Abstract
Li-Fraumeni Syndrome (LFS) is characterized by risk of multiple primary malignancies in diverse sites, pediatric onset, near complete penetrance by age 70 years, limited options for prevention, and substantial uncertainty regarding disease manifestation and prognosis. Forty-five families, including 117 individuals aged 13–81 years, enrolled in the US National Cancer Institute’s Li-Fraumeni Syndrome Study completed 66 interviews regarding their LFS experiences. An interdisciplinary team used modified grounded theory to examine family distress regarding expectations of loss and change due to likely cancer diagnoses, and the consequences of this likelihood across physical, social, and emotional domains. Disease-free periods were characterized by fearful anticipation of diagnosis or recurrence, uncertainty regarding post-treatment quality of life, and planning for shifts in family dynamics to enable caregiving. The chronicity of waiting for these changes incited dread and inhibited effective coping with the pragmatic, emotional, and existential challenges of the syndrome. Consequently, families reported high burden on roles and resources and limited guidance to prepare for, or achieve resolution with, grief. Anticipatory loss, the experience of bereavement prior to an expected change, distinguishes hereditary cancer risk from a sporadic diagnosis. Such grief is often incomplete in impact or meaning, subjected to rapid or profound change as conditions worsen, and poorly understood. In this study, losses were compounded by profound uncertainty, a chronic feature of LFS, which compromised mourning. Long-term engagement of mental health providers with bereavement training, in partnership with genetics providers, can provide invaluable educational and psychological support to families as they navigate these implacable challenges.
Keywords: Li-fraumeni syndrome, Hereditary cancer, Family, Bereavement, Family therapy, Anticipatory loss
Introduction
Li-Fraumeni Syndrome (LFS) is a rare heritable cancer predisposition disorder primarily caused by germline pathogenic variants in the TP53 tumor suppressor gene, known as the “guardian of the genome” [1]. These variants are inherited in an autosomal dominant fashion, although de novo cases are discovered through familial cascade genetic testing and genomic sequencing. LFS is characterized by nearly 100% lifetime cancer penetrance, with syndrome-related malignancies occurring at all ages, from childhood to older adulthood. The most commonly occurring cancers include those arising from soft-tissue, bone, premenopausal breast, brain, and the adrenal gland. Less frequent, but still higher-than-population risks are observed for gastrointestinal, lung, kidney, thyroid, hematological, and skin neoplasms, among others. By age 31 for women and 46 for men, approximately 50% of individuals with LFS have developed at least one cancer. Multiple, independent, primary malignant tumors frequently occur in the same individual during their lives.
In the US context, pre-and post-test genetic counseling is recommended for all individuals and families referred for germline TP53 testing. This counseling includes eliciting a personal and family cancer history to identify testing eligibility, conducting a psychosocial assessment, and communicating detailed recommendations for cancer risk management. Cancer surveillance is conducted either as part of a research study or organized clinically through local healthcare providers. Given the heterogeneity of LFS-associated malignancies, current cancer surveillance recommendations involve an intense, comprehensive regimen of radiological and biochemical surveillance methods centered around whole-body MRI imaging in addition to brain, breast, and abdominal imaging [2]. Carriers of germline pathogenic TP53 variants have only limited options for true primary cancer prevention. Among LFS-related cancer types for which there exist objectively proven screening strategies, e.g., breast cancer, surgical risk reduction can be offered, with a reasonable expectation of diminishing cancer site-specific morbidity and mortality. But LFS-associated cancers are a heterogeneous group, for most of which effective prevention is largely unavailable. Risk-reducing surgery (primarily bilateral mastectomy) is of modest potential utility, given the wide spectrum of cancers that characterize the LFS phenotype.
Given the broad range of syndrome-related cancers and the limited prevention options, families may experience multiple concurrent diagnoses, both within and across generations, leading to high cancer burden and substantial physical and emotional distress [3]. Varying periods of respite between new diagnoses may be characterized by frequent cancer screening. Current cancer screening recommendations do not specifically address psychosocial support, and this is often sought outside of screening, based on the individual and family’s needs. Within each family, normative developmental and daily tasks may overlap with periods of regular cancer screening, new diagnoses, intensive treatment (surgery, radiation, chemotherapy), recovery, caregiving, and end-of life preparations.
Identifying the constellation of losses in sporadic versus inherited cancer
The bereavement literature distinguishes between the death of a loved one and the additional, often unrecognized, losses provoked by that death [4]. These may include the loss of family roles, financial and employment status, faith, and lifestyle adaptations. Death is identified as the “primary loss” and its consequences are “secondary losses.”
In the context of sporadic (non-hereditary) cancer, losses begin at, or leading up to diagnosis and occur in both physical and emotional realms [5]. Primary losses in this context include the cancer diagnosis or its treatment. Secondary losses then ripple through the domains of physical, emotional, and social life. These are driven by the diagnosis and/ or treatment, and include diminished tangible resources, such as health insurance, salary lost to treatment and recovery, as well as existential losses including identity or security, or family roles and structures.
Wide-ranging secondary losses are not mutually exclusive and may be cumulative in their effects. Further, secondary losses may be unrecognized (or not attributed to the primary event) by familial or social networks, providers, or even the patient [6]. Persons with sporadic cancer diagnoses often have little proximal or expert knowledge about the consequences of their illness; its course and implications may be unfamiliar and only understood incrementally as their own care proceeds. Recognition of the losses that might follow a sporadic cancer diagnosis, thus, may take time to manifest [7].
Anticipatory loss
In sharp contrast to sporadic cancer, cancer risk information regarding the course and consequences of an inherited disease are often part of family members’ common knowledge and shared experience. This knowledge is acquired both through personal experience (i.e., their own illnesses) and by witnessing disease impact on affected family members [8, 9]. Such family-based knowledge forms a lens through which the uncertainties of disease risk are interpreted [10].
Anticipatory loss was first conceptualized to explore the experiences of caregivers [11]. Original formulations were subsequently expanded to include primary losses other than loss of life. This enabled application of the concept, and identification of targets for intervention, among individuals whose primary loss was the diagnosis of a life-limiting condition. Initially, the concept was widely applied to explain the experiences of caregivers for loved ones with Alzheimer’s disease, in recognition of their high caregiving burden and burn-out [12]. Variability of disease-associated decline (and death), in combination with limited control and limitations in predicting disease course or severity, led caregivers to rehearse and experience bereavement prior to expected losses. These ambiguities left families with tenuous hopes that levels of functioning might be sustained longer than predicted or that a treatment or cure might be discovered. Thus, the experience of bereavement was complicated, delayed by hope, and left incomplete in meaning and impact [13].
The concept of anticipatory loss is not new in the inherited disease context, having been used to describe family experiences with Huntington’s Disease [14], the challenges faced by young, BRCA1/2-positive women contemplating bilateral prophylactic mastectomy [15], and the death of a child from Tay Sachs disease [16]. Loss may begin with awareness of familial or genetic disease risk, a diagnosis, or the death of a loved one. Through empathic connection with ill loved ones, education regarding cancer risk, and screening-related distress, members of high-risk families may anticipate person or family-level changes before they are experienced [see Fig. 1].
Fig. 1.
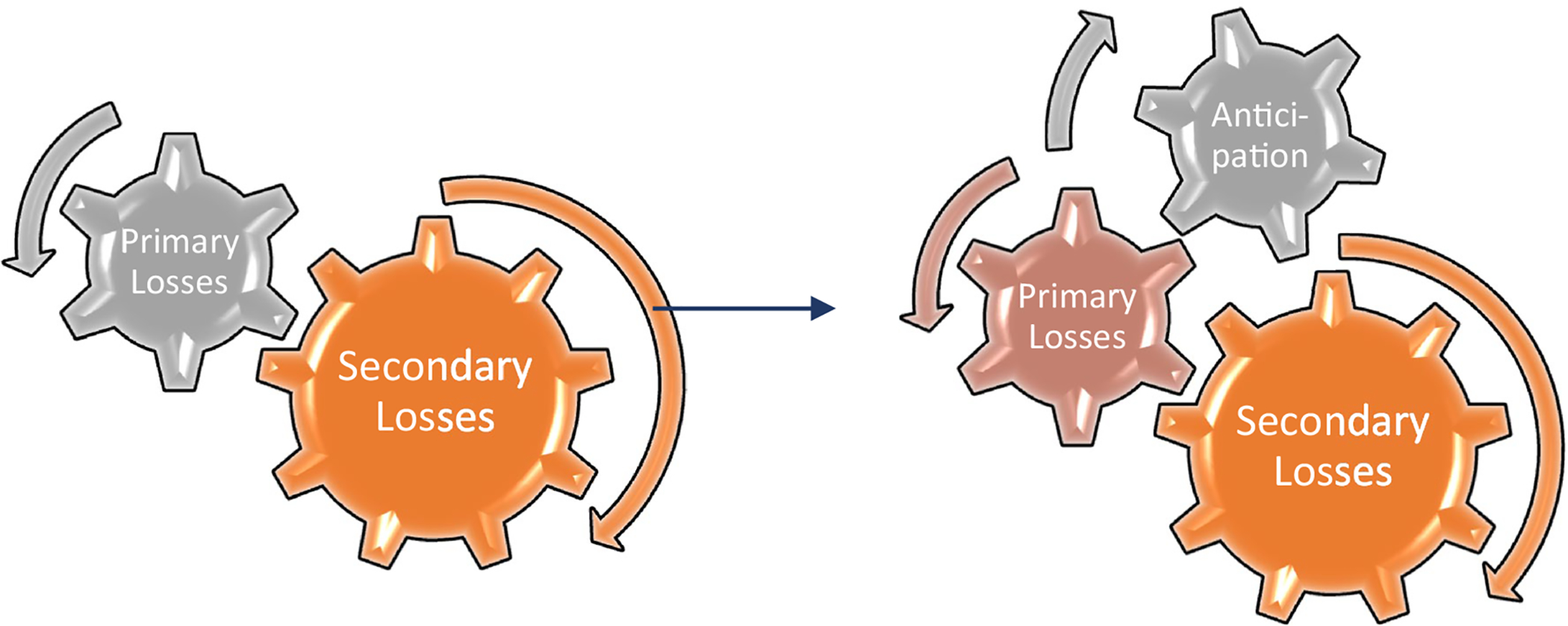
The gears of anticipatory loss in hereditary cancer
Study aims
This paper reports on an emergent set of findings from our qualitative efforts to evaluate the psychological, social, and behavioral impact of LFS, and to refine evidence-based counseling strategies. We explore family distress regarding cancer diagnoses, and more specifically distress about anticipating diagnoses, and the consequences of this anticipation across physical, social, and emotional domains.
Methods
Compliance with ethical standards
This study was part of the National Cancer Institute’s (NCI) Institutional Review Board-approved longitudinal study of Li-Fraumeni Syndrome (NIH Protocol 11-C-0255, Clinical-Trails.gov; Identifier NCT01443468; www.lfs.cancer.gov). This protocol was nested in a broader effort to investigate the clinical, epidemiologic, and genetic etiology of LFS, and to develop and refine evolving global cancer screening standards.
Li‑Fraumeni Family Study Protocol
The NCI’s LFS study opened to accrual in 2011. Eligibility criteria are based on personal and family histories of cancer consistent with criteria for classic LFS or Li-Fraumeni-like Syndrome. This includes individuals with a confirmed germline pathogenic TP53 variant, a personal history of three or more LFS-related cancers, or adrenal cortical carcinoma or choroid plexus carcinoma at any age, regardless of family history [1]. The study offers genetic counseling, TP53 testing, and study enrollment to at-risk family members.
A subset of the study participants older than 3 years of age and with confirmed germline pathogenic TP53 variant, regardless of personal history of cancer, were invited to join the cancer screening arm of the study. Participation in the screening arm involves annual visits to the NIH Clinical Center to complete whole body, brain, and breast MRIs (for female participants > 20 years of age with at least one intact breast), physical exams, and psychosocial interviews, in addition to any interval screening completed with local providers outside the NIH. Funding for a family member to accompany the participant(s) to their screening visit is provided for all minors and for adults on a case-by-case basis.
Family interview recruitment and sample
This particular analysis includes data from families enrolled in the cancer screening arm of the LFS study. A specific objective of the LFS study is to better understand, characterize and address the psychosocial impact of LFS on individuals and families. Consequently, upon enrollment, participants complete a rigorous battery of baseline mental health history questionnaires. Family interviews were conducted during annual screening visits with all presenting members together as a group regardless of TP53 mutation status. Forty-five families completed 66 interviews between 2012 and 2017, including 117 family members aged 13–81 years, with 19 parent–child groups, 26 partner dyads, eleven sibling groups, and ten mixed groups. Participants with LFS varied in their cancer history, with some having no personal history of cancer, and some having been diagnosed with multiple cancers. Interviews lasted between 21 and 81 min (mean = 50 min).
The NCI’s LFS Study is one of the largest international cohorts of families with LFS, and the largest longitudinal cancer screening cohort. It is also the only known data set targeted at family-level processes and experiences. Previous published studies on this research cohort have established that the sample is well-educated and predominantly white [17]. The protocol does not provide cancer treatment, so individuals diagnosed with cancer during the course of their research participation received that care outside the NCI, with the NCI team serving as expert consultants and partners in care. Once they were deemed cancer-free, they were eligible to return for cancer screening. So, individuals with active cancer diagnoses were not eligible for inclusion in family interviews. While ongoing psychotherapy is not offered as a part of the protocol, psychosocial needs are discussed during annual visits, and referrals to local resources are made, when appropriate.
Data collection
Multiple members of the interprofessional LFS research team collaborated to build the interview guide, including a family therapist, genetic counselor, social work consultant, and oncology medical staff. The interview guide included a semi-structured protocol, modified for the constellation of family members attending the annual visit. The goal of the family interview protocol was to focus on within-family communication issues, reproductive decision-making, couple relationships, cancer prevention behaviors, and choices regarding genetic testing for adults and children.
All family members aged 13 or older attending the Clinical Center evaluation were invited to complete the family group interview. Interviews began with obtaining written consent, and were audio-recorded with the permission of the participants. Names were anonymized by substituting participant-selected pseudonyms; all personal identifying information was removed from transcripts. Transcripts were stored on a secure, password-protected NIH computer, as was the Dedoose™ data analysis file. Access to research databases remains strictly limited to pre-approved investigators.
Design: grounded theory and interpretive description
Our data analysis employed the tenets of grounded theory [18, 19] and interpretive description [20]. Grounded theory is an iterative research method in which data collection and analysis occur simultaneously to inform one another. Interpretive description uses grounded theory as its foundation, and has its methodological roots in nursing research, which contextualizes biomedical and psychosocial experiences in preexisting empirical and practice knowledge.
Data analysis
During the first three study years, the primary interviewer conducted 66 interviews with 45 families of multiple configurations, including siblings, parent/child dyads, partners, and extended family units. Families completed 1–5 interviews during this period. The constellation of family members who attended clinic varied annually, based on study funding parameters and the family’s priorities. Consequently, the group completing their family’s interviews varied from year-to-year. Researchers created a family case file, including all interview transcripts and the family pedigree.
The coding team was comprised of three researchers trained in qualitative methods with experience working with medical transcript data, in addition to a genetic counselor with experience in LFS counseling and care, and the primary interviewer. To establish a preliminary codebook, three coders independently conducted open coding on the same participant’s transcript, identifying in vivo codes that emerged organically during interviews [21] and a priori codes originating from sensitizing concepts [18]. They met to discuss codes, resolve discrepancies, and identify avenues for inquiry in other transcripts. They then selected three additional case files for maximum variation [22] and the same three investigators coded them in round- robin style, with each coding one transcript and then passing it to a second coder to analyze. All three coders examined all three transcripts. The three researchers then met to examine the set of coded documents, compiled a working list of codes, defined decision rules for the application of codes, and clustered codes into thematic categories. All transcripts were then loaded into Dedoose software and two of the three investigators double-coded all 66 interviews. In the final analysis phase, the coding team met to discuss interpretations and synthesize findings into recurring patterns.
Data quality
The LFS Study clinical research team met weekly to discuss recent and upcoming Clinical Center family visits. The family researcher-interviewer for this project participated in these weekly debriefing sessions and consulted regularly with the genetic counselor who conducted individual family member interviews. These meetings provided a forum to discuss any concerns regarding individual participant’s mental health, follow-up on unresolved questions, and cross-checking data. In addition, two researchers coding in tandem checked each other’s findings to establish consistency with the codebook. A senior qualitative researcher and mental health provider with expertise in hereditary cancer genetics provided mentorship and additional feedback regarding the thematic findings of the study. These strategies facilitated the validation of our findings through cross-verification from multiple sources. Prolonged exposure [23], interprofessional collaboration on coding and interpretation, and triangulation with pedigree data facilitated rigorous analysis.
Findings
Families described LFS as a chronic condition with an intensive screening and treatment burden, significant caregiver involvement, and ongoing major loss. Participants’ stories included anticipation, dread, the perceived certainty of a cancer-related event, fatalism related to limited predictability or control over the event itself, and expectations of change and grief. The experiences of anticipatory loss in this sample were omnipresent.
It’s literally waiting for the shoe to drop every single day.
You get the feeling that, if you get tested and you have it, then your life is over. It has to irrevocably change. With LFS it’s [cancer] always in the back of your mind.
It’s tough, every three to four months being [screened.] You feel like you’re on death row.
Families described waiting for various cancer-related events and discriminated those over which they had some control from those not amenable to control. Participants could elect to schedule, complete, and/or receive findings from LFS-related genetic tests. Individuals in whom a pathogenic TP53 variant was detected could then elect to engage in screening protocols aimed at early cancer detection (see Table 1). Beyond that, however, living with LFS was characterized by prolonged waiting for an LFS-event to occur with limited, if any, avenues for prevention or control. This waiting was described as “unending.”
Table 1.
Experiences of waiting and selected corresponding losses
| Experience of waiting | Potential losses | Control |
|---|---|---|
| TP53 genetic test results | Possible futures | High control |
| Scheduling, completing, returning for screening or surveillance findings | Faith in body | |
| One’s own cancer diagnosis | Healthy identity | Low/no control |
| Change in physical aesthetic, suffering a subsequent diagnosis or treatment or risk- reducing surgery | Physicality, agility, fertility | |
| Child(ren)’s mutation status, cancer diagnosis or diagnoses at a ‘young age’ | Future | |
| Time to next cancer diagnosis in an individual or family group | Feeling “normal” | |
| Failure of treatment availability or clinical trials | Possibility, vitality |
Early detection
In this cohort, participants elected to join the NCI study to chart a course towards early detection via regular screening. They discussed degrees of agency related to participating in screening practices, including breast and abdominal screening outside the NCI protocol. Screening provoked distress as participants waited for appointments, reports, biopsy results, and diagnoses for themselves and mutation-positive loved ones. Return of a negative screen or biopsy led to a brief reprieve from distress followed by the return of dread.
Rigorous protocols for screening regularly foregrounded the specter of a diagnosis and created constant reminders of vulnerability. Each cancer screening event opened the door to a new diagnosis and, possibly, a new primary loss. The potential for an LFS-event across the bloodline amplified perceptions of fragility. Each experience of waiting: for one’s own diagnosis; the first or subsequent diagnosis of a loved one; information regarding treatment availability or success; constituted its own primary loss. And each primary loss incurred unique, overlapping, or additional secondary sequalae. In families with multiple, living TP53 mutation-positive individuals, the possibility of overlapping primary losses and compounded secondary losses left families struggling to find time free from LFS-related cancer worry.
Balancing certainty with uncertainty, grief and hope
Families described living betwixt and between, living with and without cancer, waiting and “weighted down.” Although early detection conferred a meaningful measure of agency, screening protocols were coupled with significant distress in balancing hope for a negative scan with the expectation of finding a malignancy (cancer worry). With near-certain lifetime cancer penetrance (all cancers combined) approaching 100%, families perceived a diagnosis within their kindred as a certainty. Families held this belief in tension with the uncertainty about the timing, course, incapacitation potential, or prognosis of a diagnosis, informing the need to remain rigorous in screening.
You know where it’s gonna end up. You just don’t know when it’s gonna end up there. You know where you finish the race, you just don’t know when you’re finishing the race.
I knew I was going to, you know, I might have to deal with it again, and that we’d have to be even more vigilant.
The latter quote suggests that mutation-positive family members developed a level of personal responsibility for monitoring their bodies between appointments. Like many in this sample, constant foregrounding of LFS risk through screening and surveillance led many participants to mis-interpret often innocuous physical symptoms as evidence of a new cancer and to then consider pursuit of immediate medical attention to ward off a late-stage diagnosis.
Every little ache and pain makes you wonder, “Well, do I wait or do I go?” Then, the most stressful piece of the whole thing is waiting for the results…you’re on pins and needles, but you’ve been on pins and needles since 2012.
Families discussed active efforts to cope by balancing the demands of loss related to screening protocols, cancer treatment and caregiving, and expectations regarding new diagnoses with typical age-appropriate developmental tasks and activities of daily life. Some families discussed challenges related to experiencing or recognizing joy or celebrating successes because they were awaiting the next diagnosis.
I don’t see light at the end of the tunnel. I guess I don’t have hope because if it’s not me, it could be one of our kids. And if it’s not one of the kids, it could be one of the grandkids.
I am waiting for the shoe to fall. I do hate that. It’s almost like things are going too good. I was focusing on things that we want to have now, or do now, or be together and make memories now. And recently, it’s just been hard for me to not think about the not having the later part.
Some of these families developed philosophies of daily living that supported coping in this context. These prized “living in the moment,” a philosophy in service of not having a future. However, reprieve from anticipating loss was often temporary.
Anticipating, rehearsing, and planning for loss
Waiting in a context that combines a likely cancer diagnosis with uncertainty regarding the potential impact of that diagnosis left families with limited cues to interpret individual risk. Families discussed difficulty preparing for possible disease trajectories in a way that might proffer a sense of agency. Participants used family heuristics quite differently from other inherited cancer predisposition syndromes; rather than identifying a specific organ vulnerability or disease trajectory, the holistic cancer experience of physical and existential pain, coupled with logistical caregiver burden, foreshadowed the future.
It’s emotionally upsetting, because it’s like glimpsing in the mirror. You see your own mortality through family members.
The more people that have it, the more all the people will be thinking when it’s their turn.
Families reported high burden on roles and resources, particularly for mutation-negative members of the family, who discussed the challenges of assuming or preparing for caregiver roles.
(My younger sister) didn’t see our dad sitting there having conversations with you when you were 13 years old, being like, “You’re going to have to take care of your family.”
Such directives left family members feeling that distinct life pathways were beyond their control. One mechanism for maximizing agency/control, and thus coping with the lack of predictability in diagnosis or death, was to plan for these events. Though at times unsettling for healthy family members, planning for not having a future yielded some comfort for those with LFS. Such plans included making financial arrangements for dependents, establishing legal proxies, and identifying child guardians. This approach established an explicit dialogue around end of life, death rituals, and bereavement to support continuity in family life. Though this approach was not always welcome across family groups:
It drives my wife crazy. I have a file I call my death folder. It’s got a little skull-and-cross bone border. I say, “If this happens, this is what you do.” She doesn’t like to see when I’m working on the death folder.
To prepare for a potential or poor prognosis, some families openly discussed caregiving, end of life preparations, and future needs of a family to be left behind.
Me being a single parent worried [my mother]. And then, that I have a high risk of cancer recurrence, that worried her. And then she’d possibly need to raise my child.
Such conversations enabled families to plan for likely eventualities, yet they also incurred the loss of possible futures or role expectations, earmarked family resources towards those eventualities, and constrained future planning. As families planned and rehearsed for change, some inadvertently instigated premature loss of their family role:
I looked in the future but as far as picturing myself in retirement - no. I don’t see grandchildren. My mother-in-law actually said I wasn’t going to be around. It was—it was—it was like, “Well, Jane won’t be around much longer, so we’ll step in and take over.”
Discussion
The experience of loss is theorized to be linked with disruption to, or breaking down, of the assumptive world. This captures core beliefs that provide stability and continuity through the events of daily life, symbolic and meaningful perceptions of the self and others through confirmed expectations and repetition, and security through ongoing connection to loved ones [24]. For individuals and families living with LFS, however, the assumptive world is replete with beliefs about vulnerability to cancer, the likely devastating impact of illness and caregiving, and the bleak reality of separation from loved ones. Loss is not a single event, definitive and finite in its impact. Rather, losses past, present, and future inform assumptions about the world in the LFS context.
Considering the relapsing, remitting chronicity of LFS cancer
The ability of comprehensive whole-body MRI to detect early cancers in LFS has been established [2]. Though screening offers the possibility of early cancer detection and the hope for better outcomes, screening does not prevent cancer. The only prospect for cancer prevention as a consequence of a screening examination lies in the occasional identification of a not-yet-malignant precursor lesion, such as an adenomatous polyp of the colon.
Families in this study reported periods of acute and chronic distress, characterized by varying degrees of control, that led to unrelenting expectations of grief. The chronicity of cancer events and risk, in addition to the lack of proven utility of long-term screening, distinguishes LFS from other, more common, hereditary cancer syndromes, such as Hereditary Breast and Ovarian Cancer or Lynch syndromes. The intensified medical and psychosocial burdens associated with LFS merit tailored psychosocial recommendations. The inability to prevent expected losses requires clinicians to help families tolerate expectations of a cancer-related event, build effective coping mechanisms for individuals and families to tolerate risk, and design unique interventions that help families prepare for expected losses.
Interventions must support families moving forward, living with the tension between certainty and uncertainty, of anticipating a diagnosis and hope that health/remission might be sustained [25]. This tension must be named as its own phenomenon, worthy of clinical attention, to identify associated grief-related distress and enable healing through syndrome-related meaning-making.
Clinical implications
The impact of chronic, anticipatory loss in the LFS-context has existential, psychological, relational implications across family systems that suggest the need for theoretically and empirically driven intervention design. Theories of grief and bereavement care have evolved over the last half century from stage-based models [26] to meaning centered approaches [27] but have largely not accounted for the complications associated with anticipation of loss. Further, community bereavement programs are often structured to begin after the death of a loved one, and may not provide services during the end of life, limiting their utility to address anticipatory loss.
Family-based [28] or supportive-expressive group interventions [29] have been successfully implemented with individuals and families affected by more common hereditary cancer syndromes and may be appropriate for people with LFS. Additionally, individual and family psychotherapeutic approaches that involve long-term alliances built with providers trained in bereavement care may be most effective in supporting families with LFS. Often, private practice or agency-based mental health providers are trained to work with only one member of the family at a time or with the entire family together. However, to optimally support people with LFS, we recommend mental health professionals work flexibly with family members individually and in the relevant and shifting combinations in need of care to address temporal concerns, ongoing grief reactions, and family capacity to access care due to illness. Specifically, we echo the work of early psychosocial research on families with hereditary cancer syndromes—multigenerational family systems approaches [30], from which the concept of anticipatory loss emerged [31], to address legacies of loss over generations [32].
We further suggest providers incorporate psychoeducational techniques into the care of families with LFS. Psychoeducation incorporates teaching of condition-related experiences with targeted emotional support, often in family or group settings. Initially, psychoeducation evolved from efforts to include family members in the care of loved ones diagnosed with schizophrenia, to reduce relapse and hospitalizations [33, 34]. In the last 40 years, clinical research has built a strong evidence base for psychoeducation as a family-level intervention to address multifaceted needs of individuals with cancer [35] to increase quality of life, reduce burden on patients and caregivers, and support families across cultural and institutional contexts [36].
Regarding LFS, educational content might target the varied types and presentations of grief along the trajectory of inherited disease [26]. Psychotherapeutic support offered by skilled mental health providers with bereavement training may offer families a safe place to identify anticipatory reactions and rehearsals related to diagnosis. Therapeutic strategies are designed to set realistic expectations about disease treatment and outcomes, to improve coping and adaptation, and to shore up family communication and flexibility [37].
A psychoeducational approach to grief support would be potentiated by partnerships between mental health providers and clinical genetics providers. Among kindreds with inherited cancer predisposition syndromes, distress often increases during points of potential transition, including genetic testing, screening or surveillance, and symptom exploration. Psychoeducational interventions during these definable moments, paired with genetic testing, annual screening, or in the peri-diagnostic period may be an economical way (due to the group versus individual focus) to fill a critical gap in ongoing care (see Table 2). These points along the LFS trajectory may be particularly critical to combine education with psychotherapy regarding anticipatory losses. Through professional education, this intervention could offer families information and skills to cope with expectations and loss regarding biomedical and psychosocial risk.
Table 2.
Therapeutic interventions related to the gears of anticipatory loss in hereditary cancer
| Intervention component | Therapeutic goals | Lead provider(s) |
|---|---|---|
| Psychoeducation: educational support | Define and distinguish primary, secondary, and anticipatory loss from other forms of grief | Mental health provider Genetics provider(s) |
| Revisit LFS-related cancer risk estimates | ||
| Outline procedural steps from cancer screening to diagnosis and treatment | ||
| Practice hard conversations | ||
| Teach and practice tools for coping and stress-reduction | ||
| Introduce conversations about family histories and rehearsals of loss | ||
| Assess family communication for immediate and long-term coping and intervention | ||
| Psychoeducation: emotional support | Scaffold meaning-making for bereavement | Mental health provider |
| Process anticipatory reactions | ||
| Reconcile family histories and risk estimates with fatalistic thoughts | ||
| Introduce ways to maintain hope | ||
| Discuss challenging decisions | ||
| Family interventions | Identify ways family histories inform anticipation about illness and loss | Mental health provider |
| Identify ways families rehearse or prepare for primary and secondary losses | ||
| Distinguish the nature and impact of secondary losses for individuals versus family groups | ||
| Support flexibility in family roles to meet pragmatic, emotional, financial needs of patients and caregivers | ||
| Support family communication to facilitate coping with anticipatory loss |
Study limitations
This is the first known study addressing the psychosocial needs of family groups living with LFS. Consistent with much of the genomics literature, this set of LFS families was largely homogenous, consisting mostly of white, highly educated, employed, and mostly insured individuals and families. As a publicly funded, government-sponsored study, enrollment is not likely representative of all U.S. families living with LFS. Most study probands were ascertained following diagnosis of an LFS cancer and were aware of their LFS mutation status upon enrollment in the study, though many at-risk family members were untested at the time of enrollment and subsequently found to harbor the familial variant. The voices of individuals undergoing treatment for an LFS-related cancer event would have added important perspectives to this dialogue about anticipatory grief, cancer treatment and end of life planning, and family life with LFS. Though adolescent minors aged 13–17 were present for some family interviews, they rarely participated in dialogue around anticipatory grief and loss. Additional research targeted at their developmental concerns is warranted and are presently underway with adolescent and young adults in enrolled in the protocol.
Future directions
Family interviews elicited critical data about how families understand, process, and manage living with LFS. A growing literature on protective buffering, or the act of withholding distress from loved ones to prevent increasing their emotional burden [38], suggests traditional individual interviews are also warranted. Using a life span model, an interprofessional team at the NCI is completing the first round of data collection on a longitudinal study of adolescent and young adults with LFS. This study aims to identify the ways living with LFS impacts individual development, social and provider networks, and overall wellbeing. We plan to analyze distress and coping, social and physical functioning, family loss and development, and existential impacts over time for individuals enrolled in the screening arm and the control arm of the protocol. Further, we aim to identify unmet needs and barriers to care across a number of domains to identify best practices for health, mental health, and allied health professionals serving families with LFS.
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee of The National Cancer Institute, NIH Protocol 11-C-0255, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1.Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, Bremer RC, Rosenberg PS, Savage SA (2016) Risks of first and subsequent cancers among TP53 mutation carriers in the National Cancer Institute Li-Fraumeni syndrome cohort. Cancer 122(23):3673–3681. 10.1002/cncr.30248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ballinger ML, Best A, Mai PL, Khincha PP, Loud JT, Peters JA, Achatz MI, Chojniak R, da Costa AB, Santiago KM, Garber J (2017) Baseline surveillance in Li-Fraumeni syndrome using whole-body magnetic resonance imaging: a meta-analysis. JAMA Oncol 3(12):1634–1639. 10.1001/jamaoncol.2017.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson SK, Pentz RD, Marani SK, Ward PA, Blanco AM, LaRue D, Vogel K, Solomon T, Strong LC (2008) Psychological functioning in persons considering genetic counseling and testing for Li–Fraumeni syndrome. Psycho-Oncology 17(8):783–789. 10.1002/pon.1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corr C (2002) Revisiting the concept of disenfranchised grief In: Doka KJ (ed) Disenfranchised grief: new directions, challenges, strategies for practice. Research Press, Champaign, pp 39–60 [Google Scholar]
- 5.Zebrack B, Jones BL, Smolinski KM (2015) Oncology social work interventions throughout the continuum of cancer care In: Christ G, Messner C, Behar L (eds) Handbook of oncology social work: psychosocial care for people with cancer. Oxford University Press, New York, pp 35–42 [Google Scholar]
- 6.Doka KJ (ed) (2002) Disenfranchised grief: New directions, challenges, and strategies for practice. Research Press, Champaign [Google Scholar]
- 7.Coelho A, Barbosa A (2017) Family anticipatory grief: an integrative literature review. Am J Hosp Palliat Med 34(8):774–785. 10.1177/1049909116647960 [DOI] [PubMed] [Google Scholar]
- 8.Esplen MJ, Urquhart C, Butler K, Gallinger S, Aronson M, Wong J (2003) The experience of loss and anticipation of distress in colorectal cancer patients undergoing genetic testing. J Psychosom Res 55(5):427–435. 10.1016/S0022-3999(03)00511-7 [DOI] [PubMed] [Google Scholar]
- 9.Forbes Shepherd R, Lewis A, Keogh LA, Werner-Lin A, Delatycki MB, Forrest LE (2018) A systematic review of how young people live with inherited disease: what can we learn for Li-Fraumeni Syndrome? J Adolesc Young Adult Oncol 7(5):525–545. 10.1089/jayao.2018.0028 [DOI] [PubMed] [Google Scholar]
- 10.Werner-Lin AV (2007) Danger zones: Risk perceptions of young women from families with hereditary breast and ovarian cancer. Fam Process 46(3):335–349. 10.1111/j.1545-5300.2007.00215.x [DOI] [PubMed] [Google Scholar]
- 11.Fulton R, Fulton J (1971) A psychosocial aspect of terminal care: anticipatory grief. OMEGA J Death Dying 2(2):91–100. 10.2190/WE4J-9CJG-GJH5-R3VA [DOI] [Google Scholar]
- 12.Chan D, Livingston G, Jones L, Sampson EL (2013) Grief reactions in dementia carers: a systematic review. Int J Geriatr Psychiatry 28(1):1–7. 10.1002/gps.3795 [DOI] [PubMed] [Google Scholar]
- 13.Large S, Slinger R (2015) Grief in caregivers of persons with Alzheimer’s disease and related dementia: a qualitative synthesis. Dementia 14(2):164–183. 10.1177/1471301213494511 [DOI] [PubMed] [Google Scholar]
- 14.Domaradzki J (2015) The impact of Huntington disease on family carers: a literature overview. Psychiatr Pol 49(5):931–944. 10.12740/PP/34496 [DOI] [PubMed] [Google Scholar]
- 15.Hoskins LM, Greene MH (2012) Anticipatory loss and early mastectomy for young female BRCA1/2 mutation carriers. Qual Health Res 22(12):1633–1646. 10.1177/1049732312458182 [DOI] [PubMed] [Google Scholar]
- 16.Williams E (2014) Death of a Child to Tay-Sachs and other progressive neurological disorders: long-term impact on parents’ emotional and personal lives. Dissertation, Brandeis University. [Google Scholar]
- 17.Peters JA, Kenen R, Bremer R, Givens S, Savage SA, Mai PL (2016) Easing the burden: describing the role of social, emotional and spiritual support in research families with Li-Fraumeni syndrome. J Genet Couns 25(3):529–542. 10.1007/s10897-015-9905-x [DOI] [PubMed] [Google Scholar]
- 18.Charmaz K (2006) Constructing grounded theory: a practical guide through qualitative analysis. Sage, Thousand Oaks [Google Scholar]
- 19.LaRossa R (2005) Grounded theory methods and qualitative family research. J Marriage Fam 67(4):837–857. 10.1111/j.1741-3737.2005.00179.x [DOI] [Google Scholar]
- 20.Thorne S (2016) Interpretive description: qualitative research for applied practice. Routledge, New York [Google Scholar]
- 21.Denzin NK, Lincoln YS (2008) Collecting and interpreting qualitative materials. Sage, Thousand Oaks, CA [Google Scholar]
- 22.Patton MQ (2005) Qualitative research Encyclopedia of statistics in behavioral science. Wiley, Hoboken [Google Scholar]
- 23.Roy K, Zvonkovic A, Goldberg A, Sharp E, LaRossa R (2015) Sampling richness and qualitative integrity: challenges for research with families. J Marriage Fam 77(1):243–260. 10.1111/jomf.12147 [DOI] [Google Scholar]
- 24.Beder J (2005) Loss of the assumptive world—how we deal with death and loss. OMEGA J Death Dying 50(4):255–265. 10.2190/GXH6-8VY6-BQ0R-GC04 [DOI] [Google Scholar]
- 25.Boss P (2007) Ambiguous loss theory: challenges for scholars and practitioners. Fam Relat 56(2):105–111. 10.1111/j.1741-3729.2007.00444.x [DOI] [Google Scholar]
- 26.Kübler-Ross E, Wessler S, Avioli LV (1972) On death and dying. JAMA 221(2):174–179 [DOI] [PubMed] [Google Scholar]
- 27.Lichtenthal WG, Catarozoli C, Masterson M, Slivjak E, Schofield E, Roberts KE et al. (2019) An open trial of meaning-centered grief therapy: rationale and preliminary evaluation. Pall Support Care 17(1):2–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mendes Á, Chiquelho R, Santos TA, Sousa L (2010) Family matters: examining a multi-family group intervention for women with BRCA mutations in the scope of genetic counselling. J Community Genet 1(4):161–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esplen MJ, Leszcz M, Hunter J, Wong J, Leung YW, Toner B et al. (2018) A randomized controlled trial of a supportive expressive group intervention for women with a family history of breast cancer. Psycho-Oncology 27(11):2645–2653 [DOI] [PubMed] [Google Scholar]
- 30.Baumann SL (2006) Family systems genetic illness model-breast cancer. Clin J Oncol Nurs 10(3):377. [DOI] [PubMed] [Google Scholar]
- 31.Sobel S, Cowan CB (2003) Ambiguous loss and disenfranchised grief: the impact of DNA predictive testing on the family as a system. Fam Process 42(1):47–57 [DOI] [PubMed] [Google Scholar]
- 32.Werner-Lin A (2007) Danger zones: risk perceptions of young women from families with hereditary breast and ovarian cancer. Fam Process 46(3):335–349 [DOI] [PubMed] [Google Scholar]
- 33.Anderson CM, Hogarty GE, Reiss DJ (1980) Family treatment of adult schizophrenic patients: a psycho-educational approach. Schizophrenia Bull 6(3):490 10.1093/schbul/6.3.490 [DOI] [PubMed] [Google Scholar]
- 34.Sin J, Gillard S, Spain D, Cornelius V, Chen T, Henderson C (2017) Effectiveness of psychoeducational interventions for family carers of people with psychosis: a systematic review and meta-analysis. Clin Psychol Rev 56:13–24. 10.1016/j.cpr.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 35.Faller H, Schuler M, Richard M, Heckl U, Weis J, Küffner R (2013) Effects of psycho-oncologic interventions on emotional distress and quality of life in adult patients with cancer: systematic review and meta-analysis. J Clin Oncol 31(6):782–793. 10.1200/JCO.2011.40.8922 [DOI] [PubMed] [Google Scholar]
- 36.Jordan C, Lewellen A, Vandiver V (1994) Psychoeducation for minority families: a social work perspective. Int J Ment Health 23(4):27–43. 10.1080/00207411.1994.11449291 [DOI] [Google Scholar]
- 37.Lukens EP, McFarlane WR (2006) Psychoeducation as evidence-based practice: Considerations for practice, research, and policy In: Roberts AR, Yaeger KR (eds) Foundations of evidence-based social work practice. Oxford University Press, New York, pp 291–313 [Google Scholar]
- 38.Langer SL, Brown JD, Syrjala KL (2009) Intrapersonal and interpersonal consequences of protective buffering among cancer patients and caregivers. Cancer 115(S18):4311–4325 [DOI] [PMC free article] [PubMed] [Google Scholar]


