Abstract
OBJECTIVE
The first-appearing β-cell autoantibody has been shown to influence risk of type 1 diabetes (T1D). Here, we assessed the risk of autoantibody spreading to the second-appearing autoantibody and further progression to clinical disease in The Environmental Determinants of Diabetes in the Young (TEDDY) study.
RESEARCH DESIGN AND METHODS
Eligible children with increased HLA-DR-DQ genetic risk for T1D were followed quarterly from age 3 months up to 15 years for development of a single first-appearing autoantibody (GAD antibody [GADA], insulin autoantibody [IAA], or insulinoma antigen-2 autoantibody [IA-2A]) and subsequent development of a single second-appearing autoantibody and progression to T1D. Autoantibody positivity was defined as positivity for a specific autoantibody at two consecutive visits confirmed in two laboratories. Zinc transporter 8 autoantibody (ZnT8A) was measured in children who developed another autoantibody.
RESULTS
There were 608 children who developed a single first-appearing autoantibody (IAA, n = 282, or GADA, n = 326) with a median follow-up of 12.5 years from birth. The risk of a second-appearing autoantibody was independent of GADA versus IAA as a first-appearing autoantibody (adjusted hazard ratio [HR] 1.12; 95% CI 0.88–1.42; P = 0.36). Second-appearing GADA, IAA, IA-2A, or ZnT8A conferred an increased risk of T1D compared with children who remained positive for a single autoantibody, e.g., IAA or GADA second (adjusted HR 6.44; 95% CI 3.78–10.98), IA-2A second (adjusted HR 16.33; 95% CI 9.10–29.29; P < 0.0001), or ZnT8A second (adjusted HR 5.35; 95% CI 2.61–10.95; P < 0.0001). In children who developed a distinct second autoantibody, IA-2A (adjusted HR 3.08; 95% CI 2.04–4.65; P < 0.0001) conferred a greater risk of progression to T1D as compared with GADA or IAA. Additionally, both a younger initial age at seroconversion and shorter time to the development of the second-appearing autoantibody increased the risk for T1D.
CONCLUSIONS
The hierarchical order of distinct autoantibody spreading was independent of the first-appearing autoantibody type and was age-dependent and augmented the risk of progression to T1D.
Introduction
β-cell autoantibodies are predictors of type 1 diabetes (T1D) (1,2) and are currently the most reliable indicator of preclinical disease. The presence of two or more autoantibodies against the four major autoantigens (insulin, GAD, insulinoma antigen-2 [IA-2], and zinc transporter 8 [ZnT8]) have been shown to confer the highest risk of T1D and was recently defined as stage 1 T1D (3). β-cell autoantibodies develop prior to and usually persist over time in the progression to clinical onset of T1D (stage 3). The age at appearance of specific β-cell autoantibodies varies (4–6). GAD antibody (GADA) or insulin autoantibody (IAA) predominate as first-appearing autoantibodies (7,8). IAA appearance peaks within the first 2 years of life. In contrast, GADA appears between ages 3 and 5 years in children with either increased genetic risk or family history of T1D (7–9). IA-2 autoantibody (IA-2A) and ZnT8 autoantibody (ZnT8A) generally appear after the initial islet autoantibody seroconversion (6,8,10,11) in more advanced stages of the disease process (3,12–14).
Recent studies have focused on the appearance of the first-appearing autoantibody (7,8) with limited discussion of the specific second-appearing autoantibody (8). The Finnish T1D Prediction and Prevention (DIPP) study (8) reported that IAA as a second-appearing autoantibody peaked in the first 5 years of life and followed a similar pattern to the first-appearing GADA, whereas GADA peaked within the first 2 years of life and mostly occurred after IAA. More recently, the DIPP study reported that the initial age at seroconversion was only associated with IAA-initiated seroconversion and further progression to T1D (15). However, because of a varied autoantibody screening interval, further exploration after the first 2 years of life was limited. Other studies assessing the presence of autoimmunity and combinations of autoantibodies were carried out where the actual order of appearance could not be distinguished because of annual screening (16,17) or capture was at the time of or just prior to diagnosis (1,11,18–22). These previous studies assessing risk from single to multiple autoantibodies showed that increased risk was associated with a younger age, HLA-DR-DQ, and IAA as the first-appearing autoantibody (16,17). In addition to HLA class II risk, a recent study from the Belgian Diabetes Registry (23) reported that specific HLA class I alleles accelerated progression from multiple autoantibody positivity to T1D in those <40 years with a family history of T1D. Specifically, HLA-A*24 was associated with an accelerated progression in HLA-DQ8+ relatives who developed either IA-2A or ZnT8A, whereas HLA-B*18 was associated with accelerated progression in HLA-DQ2+ relatives who developed both IAA and GADA (23). Moreover, non-HLA single nucleotide polymorphisms (SNPs), such as those in the INS-VNTR gene region (rs1004446) and protein tyrosine phosphatase nonreceptor type 22 (PTPN22, rs2476601) have been shown to associate with a decreased and increased risk of progression to islet autoimmunity and T1D, respectively (24,25). Thus, understanding the order and time of appearance of each distinct autoantibody will allow for better stratification of risk.
The aims of this study were 1) to assess the risk of developing a distinct second islet autoantibody in children at increased genetic risk for T1D who newly seroconverted for either IAA-only or GADA-only first-appearing autoantibodies and 2) to determine the risk associated with the specific target of the first-and second-appearing autoantibody combination on further progression to clinical T1D.
Research Design and Methods
Study Population
The Environmental Determinants of Diabetes in the Young (TEDDY) is a prospective cohort study of children at an increased genetic risk for T1D funded by the National Institutes of Health. The TEDDY study seeks to identify environmental causes of T1D. There are six clinical research centers: three in the U.S. (Colorado, Georgia/Florida, and Washington) and three in Europe (Finland, Germany, and Sweden). The high-risk genotypes for subjects screened from the general population with no family history of T1D (89%) were as follows: DRB1*04-DQA1*03-DQB1*03:02/DRB1*03-DQA1*05-DQB1*02:01 (DR3/4-Q2/8), DRB1*04-DQA1*03-DQB1*03:02/DRB1*04-DQA1*03-DQB1*03:02 (DR4/4-DQ8/8), DRB1*04-DQA1*03-DQB1*03:02/DRB1*08-DQA1*04-DQB1*04:02 (DR4/8-DQ8/4), and DRB1*03-DQA1*05-DQB1*02:01/DRB1*03-DQA1*05-DQB1*02:01 (DR3/3-DQ2/2) and six additional genotypes in first-degree relatives (FDRs) of those with a family history of T1D as previously described (26). There were 8,676 children enrolled from September 2004 to February 2010 and currently followed prospectively from age 3 months to 15 years with study visits every 3 months until 4 years and every 3 or 6 months, thereafter, depending on autoantibody positivity. All children who are persistently positive for any autoantibody are followed every 3 months until the age of 15 years or onset of T1D. Detailed study design and methods have been published previously (26–28). The protocol was approved by institutional review boards at participating centers, and all participants provided written informed consent before participation in the genetic screening and enrollment.
β-Cell Autoantibodies
β-cell autoantibodies to IAA, GADA, or IA-2A were measured in two laboratories by radiobinding assays (27,29). In the U.S., all sera were assayed at the Barbara Davis Center for Childhood Diabetes at the University of Colorado Denver; in Europe, all sera were assayed at the University of Bristol, in the U.K. Both laboratories reported high sensitivity, specificity, and concordance (29,30). All positive β-cell autoantibodies and 5% of negative samples were retested in the other reference laboratory and deemed confirmed if concordant. Autoantibody positivity (persistent confirmed) was defined as a specific autoantibody presence on greater than or equal to two consecutive visits 3 months apart and confirmed in two TEDDY laboratories. The date of positivity was the draw date of the first sample of the two consecutive positive samples that deemed a child persistent confirmed positive for a specific autoantibody. The age of seroconversion was the age of the child on the initial date of seroconversion to the first persistent confirmed autoantibody. The age of second autoantibody seroconversion was the age of the child on the date of autoantibody seroconversion of a second persistent confirmed autoantibody. Stage 1 T1D was defined as the presence of multiple autoantibodies (3). Symptomatic T1D (stage 3) was defined according to American Diabetes Association criteria for diagnosis (3,31). There were 8,502 of the 8,676 children eligible for this analysis; 174 were ineligible for this analysis and excluded due to not having an autoantibody test result (n = 54) or were deemed HLA-DR-DQ ineligible based on study criteria (n = 120) (Supplementary Fig. 1 and Supplementary Table 1). As the focus of this analysis was to examine autoantibody spreading, the analyses were restricted to the development of the first-appearing IAA single (n = 282) or GADA single (n = 326) as of 31 December 2018 and followed through 31 December 2019, excluding children who developed multiple autoantibodies (greater than or equal to two autoantibodies) at their initial seroconversion (n = 156) or IA-2A only (n = 15), or ZnT8A only (n = 12). There were 608 children included in these analyses.
Zinc Transporter 8 Autoantibodies
ZnT8A radioimmunoassay was performed as previously described (19). The ZnT8 protein was produced by in vitro transcription and translation (TNT kit; Promega, Madison, WI) and labeled with [35S]methionine (Perkin Elmer, Waltham, MA). [35S]ZnT8 (20,000 cpm) was mixed and incubated overnight at 4°C with 2-µL serum in an assay buffer (20 mmol/L Tris HCl, pH 7.4, supplemented with 150 mmol/L NaCl, 0.1% [w/v] BSA, 0.15% [v/v] Tween-20 and 0.1% [w/v] sodium azide) at a final 1:25 dilution. Autoantibody-bound antigen was precipitated with 25-µL 50% protein A-Sepharose (GE HealthCare, Piscataway, NJ) in an opaque 96-well filtration plate (Corning, Tewksbury, MA) and washed with two cycles of four washes per each cycle of cold assay buffer using a vacuum-operated 96-well plate washer (BioTek, Winooski, VT). After washing, scintillation fluid, MicroScint-20 (Perkin Elmer), was added directly to the 96-well plate, and radioactivity was counted on a TopCount 96-well plate β-counter (Perkin Elmer). The results are expressed as an index: index = (sample CPM − negative control CPM)/(positive control CPM − negative control CPM). The upper limit of normal (0.020) was established as the 99th percentile from the receiver operating characteristic curves in 100 healthy control subjects and 50 patients with new-onset diabetes. In the most recent Islet Autoantibody Standardization Program (2017) workshop, the sensitivity and specificity were 66 and 100%, respectively. ZnT8A was run on all available samples at the TEDDY Barbara Davis Laboratory, if a child was deemed antibody positive for IAA, GADA, and/or IA-2A (any number of autoantibodies, any autoantibody, persistence or confirmation not required) at a visit. Persistent ZnT8A was defined as the presence of ZnT8A on greater than or equal to two consecutive visits 3 months apart in a single laboratory. Protocol compliance for measurement of ZnT8A prior to the first autoantibody positive sample in children with persistent IAA, IA-2A, or GADA was 99%. For children who did not meet protocol compliance (n = 9), the median months following the first autoantibody positive sample was 3.7 months.
Statistical Analysis
Cumulative incidence of the appearance of the second autoantibody and the risk of progression to T1D were examined in Kaplan-Meier analyses. Multivariable proportional hazards (PH) models were applied to assess both the cause-specific risk of autoantibody spreading to a second-appearing autoantibody and the risk of progression to T1D from the second-appearing autoantibody. Each specific second-appearing autoantibody was also examined as a time-varying predictor on risk of T1D. To ensure that the second-appearing autoantibody was compared with single autoantibody–positive children in each risk set, the other single second-appearing autoantibodies or multiple second-appearing autoantibodies (two autoantibodies appearing during the same 3-month interval) were censored at the time of their appearance as defined above. The strength of the associations with T1D was described by hazard ratios (HRs) and with second-appearing autoantibodies by cause-specific hazard ratios (CHRs) with 95% CIs. All multivariable analyses were adjusted for HLA-DR-DQ, sex, family history of T1D, age at initial seroconversion, and first-appearing autoantibody (IAA or GADA). The following also considered for adjustment (and remained in the final model if P < 0.10) were HLA class I alleles (HLA-A*24:X, -B*18:X:X) (23), SLC30A8 (rs13266634) (32), SNPs previously shown to be associated with islet autoimmunity and T1D in TEDDY (INS [rs1004446], PTPN22 [rs2476601], ERBB3 [rs2292239], SH2B3 [rs3184504], TNFAIP3 [rs2327832], rs1534422, and rs10517086) (5,24,25,33) and characteristics found to be associated with islet autoimmunity and T1D (34–37), unless otherwise stated. Country of residence was included in all models as a stratified variable. Adjustments for population stratification were made by using the top two principal components from the Immunochip SNP data as covariates in the PH model (38). Data were analyzed using the SAS Software (version 9.4; SAS Institute, Cary, NC) and GraphPad PRISM 7.04 (GraphPad Software Inc., San Diego, CA) for figures. Two-tailed P values <0.05 were considered significant.
Results
As of 31 December 2018, 608 of the 8,502 enrolled children with HLA-DR-DQ genetic risk for T1D in TEDDY developed a single positive IAA or GADA at their initial seroconversion and met the inclusion criteria for this analysis: IAA only (n = 282) and GADA only (n = 326). Of those who developed first-appearing IAA or GADA only (n = 608), 272 (44.7%) remained positive for a single autoantibody for the duration of follow-up through 31 December 2019, and 10.3% (n = 28/272) of these single autoantibody–positive children progressed to T1D within 0.95 (0.28–3.37) years; 55.3% (n = 336/608) developed another autoantibody, and 53.0% (n = 178/336) of these children progressed to T1D within 3.53 (1.56–5.83) years. The median (interquartile range [IQR]) age at initial GADA- or IAA-only seroconversion was 4.28 (2.27–7.51) and 1.83 (1.00–3.82) years, respectively. The median (IQR) follow-up from the initial seroconversion was 5.7 (3.2–8.7) years with the majority (98%) followed for ≥2 years.
Time From First- to Second-Appearing Autoantibody
The characteristics of the children by specific second-appearing autoantibody are shown in Supplementary Table 2, and the age-descriptive patterns of the second-appearing autoantibody are shown in Supplementary Fig. 2. GADA as a second-appearing autoantibody developed at a median (IQR) of 3.7 (3.0–9.7) months after the first-appearing autoantibody; IAA as a second-appearing autoantibody developed at a median of 5.9 (3.0–25.9) months; IA-2A as a second-appearing autoantibody developed at a median of 6.9 (3.7–17.6) months; and ZnT8A as a second-appearing autoantibody developed at a median of 13.3 (6.3–23.7) months after the first-appearing autoantibody. The overall median (Q1-Q3) time after the first-appearing autoantibody to the appearance of the second autoantibody was 6.8 (3.2–17.0) months. There was no significant difference in the time from the first- to second-appearing autoantibody by the type of first-appearing IAA or GADA, taking into account the age at the initial seroconversion.
Risk Factors Associated With the Development of a Second-Appearing Autoantibody
Known T1D risk factors were examined for univariate association with developing a second autoantibody (Supplementary Table 3). Family history of T1D was positively associated with the development of a second autoantibody, whereas having a minor allele in the INS-VNTR gene region (rs1004446) or HLA-DR3/3 was inversely associated with developing IAA or GADA as a second autoantibody. The age at initial seroconversion was inversely associated with the development of a second autoantibody in the multivariable analyses (Table 1). Of interest, the risk of developing a second autoantibody declined with increasing age at initial seroconversion (for each additional month older, adjusted HR 0.986; 95% CI 0.983–0.990; P < 0.0001).
Table 1.
Multivariable CHRs in children with a single first-appearing IAA or GADA positivity (n = 608) for progression to second autoantibody positivity (n = 336ǂ)
| Second-appearing autoantibody | ||||||
|---|---|---|---|---|---|---|
| GADA or IAA | IA-2A | ZnT8A | ||||
| CHR* (95% CI) | P value | CHR* (95% CI) | P value | CHR* (95% CI) | P value | |
| Female vs. male | 0.90 (0.65–1.23) | 0.50 | 1.12 (0.67–1.87) | 0.67 | 0.64 (0.38–1.07) | 0.09 |
| Family history of T1D (reference = no family history) | ||||||
| Sibling with T1D | 2.22 (1.12–4.40) | 0.023 | 0.86 (0.12–6.43) | 0.89 | 1.93 (0.57–6.48) | 0.29 |
| Father with T1D | 1.35 (0.82–2.23) | 0.24 | 1.54 (0.71–3.35) | 0.27 | 2.15 (1.04–4.46) | 0.039 |
| Mother with T1D | 0.80 (0.31–2.10) | 0.65 | 2.88 (1.06–7.84) | 0.039 | 2.15 (0.66–7.08) | 0.21 |
| HLA-DR-DQ genotype (reference = DR4/4) | ||||||
| DR3/4 | 1.26 (0.83–1.93) | 0.28 | 1.21 (0.58–2.52) | 0.62 | 1.20 (0.56–2.54) | 0.64 |
| DR4/8 | 0.55 (0.29–1.01) | 0.05 | 1.74 (0.76–4.01) | 0.19 | 1.01 (0.40–2.57) | 0.98 |
| DR3/3 | 0.32 (0.16–0.67) | 0.003 | 0.69 (0.26–1.85) | 0.46 | 0.96 (0.40–2.30) | 0.93 |
| FDR-specific** | 1.01 (0.35–2.89) | 0.98 | 0.38 (0.04–3.30) | 0.38 | NA | 0.98 |
| SNP rs1004446_A (INS) | 0.70 (0.51–0.96) | 0.025 | 0.94 (0.56–1.57) | 0.81 | 1.03 (0.62–1.72) | 0.90 |
| First-appearing Ab (GADA vs. IAA) | 0.94 (0.67–1.33) | 0.73 | 1.23 (0.69–2.20) | 0.48 | 1.62 (0.91–2.86) | 0.09 |
| Age at first-appearing autoantibody | 0.99 (0.98–0.99) | <0.0001 | 0.98 (0.97–0.99) | 0.0002 | 0.99 (0.98–99) | 0.003 |
Boldface type indicates statistical significance. NA, not applicable.
Stratified Cox regression with country of residence.
FDR-specific HLA-DR-DQ genotypes are the following: DR4/1, DR4/9, DR4/13, DR3/9, DR4/4-DQB1*20X, and DR4/4-DQB1*304.
47 children developed two autoantibodies within the same 3-month interval as second-appearing autoantibodies and were censored at time of second-appearing autoantibody positivity. Data were not presented due to difficulty in determining order of appearance.
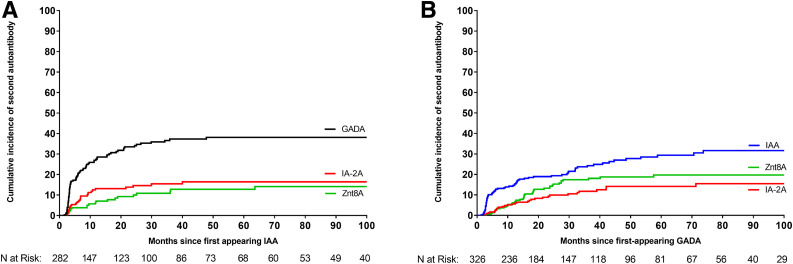
From the time of the first-appearing autoantibody, the risk of appearance of any second autoantibody did not differ if GADA or IAA was the first-appearing autoantibody (adjusted HR 1.12; 95% CI 0.88–1.42; P = 0.36), independent of age at initial seroconversion. Further assessment did not find any specific second autoantibody associated with a specific first-appearing autoantibody (IAA or GADA) after the Bonferroni correction for significance at P < 0.008 for multiple comparisons (Fig. 1A and B). Overall, there was no significant difference based on either GADA only or IAA only as the first-appearing autoantibody on the risk of developing IA-2A (adjusted CHR 1.23; 95% CI 0.69–2.20; P = 0.48) or ZnT8A (adjusted CHR 1.62; 95% CI 0.91–2.86; P = 0.09) as a second-appearing autoantibody.
Figure 1.
Cumulative incidence in children with distinct single first-appearing autoantibody positivity (IAA or GADA) for progression to distinct single second-appearing autoantibody positivity. A: The cumulative incidence of the second-appearing autoantibody by months since first-appearing IAA. B: The cumulative incidence of the second-appearing autoantibody by months since first-appearing GADA. The lines represent IAA (blue line), GADA (black line), IA-2A (red line), and ZnT8A (green line).
Influence of a Second Autoantibody on Risk of Progression to T1D
We next asked if the appearance of the second autoantibody (time-varying predictor) from the time of the first-appearing autoantibody influenced progression to T1D. Children who developed a distinct second-appearing autoantibody had an increased risk of progression to T1D as compared with children who remained positive for a single autoantibody for IAA or GADA second (adjusted HR 6.44; 95% CI 3.78–10.98; P < 0.0001), IA-2A second (adjusted HR 16.33; 95% CI 9.10–29.29; P < 0.0001), and ZnT8A second (adjusted HR 5.35; 95% CI 2.61–10.95; P < 0.0001) from age of first-appearing autoantibody, adjusting for age at initial seroconversion, sex, family history of T1D, HLA-DR-DQ, INS-VNTR gene region (rs1004446), and type of first-appearing autoantibody. No other HLA class I alleles or non-HLA SNPs assessed were found to significantly associate with developing a specific second autoantibody.
Risk Factors Associated With T1D in Children With Multiple Autoantibodies
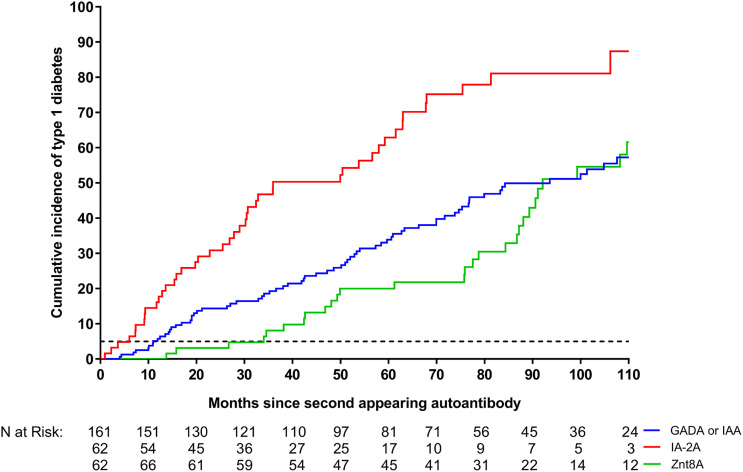
In children who developed multiple autoantibodies, IA-2A, as a second-appearing autoantibody, conferred a significantly greater risk of progression to T1D (adjusted HR 3.08; 95% CI 2.04–4.65; P < 0.0001) (Table 2 and Fig. 2) as compared with IAA or GADA. There was no statistical evidence that the appearance of ZnT8A as a second autoantibody as compared with IAA or GADA modified risk of progression to T1D (adjusted HR 0.81; 95% CI 0.51–1.29; P = 0.37). Other covariates significantly associated with progression in children who developed multiple autoantibodies were age at the appearance of the first autoantibody (adjusted HR 0.98; 95% CI 0.97–0.99; P = 0.001), time in months from the first to second autoantibody (adjusted HR 0.97; 95% CI 0.96–0.99; P = 0.003), and sex of the child (female versus male, adjusted HR 1.52; 95% CI 1.09–2.13; P = 0.014). Further assessment of specific HLA class I alleles and non-HLA SNPs (including interactions) previously shown to be associated with T1D were not found significantly associated with progression in this population with increased HLA-DR-DQ genetic risk.
Table 2.
Multivariable proportional HRs in children with a distinct second-appearing autoantibody (n = 289) for progression to T1D (n = 149)
| HR (95% CI) | P values | |
|---|---|---|
| Type of second antibody | ||
| IAA or GADA | 1 (reference) | |
| IA-2A | 3.08 (2.04–4.65) | <0.0001 |
| ZnT8A | 0.81 (0.51–1.29) | 0.37 |
| Age at first-appearing autoantibody (months) | 0.98 (0.97–0.99) | 0.001 |
| Time from first to second autoantibody (months) | 0.97 (0.96–0.99) | 0.003 |
| Sex | ||
| Female | 1.52 (1.09–2.13) | 0.014 |
| Male | 1 (reference) | |
| Family history of T1D | ||
| Mother with T1D | 1.55 (0.65–3.67) | 0.32 |
| Father with T1D | 1.01 (0.60–1.70) | 0.97 |
| Sibling with T1D | 0.92 (0.46–1.76) | 0.82 |
| General population (no T1D history) | 1 (reference) | |
| HLA-DR-DQ genotype | ||
| DR3/4 | 1.70 (0.99–2.93) | 0.06 |
| DR4/4 | 1 (reference) | |
| DR4/8 | 1.32 (0.69–2.54) | 0.41 |
| DR3/3 | 0.97 (0.44–2.15) | 0.94 |
| FDR-specific* | 1.28 (0.35–4.63) | 0.71 |
| Type of first antibody | ||
| GADA only | 0.97 (0.66–1.43) | 0.89 |
| IAA only | 1 (reference) |
FDR-specific HLA-DR-DQ genotypes are the following: DR4/4b, DR4/1, DR4/9, DR4/13, and DR3/9.
Figure 2.
Cumulative incidence in children with a distinct single second-appearing autoantibody for progression to T1D: GADA or IAA (blue line), IA-2A (red line), and ZnT8A (green line). The 5-year (60-month) cumulative risk of T1D among the children who remained single autoantibody positive is shown (black dotted line). The median (IQR) time between the first- and second-appearing autoantibody seroconversions was 5.3 (3.0–13.3) months for GADA or IAA, 6.9 (3.7–17.6) months for IA-2A, and 13.3 (6.3–23.7) months for ZnT8A.
Time Effect of First- to Second-Appearing Autoantibody on Risk of T1D
Finally, we examined if the time duration between the first and second autoantibody influenced the risk of progression to T1D after the development of the second autoantibody. The risk of progression from the second-appearing autoantibody to T1D declined with increasing time duration in months between the first- and second-appearing autoantibody (for every additional month longer, adjusted HR 0.97; 95% CI 0.96–0.99; P = 0.006) (Table 2). Such that there was a twofold increased risk of progression to T1D in children who developed a second autoantibody within 1 year of their first-appearing autoantibody as compared with those who developed it after 1 year after the initial seroconversion (adjusted HR 2.24; 95% CI 1.45–3.46; P = 0.0003).
Conclusions
In this large cohort of children at increased genetic risk for T1D with either IAA or GADA as a first-appearing autoantibody, we found that the appearance of a second autoantibody, regardless of type, conferred at least a fivefold increased risk of progression to T1D as compared with children who remained single autoantibody positive. This increased risk of progression varied by type of second-appearing autoantibody with IA-2A conferring the highest risk as compared with GADA, IAA or ZnT8A and was altered by how rapid the second autoantibody appeared. Development within 1 year doubled the risk of progression to T1D. Furthermore, a younger age at the initial seroconversion increased the risk of developing a second autoantibody, and the type of second-appearing autoantibody was independent of the type of the first-appearing autoantibody. Our results showed that having a family member with T1D at the time of screening (sibling, father, or mother) was associated with the risk of a specific second autoantibody. Also, carrying HLA-DR3/3 or the minor allele in rs1004446, a SNP in the INS-VNTR gene region, decreased the chance of IAA or GADA as a second-appearing autoantibody. Knowing the type of second autoantibody and how quickly it develops would increase our ability to identify subjects who are more likely to have a rapid versus a slow progression to T1D.
The strengths of this study include the ability to assess the risk of the appearance of another autoantibody and the temporal impact of this second autoantibody after the time of initial seroconversion. Previous studies have focused on assessing the risk of combinations of autoantibodies in cross-sectional screening designs when the actual timing of the initial seroconversion was unknown or in longitudinal studies with varied screening intervals or at/near diagnosis with T1D in those with familial risk. The relatively short interval for autoantibody screening in this study captured the sequential time of autoantibody appearance in TEDDY children from both the general population (89%) and those with a family history.
The majority of TEDDY children (77%) developed first-appearing IAA or GADA very early in life (7), as also observed in the Finnish DIPP study (8). Consistent with other studies (6,8,9), both IA-2A and ZnT8A were less likely to be a first-appearing autoantibody. Overall, ZnT8A appeared around median 3–4 years of age in the German BABYDIAB study (11) and 4–5 years of age in TEDDY, a difference that is likely due to the predominance of FDRs in the former and a general population with increased genetic risk in the latter. The reason for this later appearance of ZnT8A is not clear; however, previous studies have reported that ZnT8A is frequently detected in those patients who progress to T1D more slowly (21,39). In TEDDY, GADA or IAA were more likely to present as a second-appearing autoantibody in children under 10 years of age. It cannot be excluded that the perceived delay in the appearance of a specific second autoantibody as it relates to the age when the first autoantibody was triggered could mark a more environmentally driven, such as a prolonged enterovirus infection (40), as compared with a genetically driven disease process.
Unlike for IAA, GADA, and IA-2A, questions remain as to how much ZnT8A contributes to risk of stage 3 T1D, particularly in those who are young with increased genetic risk. In this study, ZnT8A conferred a fivefold greater risk of progression to T1D as compared with those autoantibody-positive children who did not develop multiple autoantibodies. It is evident from our findings and other studies that ZnT8A can stratify the risk of T1D progression in familial risk (11,14,32), ethnically diverse (41), and young, high-genetic-risk populations with or without familial risk (TEDDY). However, a large study of islet cell cytoplasmic autoantibody (ICA)-positive relatives of T1D probands did not find ZnT8A as a contributing marker of T1D in those <20 years at increased genetic risk (22). The likely reason for this inconsistency with our finding may be due to the preference of IAA, GADA, or IA-2A over ZnT8A in those with familial and genetic risk; the use of ICAs versus IAA, GADA, and IA-2A in risk determination; the high degree of concordance between GADA and ICA; and the small number (n = 69) of subjects with increased genetic risk <20 years of age (22).
Understanding the transition from being positive for a single autoantibody to being stage 1 (multiple autoantibodies) has both research and clinical implications. It has been shown in multiple studies that the first-appearing autoantibody is age-dependent and likely associates with different environmental triggers (5,7–9,33). Assessment of previously published environmental risk factors (34–37) shown to associate with the induction of islet autoimmunity in TEDDY were not found to be involved in further development of a second autoantibody. We, therefore, suggest that an environmental factor, such as prolonged viral shedding (40), may be sought as the etiological agent for the autoimmune reaction that is triggering the appearance of the first autoantibody and, thereby, the commencement of the autoimmune pathogenesis. The present analysis suggests that the appearance of a second autoantibody is related to other yet unknown mechanisms often referred to as epitope spreading. The present data expand our understanding of the temporal pattern by which the second autoantibody appears. Although the mechanisms need to be clarified, at least it improves prediction of progression to T1D, which advances our clinical practices for those at greatest risk and recruitment to secondary prevention clinical trials (42). Ultimately, this will result in better age-dependent autoantibody screening approaches, a reduction in symptomatic diagnoses and more strategic treatment interventions. Such specific autoantibody risk profiling will reduce heterogeneity and improve selection criteria to evaluate study objectives and interventions.
The main limitations are the select population of children at a high HLA‐DR-DQ genetic risk, the lack of extended follow-up past the age of 10 years, and the screening protocol for ZnT8A, which only measured ZnT8A in those children who developed another autoantibody (GADA, IA-2A, or IAA) further limiting our ability to determine the incidence of ZnT8A as a first-appearing autoantibody. A potential weakness of the current study is the inclusion criteria of children in four different countries with increased genetic risk for diabetes (26). The HLA-DR-DQ genotypes included 4–7.5% of newborns. However, the expected number of children reaching T1D by 18 years of age would represent <50% of the total number of children developing T1D. Certain HLA-DQ haplotypes, such as DQ5.1 and DQ6.4, are missing from the TEDDY cohort. It cannot be excluded that the pattern of IA-2A and ZnT8A as a second-appearing autoantibody may differ from the present observations.
Taken together, in children <10 years of age with genetic risk for T1D, the type of second-appearing autoantibody was associated with both genetic and familial risk, as well as varying risks of progression to T1D. All four autoantibodies under study increased the risk of stage 3 T1D. Age continues to play a critical role in the initiation of autoimmunity, development of multiple autoantibodies and progression to T1D. The importance of understanding what triggers epitope spreading and the type of autoantibody is critical in the prevention of disease progression. Further analyses including the adolescent years (>10 years) are required to better understand the transition to stage 1 (multiple autoantibodies) and how it affects the risk of stage 3 T1D, especially, in a more diverse general population with low-to-medium genetic risk.
Article Information
Acknowledgments. The authors thank Sarah Austin-Gonzalez ( Health Informatics Institute, University of South Florida) for assistance with preparing the figures. A special acknowledgment to the TEDDY families for their continued participation in this wonderful study.
Funding. This work is funded by the National Institute of Diabetes and Digestive and Kidney Diseases grants U01-DK-63829, U01-DK-63861, U01-DK-63821, U01-DK-63865, U01-DK-63863, U01-DK-63836, U01-DK-63790, U01-DK-100238, U01-DK-106955, U01-DK-112243, U01-DK-117483, U01-DK-95300, UC4-DK-63829, UC4-DK-63861, UC4-DK-63821, UC4-DK-63865, UC4-DK-63863, UC4-DK-63836, UC4-DK-95300, UC4-DK-100238, UC4-DK-106955, UC4-DK-112243, and UC4-DK-117483 and contract no. HHSN267200700014C from the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Environmental Health Sciences, JDRF, and Centers for Disease Control and Prevention. This work is supported, in part, by the National Institutes of Health National Center for Advancing Translational Sciences Clinical and Translational Science Awards to the University of Florida (UL1-TR-000064) and the University of Colorado (UL1-TR-001082).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.V. designed the study, proposed and performed the analysis, contributed to the manuscript, interpreted the findings, and wrote the manuscript. E.B., A.L., and J.P.K. designed the study, interpreted the findings, and reviewed and edited the manuscript. L.Y. and A.W. reviewed and edited the manuscript. D.S., M.R., J.-X.S., J.T., W.H., B.A., and A.G.Z. designed the study and reviewed and edited the manuscript. All authors attest to meeting International Committee of Medical Journal Editors’ uniform requirements for authorship by making substantial contributions to conception and design of this paper, acquisition, analysis, and interpretation of the data, drafting or revising the article for intellectual content, and giving final approval of the published version. K.V. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 16th Immunology of Diabetes Society Congress, London, U.K., 25–29 October 2018.
Footnotes
A complete list of the TEDDY Study Group members is provided in the supplementary material online.
This article contains supplementary material online at https://doi.org/10.2337/figshare.12302405.
Contributor Information
Collaborators: The TEDDY Study Group, Marian Rewers, Aaron Barbour, Kimberly Bautista, Judith Baxter, Daniel Felipe-Morales, Kimberly Driscoll, Brigitte I. Frohnert, Marisa Stahl, Patricia Gesualdo, Michelle Hoffman, Rachel Karban, Edwin Liu, Jill Norris, Stesha Peacock, Hanan Shorrosh, Andrea Steck, Megan Stern, Erica Villegas, Kathleen Waugh, Jorma Toppari, Olli G . Simell, Annika Adamsson, Suvi Ahonen, Mari Åkerlund, Leena Hakola, Anne Hekkala, Henna Holappa, Heikki Hyöty, Anni Ikonen, Jorma Ilonen, Sinikka Jäminki, Sanna Jokipuu, Leena Karlsson, Jukka Kero, Miia Kähönen, Mikael Knip, Minna-Liisa Koivikko, Merja Koskinen, Mirva Koreasalo, Kalle Kurppa, Jarita Kytölä, Tiina Latva-aho, Katri Lindfors, Maria Lönnrot, Elina Mäntymäki, Markus Mattila, Maija Miettinen, Katja Multasuo, Teija Mykkänen, Tiina Niininen, Sari Niinistö, Mia Nyblom, Sami Oikarinen, Paula Ollikainen, Zhian Othmani, Sirpa Pohjola, Petra Rajala, Jenna Rautanen, Anne Riikonen, Eija Riski, Miia Pekkola, Minna Romo, Satu Ruohonen, Satu Simell, Maija Sjöberg, Aino Stenius, Päivi Tossavainen, Mari Vähä-Mäkilä, Sini Vainionpää, Eeva Varjonen, Riitta Veijola, Irene Viinikangas, Suvi M. Virtanen, Jin-Xiong She, Desmond Schatz, Diane Hopkins, Leigh Steed, Jennifer Bryant, Katherine Silvis, Michael Haller, Melissa Gardiner, Richard McIndoe, Ashok Sharma, Stephen W. Anderson, Laura Jacobsen, John Marks, P.D. Towe, Anette G. Ziegler, Ezio Bonifacio, Cigdem Gezginci, Anja Heublein, Eva Hohoff, Sandra Hummel, Annette Knopff, Charlotte Koch, Sibylle Koletzko, Claudia Ramminger, Roswith Roth, Jennifer Schmidt, Marlon Scholz, Joanna Stock, Katharina Warncke, Lorena Wendel, Christiane Winkler, Åke Lernmark, Daniel Agardh, Carin Andrén Aronsson, Maria Ask, Rasmus Bennet, Corrado Cilio, Susanne Dahlberg, Helene Engqvist, Emelie Ericson-Hallström, Annika Björne Fors, Lina Fransson, Thomas Gard, Monika Hansen, Hanna Jisser, Fredrik Johansen, Berglind Jonsdottir, Helena Elding Larsson, Marielle Lindström, Markus Lundgren, Marlena Maziarz, Maria Månsson-Martinez, Jessica Melin, Zeliha Mestan, Caroline Nilsson, Karin Ottosson, Kobra Rahmati, Anita Ramelius, Falastin Salami, Anette Sjöberg, Birgitta Sjöberg, Carina Törn, Åsa Wimar, William A. Hagopian, Michael Killian, Claire Cowen Crouch, Jennifer Skidmore, Masumeh Chavoshi, Arlene Meyer, Jocelyn Meyer, Denise Mulenga, Nole Powell, Jared Radtke, Matei Romancik, Shreya Roy, Davey Schmitt, Sarah Zink, Dorothy Becker, Margaret Franciscus, MaryEllen Dalmagro-Elias Smith, Ashi Daftary, Mary Beth Klein, Chrystal Yates, Jeffrey P. Krischer, Sarah Austin-Gonzalez, Maryouri Avendano, Sandra Baethke, Brant Burkhardt, Martha Butterworth, Joanna Clasen, David Cuthbertson, Christopher Eberhard, Steven Fiske, Jennifer Garmeson, Veena Gowda, Kathleen Heyman, Belinda Hsiao, Christina Karges, Francisco Perez Laras, Qian Li, Shu Liu, Xiang Liu, Kristian Lynch, Colleen Maguire, Jamie Malloy, Cristina McCarthy, Hemang Parikh, Cassandra Remedios, Chris Shaffer, Laura Smith, Susan Smith, Noah Sulman, Roy Tamura, Dena Tewey, Michael Toth, Ulla Uusitalo, Kendra Vehik, Ponni Vijayakandipan, Jimin Yang, Michael Abbondondolo, Lori Ballard, Rasheedah Brown, Stephen Dankyi, David Hadley, Hye-Seung Lee, Wendy McLeod, Aubrie Merrell, Steven Meulemans, Ryan Quigley, Beena Akolkar, Liping Yu, Dongmei Miao, Polly Bingley, Alistair Williams, Kyla Chandler, Ilana Kelland, Yassin Ben Khoud, Huma Zahid, Matthew Randell, William Hagopian, Masumeh Chavoshi, Jared Radtke, Sarah Zink, Henry Erlich, Steven J. Mack, Anna Lisa Fear, Stephen S. Rich, Wei-Min Chen, Suna Onengut-Gumuscu, Emily Farber, Rebecca Roche Pickin, Jonathan Davis, Jordan Davis, Dan Gallo, Jessica Bonnie, Paul Campolieto, Sandra Ke, Niveen Mulholland, Kasia Bourcier, Thomas Briese, Suzanne Bennett Johnson, and Eric Triplett
References
- 1.Bingley PJ, Bonifacio E, Williams AJ, Genovese S, Bottazzo GF, Gale EA. Prediction of IDDM in the general population: strategies based on combinations of autoantibody markers. Diabetes 1997;46:1701–1710 [DOI] [PubMed] [Google Scholar]
- 2.Bonifacio E, Bingley PJ. Islet autoantibodies and their use in predicting insulin-dependent diabetes. Acta Diabetol 1997;34:185–193 [DOI] [PubMed] [Google Scholar]
- 3.Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care 2015;38:1964–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vehik K, Beam CA, Mahon JL, et al.; TrialNet Natural History Study Group . Development of autoantibodies in the TrialNet Natural History Study. Diabetes Care 2011;34:1897–1901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krischer JP, Liu X, Vehik K, et al.; TEDDY Study Group . Predicting islet cell autoimmunity and type 1 diabetes: an 8-year TEDDY study progress report. Diabetes Care 2019;42:1051–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziegler AG, Hummel M, Schenker M, Bonifacio E. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 1999;48:460–468 [DOI] [PubMed] [Google Scholar]
- 7.Krischer JP, Lynch KF, Schatz DA, et al.; TEDDY Study Group . The 6 year incidence of diabetes-associated autoantibodies in genetically at-risk children: the TEDDY study. Diabetologia 2015;58:980–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilonen J, Hammais A, Laine AP, et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes 2013;62:3636–3640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ziegler AG, Bonifacio E; BABYDIAB-BABYDIET Study Group . Age-related islet autoantibody incidence in offspring of patients with type 1 diabetes. Diabetologia 2012;55:1937–1943 [DOI] [PubMed] [Google Scholar]
- 10.Wenzlau JM, Moua O, Sarkar SA, et al. SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Ann N Y Acad Sci 2008;1150:256–259 [DOI] [PubMed] [Google Scholar]
- 11.Achenbach P, Lampasona V, Landherr U, et al. Autoantibodies to zinc transporter 8 and SLC30A8 genotype stratify type 1 diabetes risk. Diabetologia 2009;52:1881–1888 [DOI] [PubMed] [Google Scholar]
- 12.Ziegler AG, Rewers M, Simell O, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA 2013;309:2473–2479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu L, Rewers M, Gianani R, et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab 1996;81:4264–4267 [DOI] [PubMed] [Google Scholar]
- 14.De Grijse J, Asanghanwa M, Nouthe B, et al.; Belgian Diabetes Registry . Predictive power of screening for antibodies against insulinoma-associated protein 2 beta (IA-2beta) and zinc transporter-8 to select first-degree relatives of type 1 diabetic patients with risk of rapid progression to clinical onset of the disease: implications for prevention trials. Diabetologia 2010;53:517–524 [DOI] [PubMed] [Google Scholar]
- 15.Bauer W, Veijola R, Lempainen J, et al. Age at seroconversion, HLA genotype, and specificity of autoantibodies in progression of islet autoimmunity in childhood. J Clin Endocrinol Metab 2019;104:4521–4530 [DOI] [PubMed] [Google Scholar]
- 16.Gorus FK, Balti EV, Messaaoui A, et al.; Belgian Diabetes Registry . Twenty-year progression rate to clinical onset according to autoantibody profile, age, and HLA-DQ genotype in a registry-based group of children and adults with a first-degree relative with type 1 diabetes. Diabetes Care 2017;40:1065–1072 [DOI] [PubMed] [Google Scholar]
- 17.Bingley PJ, Boulware DC, Krischer JP; Type 1 Diabetes TrialNet Study Group . The implications of autoantibodies to a single islet antigen in relatives with normal glucose tolerance: development of other autoantibodies and progression to type 1 diabetes. Diabetologia 2016;59:542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Achenbach P, Warncke K, Reiter J, et al. Stratification of type 1 diabetes risk on the basis of islet autoantibody characteristics. Diabetes 2004;53:384–392 [DOI] [PubMed] [Google Scholar]
- 19.Yu L, Boulware DC, Beam CA, et al.; Type 1 Diabetes TrialNet Study Group . Zinc transporter-8 autoantibodies improve prediction of type 1 diabetes in relatives positive for the standard biochemical autoantibodies. Diabetes Care 2012;35:1213–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orban T, Sosenko JM, Cuthbertson D, et al.; Diabetes Prevention Trial-Type 1 Study Group . Pancreatic islet autoantibodies as predictors of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 2009;32:2269–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long AE, Gooneratne AT, Rokni S, Williams AJ, Bingley PJ. The role of autoantibodies to zinc transporter 8 in prediction of type 1 diabetes in relatives: lessons from the European Nicotinamide Diabetes Intervention Trial (ENDIT) cohort. J Clin Endocrinol Metab 2012;97:632–637 [DOI] [PubMed] [Google Scholar]
- 22.Long AE, Gillespie KM, Rokni S, Bingley PJ, Williams AJ. Rising incidence of type 1 diabetes is associated with altered immunophenotype at diagnosis. Diabetes 2012;61:683–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balke EM, Balti EV, Van der Auwera B, et al.; Belgian Diabetes Registry . Accelerated progression to type 1 diabetes in the presence of HLA-A*24 and -B*18 is restricted to multiple islet autoantibody-positive individuals with distinct HLA-DQ and autoantibody risk profiles. Diabetes Care 2018;41:1076–1083 [DOI] [PubMed] [Google Scholar]
- 24.Törn C, Hadley D, Lee HS, et al.; TEDDY Study Group . Role of type 1 diabetes-associated SNPs on risk of autoantibody positivity in the TEDDY study. Diabetes 2015;64:1818–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Törn C, Liu X, Hagopian W, et al.; TEDDY Study Group . Complement gene variants in relation to autoantibodies to beta cell specific antigens and type 1 diabetes in the TEDDY Study. Sci Rep 2016;6:27887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hagopian WA, Erlich H, Lernmark A, et al.; TEDDY Study Group . The Environmental Determinants of Diabetes in the Young (TEDDY): genetic criteria and international diabetes risk screening of 421 000 infants. Pediatr Diabetes 2011;12:733–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study. Ann N Y Acad Sci 2008;1150:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.TEDDY Study Group The Environmental Determinants of Diabetes in the Young (TEDDY) study: study design. Pediatr Diabetes 2007;8:286–298 [DOI] [PubMed] [Google Scholar]
- 29.Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for national institute of diabetes and digestive and kidney diseases consortia. J Clin Endocrinol Metab 2010;95:3360–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A 2000;97:1701–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl. 1):S81–S90 [DOI] [PubMed] [Google Scholar]
- 32.Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A 2007;104:17040–17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krischer JP, Lynch KF, Lernmark Å, et al.; TEDDY Study Group . Genetic and environmental interactions modify the risk of diabetes-related autoimmunity by 6 years of age: the TEDDY study. Diabetes Care 2017;40:1194–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uusitalo U, Liu X, Yang J, et al.; TEDDY Study Group . Association of early exposure of probiotics and islet autoimmunity in the TEDDY study. JAMA Pediatr 2016;170:20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lönnrot M, Lynch KF, Elding Larsson H, et al.; TEDDY Study Group . Respiratory infections are temporally associated with initiation of type 1 diabetes autoimmunity: the TEDDY study. Diabetologia 2017;60:1931–1940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elding Larsson H, Vehik K, Haller MJ, et al.; TEDDY Study Group . Growth and risk for islet autoimmunity and progression to type 1 diabetes in early childhood: The Environmental Determinants of Diabetes in the Young study. Diabetes 2016;65:1988–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lynch KF, Lee HS, Törn C, et al.; TEDDY Study Group . Gestational respiratory infections interacting with offspring HLA and CTLA-4 modifies incident β-cell autoantibodies. J Autoimmun 2018;86:93–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Concannon P, Rich SS, Nepom GT. Genetics of type 1A diabetes. N Engl J Med 2009;360:1646–1654 [DOI] [PubMed] [Google Scholar]
- 39.Long AE, Wilson IV, Becker DJ, et al. Characteristics of slow progression to diabetes in multiple islet autoantibody-positive individuals from five longitudinal cohorts: the SNAIL study. Diabetologia 2018;61:1484–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vehik K, Lynch KF, Wong MC, et al.; TEDDY Study Group . Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat Med 2019;25:1865–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gomes KF, Semzezem C, Batista R, et al. Importance of zinc transporter 8 autoantibody in the diagnosis of type 1 diabetes in Latin Americans. Sci Rep 2017;7:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Endesfelder D, Zu Castell W, Bonifacio E, et al.; TEDDY Study Group . Time-resolved autoantibody profiling facilitates stratification of preclinical type 1 diabetes in children. Diabetes 2019;68:119–130 [DOI] [PMC free article] [PubMed] [Google Scholar]