Glucagon-like peptide 1 receptor agonists (GLP-1RAs) reduce cardiovascular (CV) events among patients with type 2 diabetes and high CV risk. Because consensus professional society recommendations endorse metformin as the first-line medication for type 2 diabetes, the CV efficacy of GLP-1RAs has primarily been studied with background metformin therapy (1). However, the European Society of Cardiology now recommends GLP-1RAs as a first-line type 2 diabetes treatment for patients at high CV risk (2). These discordant recommendations raise the question of how background metformin might influence the CV benefits of GLP-1RAs. Using data from the LEADER trial, we sought to answer this question by exploring possible heterogeneity in the CV efficacy of liraglutide related to baseline metformin treatment (3).
The Liraglutide Effect and Action in Diabetes: Evaluation of Cardiovascular Outcome Results (LEADER) trial (NCT01179048, ClinicalTrials.gov) has been described elsewhere (3). Patients with type 2 diabetes and high CV risk underwent double-blind randomization to daily liraglutide versus placebo in addition to existing care.
Each patient contributed data from randomization until censoring. The primary outcome was time from randomization to first occurrence of a composite of CV death, myocardial infarction, or stroke. Prespecified secondary outcomes included an expanded composite (primary plus coronary revascularization or hospitalization for unstable angina or heart failure), the composite components, and all-cause death. All outcomes were centrally adjudicated.
Metformin treatment was identified at the baseline trial visit and each visit thereafter. Within each baseline metformin treatment subgroup, effect estimates for liraglutide versus placebo (hazard ratio [HR], 95% CI) were derived using Cox proportional hazards regression models; multivariable adjustment for baseline demographic and clinical factors was performed. Inverse probability for treatment weighting (IPTW) was used to account for imbalances in covariates between baseline metformin treatment subgroups (4). For multivariable analyses with IPTW, heterogeneity in the association between baseline metformin treatment and the effect of liraglutide was estimated using stabilized, weighted Cox proportional hazards models with randomization group, baseline metformin treatment, and the interaction of both as fixed factors (5). A Pinteraction <0.05 was held to indicate a statistically significant difference in the treatment effect of liraglutide across baseline metformin subgroups. In order to explore the impact of postrandomization changes in metformin use, a sensitivity analysis repeated the main IPTW analysis for the primary outcome with censoring for initiation and discontinuation of metformin during the study. All data, methods, and study materials are available on request.
Primary results of the LEADER trial are presented elsewhere (3). Of 9,340 randomized participants, 7,144 (76%) used metformin at baseline. There were multiple differences between baseline metformin subgroups; notably, metformin-treated patients had shorter diabetes duration, higher estimated glomerular filtration rate (eGFR), lower heart failure prevalence, and less insulin use. All differences were attenuated by IPTW adjustment. Irrespective of randomization group, baseline metformin users had lower risk of the primary outcome than nonusers in multivariable analyses with IPTW (HRadjusted [95% CI] 0.72 [0.64; 0.81]).
In multivariable analyses with IPTW (Fig. 1), liraglutide did not significantly reduce incidence of the primary outcome versus placebo among baseline metformin users (HRadjusted [95% CI] 0.97 [0.85; 1.10]). Liraglutide did reduce incidence of the primary outcome among baseline metformin nonusers (HRadjusted [95% CI] 0.79 [0.64; 0.97]). Similar results were seen for the expanded composite. The P value for the interaction between randomization group and baseline metformin treatment did not achieve statistical significance for the primary outcome (Pinteraction = 0.10), the expanded composite (Pinteraction = 0.09), or other outcomes (Fig. 1).
Figure 1.
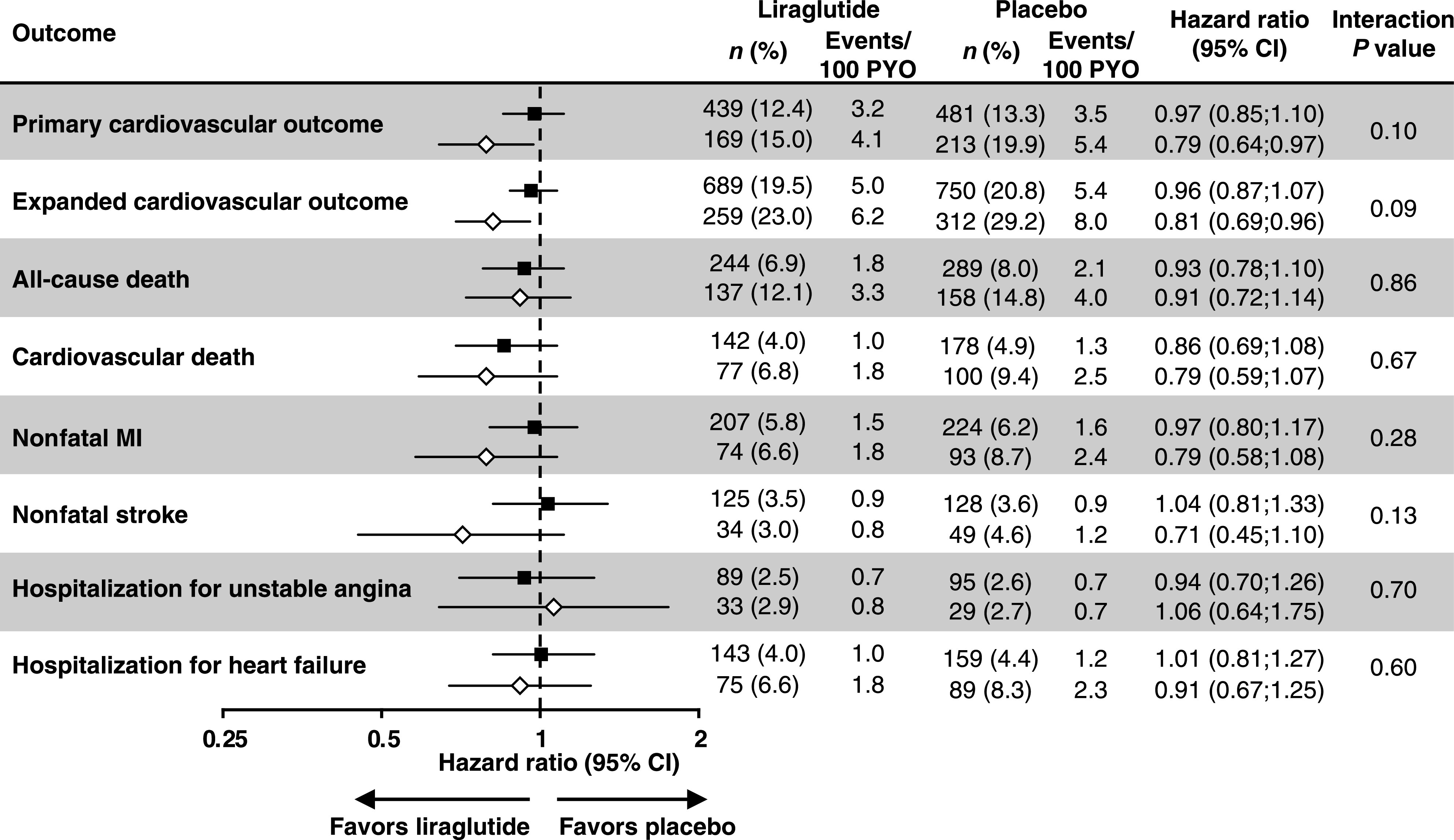
Effects of liraglutide (vs. placebo) on CV outcomes among patients with and without baseline metformin use, adjusted for baseline covariates with inverse probability weighting. Shaded squares = metformin use at baseline; empty diamonds = no metformin use at baseline. HRs derived using a Cox proportional hazards regression model with randomization group, baseline metformin exposure, and the interaction of both as factors, and diabetes duration, eGFR, and age at baseline as additional covariates, adjusted for baseline covariates: age, sex, region, diabetes duration, HbA1c, antihyperglycemic medication, eGFR, smoking, prior myocardial infarction, heart rhythm disorders, heart failure, left ventricular systolic dysfunction, left ventricular diastolic dysfunction, prior ischemic stroke, prior transient ischemic attack, prior hemorrhagic stroke, prior percutaneous coronary intervention, prior coronary bypass surgery, intracranial artery stenosis, carotid artery stenosis, peripheral arterial disease, and ≥50% stenosis of coronary, carotid, or other arteries. MI, myocardial infarction; n, number of patients with event; PYO, patient-years of observation; %, proportion of patients in subgroup with event.
Among baseline metformin users, 658 (18.6%) receiving liraglutide and 585 (16.2%) receiving placebo discontinued metformin during the trial. Among baseline nonusers, 249 (22.1%) receiving liraglutide and 299 (28.0%) receiving placebo initiated metformin. Sensitivity analysis results were similar to the main analysis for the primary outcome within baseline metformin user (HRadjusted [95% CI] 0.94 [0.82; 1.08]) and nonuser (HRadjusted [95% CI] 0.71 [0.55; 0.90]) subgroups. The interaction between randomization group and baseline metformin treatment did achieve statistical significance in the sensitivity analysis (Pinteraction = 0.046).
Whether background metformin treatment modifies the CV effects of GLP-1RAs is a foundational question underlying debates as to the optimal first-line type 2 diabetes treatment for patients with high CV risk. Although the effects of liraglutide appeared greater in the subgroup without baseline metformin treatment, our main analyses did not show statistically significant interactions between liraglutide treatment and metformin use. These findings may indicate that the CV benefits of liraglutide do not rely upon prior metformin treatment. As such, discussions regarding first-line treatment for type 2 diabetes in individuals with high CV risk should continue to focus on the absolute effectiveness of the agents in question, with appropriate consideration of costs to patients and society.
We conducted a sensitivity analysis exploring how postrandomization changes in metformin treatment impacted the interaction between randomization group and baseline metformin use. While CV effect estimates for liraglutide within the baseline metformin subgroups were similar to the main analysis, the interaction did achieve statistical significance in the sensitivity analysis. Analyzing nonrandomized exposures is challenging, and postrandomization changes in metformin use likely occurred for cause; interpreting this sensitivity analysis thus requires caution.
The present analyses add to existing evidence regarding the potential influence of metformin on the CV efficacy of newer diabetes agents. Previously, one GLP-1RA trial reported an unadjusted secondary analysis showing that the CV effects of albiglutide did not differ significantly across baseline metformin subgroups (6). A secondary analysis incorporating adjustment for baseline differences found that empagliflozin was associated with CV benefits irrespective of baseline metformin use (7). A trial-level meta-analysis without adjustment for baseline differences suggested possible variability in CV outcomes with dipeptidyl peptidase 4 inhibitors favoring baseline metformin users (8). Because potentially confounding factors like eGFR and heart failure prevalence may be independently associated with both baseline metformin use and CV outcomes, adjustment for propensity to receive metformin (as well as baseline differences) is critical and was a particular strength of our approach.
Importantly, the LEADER trial was not explicitly designed to evaluate the present research question. An appropriately powered randomized trial would be required to definitively ascertain heterogeneity in the CV efficacy of liraglutide.
In conclusion, we identified no clear evidence for heterogeneity in the CV efficacy of liraglutide based on background metformin use.
Article Information
Funding. M.J.C. receives support from the Durham Center of Innovation to Accelerate Discovery and Practice Transformation (ADAPT) (Health Services Research and Development grant CIN 13-410) at the Durham Veterans Affairs Health Care System. A.-S.A. is supported by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases under award number T32DK007012. S.V. holds a Canada Research Chair Tier 1 Chair in Cardiovascular Surgery. J.B.B. is supported in this work from grants from the National Institutes of Health (UL1TR002489 and P30DK124723).
The views expressed in this article are the authors’ and do not necessarily represent those of their employers or funding agencies.
Duality of Interest. Editorial support was provided by Watermeadow Medical under a contract from Novo Nordisk. D.K.M. reports personal fees for clinical trial leadership from AstraZeneca, Sanofi, Janssen, Boehringer Ingelheim, Merck & Co., Pfizer, Lilly USA, Novo Nordisk, Lexicon, Eisai Inc., GlaxoSmithKline, and Esperion and personal fees for consultancy from AstraZeneca, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, Merck & Co., Pfizer, Novo Nordisk, Dynavax, Metavant, Applied Therapeutics, and Afimmune. T.J.J. is a full-time employee of and stockholder in Novo Nordisk. S.R. is a full-time employee of and stockholder in Novo Nordisk (Significant). H.A.S. is a full-time employee of Novo Nordisk. S.V. has received grants and research support from Amgen, Abbott, Bayer, Boehringer Ingelheim, Lilly, AstraZeneca, Janssen, Sanofi, HLS Therapeutics, and Merck. J.B.B.’s contracted consulting fees are paid to the University of North Carolina by Adocia, AstraZeneca, Dance Biopharm, Dexcom, Eli Lilly, Fractyl, GI Dynamics, Intarcia Therapeutics, Lexicon, MannKind, Metavention, NovaTarg, Novo Nordisk, Orexigen, PhaseBio, Sanofi, Senseonics, vTv Therapeutics, and Zafgen. He reports grant support from AstraZeneca, Eli Lilly and Company, Intarcia Therapeutics, Johnson & Johnson, Lexicon, Medtronic, Novo Nordisk, Sanofi, Theracos, Tolerion, and vTv Therapeutics; is a consultant to Cirius Therapeutics Inc., CSL Behring, Mellitus Health, Neurimmune AG, Pendulum Therapeutics, and Stability Health; and holds stock/options in Mellitus Health, Pendulum Therapeutics, PhaseBio, and Stability Health. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.J.C. helped develop the analyses, interpreted data, and wrote the manuscript. All other authors contributed to developing the analyses, interpreting data, and reviewing and editing the manuscript. S.R. was responsible for conducting the analyses. M.J.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Clinical trial reg. no. NCT01179048, clinicaltrials.gov
References
- 1.Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018;41:2669–2701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323 [DOI] [PubMed] [Google Scholar]
- 3.Marso SP, Daniels GH, Brown-Frandsen K, et al.; LEADER Steering Committee; LEADER Trial Investigators . Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2016;375:311–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Austin PC. The use of propensity score methods with survival or time-to-event outcomes: reporting measures of effect similar to those used in randomized experiments. Stat Med 2014;33:1242–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Austin PC. The performance of different propensity score methods for estimating marginal hazard ratios. Stat Med 2013;32:2837–2849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez AF, Green JB, Janmohamed S, et al.; Harmony Outcomes committees and investigators . Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet 2018;392:1519–1529 [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Fitchett D, Jurišić-Eržen D, et al.; EMPA-REG OUTCOME® Investigators . Are the cardiovascular and kidney benefits of empagliflozin influenced by baseline glucose-lowering therapy? Diabetes Obes Metab 2020;22:631–639 [DOI] [PubMed] [Google Scholar]
- 8.Crowley MJ, Gokhale M, Pate V, Stürmer T, Buse JB. Impact of metformin use on the cardiovascular effects of dipeptidyl peptidase-4 inhibitors: an analysis of Medicare claims data from 2007 to 2015. Diabetes Obes Metab 2019;21:854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]


