Abstract
OBJECTIVE
To assess the relationship between subclinical cardiac dysfunction and aerobic exercise capacity (peak VO2) in adults with type 2 diabetes (T2D), a group at high risk of developing heart failure.
RESEARCH DESIGN AND METHODS
Cross-sectional study. We prospectively enrolled a multiethnic cohort of asymptomatic adults with T2D and no history, signs, or symptoms of cardiovascular disease. Age-, sex-, and ethnicity-matched control subjects were recruited for comparison. Participants underwent bioanthropometric profiling, cardiopulmonary exercise testing, and cardiovascular magnetic resonance with adenosine stress perfusion imaging. Multivariable linear regression analysis was undertaken to identify independent associations between measures of cardiovascular structure and function and peak VO2.
RESULTS
A total of 247 adults with T2D (aged 51.8 ± 11.9 years, 55% males, 37% black or south Asian ethnicity, HbA1c 7.4 ± 1.1% [57 ± 12 mmol/mol], and duration of diabetes 61 [32–120] months) and 78 control subjects were included. Subjects with T2D had increased concentric left ventricular remodeling, reduced myocardial perfusion reserve (MPR), and markedly lower aerobic exercise capacity (peak VO2 18.0 ± 6.6 vs. 27.8 ± 9.0 mL/kg/min; P < 0.001) compared with control subjects. In a multivariable linear regression model containing age, sex, ethnicity, smoking status, and systolic blood pressure, only MPR (β = 0.822; P = 0.006) and left ventricular diastolic filling pressure (E/e′) (β = −0.388; P = 0.001) were independently associated with peak VO2 in subjects with T2D.
CONCLUSIONS
In a multiethnic cohort of asymptomatic people with T2D, MPR and diastolic function are key determinants of aerobic exercise capacity, independent of age, sex, ethnicity, smoking status, or blood pressure.
Introduction
Heart failure (HF) has emerged as one of the commonest and deadliest complications of type 2 diabetes (T2D) (1). Even in asymptomatic individuals with T2D, there is a high prevalence of left ventricular (LV) systolic and diastolic dysfunction and/or cardiac remodeling (2,3). The American Heart Association has classified such individuals as having stage B HF (4), and this group is at high risk of developing clinical symptoms. Earlier identification of the cardiovascular manifestations of stage B HF may permit earlier diagnosis and treatment of those patients most at risk (5).
Individuals with T2D are recognized to have limitations in aerobic exercise capacity, even in the absence of overt cardiovascular disease (6,7), and this may be the first manifestation of stage B HF. VO2 is the gold standard method of assessing maximal aerobic capacity (8), and reduced peak VO2 is a strong risk factor for the development of cardiovascular disease and mortality (9), including HF (10). However, the relationship between cardiovascular structure, function, and aerobic exercise capacity in asymptomatic people with T2D is not fully understood.
Cardiovascular magnetic resonance imaging (CMR) is the gold standard imaging modality for assessment of cardiac volumes, mass, and ejection fraction (EF) and, with the addition of stress perfusion imaging, has the ability to provide accurate quantification of myocardial blood flow. No studies to date have used this technique to assess the associations of cardiovascular structure and function with aerobic exercise capacity in people with T2D.
The aims of this study were: 1) to determine the presence and nature of subclinical cardiovascular dysfunction in adults with T2D using multiparametric CMR, and 2) to evaluate whether markers of subclinical cardiovascular dysfunction are independently associated with peak VO2.
Research Design and Methods
Participants
This was a pooled analysis of individual baseline patient data from participants recruited to one of four studies evaluating the impact of T2D on cardiovascular structure and function (11–14). Adults with T2D were prospectively enrolled into these studies from primary and specialist care services in Leicestershire, U.K., with support from the National Institute for Health Research (NIHR) Clinical Research Network East Midlands. Participants included in the current analyses were aged 18–75 years, with no prior history, clinical signs or symptoms of cardiovascular disease, and no contraindications to CMR or cardiopulmonary exercise testing (CPET). Exclusion criteria were: type 1 diabetes, stage 4 or 5 chronic kidney disease (estimated glomerular filtration rate <30 mL/min/1.73 m2), known macrovascular disease (including myocardial infarction, transient ischemic attack, stroke, or peripheral artery disease), presence of arrhythmia, history of HF, moderate or worse valvular heart disease, and cardiovascular symptoms (such as angina or limiting dyspnea during normal physical activity). Age-, sex-, and ethnicity-matched control subjects without dysglycemia and free of prevalent cardiovascular disease were recruited for comparison. Ethical approval for each study was granted by the National Research Ethics Service, conducted according to the Declaration of Helsinki, and all participants provided written informed consent prior to any testing.
Assessments
Demographics, medical history, and anthropometric measures were collected at baseline assessment visits. Smoking status was categorized as: never smoked, ex-smoker, or current smoker. A fasting blood sample was collected for biochemical profile including diabetes control, lipids, and liver and kidney function.
CMR
CMR scanning was performed using a standardized protocol on Siemens scanners (Erlangen, Germany) at either 1.5T (Siemens Aera) or 3T (Siemens Skyra). In brief, after localizers, steady-state free precession cine images were acquired in four-, three-, and two-chamber views. Perfusion images were then acquired after vasodilatory stress with adenosine (140 to 210 μg/kg/min, infused intravenously for 3 min). At peak stress, a gadolinium-based contrast agent was injected followed by a 20-mL bolus of normal saline, at a rate of 5 mL/s, and perfusion images were acquired at three short-axis slices (basal, mid, and apex). Rest imaging was performed ∼10 min after stress. In between rest and stress imaging, a stack of short-axis cine slices was obtained with coverage of the entire LV. Late gadolinium enhancement (LGE) images were acquired ∼10 min after the rest perfusion contrast dose for assessment of focal myocardial fibrosis.
CMRs were analyzed offline blinded to all patient details. Cardiac chamber volumes, function, and strain were assessed by a single experienced observer (G.S.G.) using cmr42 version 5 (Circle Cardiovascular Imaging, Calgary, Alberta, Canada). Myocardial strain measurement was performed using cmr42 Tissue Tracking from balanced steady-state free-precession short-axis cine images (to calculate peak early diastolic strain rate [PEDSR]) and from long-axis cine images (to calculate global longitudinal strain [GLS]). Perfusion images were qualitatively assessed for focal and subendocardial perfusion defects, and individuals with reversible perfusion defects indicative of ischemia due to epicardial coronary artery disease were excluded from further analyses. Quantitative myocardial perfusion analysis was performed using a saturation recovery gradient echo-pulse sequence (at 1.5 T) (13), with signal intensity versus time curves converted to concentration curves using a linear signal response to contrast agent with Fermi-constrained deconvolution (15) or using a dual-sequence gradient echo method with inline automated reconstruction and postprocessing for myocardial blood flow quantification (at 3 T) (16) at base, mid, and apical slice positions. LGE images were assessed for focal fibrosis, categorized as present or absent, and individuals with a subendocardial pattern of late enhancement indicative of previous myocardial infarction were excluded from further analyses.
Transthoracic Echocardiography
Transthoracic echocardiography was performed in a subset of participants (175 subjects with T2D and 72 control subjects) by two accredited operators (A.-M.M. and Manjit Singh Sian) using an iE33 system with S5–1 transducer (Philips Medical Systems, Best, the Netherlands). Images were acquired and reported as per American Society of Echocardiography guidelines (17). Early diastolic transmitral flow velocities (E) and early diastolic mitral annular velocities (e′) to estimate LV filling pressures (E/e′) were assessed by Doppler echocardiography per current recommendations (18).
CPET
A symptom-limited incremental CPET was performed on a stationary electromagnetically braked cycle ergometer with expired gas analysis to determine peak VO2 (19). One-minute workload increments were based on participant age, sex, height, and weight (19). Each test was physician supervised with continuous electrocardiogram (ECG) monitoring and blood pressure recording at 2-min intervals. Indications for medical termination were as previously described (20). Subjects with ST-segment ECG changes indicative of myocardial ischemia during exercise testing were excluded from subsequent analyses. Breath-by-breath data were smoothed using a 30-s rolling mean, and peak VO2 was determined as the highest value.
Statistical Analysis
Normality was assessed using histograms, the Shapiro-Wilk test, and Q-Q plots. Continuous data are expressed as mean (± SD), if normally distributed, or median (interquartile range) if not. At baseline, patient and control groups were compared by independent t tests or Mann-Whitney tests as appropriate. Categorical variables are presented as absolute and relative frequency and were compared using the χ2 test or Fisher exact test as appropriate. Biochemical, CMR, echocardiography, and CPET variable between-group comparisons were undertaken using a general linear univariate ANOVA, with adjustments for variables age, sex, and ethnic group. Multiple imputation was used to impute missing CMR and echocardiography data. Correlations with peak VO2 were assessed using Pearson correlation coefficient separately in participants with and without T2D. Generalized linear modeling was performed to identify independent associations of aerobic exercise capacity separately in patients with and without T2D. The dependent variable was peak VO2 corrected for body weight. Only patients who achieved a respiratory exchange ratio (RER) ≥1 on CPET were included in correlation and regression analyses (total of 23 subjects with T2D excluded) to mitigate the confounding effects of tests in which reaching of peak VO2 was highly unlikely. A base model was adjusted for age, sex, ethnicity, smoking status, and systolic blood pressure, factors that are recognized for their associations with aerobic exercise capacity (21). CMR and echocardiographic variables that significantly correlated with peak VO2 were first analyzed individually in the base model. Those CMR or echocardiographic variables found to be individually associated with peak VO2 in the base model were then further selected and simultaneously entered into the base model to provide an assessment of whether these were associated with peak VO2 independently of one another. A correlation matrix of included factors was assessed for potential multicollinearity; variables correlated with a magnitude ≥0.5 or ≤ −0.5 were not included in the same regression model. Regression coefficients (β) are presented as point estimate and 95% CIs. Statistical analysis was performed by G.S.G., E.M.B., and T.Y. using SPSS version 25.0 (SPSS Inc., Chicago, IL). A P value <0.05 was considered statistically significant.
Results
The study profile is displayed in Fig. 1. At baseline, 259 subjects with T2D and 85 control subjects were recruited. Twelve subjects with T2D were found to be ineligible after consent. Reasons for ineligibility are shown in Fig. 1. A total of 247 subjects with T2D were therefore included in this analysis. Eighty-five healthy volunteers were enrolled for case-control comparison. Seven of these were subsequently excluded (three after blood sampling revealed a glycated hemoglobin level ≥6.0% and <6.5%, indicating the presence of prediabetes, three who were unable to undergo CMR scanning due to claustrophobia, and one who developed arrhythmia during CPET). A total of 78 healthy volunteers were therefore included in case-control comparisons.
Figure 1.
Study profile. MI, myocardial infarction.
Case-Control Comparisons
Bioanthropometric Characteristics
The baseline demographic characteristics of subjects with T2D and control subjects are shown in Table 1. Mean age of participants with T2D was 51.8 ± 11.9 years, mean BMI was 34.2 ± 6.0 kg/m2, median duration of diabetes was 61 (32–120) months, 45% were women, and 37% were from a black or minority ethnic group. The control group subjects were similar for age, sex, and ethnicity but had lower overall body weight and BMI. Those with T2D had a higher proportion of individuals with a history of smoking, hypertension, and dyslipidemia compared with control subjects. Antihypertensive and lipid-lowering medication use was therefore higher in those with T2D compared with control subjects.
Table 1.
Demographic, clinical, and bioanthropometric characteristics of subjects with T2D and control subjects
| Subjects with T2D (n = 247) | Control subjects (n = 78) | P value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 51.8 ± 11.9 | 51.5 ± 12.3 | 0.898 |
| Sex, n (%) | |||
| Male | 136 (55) | 42 (54) | 0.851 |
| Female | 111 (45) | 36 (46) | |
| Ethnic origin, n (%) | |||
| Caucasian | 155 (63) | 53 (68) | 0.405 |
| Black or other minority ethnicity | 92 (37) | 25 (32) | |
| Anthropometrics | |||
| Height, cm | 168 ± 10 | 170 ± 10 | 0.111 |
| Weight, kg | 96.9 ± 19.1 | 72.0 ± 13.6 | <0.001 |
| BMI, kg/m2 | 34.2 ± 6.0 | 24.8 ± 3.1 | <0.001 |
| Systolic blood pressure, mmHg | 138 ± 16 | 129 ± 18 | <0.001 |
| Diastolic blood pressure, mmHg | 87 ± 8 | 81 ± 9 | <0.001 |
| Heart rate, bpm | 76 ± 12 | 63 ± 11 | <0.001 |
| Medical history | |||
| Diabetes duration, months | 61 (32–120) | N/A | N/A |
| Smoking history, n (%) | |||
| Never smoked | 140 (56) | 50 (64) | 0.023 |
| Ex-smoker | 68 (28) | 25 (32) | |
| Current smoker | 39 (16) | 3 (4) | |
| Hypertension, n (%) | 121 (49) | 5 (6) | <0.001 |
| Dyslipidemia, n (%) | 148 (60) | 7 (9) | <0.001 |
| Medications | |||
| ACE inhibitor, n (%) | 67 (27) | 4 (5) | <0.001 |
| ARB, n (%) | 28 (11) | 0 (0) | 0.002 |
| β-Blocker, n (%) | 16 (6) | 0 (0) | 0.024 |
| Calcium channel blocker, n (%) | 50 (20) | 1 (1) | 0.001 |
| Statin, n (%) | 144 (58) | 7 (9) | <0.001 |
| Metformin, n (%) | 214 (87) | N/A | N/A |
| Sulfonylurea, n (%) | 50 (20) | N/A | N/A |
| DPP-4 inhibitor, n (%) | 16 (6) | N/A | N/A |
| SGLT2 inhibitor, n (%) | 36 (15) | N/A | N/A |
| GLP-1 receptor agonist, n (%) | 17 (7) | N/A | N/A |
| Insulin, n (%) | 20 (8) | ||
| Fasting blood tests | |||
| Urea, mmol/L | 5.3 ± 1.3 | 5.4 ± 1.4 | 0.656 |
| Creatinine, mmol/L | 74 ± 16 | 76 ± 15 | 0.147 |
| Estimated GFR, mL/min | 84 ± 10 | 83 ± 9 | 0.811 |
| Glucose, mmol/L | 7.7 (6.7–9.5) | 5.0 (4.8–5.3) | <0.001 |
| HbA1c, % | 7.4 ± 1.1 | 5.4 ± 0.3 | <0.001 |
| HbA1c, mmol/mol | 57 ± 12 | 36 ± 3 | <0.001 |
| Total cholesterol, mmol/L | 4.5 ± 1.0 | 5.5 ± 1.0 | <0.001 |
| Triglycerides, mmol/L | 1.8 (1.2–2.6) | 1.0 (0.7–1.4) | <0.001 |
| LDL, mmol/L | 2.4 ± 0.8 | 3.2 ± 0.9 | <0.001 |
| Hemoglobin, g/L | 144 ± 15 | 144 ± 13 | 0.985 |
Data are n (%), mean ± SD, or median (interquartile range). Boldface type indicates P < 0.05.
ARB, angiotensin receptor blocker; bpm, beats per minute; DPP-4, dipeptidyl peptidase 4; GFR, glomerular filtration rate; GLP-1, glucagon-like peptide 1; N/A, not applicable; SGLT2, sodium–glucose cotransporter 2.
Fasting blood test results, adjusted for age, sex, and ethnicity, are displayed in Table 1. Both groups had similar renal function. Subjects with T2D had higher overall glycated hemoglobin and lower total cholesterol and LDL cholesterol than control subjects.
Cardiovascular Structure, Function, and Fitness
Baseline CMR, echocardiography, and CPET and echocardiography data comparing subjects with T2D and control subjects with adjustment for age, sex, and ethnicity are displayed in Supplementary Table 1. Patients with T2D had similar absolute LV volumes but smaller indexed LV volumes and higher LV mass, with increased concentric LV remodeling (LV mass/volume 0.84 ± 0.14 vs. 0.76 ± 0.11 g/mL; P < 0.001) compared with control subjects. Similarly, there was no difference in absolute left atrial (LA) volumes, but indexed LA volumes were smaller in subjects with T2D versus control subjects.
Overall, there was no difference in LV EF between groups; however, LV GLS was lower in subjects with T2D versus control subjects (−16.2 ± 2.4 vs. −17.4 ± 1.9%; P < 0.001). LA EF was similar in both groups (P = 0.278). Concerning diastolic function, there was no significant difference in LV PEDSR (1.02 ± 0.23 vs. 1.05 ± 0.22; P = 0.206) or average E/e′ (7.1 [3.1–9.4] vs. 7.1 [5.2–8.3]; P = 0.438) between groups, but E/A ratio was significantly lower in subjects with T2D (0.84 [0.66–1.05] vs. 1.10 [0.83–1.23]; P = 0.006).
Aortic distensibility was significantly lower in those with diabetes compared with control subjects (2.75 [1.74–4.03] vs. 4.92 [2.65–7.13] mmHg−1 × 10−3; P < 0.001). Stress and rest perfusion imaging was performed in 208 subjects with T2D and 77 control subjects, and overall myocardial perfusion reserve (MPR) was lower in subjects with T2D (2.60 ± 1.24 vs. 3.54 ± 1.15, respectively; P < 0.001). Prevalence of nonischemic LGE was low, and there was no significant difference in the presence of LGE between subjects with T2D and control subjects (14 vs. 15%; P = 0.740).
After adjustment for age, sex, and ethnicity, both absolute and body weight–corrected peak VO2 were significantly lower in the subjects with T2D versus control subjects (18.0 ± 6.6 vs. 27.8 ± 9.0 mL/kg/min; P < 0.001).
Correlations With Aerobic Exercise Capacity
Correlations of participant characteristics and CMR measures of cardiac structure and function, with peak VO2 separately in subjects with and without T2D, are displayed in Supplementary Table 2.
In subjects with T2D, significant correlations were observed between peak VO2 and age, T2D duration, systolic blood pressure, absolute and indexed LV volumes, LV EF, LV mass, LV GLS, average E/e′, and MPR. In control subjects, significant correlations were observed between peak VO2 and absolute and indexed LV volumes, LV EF, LV mass, absolute and indexed LA volumes, LV PEDSR, E/e′, MPR, and aortic distensibility.
Multivariable Associations With Aerobic Exercise Capacity
Participant Characteristics
Multivariable associations between participant characteristics and peak VO2 in subjects with and without T2D are displayed in Supplementary Table 3. In both groups with and without T2D, variables significantly associated with peak VO2 were age (participants with T2D: β = −0.195, P < 0.001; control subjects: β = −0.448, P < 0.001), male sex (subjects with T2D: β = 3.5437, P < 0.001; control subjects: β = 3.310, P = 0.029), and white ethnicity (subjects with T2D: β = 1.878, P = 0.011; control subjects: β = 4.915, P = 0.003). Smoking status and resting systolic blood pressure were not significantly associated with peak VO2 in either subjects with T2D or control subjects.
CMR and Echocardiographic Measures of Cardiovascular Structure and Function
Associations of CMR measures of cardiovascular structure and function with peak VO2, tested individually against the base model of bioanthropometric characteristics in participants with T2D and control subjects, are shown in Supplementary Table 3. In patients with T2D, LV EF (β = −0.108; P = 0.037), LV GLS (β = 0.265; P = 0.046), MPR (β = 0.798; P = 0.005), and E/e′ (β = −0.385; P < 0.001) had significant individual associations with peak VO2. In control subjects, only LV EDV (β = 0.082; P < 0.001), LV EF (β = −0.297; P = 0.012), and LV mass (β = 0.129; P < 0.001) were significantly associated with peak VO2.
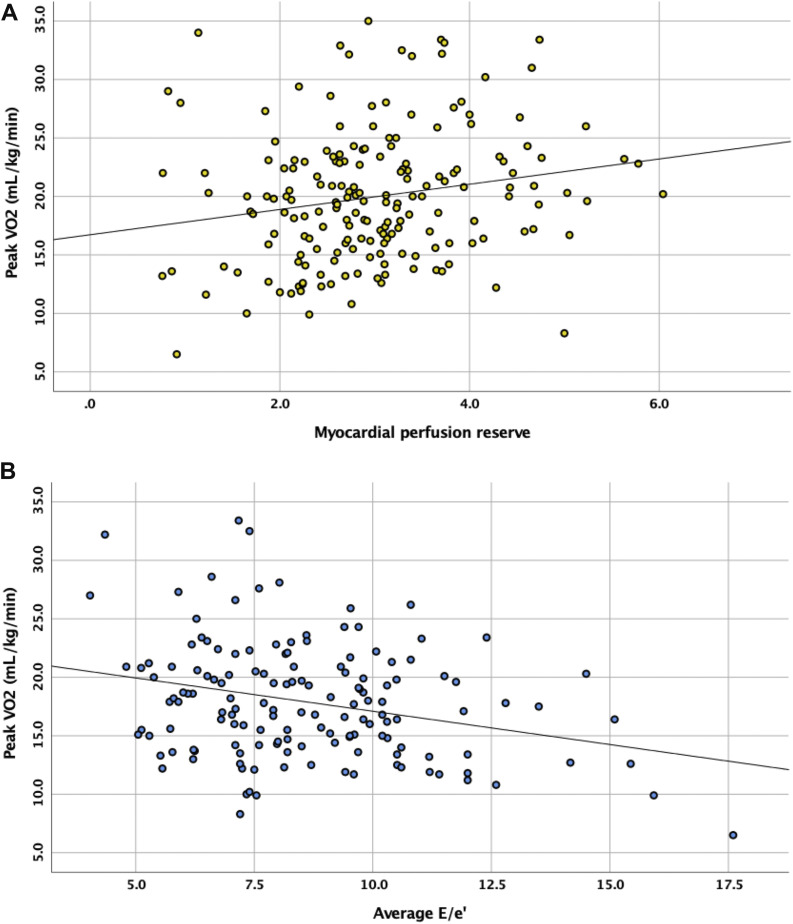
Multivariable associations between CMR measures of cardiovascular structure and function with significant individual associations with peak VO2, simultaneously added to the base model of bioanthropometric characteristics, are shown in Table 2. In subjects with T2D, only E/e′ (β = −0.388; P < 0.001) and MPR (β = 0.822; P = 0.006) were significantly associated with peak VO2 independent of age, sex, ethnicity, smoking status, and systolic blood pressure (Fig. 2). Addition of HbA1c to the model did not significantly affect these associations (Supplementary Table 4). In control subjects, only LV mass was significantly associated with peak VO2 (β = 0.116; P = 0.012).
Table 2.
Multivariable associations between measures of cardiovascular structure and function with peak VO2 in people with T2D and control subjects
| β | 95% CI | P value | |
|---|---|---|---|
| Subjects with T2D (n = 224)* | |||
| Variable | |||
| Age | −0.104 | −0.172 to −0.036 | 0.003 |
| Male sex | 2.345 | 0.909 to 3.781 | 0.001 |
| White ethnicity | 1.415 | −0.041 to 2.871 | 0.057 |
| Never smoked | 2.034 | 0.193 to 3.874 | 0.030 |
| Systolic blood pressure | −0.017 | −0.062 to 0.027 | 0.443 |
| LV EF | −0.041 | −0.150 to 0.067 | 0.453 |
| LV GLS | 0.214 | −0.072 to 0.499 | 0.142 |
| Myocardial perfusion reserve | 0.822 | 0.235 to 1.409 | 0.006 |
| Average E/e′ | −0.388 | −0.595 to −0.180 | <0.001 |
| Control subjects (n = 78) | |||
| Variable | |||
| Age | −0.446 | −0.563 to −0.329 | <0.001 |
| Male sex | −0.461 | −3.596 to 2.675 | 0.773 |
| White ethnicity | 2.929 | −0.220 to 6.078 | 0.068 |
| Never smoked | −5.636 | −12.185 to 0.914 | 0.092 |
| Systolic blood pressure | −0.037 | −0.125 to 0.052 | 0.417 |
| LV EDV | <0.001 | −0.072 to 0.072 | 0.998 |
| LV EF | −0.143 | −0.375 to 0.089 | 0.227 |
| LV mass | 0.116 | 0.026 to 0.206 | 0.012 |
Boldface type indicates P < 0.05.
EDV, end-diastolic volume.
Excluding subjects with peak RER <1 on CPET.
Figure 2.
Scatterplots displaying the correlations of peak VO2 in subjects with T2D with MPR (A) and E/e′ (B).
Conclusions
This is the first study to comprehensively describe the associations of aerobic exercise capacity with cardiac structure and function in asymptomatic people with T2D, using a combination of multiparametric CMR and echocardiography. Compared with control subjects, we have confirmed several markers of LV dysfunction in those with T2D, and of these, LV diastolic filling pressure (E/e′) and MPR were independently associated with peak VO2. By contrast, only LV mass was associated with peak VO2 in control subjects. Moreover, those with T2D displayed markedly lower levels of exercise capacity compared with control subjects in the presence of overall normal LV EF.
To our knowledge, only one other (smaller, n = 170) study published >15 years ago has assessed the cardiac determinants of exercise capacity in people with T2D (22). In a model containing age, male sex, BMI, and HbA1c, the only independent cardiac determinant of exercise capacity was basal early diastolic velocity. However, no measures of myocardial perfusion were performed. Exercise capacity was measured during treadmill stress testing performed for assessment of coronary artery disease and was estimated in metabolic equivalents and not peak VO2. Furthermore, we assessed cardiovascular structure and function by multiparametric CMR, which is not limited by poor acoustic windows and operator dependency as in echocardiography.
Although there is a high prevalence of diabetes in both common forms of HF—HF with preserved EF (HFpEF) and HF with reduced EF (HFrEF)—emerging evidence suggests that people with T2D are particularly prone to developing HFpEF (23,24). Recent secondary analyses of the Look AHEAD (Action for Health in Diabetes) trial have shown that baseline cardiorespiratory fitness is an independent predictor of incident HFpEF (but not HFrEF) in T2D, after adjustment for traditional cardiovascular risk factors and interval myocardial infarction. Even though our group with T2D overall had normal resting LV filling pressures, these were associated with peak VO2. It is well recognized that even in patients with HFpEF, in whom resting E/e′ may be within the normal range, exercise leads to abnormal elevations in LV filling pressures coupled with a diminished cardiac output reserve (25). A similar pattern has recently been observed in a cohort of asymptomatic people with T2D, in whom exercise echocardiography unmasked subclinical diastolic dysfunction and early HF even though resting filling pressures were within normal limits (26). We speculate that, because people with diabetes have less compliant ventricles, ventricular filling pressure rises faster on exercise than in control subjects.
While diastolic dysfunction has long been considered a central mechanism driving HFpEF, the role of microvascular inflammation and endothelial dysfunction is now increasingly being recognized (27). Subclinical alterations in myocardial perfusion could therefore be key drivers for the development of HFpEF in T2D (27), although studies evaluating the relationship between myocardial perfusion and diastolic function have to date yielded inconsistent findings (28,29), possibly due to different selection criteria and methods of assessment. Nevertheless, impaired MPR has been associated with increased cardiovascular mortality (30), and it is possible that targeting even subclinical impairments in myocardial perfusion might lower the risk of incident HF development in people with T2D. A striking finding in our cohort is that, even after excluding subjects with reversible perfusion defects, previous myocardial infarction on CMR, and myocardial ischemia on exercise ECG, subjects with T2D had lower overall MPR than control subjects, as has been shown in several other cohorts (31,32), and this was independently associated with exercise capacity. This finding is also physiologically plausible, as myocardial perfusion must increase during incremental exercise to meet myocardial oxygen demands, driven by increased heart rate and blood pressure. We have shown a similar relationship in pressure-overload hypertrophy in patients with aortic stenosis (33,34).
Interventions to improve diastolic function and myocardial blood flow in asymptomatic people with T2D could therefore attenuate progression from stage B HF to overt HFpEF. For example, we have recently shown in a randomized trial that improvements in diastolic function occurred with exercise but not dietary weight loss (35). Limited and conflicting data exist regarding the impact of newer glucose-lowering therapies (sodium–glucose cotransporter 2 inhibitors and glucagon-like peptide 1 receptor agonists) on diastolic function (36–38) in people with T2D, and these warrant further investigation. By contrast, few studies have evaluated treatment options for coronary microvascular dysfunction in T2D. In general, optimization of traditional cardiovascular risk factors is advocated in the first instance (39), although good glycemic control is not itself convincingly associated with improved coronary microvascular function (40). Few to no data exist to demonstrate the efficacy of ACE inhibition, β-blockade, calcium-channel inhibition, ranolazine, and nitrates on improving coronary microvascular function in T2D (39), although mineralocorticoid receptor antagonists may be beneficial (41). In a recent randomized, open-label, active comparator trial of 26-week treatment with liraglutide or sitagliptin in young obese adults with T2D, we found no improvement in MPR with either study drug, suggesting that targeting the incretin pathway may not improve microvascular dysfunction in the medium term (36). However, MPR was a secondary outcome measure, and the study was not therefore powered for this end point. Further studies are needed in people with T2D and stage B HF targeting both lifestyle and pharmacological interventions that improve diastolic function and/or MPR.
Strengths and Limitations
The major strengths of the study are the detailed cardiac phenotyping (including absolute quantification of myocardial perfusion), the large sample size, use of CPET for absolute quantification of exercise capacity, and close matching of patient and control groups. In addition, we rigorously excluded those with established cardiovascular disease or low RER, which may have confounded the results. Lastly, there was a high proportion of both females and ethnic minorities, which makes the results more generalizable.
Our study also has several limitations. This was a pooled cohort of baseline CPET and CMR data from participants of studies in our unit, with minor differences in recruitment criteria. However, we used prespecified inclusion and exclusion criteria for the present analyses to unify the study cohort, and all imaging was performed with standardized protocols and analysis techniques. We acknowledge that invasive angiography remains the gold-standard modality for assessment of coronary artery disease, and subjects with diffuse, three-vessel coronary disease may not have regional perfusion defects detectable by CMR. Different perfusion acquisition and analysis methods were used between the different pooled studies, which may have introduced systematic differences in MPR values (42). Each substudy had its own case subjects with T2D and control subjects, whom were analyzed with a common method, so differences in MPR between groups were not affected by analysis method.
As with any multiple regression model, there is a risk that omitted variables (which influence peak VO2) may have sloped the estimates for those variables that were included in model. To minimize this risk, we exercised a rigorous approach for selection of variables to be included in our final regression models. We first tested for correlations with both the dependent variable and assessed for potential multicollinearity, then individually tested correlated imaging variables against the base model before selecting the final model. We did not have data on markers of insulin resistance (such as the HOMA of insulin resistance), dietary intake, physical activity levels, etc., which may influence aerobic exercise capacity, and acknowledge this may have led to omitted variable bias and exaggerated the effect size of diastolic function and MPR. There is also the risk of measurement errors occurring in both our dependent variable (peak VO2) and imaging variables, which may have been a source of imprecision. Every effort was made to minimize this risk. All CPET studies were performed according to a standardized protocol, and a quality-control CPET is undertaken every 6 weeks using a biological control in our unit. Image analysis was performed using standard protocols by experienced observers blinded to patient details (to minimize observer bias), with excellent test-retest reproducibility in our laboratory (43–46).
Conclusion
In asymptomatic people with T2D, diastolic function and reduced MPR are key determinants of aerobic exercise capacity, independent of age, sex, ethnicity, smoking status, blood pressure, or glycemic control and may drive the progression of stage B HF. Further studies are needed to determine whether strategies to reverse subclinical abnormalities in cardiovascular function lead to improvements in exercise capacity and prevent HF development in T2D.
Article Information
Acknowledgments. The authors thank Susan Mackness (NIHR Leicester Biomedical Research Centre, Leicester, U.K.) for research nurse support, Joanne Wormleighton and Kelly Parke (University Hospitals of Leicester NHS Trust, Leicester, U.K.) for support with CMR protocol design and scanning, and the study participants. The authors acknowledge support from the NIHR Leicester Biomedical Research Centre, NIHR Leicester Clinical Research Facility, and the NIHR Collaboration in Leadership Applied Health Research and Care East Midlands.
Funding. This study was funded by the NIHR Research Trainees Coordinating Centre through a career development fellowship (CDF 2014-07-045 to G.P.M.), the British Heart Foundation through a Clinical Research Training Fellowship (FS/16/47/32190 to G.S.G.), the Medical Research Council through an Interdisciplinary Bridging Award, and Novo Nordisk.
Study funders provided financial support but had no role in study design (other than the external review process), data collection, data analysis, data interpretation, or writing of reports (including the current manuscript).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. E.M.B., K.K., D.W., M.J.D., T.Y., and G.P.M. contributed to the design of the study. G.S.G., J.H., J.A.S., E.G.W., L.A., and Z.Z.H. recruited study participants and supervised assessment visits and clinical reviews. A.-M.M. performed the echocardiograms and CPET. G.S.G., J.D.B., and P.K. analyzed the data. G.S.G., E.M.B., and T.Y. performed the statistical analyses. G.S.G. drafted the report, which was critically revised by E.M.B., K.K., M.J.D., T.Y., and G.P.M. All of the authors have read and approved the final version. G.P.M. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12490427.
References
- 1.Gulsin GS, Athithan L, McCann GP. Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Ther Adv Endocrinol Metab 2019;10:1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyer JK, Thanigaraj S, Schechtman KB, Pérez JE. Prevalence of ventricular diastolic dysfunction in asymptomatic, normotensive patients with diabetes mellitus. Am J Cardiol 2004;93:870–875 [DOI] [PubMed] [Google Scholar]
- 3.Yazici M, Ozdemir K, Gonen MS, et al. . Is there any relationship between metabolic parameters and left ventricular functions in type 2 diabetic patients without evident heart disease? Echocardiography 2008;25:675–682 [DOI] [PubMed] [Google Scholar]
- 4.Goldberg LR, Jessup M. Stage B heart failure: management of asymptomatic left ventricular systolic dysfunction. Circulation 2006;113:2851–2860 [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Yang H, Huynh Q, Nolan M, Negishi K, Marwick TH. Diagnosis of nonischemic stage B heart failure in type 2 diabetes mellitus: optimal parameters for prediction of heart failure. JACC Cardiovasc Imaging 2018;11:1390–1400 [DOI] [PubMed] [Google Scholar]
- 6.Reusch JE, Bridenstine M, Regensteiner JG. Type 2 diabetes mellitus and exercise impairment. Rev Endocr Metab Disord 2013;14:77–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Regensteiner JG, Bauer TA, Reusch JE, et al. . Cardiac dysfunction during exercise in uncomplicated type 2 diabetes. Med Sci Sports Exerc 2009;41:977–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Thoracic Society; American College of Chest Physicians ATS/ACCP statement on cardiopulmonary exercise testing [published correction appears in Am J Respir Crit Care Med 2003;1451–1452] Am J Respir Crit Care Med 2003;167:211–277 [DOI] [PubMed] [Google Scholar]
- 9.Laukkanen JA, Kurl S, Salonen R, Rauramaa R, Salonen JT. The predictive value of cardiorespiratory fitness for cardiovascular events in men with various risk profiles: a prospective population-based cohort study. Eur Heart J 2004;25:1428–1437 [DOI] [PubMed] [Google Scholar]
- 10.Khan H, Kunutsor S, Rauramaa R, et al. . Cardiorespiratory fitness and risk of heart failure: a population-based follow-up study. Eur J Heart Fail 2014;16:180–188 [DOI] [PubMed] [Google Scholar]
- 11.Gulsin GS, Brady EM, Swarbrick DJ, et al. . Rationale, design and study protocol of the randomised controlled trial: Diabetes Interventional Assessment of Slimming or Training tO Lessen Inconspicuous Cardiovascular Dysfunction (the DIASTOLIC study). BMJ Open 2019;9:e023207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Htike ZZ, Yates T, Brady EM, et al. . Rationale and design of the randomised controlled trial to assess the impact of liraglutide on cardiac function and structure in young adults with type 2 diabetes (the LYDIA study). Cardiovasc Diabetol 2016;15:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan JN, Wilmot EG, Leggate M, et al. . Subclinical diastolic dysfunction in young adults with type 2 diabetes mellitus: a multiparametric contrast-enhanced cardiovascular magnetic resonance pilot study assessing potential mechanisms. Eur Heart J Cardiovasc Imaging 2014;15:1263–1269 [DOI] [PubMed] [Google Scholar]
- 14.National Institute of Mental Health Prevalence and determinants of subclinical cardiovascular dysfunction in adults with type 2 diabetes mellitus (PREDICT). In ClinicalTrials.gov [Internet]. Bethesda, MD, National Library of Medicine, 2017. Available from https://clinicaltrials.gov/ct2/show/NCT03132129. Accessed 21 June 2020 [Google Scholar]
- 15.Biglands JD, Magee DR, Sourbron SP, Plein S, Greenwood JP, Radjenovic A. Comparison of the diagnostic performance of four quantitative myocardial perfusion estimation methods used in cardiac MR imaging: CE-MARC substudy. Radiology 2015;275:393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellman P, Hansen MS, Nielles-Vallespin S, et al. . Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson 2017;19:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Picard MH, Adams D, Bierig SM, et al.; American Society of Echocardiography . American Society of Echocardiography recommendations for quality echocardiography laboratory operations. J Am Soc Echocardiogr 2011;24:1–10 [DOI] [PubMed] [Google Scholar]
- 18.Nagueh SF, Smiseth OA, Appleton CP, et al.; Houston, Texas; Oslo, Norway; Phoenix, Arizona; Nashville, Tennessee; Hamilton, Ontario, Canada; Uppsala, Sweden; Ghent and Liège, Belgium; Cleveland, Ohio; Novara, Italy; Rochester, Minnesota; Bucharest, Romania; and St. Louis, Missouri . Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2016;17:1321–1360 [DOI] [PubMed] [Google Scholar]
- 19.Wasserman K. Principles of Exercise Testing and Interpretation: Including Pathophysiology and Clinical Applications. Philadelphia, PA, Lippincott Williams & Wilkins, 2005 [Google Scholar]
- 20.Das P, Rimington H, Smeeton N, Chambers J. Determinants of symptoms and exercise capacity in aortic stenosis: a comparison of resting haemodynamics and valve compliance during dobutamine stress. Eur Heart J 2003;24:1254–1263 [DOI] [PubMed] [Google Scholar]
- 21.Zeiher J, Ombrellaro KJ, Perumal N, Keil T, Mensink GBM, Finger JD. Correlates and determinants of cardiorespiratory fitness in adults: a systematic review. Sports Med Open 2019;5:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang ZY, Sharman J, Prins JB, Marwick TH. Determinants of exercise capacity in patients with type 2 diabetes. Diabetes Care 2005;28:1643–1648 [DOI] [PubMed] [Google Scholar]
- 23.Solomon SD, McMurray JJV, Anand IS, et al.; PARAGON-HF Investigators and Committees . Angiotensin-neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620 [DOI] [PubMed] [Google Scholar]
- 24.McMurray JJ, Packer M, Desai AS, et al.; PARADIGM-HF Investigators and Committees . Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004 [DOI] [PubMed] [Google Scholar]
- 25.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishi T, Kobayashi Y, Christle JW, et al. . Incremental value of diastolic stress test in identifying subclinical heart failure in patients with diabetes mellitus. Eur Heart J Cardiovasc Imaging 2020;21:876–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bayes-Genis A, Bisbal F, Núñez J, et al. . Transitioning from preclinical to clinical heart failure with preserved ejection fraction: a mechanistic approach. J Clin Med 2020;9:1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sørensen MH, Bojer AS, Broadbent DA, Plein S, Madsen PL, Gæde P. Cardiac perfusion, structure, and function in type 2 diabetes mellitus with and without diabetic complications. Eur Heart J Cardiovasc Imaging 2020;21:887–895 [DOI] [PubMed] [Google Scholar]
- 29.Korosoglou G, Humpert PM, Ahrens J, et al. . Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging 2012;35:804–811 [DOI] [PubMed] [Google Scholar]
- 30.Murthy VL, Naya M, Foster CR, et al. . Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation 2012;126:1858–1868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levelt E, Rodgers CT, Clarke WT, et al. . Cardiac energetics, oxygenation, and perfusion during increased workload in patients with type 2 diabetes mellitus. Eur Heart J 2016;37:3461–3469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cosson E, Pham I, Valensi P, Pariès J, Attali JR, Nitenberg A. Impaired coronary endothelium-dependent vasodilation is associated with microalbuminuria in patients with type 2 diabetes and angiographically normal coronary arteries. Diabetes Care 2006;29:107–112 [DOI] [PubMed] [Google Scholar]
- 33.Singh A, Greenwood JP, Berry C, et al. . Comparison of exercise testing and CMR measured myocardial perfusion reserve for predicting outcome in asymptomatic aortic stenosis: the PRognostic Importance of MIcrovascular Dysfunction in Aortic Stenosis (PRIMID AS) study. Eur Heart J 2017;38:1222–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steadman CD, Jerosch-Herold M, Grundy B, et al. . Determinants and functional significance of myocardial perfusion reserve in severe aortic stenosis. JACC Cardiovasc Imaging 2012;5:182–189 [DOI] [PubMed] [Google Scholar]
- 35.Gulsin GS, Swarbrick DJ, Athithan L, et al. . Effects of low-energy diet or exercise on cardiovascular function in working-age adults with type 2 diabetes: a prospective, randomized, open-label, blinded end point trial. Diabetes Care 2020;43:1300–1310 [DOI] [PubMed] [Google Scholar]
- 36.Webb DR, Htike ZZ, Swarbrick DJ, et al. . A randomized, open-label, active comparator trial assessing the effects of 26 weeks of liraglutide or sitagliptin on cardiovascular function in young obese adults with type 2 diabetes. Diabetes Obes Metab. 2020;22:1187–1196 [DOI] [PubMed] [Google Scholar]
- 37.Verma S, Garg A, Yan AT, et al. . Effect of empagliflozin on left ventricular mass and diastolic function in individuals with diabetes: an important clue to the EMPA-REG OUTCOME trial? Diabetes Care 2016;39:e212–e213 [DOI] [PubMed] [Google Scholar]
- 38.Bizino MB, Jazet IM, Westenberg JJM, et al. . Effect of liraglutide on cardiac function in patients with type 2 diabetes mellitus: randomized placebo-controlled trial [published correction appears in Cardiovasc Diabetol 2019;18:101] Cardiovasc Diabetol 2019;18:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kibel A, Selthofer-Relatic K, Drenjancevic I, et al. . Coronary microvascular dysfunction in diabetes mellitus. J Int Med Res 2017;45:1901–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valenzuela-Garcia LF, Matsuzawa Y, Sara JD, et al. . Lack of correlation between the optimal glycaemic control and coronary micro vascular dysfunction in patients with diabetes mellitus: a cross sectional study. Cardiovasc Diabetol 2015;14:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garg R, Rao AD, Baimas-George M, et al. . Mineralocorticoid receptor blockade improves coronary microvascular function in individuals with type 2 diabetes. Diabetes 2015;64:236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Broadbent DA, Biglands JD, Ripley DP, et al. . Sensitivity of quantitative myocardial dynamic contrast-enhanced MRI to saturation pulse efficiency, noise and t1 measurement error: comparison of nonlinearity correction methods. Magn Reson Med 2016;75:1290–1300 [DOI] [PubMed] [Google Scholar]
- 43.Shetye AM, Nazir SA, Razvi NA, et al. . Comparison of global myocardial strain assessed by cardiovascular magnetic resonance tagging and feature tracking to infarct size at predicting remodelling following STEMI. BMC Cardiovasc Disord 2017;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan JN, Singh A, Nazir SA, Kanagala P, Gershlick AH, McCann GP. Comparison of cardiovascular magnetic resonance feature tracking and tagging for the assessment of left ventricular systolic strain in acute myocardial infarction. Eur J Radiol 2015;84:840–848 [DOI] [PubMed] [Google Scholar]
- 45.Graham-Brown MP, Gulsin GS, Parke K, et al. . A comparison of the reproducibility of two cine-derived strain software programmes in disease states. Eur J Radiol 2019;113:51–58 [DOI] [PubMed] [Google Scholar]
- 46.Singh A, Steadman CD, Khan JN, et al. . Intertechnique agreement and interstudy reproducibility of strain and diastolic strain rate at 1.5 and 3 Tesla: a comparison of feature-tracking and tagging in patients with aortic stenosis. J Magn Reson Imaging 2015;41:1129–1137 [DOI] [PubMed] [Google Scholar]