Abstract
OBJECTIVE
Sulfonylureas are first-line treatment of hepatocyte nuclear factor 1-α (HNF1A) diabetes (maturity-onset diabetes of the young type 3), but many patients do not achieve optimal glycemic control without episodes of hypoglycemia. We investigated the combination of the sulfonylurea glimepiride and the dipeptidyl peptidase 4 inhibitor linagliptin versus glimepiride monotherapy with respect to glycemic variability, glycemic control, and risk of hypoglycemia.
RESEARCH DESIGN AND METHODS
In a randomized, double-blinded, crossover trial, patients with HNF1A diabetes (n = 19; mean ± SD age 43 ± 14 years, BMI 24.8 ± 2.8 kg/m2, and glycated hemoglobin [HbA1c] 7.4 ± 0.2% [57.1 ± 7.3 mmol/mol]) were randomly assigned to treatment with glimepiride + linagliptin 5 mg (16 weeks), washout (4 weeks), and glimepiride + placebo (16 weeks) (or vice versa). Glimepiride was titrated targeting a fasting plasma glucose of 4.5–6.0 mmol/L without hypoglycemia. Treatments were evaluated by continuous glucose monitoring (CGM), HbA1c, and meal test.
RESULTS
Compared with glimepiride + placebo, glimepiride + linagliptin did not significantly improve the primary end point, mean amplitude of glycemic excursions (MAGE) (mean difference −0.7 mmol/L, P = 0.1540), but displayed significant reductions in coefficient of variation on CGM (−3.6%, P = 0.0401), HbA1c (−0.5%, P = 0.0048), and glimepiride dose (−0.7 mg/day, P = 0.0099). β-cell glucose sensitivity (assessed as C-peptide–to–glucose ratio) during meal test improved with glimepiride + linagliptin. Incidences of hypoglycemia were similar with both treatments.
CONCLUSIONS
Linagliptin as add-on treatment to glimepiride improved glycemic variability and control without increasing risk of hypoglycemia in patients with HNF1A diabetes.
Introduction
Hepatocyte nuclear factor 1-α (HNF1A) diabetes is a monogenic diabetes subtype that belongs to the heterogenous group of diabetes subtypes collectively known as maturity-onset diabetes of the young (MODY). Approximately 1%–2% of all diabetes cases have a monogenic cause. HNF1A diabetes (MODY3) is the most prevalent MODY subtype, accounting for ∼50% of all MODY cases (1–3). Similar to type 1 diabetes, tight glycemic control is critical for reducing microvascular and macrovascular complications in patients with HNF1A diabetes (4,5). A key characteristic of HNF1A diabetes is impaired glucose-stimulated insulin secretion. This is due to a reduced β-cell glucose uptake and metabolism resulting in low intracellular ATP levels (6,7), which, in turn, leave KATP channels open, preventing depolarization and insulin release (8). By binding to the sulfonylurea receptor, a subunit of the KATP channel, sulfonylureas close these channels, causing depolarization and insulin release (7,9). The potent insulinotropic effect of sulfonylureas and the normal-to-high insulin sensitivity typical for patients with HNF1A diabetes (7,10) explain their potent glucose-lowering effect in these patients (11,12). Accordingly, the sulfonylurea gliclazide resulted in a 5.2-fold greater reduction in fasting plasma glucose in patients with HNF1A diabetes as compared with metformin (11). Thus, sulfonylureas are recommended as first-line therapy in patients with HNF1A diabetes (13,14). However, sulfonylurea treatment has limitations: first, sulfonylureas do not provide sustained glycemic control in all patients, and additional glucose-lowering treatment is often needed; second, sulfonylurea increases risk of hypoglycemia (15,16); and third, sulfonylurea may lead to increased body weight (17). Accordingly, there is a need for studies investigating efficient glucose-lowering agents as add-on to sulfonylurea treatment, when patients lack glycemic control, or an alternative treatment approach, when patients do not achieve glycemic targets due to recurrent hypoglycemia with sulfonylureas.
Glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide 1 (GLP-1) are gut-derived incretin hormones that stimulate insulin release in a glucose-dependent manner (i.e., only at plasma glucose levels >4–5 mmol/L) (18,19). Both hormones are degraded by the enzyme dipeptidyl peptidase-4 (DPP-4). Inhibitors of DPP-4 increase endogenous levels of GIP and GLP-1 and are well-known for their glucose-lowering actions with low risk of hypoglycemia in patients with type 2 diabetes (20,21). In patients with type 2 diabetes, the glucose-lowering effect of DPP-4 inhibition is mainly attributed to the increase of intact GLP-1 levels because insulinotropic effect of GIP is severely diminished in acute studies (22). However, positive GIP-mediated effects may occur during prolonged DPP-4 inhibitor therapy (23). Recent work from our group has shown additive–to–supra-additive insulinotropic effects of GIP and GLP-1 in patients with HNF1A diabetes treated with sulfonylureas (24). In this study, we evaluated the combination of the sulfonylurea glimepiride and the DPP-4 inhibitor linagliptin versus glimepiride monotherapy with respect to glycemic variability and control (glycated hemoglobin [HbA1c]) and risk of hypoglycemia in patients with HNF1A diabetes.
Research Design and Methods
Approvals
The study received approval by the Danish Medicines Agency, the Scientific-Ethical Committees of the Central and Capital Region of Denmark, and the Danish Data Protection Agency. The trial was initiated September 2017 and concluded in June 2019. The trial was registered in the European Union Clinical Trials Register (EudraCT) (reg. no. 2017-000204-15) prior to initiation of the trial, and a published trial protocol is available (25). The study was conducted in accordance with the Declaration of Helsinki and International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use guidelines for good clinical practice (GCP). The study was monitored by the GCP unit at the University of Copenhagen and the GCP unit at the Aarhus University Hospital and University Hospital of Aalborg, Aarhus, Denmark.
Trial Design and End Points
This was a randomized, double-blinded, placebo-controlled, crossover trial conducted at two sites: Steno Diabetes Center Copenhagen (Gentofte Hospital) and Steno Diabetes Center Aarhus (Aarhus University Hospital). After a 1-week baseline evaluation, patients were randomly assigned in a 1:1 ratio to the following two treatment sequences: 1) glimepiride + linagliptin (16 weeks), washout (4 weeks), and glimepiride + placebo (16 weeks) or 2) glimepiride + placebo (16 weeks), washout (4 weeks), and glimepiride + linagliptin (16 weeks) (Supplementary Fig. 1). The primary end point was the difference between treatments in mean amplitude of glycemic excursions (MAGE) calculated from 6 days of continuous glucose monitoring (CGM) at the end of each treatment period. Secondary end points were differences between treatments in regard to 1) other variables of glycemic variability calculated from CGM (coefficient of variation [CV], SD, mean glucose, and low blood glucose index), 2) HbA1c, 3) fasting plasma glucose, 4) body weight, 5) episodes of hypoglycemia (assessed with both CGM and self-measured blood glucose), 6) quality of life, and 7) plasma glucose excursions, pancreatic hormone responses (C-peptide and glucagon), and acetaminophen absorption during a 4-h combined meal and bicycle test.
Patient Population
Potential participants were screened after receiving detailed information about the trial and signing informed consent. Eligibility was evaluated according to inclusion and exclusion criteria. Inclusion criteria were 1) diagnosis of HNF1A diabetes caused by a heterozygous mutation in HNF1A confirmed by Sanger sequencing of the gene, 2) monotherapy with a stable dose of glimepiride of (≥0.5 mg per day) during 4 weeks or no glucose-lowering agent, 3) HbA1c ≥6.5% (≥48 mmol/mol) (patients on glimepiride treatment) or HbA1c ≥7.0% (≥53 mmol/mol) (patients receiving no glucose-lowering agents), 4) age ≥18 years, 5) capability to perform a 30-min light bicycle test at a heart rate of 100–120 bpm, and 6) use of intrauterine contraceptive devices or hormonal contraception (females). Exclusion criteria were 1) use of glucose-lowering drugs other than glimepiride; 2) uremia, end-stage renal disease, or estimated glomerular filtration rate <30 mL/min/1.73 m2 and/or macroalbuminuria; 3) liver disease, serum alanine aminotransferase, and/or serum AST >2 × upper normal serum levels; 4) anemia (males, hemoglobin <8.0 mmol/L; females, <7.0 mmol/L); 5) history of acute and/or chronic pancreatitis; 6) pregnancy, breastfeeding, or intention to become pregnant; 7) inability to complete the study; and 8) known allergic reaction to study medication.
Randomization
Randomization codes were developed by a computer-generated random list of numbers. and the patients were allocated to treatment group by an unblinded employee who was otherwise uninvolved in the study. A.S.C. enrolled the patients, but both patients and A.S.C. remained blinded throughout the trial period and data analyses.
Trial Medication
Tablets with linagliptin 5 mg and placebo had identical appearance and were provided by Boehringer Ingelheim. Tablets of glimepiride 1 mg (with the possibility of splitting in half) was not blinded and were delivered by the Hospital Pharmacy of the Capital Region, Herlev, Denmark. Patients treated with glimepiride at screening continued the same dosage during the 1-week baseline evaluation and during washout. One treatment-naïve patient started on 0.5 mg glimepiride after the baseline evaluation. Each treatment period was divided into a drug titration period (weeks 1–4/weeks 16–20) and a maintenance period (weeks 5–15/weeks 25–35). During the titration period, glimepiride was uptitrated once weekly (0.5 mg per week) in a treat-to-target manner with a maximum daily dose of 6 mg in both groups (Supplementary Fig. 1). A total daily dose of glimepiride ≥1 mg was administered in two doses: one dose in the morning and one dose in the evening. Target fasting plasma glucose (average for 5 days) was between 4.5 and 6.0 mmol/L (both inclusive) without episodes of symptomatic or biochemical hypoglycemia (plasma glucose ≤3.9 mmol/L). During the maintenance period, all medication doses remained unaltered unless patients had recurrent (two or more) hypoglycemic episodes, and in this case, the dose of glimepiride was downtitrated adequately. If target fasting plasma glucose was not achieved during the drug titration period, the dose of glimepiride was adjusted at the investigators’ discretion. Once-daily linagliptin 5 mg or placebo was initiated at the start of each treatment period and the dose kept stable throughout the study. During washout, the dose of glimepiride at randomization was immediately reintroduced and linagliptin/placebo was terminated. Participants were encouraged not to change their lifestyle (i.e., diet, exercise, and smoking status) during the trial. Episodes of hypoglycemia were defined according to guidelines and are reported separately for the maintenance phase and during CGM and combined meal and bicycle test. Patient-reported episodes of hypoglycemia are defined as blood glucose <4.0 mmol/L confirmed by self-measured blood glucose measurements on a glucometer (26). A hypoglycemic episode during CGM was defined as glucose measured ≤3.9 mmol/L in at least 20 consecutive minutes as recommended in guidelines (27). Hypoglycemia was categorized as level 1 hypoglycemia (plasma glucose 3.0–3.9 mmol/L), level 2 hypoglycemia (plasma glucose <3.0 mmol/L), and level 3 hypoglycemia (severe hypoglycemia with need of third-party assistance for recovery) (26).
Clinical Visits
Six types of visits were performed: screening, randomization, mounting of CGM, telephone visits, clinical visits, and a combined meal and bicycle test. Specification of blood samples taken at the visits has previously been published (25). A study timeline is depicted in Supplementary Table 1. At all onsite visits, the following were registered: adverse events, episodes of hypoglycemia, and compliance. CGM devices were placed on the lower left or right quadrant of the abdomen, and patients were instructed to measure plasma glucose four times daily, preferably before the three main meals and before bedtime.
Meal and Bicycle Test
A combined meal and bicycle test was applied to make our test similar to that of two other studies of patients with HNF1A diabetes (15,16). The combined meal and bicycle test provides a greater risk of hypoglycemia (due to exercise-induced reduction of plasma glucose) compared with a traditional meal test executed in an inactive semirecumbent state. In addition, it also mimics daily life better, as it takes into account that most patients are not totally inactive 4 h following a meal. The combined 4-h meal and bicycle test consisted of a liquid mixed meal (525 kcal: 65 g carbohydrate, 20 g fat, and 21 g protein) to which was added 1.5 g acetaminophen (Panodil; GlaxoSmithKline A/S, Copenhagen, Denmark). Patients arrived in the clinical research facility after an overnight fast of at least 10 h. Trial medication was given at time −60 min, and the meal was served at time 0 min. At time 60–90 min, patients cycled with a target heart rate of 90–110 bpm. Patients were monitored for hypoglycemic symptoms, and blood was sampled for measurements of plasma glucose, C-peptide, glucagon, and acetaminophen at prespecified time points (see Supplementary Fig. 3).
Analytical Procedures
For CGM measurements, iPro2 CGM (Medtronic, Northridge, CA) was used, which has previously been validated (28). For calibration of CGM and measurements of blood glucose during the trial, participants were given Contour XT glucometers (Ascencia Diabetes Care, Copenhagen, Denmark). A description of analytical procedures regarding the samples from the meal test is presented in the Supplementary Material.
Statistical Analysis and Calculations
All analyses were performed on the intention-to-treat population, who were randomized and received at least one dose of linagliptin or placebo. Normally distributed data were summarized by number of observations (n) and mean and SD. Data that were not normally distributed are presented as median and interquartile ranges. Categorical data were summarized in numbers and percentages. Comparisons between treatment outcomes (except body weight) were performed in a linear mixed model including treatment as a fixed effect and with an unstructured covariance pattern. P values were evaluated using Kenward-Roger approximation of the degrees of freedom. Missing data were implicitly imputed by performing likelihood inference in the linear mixed model. To investigate potential carryover effects, we tested the treatment * period interaction, but no evidence was detected for any variable. To test whether relatedness had an effect on the outcome, family was added to the model as a random effect; however, this did not alter any of the statistical results. Body weight changes (Δ values) were compared between the treatments using a paired t test. All tests were carried out with a significance level of 5% without adjustment for multiple comparisons. Adverse events are summarized qualitatively. All statistical analyses were performed in SAS studio, version 9.4, and graphical presentation was in GraphPad Prism 8. For calculation of CGM-derived variables, the program EasyGV was used (29). Glucose variables included MAGE, CV, SD, mean, low blood glucose index, and time spent in hypoglycemia, near normoglycemia, and hyperglycemia. Treatment responses during the meal and bicycle test were quantified as area under the curve (AUC) calculated using the trapezoidal rule or baseline-subtracted AUC (bsAUC). For bsAUC, the average of values at times −10, −5, and 0 min was defined as baseline. Sample size calculation was based on the primary outcome MAGE (difference between glimepiride + linagliptin and glimepiride + placebo). The power of our study (1 − β) was set at 0.8, and, thus, the risk of accepting a false hypothesis is 0.20 (β = 0.20) with a two-sided significance level at 0.05 (α = 0.05). A minimal relevant difference of 2 mmol/L between treatments in MAGE was chosen with an expected SD of 2.8 mmol/L based on the work by Saisho et al. (30). Thus, a total of 16 patients were needed, but due to risk of dropout and to increase power of secondary end points, 20 patients were included. A detailed power calculation has previously been published (25).
Results
Patient Disposition and Baseline Characteristics
We assessed 224 patients with HNF1A diabetes for eligibility to enter the study (Supplementary Fig. 2). A total of 22 patients were screened: 1 was excluded due to albuminuria, and 1 withdrew consent before randomization. One participant was excluded from data analysis due to withdrawal of consent after randomization but before exposure to study drugs. Two other participants dropped out during the study in week 4 and week 13 (due to difficulty adhering to study protocol and time concerns, respectively), and both patients are included in the analysis. Participant baseline characteristics are presented in Table 1 and the specific HNF1A mutations in Supplementary Table 2. Overall compliance was good: 97% with both glimepiride and linagliptin/placebo.
Table 1.
Baseline characteristics
| Participants, n | 19 |
| Female sex, n (%) | 11 (58) |
| Age (years) | 43 (14) |
| Caucasian, n (%) | 19 (100) |
| Diabetes duration (years) | 20 (8–34) |
| BMI (kg/m2) | 24.8 (22.0–25.7) |
| Fasting plasma glucose (mmol/L) | 8.9 (2.3) |
| HbA1c (%) | 7.4 (0.7) |
| HbA1c (mmol/mol) | 57.1 (7.3) |
| HOMA-IR | 0.9 (0.7–1.3) |
| Complications, n | |
| Retinopathy | 5 |
| Microalbuminuria | 1 |
| Transient ischemic attack | 1 |
| Comorbidities, n | |
| Hypertension | 3 |
| Hypercholesterolemia | 9 |
| Multiple sclerosis | 1 |
| Treatment, n | |
| Glimepiride | 18 |
| Diet | 1 |
Data are mean (SD) or median (interquartile range) unless otherwise indicated. Diabetes duration is from manifest diabetes (first measurement of HbA1c ≥6.5% [≥48 mmol/mol]). HOMA-IR, HOMA of insulin resistance.
Glimepiride Dose
The mean glimepiride dose increased from 1.9 mg at baseline to 2.7 mg and 3.4 mg with glimepiride + linagliptin and glimepiride + placebo, respectively (mean difference −0.7 mg [95% CI −1.2 to −0.2], P = 0.0099) (Table 2). Nine of 17 patients received a lower glimepiride dose during the treatment period with glimepiride + linagliptin compared with glimepiride + placebo (Supplementary Table 2). The remaining eight patients received the same dose of glimepiride regardless of linagliptin/placebo; three of these received the maximal glimepiride dose (6.0 mg glimepiride), while the remaining patients received submaximal doses of glimepiride.
Table 2.
Primary and secondary outcomes
| Baseline | Glimepiride + placebo vs. baseline | Glimepiride + linagliptin vs. baseline | Glimepiride + linagliptin vs. glimepiride + placebo | |
|---|---|---|---|---|
| CGM | ||||
| MAGE (mmol/L) | 5.5 (4.6–6.4) | 0.3 (−0.9 to 1.5), P = 0.5616 | −0.4 (−1.6 to 0.7), P = 0.4286 | −0.7 (−1.9 to 0.3), P = 0.1540 |
| CV (%) | 27.2 (24.0–30.5) | 4.2 (−3.0 to 4.2), P = 0.0438 | 0.6 (−3.0 to 4.2), P = 0.7364 | −3.6 (−7.0 to −0.2), P = 0.0401 |
| SD (mmol/L) | 2.6 (2.3–3.0) | 0.1 (−0.3 to 0.6), P = 0.5192 | −0.4 (−0.7 to 0.1), P = 0.1000 | −0.4 (−0.8 to −0.1), P = 0.0210 |
| Mean (mmol/L) | 9.6 (8.9–10.4) | −0.9 (−1.8 to 0.0), P = 0.0548 | −1.4 (−2.2 to −0.6), P = 0.0012 | −0.5 (−1.4 to 0.4), P = 0.2341 |
| Low blood glucose index | 0.9 (0.4–1.5) | 0.7 (0.1–1.4), P = 0.0364 | 0.4 (−0.3 to 1.2), P = 0.2458 | −0.3 (−1.0 to 0.4), P = 0.3740 |
| % time in hyperglycemia (≥10.0 mmol/L) | 38.9 (29.2–48.6) | −9.8 (−21.2 to 1.7), P = 0.0894 | −15.7 (−26.0 to −5.5), P = 0.0047 | −6.0 (−16.4 to 4.5), P = 0.2470 |
| % time in near normoglycemia (4.0–9.9 mmol/L) | 60.7 (50.9–70.4) | 8.5 (−2.9 to 20.0), P = 0.1358 | 15.3 (4.7–25.8), P = 0.0068 | 6.7 (−3.9 to 17.4), P = 0.1990 |
| Biochemistry and other | ||||
| HbA1c (%) | 7.4 (7.0–7.7) | −0.4 (−0.8 to 0.1), P = 0.1165 | −0.9 (−1.3 to −0.5), P = 0.0001 | −0.5 (−0.9 to −0.2), P = 0.0048 |
| HbA1c (mmol/mol) | 57.1 (53.6–60.5) | −4.0 (−9.0 to 1.0), P = 0.1116 | −9.6 (−13.7 to −5.4), P = 0.0001 | −5.6 (−9.3 to −1.8), P = 0.0055 |
| Fasting plasma glucose (mmol/L) | 9.0 (7.9–10.0) | −1.4 (−2.5 to −0.3), P = 0.0182 | −2.1 (−3.2 to −1.0), P = 0.0008 | −0.7 (−1.4 to −0.0), P = 0.0492 |
| Mental component score (SF-36) | 53.6 (49.5–57.8) | −3.2 (−6.7 to 0.4), P = 0.0765 | 0.6 (−2.5 to 3.8), P = 0.6751 | 3.8 (−1.7 to 9.3), P = 0.1655 |
| Physical component score (SF-36) | 55.2 (52.2–58.2) | 0.8 (−0.8 to 2.4), P = 0.2891 | 2.1 (0.6–3.5), P = 0.0087 | 1.2 (−0.0 to 2.5), P = 0.0569 |
| Glimepiride dose (mg) | 1.9 (1.3–2.4) | 1.5 (0.9–2.1), P < 0.0001 | 0.8 (0.2–1.5), P = 0.0166 | −0.7 (−1.2 to −0.2), P = 0.0099 |
| Meal and bicycle test | ||||
| Plasma glucose | ||||
| AUC (mol/L × min) | 2.9 (2.5–3.4) | −0.3 (−0.5 to −0.0), P = 0.0463 | −0.6 (−0.9 to −0.3), P = 0.008 | −0.3 (−0.6 to −0.0), P = 0.0466 |
| bsAUC (mol/L × min) | 0.9 (0.6–1.2) | −0.1 (−0.3 to 0.2), P = 0.6066 | −0.2 (−0.4 to 0.0), P = 0.0975 | −0.1 (−0.4 to 0.1), P = 0.2418 |
| Excursion (mmol/L) | 7.8 (6.3–9.3) | −0.1 (−1.3 to 1.1), P = 0.8478 | −0.6 (−1.8 to 0.7), P = 0.3396 | −0.5 (−1.9 to 1.0), P = 0.4976 |
| Peak (mmol/L) | 15.3 (13.2–17.5) | −0.9 (−2.2 to 0.4), P = 0.1482 | −2.3 (−3.8 to −0.8), P = 0.0042 | −1.4 (−3.2 to 0.3), P = 0.1090 |
| C-peptide | ||||
| AUC (nmol/L × min) | 226 (174–278) | −7 (−23 to 93), P = 0.3847 | 2 (−15 to 19), P = 0.7813 | 9 (−3.5 to 22), P = 0.1453 |
| bsAUC (nmol/L × min) | 142 (107–176) | −21 (−39 to −4), P = 0.0174 | −5 (−25 to 15), P = 0.6073 | 17 (−3 to 36), P = 0.0940 |
| C-peptide–to–glucose ratio | ||||
| AUC (pmol/L × mmol−1 × 10−3 × min) | 20 (14–26) | 2 (−2 to 6), P = 0.2588 | 7 (3–12), P = 0.0018 | 5 (2–8), P = 0.0008 |
| bsAUC (pmol/L × mmol−1 × 10−3 × min) | 9 (5–13) | 1 (−3 to 2), P = 0.5979 | 4 (−2 to 9), P = 0.1603 | 4 (1–8), P = 0.0261 |
| Glucagon | ||||
| Fasting levels (pmol/L) | 7.4 (5.6–9.1) | 0.1 (−1.3 to 1.6), P = 0.8473 | −0.7 (−2.4 to 1.0), P = 0.4108 | −0.8 (−2.3 to 0.7), P = 0.2661 |
| AUC (nmol/L × min) | 3.1 (2.6–3.7) | −0.0 (−0.4 to 0.4), P = 0.8962 | −0.2 (−0.5 to 0.1), P = 0.1535 | −0.2 (−0.5 to 0.1), P = 0.2458 |
| bsAUC (nmol/L × min) | 1.3 (0.9–1.7) | −0.0 (−0.4 to 0.4), P = 0.9280 | 0.0 (−0.4 to 0.4), P = 0.9731 | 0.0 (−0.2 to 0.3), P = 0.8538 |
| Acetaminophen | ||||
| AUC (µmol/L × min) | 15.4 (14.1–16.8) | 0.0 (−0.7 to 0.8), P = 0.9159 | −0.6 (−1.4 to 0.1), P = 0.0879 | −0.7 (−1.4 to −0.0), P = 0.0479 |
| Time to peak (min) | 122 (110–133) | −3 (−18 to 13), P = 0.7337 | 11 (−2 to 25), P = 0.0936 | 14 (2–26), P = 0.0238 |
| Peak (µmol/L) | 0.10 (0.09–0.11) | 0.01 (−0.05 to 0.02), P = 0.2448 | 0.00 (−0.01 to 0.01), P = 0.8526 | −0.01 (−0.01 to −0.00), P = 0.0439 |
Data are mean (95% CI). SF-36, 36-Item Short Form Health Survey, version 2.
Glycemic Variability From CGM
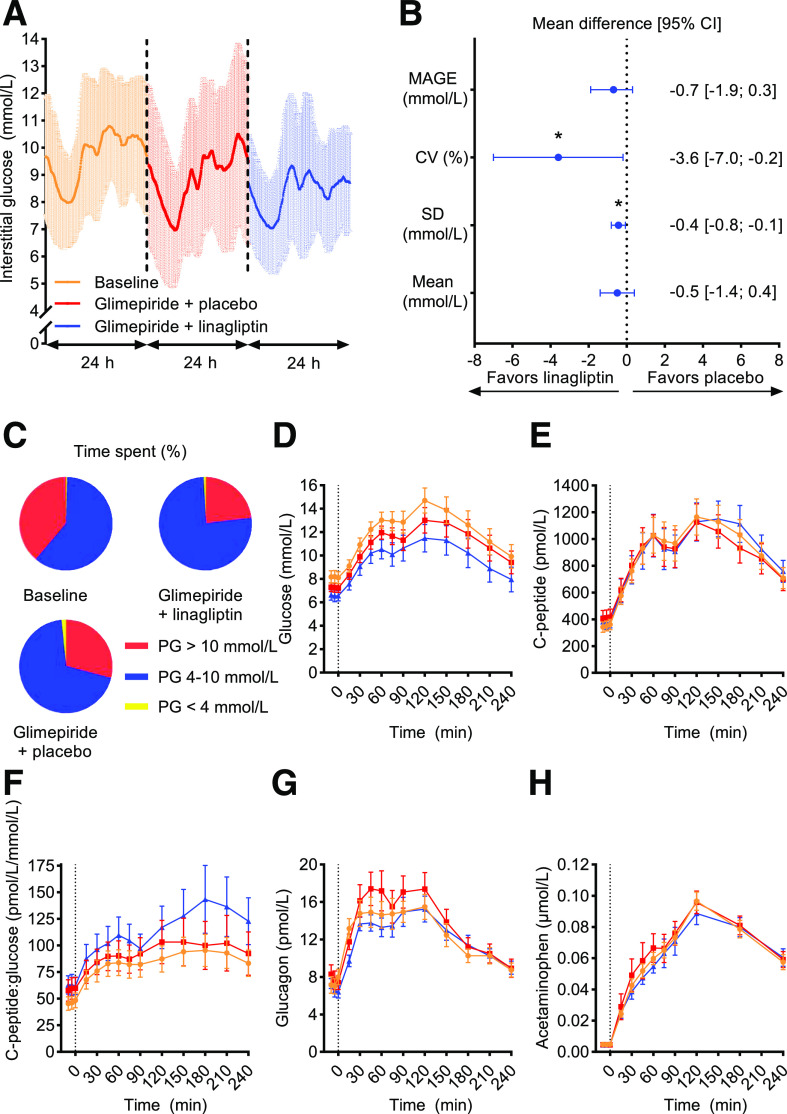
No significant reduction in the primary end point, MAGE, was observed with glimepiride + linagliptin compared with glimepiride + placebo (−0.7 mmol/L [−1.9 to 0.4], P = 0.1540) (Table 2, Fig. 1A–C, and Supplementary Table 3). Compared with glimepiride + placebo, glimepiride + linagliptin resulted in significant reductions in both CV (−3.6% [−7.0 to −0.2], P = 0.0401) and SD (−0.4 mmol/L [−0.8 to −0.1], P = 0.0210), while mean plasma glucose did not change significantly (−0.5 mmol/L [−1.4 to 0.4], P = 0.2341). A trend toward a reduction in time spent in hyperglycemia and an increase in time spent in normoglycemia with glimepiride + linagliptin versus glimepiride + placebo was also observed (Table 2).
Figure 1.
CGM and meal and bicycle test. A: Mean ± SD values from CGM during 24 h at baseline and at the end of two 16-week treatment periods of glimepiride + linagliptin and glimepiride + placebo in 19 patients with HNF1A diabetes. B: Differences in means and 95% CI in glycemic variability calculated from CGM data between glimepiride + linagliptin and glimepiride + placebo. C: Mean percent time spent in different plasma glucose (PG) ranges, calculated from CGM. D–H: Combined meal and bicycle test concentration versus time for plasma/serum glucose (D), C-peptide (E), C-peptide–to–glucose ratio (F), glucagon (G), and acetaminophen (H). Graphics data are mean ± SEM if not otherwise indicated. Orange circle, baseline; red square, glimepiride + placebo; blue triangle, glimepiride + linagliptin. *P < 0.05.
Glycemic Control
A reduction in HbA1c was observed with glimepiride + linagliptin compared with glimepiride + placebo (−0.5% [−0.9 to −0.2] (−5.6 mmol/mol [−9.3 to −1.8]), P = 0.0048) (Table 2). The proportions of patients achieving an HbA1c ≤6.5% (48 mmol/mol) or a decrement ≥0.5% (5 mmol/mol) without hypoglycemia (during the maintenance phase) were 53% (9 of 17) with glimepiride + linagliptin and 44% (8 of 18) with glimepiride + placebo (Supplementary Table 2).
Fasting Plasma Glucose
Compared with glimepiride + placebo, glimepiride + linagliptin reduced fasting plasma glucose (−0.7 mmol/L [−1.4 to −0.0], P = 0.0492), and both treatments reduced fasting plasma glucose compared with baseline (Table 2).
Hypoglycemia
During CGM, 15 vs. 32 episodes of hypoglycemia were observed with glimepiride + linagliptin versus glimepiride + placebo, respectively (Table 3 and Supplementary Table 3). The median time spent in a state of hypoglycemia was in general short: 0.0% (baseline), 0.1% (glimepiride + linagliptin), and 0.3% (glimepiride + placebo), respectively (Table 3). In contrast, when hypoglycemic events were assessed from self-reported and self-measured blood glucose during the maintenance period, a total of 16 and 10 episodes of hypoglycemia were observed with glimepiride + linagliptin versus glimepiride + placebo, respectively (Table 3 and Supplementary Table 2). Dose reductions of glimepiride due to recurrent hypoglycemia occurred more frequently during the maintenance period in the glimepiride + linagliptin arm (two reductions in one patient and one reduction in two patients) compared with the glimepiride + placebo arm (one reduction in one patient). No severe hypoglycemia was reported. Two events of hypoglycemia were observed during the meal and bicycle test in both treatment arms.
Table 3.
Hypoglycemia
| Baseline (n = 19) | Glimepiride + placebo (n = 18) | Glimepiride + linagliptin (n = 17) | |
|---|---|---|---|
| Hypoglycemia during CGM | |||
| Total episodes | 8 | 32 | 15 |
| Level 1 episodes | 6 | 30 | 14 |
| Level 2 episodes | 2 | 2 | 1 |
| Patients without episode | 17 | 6 | 12 |
| Percent time spend in hypoglycemia during CGM | |||
| Total | 0.0 (0.0–0.0) | 0.3 (0–3.4) | 0.1 (0–3.4) |
| Level 1 | 0.0 (0.0–0.0) | 0.3 (0.0–3.0) | 0.1 (0.0–0.7) |
| Level 2 | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) |
| Patient-reported hypoglycemia in maintenance phase (weeks 5–15 and 24–35) | |||
| Total episodes | N/A | 10 | 16 |
| Level 1 episodes | 10 | 15 | |
| Level 2 episodes | 0 | 1 | |
| Patients without episode | 11 | 10 | |
| Hypoglycemia during meal and bicycle test | |||
| Level 1 episodes | 1 | 2 | 2 |
| Level 2 episodes | 0 | 0 | 0 |
Data are n (counts) or median (interquartile range). A hypoglycemic episode verified by CGM is defined as glucose measurements ≤3.9 mmol/L in at least 20 consecutive minutes. Patient-reported episodes of hypoglycemia are defined as blood glucose <4.0 mmol/L confirmed by self-measured blood glucose measurements on a glucometer. Level 1 hypoglycemia is a glucose value of 3.0–3.9 mmol/L and level 2 hypoglycemia is defined as a glucose value <3.0 mmol/L. Both level 1 and level 2 hypoglycemia are per definition nonsevere hypoglycemia (no need of third-party assistance for recovery of hypoglycemia).
Body Weight
There was a significant increase in body weight with glimepiride + placebo (1.2 kg [0.4–2.0], P = 0.0078). Glimepiride + linagliptin was body weight neutral (0.2 kg [−0.7 to 1.0], P = 0.7025). The mean difference in body weight changes between treatment arms was −1.0 kg (−2.3 to 0.2), P = 0.0961.
Meal and Bicycle Test
Time courses for plasma/serum glucose, glucagon, C-peptide, C-peptide–to–glucose ratio, and acetaminophen are presented in Fig. 1. Glimepiride + linagliptin resulted in a greater reduction in plasma glucose AUC compared with glimepiride + placebo, and both treatment regimens reduced AUC for plasma glucose compared with baseline (Table 2). Likewise, the β-cell sensitivity for glucose assessed by AUC for C-peptide–to–glucose ratio was significantly greater with glimepiride + linagliptin compared with glimepiride + placebo (Fig. 1 and Table 2). An increase in postprandial glucagon levels from time 0 min was observed regardless of treatment (Fig. 1). Gastric emptying assessed by acetaminophen absorption was slower with glimepiride + linagliptin compared with glimepiride + placebo assessed by AUC, time to peak, and peak concentration (Fig. 1 and Table 2).
Quality of Life
No significant changes were observed in mental component scores or physical component scores between treatment arms (Table 2).
Adverse Events
No serious adverse events occurred during the trial. Hypoglycemia was the most frequently reported adverse event. Nonhypoglycemic adverse event counts and types were similar with both treatments (Supplementary Table 4).
Conclusions
This randomized, double-blinded, crossover study is the first to evaluate add-on treatment to sulfonylurea in patients with HNF1A diabetes. We find that addition of the DPP-4 inhibitor linagliptin to the sulfonylurea glimepiride does not improve MAGE but improves CV, SD, HbA1c, and β-cell glucose sensitivity without increasing the risk of hypoglycemia.
A crossover design was chosen to ensure enough power given the low prevalence of HNF1A diabetes and lessen recruitment struggles. For reduction of a potential carryover effect, a 4-week washout period was introduced between treatments. A limitation to our study is that four patients were related (mother and son in two families); however, our statistical analyses indicate no effect of family. Another limitation is that our study investigated linagliptin given as an add-on therapy before the therapeutic potential of a dose escalation of glimepiride had been fully explored in all patients. Based on number of participants and time span, our study is the largest interventional study performed in patients with HNF1A diabetes; nevertheless, sample size is small and may reduce generalizability of our results. Of note, we only included adults (≥18 years), and our results may not apply to pediatric and adolescent populations with HNF1A diabetes. Three patients dropped out during the trial, but our statistical approach with linear mixed models implicitly imputes missing data by performing likelihood inference.
We chose MAGE as the primary end point, as it previously has been seen as a gold standard measurement of glycemic variability (31), though without a clear consensus on the topic. During the conduct of our study, a consensus report on how to report results on glycemic variability obtained from CGM was published. This strongly recommends CV and SD as primary and secondary key metrics, respectively, of glucose variability (27). Considering this publication, it is fair to say that linagliptin added to glimepiride treatment in the current study improved glycemic variability as assessed by CV and SD.
In interpretation of the results from our study, it is key to bear in mind that we used a treat-to-target approach. This resulted in a dose reduction of glimepiride in 9 of 17 patients (a significant mean dose reduction of 0.7 mg) during the glimepiride + linagliptin period compared with the glimepiride + placebo period. Given the high efficacy of sulfonylureas in patients with HNF1A diabetes, demonstrated in other clinical studies (11,15), we believe that this is a clinically relevant dose reduction.
The rationale for investigating DPP-4 inhibition in the current study originates from clinical experience with the inhibitors in patients with HNF1A diabetes and their well-known mode of action on the β-cells (32,33). Furthermore, our group has previously shown that patients with HNF1A diabetes have an impaired incretin effect (34) and that sulfonylurea-induced insulin secretion can be potentiated by addition of exogenous GIP and GLP-1 infusions, respectively (24). In line with these observations, we here demonstrate an improved β-cell sensitivity to glucose (as evaluated from the increase in AUC for C-peptide–to–glucose ratio during the combined meal and bicycle test) with glimepiride + linagliptin compared with glimepiride + placebo. This finding is in line with the effect of DPP-4 inhibitors in patients with type 2 diabetes (35,36). Clinically, this may translate into improvements of long-term glycemic control and low glycemic variability. Given the previous study showing insulinotropic actions of both GIP and GLP-1, it seems likely that the elevated levels of both hormones due to DPP-4 inhibition contribute to the insulinotropic effect observed in the current study.
We observed a discrepancy in the number of episodes of hypoglycemia measured with CGM (glimepiride + linagliptin vs. glimepiride + placebo: 15 vs. 32 events, respectively) and self-reported events during the maintenance phase (glimepiride + linagliptin vs. glimepiride + placebo: 16 vs. 10 events). Our interpretation of these findings is that the combination therapy was indeed efficacious and that an increased number of HNF1A patients needed dose reductions of glimepiride (due to recurrent hypoglycemia during the maintenance phase) when treated with glimepiride + linagliptin versus glimepiride + placebo. A dose titration targeted on fasting blood glucose levels of 4.5–6.0 mmol/L and absence of hypoglycemia over a time course of 4 weeks may have been too short to fully evaluate the risk of hypoglycemia. This is probably why some patients had recurrent hypoglycemia and dose reductions of glimepiride during the maintenance phase. Given the differences in observed hypoglycemia between the methods used, we believe it is fair to conclude that the risk of hypoglycemia is similar with both treatments.
We found that linagliptin added to glimepiride treatment exerted small but statistically significant reductions in gastric emptying (evaluated by the acetaminophen absorption test) (Table 2). Studies in patients with type 2 diabetes have not shown any effect of DPP-4 inhibition on gastric emptying as assessed by radioactively labeled meals (37) or the acetaminophen absorption test (35), and the present finding is likely of no or little clinical relevance and may represent a chance finding. Our finding could also be attributable to the timing of blood samples with infrequent blood sampling near time to peak, which may reduce the precision of the acetaminophen absorption test.
In conclusions, the DPP-4 inhibitor linagliptin added to treatment with the sulfonylurea glimepiride in patients with HNF1A diabetes reduced glycemic variability (as evaluated from CV and SD but not MAGE) and HbA1c without increasing the risk of hypoglycemia or other adverse events.
Article Information
Acknowledgments. The authors thank the study participants for commitment and loyalty. The authors thank Sisse Marie Schmidt, Inass Al Nachar, Kirsten Abelin (all from Steno Diabetes Center Copenhagen, Gentofte Hospital, Hellerup, Denmark), and Eva Schriver (Steno Diabetes Center Aarhus, Aarhus University Hospital, Aarhus, Denmark) for laboratory assistance. The authors thank coworkers in their departments for valuable advice and support.
Duality of Interest. The study was funded by an unrestricted grant from Boehringer Ingelheim, Copenhagen, Denmark. H.S. has served on advisory panels for Novo Nordisk and has given paid lectures for Novo Nordisk. J.J.H. has served on scientific advisory panels for Novo Nordisk and has given paid lectures for Novo Nordisk and Merck Sharp & Dohme (MSD). F.K.K. has served on scientific advisory panels for, been part of speakers bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Carmot Therapeutics, Eli Lilly, Gubra, MedImmune, MSD/Merck, Mundipharma, Norgine, Novo Nordisk, Sanofi, and Zealand Pharma. T.V. has served on scientific advisory panels for, served on speakers bureaus for, served as a consultant to, and/or received research support from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, MSD, Mundipharma, Novo Nordisk, Sanofi, and Sun Pharma. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. A.S.C., S.H., H.S., T.H., F.K.K., and T.V. designed the study and wrote the study protocol. A.S.C., J.S., and M.S. performed the study. J.J.H. measured glucagon. J.L.F. assisted with the statistical analyses. A.S.C. did the data analysis and wrote the first manuscript draft. All authors critically edited the manuscript and approved the final version. A.S.C. and T.V. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
Clinical trial reg. no. 2017-000204-15, https://eudract.ema.europa.eu
This article contains supplementary material online at https://doi.org/10.2337/figshare.12515230.
References
- 1.Pihoker C, Gilliam LK, Ellard S, et al.; SEARCH for Diabetes in Youth Study Group . Prevalence, characteristics and clinical diagnosis of maturity onset diabetes of the young due to mutations in HNF1A, HNF4A, and glucokinase: results from the SEARCH for Diabetes in Youth. J Clin Endocrinol Metab 2013;98:4055–4062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shields BM, Hicks S, Shepherd MH, Colclough K, Hattersley AT, Ellard S. Maturity-onset diabetes of the young (MODY): how many cases are we missing? Diabetologia 2010;53:2504–2508 [DOI] [PubMed] [Google Scholar]
- 3.Colclough K, Bellanne-Chantelot C, Saint-Martin C, Flanagan SE, Ellard S. Mutations in the genes encoding the transcription factors hepatocyte nuclear factor 1 alpha and 4 alpha in maturity-onset diabetes of the young and hyperinsulinemic hypoglycemia. Hum Mutat 2013;34:669–685 [DOI] [PubMed] [Google Scholar]
- 4.Isomaa B, Henricsson M, Lehto M, et al. Chronic diabetic complications in patients with MODY3 diabetes. Diabetologia 1998;41:467–473 [DOI] [PubMed] [Google Scholar]
- 5.Steele AM, Shields BM, Shepherd M, Ellard S, Hattersley AT, Pearson ER. Increased all-cause and cardiovascular mortality in monogenic diabetes as a result of mutations in the HNF1A gene. Diabet Med 2010;27:157–161 [DOI] [PubMed] [Google Scholar]
- 6.Stride A, Vaxillaire M, Tuomi T, et al. The genetic abnormality in the beta cell determines the response to an oral glucose load. Diabetologia 2002;45:427–435 [DOI] [PubMed] [Google Scholar]
- 7.Pearson ER, Velho G, Clark P, et al. beta-cell genes and diabetes: quantitative and qualitative differences in the pathophysiology of hepatic nuclear factor-1alpha and glucokinase mutations. Diabetes 2001;50(Suppl. 1):S101–S107 [DOI] [PubMed] [Google Scholar]
- 8.Wollheim CB. Beta-cell mitochondria in the regulation of insulin secretion: a new culprit in type II diabetes. Diabetologia 2000;43:265–277 [DOI] [PubMed] [Google Scholar]
- 9.Hansen T, Eiberg H, Rouard M, et al. Novel MODY3 mutations in the hepatocyte nuclear factor-1α gene: evidence for a hyperexcitability of pancreatic β-cells to intravenous secretagogues in a glucose-tolerant carrier of a P447L mutation. Diabetes 1997;46:726–730 [DOI] [PubMed] [Google Scholar]
- 10.Tripathy D, Carlsson Å-L, Lehto M, Isomaa B, Tuomi T, Groop L. Insulin secretion and insulin sensitivity in diabetic subgroups: studies in the prediabetic and diabetic state. Diabetologia 2000;43:1476–1483 [DOI] [PubMed] [Google Scholar]
- 11.Pearson ER, Starkey BJ, Powell RJ, Gribble FM, Clark PM, Hattersley AT. Genetic cause of hyperglycaemia and response to treatment in diabetes. Lancet 2003;362:1275–1281 [DOI] [PubMed] [Google Scholar]
- 12.Pearson ER, Liddell WG, Shepherd M, Corrall RJ, Hattersley AT. Sensitivity to sulphonylureas in patients with hepatocyte nuclear factor-1α gene mutations: evidence for pharmacogenetics in diabetes. Diabet Med 2000;17:543–545 [DOI] [PubMed] [Google Scholar]
- 13.ISPAD Clinical Practice Consensus Guidelines 2018. [Internet], 2018. Available from https://www.ispad.org/page/ISPADGuidelines2018. Accessed 13 December 2019
- 14.American Diabetes Association 9. Pharmacologic approaches to glycemic treatment: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019;42(Suppl. 1):S90–S102 [DOI] [PubMed] [Google Scholar]
- 15.Østoft SH, Bagger JI, Hansen T, et al. Glucose-lowering effects and low risk of hypoglycemia in patients with maturity-onset diabetes of the young when treated with a GLP-1 receptor agonist: a double-blind, randomized, crossover trial. Diabetes Care 2014;37:1797–1805 [DOI] [PubMed] [Google Scholar]
- 16.Tuomi T, Honkanen EH, Isomaa B, Sarelin L, Groop LC. Improved prandial glucose control with lower risk of hypoglycemia with nateglinide than with glibenclamide in patients with maturity-onset diabetes of the young type 3. Diabetes Care 2006;29:189–194 [DOI] [PubMed] [Google Scholar]
- 17.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) UK Prospective Diabetes Study (UKPDS) Group [published correction appears in Lancet 1999;354:602]. Lancet 1998;352:837–853 [PubMed] [Google Scholar]
- 18.Christensen MB, Calanna S, Holst JJ, Vilsbøll T, Knop FK. Glucose-dependent insulinotropic polypeptide: blood glucose stabilizing effects in patients with type 2 diabetes. J Clin Endocrinol Metab 2014;99:E418–E426 [DOI] [PubMed] [Google Scholar]
- 19.Weir GC, Mojsov S, Hendrick GK, Habener JF. Glucagonlike peptide I (7-37) actions on endocrine pancreas. Diabetes 1989;38:338–342 [DOI] [PubMed] [Google Scholar]
- 20.Mari A, Sallas WM, He YL, et al. Vildagliptin, a dipeptidyl peptidase-IV inhibitor, improves model-assessed β-cell function in patients with type 2 diabetes. J Clin Endocrinol Metab 2005;90:4888–4894 [DOI] [PubMed] [Google Scholar]
- 21.McGill JB. Linagliptin for type 2 diabetes mellitus: a review of the pivotal clinical trials. Ther Adv Endocrinol Metab 2012;3:113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mentis N, Vardarli I, Köthe LD, et al. GIP does not potentiate the antidiabetic effects of GLP-1 in hyperglycemic patients with type 2 diabetes. Diabetes 2011;60:1270–1276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aaboe K, Akram S, Deacon CF, Holst JJ, Madsbad S, Krarup T. Restoration of the insulinotropic effect of glucose-dependent insulinotropic polypeptide contributes to the antidiabetic effect of dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab 2015;17:74–81 [DOI] [PubMed] [Google Scholar]
- 24.Christensen AS, Hædersdal S, Storgaard H, et al. GIP and GLP-1 potentiate sulfonylurea-induced insulin secretion in hepatocyte nuclear factor 1-α mutation carriers. Diabetes. 9 June 2020. [Epub ahead of print]. DOI: 10.2337/db20-0074 [DOI] [PMC free article] [PubMed]
- 25.Sidelmann Christensen A, Storgaard H, Hædersdal S, Hansen T, Krag Knop F, Vilsbøll T. Glimepiride monotherapy versus combination of glimepiride and linagliptin therapy in patients with HNF1A-diabetes: a protocol for a randomised, double-blinded, placebo-controlled trial. BMJ Open 2018;8:e022517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.International Hypoglycaemia Study Group Glucose concentrations of less than 3.0 mmol/L (54 mg/dL) should be reported in clinical trials: a joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017;40:155–157 [DOI] [PubMed] [Google Scholar]
- 27.Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liebl A, Henrichs HR, Heinemann L, Freckmann G, Biermann E, Thomas A; Continuous Glucose Monitoring Working Group of the Working Group Diabetes Technology of the German Diabetes Association . Continuous glucose monitoring: evidence and consensus statement for clinical use. J Diabetes Sci Technol 2013;7:500–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill NR, Oliver NS, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther 2011;13:921–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saisho Y, Tanaka C, Tanaka K, et al. Relationships among different glycemic variability indices obtained by continuous glucose monitoring. Prim Care Diabetes 2015;9:290–296 [DOI] [PubMed] [Google Scholar]
- 31.Baghurst PA. Calculating the mean amplitude of glycemic excursion from continuous glucose monitoring data: an automated algorithm. Diabetes Technol Ther 2011;13:296–302 [DOI] [PubMed] [Google Scholar]
- 32.Katra B, Klupa T, Skupien J, et al. Dipeptidyl peptidase-IV inhibitors are efficient adjunct therapy in HNF1A maturity-onset diabetes of the young patients--report of two cases. Diabetes Technol Ther 2010;12:313–316 [DOI] [PubMed] [Google Scholar]
- 33.Misra S, Owen KR. Genetics of monogenic diabetes: present clinical challenges. Curr Diab Rep 2018;18:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Østoft SH, Bagger JI, Hansen T, et al. Incretin effect and glucagon responses to oral and intravenous glucose in patients with maturity-onset diabetes of the young--type 2 and type 3. Diabetes 2014;63:2838–2844 [DOI] [PubMed] [Google Scholar]
- 35.DeFronzo RA, Okerson T, Viswanathan P, Guan X, Holcombe JH, MacConell L. Effects of exenatide versus sitagliptin on postprandial glucose, insulin and glucagon secretion, gastric emptying, and caloric intake: a randomized, cross-over study. Curr Med Res Opin 2008;24:2943–2952 [DOI] [PubMed] [Google Scholar]
- 36.Ahrén B, Landin-Olsson M, Jansson P-A, Svensson M, Holmes D, Schweizer A. Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J Clin Endocrinol Metab 2004;89:2078–2084 [DOI] [PubMed] [Google Scholar]
- 37.Vella A, Bock G, Giesler PD, et al. Effects of dipeptidyl peptidase-4 inhibition on gastrointestinal function, meal appearance, and glucose metabolism in type 2 diabetes. Diabetes 2007;56:1475–1480 [DOI] [PubMed] [Google Scholar]