Abstract
Background
The choice of treatment in patients with metastatic colorectal cancer (mCRC) is generally influenced by tumour and patient characteristics, treatment efficacy and tolerability, and quality of life. Better patient selection might lead to improved outcomes.
Methods
This post hoc exploratory analysis examined the effect of prognostic factors on outcomes in the Randomized, Double-blind, Phase 3 Study of trifluridine tipiracil (FTD/TPI) plus Best Supportive Care (BSC) versus Placebo plus BSC in Patients with mCRC Refractory to Standard Chemotherapies (RECOURSE) trial. Patients were redivided by prognosis into two subgroups: those with <3 metastatic sites at randomisation (low tumour burden) and ≥18 months from diagnosis of metastatic disease to randomisation (indolent disease) were included in the good prognostic characteristics (GPC) subgroup; the remaining patients were considered to have poor prognostic characteristics (PPC).
Results
GPC patients (n=386) had improved outcome versus PPC patients (n=414) in both the trifluridine/tipiracil and placebo arms. GPC patients receiving trifluridine/tipiracil (n=261) had an improved median overall survival (9.3 vs 5.3 months; HR (95% CI) 0.46 (0.37 to 0.57), p<0.0001) and progression-free survival (3.3 vs 1.9 months; HR (95% CI) 0.56 (0.46 to 0.67), p<0.0001) than PPC patients receiving trifluridine/tipiracil (n=273). Improvements in survival were irrespective of age, Eastern Cooperative Oncology Group Performance Status (ECOG PS), KRAS mutational status, and site of metastases at randomisation. In the trifluridine/tipiracil arm, time to deterioration of ECOG PS to ≥2 and proportion of patients with PS=0–1 discontinuing treatment were longer for GPC than for PPC patients (7.8 vs 4.2 months and 89.1% vs 78.4%, respectively).
Conclusion
Low tumour burden and indolent disease were factors of good prognosis in late-line mCRC, with patients experiencing longer progression-free survival and greater overall survival.
Keywords: chemotherapy, metastases, performance status, prognosis, tumour burden
Key questions.
What is already known about this subject?
The choice of treatment in patients with metastatic colorectal cancer (mCRC) is generally influenced by tumour characteristics and patient factors, as well as treatment characteristics such as tolerability, efficacy and quality-of-life effects. Trifluridine/tipiracil is indicated in pretreated patients with mCRC, based on results of the pivotal Randomised, Double-blind, Phase 3 Study of trifluridine tipiracil (FTD/TPI) plus Best Supportive Care (BSC) versus Placebo plus BSC in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapies (RECOURSE) trial, which demonstrated significantly improved overall survival (OS) compared with placebo with a manageable safety profile.
What does this study add?
In RECOURSE, classification of patients as having good prognostic characteristics (GPC, defined as those with low tumour burden (<3 metastatic sites at randomisation) and less aggressive disease (≥18 months from diagnosis of first metastasis at randomisation)) identified a subgroup of patients with improved OS and progression-free survival with trifluridine/tipiracil compared with patients with poor prognostic characteristics treated with trifluridine/tipiracil and GPC patients treated with placebo.
How might this impact on clinical practice?
Low tumour burden and indolent disease were shown to be factors of good prognosis in late-line mCRC, with these patients experiencing longer time on treatment and greater OS. This suggests that these patients could be candidates to receive further lines of therapy post trifluridine/tipiracil.
Introduction
Inclusion of new therapeutic options into the current treatment landscape in metastatic colorectal cancer (mCRC) has led to an increased survival in the last couple of decades.1–3 First-line treatment of patients typically involves the use of vascular endothelial growth factor (VEGF)- or epidermal growth factor receptor (EGFR)-targeted agents (eg, bevacizumab, cetuximab, panitumumab) to fluoropyrimidine-based (fluorouracil or capecitabine) chemotherapy regimens, depending on the presence or absence of RAS mutation-positive disease.2 4 In the USA, immunotherapies (nivolumab±ipilimumab or pembrolizumab) are also recommended for the treatment of patients with mismatch repair deficient or microsatellite instability-high disease.4 In the second-line setting, VEGF-targeted treatments (eg, aflibercept, ramucirumab) can also be used in combination with chemotherapy.2 4 The optimal chemotherapeutic regimen for use beyond third line remains unclear, where resistant/refractory disease and residual toxicity potentially limit the treatment options with only two possible candidates at present.5
The general condition and performance status of a patient are strong prognostic and predictive factors for mCRC treatment.2 Fitter patients are typically assigned to a more intensive treatment approach (ie, a combination of 2–3 cytotoxic agents with a biological agent) than less fit patients.2 4 The choice of treatment in the metastatic setting is generally influenced by tumour characteristics (tumour burden, localisation and biology), patient characteristics (age, Eastern Cooperative Oncology Group performance status (ECOG PS), organ function and comorbidities) and treatment characteristics (efficacy, toxicity profile, administration and quality of life (QoL) effects).2
The proportion of patients with mCRC receiving active treatment decreases from line to line, leaving more than half of patients who received an active treatment in the first line without treatment in the third-line setting, even in randomised clinical trials (in FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer only 43% of patients reached third line).6 Data from the USA indicate that only 53% of patients receiving a first line of treatment move into the second line, 28% move to the third line and only 13% will receive a fourth line of treatment.7 Being unable to receive a subsequent line of treatment therefore appears to have a negative impact on the patient’s survival.
Trifluridine/tipiracil (FTD/TPI, Lonsurf) is indicated for the treatment of adult patients with mCRC who have been previously treated with, or are not considered candidates for, available therapies including fluoropyrimidine-based, oxaliplatin-based and irinotecan-based chemotherapies, anti-VEGF agents and anti-EGFR agents for eligible patient (RAS wild type). Combination of tipiracil hydrochloride with the nucleoside metabolic inhibitor trifluridine improves its bioavailability by inhibiting its catabolism by thymidine phosphorylase.8 9 The relatively limited non-haematological toxicity of trifluridine/tipiracil makes it a good option in the third-line and refractory settings.2 5
In the pivotal phase III Randomised, Double-blind, Phase 3 Study of trifluridine tipiracil (FTD/TPI) plus Best Supportive Care (BSC) versus Placebo plus BSC in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapies (RECOURSE) trial conducted in 800 patients with mCRC eligible for treatment in the third line and beyond, treatment with trifluridine/tipiracil versus placebo extended overall survival (median OS 7.1 vs 5.3 months; HR 0.68, p<0.001) and progression-free survival (median PFS 2.0 vs 1.7 months; HR 0.48, p<0.001).10 This effect was shown in all subgroups regardless of age, ECOG PS (0–1), geographical region, race and KRAS mutational status.10 Furthermore, trifluridine/tipiracil was well tolerated, with few serious adverse events (AEs) reported; haematological toxicities were the most frequently observed AEs.10 Also, time to deterioration of ECOG PS to ≥2 was significantly improved (median 5.7 vs 4.0 months; HR 0.66, p<0.001)10 with 84% of patients treated with trifluridine/tipiracil remaining at PS 0–1 at discontinuation.11 Remaining at ECOG PS 0–1 is important as it could allow patients to further benefit from subsequent therapy and potentially extend their survival. In RECOURSE, 57.8% and 26.6% of patients treated with trifluridine/tipiracil remained alive at 6 and 12 months, respectively, in the refractory setting. In the post hoc analysis described here, we set out to explore other factors that could extend survival in the RECOURSE population. For the purposes of our exploratory analysis, we defined the characteristics of good prognosis as low tumour burden (<3 metastatic sites by Response Evaluation Criteria in Solid Tumors (RECIST) evaluation at randomisation) and less aggressive/indolent disease (≥18 months from diagnosis of first metastasis to randomisation), which are known to be strong prognostic factors in patients with mCRC with good ECOG PS.12 13 Our ultimate aim is to explore how clinicians can better predict individual treatment outcomes and support treatment selection through the continuum of care.
Materials and methods
Study design and patients
The study design and methodology of the RECOURSE trial (ClinicalTrials.gov number, NCT01607957) have been previously published.10 In brief, RECOURSE was a phase III, randomised, double-blind, placebo-controlled study comparing the efficacy and safety of trifluridine/tipiracil plus best supportive care with those of placebo plus best supportive care.10 This study included patients with metastatic biopsy-proven/documented adenocarcinoma of the colon or rectum who were previously treated with ≥2 standard chemotherapy regimens or who had tumour progression within 3 months of their most recent chemotherapy or who had clinically significant AEs precluding readministration of standard chemotherapies. Patients were randomised 2:1 to trifluridine/tipiracil 35 mg/m2 two times a day on days 1–5 and 8–12 every 4 weeks or matching placebo.10 Randomisation was stratified according to KRAS mutation status (wild type vs mutant), time from diagnosis of first metastasis to randomisation (<18 vs ≥18 months) and geographical region (Japan vs USA, European Union and Australia).10 All patients had adequate organ function and were ECOG PS of 0–1 at inclusion.10 The primary endpoint of the study was OS and secondary endpoints included PFS, objective response rate, clinical benefit rate and safety.10
Patient subgroups
In examining the effects of prognostic factors on treatment outcomes in the current analysis, several subgroups of RECOURSE patients were considered. Patients from RECOURSE (n=800) were divided according to good prognostic characteristics (GPC) and poor prognostic characteristics (PPC). Good prognosis was considered to be defined by low tumour burden (<3 metastatic sites by RECIST tumour evaluation at randomisation) and less aggressive/indolent disease (≥18 months from diagnosis of first metastasis to randomisation).12 13 Of the GPC subgroup (n=386), 261 (67.7%) patients received trifluridine/tipiracil and 125 received placebo. The remaining patients were included in the complementary PPC subgroup (n=414); of these, 273 received trifluridine/tipiracil and 141 received placebo.
Analysis outcomes
OS and PFS in the GPC subgroup were compared with those in the PPC subgroup. These subgroups were then analysed according to other tumour and patient characteristics, that is, metastatic site at randomisation for those sites present in >10% of the population (liver, lung, lymph, or peritoneum), ECOG PS (0 vs 1), KRAS mutation status (wild type vs mutant) and age (<65 vs ≥65 years). OS and PFS with trifluridine/tipiracil were compared with placebo, and were analysed according to prognostic subgroups within each of the two arms. Finally, the effect of prognostic classification of patients on ECOG PS deterioration was analysed for all patients and subgroups.
Statistical methods
Demographic and baseline characteristics of patients were summarised by treatment arm and subgroups using descriptive statistics (n, mean, SD, median, minimum and maximum) and/or frequency distributions, as appropriate.
The differences in OS, PFS and time to ECOG PS deterioration between trifluridine/tipiracil and placebo patients (or between subgroups of patients in a specific arm of treatment) were assessed using the stratified log-rank test (stratification factors used for the randomisation) from a Cox proportional hazards model. For each arm (or each subgroup), survival was summarised using Kaplan-Meier curves and was further characterised in terms of the median with the corresponding two-sided 95% CIs.
Results
Patients
Baseline patient demographics and clinical characteristics were generally similar between GPC and PPC patients (table 1). In the trifluridine/tipiracil arm, slight imbalances were seen in ECOG PS (8.1% more GPC than PPC patients had an ECOG PS of 0) and KRAS status (10.4% more GPC than PPC patients were KRAS wild type). Also, 21.4% more GPC than PPC patients had received ≥4 prior regimens. Among the PPC group treated with trifluridine/tipiracil, 59.3% of patients had ≥18 months from diagnosis of first metastasis to randomisation, but had ≥3 metastatic sites, and 23.1% of patients had <3 metastatic sites, but <18 months from diagnosis of first metastasis. Similar differences were observed in the placebo arm, with the exception of KRAS status, which was comparable in the GPC and PPC subgroups.
Table 1.
Baseline patient demographics and clinical characteristics, according to prognosis
| Trifluridine/tipiracil | Placebo | |||
| GPC subgroup* (n=261) | PPC subgroup (n=273) | GPC subgroup* (n=125) | PPC subgroup (n=141) | |
| Median age, years | 64.0 | 62.0 | 63.0 | 64.0 |
| Patient age, n (%) | ||||
| <65 years | 137 (52.5) | 163 (59.7) | 72 (57.6) | 76 (53.9) |
| 65 to <75 years | 105 (40.2) | 93 (34.1) | 43 (34.4) | 51 (36.2) |
| ≥75 years | 19 (7.3) | 17 (6.2) | 10 (8.0) | 14 (9.9) |
| Gender, n (%) | ||||
| Females | 97 (37.2) | 111 (40.7) | 47 (37.6) | 54 (38.3) |
| Male | 164 (62.8) | 162 (59.3) | 78 (62.4) | 87 (61.7) |
| Ethnicity, n (%) | ||||
| Asian | 91 (34.9) | 93 (34.1) | 43 (34.4) | 51 (36.2) |
| Other | 170 (65.1) | 180 (65.9) | 82 (65.6) | 90 (63.8) |
| ECOG PS, n (%) | ||||
| 0 | 158 (60.5) | 143 (52.4) | 77 (61.6) | 70 (49.6) |
| 1 | 103 (39.5) | 130 (47.6) | 48 (38.4) | 71 (50.4) |
| KRAS status, n (%) | ||||
| Mutant | 119 (45.6) | 153 (56.0) | 64 (51.2) | 71 (50.4) |
| Wild type | 142 (54.4) | 120 (44.0) | 61 (48.8) | 70 (49.6) |
| Time since diagnosis of metastasis, n (%) | ||||
| <18 months | 0 | 111 (40.7) | 0 | 55 (39.0) |
| ≥18 months | 261 (100.0) | 162 (59.3) | 125 (100.0) | 86 (61.0) |
| Number of prior regimens, n (%) | ||||
| 2 | 26 (10.0) | 69 (25.3) | 15 (12.0) | 30 (21.3) |
| 3 | 50 (19.2) | 69 (25.3) | 18 (14.4) | 36 (25.5) |
| ≥4 | 185 (70.9) | 135 (49.5) | 92 (73.6) | 75 (53.2) |
| Number of metastatic sites, n (%) | ||||
| 1–2 | 261 (100.0) | 63 (23.1) | 125 (100.0) | 28 (19.9) |
| ≥3 | 0 | 210 (76.9) | 0 | 113 (80.1) |
| Site of metastatic lesion, n (%)† | ||||
| Liver | 164 (62.8) | 238 (87.2) | 69 (55.2) | 120 (85.1) |
| Lung | 172 (65.9) | 219 (80.2) | 100 (80.0) | 117 (83.0) |
| Lymph | 53 (20.3) | 157 (57.5) | 32 (25.6) | 88 (62.4) |
| Peritoneum | 19 (7.3) | 70 (25.6) | 6 (4.8) | 41 (29.1) |
| Primary site of disease, n (%) | ||||
| Colon | 171 (65.5) | 167 (61.2) | 63 (50.4) | 98 (69.5) |
| Rectum | 90 (34.5) | 106 (38.8) | 62 (49.6) | 43 (30.5) |
*Defined as <3 metastatic sites and ≥18 months since first metastasis.
†Only those in more than 10% of the intent-to-treat population are included (liver, lung, lymph and peritoneum).
ECOG PS, Eastern Cooperative Oncology Group performance status; GPC, good prognostic characteristics; PPC, poor prognostic characteristics.
Treatment
Among trifluridine/tipiracil-treated patients, those in the GPC group received more treatment cycles (mean (SD) 4.1 (2.9)) compared with patients in the PPC group (mean (SD) 2.8 (2.0); online supplementary table S1). A higher proportion of GPC patients than PPC patients receiving trifluridine/tipiracil had a dose delay (53.6% vs 38.6%, respectively) or dose reduction (18.0% vs 9.6%, respectively), which is consistent with a longer duration of treatment (online supplementary table S1). However, median dose intensity in the first four cycles was high (≥80%) and did not differ markedly between the groups (cycle 1: 94.3% in the GPC group and 95.2% the PPC group; cycle 2: 89.7% and 93.0%, respectively; cycle 3: 82.4% and 87.4%, respectively; cycle 4: 84.0% and 88.3%, respectively).
esmoopen-2020-000752supp001.pdf (354.9KB, pdf)
The effect of good versus poor prognosis classifications on survival
Survival curves for the GPC versus PPC subgroups are shown in figure 1. Median OS was longer in the GPC subgroup than the PPC subgroup for both trifluridine/tipiracil (9.3 vs 5.3 months; HR (95% CI) 0.46 (0.37 to 0.57), p<0.0001, figure 1A) and placebo (6.8 vs 4.4 months; HR (95% CI) 0.55 (0.42 to 0.73), p<0.0001, figure 1B). Rates of 6 month OS (71.7% and 53.9% in GPC, and 44.4% and 34.1% in PPC for trifluridine/tipiracil and placebo, respectively) and 12 month OS (37.5% and 25.2% in GPC, and 15.3% and 10.9% in PPC for trifluridine/tipiracil and placebo, respectively) were also higher in GPC subgroups compared with PPC subgroups. Median PFS with trifluridine/tipiracil was also longer in the GPC subgroup versus the PPC subgroup (3.3 vs 1.9 months; HR (95% CI) 0.56 (0.46 to 0.67), p<0.0001); respective values for GPC versus PPC in the placebo arm were 1.8 versus 1.7 months (HR 0.79, p=0.0699). PFS at 3 and 6 months in the PPC subgroup was 31.0% and 7.9% for trifluridine/tipiracil and 11.6% and 0.9% for placebo, respectively. In the GPC subgroup, these were 51.1% and 22.4% with trifluridine/tipiracil, and 22.4% and 1.9% with placebo, respectively.
Figure 1.
Overall survival (OS) for the good prognostic characteristics (GPC) and poor prognostic characteristics (PPC) subgroups in patients receiving (A) trifluridine/tipiracil or (B) placebo. ap<0.001 (one sided). bp<0.001 (two sided). FTD/TPI, trifluridine/tipiracil; mOS, median overall survival; NR, not reached.
Effects of good prognostic factors on the relative efficacy of trifluridine/tipiracil
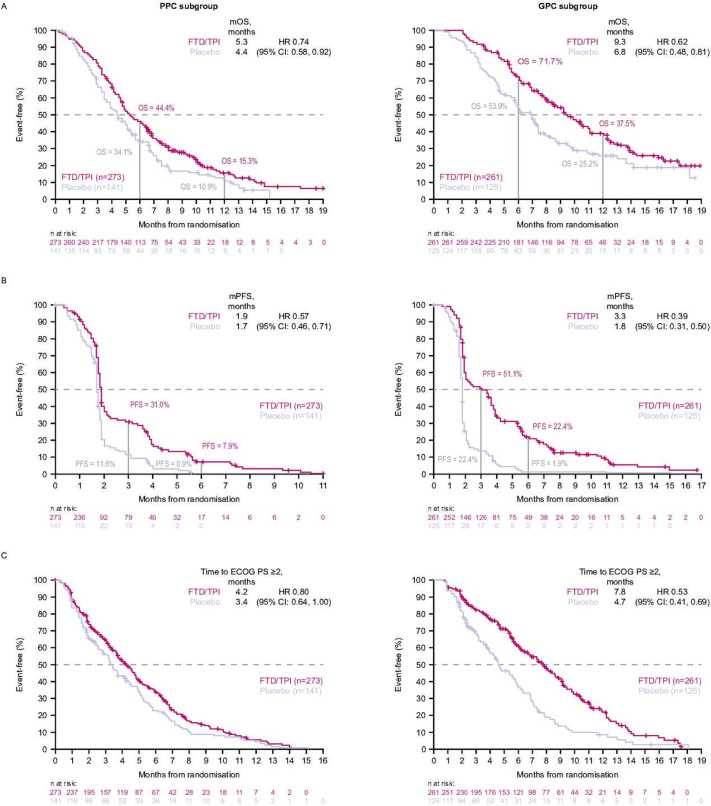
Median OS was prolonged with trifluridine/tipiracil versus placebo in both subgroups, but to a greater extent in the GPC subgroup than in the PPC subgroup (figure 2A). Similarly, median PFS was prolonged with trifluridine/tipiracil versus placebo in both subgroups, with the greatest magnitude of benefit observed in the GPC patients (figure 2B).
Figure 2.
(A) Overall survival (OS), (B) progression-free survival (PFS) and (C) time to Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2 with trifluridine/tipiracil versus placebo in the good prognostic characteristics (GPC; n=386) and poor prognostic characteristics (PPC; n=414) subgroups. FTD/TPI, trifluridine/tipiracil; mOS, median overall survival.
Analysis of prognostic factors
The effect of various prognostic factors on median OS and PFS is shown in table 2; their effect on 6-month and 12-month OS, and 3-month, 6-month, and 9-month PFS is shown in online supplementary tables 2 and 3. For both trifluridine/tipiracil and placebo, the GPC subgroup had better median OS and PFS than the PPC subgroup, irrespective of patient age (≥65 vs <65 years), ECOG PS (0 vs 1), KRAS mutation status (mutant vs wild type) and liver metastases (yes vs no).
Table 2.
The effect of various prognostic factors on median overall survival (OS) and progression-free survival (PFS)
| Number of patients FTD/TPI /placebo |
Median survival, months | HR (95% CI) | Number of patients FTD/TPI/placebo |
Median survival, months | HR (95% CI) | |
| OS | ||||||
| No liver metastases | Liver metastases | |||||
| GPC subgroup | n=97/n=56 | 16.4 vs 8.6 | 0.47 (0.29 to 0.77) | n=164/n=69 | 7.7 vs 4.7 | 0.61 (0.44 to 0.85) |
| PPC subgroup | n=35/n=21 | 7.6 vs 7.2 | 0.70 (0.35 to 1.37) | n=238/n=120 | 5.0 vs 3.7 | 0.7 (0.55 to 0.89) |
| No lung metastases | Lung metastases | |||||
| GPC subgroup | n=89/n=25 | 9.2 vs 7.3 | 0.77 (0.45 to 1.31) | n=172/n=100 | 9.8 vs 6.3 | 0.57 (0.41 to 0.78) |
| PPC subgroup | n=54/n=24 | 5.0 vs 3.7 | 0.93 (0.52 to 1.64) | n=219/n=117 | 5.3 vs 4.4 | 0.71 (0.55 to 0.91) |
| No lymph metastases | Lymph metastases | |||||
| GPC subgroup | n=208/n=93 | 9.2 vs 6.3 | 0.65 (0.48 to 0.88) | n=53/n=32 | 10.3 vs 6.9 | 0.53 (0.30 to 0.93) |
| PPC subgroup | n=116/n=53 | 5.0 vs 4.2 | 0.75 (0.52 to 1.08) | n=157/n=88 | 5.4 vs 4.5 | 0.72 (0.54 to 0.97) |
| No peritoneal metastases | Peritoneal metastases | |||||
| GPC subgroup | n=242/n=119 | 9.1 vs 6.8 | 0.66 (0.51 to 0.87) | n=19/n=6 | 14.2 vs 5.6 | 0.23 (0.06 to 0.90) |
| PPC subgroup | n=203/n=100 | 5.3 vs 4.7 | 0.74 (0.57 to 0.97) | n=70/n=41 | 4.9 vs 3.3 | 0.70 0.45 to 1.07) |
| ECOG PS=0 | ECOG PS=1 | |||||
| GPC subgroup | n=158/n=77 | 10.5 vs 7.1 | 0.66 (0.47 to 0.94) | n=103/n=48 | 8.0 vs 5.9 | 0.54 (0.36 to 0.81) |
| PPC subgroup | n=143/n=70 | 6.7 vs 5.2 | 0.77 (0.55 to 1.07) | n=130/n=71 | 4.2 vs 3.5 | 0.69 (0.50 to 0.95) |
| Age <65 years | Age≥65 years | |||||
| GPC subgroup | n=137/n=72 | 9.8 vs 6.9 | 0.64 (0.45 to 0.92) | n=124/n=53 | 9.1 vs 6.3 | 0.57 (0.39 to 0.84) |
| PPC subgroup | n=163/n=76 | 4.9 vs 5.0 | 0.80 (0.59 to 1.09) | n=110/n=65 | 5.6 vs 3.9 | 0.68 (0.48 to 0.97) |
| KRAS wild type | KRAS mutant | |||||
| GPC subgroup | n=142/n=61 | 9.3 vs 6.9 | 0.60 (0.42 to 0.86) | n=119/n=64 | 6.4 vs 4.7 | 0.61 (0.43 to 0.84) |
| PPC subgroup | n=120/n=70 | 6.4 vs 4.7 | 0.61 (0.43 to 0.84) | n=153/n=71 | 4.7 vs 3.9 | 0.87 (0.64 to 1.19) |
| PFS | ||||||
| No liver metastases | Liver metastases | |||||
| GPC subgroup | n=97/n=56 | 5.4 vs 1.9 | 0.35 (0.24 to 0.52) | n=164/n=69 | 2.1 vs 1.8 | 0.32 (0.23 to 0.45) |
| PPC subgroup | n=35/n=21 | 1.9 vs 1.9 | 0.84 (0.44 to 1.60) | n=238/n=120 | 1.9 vs 1.7 | 0.54 (0.42 to 0.68) |
| No lung metastases | Lung metastases | |||||
| GPC subgroup | n=89/n=25 | 2.3 vs 1.9 | 0.57 (0.35 to 0.95) | n=172/n=100 | 3.6 vs 1.8 | 0.33 (0.25 to 0.44) |
| PPC subgroup | n=54/n=24 | 1.8 vs 1.6 | 0.62 (0.36 to 1.08)) | n=219/n=117 | 1.9 vs 1.7 | 0.54 (0.42 to 0.69) |
| No lymph metastases | Lymph metastases | |||||
| GPC subgroup | n=208/n=93 | 3.0 vs 1.8 | 0.38 (0.29 to 0.49) | n=53/n=32 | 3.5 vs 1.8 | 0.40 (0.24 to 0.67) |
| PPC subgroup | n=116/n=53 | 1.9 vs 1.7 | 0.49 (0.34 to 0.69) | n=157/n=88 | 1.9 vs 1.7 | 0.65 (0.49 to 0.86) |
| No peritoneal metastases | Peritoneal metastases | |||||
| GPC subgroup | n=242/n=119 | 2.6 vs 1.8 | 0.41 (0.32 to 0.52) | n=19/n=6 | 5.5 vs 1.8 | 0.19 (0.05 to 0.69) |
| PPC subgroup | n=203/n=100 | 1.9 vs 1.7 | 0.56 (0.43 to 0.72) | n=70/n=41 | 1.7 vs 1.6 | 0.61 (0.40 to 0.93) |
| ECOG PS=0 | ECOG PS=1 | |||||
| GPC subgroup | n=158/n=77 | 3.5 vs 1.8 | 0.44 (0.32 to 0.59) | n=103/n=48 | 2.4 vs 1.7 | 0.33 (0.22 to 0.49) |
| PPC subgroup | n=143/n=70 | 1.9 vs 1.7 | 0.53 (0.38 to 0.72) | n=130/n=71 | 1.8 vs 1.6 | 0.61 (0.45 to 0.83) |
| Age <65 years | Age ≥65 years | |||||
| GPC subgroup | n=137/n=72 | 3.1 vs 1.8 | 0.42 (0.30 to 0.58) | n=124/n=53 | 3.5 vs 1.8 | 0.35 (0.24 to 0.51) |
| PPC subgroup | n=163/n=76 | 1.8 vs 1.7 | 0.62 (0.46 to 0.83) | n=110/n=65 | 1.9 vs 1.7 | 0.49 (0.35 to 0.69) |
| KRAS wild type | KRAS mutant | |||||
| GPC subgroup | n=142/n=61 | 3.3 vs 1.8 | 0.41 (0.29 to 0.57) | n=119/n=64 | 3.3 vs 1.8 | 0.38 (0.27 to 0.53) |
| PPC subgroup | n=120/n=70 | 1.9 vs 1.7 | 0.57 (0.41 to 0.79) | n=153/n=71 | 1.8 vs 1.7 | 0.57 (0.42 to 0.77) |
Good prognostic characteristics (GPC) were defined as <3 metastatic sites at randomisation and ≥18 months from first metastasis to randomisation.
FTD/TPI, trifluridine/tipiracil; PPC, poor prognostic characteristics; ECOG PS, Eastern Cooperative Oncology Group performance status.
When analysing the GPC subgroup, the absence of liver metastasis at randomisation (n=153; representing 39.6% of the GPC and 19.1% of the intent-to-treat population) was found to be the best factor of prognosis; further information on this group of patients is available in online supplementary table S4 and online supplementary figures S1-S3. Among GPC patients treated with trifluridine/tipiracil, median OS was 8.7 months longer in patients with no liver metastases compared with those with liver metastases (16.4 vs 7.7 months; table 2). The 6-month OS rate in GPC patients treated with trifluridine/tipiracil was 83.4% in those without liver metastases and 64.8% in those with liver metastases; corresponding 12-month OS rates in these groups were 65.1% and 22.1%, respectively (online supplementary table S2). Median OS was also longer in patients with no liver metastases compared with those with liver metastases in the trifluridine/tipiracil PPC subgroup (7.6 vs 5.0 months), and both the GPC and PPC subgroups of the placebo arm (8.6 vs 4.7 months, and 7.2 vs 3.7 months, respectively; table 2). In the group of PPC patients treated with trifluridine/tipiracil, the 6-month and 12-month OS rates were 62.7% and 36.1%, respectively, in those without liver metastases compared with 41.7% and 12.4%, respectively, in those with liver metastases (online supplementary table S2). For the trifluridine/tipiracil and placebo arms, patients with baseline ECOG PS 0 had higher median OS compared with ECOG PS 1 patients in both the GPC and PPC subgroups (table 2). In the trifluridine/tipiracil arm, age (<65 or ≥65 years) and KRAS status did not seem to affect the treatment outcome (table 2).
Similar results were found for PFS with an effect for all trifluridine/tipiracil GPC and PPC subgroups with median PFS values ranging from 1.7 to 5.4 months (table 2). Among GPC patients treated with trifluridine/tipiracil, the 6-month PFS rate was 35.9% in those with no liver metastases compared with 14.5% in those with liver metastases. Corresponding 6-month PFS rates in the PPC group of patients treated with trifluridine/tipiracil were 17.6% and 6.6%, respectively (online supplementary table S3). No such effect was observed in the placebo arm, with values ranging 1.6–1.9 months whatever the prognosis at the outset. For almost all subgroups, median PFS was longer and all HRs favoured treatment with trifluridine/tipiracil (table 2).
Effects of prognostic classification of patients on ECOG PS
Data relative to the effect of ECOG PS are presented in table 3. The proportion of GPC patients treated with trifluridine/tipiracil with an ECOG PS of 0–1 at treatment discontinuation was 89.1%. Among GPC patients with an ECOG PS of 0 at baseline, 95.2% had not deteriorated beyond a PS of 0–1 at treatment discontinuation. Similarly, among GPC patients with an ECOG PS of 1 at baseline, 78.0% had not deteriorated beyond a PS of 0–1 at treatment discontinuation. The median time to deterioration of ECOG PS to ≥2 in patients receiving trifluridine/tipiracil was 7.8 months in the GPC subgroup and 4.2 months in the PPC subgroup (figure 2C).
Table 3.
Effects of prognostic classification of patients on Eastern Cooperative Oncology Group performance status (ECOG PS)
| Median time to deterioration to ECOG PS ≥2, months | HR (95% CI) | P value | ECOG PS 0–1 at treatment discontinuation, % | |||
| FTD/TPI | Placebo | FTD/TPI | Placebo | |||
| ITT population (n=800)11 | 5.7 | 4.0 | 0.66 (0.56 to 0.78) | <0.001 | 84.0 | 81.0 |
| Good prognosis patients (n=386) | 7.8 | 4.7 | 0.53 (0.41 to 0.69) | <0.0001 | 89.1 | 83.1 |
| Poor prognosis patients (n=414) | 4.2 | 3.4 | 0.80 (0.64 to 1.00) | 0.048 | 78.4 | 75.1 |
FTD/TPI, trifluridine/tipiracil; ITT, intent to treat.
Tolerability and safety
The most common AEs in patients receiving trifluridine/tipiracil were nausea, anaemia, neutropenia/neutrophil count decrease, diarrhoea, fatigue and reduced appetite (online supplementary table S5). The most common grade ≥3 AEs experienced by patients receiving trifluridine/tipiracil were haematological (anaemia, neutropenia/neutrophil count decrease, white blood cell count decrease). There was no evidence of a higher incidence of AEs in patients with PPC versus GPC in the group receiving trifluridine/tipiracil, but there was a trend towards a higher incidence of AEs in placebo recipients with PPC compared with GPC (online supplementary table S5).
Discussion
The results of our analysis show that patients in the GPC subgroup consistently performed better than those in the PPC subgroup in both the trifluridine/tipiracil and placebo arms. Within the same subgroups, patients treated with trifluridine/tipiracil performed better than placebo. Trifluridine/tipiracil has consistently been shown to provide a significant survival benefit to patients with mCRC refractory to standard therapy with a well-tolerated safety profile in three large-scale, randomised clinical trials.10 14–16 A previous subanalysis of RECOURSE showed that trifluridine/tipiracil was more effective than placebo in patients, irrespective of region, age, racial/ethnic differences or KRAS mutation status.17 In the current analysis, further categorisation of patients as having good prognosis (using the criteria of <3 metastatic sites by RECIST tumour evaluation at randomisation and ≥18 months from diagnosis of first metastasis to randomisation12 13) identified a subgroup of patients with improved OS and PFS with trifluridine/tipiracil compared with poorer prognosis patients (ie, those with ≥3 metastatic sites and <18 months from first metastasis). PFS and OS were also improved in GPC patients treated with trifluridine/tipiracil compared with GPC patients who received placebo.
Patients with GPC received more cycles of treatment than patients with PPC, because progression was delayed in this group, which may have contributed to the better survival outcomes. The difference cannot be explained by a difference in dose intensity, since this was high and similar in both the PPC and GPC subgroups of patients receiving trifluridine/tipiracil. In addition, there was no evidence for higher toxicity in the PPC than the GPC group. In fact, the haematological AEs occurred at a slightly higher rate in GPC patients than in PPC patients who received trifluridine/tipiracil, which probably reflects a longer exposure to treatment in the GPC group. More patients in the GPC than in the PPC subgroup had dose delays, which suggests that grade ≥3 haematological AEs were appropriately managed during treatment.
It is thought that the availability of more treatment options for mCRC has contributed to an improvement in OS over the last 20 years.3 Indeed, a retrospective study in elderly patients aged ≥70 years, a patient population more prone to comorbidities, poor performance status and the development of treatment-related toxicity, reported a correlation between OS and the number of treatment lines received.18 Thus, maintaining the general condition and performance status of a patient throughout the continuum of care is of great importance, especially beyond the second line, to ensure patients remain fit, with good QoL.5 Our analysis showed that the majority of patients in the GPC subgroup (89.1%) discontinued treatment with an ECOG PS of 0–1 at the time of disease progression, suggesting that these patients could be candidates to receive further lines of therapy post trifluridine/tipiracil; this is important when sequencing through the continuum of care. This is in line with other analyses indicating preservation of health-related QoL on treatment of patients with mCRC with trifluridine/tipiracil.19 20
While the post hoc nature of this analysis limits it to an exploratory analysis, the relatively large number of patients analysed make these data a good tool to estimate the expected outcomes when treating patients with refractory mCRC with trifluridine/tipiracil. The smaller size of some of the subgroups may limit the conclusions that can be drawn, thus preventing an evaluation of other parameters that might impact on outcomes (such as lactate dehydrogenase levels). The exact definition of good and poor prognostic factors12 13 may require further validation in a prospective cohort.
Conclusion
The current analysis shows that compared with poor prognosis patients treated with either trifluridine/tipiracil or placebo, and good prognosis patients treated with placebo, patients with GPCs treated with trifluridine/tipiracil (adequate organ function, ECOG PS 0–1, <3 metastatic sites by RECIST tumour evaluation at randomisation, and ≥18 months from diagnosis of first metastasis) have an increased survival in terms of median OS and 6-month and 12 month survival rates. Treatment with trifluridine/tipiracil is effective and provides the majority of patients the opportunity to maintain ECOG PS and the possibility to receive further treatment options through the continuum of care.
Acknowledgments
The authors would like to thank Andrea Bothwell who wrote the first draft of this manuscript on behalf of Springer Healthcare Communications. This medical writing assistance was funded by Institut de Recherches Internationales Servier, Suresnes, France.
Footnotes
Correction notice: This paper has been updated since first published to amend supplemental material.
Contributors: JT and SRMV contributed to the conception and design of the study. All authors were involved in the acquisition, analysis and interpretation of data, and in writing and/or revising drafts of the manuscript. All authors have read and approved the final draft of the manuscript and accept responsibility for the finished article and the decision to submit the manuscript for publication.
Funding: The RECOURSE study was funded by Taiho Oncology and Taiho Pharmaceutical Co.; this analysis was funded by Servier in partnership with Taiho.
Competing interests: JT has received personal fees from Array Biopharma, AstraZeneca, Bayer AG, BeiGene, Boehringer Ingelheim, Chugai, Genentech, Genmab A/S, Halozyme, Imugene Limited, Inflection Biosciences Limited, Ipsen, Kura Oncology, Eli Lilly and Company, Merck, Menarini, Merck Serono, Merrimack Pharmaceuticals, Merus, Molecular Partners, Novartis, Peptomyc, Pfizer, Pharmacyclics, ProteoDesign SL, Rafael Pharmaceuticals, F. Hoffmann-La Roche, Sanofi, Seattle Genetics, Servier, Symphogen, Taiho Pharmaceutical, VCN Biosciences, Biocartis, Foundation Medicine, HalioDX, SAS Pharmaceuticals and Roche Diagnostics. GA has had an advisory role, or received honoraria or travel grants from Hoffmann-La Roche, Merck Serono, Amgen, Sanofi, Bayer, Servier and Bristol-Myers Squibb. AFS has had an advisory role for Amgen, Bayer, Celgene, Roche, Merck Serono, Sanofi, and Servier, and has attended a speakers’ bureau for Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Celgene, Lilly, Merck Serono, Roche, Sanofi and Takeda. EVC has received research funding from Amgen, Bayer, Boehringer Ingelheim, Celgene, Ipsen, Lilly, Merck, Merck KgA, Novartis, Roche, Sanofi and Servier, and has attended advisory boards for Astellas, Astrazeneca, Bayer, Bristol-Myers Squibb, Celgene, Lilly, Merck Sharp & Dohme, Merck KGaA, Novartis, Roche, and Servier. CB has attended advisory boards for Roche, Servier and Sanofi, and has received a research grant from Roche. AO has received honoraria from Ono, BMS, Chugai, Taiho, Eisai and Amgen, and has received research funding from Bristol-Myers Squibb. An immediate family member of AO has been employed by Celgene. RJM declares no conflicts of interest. LV and SRMV are employees of Servier.
Patient consent for publication: Not required.
Ethics approval: The RECOURSE study was approved by the review boards at each participating institution and the study was conducted according to the principles of the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. All patients provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1.Martini G, Troiani T, Cardone C, et al. Present and future of metastatic colorectal cancer treatment: a review of new candidate targets. World J Gastroenterol 2017;23:4675–88. 10.3748/wjg.v23.i26.4675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386–422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 3.Vogel A, Hofheinz RD, Kubicka S, et al. Treatment decisions in metastatic colorectal cancer - Beyond first and second line combination therapies. Cancer Treat Rev 2017;59:54–60. 10.1016/j.ctrv.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network NCCN clinical practice guidelines in oncology: colon cancer. version 3, 2018. Available: http://www.nccn.org/professionals/physician_gls/default.aspx#site [Accessed 21 Sep 2018].
- 5.Arnold D, Prager GW, Quintela A, et al. Beyond second-line therapy in patients with metastatic colorectal cancer: a systematic review. Ann Oncol 2018;29:835–56. 10.1093/annonc/mdy038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modest DP, Stintzing S, von Weikersthal LF, et al. Impact of subsequent therapies on outcome of the FIRE-3/AIO KRK0306 trial: first-line therapy with FOLFIRI plus cetuximab or bevacizumab in patients with KRAS wild-type tumors in metastatic colorectal cancer. J Clin Oncol 2015;33:3718–26. 10.1200/JCO.2015.61.2887 [DOI] [PubMed] [Google Scholar]
- 7.Abrams TA, Meyer G, Schrag D, et al. Chemotherapy usage patterns in a US-wide cohort of patients with metastatic colorectal cancer. J Natl Cancer Inst 2014;106:djt371. 10.1093/jnci/djt371 [DOI] [PubMed] [Google Scholar]
- 8.Lenz H-J, Stintzing S, Loupakis F. Tas-102, a novel antitumor agent: a review of the mechanism of action. Cancer Treat Rev 2015;41:777–83. 10.1016/j.ctrv.2015.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Temmink OH, Emura T, de Bruin M, et al. Therapeutic potential of the dual-targeted TAS-102 formulation in the treatment of gastrointestinal malignancies. Cancer Sci 2007;98:779–89. 10.1111/j.1349-7006.2007.00477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayer RJ, Van Cutsem E, Falcone A, et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N Engl J Med 2015;372:1909–19. 10.1056/NEJMoa1414325 [DOI] [PubMed] [Google Scholar]
- 11.Van Cutsem E, Falcone A, Garcia-Carbonero R, et al. Proxies of quality of life in metastatic colorectal cancer: analyses in the recourse trial. ESMO Open 2017;2:e000261. 10.1136/esmoopen-2017-000261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adenis A, de la Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016;16:412. 10.1186/s12885-016-2440-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka A, Sadahiro S, Suzuki T, et al. Retrospective study of regorafenib and trifluridine/tipiracil efficacy as a third-line or later chemotherapy regimen for refractory metastatic colorectal cancer. Oncol Lett 2018;16:6589–97. 10.3892/ol.2018.9421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayer RJ, Ohtsu A, Yoshino T, et al. Tas-102 versus placebo plus best supportive care in patients with metastatic colorectal cancer refractory to standard therapies: final survival results of the phase III recourse trial. JCO 2016;34:634 10.1200/jco.2016.34.4_suppl.634 [DOI] [Google Scholar]
- 15.Xu J, Kim TW, Shen L, et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. J Clin Oncol 2018;36:350–8. 10.1200/JCO.2017.74.3245 [DOI] [PubMed] [Google Scholar]
- 16.Yoshino T, Mizunuma N, Yamazaki K, et al. Tas-102 monotherapy for pretreated metastatic colorectal cancer: a double-blind, randomised, placebo-controlled phase 2 trial. Lancet Oncol 2012;13:993–1001. 10.1016/S1470-2045(12)70345-5 [DOI] [PubMed] [Google Scholar]
- 17.Van Cutsem E, Mayer RJ, Laurent S, et al. The subgroups of the phase III recourse trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. Eur J Cancer 2018;90:63–72. 10.1016/j.ejca.2017.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geva R, Sarid N, Shacham-Shmueli E. Retrospective analysis of efficacy and safety of third-line chemotherapy for metastatic colorectal cancer among elderly patients receiving targeted therapy in early lines. J Clin Gerontol Geriatrics 2015;6:95–9. 10.1016/j.jcgg.2015.02.006 [DOI] [Google Scholar]
- 19.Moiseyenko V, Saunders MP, Wasan HS, et al. Qol from TASCO1: health-related quality of life of trifluridine/tipiracil-bevacizumab and capecitabine-bevacizumab as first-line treatments in metastatic colorectal cancer patients not eligible for intensive chemotherapy—Results from the TASCO1 phase II study. JCO 2019;37:676 10.1200/JCO.2019.37.4_suppl.676 [DOI] [Google Scholar]
- 20.Taieb J, Price TJ, Ciardiello F, et al. Health-Related quality of life in the early-access phase IIIB study of trifluridine/tipiracil in pretreated metastatic colorectal cancer (mCRC): results from PRECONNECT study. JCO 2019;37:638 10.1200/JCO.2019.37.4_suppl.638 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2020-000752supp001.pdf (354.9KB, pdf)