Abstract
Purpose
Succinate dehydrogenase B (SDHB) associated pheochromocytomas (PHEOs) are associated with a higher risk of tumor aggressiveness and malignancy. The aim of the present study was to evaluate (1) the frequency of germline SDHB mutations in apparently sporadic patients with PHEO who undergo preoperative genetic testing and (2) the ability to predict pathogenic mutations.
Methods
From 2012 to 2016, 82 patients underwent a PHEO surgical resection. Sixteen were operated in the context of hereditary PHEO and were excluded from analysis. Among the 66 remaining cases, 48 were preoperatively screened for an SDHB mutation. In addition to imaging studies with specific radiopharmaceuticals (123I-MIBG or 18F-FDOPA) for exclusion of multifocality/metastases, 36 patients underwent 18F-FDG PET/CT.
Results
From the 48 genetically screened patients, genetic testing found a germline SDHB variant in two (4.2%) cases: a variant of unknown significance, exon 1, c.14T>G (p.Val5Gly), and a most likely pathogenic mutation, exon 5, c.440A>G (p.Tyr147Cys), according to in silico analysis. Structural and functional analyses of the protein predicted that p.Tyr147Cys mutant was pathogenic. Both tumors exhibited moderate 18F-FDG PET uptake with similar uptake patterns to non-SDHB mutated PHEOs. The two patients underwent total laparoscopic adrenalectomies. Of the remaining patients, 44 underwent a laparoscopic adrenalectomy, and two had an open approach. Pathological analysis of the tumors from patients bearing two germline SDHB variants revealed a typical PHEO (PASS 0 and 2). Ex-vivo analyses (metabolomics, SDHB immunohistochemistry, loss of heterozygosity analysis) allowed a reclassification of the two SDHB variants as probably non-pathogenic variants.
Conclusions
This study illustrates that SDHx mutational analysis can be misleading, even if structural and functional analyses are done. Surgeons should be aware of the difficulty of classifying new SDHB variants prior to implementing SDHB mutation status into a tailored surgical management strategy of a patient.
Keywords: Pheochromocytomas, SDHB, Radionuclide imaging
Introduction
Pheochromocytomas/paragangliomas (PPGLs) are rare tumors arising from the adrenal medulla with an annual incidence of one to eight patients per million [1]. They account for about 4% of adrenal incidentalomas, with an even higher prevalence in autopsy series. Pheochromocytomas (PHEOs) usually cause symptoms of catecholamine (norepinephrine or epinephrine) over secretion (e.g., sustained or paroxysmal elevations in blood pressure, headache, episodic profuse sweating, palpitations, pallor, nervousness, or anxiety). PPGLs can be sporadic, or occur as components of hereditary syndromes in up to 30–40%, with variable penetrance and increased risk for recurrent behavior and tumor multiplicity [2, 3]. Hereditary PHEOs develop primarily in the context of three familial tumor syndromes: von Hippel-Lindau disease (VHL), multiple endocrine neoplasia type 2 (RET), and familial PPGL (related to mutations in one of the succinate dehydrogenase (SDH) subunits genes, collectively named SDHx). Other genes account for a small minority of cases (e.g., NF1, MAX, and TMEM127). Most PHEOs occur sporadically, but can be related to germline driver mutations in less than 10% of cases. Metastatic PHEOs are rare and more often seen with large tumors (usually over 5–6 cm) and those presenting with significant necrosis on histopathological examination. PHEOs with an underlying SDHB mutation are also associated with a higher risk of aggressive behavior, development of metastatic disease, and ultimately, death [4]. Overall, the malignancy risk for SDHB mutation-associated tumors has been estimated at 30% (range 20–70%). At least 40% of SDHB mutation carriers develop metastatic disease [5]. It is widely accepted that the identification of a mutation in SDHB is a marker of poor prognosis and more close clinically monitoring of the patient is required. Therefore, all patients with PPGLs should be engaged in shared decision making for genetic testing. Immunohistochemical studies can be used as a screening method to guide genetic testing. The absence of SDHB immunoexpression is indicative of a germline mutation in one of the SDH genes [6].
Currently, an adrenalectomy is the only curative treatment for patients with PHEOs. Anatomical imaging can guide surgeons towards the most appropriate surgical route (laparoscopic, retroperitoneoscopic, laparotomy) and strategy (subtotal vs total). Depending on the situation, a total adrenalectomy can also be extended to the retroperitoneal fat or adjacent organs. As of now, knowledge of the mutational status is not mandatory prior to an adrenalectomy for an apparently sporadic PHEO. However, based on what we know in terms of the increased risk of aggressiveness of SDHB-related PHEOs as well as their pathogenesis related to adrenomedullary hyperplasia [7], it seems logical to perform a total adrenalectomy after gaining the knowledge of an SDHB genetic status. A study from the National Institutes of Health recently suggested that a more aggressive surgical approach would be warranted in patients with SDHB-related carotid body paragangliomas [8]. It is therefore possible that such an attitude may also be applied to SDHB-related PHEOs, although properly designed studies have not been done. Lack of recommendation regarding the need to get the results of genetic status prior to adrenalectomy is probably due to a number of reasons: (1) fear of prolonged delay of adrenalectomy while waiting for the results of genetic tests; (2) uncertainty regarding the pathogenesis of new genetic variants, which could not be fully investigated in the absence of evaluating tumor tissue; and (3) a low frequency of germline mutations, (especially SDHB mutations) in this population of patients, further complicated by the low penetrance of PHEO in SDHB mutation carriers.
The aim of the present study was to evaluate the frequency of germline SDHB mutations in patients who underwent preoperative genetic testing and the ability to predict potentially pathogenic mutations.
Material and methods
Patient population
Among all patients who underwent an adrenalectomy between January 2012 and June 2016 for a presumed non-metastatic PHEO (based on radiological and functional imaging studies) in the Department of Endocrine Surgery, only those who fulfilled the following criteria were included: (1) initial surgery for PHEO, (2) apparently sporadic cases, and (3) underwent preoperative genetic testing for SDHB mutation. The criteria used to define the apparently sporadic cases were the absence of a family history of PHEO and/or PHEO syndrome-related tumors. Preoperative evaluation for malignancy and multifocality was performed with a whole-body 123I-MIBG SPECT/CT or 18F-FDOPA PET/CT. Patients with multifocal disease or metastases were excluded.
In addition to specific radiopharmaceuticals, 36 patients underwent 18F-FDG PET/CT according to our previous institutional guidelines.
Genetic testing
After a blood sample was obtained, results of SDHB testing were received within 2 weeks and were given to a surgeon prior to adrenalectomy. Genetic testing for other germline mutations for PHEO susceptibility genes (VHL, SDHB, SDHC, SDHD, MAX, and TMEM127) was obtained in a few weeks following surgery. RET genetic screening was not performed due to normal preoperative serum calcitonin levels and no family history of multiple endocrine neoplasia type 2 in our patients.
Genomic DNA from peripheral blood leukocytes was extracted and the coding exons and exon-intron boundaries of genes (SDHB NM_003000.2, SDHD NM_003002.3, SDHC NM_003001.3, VHL NM_000551.3, MAX NM_002382.3, TMEM127 NM_017849.3) were PCR-amplified and screened by direct sequencing. The potential effect of each missense or silent variation on genes was evaluated in silico by using a battery of different bioinformatics algorithms, i.e., Polyphen2, UMD-predictor® [9], Alamut software (including SpliceSiteFinder, MaxEntScan, MNSPLICE, GeneSplicer, Human Splicing finder, RESCUE-ESE), and SIFT. Then the variants were also classified following the recommendation of the American College of Medical Genetics [10].
Surgical strategy
Laparoscopic adrenalectomy was performed via a transperitoneal lateral laparoscopic approach. The technique consisted in the mobilization of a tumor together with surrounding fatty tissue. The mobilization was performed with minimal manipulation of a tumor with progressive vascular controls, in coordination with the anesthesiology team. The adrenal glands were excised with all surrounding fatty tissue (i.e., radical adrenalectomy) to allow safe tumor margins. PHEOs were removed in one piece or morcellated to allow excision via a laparoscopic port. Drainage was used selectively. All specimens were placed in a surgical bag. In other cases, a midline laparotomy was performed. Surgical strategy was tailored to individual cases depending on patient and tumor characteristics (size, relation to adjacent organ). If performed, the surgeon had the knowledge of the SDHB mutation status in all cases.
Immunohistochemistry
In addition to standard histological analysis, immunohistochemical analysis of SDHB protein was performed on paraffin-embedded tumors for all cases (polyclonal Anti-SDHB antibody, dilution 1/150, Sigma-Aldrich, HPA002868). Non-SDHx mutated PHEOs typically exhibit a granular cytoplasmic positive SDHB staining (mitochondrial pattern) whereas SDHx-related PHEOs are SDHB protein negative (normal tissue is used as positive control).
In vitro studies
Generation of SDHB expression vectors
Normal SDHB cDNAwas generated from human adrenal total RNA and inserted into pEGFP-N1, using EcoRI and BamHI restriction sites, as previously described [11]. P.Tyr147Cys SDHB plasmid was generated by performing site-directed mutagenesis using Quikchange mutagenesis kit (Agilent Stratagene, CA, USA) with 5′-TTTGTACTGTGCAC AGAAGTTGCTCAAATCGGGAACAAG-3′ and its reverse compliment primer, according to the manufacturer’s guidelines. Sanger sequencing was used to confirm the presence of wildtype or mutant sequences.
Cell culture and transfections
Human embryonic kidney 293 (HEK293) cells (American Type Culture Collection, VA, USA) cultured in DMEM (Life Technologies, CA, USA) with 10% fetal bovine serum (Life Technologies) were seeded at 1.0 × 106 cells/25 cm2 flask and left to settle over night at 37 °C and 5% CO2. Cells were transfected with 7.5 μg of SDHB plasmids under presence of 15 μl of Lipofectamine 2000 (Life Technologies) and 650 μl Opti-MEM (Life Technologies) over 24 h post seeding. The cells were then washed with PBS and lysed using a subcellular fractioning kit or immunoprecipitation lysis buffer.
Subcellular localization and Western blotting
A Qproteome Kit (Qiagen, Hilden, Germany) was used to separate and extract cytosolic or membrane proteins from HEK293 cells post transfection in accordance with the manufacturer’s guidelines. Proteins were then homogenized by brief sonication, and protein concentration was determined using BCA Protein Assay Kit (Pierce Biotechnology, IL, USA). Cell lysates were mixed with NuPAGE ® LDS sample buffer (Invitrogen CA, USA) and dithiothreitol (Sigma, MO, USA), incubated at 95 °C over 5 min, and then cooled on ice for 5 min. Proteins were separated by SDS-PAGE (4–12% NuPAGE Bis-Tris Gels, Invitrogen) and then transferred to nitrocellulose membranes and membranes blocked with 5% skim milk (in TBST) for 1 h at room temperature. GFP monoclonal mouse antibody (dilution 1:2000, Roche (11814460001), Basel, Switzerland), MT-CO2 (dilution 1:2000, Abcam (ab3298), Cambridge, UK) and GAPDH (dilution 1:5000, Cell Signaling (D16H11), MA, USA) were used to probe membranes overnight at 4 °C. Blotted membranes were washed 3 × 10 min with TBST and incubated with the relevant secondary antibody conjugated to HRP. After another 3 × 10 min with TBST, proteins were detected with ECL Plus Western Blotting Detection Reagent (GE Healthcare, Little Chalfont, UK) on a LAS-3000 (Fujifilm, NSW, Australia). Quantitation was performed using the Multi-Gauage 3.11 Software (Fujifilm).
Immunoprecipitation and SDH activity assay
After 24 h of transfection, HEK293 cells were washed with PBS, pelleted, and lysed using immunoprecipitation (IP) buffer consisting of 20 mM Tris pH 7.5, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 0.1% Triton X100. Dynabeads® M-280 sheep anti-mouse IgG (Life Technologies) were incubated with either GFP monoclonal mouse antibody (dilution 1:2000, Roche, Basel, Switzerland) or mouse IgG antibody (dilution 1:2000, Thermo-Fisher, MA, USA) for negative control for 2 h prior to washing, followed by overnight incubation with cell lysates at 4 °C under gentle agitation. Proteins not associated with GFP-tagged SDHB were removed with 3 × 10 min gentle agitation washes from the beads using IP buffer with a higher salt concentration (500 mM NaCl), then fresh IP lysis buffer was re-introduced to prevent drying and degradation of samples.
For SDH activity assay, reagent containing 25 mM potassium phosphate buffer, 5 mM MgCl2, pH 7.2, 20 mM sodium succinate, 50 mM 2, 6-dichlorophenolindophenol (DCPIP), 2 mM KCN, 2 mg/ml antimycin A, 2 mg/ml rotenone, and water was incubated with immunoprecipitated GFP-tagged products for 10 mins at 30 °C. Reaction was initiated with addition of 120 μM phenazine methosulfate and 120 μM DCPIP. Decrease in absorbance at 600 nm over time was measured and normalized by densitometry of SDHB-GFP immunoblot from IP beads as SDH function [11]. Genetic screening for loss of heterozygosity (LOH) was performed in SDHB PHEOs. 1H-high-resolution magic angle spinning nuclear magnetic resonance spectroscopy (HRMAS NMR) was performed in one tumor.
In silico homology modeling of SDHB proteins
Theoretical 3D models were built for human mitochondrial succinate dehydrogenase subunits A and B (Proteins SDHA and SDHB corresponding to Uniprot accession numbers P31040 and P21912, respectively) using homology modeling. When no experimental structural data is available for a protein, 3D models can be generated using this approach, which relies on using a template with a known 3D structure. Quality of theoretical 3D models is directly linked to the identity between template and target sequences. It is generally accepted that accurate models, helpful in designing or interpreting mutagenesis experiments, can be built for proteins sharing at least 30% sequence identities. It is important to mention that we consider strict identity here (and not just similarity). In the present case study, the human and porcine proteins share a very high sequence identity (98.4 and 97.5% for SDHA and SDHB, respectively). This extremely high percentage of sequence identity between the template and the target allows for the construction of high quality 3D models. It is also important to note that all residues involved in the interaction between SDHA and SDHB are strictly conserved between the porcine and human proteins, in particular, residues Tyr147 (SDHB), Arg97, and Phe94 (SDHA), which are discussed in Fig. 2.
Fig. 2.
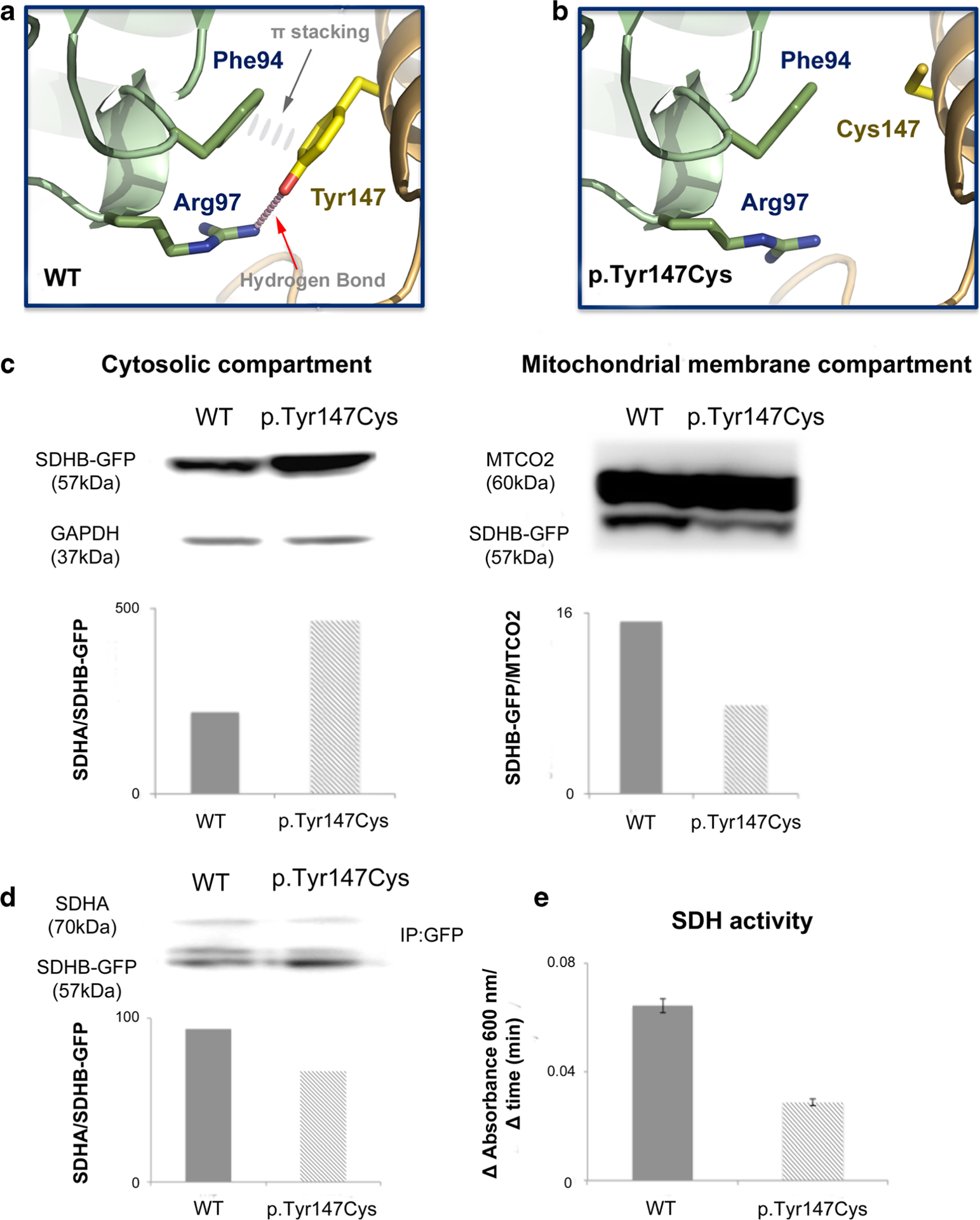
Cartoon representation of the 3D models, structural and functional analyses. a, b Homology 3D models of human mitochondrial succinate dehydrogenase subunits a and b. Homology models of wildtype (WT) (a) and Tyr147Cys mutant (b) human SDHA/SDHB complexes focusing on the region of the mutation. Residue Tyr147 (SDHB), located at the interface between SDHA and SDHB, is engaged in a hydrogen bond with Arg97 and aromatic stacking interactions with Phe94 (both from SDHA). These interactions that stabilize the dimer interface in WT proteins are lost in the mutant leading to a weaker interface. Y147C loses these two interactions with inevitable destabilization of the SDH complex. It is worth pointing out that tyrosine is known to be a frequent hotspot at protein-protein interfaces. The figure was generated with pymol (http://www.pymol.org/). c–e WT and p.Tyr147Cys SDHB in PEGFPN1 vector were transfected into HEK293 over 24 h before lysis. c Compartmentalization of GFP-tagged SDHB WT and p.Tyr147Cys was assessed by western blotting of GFP in fractionized cell lysate with corresponding cytosolic (GAPDH) and mitochondrial membrane (MTCO2) compartment housekeeper proteins. d–e Immunoprecipitation (IP) using GFP-tag was performed to isolate product of transfection from endogenous SDHB. Association between GFP-tagged SDHB products and endogenous SDHAwas assessed with western blotting (d). SDH enzyme activity was determined by measuring the reduction of the artificial electron acceptor DCPIP during conversion of succinate to fumarate using an absorbance assay over time in presence of immunoprecipitated GFP-tagged SDHB and its associated subunits [41], reduced conversion rate of succinate to fumarate was observed with mutant SDHB relative to WT (e)
Protein sequence alignments were obtained with Clustal Omega [12] and Emboss Needle (http://www.ebi.ac.uk/Tools/psa/emboss_needle/). Homology models of human SDHA and SDHB were built with MODELLER 9v14 [13, 14]. Crystal structure of mitochondrial respiratory complex II from porcine heart (PDB code 1ZOY) was used as a template [15]. Chain A in 1ZOY corresponds to porcine SDHA (accession number Q0QF01) and chain B to porcine SDHB (accession number Q007T0). Tyr147Cys mutant was generated by replacing residue Tyr147 by a cysteine in the sequence of human SDHB before performing the homology modeling.
1H-high-resolution magic angle spinning nuclear magnetic resonance spectroscopy (1H-HRMAS NMR)
Metabolomics investigations were performed in one tumor by 1H-HRMAS NMR spectroscopy using a Bruker Avance III 500 spectrometer. Fifteen milligrams of frozen tumor was used for NMR analysis. A one-dimensional (1D) proton spectrum using a Carr-Purcell-Meiboom-Gill pulse sequence with water signal pre-saturation followed by a two-dimensional (2D) heteronuclear (1H-13C) spectrum was acquired. Metabolites were assigned using standard chemical shift tables. The procedure for metabolite quantification has been previously described [16, 17]. Briefly, quantification was performed using an external reference standard of lactate, scanned under the same analytical conditions. Spectra within the range of 8.65–1 ppm were normalized according to sample weight and peaks of interest were automatically defined. Succinate concentration in tissue samples is estimated by integrating the area comprised between 2.39 and 2.43 ppm [16, 17].
PHEO SDHB loss of heterozygosity
Analysis of loss of heterozygosity (LOH) of SDHB was performed in PHEOs from patients bearing the p.Val5Gly and p.Tyr147Cys SDHB variants at germline level. Specimens were obtained from formalin-fixed paraffin-embedded (FFPE) PHEO tissues. The fragment used for LOH analysis was selected by the pathologist and contained more than 90% of tumor cells. After DNA extraction, samples were analyzed for somatic allelic SDHB variants using Sanger sequencing. Using an electropherogram, the presence or absence of heterozygosity on the mutated site was checked.
Statistical analyses
Data are presented as median (inter-quartile range) for continuous variable and as counts (percentages) for categorical data. A Mann-Whitney test was used to compare continuous data. Chi2 or Fisher’s exact tests were used to compare categorical data. P values of less than 0.05 were taken to be statistically significant. All statistical analyses were two-sided and performed using SPSS PAWS Statistics 24.0 (IBM Inc., NY, USA).
Results
Patients
From 2012 to 2016, 82 patients were operated for a presumed PHEO. Sixteen cases were operated under the context of hereditary PHEO and were excluded from this study (8 MEN2, 3 NF1, 2 VHL, 1 MAX, 1 SDHD, 1 SDHB). Among the 66 remaining cases, 48 were preoperatively screened for a SDHB mutation (Fig. 1). Patients and tumor characteristics are detailed in Table 1.
Fig. 1.
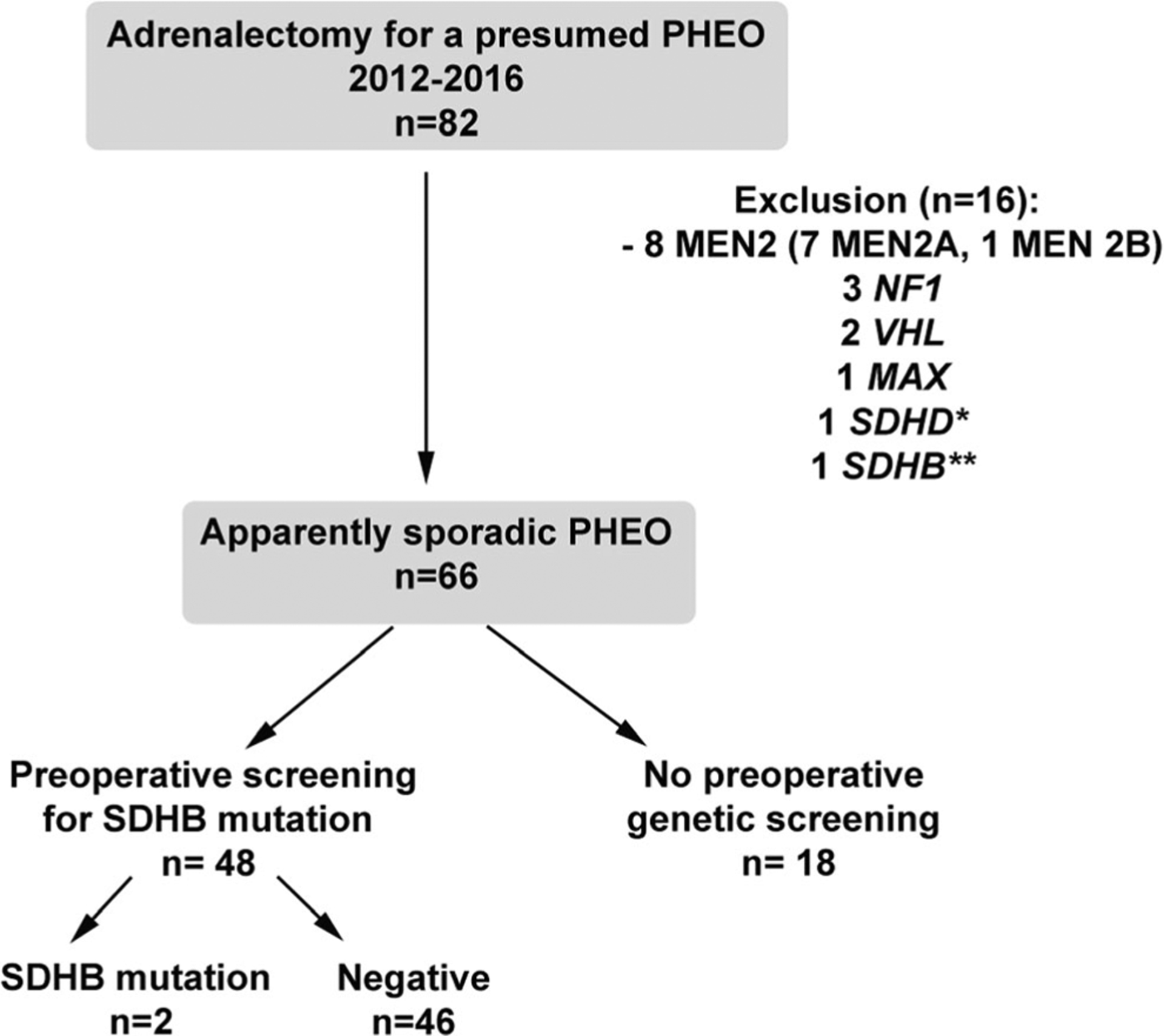
Flow chart
Table 1.
Patients and tumor characteristics. Median values (min-max)
| Preop testing for SDHB | No preop SDHB testing | p | ||
|---|---|---|---|---|
| A—negative | B—positive | C | A+B vs C | |
| N | 46 | 2 | 18 | |
| Age | 49 (30–75) | 69 (63–75) | 52 (36–75) | 0.624 |
| Sex ratio (M/W) | 0.92 | 1 | 1 | 0.880 |
| Tumor size (mm) | 40 (12–110) | 31.5 (26–37) | 32 (15–66) | 0.433 |
| Side | 0.313 | |||
| - Right | 27 (58.7%) | 1 (50%) | 8 (44.4%) | |
| - Left | 19 (41.3%) | 1(50%) | 10 (55.6%) | |
| Single location | 45 (97.8%) | 2 (100%) | 18 (100%) | 0.999 |
| Metanepherine U (URL) | 2.1 (0.1–24.5) | 1.8 (1.2–2.3) | 2.47 (0.4–22) | 0.664 |
| Normetanephrine U (URL) | 3.3 (0.3–48.4) | 0.9 (0.9–1) | 2.35 (1.6–28.4) | 0.962 |
| PASS score | ||||
| - <4 | 40 (87%) | 2 (100%) | 14 (n = 17) (82.4%) | |
| - >3 | 6 (13%) | 0 | 3 (n = 17) (17.7%) | 0.689 |
| FDG uptake (tumor/liver SUVmax radtio) | N = 34 | N = 2 | N = 11 | 0.562 |
| 1.6 (0.7–8.5) | 1.25 (1.2–1.3) | 1.6 (1–3.5) | ||
SDHB genetic status
Genetic testing revealed new germline SDHB variants in two patients: exon 5, c.440A>G (p.Tyr147Cys); and exon 1, c.14T>G (p.Val5Gly). Before surgery and based on in silico analysis, p.Tyr147Cys variant was classified as probably pathogenic and p.Val5Gly as a variant of unknown significance (Table 2).
Table 2.
In silico analysis of the two new SDHB variants p.Val5Gly and p.Tyr147Cys
| SDHB variants | Nucleotide conservationa |
Amino acid conservationa |
Physico-chemical gapa (Grantham score) |
LOVD databaseb |
1000 genome |
ESP database |
Exac database |
Polyphen (score) | SIFT | UMD-predictor® (score) |
Our classification before surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| c.14A>G (p.Val5Gly) |
Low | Middle | Strong (109) | Absent | Absent | Absent | 1/121218 | Benign (0.010) | Tolerated | Probably polymorphism (60) | Unknown significance |
| c.440A>G (p.Tyrl47Cys) |
Strong | Very strong | Very strong (194) | Absent | Absent | Absent | 1/121410 | Probably damaging (0.98) | Deleterious | Pathogenic (90) | Probably pathogenic |
Alamut software
In vitro functional analysis
Lysates from cytosolic and mitochondrial membrane compartments were isolated to assess impact of p.Tyr147Cys change on cellular localization of the mutant SDHB product. Western blotting analysis shows higher level of p.Tyr147Cys SDHB-GFP in cytosol and lower level in mitochondrial membrane compared to wildtype SDHB-GFP (Fig. 2c). Immunopre cipitation of SDHA through GFP pulldown showed reduced association between endogenous SDHA and SDHB-GFP products (Fig. 2d). Also, SDH activity measured from GFP pulldown SDHB products showed noticeable reduction in enzymatic activity (Fig. 2e). Taken together, these data suggest certain level of impairment in SDH maturation arising from p.Tyr147Cys SDHB mutant in context of compartmentalization and association with SDHA subunit leading to reduced SDH function.
Surgical approaches and pathological findings
From the 48 patients, all but two were operated via a laparoscopic approach, including the patients presenting with new SDHB variants. Based on preoperative imaging studies, a surgical approach consisted of total adrenalectomy without resection of adjacent organs in both patients bearing new SDHB variants. The other 44 patients were also operated via a laparoscopic approach (43 total and 1 subtotal) and open surgery was performed in the remaining two cases. Among the 44 patients operated via a laparoscopic approach, surgical conversion to laparotomy was performed in one case (nephrectomy needed to be performed to achieve hemostasis). Partial (subtotal) and total adrenalectomies (with removal of periadrenal fat) were performed in one and 43 cases, respectively. In three cases, an adrenalectomy was associated with resection of ipsilateral kidney in two cases or extensive lymph node resection in one case (Table 3).
Table 3.
Surgical procedures
| Preop testing for SDHB | No preop SDHB testing | p | ||
|---|---|---|---|---|
| A—negative | B—positive | C | A+B vs C | |
| N | 46 | 2 | 18 | |
| Open surgery | 2 (4.4%) | 0 | 0 | 0.999 |
| Total laparoscopic surgery | 43 (93.5%) | 2 (100%) | 17 (94.4%) | |
| Surgical conversion during laparoscopic surgery | 1 (2.2%) | 0 | 1 (5.6%) | 0.487 |
| Type of surgery | ||||
| - Partial adrenalectomy | 1 (2.2%) | 0 | 0 | 0.999 |
| - Total adrenalectomy | 45 (97.8%) | 2 (100%) | 18(100%) | 0.999 |
| Adrenalectomy with resection of adjacent organs (extended Adx) | 3 (6.5%) | 0 | 0 | 0.556 |
| Final genetic status | 0.999 | |||
| - SDHB variant | 0 | 2 | 0 | |
| - Other mutation | 0 | 0 | 0 | |
Final genetic status at germline level
The genetic screening of other PHEO susceptibility genes (SDHD, SDHC, VHL, MAX TMEM127) was negative for all patients, including the two patients bearing new SDHB variants. These analyses were performed after surgery.
Description of the two patients bearing a SDHB variant at germline level
Case #1
A 75-year-old male was admitted to our center in 2012 for evaluation of an incidentally discovered adrenal mass in 2008. The patient had no personal or family history of endocrine tumors. He was asymptomatic. Preoperative work-up on two different occasions found mild elevation of metanephrines (3740/3630 nmol/24 h, upper reference limit URL <1600) and normetanephrine levels within the upper normal range (1890/1790, nmol/24 h, URL <1900 nmol/24 h). Serum chromogranin A was elevated (414 μg/l, URL <100 μg/l). Computed tomography (CT) and 123I-metaiodobenzy lguanidine (MIBG) findings were consistent with a solitary 37-mm left PHEO. 18F-FDG PET/CT found moderate tracer uptake (tumor SUVmax/liver SUVmax = 1.3). 18F-fluoro-L-dihydroxyphenylalanine (18F-FDOPA) PET/CT was also positive. Preoperative genetic screening found a SDHB variant (exon 5, p.Tyr147Cys (c.440A>G)), classified as probably pathogenic variant. Structural and functional analyses (as outlined in previous work) [11] found reduced mitochondrial localization (accumulation in cytoplasm) of the mutant with reduced SDH (succinate dehydrogenase) activity relative to wildtype (Fig. 2). A radical adrenalectomy (without cortical sparing) via a laparoscopic approach was indicated. Pathological analysis found a typical PHEO without aggressive features (PASS 0, Ki-67 <1%). In silico modeling of SDHB protein bearing the variant p.Tyr147Cys predicted a pathogenic variant (Fig. 3a, b). However, tumor cells exhibited positive SDHB staining (Fig. 3c). Furthermore, succinate level assessed by HRMAS NMR was normal, a finding which excludes a SDH deficiency in the tumor (Fig. 3d). Finally, LOH in SDHB was not observed and, therefore, it was in agreement with the pathological and metabolomic patterns.
Fig. 3.
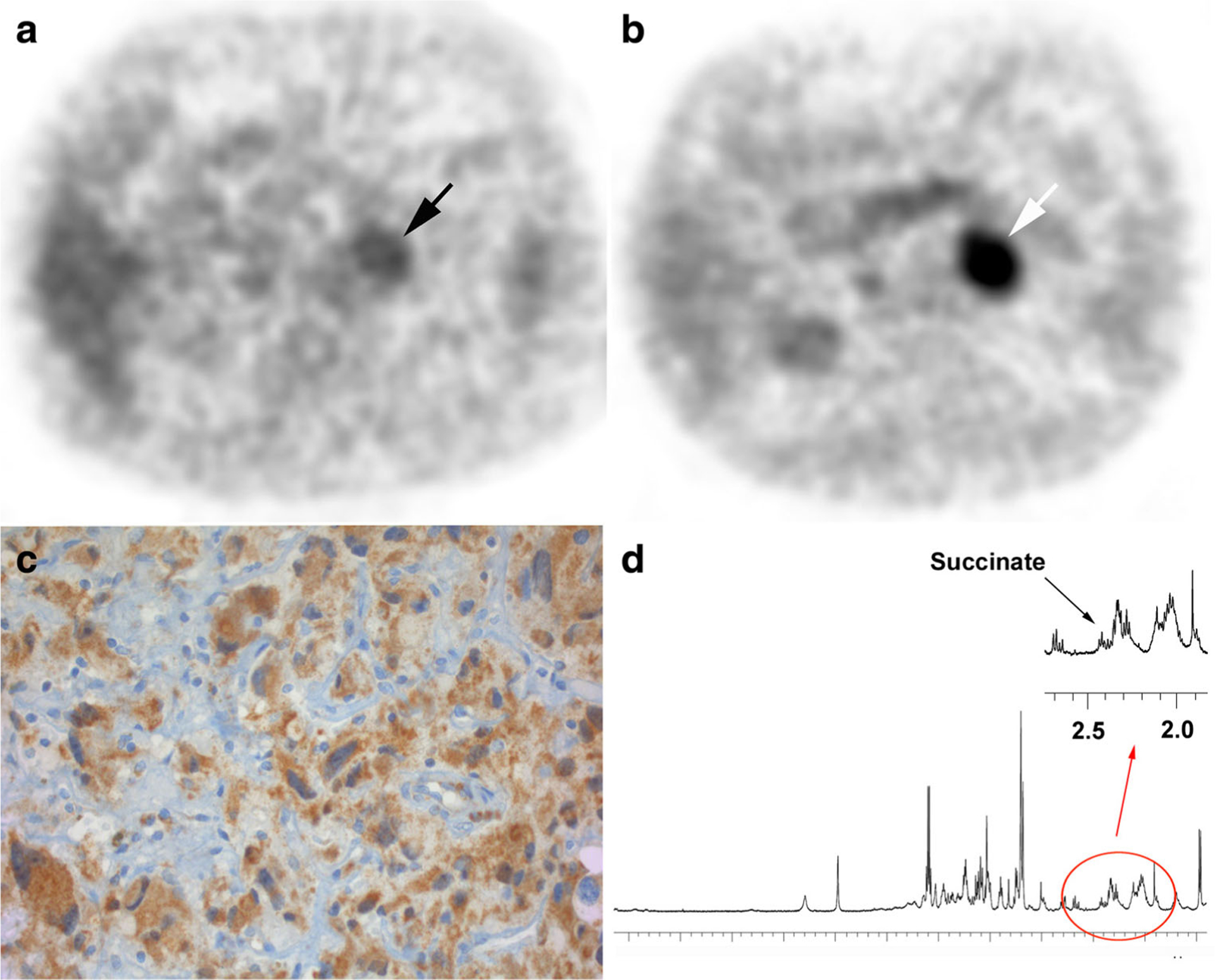
PET/CT imaging, immunohistochemistry, and metabolomics. a 18F-FDG PET (correction-attenuation axial image) showing an adrenal mass with relatively moderate tracer uptake. b 18F–FDOPA PET/CT (correction-attenuation axial image) showing a highly-avid adrenal tumor, highly suggestive of PHEO. c The tumor exhibits positive SDHB immunostaining. d 1H-high-resolution magic angle spinning (HRMAS) NMR spectroscopy results from the analysis of the tumor showing low succinate amount, confirming the absence of an SDH complex deficiency. Norepinephrine and epinephrine signal were not detected in the examined tissue sample
Case #2
A 62-year-old woman was admitted to our center in 2013 for an incidentally discovered adrenal mass on an abdominal CT scan performed for the evaluation of prolonged fever. She had a history of non-secreting pituitary adenoma (8 mm) and a gastric neuroendocrine tumor associated with pernicious anemia. She had no other family history of endocrine tumors. She had symptoms of catecholamine oversecretion, with sustained and paroxysmal elevations in blood pressure, palpitations, and episodic profuse sweating. Preoperative work-up found an elevation of urinary (1890 nmol/24 h, upper reference limit URL <1600 nmol/24 h) and plasma (0.98 and 0.89 nmol/l, URL <0.37 nmol/l) metanephrines with normal levels of urinary (1570 nmol/24 h, URL <1900 nmol/24 h) and plasma (0.58 and 0.65 nmol/l, URL <0.94 nmol/l) normetanephrine. Serum chromogranin A was elevated (240 μg/l, URL <100). CTand 123I-MIBG findings were consistent with a solitary 26-mm right PHEO. 18F-FDG PET/CT found moderate tracer uptake (tumor SUVmax/liver SUVmax = 1.2). 18F-FDOPA PET/CT was also positive. Preoperative genetic screening found a SDHB variant (exon 1, c.14T>G p.Val5Gly) of unknown significance. Homology modeling was not possible due to the lack of published 3D representation of the N-terminus region of SDHB. In vitro functional analysis was not performed. A radical adrenalectomy (without cortical sparing) via a laparoscopic approach was indicated. Pathological analysis found a typical PHEO without aggressive features (PASS 0, Ki-67 <1%). HRMAS NMR was not performed due to the absence of available frozen tissue sample. Immunostaining for SDHB staining was strongly positive, a finding that is consistent with a sporadic (non-SDH deficient) PHEO. Finally, LOH in SDHB was not observed.
Discussion
To our best knowledge, this is the first study that evaluates the use of preoperative SDHB mutation screening for the management of apparently sporadic PHEOs under real life conditions. The principal conclusions that can be drawn from this study include the following: first, the low frequency of SDHB mutations occurring in a selected large population of patients presenting with a single, apparently sporadic PHEO; second, the difficulty of reclassifying new SDHB variants based on the currently accepted structural (mitochondrial vs cytoplasmic localization of SDHB protein) or functional (relative SDH activity) ex-vivo methods; and third, the excellent help of immunohistochemical analysis (detecting the presence of SDHB protein), LOH, and functional imaging using 18F-FDG PET/CT for better characterization of SDHB-related PHEOs. In clinical practice, 18F-FDG PET/CT should be performed in PPGLs patients with SDHx mutations/variants. Thus, present and previously published results suggest that comprehensive molecular-structural-functional-imaging analysis of PHEOs labeled as “SDHB” should be performed when variants of unknown clinical significance are identified (not truncating variants).
The frequency of germline mutations in apparently sporadic PHEOs is roughly 5 to 8% [18–20]. Nevertheless, SDHB gene analysis should always be performed in PHEOs because of their aggressive, especially metastatic, behavior, regardless of family history since the penetrance of the disease is low. Furthermore, SDHB mutations are associated with other tumors, including renal cell carcinoma, which has an extremely high metastatic potential [21, 22]. In the case of epinephrine or metanephrine-producing PHEOs, SDHB genetic analysis may not need to be performed, as these tumors are unlikely to be associated with an SDHB mutation [23, 24]. In the present study, both patients presented with a noradrenergic biochemical phenotype.
The present study also illustrates that SDHx mutational analysis can be misleading, even when structural and functional analyses of SDHB protein are performed. This could be due to lack of a particular assay performance despite its validation for clinical use.
The study indicates the importance of additional tests, including immunohistochemistry, metabolomics, or LOH, which are absolutely necessary to correctly label a patient as an SDHB mutation carrier. Higher cytoplasmic and lower membrane localization of GFP-tagged p.Tyr147Cys SDHB relative to wildtype suggest that this variant is potentially compromising the integrity of SDH cellular compartmentalization leading to SDH dysfunction.
PPGLs can be associated with germline mutations in one of the succinate dehydrogenase subunit genes (A through D). The four SDHx genes encode the four subunits of the SDH enzyme (also named mitochondrial complex II). This membrane complex catalyzes the oxidation of succinate to fumarate in the tricarboxylic acid cycle (TCA) and the respiratory chain. SDH acts as a tumor suppressor in the paraganglionic system. According to Knudson’s classical “two-hit hypothesis,” tumorigenesis requires the combination of an inactivating germline mutation as a first hit, resulting in somatic loss of function of the WT allele. The nature of the second hit has been reported in a recent study for 85 SDH mutations and includes inactivation of the WT allele by LOH (in 73% of cases), or more rarely, somatic mutations (in 14% of cases). Exceptionally, an epigenetic inactivation of the second allele might be observed [25]. Biallelic inactivation in any of the SDH genes results in decreased SDHB expression and accumulation of succinate [6, 26]. Immunohistochemical studies against SDHB [6], SDHA [27], and SDHD [28] are well-established methods and are currently well incorporated in the pathological analysis of these tumors. The present study further testifies that this approach should be used in all sporadic PHEOs, especially those labeled as potentially SDHB-related. Immunohistochemical analysis is a standardized technique with good inter-observer reproducibility [6]. Non-SDHx mutated PHEOs typically exhibit a granular cytoplasmic positive immunostaining (mitochondrial pattern) whereas SDHx-related PHEOs have negative immunostaining (normal tissue is used as positive control). The main pitfalls of LOH analysis is the number of tumor cells in the analyzed tumor fragment. In both cases presented here, the fragments used for LOH analysis were selected by a pathologist and contained more than 90% of tumor cells.
Furthermore, several excellent ex-vivo studies showed the importance of metabolomics in the assessment of these tumors. This is based on the most important fact that SDHB mutations are associated with high succinate and low fumarate levels, respectively [16, 17, 29, 30]. More recently, in vivo detection of succinate was also achieved by proton Magnetic Resonance Spectroscopy (1H-MRS) in patients with SDHx-related PPGL [31, 32]. Respiratory-triggered 1H-MRS in addition to standard MR acquisition could be also performed for the in vivo assessment of catecholamines for the diagnosis of PHEO in difficult situations [33].
Finally, several previous and recent studies showed the important value of functional imaging in the evaluation of PHEO including those that are SDHB-related [34–40].
This study shows that a proper comprehensive evaluation should include immunohistochemistry and/or metabolomics of PHEO in patients with SDHB variants. Evaluations are necessary to provide the patient with the correct genetic diagnosis, appropriate treatment, follow-up protocol, prognosis, and genetic counseling.
Conclusion
Surgeons should be aware of the difficulty of classifying new SDHB variants prior to implementing SDHB mutation status into a tailored surgical management strategy of a patient. The impact of preoperative SDHB testing needs to be further evaluated in a multicenter study with a cost-benefit analysis due to low incidence of SDHB mutations in apparently sporadic PHEOs.
Funding
This research did not receive a specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Footnotes
Conflicts of interest The authors declare that they have no conflict of interest.
References
- 1.Stenstrom G, Svardsudd K (1986) Pheochromocytoma in Sweden 1958–1981. An analysis of the National Cancer Registry Data. Acta Med Scand 220(3):225–232 [PubMed] [Google Scholar]
- 2.Pacak K, Wimalawansa SJ (2015) Pheochromocytoma and paraganglioma. Endocr Pract 21(4):406–412. doi: 10.4158/EP14481.RA [DOI] [PubMed] [Google Scholar]
- 3.Jochmanova I, Yang C, Zhuang Z, Pacak K (2013) Hypoxiainducible factor signaling in pheochromocytoma: turning the rudder in the right direction. J Natl Cancer Inst 105(17):1270–1283. doi: 10.1093/jnci/djt201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turkova H, Prodanov T, Maly M, Martucci V, Adams K, Widimsky J Jr, Chen CC, Ling A, Kebebew E, Stratakis CA, Fojo T, Pacak K (2016) Characteristics and outcomes of metastatic Sdhb and sporadic pheochromocytoma/paraganglioma: an National Institutes of Health study. Endocr Pract 22(3):302–314. doi: 10.4158/EP15725.OR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF Jr (2014) Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99(6):1915–1942. doi: 10.1210/jc.2014-1498 [DOI] [PubMed] [Google Scholar]
- 6.van Nederveen FH, Gaal J, Favier J, Korpershoek E, Oldenburg RA, de Bruyn EM, Sleddens HF, Derkx P, Riviere J, Dannenberg H, Petri BJ, Komminoth P, Pacak K, Hop WC, Pollard PJ, Mannelli M, Bayley JP, Perren A, Niemann S, Verhofstad AA, de Bruine AP, Maher ER, Tissier F, Meatchi T, Badoual C, Bertherat J, Amar L, Alataki D, Van Marck E, Ferrau F, Francois J, de Herder WW, Peeters MP, van Linge A, Lenders JW, Gimenez-Roqueplo AP, de Krijger RR, Dinjens WN (2009) An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: a retrospective and prospective analysis. Lancet Oncol 10(8):764–771. doi: 10.1016/S1470-2045(09)70164-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grogan RH, Pacak K, Pasche L, Huynh TT, Greco RS (2011) Bilateral adrenal medullary hyperplasia associated with an SDHB mutation. J Clin Oncol 29(8):e200–e202. doi: 10.1200/JCO.2010.32.2156 [DOI] [PubMed] [Google Scholar]
- 8.Ellis RJ, Patel D, Prodanov T, Nilubol N, Pacak K, Kebebew E (2013) The presence of SDHB mutations should modify surgical indications for carotid body paragangliomas. Ann Surg. doi: 10.1097/SLA.0000000000000283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frederic MY, Lalande M, Boileau C, Hamroun D, Claustres M, Beroud C, Collod-Beroud G (2009) UMD-predictor, a new prediction tool for nucleotide substitution pathogenicity—application to four genes: FBN1, FBN2, TGFBR1, and TGFBR2. Hum Mutat 30(6):952–959. doi: 10.1002/humu.20970 [DOI] [PubMed] [Google Scholar]
- 10.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL, Committee ALQA (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim E, Rath EM, Tsang VH, Duff AP, Robinson BG, Church WB, Benn DE, Dwight T, Clifton-Bligh RJ (2015) Structural and functional consequences of succinate dehydrogenase subunit B mutations. Endocr Relat Cancer 22(3):387–397. doi: 10.1530/ERC-15-0099 [DOI] [PubMed] [Google Scholar]
- 12.Sievers F, Higgins DG (2014) Clustal omega. Curr Protoc Bioinformatics 48:3.13.11–3.13.16. doi: 10.1002/0471250953.bi0313s48 [DOI] [PubMed] [Google Scholar]
- 13.Webb B, Sali A (2016) Comparative protein structure modeling using MODELLER. Curr Protoc Bioinformatics 54:5.6.1–5.6.37. doi: 10.1002/cpbi.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Webb B, Sali A (2014) Protein structure modeling with MODELLER. Methods Mol Biol 1137:1–15. doi: 10.1007/978-1-4939-0366-5_1 [DOI] [PubMed] [Google Scholar]
- 15.Sun F, Huo X, Zhai Y, Wang A, Xu J, Su D, Bartlam M, Rao Z (2005) Crystal structure of mitochondrial respiratory membrane protein complex II. Cell 121(7):1043–1057 [DOI] [PubMed] [Google Scholar]
- 16.Imperiale A, Moussallieh FM, Sebag F, Brunaud L, Barlier A, Elbayed K, Bachellier P, Goichot B, Pacak K, Namer IJ, Taieb D (2013) A new specific succinate-glutamate metabolomic hallmark in SDHx-related paragangliomas. PLoS One 8(11):e80539. doi: 10.1371/journal.pone.0080539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Imperiale A, Moussallieh FM, Roche P, Battini S, Cicek AE, Sebag F, Brunaud L, Barlier A, Elbayed K, Loundou A, Bachellier P, Goichot B, Stratakis CA, Pacak K, Namer IJ, Taieb D (2015) Metabolome profiling by HRMAS NMR spectroscopy of pheochromocytomas and paragangliomas detects SDH deficiency: clinical and pathophysiological implications. Neoplasia 17(1):55–65. doi: 10.1016/j.neo.2014.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Welander J, Andreasson A, Juhlin CC, Wiseman RW, Backdahl M, Hoog A, Larsson C, Gimm O, Soderkvist P (2014) Rare germline mutations identified by targeted next-generation sequencing of susceptibility genes in pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 99(7):E1352–E1360. doi: 10.1210/jc.2013-4375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buffet A, Venisse A, Nau V, Roncellin I, Boccio V, Le Pottier N, Boussion M, Travers C, Simian C, Burnichon N, Abermil N, Favier J, Jeunemaitre X, Gimenez-Roqueplo AP (2012) A decade (2001–2010) of genetic testing for pheochromocytoma and paraganglioma. Horm Metab Res 44(5):359–366. doi: 10.1055/s-0032-1304594 [DOI] [PubMed] [Google Scholar]
- 20.Curras-Freixes M, Inglada-Perez L, Mancikova V, Montero-Conde C, Leton R, Comino-Mendez I, Apellaniz-Ruiz M, Sanchez-Barroso L, Aguirre Sanchez-Covisa M, Alcazar V, Aller J, Alvarez-Escola C, Andia-Melero VM, Azriel-Mira S, Calatayud-Gutierrez M, Diaz JA, Diez-Hernandez A, Lamas-Oliveira C, Marazuela M, Matias-Guiu X, Meoro-Aviles A, Patino-Garcia A, Pedrinaci S, Riesco-Eizaguirre G, Sabado-Alvarez C, Saez-Villaverde R, Sainz de Los Terreros A, Sanz Guadarrama O, Sastre-Marcos J, Scola-Yurrita B, Segura-Huerta A, Serrano-Corredor Mde L, Villar-Vicente MR, Rodriguez-Antona C, Korpershoek E, Cascon A, Robledo M (2015) Recommendations for somatic and germline genetic testing of single pheochromocytoma and paraganglioma based on findings from a series of 329 patients. J Med Genet 52(10):647–656. doi: 10.1136/jmedgenet-2015-103218 [DOI] [PubMed] [Google Scholar]
- 21.Ricketts CJ, Forman JR, Rattenberry E, Bradshaw N, Lalloo F, Izatt L, Cole TR, Armstrong R, Kumar VK, Morrison PJ, Atkinson AB, Douglas F, Ball SG, Cook J, Srirangalingam U, Killick P, Kirby G, Aylwin S, Woodward ER, Evans DG, Hodgson SV, Murday V, Chew SL, Connell JM, Blundell TL, Macdonald F, Maher ER (2010) Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat 31(1):41–51. doi: 10.1002/humu.21136 [DOI] [PubMed] [Google Scholar]
- 22.Saxena N, Maio N, Crooks DR, Ricketts CJ, Yang Y, Wei MH, Fan TW, Lane AN, Sourbier C, Singh A, Killian JK, Meltzer PS, Vocke CD, Rouault TA, Linehan WM (2016) SDHB-deficient cancers: the role of mutations that impair iron sulfur cluster delivery. J Natl Cancer Inst 108(1). doi: 10.1093/jnci/djv287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sue M, Martucci V, Frey F, Lenders JM, Timmers HJ, Peczkowska M, Prejbisz A, Swantje B, Bornstein SR, Arlt W, Fassnacht M, Beuschlein F, Robledo M, Pacak K, Eisenhofer G (2015) Lack of utility of SDHB mutation testing in adrenergic metastatic phaeochromocytoma. Eur J Endocrinol 172(2):89–95. doi: 10.1530/EJE-14-0756 [DOI] [PubMed] [Google Scholar]
- 24.Eisenhofer G, Lenders JW, Timmers H, Mannelli M, Grebe SK, Hofbauer LC, Bornstein SR, Tiebel O, Adams K, Bratslavsky G, Linehan WM, Pacak K (2011) Measurements of plasma methoxytyramine, normetanephrine, and metanephrine as discriminators of different hereditary forms of pheochromocytoma. Clin Chem 57(3):411–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evenepoel L, Papathomas TG, Krol N, Korpershoek E, de Krijger RR, Persu A, Dinjens WN (2014) Toward an improved definition of the genetic and tumor spectrum associated with SDH germ-line mutations. Genet Med. doi: 10.1038/gim.2014.162 [DOI] [PubMed] [Google Scholar]
- 26.Yang C, Matro JC, Huntoon KM, Ye DY, Huynh TT, Fliedner SM, Breza J, Zhuang Z, Pacak K (2012) Missense mutations in the human SDHB gene increase protein degradation without altering intrinsic enzymatic function. FASEB J 26(11):4506–4516. doi: 10.1096/fj.12-210146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korpershoek E, Favier J, Gaal J, Burnichon N, van Gessel B, Oudijk L, Badoual C, Gadessaud N, Venisse A, Bayley JP, van Dooren MF, de Herder WW, Tissier F, Plouin PF, van Nederveen FH, Dinjens WN, Gimenez-Roqueplo AP, de Krijger RR (2011) SDHA immunohistochemistry detects germline SDHA gene mutations in apparently sporadic paragangliomas and pheochromocytomas. J Clin Endocrinol Metab 96(9):E1472–E1476. doi: 10.1210/jc.2011-1043 [DOI] [PubMed] [Google Scholar]
- 28.Menara M, Oudijk L, Badoual C, Bertherat J, Lepoutre-Lussey C, Amar L, Iturrioz X, Sibony M, Zinzindohoue F, de Krijger R, Gimenez-Roqueplo AP, Favier J (2015) SDHD immunohistochemistry: a new tool to validate SDHx mutations in pheochromocytoma/paraganglioma. J Clin Endocrinol Metab 100(2):E287–E291. doi: 10.1210/jc.2014-1870 [DOI] [PubMed] [Google Scholar]
- 29.Lendvai N, Pawlosky R, Bullova P, Eisenhofer G, Patocs A, Veech RL, Pacak K (2014) Succinate-to-fumarate ratio as a new metabolic marker to detect the presence of SDHB/D-related paraganglioma: initial experimental and ex vivo findings. Endocrinology 155(1): 27–32. doi: 10.1210/en.2013-1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richter S, Peitzsch M, Rapizzi E, Lenders JW, Qin N, de Cubas AA, Schiavi F, Rao JU, Beuschlein F, Quinkler M, Timmers HJ, Opocher G, Mannelli M, Pacak K, Robledo M, Eisenhofer G (2014) Krebs cycle metabolite profiling for identification and stratification of pheochromocytomas/paragangliomas due to succinate dehydrogenase deficiency. J Clin Endocrinol Metab 99(10):3903–3911. doi: 10.1210/jc.2014-2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lussey-Lepoutre C, Bellucci A, Morin A, Buffet A, Amar L, Janin M, Ottolenghi C, Zinzindohoue F, Autret G, Burnichon N, Robidel E, Banting B, Fontaine S, Cuenod CA, Benit P, Rustin P, Halimi P, Fournier L, Gimenez-Roqueplo AP, Favier J, Tavitian B (2016) In vivo detection of succinate by magnetic resonance spectroscopy as a Hallmark of SDHx mutations in paraganglioma. Clin Cancer Res 22(5):1120–1129. doi: 10.1158/1078-0432.CCR-15-1576 [DOI] [PubMed] [Google Scholar]
- 32.Varoquaux A, le Fur Y, Imperiale A, Reyre A, Montava M, Fakhry N, Namer IJ, Moulin G, Pacak K, Guye M, Taieb D (2015) Magnetic resonance spectroscopy of paragangliomas: new insights into in vivo metabolomics. Endocr Relat Cancer 22(4):M1–M8. doi: 10.1530/ERC-15-0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Imperiale A, Battini S, Averous G, Mutter D, Goichot B, Bachellier P, Pacak K, Taieb D, Namer IJ (2016) In vivo detection of catecholamines by magnetic resonance spectroscopy: a potential specific biomarker for the diagnosis of pheochromocytoma. Surgery 159(4):1231–1233. doi: 10.1016/j.surg.2015.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taieb D, Sebag F, Barlier A, Tessonnier L, Palazzo FF, Morange I, Niccoli-Sire P, Fakhry N, De Micco C, Cammilleri S, Enjalbert A, Henry JF, Mundler O (2009) 18F-FDG avidity of pheochromocytomas and paragangliomas: a new molecular imaging signature? J Nucl Med 50(5):711–717. doi: 10.2967/jnumed.108.060731 [DOI] [PubMed] [Google Scholar]
- 35.Taieb D, Timmers HJ, Shulkin BL, Pacak K (2014) Renaissance of (18)F-FDG positron emission tomography in the imaging of pheochromocytoma/paraganglioma. J Clin Endocrinol Metab 99(7): 2337–2339. doi: 10.1210/jc.2014-1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Eisenhofer G, King KS, Rao JU, Wesley RA, Adams KT, Pacak K (2012) Staging and functional characterization of pheochromocytoma and paraganglioma by 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography. J Natl Cancer Inst 104(9): 700–708. doi: 10.1093/jnci/djs188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K (2009) Comparison of 18F-fluoro-L-DOPA, 18F-fluorodeoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. J Clin Endocrinol Metab 94(12):4757–4767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Ling A, Eisenhofer G, Adams KT, Solis D, Lenders JW, Pacak K (2007) Superiority of fluorodeoxyglucose positron emission tomography to other functional imaging techniques in the evaluation of metastatic SDHB-associated pheochromocytoma and paraganglioma. J Clin Oncol 25(16):2262–2269 [DOI] [PubMed] [Google Scholar]
- 39.van Berkel A, Rao JU, Kusters B, Demir T, Visser E, Mensenkamp AR, van der Laak JA, Oosterwijk E, Lenders JW, Sweep FC, Wevers RA, Hermus AR, Langenhuijsen JF, Kunst DP, Pacak K, Gotthardt M, Timmers HJ (2014) Correlation between in vivo 18F-FDG PET and immunohistochemical markers of glucose uptake and metabolism in pheochromocytoma and paraganglioma. J Nucl Med 55(8):1253–1259. doi: 10.2967/jnumed.114.137034 [DOI] [PubMed] [Google Scholar]
- 40.Zelinka T, Timmers HJ, Kozupa A, Chen CC, Carrasquillo JA, Reynolds JC, Ling A, Eisenhofer G, Lazurova I, Adams KT, Whatley MA, Widimsky J Jr, Pacak K (2008) Role of positron emission tomography and bone scintigraphy in the evaluation of bone involvement in metastatic pheochromocytoma and paraganglioma: specific implications for succinate dehydrogenase enzyme subunit B gene mutations. Endocr Relat Cancer 15(1):311–323 [DOI] [PubMed] [Google Scholar]
- 41.Kirby DM, Thorburn DR, Turnbull DM, Taylor RW (2007) Biochemical assays of respiratory chain complex activity. Methods Cell Biol 80:93–119. doi: 10.1016/S0091-679X(06)80004-X [DOI] [PubMed] [Google Scholar]


