Abstract
OBJECTIVE
To study the effect of 12 weeks of high-intensity interval training (HIIT) on glycemic control in adults with type 1 diabetes and overweight or obesity.
RESEARCH DESIGN AND METHODS
Thirty inactive adults with type 1 diabetes who had BMI ≥25 kg/m2 and HbA1c ≥7.5% were randomized to 12 weeks of either HIIT exercise intervention consisting of 4 × 4-min HIIT (85–95% peak heart rate) performed thrice weekly or usual care control. In a partial crossover design, the control group subsequently performed the 12-week HIIT intervention. The primary end point was the change in HbA1c from baseline to 12 weeks. Glycemic and cardiometabolic outcomes were measured at 0, 12, and 24 weeks.
RESULTS
Participants were aged 44 ± 10 years with diabetes duration 19 ± 11 years and BMI 30.1 ± 3.1 kg/m2. HbA1c decreased from 8.63 ± 0.66% at baseline to 8.10 ± 1.04% at 12 weeks in the HIIT intervention group (P = 0.01); however, this change was not significantly different from the control group (HIIT −0.53 ± 0.61%, control −0.14 ± 0.48%, P = 0.08). In participants who undertook at least 50% of the prescribed HIIT intervention, the HbA1c reduction was significantly greater than control (HIIT −0.64 ± 0.64% [n = 9], control −0.14 ± 0.48% [n = 15], P = 0.04). There were no differences in insulin dose, hypoglycemia on continuous glucose monitoring, blood pressure, blood lipids, body weight, or body composition between groups.
CONCLUSIONS
Overall, there was no significant reduction in HbA1c with a 12-week HIIT intervention in adults with type 1 diabetes. However, glycemic control may improve for people who undertake HIIT with greater adherence.
Introduction
Regular exercise is recommended for people with type 1 diabetes (1,2) and can provide multiple health benefits, including improvements in body weight, cardiorespiratory fitness, and lipid profile (3). Physical activity is associated with better glycemic control in cross-sectional studies of people with type 1 diabetes (2,4). However, HbA1c reduction with regular exercise has not been consistently seen in randomized controlled trials in type 1 diabetes (3,5–7), which contrasts with type 2 diabetes (8–10).
Despite the known benefits of exercise, many people with type 1 diabetes do not undertake sufficient exercise because of barriers, including exercise-related hypoglycemia, increased glucose variability, and the fear of hypoglycemia (9). High-intensity interval training (HIIT) is characterized by repeated short bursts of vigorous exercise interspersed with recovery periods. The risk of acute exercise-related hypoglycemia is generally lower during HIIT compared with traditional moderate-intensity continuous aerobic exercise (10). Randomized controlled trials of HIIT in people with type 2 diabetes have demonstrated improvements in insulin resistance, HbA1c, and cardiovascular risk factors after study durations of 12 weeks (11,12). Thus, HIIT may be a relatively safe type of exercise, with less hypoglycemia and potential benefits on glycemic control and cardiometabolic risk factors in type 1 diabetes; however, randomized controlled trials are lacking.
Many people with type 1 diabetes are overweight or obese (13), which can be associated with insulin resistance (14) and a greater risk of developing microvascular complications and cardiovascular disease (15,16). Insulin resistance can be improved with exercise (17), and prior exercise studies have not specifically targeted this common high-risk group. In this randomized controlled trial, we studied whether a 12-week HIIT exercise intervention can reduce HbA1c and improve cardiometabolic risk factors in people with type 1 diabetes who are overweight or obese.
Research Design and Methods
Study Design
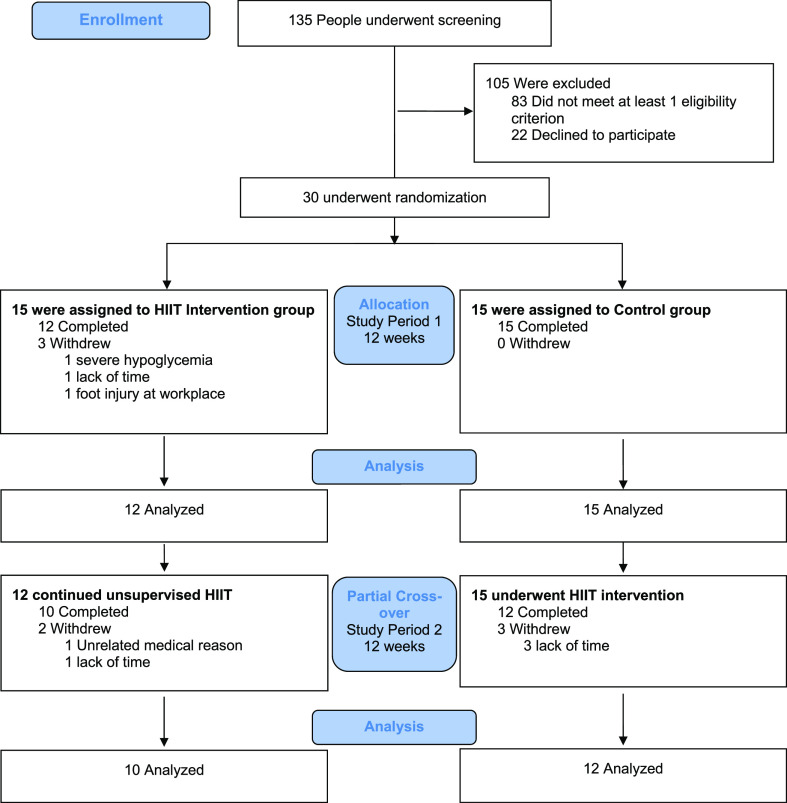
A randomized controlled trial was undertaken that involved 12 weeks of exercise intervention with partial crossover of the control group to the intervention arm for a subsequent 12 weeks (Fig. 1). Study outcomes were measured at baseline, 12 weeks, and 24 weeks. The primary outcome was the change in HbA1c between intervention and control groups at the conclusion of the randomized controlled trial, occurring at the 12-week time point. The rationale for the partial crossover design was twofold. First, it enabled all recruited participants to participate in the 12-week HIIT intervention, giving an incentive for retention in the control group, and second, as prespecified secondary outcomes, it allowed the longitudinal study of changes from preexercise to postexercise in all participants completing the 12-week HIIT intervention (occurring in study period 1 for the intervention group and study period 2 in the crossover group). The initial intervention group was studied longitudinally for 24 weeks of exercise intervention. There were no changes to the study design after trial commencement. All study visits and supervised exercise sessions were conducted in the Charles Perkins Centre, University of Sydney. The study was approved by the Sydney Local Health District Ethics Committee (HREC/17/RPAH/74) and prospectively registered in the Australian New Zealand Clinical Trials Registry.
Figure 1.
CONSORT diagram of the randomized controlled trial with partial crossover.
Study Participants
Participants were recruited from local diabetes specialist services. Inclusion criteria required each of: age between 18 and 70 years, type 1 diabetes (with presence of at least one islet autoantibody and/or low C-peptide), diabetes duration >1 year, HbA1c between 7.5 and 10.5%, BMI ≥25 kg/m2, self-management of diabetes with multiple daily insulin injections or subcutaneous insulin pump, and self-reported exercise of <150 min per week of moderate intensity for the past 6 months. Exclusion criteria were any of the following: medical condition limiting exercise participation (e.g., arthritis, unstable cardiac conditions, active foot ulcers, untreated severe retinopathy), inability to undergo an MRI scan, and in women, pregnancy or breastfeeding. Participants were fully informed of the experimental protocol and gave their written consent.
Participants were randomized 1:1 to intervention or control in blocks of 10 using computer-generated randomization by www.randomization.com. Allocation was concealed in sequentially numbered sealed opaque envelopes that were opened only once participants were enrolled. Neither participants nor study investigators were blinded to the exercise intervention allocation.
Exercise Intervention
The exercise intervention consisted of thrice-weekly HIIT for 12 weeks. At least one session per week was supervised at the Royal Prince Alfred Charles Perkins Centre research gymnasium where participants also received individualized diabetes management advice for exercise by an endocrinologist on the basis of their self-monitored blood glucose readings. HIIT was done on a cycle ergometer or treadmill at the research facility and as walking or jogging at home.
Each HIIT exercise session lasted 33 min and consisted of 5 min of warm-up at 60% peak heart rate (HRpeak), then four bouts of 4-min high-intensity intervals at 85–95% HRpeak interspersed with three bouts of 3-min recovery intervals at 50–70% HRpeak, and concluded with 3 min of cooldown. An HR monitor (Polar H7) was used during all exercise sessions (supervised and unsupervised) to first ensure that the prescribed exercise intensity was achieved and second, record adherence in an electronic diary using a Bluetooth-linked smartphone application (Polar Beat). Exercise adherence was calculated as the percentage of exercise sessions completed out of the total 36 sessions over 12 weeks. Participants were instructed to maintain their usual dietary habits throughout the study.
The control group participants continued their usual daily activities and diet, with no recommendations or restrictions given for exercise. They were offered the opportunity for weekly endocrinologist reviews for diabetes management advice to match the frequency of review received by the intervention group.
In the partial crossover design in study period 2, participants in the original control group completed the 12-week HIIT exercise intervention as described. Participants in the original intervention group continued a further 12 weeks of HIIT exercise unsupervised at home or in their own gym.
Blood and Urine Samples
Antecubital venous blood samples and spot urine samples were collected in the morning after at least 8 h of fasting. All samples were analyzed by NSW Health Pathology, with the HbA1c analyzed at a single laboratory at Royal Prince Alfred Hospital using cation-exchange high-performance liquid chromatography in an automated analyzer (D-100 System; Bio-Rad). HbA1c units were converted from % NGSP units to mmol/mol International Federation of Clinical Chemistry and Laboratory Medicine units using www.ngsp.org/convert1.asp.
Continuous Glucose Monitoring
Continuous glucose monitoring (CGM) was measured using FreeStyle Libre Pro glucose sensors (Abbott Diabetes Care, Witney, U.K.) over 14 days at baseline, weeks 11–12, and weeks 23–24. Participants were blinded to the CGM data, and calibration was not required. Total daily insulin dose was calculated from the mean of a 7-day participant-recorded diary or insulin pump download data.
Cardiovascular Measures
Cardiovascular measures were taken with the participant supine after 15 min of rest and included brachial artery blood pressure, mean arterial pressure, aortic pulse wave velocity, and augmentation pressure using a semiautomated device (SphygmoCor; ATCOR Medical, Sydney, New South Wales, Australia). Cardiac autonomic neuropathy was assessed using ECG software (Cardiosys Extra; MDE Diagnostics, Walldorf, Germany) involving cardiovascular reflex tests as described by Ewing and Clarke (18), with a total score from 0 (normal) to 10 (abnormal), and all participants were confirmed to be free of ECG changes of myocardial ischemia to proceed in the study.
Anthropometric and Body Composition Assessment
Anthropometric measures included body weight, height, BMI, waist circumference, and body composition by DXA (Hologic, Bedford, MA).
Exercise Testing
Cardiorespiratory fitness was assessed by a graded maximal exercise test on a treadmill using modified Bruce protocol. Participants wore an HR monitor (Polar H7) to determine HRpeak. Expired respiratory gases were collected through breath-by-breath analysis (Ultima PFX pulmonary function/stress testing system; MGC Diagnostics) to measure VO2peak. Joint flexibility was inferred through the sit and reach test, and muscle strength was measured by determining the one repetition maximum on chest press and leg press machines (Keiser, Fresno, CA).
Questionnaires
Participants completed two questionnaires: the Hypoglycemia Fear Survey-II (19,20), where greater scores indicate greater fear of hypoglycemia, and the Diabetes Quality of Life (21), where lower scores indicate greater quality of life.
Adverse Events
Severe hypoglycemia was defined as low blood glucose levels requiring external assistance for recovery. Participants were specifically asked about any occurrence of cardiac and respiratory conditions and musculoskeletal injuries during the study.
Sample Size and Power
Sample size calculation was based on the primary outcome of change in HbA1c between HIIT intervention and control groups at 12 weeks, with an estimated effect size of −0.7% on the basis of a meta-analysis of exercise training in people with type 2 diabetes (8). With a predicted baseline HbA1c mean ± SD of 9.0 ± 0.6%, significance level of 5%, and statistical power of 80% for a two-sided test, the calculated sample size was 12 participants per group. After allowing for a 20% dropout rate, the planned sample size was 15 participants per group.
Statistical Analysis
Data distribution was evaluated by the Shapiro-Wilk test. Participant baseline characteristics were compared using χ2 test for categorical variables and independent samples t test or Mann-Whitney U test according to normality of distribution for continuous variables. All participants with outcome measures completed at 12 weeks were included for analysis according to their randomized group allocation (modified intention-to-treat analysis). Study end points comparing groups at the end of the randomized controlled trial (study period 1) were analyzed using two-sided independent samples t tests or Mann Whitney U tests for the modified intention-to-treat analysis and a post hoc subgroup analysis of participants who completed at least 50% of the intervention. Within-group differences were analyzed using paired samples t test or Wilcoxon signed rank test for changes from baseline to 12 and 24 weeks in the intervention group, baseline to 12 weeks in the control group, and pre- to postexercise intervention in the entire cohort. All analyses were performed using SPSS version 22.0 (IBM Corporation, Armonk, NY). Data are presented as mean ± SD, and the significance level was set at P < 0.05.
Results
Baseline Characteristics
Thirty participants were recruited from July 2017 to March 2018. The last study visit was in October 2018 with the completion of the planned study protocol. Three participants dropped out of the intervention group within the first 2 weeks. One withdrew following a study-related adverse event involving a severe hypoglycemia episode (detailed below). The other two withdrawals were unrelated to the exercise intervention. At the end of study period 1, 12 and 15 participants completed the HIIT intervention and control, respectively, and had outcome data for analysis (Fig. 1). Twelve participants from the control group completed the HIIT intervention in the crossover period, allowing for the longitudinal analysis of a total of 24 participants.
Participant baseline characteristics are shown in Table 1 and were balanced between the groups, with the exception of BMI, which was slightly lower in the intervention group (P = 0.03). The control group had an average greater diabetes duration and diabetes complications prevalence, each of which were not significantly different from the intervention group. All participants used finger-prick capillary glucose testing multiple times daily, and none routinely used CGM systems or flash glucose monitoring. At study baseline, the majority of participants reported not undertaking any regular exercise, and no participants reported regular vigorous exercise.
Table 1.
Baseline characteristics of participants
| Characteristic | HIIT (n = 12) | Control (n = 15) |
|---|---|---|
| Age (years) | 40.5 ± 10.0 | 46.1 ± 10.5 |
| Male | 6 (50) | 10 (67) |
| Female | 6 (50) | 5 (33) |
| Diabetes characteristics | ||
| HbA1c (% NGSP units) | 8.63 ± 0.66 | 8.37 ± 0.71 |
| HbA1c (mmol/mol) | 70.8 ± 7.2 | 68.0 ± 7.8 |
| Duration diabetes (years) | 15.8 ± 12.2 | 22.5 ± 10.0 |
| Insulin delivery, n | ||
| MDI | 9 | 7 |
| CSII | 3 | 8 |
| Insulin dose (units/kg/day) | 0.70 ± 0.22 | 0.72 ± 0.21 |
| Impaired awareness of hypoglycemia | 2 (17) | 1 (7) |
| Diabetes complications | ||
| Macrovascular disease | 0 | 0 |
| Retinopathy | 3 (25) | 9 (60) |
| Microalbuminuria | 2 (17) | 5 (33) |
| Peripheral neuropathy | 0 (0) | 1 (7) |
| Anthropometric measures | ||
| Weight (kg) | 88.6 ± 10.0 | 92.2 ± 16.7 |
| Height (cm) | 174.7 ± 6.9 | 170.4 ± 11.8 |
| BMI (kg/m2) | 29.0 ± 2.1 | 31.6 ± 3.4* |
| Waist circumference (cm) | 102.2 ± 10.7 | 106.6 ± 12.5 |
| Fat mass (%) | 37.8 ± 5.7 | 37.2 ± 6.3 |
| Blood pressure | ||
| Systolic (mmHg) | 133 ± 16 | 137 ± 9 |
| Diastolic (mmHg) | 79 ± 10 | 78 ± 9 |
| Lipids | ||
| Total cholesterol (mmol/L) | 4.8 ± 1.0 | 4.6 ± 1.0 |
| LDL (mmol/L) | 2.7 ± 0.9 | 2.2 ± 0.7 |
| HDL (mmol/L) | 1.6 ± 0.3 | 1.8 ± 0.7 |
| Triglycerides (mmol/L) | 1.3 ± 0.5 | 1.4 ± 1.4 |
| Exercise testing | ||
| Habitual exercise participation (min/week) | 29 ± 36 | 25 ± 37 |
| VO2peak (mL/kg/min) | 30.4 ± 5.3 | 28.6 ± 5.6 |
| Bruce protocol time (s) | 737 ± 124 | 711 ± 156 |
| Chest press (N) | 420 ± 169 | 504 ± 206 |
| Leg press (N) | 2,458 ± 874 | 2,987 ± 1,192 |
Data are mean ± SD or n (%) unless otherwise indicated. Microalbuminuria defined as present if urine albumin-to-creatinine ratio was >2.5 mg/mmol in males and >3.5 mg/mmol in females. Impaired awareness of hypoglycemia defined as present if Clark score ≥4. CSII, continuous subcutaneous insulin infusion; MDI, multiple daily injections; N, newton.
P = 0.03.
Mean adherence to prescribed exercise was 67 ± 20% in the intervention group and 65 ± 23% in the crossover group (P = 0.84). Overall exercise adherence was 70 ± 25% in the initial 4 weeks, 66 ± 27% in the middle 4 weeks, and 62 ± 30% in the last 4 weeks of the HIIT intervention (P = 0.15 for initial vs. last). Overall, 55% of all exercise sessions that were undertaken were performed in the research gym and 45% at home. Of the 24 participants completing HIIT, 17 were able to consistently achieve the prescribed high-intensity HR targets both in the research gym and at home and 3 in the research gym only (including 2 participants not attempting home exercise); 4 did not consistently reach HR targets but were encouraged to make their best ongoing attempt.
Glycemic Outcomes
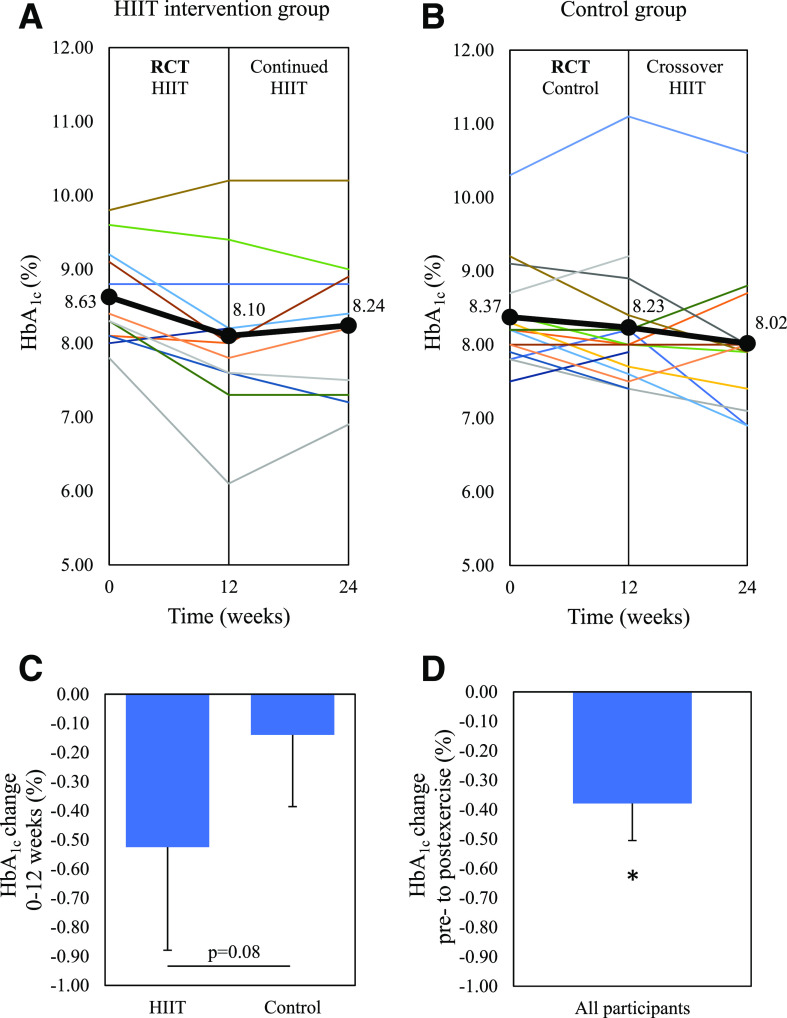
HbA1c decreased by 0.53 ± 0.61% in the intervention group after 12 weeks; however, this was not significantly different from the control group decrease of 0.14 ± 0.48% (P = 0.08), as shown in Table 2. Within the intervention group, there was a significant reduction in HbA1c at 12 weeks compared with baseline (P = 0.01) without a significant change in insulin dose or hypoglycemia on CGM. Figure 2 shows the change in HbA1c for each individual participant.
Table 2.
Study outcomes at 12 weeks in HIIT and control groups
| HIIT (n = 12) | Control (n = 15) | |||||
|---|---|---|---|---|---|---|
| Variable | Baseline | 12 weeks | Change | Baseline | 12 weeks | Change |
| Glucose control | ||||||
| HbA1c (% NGSP units) | 8.63 ± 0.66 | 8.10 ± 1.04* | −0.53 ± 0.61 | 8.37 ± 0.71 | 8.23 ± 0.95 | −0.14 ± 0.48 |
| HbA1c (mmol/mol) | 70.8 ± 7.2 | 65.0 ± 11.4* | −5.8 ± 6.7 | 68.0 ± 7.8 | 66.5 ± 10.4 | −1.5 ± 5.2 |
| Insulin dose (units/kg/day) | 0.70 ± 0.23 | 0.67 ± 0.24 | −0.02 ± 0.07 | 0.72 ± 0.21 | 0.71 ± 0.25 | −0.01 ± 0.09 |
| Mean glucose (mmol/L) | 11.8 ± 1.7 | 10.6 ± 1.8* | −1.2 ± 1.7 | 11.1 ± 1.8 | 9.7 ± 2.0* | −1.2 ± 1.8 |
| CV glucose (%) | 41.5 ± 8.0 | 42.4 ± 7.7 | 0.8 ± 7.4 | 43.1 ± 8.2 | 45.3 ± 10.5 | −2.1 ± 7.6 |
| Time in hypoglycemia (%) | 4.8 ± 6.0 | 5.9 ± 4.0 | 1.1 ± 4.4 | 6.5 ± 6.8 | 10.5 ± 9.4* | 4.0 ± 6.2 |
| Time in target (%) | 34.5 ± 9.5 | 45.5 ± 17.6 | 10.9 ± 17.3 | 39.7 ± 11.4 | 46.5 ± 12.5 | 6.8 ± 12.2 |
| Time in hyperglycemia (%) | 60.6 ± 13.8 | 48.6 ± 17.4 | −12.0 ± 19.5 | 53.8 ± 13.8 | 43.0 ± 15.6* | −10.8 ± 15.4 |
| Anthropometric measures | ||||||
| Weight (kg) | 88.6 ± 10.0 | 88.9 ± 9.9 | 0.3 ± 1.4 | 92.2 ± 16.7 | 93.5 ± 17.0* | 1.3 ± 1.8 |
| BMI (kg/m2) | 29.0 ± 2.1† | 29.1 ± 2.1† | 0.1 ± 0.5 | 31.6 ± 3.4 | 32.0 ± 3.5* | 0.4 ± 0.6 |
| Waist circumference (cm) | 102.2 ± 10.7 | 103.3 ± 9.0 | 1.1 ± 4.3 | 106.6 ± 12.5 | 109.5 ± 10.8* | 2.9 ± 4.2 |
| Fat mass (%) | 37.8 ± 5.7 | 37.6 ± 5.5 | −0.2 ± 1.5 | 37.2 ± 6.3 | 37.6 ± 5.8 | 0.4 ± 1.3 |
| Total body fat mass (kg) | 32.9 ± 6.0 | 32.7 ± 5.4 | −0.2 ± 1.6 | 33.2 ± 6.0 | 34.1 ± 6.4* | 0.9 ± 1.6 |
| Total body lean mass (kg) | 54.1 ± 8.2 | 54.4 ± 8.3 | 0.3 ± 1.4 | 57.2 ± 13.2 | 57.5 ± 13.0 | 0.3 ± 1.5 |
| Visceral adipose tissue mass (kg) | 0.8 ± 0.4 | 0.7 ± 0.3 | −0.0 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.0 ± 0.1 |
| Cardiovascular measures | ||||||
| Systolic blood pressure (mmHg) | 133 ± 16 | 135 ± 15 | 2 ± 16 | 137 ± 9 | 132 ± 11 | −4.6 ± 11.3 |
| Diastolic blood pressure (mmHg) | 79 ± 10 | 79 ± 9 | −0 ± 12 | 78 ± 9 | 80 ± 8 | 2.1 ± 8.8 |
| Mean arterial pressure (mmHg) | 96 ± 9 | 96 ± 11 | 0 ± 13 | 97 ± 9 | 97 ± 8 | −0 ± 8 |
| Augmentation index | 16.0 ± 8.1 | 17.0 ± 13.3 | 1 ± 11 | 18.4 ± 13.8 | 23.1 ± 14.0* | 5 ± 9 |
| aPWV (m/s) | 8.0 ± 3.2 | 7.8 ± 2.5 | −0.1 ± 2.7 | 8.9 ± 1.9 | 8.6 ± 1.7 | −0.3 ± 1.3 |
| Ewing score | 1.2 ± 0.9 | 0.7 ± 0.7 | −0.5 ± 0.8 | 1.9 ± 2.0 | 1.4 ± 1.3 | −0.5 ± 1.8 |
| Biochemistry | ||||||
| Total cholesterol (mmol/L) | 4.7 ± 0.9 | 4.9 ± 1.4 | 0.2 ± 1.0 | 4.6 ± 1.0 | 4.7 ± 1.0 | 0.0 ± 0.5 |
| LDL (mmol/L) | 2.7 ± 0.9 | 2.7 ± 0.9 | 0.0 ± 0.7 | 2.2 ± 0.7 | 2.5 ± 1.0 | 0.3 ± 0.7 |
| HDL (mmol/L) | 1.6 ± 0.3 | 1.6 ± 0.5 | 0.1 ± 0.2 | 1.8 ± 0.7 | 1.6 ± 0.4 | −0.1 ± 0.6 |
| Triglycerides (mmol/L) | 1.3 ± 0.5 | 1.4 ± 0.8 | 0.2 ± 0.6 | 1.4 ± 1.4 | 1.2 ± 0.8 | −0.2 ± 0.6 |
| Apolipoprotein A1 (g/L) | 1.6 ± 0.2 | 1.7 ± 0.3 | −0.0 ± 0.1 | 1.6 ± 0.2 | 1.6 ± 0.2 | −0.0 ± 0.1 |
| Apolipoprotein B (g/L) | 0.9 ± 0.3 | 1.0 ± 0.3 | −0.1 ± 0.2 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.0 ± 0.2 |
| Leptin (ng/mL) | 31.4 ± 15.4 | 42.4 ± 19.1* | 10.9 ± 15.2 | 35.2 ± 16.5 | 43.8 ± 26.1 | 8.6 ± 15.9 |
| Adiponectin (µg/mL) | 15.2 ± 8.4 | 15.8 ± 12.4 | 0.6 ± 5.4 | 14.1 ± 7.8 | 13.5 ± 8.0 | −0.6 ± 4.4 |
| hs-CRP (mg/L) | 3.6 ± 4.2 | 3.6 ± 3.4 | −0.0 ± 2.6 | 3.1 ± 3.3 | 3.4 ± 3.3 | 0.3 ± 2.3 |
| Urine ACR (mg/mmol) | 2.5 ± 4.0 | 1.9 ± 2.1 | −0.7 ± 2.1 | 6.7 ± 14.1 | 4.9 ± 9.9 | −1.7 ± 5.5 |
| Exercise parameters | ||||||
| VO2peak (mL/kg/min) | 30.4 ± 5.3 | 29.5 ± 3.6 | −0.9 ± 5.5 | 28.7 ± 5.8 | 26.6 ± 6.3 | −2.1 ± 5.0 |
| Bruce protocol time (s) | 737 ± 124 | 796 ± 120* | 59 ± 43 | 717 ± 160 | 755 ± 179 | 38 ± 87 |
| Chest press (N) | 454 ± 164 | 470 ± 183 | 16 ± 54 | 496 ± 213 | 481 ± 204 | −15 ± 5,758 |
| Leg press (N) | 2,458 ± 874 | 2,992 ± 986* | 533 ± 360† | 2,986 ± 1,237 | 3,129 ± 1,161 | 143 ± 426 |
| Sit and reach (cm) | −3.8 ± 13.7 | −2.8 ± 13.3 | 1.0 ± 3.3 | −6.7 ± 10.8 | −9.3 ± 10.1 | −2.7 ± 7.9 |
| Questionnaires | ||||||
| HFS score | 46 ± 28 | 44 ± 30 | −2.6 ± 11.8 | 37 ± 19 | 30 ± 18* | −6.8 ± 11.2 |
| DQOL score | 118 ± 26† | 117 ± 29† | −1.3 ± 17.5 | 92 ± 22 | 85 ± 18 | −7.1 ± 16.8 |
Data are mean ± SD. Time in hypoglycemia (glucose ≤3.9 mmol/L), time in target (glucose 4.0–10.0 mmol/L), time in hyperglycemia (glucose >10.0 mmol/L). ACR, albumin-to-creatinine ratio; aPWV, aortic pulse wave velocity; CV, coefficient of variation; DQOL, Diabetes Quality of Life; HFS, Hypoglycemia Fear Survey; N, newton.
P < 0.05 vs. baseline.
P < 0.05 vs. control.
Figure 2.
The change in HbA1c for individual participants. A: HIIT group where 0–12 weeks involved the HIIT intervention, and 12–24 weeks involved continued, unsupervised HIIT. B: Control group where 0–12 weeks involved inactive control, and 12–24 weeks involved the HIIT intervention as the crossover design. C: Change in HbA1c from 0 to 12 weeks between the HIIT and control groups as the primary study end point. D: Predefined secondary end point for change in HbA1c from preexercise to postexercise for all 24 participants who undertook 12 weeks of HIIT. Bold line indicates the mean HbA1c. Data are mean ± SEM. *P < 0.05 change from preexercise. RCT, randomized controlled trial.
For the nine participants in the intervention group who completed at least 50% of prescribed exercise sessions, the HbA1c decreased by 0.64 ± 0.64%, which was significantly greater than the control group (P = 0.04) (Supplementary Table 1). HbA1c decreased by 0.40 ± 0.62% (P = 0.006) in the 24 participants who completed the 12-week HIIT exercise intervention (Supplementary Table 2).
Metabolic Outcomes
Body composition was unchanged in the intervention group after 12 weeks. In contrast, there were significant increases in body weight, BMI, waist circumference, and total fat mass in the control group, as shown in Table 2. Blood pressure did not change in the intervention group; however, there was a significant increase in augmentation pressure, as a measure of increased arterial stiffness, in the control group (P = 0.049). Blood lipid profile was unchanged in both groups. An increase in circulating leptin was seen in the intervention group (P = 0.03) but not in the control group. The intervention group showed improvement in leg press strength (P < 0.001) and in maximal treadmill exercise test time (P = 0.001), although there was no significant change in measured VO2peak.
For the nine participants in the intervention group who undertook at least 50% of prescribed exercise, there was an increase in total body lean mass of 0.9 ± 1.0 kg (P = 0.04), as shown in Supplementary Table 1. There were 10 participants in the initial intervention group who completed 24 weeks of HIIT exercise, with outcomes shown in Supplementary Table 3. These participants had improvements in HbA1c, maximal treadmill exercise test time, and leg strength at 12 weeks compared with baseline (all P < 0.05). All improvements persisted at 24 weeks, and there were additional improvements seen with increases in total body lean mass and leptin compared with baseline.
Adverse Events
There was one severe hypoglycemia event occurring immediately after exercise that required short-term hospital emergency department care. There were no adverse cardiac events, respiratory events, or musculoskeletal injuries related to the HIIT exercise. There were no episodes of diabetic ketoacidosis.
Conclusions
We conducted a randomized controlled trial to assess the effect of HIIT on glycemic control and cardiometabolic risk factors in adults with type 1 diabetes and overweight or obesity. To our knowledge, this is the first study to specifically target the common high-risk phenotype of the combination of type 1 diabetes and high BMI in an exercise study involving HIIT compared with nonexercise control. While there was no significant difference in our primary end point of HbA1c change after 12 weeks between groups, there was a significant HbA1c reduction of 0.64% seen in the intervention subgroup who completed at least 50% of prescribed HIIT exercise. In the prespecified secondary analysis using the partial crossover design, HbA1c significantly decreased by 0.40% postexercise in the 24 participants who completed 12 weeks of HIIT. This HbA1c reduction was not attributed to any increase in insulin dose and, reassuringly, was not accompanied by any significantly increased hypoglycemia as measured on blinded CGM.
There have only been a few published studies of HIIT in type 1 diabetes, using different HIIT protocols (6,7,22,23). Farinha et al. (7) studied the effects of 10 weeks of three exercise programs; HIIT (10 × 60-s cycling intervals at 90% maximum HR), strength training, and combination HIIT and strength training. HbA1c decreased by 0.26% in the pooled analysis of the three exercise groups. The greater degree of HbA1c reduction seen in our intervention group may be in part related to differences in exercise dose and/or study populations, with our participants having a higher metabolic risk phenotype, including higher BMI, and higher baseline HbA1c.
In contrast to our study, Boff et al. (6) found no changes in HbA1c after 8 weeks in either HIIT or moderate-intensity continuous training interventions. Their HIIT protocol included 6 × 1-min high-intensity intervals at 85% maximum HR and, thus, had a lower volume of high-intensity exercise compared with our 4 × 4-min HIIT protocol performed over 12 weeks. Differences in HbA1c effect between studies may relate to the differences in volume of exercise, in both the HIIT sessions, and duration of intervention studied. The 4 × 4-min HIIT protocol was chosen for our study because of evidence for benefit in HbA1c in people with type 2 diabetes (24,25). A study by Harmer et al. (22) involving eight participants with type 1 diabetes undertaking 7 weeks of HIIT found a nonsignificant change in HbA1c from 8.6% preexercise to 8.1% postexercise. This trend in HbA1c change is consistent with the magnitude of HbA1c change seen in our study.
The magnitude of HbA1c reduction in the HIIT intervention group may have been limited by two significant factors: suboptimal exercise adherence in the intervention group and confounding participation in physical activity in the control group. First, participants undertook a mean of only two of the three prescribed exercise sessions per week, although this was higher than their reported habitual exercise participation, with the majority of participants reporting undertaking no exercise at study baseline. We designed the HIIT intervention to have some flexibility in an attempt to reduce exercise barriers, such as time limitations and necessity for equipment, with approximately half the exercise sessions intended to be completed at the research facility and half at home. Poor exercise participation is a common issue, with population studies reporting <20% of people with type 1 diabetes exercising more than twice per week (4). The reason for suboptimal exercise adherence in our study may not be specific to the HIIT form of exercise, given the continuing participation across 12 weeks, but it is likely multifactorial, such as personal motivation and competing time commitments as reported by others (9). While the majority of study participants were able to undertake the 4 × 4-min HIIT at the prescribed intensity in a supervised gym setting, long-term adherence, particularly in an unsupervised setting, may be challenging to maintain in some people and requires further study. In post hoc subgroup analysis, the HIIT intervention subgroup who completed at least 50% of the prescribed exercise showed a significant HbA1c change of −0.64% compared with the control group. Second, participants in the control group were not given formal exercise restrictions, and some participants randomized to the control group may have increased their exercise of their own volition; however, this was not explicitly measured.
Given the established risks of exercise-related hypoglycemia, participants were given individualized advice on insulin and carbohydrate adjustments on the basis of international consensus guidelines (2). To reduce bias associated with differences in receiving diabetes review, participants in both the intervention and the control groups were given the opportunity for weekly diabetes consultation by a single endocrinologist, although this was not blinded given the requirement to adjust diabetes management to individual exercise-glucose response. Consultations were done mostly by phone and e-mail during the control and thus were less detailed than during the intervention when participants had consultations during their weekly gym attendance. Differences in diabetes self-management behavior between groups may have influenced the HbA1c. This limitation could be minimized in future studies through the use of closed-loop systems enabling automated modulation of insulin delivery; however, there are still challenges with this technology during exercise (26). Another limitation is that we were unable to quantify dietary changes, although participants were advised to continue their routine diet throughout the study.
Our study did not find any differences in blood pressure, lipid profile, or body composition between the HIIT intervention and control groups. Improvements in body composition were seen in a study by Farinha et al. (7) but not in other type 1 diabetes HIIT exercise studies (6,23). There is some evidence in other type 1 diabetes HIIT studies for favorable changes in measures of vascular function, improvements in arterial stiffness measured by aortic pulse wave velocity (23), and improvements in endothelial function (6).
Our HIIT intervention used predominantly lower-limb–based exercise, namely walking, running, and cycling. This likely explains the improvements gained in leg strength without improvements seen in upper-body (chest press) strength. As expected, participation in the exercise intervention was associated with a significant improvement in maximal treadmill exercise test time, which is consistent with an improvement in functional capacity and aerobic fitness. Improvement in aerobic fitness has been demonstrated consistently across studies involving HIIT in many populations (12). However, improvement in aerobic fitness (as inferred by exercise test time to volitional fatigue) was not corroborated by significant changes in VO2peak (as measured by expired respiratory gas analysis), which may have been attributable to poor compliance with the face mask (as leaking of expired air), and the gas analyzer required a service repair during the middle of the study, which may have affected VO2peak data reliability. Future studies may need to consider repeated familiarization sessions at baseline. The improvements in leg strength seen in the intervention group, without improvements in VO2peak, could also suggest that the increase in maximal treadmill exercise test time was mainly due to increased muscular endurance rather than aerobic fitness. However, this would be inconsistent with other studies in type 1 diabetes that have shown improvements in VO2peak with HIIT (6,7,23). Improvements in cardiorespiratory fitness, as measured by VO2peak, are largely driven by increases in cardiac output, whereas increases in exercise capacity, as measured by the treadmill test time, is more dependent on peripheral adaptations in skeletal muscle (27). Given that the exercise-induced changes in insulin sensitivity occur peripherally in skeletal muscle, reductions in HbA1c may be more strongly correlated with treadmill test time than with the VO2peak (28).
There was one severe hypoglycemic event in the study, which occurred immediately following a supervised HIIT exercise session during the first week. The participant could not drink juice to treat hypoglycemia because of nausea so was treated with parenteral glucose and had a short observation period in the emergency department. There was no loss of consciousness. Contributing factors to the hypoglycemia include preceding mild hypoglycemia earlier that day and the challenge in managing glucose when initiating a new exercise program. Antecedent hypoglycemia increases the risk of exercise-related hypoglycemia (29). In our pilot study examining the feasibility and safety of the 4 × 4-min HIIT protocol in people with type 1 diabetes, there was no increase in hypoglycemia for 24 h following afternoon HIIT exercise, compared with a control day, when using diabetes self-management strategies, including evening basal insulin dose reduction (30). However, individual glucose responses can vary, and particular care should be taken in managing glucose when starting any new exercise program.
A strength of our study design is the incorporation of a partial crossover to incentivize retention in the control group and allow longitudinal analysis after exercise intervention, compared with the participants’ inactive state, in the entire cohort. However, this study design precluded the implementation of an active intervention in the control arm. Comparing 4 × 4-min HIIT with moderate-intensity continuous aerobic exercise and strength training, as well as exploring the longer-term effects of continued HIIT on metabolic health and diabetes complications and whether adherence to HIIT can be realized longer term, are areas that warrant future research.
In conclusion, our study did not find evidence for a significant reduction in HbA1c with a 12-week HIIT intervention compared with control in adults with type 1 diabetes and overweight or obesity. However, there were favorable HbA1c reductions in the subgroup with >50% adherence to the HIIT intervention. This suggests that HIIT may improve glycemic control when the exercise is undertaken with at least modest adherence. Our study can help people with type 1 diabetes to consider including HIIT as part of their diabetes management.
Article Information
Funding and Duality of Interest. Sydney Medical School Foundation, University of Sydney, and a Diabetes Australia Research Trust Project Grant supported consumables costs. Abbott Diabetes Care generously provided the FreeStyle Libre Pro glucose sensors. A.S.L. was supported by a National Health and Medical Research Council Postgraduate Scholarship and a JDRF Australia Postgraduate Scholarship. S.M.-H. was supported by a National Commission for Scientific and Technological Research, Becas Chile Scholarship for foreign PhD programs (Resolución Exenta #2185/2015). S.M.T. is on the Australian National Advisory Board for the FreeStyle Libre Pro Flash Glucose Monitoring System. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.S.L. conducted the study visits, collected the data, and wrote the draft manuscript. A.S.L., N.A.J., M.J.M., J.O., C.L., C.J.B., S.M.-H., J.W., J.R.F., and S.M.T. interpreted the data, contributed to the discussion, and reviewed and edited the manuscript. A.S.L., N.A.J., M.J.M., J.O., C.L., J.W., J.R.F., and S.M.T. wrote and reviewed the study protocol. A.S.L., M.J.M., J.O., C.L., J.W., and S.M.T. recruited participants. A.S.L., C.J.B., S.M.-H., and S.M.T. supervised the exercise sessions. A.S.L. and S.M.T. performed the statistical analysis. A.S.L. and S.M.T. are the guarantors of this work and, as such, had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the Australasian Diabetes Congress, Sydney, New South Wales, Australia, 21–23 August 2019, and at the 55th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 16–20 September 2019.
Footnotes
Clinical trial reg. no. ACTRN12617000478314, www.anzctr.org.au.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12515424.
References
- 1.American Diabetes Association 5. Lifestyle management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S46–S60 [DOI] [PubMed] [Google Scholar]
- 2.Riddell MC, Gallen IW, Smart CE, et al. . Exercise management in type 1 diabetes: a consensus statement. Lancet Diabetes Endocrinol 2017;5:377–390 [DOI] [PubMed] [Google Scholar]
- 3.Ostman C, Jewiss D, King N, Smart NA. Clinical outcomes to exercise training in type 1 diabetes: a systematic review and meta-analysis. Diabetes Res Clin Pract 2018;139:380–391 [DOI] [PubMed] [Google Scholar]
- 4.Bohn B, Herbst A, Pfeifer M, et al.; DPV Initiative . Impact of physical activity on glycemic control and prevalence of cardiovascular risk factors in adults with type 1 diabetes: a cross-sectional multicenter study of 18,028 patients. Diabetes Care 2015;38:1536–1543 [DOI] [PubMed] [Google Scholar]
- 5.Kennedy A, Nirantharakumar K, Chimen M, et al. . Does exercise improve glycaemic control in type 1 diabetes? A systematic review and meta-analysis. PLoS One 2013;8:e58861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boff W, da Silva AM, Farinha JB, et al. . Superior effects of high-intensity interval vs. moderate-intensity continuous training on endothelial function and cardiorespiratory fitness in patients with type 1 diabetes: a randomized controlled trial. Front Physiol 2019;10:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farinha JB, Ramis TR, Vieira AF, et al. . Glycemic, inflammatory and oxidative stress responses to different high-intensity training protocols in type 1 diabetes: a randomized clinical trial. J Diabetes Complications 2018;32:1124–1132 [DOI] [PubMed] [Google Scholar]
- 8.Umpierre D, Ribeiro PA, Kramer CK, et al. . Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: a systematic review and meta-analysis. JAMA 2011;305:1790–1799 [DOI] [PubMed] [Google Scholar]
- 9.Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care 2008;31:2108–2109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guelfi KJ, Jones TW, Fournier PA. The decline in blood glucose levels is less with intermittent high-intensity compared with moderate exercise in individuals with type 1 diabetes. Diabetes Care 2005;28:1289–1294 [DOI] [PubMed] [Google Scholar]
- 11.Jelleyman C, Yates T, O’Donovan G, et al. . The effects of high-intensity interval training on glucose regulation and insulin resistance: a meta-analysis. Obes Rev 2015;16:942–961 [DOI] [PubMed] [Google Scholar]
- 12.Weston KS, Wisløff U, Coombes JS. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: a systematic review and meta-analysis. Br J Sports Med 2014;48:1227–1234 [DOI] [PubMed] [Google Scholar]
- 13.Lee AS, Colagiuri S, Flack JR. Successful implementation of diabetes audits in Australia: the Australian National Diabetes Information Audit and Benchmarking (ANDIAB) initiative. Diabet Med 2018;35:929–936 [DOI] [PubMed] [Google Scholar]
- 14.Priya G, Kalra S. A review of insulin resistance in type 1 diabetes: is there a place for adjunctive metformin? Diabetes Ther 2018;9:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007;30:707–712 [DOI] [PubMed] [Google Scholar]
- 16.McGill M, Molyneaux L, Twigg SM, Yue DK. The metabolic syndrome in type 1 diabetes: does it exist and does it matter? J Diabetes Complications 2008;22:18–23 [DOI] [PubMed] [Google Scholar]
- 17.Mann S, Beedie C, Balducci S, et al. . Changes in insulin sensitivity in response to different modalities of exercise: a review of the evidence. Diabetes Metab Res Rev 2014;30:257–268 [DOI] [PubMed] [Google Scholar]
- 18.Ewing DJ, Clarke BF. Diagnosis and management of diabetic autonomic neuropathy. Br Med J (Clin Res Ed) 1982;285:916–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonder-Frederick LA, Schmidt KM, Vajda KA, et al. . Psychometric properties of the hypoglycemia fear survey-ii for adults with type 1 diabetes. Diabetes Care 2011;34:801–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox DJ, Irvine A, Gonder-Frederick L, Nowacek G, Butterfield J. Fear of hypoglycemia: quantification, validation, and utilization. Diabetes Care 1987;10:617–621 [DOI] [PubMed] [Google Scholar]
- 21.The DCCT Research Group Reliability and validity of a diabetes quality-of-life measure for the diabetes control and complications trial (DCCT). Diabetes Care 1988;11:725–732 [DOI] [PubMed] [Google Scholar]
- 22.Harmer AR, Chisholm DJ, McKenna MJ, et al. . High-intensity training improves plasma glucose and acid-base regulation during intermittent maximal exercise in type 1 diabetes. Diabetes Care 2007;30:1269–1271 [DOI] [PubMed] [Google Scholar]
- 23.Scott SN, Cocks M, Andrews RC, et al. . High-intensity interval training improves aerobic capacity without a detrimental decline in blood glucose in people with type 1 diabetes. J Clin Endocrinol Metab 2019;104:604–612 [DOI] [PubMed] [Google Scholar]
- 24.Støa EM, Meling S, Nyhus LK, et al. . High-intensity aerobic interval training improves aerobic fitness and HbA1c among persons diagnosed with type 2 diabetes. Eur J Appl Physiol 2017;117:455–467 [DOI] [PubMed] [Google Scholar]
- 25.Hollekim-Strand SM, Bjørgaas MR, Albrektsen G, Tjønna AE, Wisløff U, Ingul CB. High-intensity interval exercise effectively improves cardiac function in patients with type 2 diabetes mellitus and diastolic dysfunction: a randomized controlled trial. J Am Coll Cardiol 2014;64:1758–1760 [DOI] [PubMed] [Google Scholar]
- 26.Bally L, Thabit H. Closing the loop on exercise in type 1 diabetes. Curr Diabetes Rev 2018;14:257–265 [DOI] [PubMed] [Google Scholar]
- 27.Brennan AM, Lam M, Stotz P, Hudson R, Ross R. Exercise-induced improvement in insulin sensitivity is not mediated by change in cardiorespiratory fitness. Diabetes Care 2014;37:e95–e97 [DOI] [PubMed] [Google Scholar]
- 28.Sénéchal M, Swift DL, Johannsen NM, et al. . Changes in body fat distribution and fitness are associated with changes in hemoglobin A1c after 9 months of exercise training: results from the HART-D study. Diabetes Care 2013;36:2843–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of antecedent hypoglycemia on counterregulatory responses to subsequent euglycemic exercise in type 1 diabetes. Diabetes 2003;52:1761–1769 [DOI] [PubMed] [Google Scholar]
- 30.Lee AS, Way KL, Johnson NA, Twigg SM. High-intensity interval exercise and hypoglycaemia minimisation in adults with type 1 diabetes: a randomised cross-over trial. J Diabetes Complications 2020;34:107514. [DOI] [PubMed] [Google Scholar]