cell level. Biotechniques 59:74–81. doi: 10.2144/000114317. [DOI] [PubMed] [Google Scholar]
- 54.Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Simon P. 2003. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics 19:1439–1440. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- 56.Singh AK. 2010. Optimization of culture conditions for thermostable chitinase production by Paenibacillus sp. D1. African J Microbiol Res 4:2291–2298. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.