Abstract
OBJECTIVE
Previous studies have suggested that diabetes increases the risk of Parkinson disease (PD); however, this has not been conclusively established. We analyzed the risk of PD based on baseline glucose tolerance status in a large-scale cohort representative of the general Korean population.
RESEARCH DESIGN AND METHODS
This analysis was performed in a cohort of 15,168,021 adults aged ≥40 years who underwent health checkups under the National Health Insurance Service between January 2009 and December 2010. The clinical course of subjects was monitored until December 2016. Subjects were classified into the following groups: no diabetes, impaired fasting glucose (IFG), diabetes duration <5 years, and diabetes duration ≥5 years. We analyzed the adjusted hazard ratio of PD for each group.
RESULTS
During the observation period of 49,076,148.74 person-years, PD occurred in 31,577 patients. Compared with the nondiabetes group, the adjusted hazard ratio was 1.038 (95% CI, 1.009–1.067) in the IFG group, 1.185 (95% CI, 1.143–1.229) in the diabetes duration <5 years group, and 1.618 (95% CI, 1.566–1.672) in the diabetes duration ≥5 years group. These results were consistent with those of the subgroup analysis, and the presence of diabetes further increased the risk of PD regardless of comorbidities such as cardiovascular, cerebrovascular, and chronic kidney diseases.
CONCLUSIONS
This population-based cohort study suggests that diabetes is an independent risk factor for PD.
Introduction
The prevalence of diabetes and related complications is increasing worldwide (1,2). Accordingly, the burden on global health care related to diabetes continues to increase (1,3). Meanwhile, various therapeutic interventions for diabetes have been developed, and the clinical course and quality of life of people with diabetes have improved (4). However, it remains impossible to completely prevent the development of diabetes-related complications. Rather, the clinical significance of previously overlooked atypical complications has paradoxically increased (5).
Parkinson disease (PD) is also a major chronic disease, and its clinical significance is increasing worldwide. Damage to the dopaminergic neurons of the substantia nigra is known to be a major cause of PD, which is clinically characterized by a variety of neurologic symptoms (6). The prevalence of PD is expected to continue to increase as human life expectancy increases (7). However, curative treatment for this disease does not currently exist, and only symptomatic management is performed. Thus, the disease burden for PD is also expected to increase in the future (8).
Various environmental and genetic factors are known to increase the risk of PD (9). In particular, recent reports have suggested that metabolic diseases, such as obesity, metabolic syndrome, and diabetes, are important risk factors for PD (10). This is because the pathophysiologic mechanism associated with insulin resistance plays an important role in the development and worsening of PD as well as diabetes (11). However, there have been conflicting results from previous epidemiologic studies on the association between diabetes and PD (12–18). Moreover, any causal relationship between diabetes and PD needs to be more clearly defined.
Considering this background, we conducted a longitudinal study to evaluate PD risk according to baseline glucose tolerance status, diabetes disease duration, and comorbidity. Specifically, we used a large cohort representative of the Korean general population to determine the risk of developing PD in subjects with normal glucose tolerance, impaired fasting glucose (IFG), and diabetes duration <5 or ≥5 years. An additional goal of this study was to determine whether a causal relationship between dysglycemia and PD exists.
Research Design and Methods
National Health Insurance Health Examination Cohort
The Korean National Health Insurance (NHI) is a single health care insurance system that covers ∼97% of the total Korean population; the remaining 3% are “medical protection” beneficiaries. Information on individuals’ use of medical facilities, prescription records, and diagnostic codes configured in the form of ICD-10 is recorded in the National Health Insurance Service (NHIS) database (19). In addition, the NHIS provides a biennial health examination program for all beneficiaries aged ≥40 years, which consists of anthropometry, a self-administered questionnaire on past medical history or health-related behavior, and laboratory tests (20). This database has been considered to be representative of the Korean population and is used in research through anonymization and deidentification.
Study Subjects
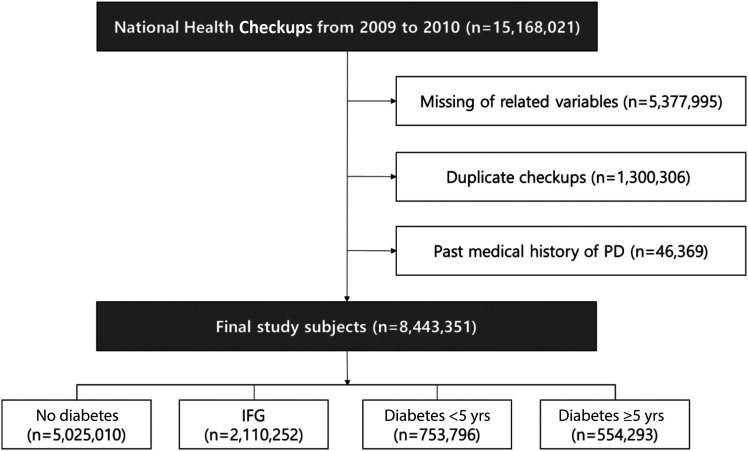
From 2009 to 2010, 15,168,021 NHIS health examinations were conducted (Fig. 1). Among these, subjects with missing variables, those with duplicate screenings, and those with a medical history of PD were excluded. As a result, 8,443,351 subjects were selected for the current analysis. Diabetes and glucose tolerance status of subjects were categorized into the groups according to ICD-10 codes (E11.x–E14.x), prescription records (oral and/or injectable antidiabetic medications), and fasting glucose measurements during the health screening. This definition was based on the consensus of relevant findings widely used in previous studies (21,22). The subjects were classified based on glycemic status as no diabetes (fasting glucose <100 mg/dL), IFG (fasting glucose 100–125 mg/dL), diabetes duration <5 years, and diabetes duration ≥5 years. IFG and diabetes were diagnosed according to the criteria of the American Diabetes Association (23). Among all of the subjects, there were 5,025,010 in the nondiabetes group, 2,110,252 in the IFG group, 753,796 in the diabetes duration <5 years group, and 554,293 in the diabetes duration ≥5 years group. Patients’ clinical course was assessed until December 2016, and the mean observation period was 6.3 years.
Figure 1.
Flowchart of the selection of study subjects.
Clinical Variables
Anthropometric assessments of subjects were performed by health care professionals. Height, weight, waist circumference, and blood pressure (systolic and diastolic) were assessed. Detailed information on the lifestyle of subjects was obtained through standardized self-reported questionnaires. Subjects were classified according to smoking status as nonsmoker, former smoker, or current smoker. Individuals who consumed 30 g of alcohol per day were classified as being heavy alcohol consumers (24). Regular physical activity was defined as performance of strenuous exercise for at least 20 min (once per week) (10). Baseline comorbidities (hypertension, dyslipidemia, cardiovascular disease, and cerebrovascular disease) of subjects were identified based on a combination of the past medical history questionnaires, ICD-10 codes, and data from the prescription database.
Blood sampling was conducted after overnight fasting, and serum glucose, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, and creatinine concentrations were measured. The estimated glomerular filtration rate (eGFR) of subjects was calculated using the MDRD Study equation: eGFR = 175 × serum creatinine − 1.154 × age − 0.203, further multiplied by 0.742 for women (25). We defined chronic kidney disease (CKD) as an eGFR <60 mL/min/1.73 m2 (26).
Identification of New PD Cases
The policy of NHI in Korea enhanced the health coverage for rare or incurable diseases, including PD, since 2006. To receive the benefits of reduced payment for PD-related management, physicians need to determine whether patients can be correctly diagnosed with PD based on their clinical condition. For this reason, new PD cases were identified as those with an ICD-10 code for PD (G20) and registered as rare or incurable disease cases for PD (V124, a special code for PD). The V124 code is independent of the ICD-10 code and is assigned at the specialist’s discretion, taking into account various clinical situations. The criteria for the V124 code are as follows: 1) satisfy the criteria for the diagnosis of parkinsonian syndrome, including mild or worse bradykinesia, 2) satisfy the exclusion criteria for PD, and 3) satisfy the supportive prospective positive criteria for PD, including responsiveness to levodopa. The V124 code criteria for PD are almost identical to the UK Parkinson’s Disease Society Brain Bank diagnostic criteria (27). Moreover, this definition for new PD cases has been used and validated in many previous studies (10,28,29).
Statistical Analysis
Basic characteristics of subjects are expressed as mean ± SD for continuous variables in each subgroup and as the number and percentage for categorical variables. The cumulative incidence of PD in each subgroup of subjects was calculated using the Kaplan-Meier curve, and statistical significance was assessed using the log-rank test. The incidence of PD among subjects was calculated by dividing the event occurrence in each group of the cohort by 1,000 person-years (PY). The hazard ratio (HR) and 95% CI for PD for each group were calculated using Cox proportional hazards analyses. Model 1 was used to calculate the unadjusted HR; model 2 was adjusted for age and sex; model 3 was further adjusted for the subject’s BMI, smoking status, drinking status, and physical activity; and model 4 was used to assess the competing risk of death (30). Further analyses were performed to consider changes in the glucose tolerance status of subjects during the observation period and to verify lagged effects on outcome. In addition, we examined the effect of diabetes on the incidence of PD by performing stratified analyses in consideration of cardiovascular disease, cerebrovascular disease, and CKD status of subjects. We also examined the differences in the incidence of PD based on baseline antidiabetic medication use in the subgroup with diabetes. Statistical analyses were performed using SAS 9.2 software (SAS Institute, Cary, NC). P < 0.05 was considered statistically significant.
Ethics Statement
This study was approved by the Kangbuk Samsung Medical Center Institutional Review Board (no. KBSMC 2018-12-016), Seoul, Korea. The Institutional Review Board waived the requirement for informed consent because deidentified information was used for the analyses.
Results
Baseline Characteristics
Baseline characteristics of the subjects are described in Table 1. The mean age increased with the deterioration of glucose tolerance. The proportion of men was higher in the IFG and diabetes groups than in the nondiabetes group. There were no pronounced differences in BMI between the groups, but waist circumference was significantly lower in the nondiabetes group. Fasting blood glucose levels of subjects significantly increased according to the deterioration of glucose tolerance. In addition, the proportion of subjects with a comorbidity, such as obesity, dyslipidemia, CKD, cardiovascular disease, and cerebrovascular disease, was also significantly different between groups. The characteristics of subjects not included in this study are also summarized in Supplementary Table 1.
Table 1.
Baseline characteristics of the study subjects
| Variables | No diabetes (n = 5,025,010) | IFG (n = 2,110,252) | Diabetes duration <5 years (n = 753,796) | Diabetes duration ≥5 years (n = 554,293) |
|---|---|---|---|---|
| Age (years) | 54.46 ± 10.74 | 56.25 ± 10.77 | 58.83 ± 10.5 | 62.78 ± 9.6 |
| Sex (male) | 2,249,965 (44.78) | 1,190,543 (56.42) | 445,971 (59.16) | 296,079 (53.42) |
| Height (cm) | 161.02 ± 8.84 | 162.2 ± 9.02 | 161.82 ± 9.08 | 160.57 ± 8.97 |
| Weight (kg) | 61.76 ± 10.36 | 64.85 ± 10.83 | 66.32 ± 11.19 | 63.79 ± 10.56 |
| BMI (kg/m2) | 23.74 ± 2.97 | 24.57 ± 3.07 | 25.26 ± 3.26 | 24.68 ± 3.14 |
| Waist circumference (cm) | 80.19 ± 8.44 | 83.2 ± 8.32 | 85.87 ± 8.32 | 85.24 ± 8.25 |
| SBP (mmHg) | 122.94 ± 15.3 | 127.55 ± 15.36 | 129.58 ± 15.69 | 129.12 ± 15.87 |
| DBP (mmHg) | 76.49 ± 10.04 | 79.03 ± 10.06 | 79.61 ± 10.08 | 77.59 ± 9.88 |
| Fasting glucose level (mg/dL) | 88.80 ± 7.20 | 108.11 ± 6.63 | 140.44 ± 40.74 | 143.7 ± 49.58 |
| Total cholesterol level (mg/dL) | 197.10 ± 35.70 | 203.45 ± 37.68 | 198.43 ± 41.57 | 187.45 ± 40.17 |
| Rural area | 2,805,985 (55.84) | 1,177,483 (55.8) | 423,630 (56.2) | 311,414 (56.18) |
| Current smoker | 918,695 (18.28) | 447,183 (21.19) | 180,542 (23.95) | 101,328 (18.28) |
| Heavy drinker | 243,283 (4.84) | 173,355 (8.21) | 66,944 (8.88) | 32,244 (5.82) |
| Regular exercise | 2,464,397 (49.04) | 1,053,945 (49.94) | 362,671 (48.11) | 260,368 (46.97) |
| Obesity | 1,575,978 (31.36) | 890,560 (42.2) | 383,711 (50.9) | 236,836 (42.73) |
| Dyslipidemia | 1,060,104 (21.1) | 609,149 (28.87) | 327,232 (43.41) | 260,207 (46.94) |
| CKD | 349,643 (6.96) | 183,634 (8.7) | 80,323 (10.66) | 96,463 (17.4) |
| Cardiovascular disease | 172,593 (3.43) | 92,993 (4.41) | 41,169 (5.46) | 42,072 (7.59) |
| Cerebrovascular disease | 82,856 (1.65) | 44,949 (2.13) | 17,601 (2.33) | 18,469 (3.33) |
| Metformin | — | — | 344,561 (45.71) | 395,444 (71.34) |
| Sulfonylurea | — | — | 311,217 (41.29) | 415,202 (74.91) |
| Meglitinide | — | — | 15,568 (2.07) | 28,137 (5.08) |
| Thiazolidinedione | — | — | 48,084 (6.38) | 74,590 (13.46) |
| Dipeptidyl peptidase 4 inhibitor | — | — | 46,881 (6.22) | 54,495 (9.83) |
| α-Glucosidase inhibitor | — | — | 63,981 (8.49) | 150,253 (27.11) |
| Insulin | — | — | 43,470 (5.77) | 101,585 (18.33) |
Data are presented as n (%) or mean ± SD. DBP, diastolic blood pressure; SBP, systolic blood pressure.
Cumulative Incidence Among Groups
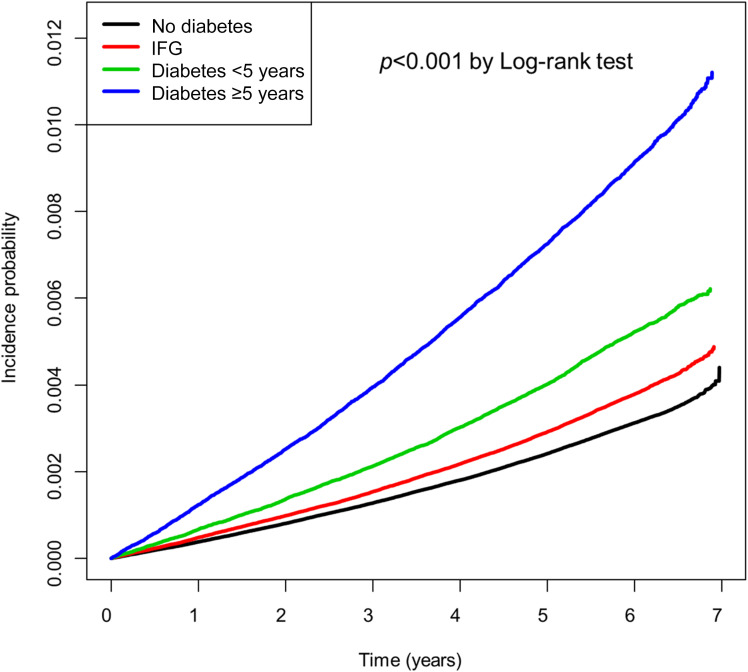
During the observation period of 49,076,148.74 PY, 362,560 deaths and 31,577 cases of PD were recorded (Table 2 and Supplementary Table 2). The incidence of death in all subjects was 7.39 per 1,000 PY, and the mortality rate among IFG and diabetes subjects was significantly higher than that among subjects without diabetes. The incidence of PD with or without diabetes was 0.558 and 1.134 per 1,000 PY, respectively (P < 0.001). When the subjects were compared according to glucose tolerance and diabetes duration, the incidence of PD was 0.521 in the nondiabetes group, 0.633 in the IFG group, 0.865 in the diabetes duration <5 years group, and 1.522 in the diabetes duration ≥5 years group. These results were statistically significant (P < 0.001). The cumulative incidence of PD in each group was calculated using the Kaplan-Meier curve (Fig. 2). The log-rank test also showed a significant difference in the incidence of PD between the groups (P < 0.001).
Table 2.
Comparison of the incidence of PD according to the subjects’ diabetes status
| Group | PYs | Events (n) | Incidence per 1,000 PY | HR (95% CI) | |||
|---|---|---|---|---|---|---|---|
| Model 1* | Model 2** | Model 3*** | Model 4**** | ||||
| No diabetes vs. diabetes | |||||||
| No diabetes | 41,773,272.48 | 23,299 | 0.55775 | 1.000 (ref.) | 1.000 (ref.) | 1.000 (ref.) | 1.000 (ref.) |
| Diabetes | 7,302,876.27 | 8,278 | 1.13353 | 2.036 (1.985–2.087) | 1.389 (1.354–1.424) | 1.372 (1.337–1.407) | 1.337 (1.304–1.374) |
| IFG vs. diabetes | |||||||
| IFG | 12,276,937.07 | 7,772 | 0.63306 | 1.000 (ref.) | 1.000 (ref.) | 1.000 (ref.) | 1.000 (ref.) |
| Diabetes | 7,302,876.27 | 8,278 | 1.13353 | 1.794 (1.739–1.850) | 1.356 (1.314–1.398) | 1.356 (1.315–1.399) | 1.329 (1.288–1.371) |
| Diabetes duration <5 years | 4,345,219.48 | 3,757 | 0.86463 | 1.366 (1.314–1.420) | 1.151 (1.107–1.197) | 1.153 (1.109–1.199) | 1.134 (1.090–1.179) |
| Diabetes duration ≥5 years | 3,122,274.66 | 4,752 | 1.52197 | 2.416 (2.331–2.505) | 1.581 (1.525–1.639) | 1.576 (1.520–1.634) | 1.538 (1.482–1.595) |
| All subgroups | |||||||
| No diabetes | 29,331,717.53 | 15,296 | 0.52148 | 1.000 (ref.) | 1.000 (ref.) | 1.000 (ref.) | 1.000 (ref.) |
| IFG | 12,276,937.07 | 7,772 | 0.63306 | 1.214 (1.181–1.248) | 1.049 (1.020–1.078) | 1.038 (1.009–1.067) | 1.037 (1.030–1.066) |
| Diabetes duration <5 years | 4,345,219.48 | 3,757 | 0.86463 | 1.657 (1.599–1.718) | 1.203 (1.161–1.247) | 1.185 (1.143–1.229) | 1.158 (1.115–1.202) |
| Diabetes duration ≥5 years | 3,122,274.66 | 4,752 | 1.52197 | 2.933 (2.839–3.030) | 1.646 (1.593–1.701) | 1.618 (1.566–1.672) | 1.571 (1.519–1.624) |
Model 1: nonadjusted.
Model 2: adjusted for age and sex.
Model 3: adjusted for factors in model 2 and BMI, smoking, drinking, and physical activity.
Model 4: considers competing risk of death in addition to the results of model 3.
Figure 2.
Cumulative incidence of PD according to glucose tolerance status.
HR and Adjusted HR for PD
The risk of PD was calculated for subjects with or without diabetes (Table 2). The nonadjusted HR for PD in the diabetes group was significantly higher (HR 2.036 [95% CI 1.985–2.087]) than in the group without diabetes. In the comparison between the four subgroups according to glucose tolerance and diabetes status, the HR was 1.214 (95% CI 1.181–1.248) for IFG, 1.657 (95% CI 1.599–1.718) for diabetes duration <5 years, and 2.933 (95% CI 2.839–3.030) for diabetes duration ≥5 years. The adjusted HRs (aHRs) for major clinical variables were also consistent. The aHR for diabetes was 1.389 (95% CI 1.354–1.424) in model 2 and 1.372 (95% CI 1.337–1.407) in model 3. In the analysis of the four subgroups, the aHR for IFG was 1.049 (95% CI 1.020–1.078) in model 2 and 1.038 (95% CI 1.009–1.067) in model 3. The aHR for diabetes duration <5 years was 1.203 (95% CI 1.161–1.247) in model 2 and 1.185 (95% CI 1.143–1.229) in model 3, whereas the aHR for diabetes duration ≥5 years was 1.646 (95% CI 1.593–1.701) in model 2 and 1.618 (95% CI 1.566–1.672) in model 3. After further consideration of the competing risk of death, these results remained consistent. The aHRs for PD of subjects with diabetes estimated based on subjects with IFG also showed consistent results (Table 2). Further analyses of changes in the glucose tolerance status of subjects and lagged effects on outcomes also showed consistent results (Supplementary Tables 3 and 4).
Additional Analyses Considering Major Comorbidities
The HR for PD was further analyzed based on the subjects’ major comorbidities and the presence of diabetes (Supplementary Table 5). These results indicated that the presence of cardiovascular disease, cerebrovascular disease, and CKD among the subjects significantly increased the HR for PD. Moreover, the presence of diabetes further increased the HR for PD regardless of the subjects’ comorbidities. The aHR for subjects without baseline cardiovascular disease but with diabetes was 1.356 (95% CI 1.296–1.419) in model 3, whereas that for subjects with baseline cardiovascular disease and diabetes was 1.622 (95% CI 1.514–1.738). Furthermore, the aHR for subjects without baseline cerebrovascular disease but with diabetes was 1.565 (95% CI 1.470–1.667) in model 3, whereas that for subjects with baseline cerebrovascular disease and diabetes was 1.816 (95% CI 1.643–2.008). In addition, the aHR for PD among subjects with a baseline eGFR ≥90 mL/min/1.73 m2 and diabetes was 1.115 (95% CI 1.080–1.151) in model 3, that for subjects with an eGFR 45–60 mL/min/1.73 m2 and diabetes was 1.512 (95% CI 1.453–1.574), and that for subjects with an eGFR <45 mL/min/1.73 m2 and diabetes was 1.934 (95% CI 1.762–2.123). In the analysis of the diabetes subgroup only, the HR for PD was significantly higher regardless of the presence of comorbidities (Supplementary Fig. 1).
aHR of PD According to Antidiabetic Medication Use
We estimated the HR and aHR for the incidence of PD based on baseline antidiabetic medication use in the diabetes subgroup (Supplementary Table 6). All antidiabetic medications significantly increased the HR for PD. In particular, the aHR with insulin was 1.599 (95% CI 1.506–1.698) in model 3, which was higher than that with other oral medications.
Conclusions
In this study, we analyzed data from 8,443,351 subjects in the NHIS cohort with an average observation period of 6.3 years and identified the risk of PD according to baseline glucose tolerance status. We found that the risk of PD significantly increased with the degree of hyperglycemia and the duration of diabetes. In particular, our findings indicated that the risk of PD was significantly increased not only in subjects with diabetes but also in subjects with IFG. These results were consistent after adjusting for various confounders and the competing risk of death.
Diabetes has been suggested to increase the risk of PD; however, a definite conclusion has not yet been reached in this regard. This is because the results of many studies are controversial. In a prospective analysis of ∼290,000 patients in the U.S. NIH-AARP Diet and Health Study, the odds ratio of PD in patients with diabetes was 1.41 (95% CI 1.20–1.66) (12). In a retrospective study using data from ∼140,000 subjects from the National Health Insurance Research Database in Taiwan, the aHR for the incidence of PD in the cohort with diabetes was 1.19 (95% CI 1.08–1.32) (13). A recent U.K. large-scale claim-based study also found that the HR for PD in a cohort with diabetes was 1.32 (95% CI 1.29–1.35) (14). However, a prospective cohort study of ∼140,000 patients in the Cancer Prevention Study II Nutrition Cohort revealed that the combined relative risk of PD among patients with a history of diabetes was 0.88 (95% CI 0.62–1.25) (15). Although these cohort findings had the advantage of identifying causality, these studies were designed with the aim of identifying any causality between diabetes and PD as the primary goal.
Similarly, conflicting results have been reported in meta-analyses. In one study, the relative risk of PD among individuals with a past history of diabetes estimated based on four cohort studies (total of 32,695 subjects with diabetes and 501,239 subjects without diabetes) was significantly higher (1.37; 95% CI 1.21–1.55) than the risk among subjects without diabetes (16). In another meta-analysis based on seven population-based cohorts (total of 1,761,632 subjects), the adjusted relative risk for PD among patients with diabetes was 1.38 (95% CI 1.18–1.62), which was significantly higher than the risk among subjects without diabetes (17). However, in a study conducted by Cereda et al. (16), the odds ratio of PD among subjects with diabetes estimated from case–control studies (1,387 subjects with diabetes and 6,953 PD subjects from a total of 22,359 subjects) was 0.75 (95% CI 0.50–1.11). In addition, another meta-analysis based on 14 studies (21,397 PD subjects and 84,579 control subjects) showed that diabetes had a negative association (odds ratio 0.75; 95% CI 0.58–0.98) with PD (18). These meta-analyses were limited in that the designs of the studies included in the analysis were heterogeneous. Thus, these results imply that more research is needed to confirm any causal relationship between diabetes and the incidence of PD.
Our results provide further evidence supporting previous findings that the risk of PD is increased in patients with diabetes. In particular, our finding that the risk of PD increases owing to IFG, a nondiabetic hyperglycemic status, is a novel finding. From this result, it is possible to deduce that the incidence of PD is proportional to the degree or duration of exposure to hyperglycemia rather than simply being dependent on the presence of diabetes per se. In fact, a longer diabetes duration has been identified as an important factor that significantly increases the risk of PD in diabetes (31). Thus, the increased risk of PD in subjects with prediabetes and diabetes is likely to vary according to the degree of glycemic burden. Therefore, our findings may indirectly explain why previous studies have found conflicting results.
The mechanism underlying the association of diabetes with the increased incidence of PD has not yet been elucidated. However, mitochondrial dysfunction, endoplasmic reticulum stress, chronic low-grade inflammation, and alterations in metabolism are thought to cause insulin resistance and ultimately neurodegenerative disorders as well as diabetes (11,32). One study confirmed the upregulation of amyloid precursor protein levels before an increase in insulin resistance and neurodegeneration, confirming the existence of a shared, dysregulated molecular pathway between the two diseases (33).
These postulated mechanisms suggest that antidiabetic interventions, including the improvement of insulin resistance, may help achieve good clinical outcomes among subjects at high risk of developing neurodegenerative disorders, including PD, among those with diabetes and prediabetes. Recently, a double-blind, randomized, controlled study on the effects of glucagon-like peptide 1 agonists in PD subjects showed a positive effect in the intervention group (34). However, these findings are still difficult to generalize, and therefore require further validation through larger-scale intervention and long-term follow-up studies. In addition, all antidiabetic medication users in this study had a high aHR for PD. However, subjects’ use of antidiabetic medications should be interpreted as reflecting their insulin resistance and high glycemic burden. Care should be taken while interpreting the results, as drug use does not imply a substantial intervention in the disease mechanism during the observation period in subjects.
This study has some limitations. First, because this study was based on claims and health checkup data, the sampling is likely to be inaccurate in contrast to that in a directly sampled cohort. Second, there were many missing variables that could affect the analysis results. Third, owing to the nature of national health screening, prediabetes status, other than IFG, was not considered sufficiently. Fourth, the use of medical services may vary based on the health priorities of subjects according to the baseline comorbid condition, thus leading to differences in the rate of diagnosis of PD. Finally, the exact mechanism underlying the increased PD risk in subjects with diabetes is difficult to ascertain based on the findings.
This study was, however, based on a large cohort representative of the entire population of Korea, and such large-scale cohort studies are extremely rare. In addition, this study overcame the simplicity of claim-based research and used detailed characteristics of the subjects based on clinical information such as anthropometric parameters, past medical history, lifestyle, and blood chemistry results. Moreover, it is possible to make accurate outcome judgments using the V124 special code, as well as the ICD-10 diagnostic code, based on characteristics of the Korean health insurance system. This system allowed us to selectively exclude PD subjects from the baseline cohort and to determine exact outcomes. In addition to the large number of subjects, an average of 6.3 years of observation allowed us to more rigorously examine the causality of dysglycemia and PD in contrast to other studies. Thus, the results of this study suggest that diabetes and prediabetes are both important factors contributing to the incidence of PD.
In conclusion, our population-based cohort study clearly suggests that diabetes is an independent risk factor for the development of PD. In the future, more detailed mechanistic studies on the relationship between diabetes and PD should be performed, and an effective method for the prevention of PD should be determined.
Article Information
Acknowledgments. The authors thank Jeong-Taek Woo and Young Seol Kim of Kyung Hee University for their exceptional teaching and inspiration, which encouraged the authors to conduct the current study. This study used the National Health Screening Cohort data (NHIS-2019-1-133) from the National Health Insurance Service.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. S.Y.R. drafted the manuscript and interpreted the results. K.-D.H. analyzed and interpreted the results. H.K., S.-E.P., Y.-G.P., Y.-H.K., and S.-J.Y. participated in the study design and interpreted the results. E.-J.R. and W.-Y.L. conceived the study and finally approved the manuscript. W.-Y.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12397889.
S.Y.R. and K.-D.H. contributed equally to this study as first authors.
References
- 1.Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271–281 [DOI] [PubMed] [Google Scholar]
- 2.Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol 2018;14:88–98 [DOI] [PubMed] [Google Scholar]
- 3.Yoon J, Oh IH, Seo H, et al. Disability-adjusted life years for 313 diseases and injuries: the 2012 Korean Burden of Disease Study. J Korean Med Sci 2016;31(Suppl. 2):S146–S157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrésdóttir G, Jensen ML, Carstensen B, et al. Improved survival and renal prognosis of patients with type 2 diabetes and nephropathy with improved control of risk factors. Diabetes Care 2014;37:1660–1667 [DOI] [PubMed] [Google Scholar]
- 5.Lamberts SW, Romijn JA, Wiersinga WM. The future endocrine patient. Reflections on the future of clinical endocrinology. Eur J Endocrinol 2003;149:169–175 [DOI] [PubMed] [Google Scholar]
- 6.Poewe W, Seppi K, Tanner CM, et al. Parkinson disease. Nat Rev Dis Primers 2017;3:17013. [DOI] [PubMed] [Google Scholar]
- 7.Kalia LV, Lang AE. Parkinson’s disease. Lancet 2015;386:896–912 [DOI] [PubMed] [Google Scholar]
- 8.GBD 2016 Parkinson’s Disease Collaborators Global, regional, and national burden of Parkinson’s disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018;17:939–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol 2016;15:1257–1272 [DOI] [PubMed] [Google Scholar]
- 10.Nam GE, Kim SM, Han K, et al. Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med 2018;15:e1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson’s disease and diabetes. Trends Mol Med 2013;19:176–186 [DOI] [PubMed] [Google Scholar]
- 12.Xu Q, Park Y, Huang X, et al. Diabetes and risk of Parkinson’s disease. Diabetes Care 2011;34:910–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang YW, Hsieh TF, Li CI, et al. Increased risk of Parkinson disease with diabetes mellitus in a population-based study. Medicine (Baltimore) 2017;96:e5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagano G, Polychronis S, Wilson H, et al. Diabetes mellitus and Parkinson disease. Neurology 2018;90:e1654–e1662 [DOI] [PubMed] [Google Scholar]
- 15.Palacios N, Gao X, McCullough ML, et al. Obesity, diabetes, and risk of Parkinson’s disease. Mov Disord 2011;26:2253–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cereda E, Barichella M, Pedrolli C, et al. Diabetes and risk of Parkinson’s disease: a systematic review and meta-analysis. Diabetes Care 2011;34:2614–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yue X, Li H, Yan H, Zhang P, Chang L, Li T. Risk of Parkinson disease in diabetes mellitus: an updated meta-analysis of population-based cohort studies. Medicine (Baltimore) 2016;95:e3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu L, Fu DL, Li HQ, Liu AJ, Li JH, Zheng GQ. Diabetes and risk of Parkinson’s disease: an updated meta-analysis of case-control studies. PLoS One 2014;9:e85781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin DW, Cho B, Guallar E. Korean National Health Insurance Database. JAMA Intern Med 2016;176:138. [DOI] [PubMed] [Google Scholar]
- 20.Seong SC, Kim YY, Park SK, et al. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017;7:e016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee YH, Han K, Ko SH, Ko KS, Lee KU; Taskforce Team of Diabetes Fact Sheet of the Korean Diabetes Association . Data analytic process of a nationwide population-based study using National Health Information Database established by National Health Insurance Service. Diabetes Metab J 2016;40:79–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ko SH, Han K, Lee YH, et al.; TaskForce Team for the Diabetes Fact Sheet of the Korean Diabetes Association . Past and current status of adult type 2 diabetes mellitus management in Korea: a National Health Insurance Service database analysis. Diabetes Metab J 2018;42:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.American Diabetes Association 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41(Suppl. 1):S13–S27 [DOI] [PubMed] [Google Scholar]
- 24.Agarwal DP. Cardioprotective effects of light-moderate consumption of alcohol: a review of putative mechanisms. Alcohol Alcohol 2002;37:409–415 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Coresh J, Greene T, et al.; Chronic Kidney Disease Epidemiology Collaboration . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate [published correction appears in Ann Intern Med 2008;149:519]. Ann Intern Med 2006;145:247–254 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Coresh J, Balk E, et al.; National Kidney Foundation . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification [published correction appears in Ann Intern Med 2003;139:605]. Ann Intern Med 2003;139:137–147 [DOI] [PubMed] [Google Scholar]
- 27.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 1988;51:745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JH, Kim DH, Park YG, et al. Cancer risk in patients with Parkinson’s disease in South Korea: a nationwide, population-based cohort study. Eur J Cancer 2019;117:5–13 [DOI] [PubMed] [Google Scholar]
- 29.Nam GE, Kim NH, Han K, et al. Chronic renal dysfunction, proteinuria, and risk of Parkinson’s disease in the elderly. Mov Disord 2019;34:1184–1191 [DOI] [PubMed] [Google Scholar]
- 30.Andersen PK, Geskus RB, de Witte T, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol 2012;41:861–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Pablo-Fernandez E, Sierra-Hidalgo F, Benito-León J, Bermejo-Pareja F. Association between Parkinson’s disease and diabetes: data from NEDICES study. Acta Neurol Scand 2017;136:732–736 [DOI] [PubMed] [Google Scholar]
- 32.Craft S, Watson GS. Insulin and neurodegenerative disease: shared and specific mechanisms. Lancet Neurol 2004;3:169–178 [DOI] [PubMed] [Google Scholar]
- 33.Santiago JA, Potashkin JA. Integrative network analysis unveils convergent molecular pathways in Parkinson’s disease and diabetes. PLoS One 2013;8:e83940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Athauda D, Maclagan K, Skene SS, et al. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet 2017;390:1664–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]