Abstract
Background:
The molecular mechanisms underpinning the progesterone-triggering mood symptoms in women with premenstrual dysphoric disorder (PMDD) are unknown. Cell metabolism is a potential source of variability. Very little is known about the effect of progesterone sensitivity on the metabolome. In this study, we aimed to characterize the effects of progesterone on the global metabolic profile and explore the differences between women with PMDD and controls.
Methods:
Plasma was obtained from 12 women with prospectively confirmed PMDD and 25 controls under two hormone conditions: (1) gonadal suppression induced by leuprolide acetate (3.75 mg IM monthly) and (2) add-back phase with leuprolide and progesterone (200 mg twice daily by vaginal suppository). The global metabolic profile was obtained using liquid and gas chromatography followed by mass spectrometry. Differences between groups and time points were tested using repeated measures analysis of variance. The false discovery rate was calculated to account for multiple testing.
Results:
Amino acids and their derivatives represented 78% (28/36) of the known compounds that were found in significantly lower plasma concentrations after progesterone administration than during gonadal suppression. The concentration of tyrosine was nominally significantly decreased after progesterone add-back in controls, but not in cases (P = 0.02).
Conclusion:
Plasma levels of some amino acids are decreased in response to progesterone. Albeit preliminary, evidence further suggests that progesterone has a different effect on the metabolic profiles of women with PMDD compared to controls. Further research is needed to replicate our findings in a larger sample and to identify the unknown compounds, especially those differentially expressed.
Keywords: amino acids, leuprolide, menstrual cycle, metabolomics, women’s health
1. INTRODUCTION
Premenstrual dysphoric disorder (PMDD) is characterized by affective, cognitive, behavioral, and somatic symptoms that are limited to the late luteal phase of most menstrual cycles, cause significant impairment, and that do not represent an exacerbation of a concurrent psychiatric disorder (American Psychiatric Association, 2013). Prevalence estimates range between 1% and 6% (Gehlert, Song, Chang, & Hart-lage, 2009; Wittchen, Becker, Lieb, & Krause, 2002) in women of reproductive age.
Evidence from naturalistic studies and hormone manipulation protocols suggests that PMDD is characterized by an abnormal response to normal concentrations of progesterone (Dubey et al., 2017; Parry, Javeed, Laughlin, Hauger, & Clopton, 2000; Roca et al., 2003; Schmidt, Nieman, Danaceau, Adams, & Rubinow, 1998; Smith, Adams, Schmidt, Rubinow, & Wassermann, 2002; Sundström et al., 1998) rather than abnormal basal hormone levels (Rubinow et al., 1988). In the context of ovarian suppression, progesterone precipitates affective symptoms in women with PMDD, but not in controls without PMDD, suggesting a vulnerability to progesterone in PMDD (Schmidt et al., 1998).
The molecular mechanisms underpinning the progesterone-triggering mood symptoms in vulnerable women are unknown. The interindividual variability in sensitivity to progesterone can be due to a number of differences at the genomic, molecular, cellular, and tissue levels. Cell metabolism is a potential source of variability. Compared to the study of genes and proteins, that of metabolites (i.e., the small molecules that are produced by cell metabolism) has several advantages: metabolites are not subject to epigenetic or posttranslational modifications and present less interindividual variability than the genome (Patti, Yanes, & Siuzdak, 2012).
Very little is known about the effect of progesterone sensitivity on the metabolome. The only study published so far focused on the steroid metabolome and did not observe any differences in the formation of progesterone-derived neurosteroids (Nguyen et al., 2017). It still remains unclear whether progesterone has effects on the general metabolome, which includes many neuroregulatory small molecules.
The mechanisms of action of progesterone in vivo are difficult to disentangle because high levels of progesterone are always concurrent with estrogen elevation and may vary between subjects. Reproductive axis suppression followed by hormone add-back is the gold standard methodology to isolate the independent effects of progesterone and to test causal inference (Stachenfeld & Taylor, 2014). The suppression-administration protocols consist of two phases: (1) a temporary suppression of the menstrual cycle with gonadotropin-releasing hormone agonists and (2) the administration of estradiol and/or progesterone.
Consequently, we have performed an opportunistic pharmaco-metabolomics study, combining a hormone suppression-administration protocol with high-throughput, untargeted metabolomics to (1) characterize the global metabolic profile of eumenorrheic women during gonadal suppression and progesterone replacement, and (2) explore the differences in the global metabolic profiles between cases of PMDD and controls.
2. MATERIAL AND METHODS
2.1. Subjects
The study was performed on 37 women, 12 affected by PMDD and 25 controls, all eumenorrheic.
Women with PMDD were recruited via advertisements in the local newspapers or through their physicians. The diagnosis of PMDD was based on the criteria outlined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 2000) and was confirmed prospectively by daily self-reports for 3 months using a three-item 100-mm visual analogue scale assessing the timing and severity of mood symptoms (Rubinow, Roy-Byrne, Hoban, Gold, & Post, 1984). Women completed also the Daily Rating Form, consisting in four questions on distress and functional disability. The Daily Rating Form was then combined with a semi-structured interview and a self-report questionnaire (both in-house tools) in order to confirm that women met the number of symptoms required by the DSM-IV criteria. Our definition of PMDD was more stringent than that proposed by both DSM-IV and 5, as it included a severity criterion for the symptoms disclosed. Our severity criterion required a 30% or greater increase in the mean ratings of negative mood symptoms in the week before the menses compared with the week after menses in at least two of the three menstrual cycles rated.
Women who reported similar or higher levels of symptoms and impairment in the follicular phase compared to the luteal phase were excluded.
Controls were recruited via advertisement. The lack of premenstrual symptoms was ascertained using the same daily ratings used for cases for 2 months.
Exclusion criteria included the following: psychiatric and medical comorbidities and being pregnant and taking medications. Women in the PMDD group were included if they had not experienced any psychiatric disorders within the previous 2 years, while none of the controls had ever had a psychiatric diagnosis, as assessed by the Structured Clinical Interview for the Diagnostic Statistic Manual of Mental Disorders (First, Spitzer, Gibbon, & Williams, 2012). All women had a normal physical examination, including laboratory testing.
We requested that all women did not take any medications or supplements for the duration of the study.
All participants provided written informed consent and were paid for their participation according to the guidelines of the National Institutes of Health. The protocol was approved by the Central Neuro-science Institutional Review Board within the NIMH IRP.
2.2. Hormone manipulation protocol
The hormone manipulation protocol consisted of two phases (Figure 1):
FIGURE 1.
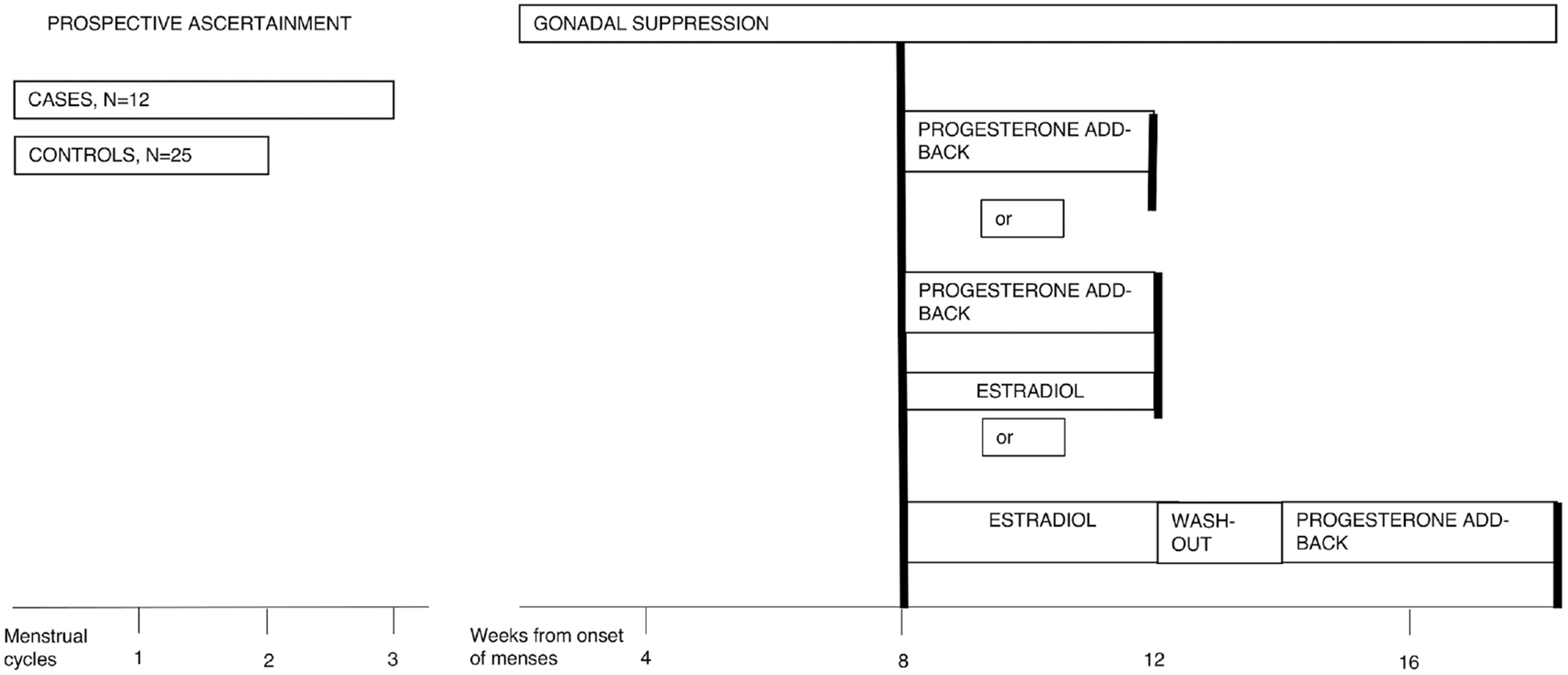
Schematic representation of the experimental hormone manipulation protocol. Between 2 and 6 days after onset of menses, participants received six monthly intramuscular injections of 3.75-mg leuprolide, a gonadotropin-releasing hormone agonist, which suppresses ovarian function after an initial stimulation. Clinic visits occurred every 2 weeks. Ovarian suppression was confirmed by plasma Follicle-stimulating hormone (FSH), Luteinizing hormone (LH), estradiol, and progesterone levels at each visit. Following 3 months of leuprolide alone, while continuing to receive monthly leuprolide injections for another 3 months, 24 participants received progesterone (200 mg vaginal suppository twice daily) only; 3 progesterone (200 mg vaginal suppository twice daily) and estradiol (100 mg daily by skin patch); and 10 women entered the progesterone add-back phase after 5 weeks of estradiol followed by 2-week washout
Gonadal suppression: subjects received monthly injections of the gonadotropin-releasing hormone agonist leuprolide acetate (3.75 mg IM), starting between 2 and 6 days after the onset of the menses for at least 8 weeks. At the end of the gonadal suppression phase, a fasting plasma sample was taken (time 1).
Progesterone add-back: the women entered a 4 week add-back phase, during which progesterone vaginal suppositories, 200 mg b.i.d., were administrated together with the monthly injection of leuprolide. At the end of the 4 weeks, another fasting plasma sample was taken (time 2). A total of 27 (10 cases and 17 controls) of the 37 women entered the progesterone add-back phase immediately following the gonadal suppression phase; for three of the 27 women, the add-back phase consisted of both progesterone and estradiol. The remaining 10 women (two cases and eight controls) entered the progesterone add-back phase after 5 weeks of estradiol, 0.1 mg per day, followed by 2-week washout. The proximity of estradiol administration to receiving progesterone did not affect mood ratings and progesterone plasma levels, which were similar across groups treated with different hormone protocols (Supporting Information Table 1).
2.3. Metabolomic analyses
Metabolomic analyses were performed by Metabolon (https://www.metabolon.com). Details on the methodology are described elsewhere (Suhre et al., 2011).
In brief, serum samples (100 μL) were extracted and analyzed in a platform including two separate ultrahigh-performance liquid chromatography/tandem mass spectrometry injections (UHPLC/MS/MS2) and one gas chromatography/mass spectrometry (GC/MS) injection.
Retention time, molecular mass to charge ratio (m/z), preferred adducts, and in-source fragments were used to identify metabolites by comparison to a standard library curated by Metabolon.
The study data met Metabolon’s quality control standards. Matrix data showed a median relative standard deviation (RSD) of 17% for GC/MS and 15% UHPLC/MS. The comparable variation in the overall study samples was 45% median RSD (excluding very sparsely detected compounds).
2.4. Statistical analyses
Data were normalized by registering the median values for each runday block. Comparisons between groups (PMDD cases and controls) and time points (gonadal suppression and progesterone add-back) were conducted using the repeated measures analysis of variance (ANOVA). For ANOVA tests, missing values were imputed with the minimum observed value for the particular compound after block-normalization procedure.
To assess the potential confounding effect of the proximity to estradiol administration, we first compared plasma concentrations of progesterone and nominally significant metabolites in the three groups undergoing different hormone manipulation protocols. Then we ran sensitivity analyses excluding the three women taking progesterone and estradiol at the same time.
The false discovery rate was calculated to account for multiple testing. Q-values are the adjusted P-values obtained using the false discovery rate approach. A Q-value below 0.1 is an indication of high confidence in a hit, whereas higher Q-values indicate diminished confidence but do not necessarily rule out the significance of a compound.
3. RESULTS
Sample characteristics are reported in Table 1. Cases and controls did not statistically differ in age, body mass index, and progesterone or estradiol concentrations.
TABLE 1.
Demographics and clinical characteristics of women with premenstrual dysphoric disorder (PMDD) and control women
| PMDD | Controls | ||
|---|---|---|---|
| N | 12 | 25 | |
| Age (years) | Mean ± SD | 38±6.9 | 34± 8.1 |
| Body mass index (Kg/m2) | Mean ± SD | 23.8 ± 4.25 | 24.3±3.27 |
| PMDD symptom severity (Steiner-Carroll scale: self-report) | |||
| Leuprolide | Median (range) |
1.5 (0–5) | 2(0–17) |
| Leuprolide + progesteronea,b | Median (range) |
11.5 (3–26) | 1 (0–9) |
Kruskal-Wallis chi-squared = 17.75, df = 1, P < 0.0001 between cases and controls
Wilcoxon signed rank test with continuity correction = 3, P = 0.00532 for leuprolide versus leuprolide + progesterone in cases.
The proximity of estradiol administration to receiving progesterone did not affect mood ratings and progesterone plasma levels that were similar across groups treated with different hormone protocols.
Although controls did not manifest mood symptoms during the manipulation protocol, in women with PMDD, we observed few affective symptoms during ovarian suppression and the recurrence of typical PMDD symptomatology after administration of progesterone. Steiner–Carroll scale self- and observer-reported symptom scores did not differ between cases and controls during leuprolide administration (suppression phase of the hormone manipulation protocol—Kruskal–Wallis chi-squared = 9.64, df = 8, P = 0.29 and Kruskal–Wallis chi-squared = 8.76, df = 10, P = 0.55, respectively). Controls remained asymptomatic during hormone replacement with progesterone (paired Wilcoxon signed rank test V = 89.5, P = 0.10 for self-reported scores, and V = 128, P = 0.67 for observer reported scores), while symptoms significantly increased in cases during treatment with leuprolide plus progesterone as compared with treatment with leuprolide alone (paired Wilcoxon signed rank test = V = 3, P = 0.005 for self-reported scores, and V = 0, P = 0.004 for observer-reported scores).
Following full data curation, the serum study yielded 407 compounds. Approximately one third of these corresponded to identifiable chemical entities, and the remaining two thirds represented unknown metabolites. More than 41% of the chemicals detected were measurable in all the samples, 82% in more than two-thirds. Among detectable compounds, there was a preponderance of amino acids and their derivatives, which represented 44% of the known compounds detected. Other types of detected compounds included the following: carbohydrates (11%), vitamins and cofactors (8%), compounds involved in energy and tricarboxylic acid cycle (6%), lipids (12%), nucleotides (9%), peptides (4%), and xenobiotics (5%).
3.1. Effects of progesterone on the metabolic profile
Of the 36 known compounds that showed nominally significantly (P < 0.05) lower plasma levels as a result of progesterone administration (Table 2), 28 were amino acids or their derivatives.
TABLE 2.
Compounds affected by the administration of progesterone. Compounds that narrowly missed statistical cutoff for significance 0.05 < P < 0.10 are also displayed. If the ratio of means time 2/time 1 is less than 1, then the compound was found in higher concentrations during gonadal suppression than after progesterone add-back
| Time ANOVA for the whole sample | Ratio of means time 2/time 1 | Time ANOVA in cases | Time ANOVA in controls | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Biochemical | Pathway | P-value | Q-value | In cases | In controls | P-value | Q-value | P-value | Q-value |
| Alanine Aspartate |
Alanine and aspartate metabolism | 0.175 0.020 |
0.537 0.187 |
0.96 0.77 |
0.75 0.63 |
0.908 0.230 |
0.989 0.858 |
0.030 0.017 |
0.153 0.126 |
| Glutamine | Glutamate metabolism |
0.014 | 0.139 | 0.68 | 0.78 | 0.123 | 0.858 | 0.029 | 0.153 |
| Threonine | Glycine, serine and threonine metabolism | <0.001 | <0.001 | 0.79 | 0.64 | 0.004 | 0.357 | <0.001 | <0.001 |
| Histamine | Histidine metabolism |
0.001 | 0.018 | 0.79 | 0.75 | 0.041 | 0.742 | 0.002 | 0.027 |
| Histidine | 0.343 | 0.661 | 1.02 | 0.88 | 0.768 | 0.989 | 0.041 | 0.173 | |
| Isobar 6 valine, betaine | Leucine, isoleucine and valine metabolism | 0.397 | 0.661 | 1.07 | 0.85 | 0.567 | 0.979 | 0.025 | 0.142 |
| Alpha- aminoadipic acid |
Lysine metabolism | 0.107 | 0.422 | 0.84 | 0.84 | 0.455 | 0.944 | 0.078 | 0.255 |
| Glutarate | <0.001 | 0.007 | 0.62 | 0.58 | 0.023 | 0.742 | <0.001 | 0.010 | |
| Lysine | <0.001 | 0.007 | 0.89 | 0.84 | 0.036 | 0.742 | <0.001 | 0.004 | |
| N(‘6)-trimethyl-L- lysine | <0.001 | 0.008 | 0.90 | 0.71 | 0.240 | 0.858 | <0.001 | 0.000 | |
| Methionine | Methionine, cysteine, SAM and taurine metabolism | 0.024 | 0.208 | 1.02 | 0.78 | 0.687 | 0.989 | 0.001 | 0.020 |
| Alpha-4- dihydroxyben zenepropanoic acid | Phenylalanine and tyrosine metabolism | 0.003 | 0.055 | 0.92 | 0.88 | 0.110 | 0.858 | 0.003 | 0.035 |
| Thyroxine | 0.206 | 0.576 | 0.81 | 1.47 | 0.834 | 0.989 | 0.015 | 0.113 | |
| Tyramine | 0.069 | 0.354 | 0.72 | 0.73 | 0.315 | 0.877 | 0.078 | 0.255 | |
| Tyrosine | 0.093 | 0.401 | 1.05 | 0.82 | 0.671 | 0.989 | 0.001 | 0.017 | |
| 5-Hydroxy- indoleacetate | Tryptophan metabolism |
0.018 | 0.169 | 0.79 | 0.88 | 0.069 | 0.749 | 0.104 | 0.281 |
| 3-Indoxyl sulfate | 0.038 | 0.284 | 0.87 | 0.84 | 0.224 | 0.857 | 0.054 | 0.212 | |
| Serotonin | 0.172 | 0.537 | 0.94 | 0.89 | 0.644 | 0.989 | 0.084 | 0.259 | |
| Arginine N(5)- (aminocarbonyl)- L-ornithine | Urea cycle; arginine and proline metabolism | <0.001 0.004 |
0.003 0.062 |
0.85 0.63 |
0.73 0.81 |
0.036 0.008 |
0.742 0.447 |
<0.001 0.186 |
<0.001 0.369 |
| Proline | 0.009 | 0.102 | 0.85 | 0.83 | 0.096 | 0.841 | 0.024 | 0.142 | |
| Urea adductof isobar 6 | 0.002 | 0.048 | 0.99 | 0.76 | 0.051 | 0.749 | 0.008 | 0.084 | |
| Cl adductof uric acid | 0.003 | 0.055 | 0.68 | 0.73 | 0.063 | 0.749 | 0.009 | 0.089 | |
| Hydroxyproline form of bradykinin | 0.014 | 0.139 | 0.78 | 0.71 | 0.382 | 0.925 | 0.002 | 0.032 | |
| Trans-4- hydroxyproline |
<0.001 | 0.008 | 0.88 | 0.60 | 0.228 | 0.858 | <0.001 | <0.001 | |
| Citric acid | TCA cycle | 0.095 | 0.403 | 0.83 | 0.81 | 0.396 | 0.926 | 0.085 | 0.259 |
| 2-deoxy-D-ribose | Pentose metabolism | 0.056 | 0.334 | 1.32 | 1.02 | 0.052 | 0.749 | 0.567 | 0.530 |
| Xylitol | 0.049 | 0.320 | 0.79 | 0.77 | 0.335 | 0.881 | 0.037 | 0.162 | |
| Alpha-D-ribose 5-phosphate | Pentose phosphate pathway |
0.431 | 0.683 | 1.03 | 0.83 | 0.834 | 0.989 | 0.097 | 0.280 |
| Pyridoxamine | Vitamin B6 metabolism | <0.001 | 0.011 | 0.82 | 0.93 | 0.001 | 0.192 | 0.075 | 0.255 |
| Isobar 2 amino- butyrates | Pyrimidine metabolism, Thymine containing | 0.002 | 0.032 | 0.84 | 0.79 | 0.068 | 0.749 | 0.002 | 0.031 |
| L-carnosine | Dipeptide derivative | 0.173 | 0.537 | 1.02 | 0.88 | 0.972 | 0.996 | 0.022 | 0.142 |
| Gamma-L- glutamyl-L- glutamine | Gamma-glutamyl amino acid | 0.025 | 0.215 | 0.91 | 0.73 | 0.332 | 0.881 | 0.011 | 0.096 |
| Gamma-L- glutamyl-L- tyrosine | 0.246 | 0.616 | 1.14 | 0.83 | 0.465 | 0.944 | 0.004 | 0.037 | |
| Tartarate | Food component/plant | 0.008 | 0.096 | 0.74 | 0.77 | 0.117 | 0.858 | 0.014 | 0.113 |
ANOVA, analysis of variance; time 1, after gonadal suppression; time 2, after progesterone add-back; TCA, tricarboxylic acid.
In bold: P ≤ 0.05 and Q < 0.1.
Amino acid pathways affected by progesterone involved the metabolism of arginine and proline, lysine, threonine, tyrosine, and methionine.
The most dramatic change after administration of progesterone was an approximately 28-fold increase in the mean concentration of an unknown compound (measured mass: 400.2). There were two other unknowns (measured masses: 398.2160 and 384.1636) that exhibited a similar response but with not so large an increase. The changes after progesterone administration were significant (P < 0.01 and Q < 0.1) and similar in controls and PMDD subjects. All these compounds had mass values that were consistent with them being steroid hormone derivatives. Mass queries in the Kyoto Encyclopedia of Genes and Genomes (Kanehisa & Goto, 2000) yielded potential identities. The top two compounds appeared to be sulfated versions of immediate progesterone metabolites (pregnanediol-sulfate, mass: 400.2278) and (3-OH-5beta-pregnane-20-one-sulfate, mass: 398.2122). The third compound’s mass was consistent with it being a sulfated version of one of several steroid derivatives, all androgens (hydroxyl-DHEA-sulfate or 7alpha-hydroxytestosterone-sulfate or 19-hydroxytestosterone sulphate, mass: 384.1601).
3.2. Differences in metabolic profile between cases with PMDD and controls
Of the 15 compounds that differed between women with PMDD and controls in both phases of the hormone manipulation protocol, seven were amino acids or their derivatives (Table 3). Differences between cases of PMDD and controls were more pronounced after progesterone add-back than during gonadal suppression (Table 3).
TABLE 3.
Compounds found in different concentrations in cases of premenstrual dysphoric disorder (PMDD) and controls. Compounds that narrowly missed statistical cutoff for significance 0.05 < P < 0.10 are also displayed
| Group ANOVA | Ratio of means cases/control | Group ANOVA at time 1 | Group ANOVA at time 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Biochemical | Pathway | P-value | Q-value | At time 1 | At time 2 | P-value | Q-value | P-value | Q-value |
| Alanine | Alanine and aspartate metabolism | 0.134 | 0.775 | 0.76 | 0.97 | 0.059 | 0.732 | 0.865 | 0.681 |
| N-acetyl-L-aspartic acid | 0.004 | 0.542 | 1.62 | 1.89 | 0.033 | 0.732 | 0.007 | 0.465 | |
| Isobar 6 valine, betaine | Leucine, isoleucine and valine metabolism | 0.364 | 0.838 | 0.86 | 1.10 | 0.065 | 0.732 | 0.632 | 0.672 |
| Tyrosine | Phenylalanine and tyrosine metabolism | 0.566 | 0.877 | 0.95 | 1.23 | 0.492 | 0.997 | 0.095 | 0.596 |
| N(5)-(Aminocarbonyl)-L-Ornithine | Urea cycle; arginine and proline metabolism | 0.102 | 0.775 | 0.94 | 0.72 | 0.633 | 0.997 | 0.028 | 0.596 |
| Trans-4-hydroxyproline | 0.254 | 0.807 | 1.07 | 1.57 | 0.906 | 0.997 | 0.056 | 0.596 | |
| Glycerol-2-phosphatase | Glycolysis, gluconeogenesis, and pyruvate metabolism | 0.012 | 0.542 | 0.85 | 0.96 | 0.067 | 0.732 | 0.227 | 0.596 |
| Glycerate | 0.125 | 0.775 | 0.83 | 0.96 | 0.065 | 0.732 | 0.290 | 0.611 | |
| Alpha-D-ribose 5-phosphate | Pentose phosphate pathway | 0.024 | 0.542 | 0.78 | 0.97 | 0.022 | 0.732 | 0.601 | 0.672 |
| Biliverdin | Hemoglobin and porphyrin metabolism | 0.325 | 0.807 | 1.35 | 0.96 | 0.996 | 0.997 | 0.084 | 0.596 |
| Pyridoxamine | Vitamin B6 metabolism | 0.015 | 0.542 | 0.91 | 0.81 | 0.219 | 0.930 | 0.003 | 0.307 |
| Isobar 2 amino-butyrates | Pyrimidine metabolism, thymine containing | 0.035 | 0.615 | 1.11 | 1.18 | 0.167 | 0.930 | 0.069 | 0.596 |
| Uridine | Pyrimidine metabolism, uracil containing | 0.051 | 0.723 | 0.74 | 0.79 | 0.054 | 0.732 | 0.189 | 0.596 |
| Gamma-L-glutamyl-L-Tyrosine | Gamma-glutamyl amino acid | 0.038 | 0.615 | 1.07 | 1.46 | 0.537 | 0.997 | 0.003 | 0.307 |
| Caffeine | Xanthine metabolism | 0.044 | 0.674 | 1.59 | 1.97 | 0.111 | 0.831 | 0.043 | 0.596 |
ANOVA, analysis of variance; time 1, after gonadal suppression; time 2, after progesterone add-back.
In bold: P ≤ 0.05.
Many compounds had a different response to progesterone in women with PMDD and in controls (Table 4). Although these differences were nominally significant (P < 0.05), the false discovery rates were above 0.1. The interaction of time by group on ANOVA yielded six nominally significant hits for amino acids and their derivatives: isoleucine (P = 0.040), N(‘6)-trimethyl-l-lysine (P = 0.047), tyrosine (P = 0.020; Figure 2), trans-4-hydroxyproline (P = 0.045), gamma-L-glutamyl-L-tyrosine (P = 0.022), and carnitine (P = 0.045). In each case, the concentrations increased on progesterone in the PMDD women and decreased in the controls (four compounds) or decreased to a lesser degree in patients compared with controls (two compounds). The concentration of citric acid, involved in the citric acid cycle in the mitochondria, was increased after progesterone add-back in cases of PMDD, but not in controls.
TABLE 4.
Compounds with different changes in concentration at time 1 and time 2 between cases of premenstrual dysphoric disorder (PMDD) and controls (i.e., time by group interaction). If time 2/time 1 ratio is greater than 1, the compound was found in higher concentrations after progesterone administration than during gonadal suppression. If PMDD/CONTR ratio is greater than 1, the compound was found in higher concentrations in cases than in controls
| Fold-change (ratios of means from scaled imputed data) | |||||||
|---|---|---|---|---|---|---|---|
| Group by time interaction ANOVA | Time 2/time 1 | PMDD/CONTR | |||||
| Biochemical | Pathway | P | Q | PMDD | Control | Time 1 | Time2 |
| Unknown compound | N/A | 0.012 | 0.850 | 1.28 | 0.89 | 0.78 | 1.12 |
| Tyrosine | Phenylalanine and tyrosine metabolism | 0.020 | 0.850 | 1.05 | 0.82 | 0.95 | 1.23 |
| Gamma-L-glutamyl-L-tyrosine | Gamma-glutamyl amino acid | 0.022 | 0.850 | 1.14 | 0.83 | 1.07 | 1.46 |
| Unknown compound | N/A | 0.030 | 0.850 | 0.70 | 1.63 | 1.90 | 0.81 |
| Citric acid | N/A | 0.033 | 0.850 | 1.21 | 0.87 | 0.73 | 1.01 |
| Unknown compound | N/A | 0.036 | 0.850 | 1.25 | 0.80 | 0.60 | 0.93 |
| Isoleucine | Leucine, Isoleucine and valine metabolism | 0.040 | 0.850 | 1.44 | 0.85 | 0.87 | 1.49 |
| Unknown compound | N/A | 0.044 | 0.850 | 0.80 | 1.51 | 1.70 | 0.89 |
| Carnitine-1 | N/A | 0.045 | 0.850 | 1.23 | 0.78 | 0.81 | 1.28 |
| Trans-4-hydroxyproline | Urea cycle; arginine and proline metabolism | 0.045 | 0.850 | 0.88 | 0.60 | 1.07 | 1.57 |
| N(‘6)-trimethyl-L-lysine | Lysine metabolism | 0.047 | 0.850 | 0.90 | 0.71 | 0.97 | 1.24 |
| Unknown compound | N/A | 0.049 | 0.850 | 0.82 | 1.00 | 1.21 | 0.99 |
ANOVA, analysis of variance; CONTR, controls; time 1, after gonadal suppression; time 2, after progesterone add-back.
In bold P ≤ 0.05.
FIGURE 2.
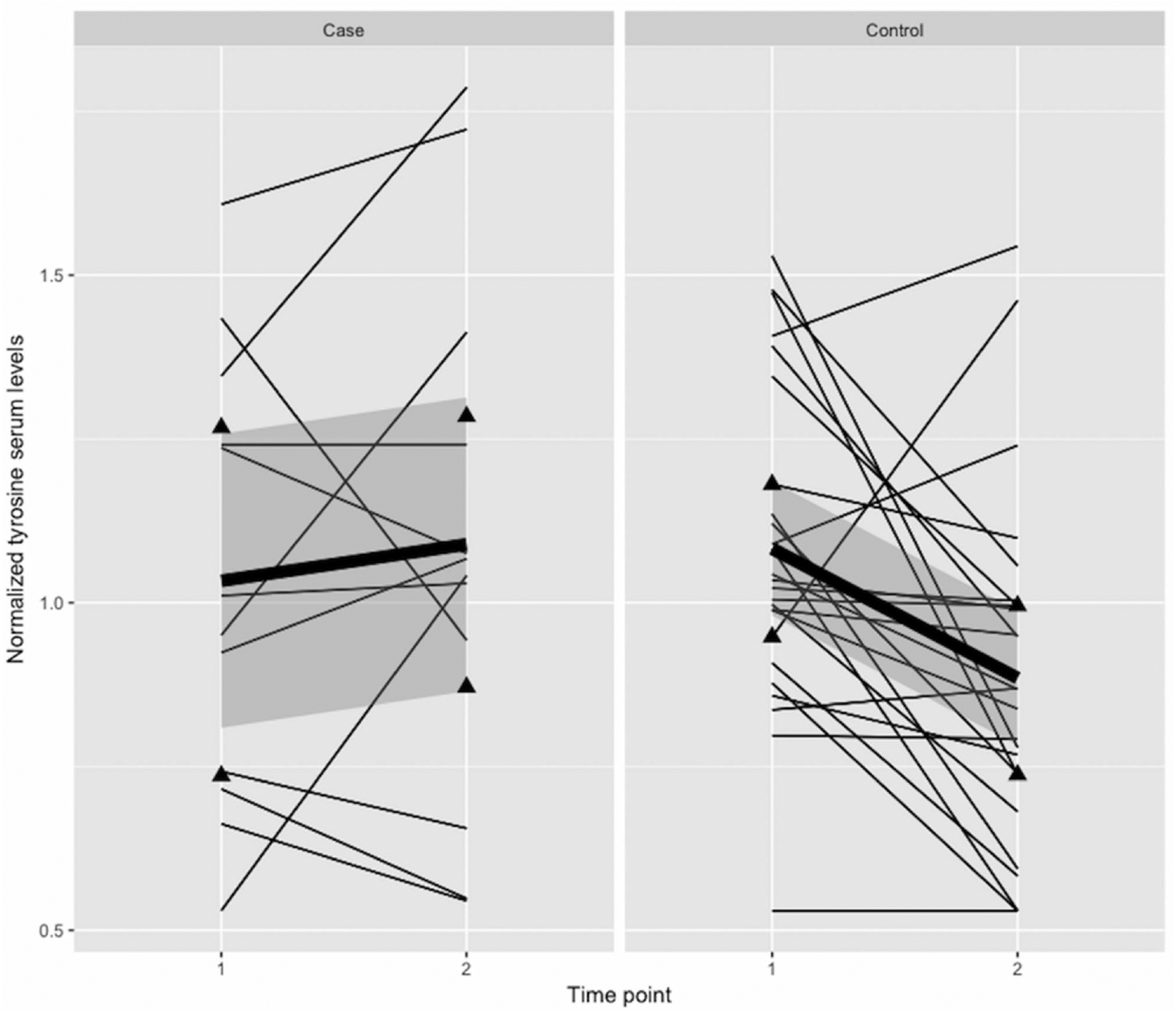
Trajectories of normalized tyrosine serum levels for all individual cases with premenstrual dysphoric disorder (PMDD) and controls. Time point 1, gonadal suppression; Time point 2, 4 weeks progesterone add-back. Average trends are represented by the bold regression line, triangles represent lower and upper quartiles and shadings standard errors
The plasma levels of several unknown compounds were also altered in response to progesterone in controls or in cases but not in both (leading to a nominally significant interaction term). Additionally, there were sparse compounds that were exclusively expressed in cases or in controls. The concentration of an unknown sparse compound (measured mass: 346.2) had an increase about eight times greater in women with PMDD than in controls following progesterone add-back. In controls (but not in cases), the concentrations of three unknown sparse compounds were increased over twofold after the administration of progesterone. Salicylate, salicylurate, serotonin, and four unknown compounds were exclusively found in women with PMDD and increased after the administration of progesterone.
4. DISCUSSION
4.1. Effect of progesterone on amino acid metabolism
Using a hormone manipulation protocol, we found that progesterone significantly decreases the concentration of amino acids and related compounds in the context of ovarian suppression. Our study confirms previous indirect, uncontrolled observations concerning the effect of gonadal steroids on the metabolism of amino acids and related compounds (Supporting Information Table 2). In contrast to our study, however, previous research has not been able to test the causal relationship and examine the effects of standardized levels of progesterone, separately from those of other hormones.
The amino acid drop following progesterone add-back could result either from faster utilization (e.g., increased protein synthesis demands) or from increased catabolism, with evidence pointing to both mechanisms (Bisdee, et al., 1989; Kriengsinyos, et al., 2004, Smith et al., 2014).
Our findings represent the first demonstration in humans that progesterone administered under controlled conditions alters the metabolome. As such, reproductive-steroid-induced changes in the metabolome, small molecules that are fundamental to cell signaling and function, may represent a significant albeit indirect means by which steroids regulate processes relevant for brain and behavior. Animal and cell culture studies, in fact, suggest both sex differences and an effect of reproductive steroids in the metabolism of proteins that affect a broad range of pathological and physiological events (Comitato, Saba, Turrini, Arganini, & Virgili, 2015; Smith et al., 2014).
4.2. Differential response to progesterone in women with PMDD compared to controls
Our study provides some of the first evidence of a different metabolic response to progesterone in women with PMDD compared to controls and requires corroboration or additional work to determine false discoveries from truly significant biomarkers. Nonetheless, this observation is consistent with other data suggesting an abnormal response to progesterone in PMDD, including the following (seen in women with PMDD compared with controls): precipitation of affective symptoms by progesterone in the context of ovarian suppression (Schmidt et al., 1998); absent luteal phase increase in the hypothalamic–pituitary–adrenal axis activity (Roca et al., 2003) and pregnanolone-stimulated sedation (Sundström et al., 1998); lack of cortical inhibition (Smith et al., 2002) and advance in cortisol acrophase (Parry et al., 2000) during the luteal phase.
We found that amino acids involved in the structure and function of collagen (trans-4-OH-proline), cell metabolism (carnitine), protein (isoleucine and tri-methyl-lysine), and catecholamine synthesis (tyrosine) had a different response to progesterone in cases and controls.
If confirmed in a larger independent sample, our results would suggest that the differential sensitivity to progesterone in women with PMDD may be reflected in, and potentially mediated by, a blunted metabolic response to progesterone, an abnormality revealed in the periphery and presumably the central nervous system.
It is not clear how the different sensitivity to progesterone in cases of PMDD compared to controls can influence the concentrations of some plasma amino acids, but not others. It is possible that women with PMDD have an impaired response to the metabolic and home-ostatic signaling of progesterone. Women with PMDD compared to controls may also have increased absorption or decreased clearance or dietary consumption of certain amino acids in response to progesterone.
Diet composition also impacts metabolomic profile (Esko et al., 2017). In this respect, it is interesting that plasma levels of exogenous substances as salicylates (found in foods) and caffeine were increased after progesterone administration in women with PMDD, but not controls. As supplements and drugs were prohibited for the duration of the study, this observation suggests that differences between cases and controls after progesterone administration may be, at least in part, due to changes in quantity and quality of ingested foods. The association between PMDD and diet, including caffeine consumption (Rossignol & Bonnlander, 1990), is, however, controversial, supported by little evidence and largely based on retrospective, cross-sectional studies.
We found that the concentration of tyrosine, a precursor of the neurotransmitters dopamine, epinephrine, and norepinephrine was decreased after progesterone administration in 80% (N = 20) of healthy controls, but only in 42% of cases with PMDD (N = 5; Figure 2). Interestingly, we did not find any differences between cases and controls in some other obvious candidate neurotransmitter precursors, such as tryptophan, whereas serotonin was detected as a sparse compound only in cases. Although the lack of an effect can be due to the limited sample size, our results are in agreement with previous evidence of a lack of association between tryptophan and premenstrual syndrome (Rapkin, Reading, Woo, & Goldman, 1991). There is a dearth of information on the effect of progesterone on the metabolism of catecholamines in humans. Animal brain studies have suggested that progesterone affects the activity of tyrosine hydroxylase, the enzyme that hydroxylates tyrosine to 3–4-dihydroxyphenylalanine, but the exact mechanisms are unknown and likely to be complex (Tekin, Roskoski, Carkaci-Salli, & Vrana, 2014). If replicated, our results may suggest that women with PMDD have a blunted or absent effect of progesterone on the availability of the catecholamine precursor tyrosine. We speculate that this leads to a reduced utilization of tyrosine for the synthesis of catecholamines during luteal phase in women with PMDD compared to controls.
4.3. Limitations
Our results should be interpreted in light of the following limitations:
Because of the small size of the sample with PMDD, the false-discovery test did not yield significantly low Q-values in the comparisons between cases and controls. While some metabolic changes observed selectively in cases or controls may be false discoveries, some were likely significant and are consistent with previous, indirect evidence of a role of progesterone in metabolism.
Although metabolite databases have largely improved in the last decade, there are still many poorly characterized metabolites with unknown chemical structure and function. In particular, we were not able to identify three steroid derivatives, whose concentrations were dramatically increased after administration of progesterone.
Women differed in the proximity of estradiol administration to receiving progesterone. However, we did not find any differences in mood ratings, progesterone or tyrosine plasma levels between groups undergoing different hormone protocols. Moreover, we were able to replicate the time-by-group interaction for tyrosine in the subanalyses accounting for differences in the proxy and in the sensitivity, analyses excluding the three women taking estradiol together with progesterone.
We did not collect information on women’s diet and therefore cannot exclude that the differences we observed between cases and controls are due to different changes in the diet in response to progesterone administration. Changes in diet in women with PMDD, but not in controls, however, are unlikely to be the sole explanation for our findings, as we found that changes in amino acid concentrations after progesterone were more pronounced in controls than cases.
5. CONCLUSION
In this pharmaco-metabolomics, hypothesis-generating study, we report a decrease in plasma levels of amino acids in response to progesterone.
Further research is needed to replicate our findings in a larger sample and to identify the unknown compounds affected by progesterone, especially those differentially expressed in response to progesterone in women with PMDD and controls.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank all of the women who gave their time to participate in the study.
Grants or fellowships supporting the writing of the paper: ADF is funded by a European Commission Marie Curie Fellowship, grant number 623932. This research was supported by the Intramural Research Program of the NIMH, NIH. NIMH Protocols NCT00001259 and NCT00001322; Project # MH002865.
Funding information
European Commission, Grant/Award Number: 623932; Intramural Research Program of the NIMH, NIH. NIMH Protocols NCT00001259 and NCT00001322; Project # MH002865
Footnotes
CONFLICT OF INTEREST
Arianna Di Florio, Danny Alexander, Peter Schmidt declare that they have no conflict of interest.
David Rubinow is an employee of Metabolon.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- American Psychiatric Association. (2000). Diagnostic criteria from DSM-IVTR. Washington, DC: American Psychiatric Association. [Google Scholar]
- American Psychiatric Association. (2013). DSM 5—Fifth edition of the Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; Retrieved from https://www.psyweb.com/content/main-pages/dsm-5-fifth-edition-of-the-diagnostic-and-statistical-manual-of-mental-disorders [Google Scholar]
- Bisdee, (1989). British Journal of Nutrition 61(3):641–650. [DOI] [PubMed] [Google Scholar]
- Comitato R, Saba A, Turrini A, Arganini C, & Virgili F (2015). Sex hormones and macronutrient metabolism. Critical Reviews in Food Science and Nutrition, 55(2), 227–241. 10.1080/10408398.2011.651177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey N, Hoffman JF, Schuebel K, Yuan Q, Martinez PE, Nieman LK, … Goldman D (2017). The ESC/E(Z) complex, an effector of response to ovarian steroids, manifests an intrinsic difference in cells from women with premenstrual dysphoric disorder. Molecular Psychiatry, 22(8), 1172–1184. 10.1038/mp.2016.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esko T, Hirschhorn JN, Feldman HA, Hsu Y-HH, Deik AA, Clish CB, … Ludwig DS (2017). Metabolomic profiles as reliable biomarkers of dietary composition. The American Journal of Clinical Nutrition, 105(3), 547–554. 10.3945/ajcn.116.144428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2012). Structured clinical interview for DSM-IV® axis I disorders (SCID-I), clinician version, administration booklet. Washington, DC: American Psychiatric Association. [Google Scholar]
- Gehlert S, Song IH, Chang C-H, & Hartlage SA (2009). The prevalence of premenstrual dysphoric disorder in a randomly selected group of urban and rural women. Psychological Medicine, 39(1), 129–136. 10.1017/S003329170800322X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, & Goto S (2000). KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Research, 28(1), 27–30. 10.1093/nar/28.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriengsinyos, et al. , (2004). Am J Physiol Endrocrinol Metab 287:E489–496. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Reuter JM, Gaikwad NW, Rotroff DM, Kucera HR, Motsinger-Reif A, … Schmidt PJ (2017). The steroid metabolome in women with premenstrual dysphoric disorder during GnRH agonist-induced ovarian suppression: Effects of estradiol and progesterone addback. Translational Psychiatry, 7(8), e1193 10.1038/tp.2017.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry BL, Javeed S, Laughlin GA, Hauger R, & Clopton P (2000). Cortisol circadian rhythms during the menstrual cycle and with sleep deprivation in premenstrual dysphoric disorder and normal control subjects. Biological Psychiatry, 48(9), 920–931. 10.1016/S0006-3223(00)00876-3 [DOI] [PubMed] [Google Scholar]
- Patti GJ, Yanes O, & Siuzdak G (2012). Innovation: Metabolomics: The apogee of the omics trilogy. Nature Reviews Molecular Cell Biology, 13(4), 263–269. 10.1038/nrm3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin AJ, Reading AE, Woo S, & Goldman LM (1991). Tryptophan and neutral amino acids in premenstrual syndrome. American Journal of Obstetrics and Gynecology, 165(6 Pt 1), 1830–1833. [DOI] [PubMed] [Google Scholar]
- Roca CA, Schmidt PJ, Altemus M, Deuster P, Danaceau MA, Putnam K, & Rubinow DR (2003). Differential menstrual cycle regulation of hypothalamic-pituitary-adrenal axis in women with premenstrual syndrome and controls. The Journal of Clinical Endocrinology and Metabolism, 88(7), 3057–3063. 10.1210/jc.2002-021570 [DOI] [PubMed] [Google Scholar]
- Rossignol AM, & Bonnlander H (1990). Caffeine-containing beverages, total fluid consumption, and premenstrual syndrome. American Journal of Public Health, 80(9), 1106–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow DR, Hoban MC, Grover GN, Galloway DS, Roy-Byrne P, Andersen R, & Merriam GR (1988). Changes in plasma hormones across the menstrual cycle in patients with menstrually related mood disorder and in control subjects. American Journal of Obstetrics and Gynecology, 158(1), 5–11. [DOI] [PubMed] [Google Scholar]
- Rubinow DR, Roy-Byrne P, Hoban MC, Gold PW, & Post RM (1984). Prospective assessment of menstrually related mood disorders. The American Journal of Psychiatry, 141(5), 684–686. 10.1176/ajp.141.5.684 [DOI] [PubMed] [Google Scholar]
- Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, & Rubinow DR (1998). Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. The New England Journal of Medicine, 338(4), 209–216. 10.1056/NEJM199801223380401 [DOI] [PubMed] [Google Scholar]
- Smith GI, Yoshino J, Reeds DN, Bradley D, Burrows RE, Heisey HD, … Mittendorfer B (2014). Testosterone and progesterone, but not estradiol, stimulate muscle protein synthesis in postmenopausal women. The Journal of Clinical Endocrinology and Metabolism, 99(1), 256–265. 10.1210/jc.2013-2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, & Wasser-mann EM (2002). Effects of ovarian hormones on human cortical excitability. Annals of Neurology, 51(5), 599–603. 10.1002/ana.10180 [DOI] [PubMed] [Google Scholar]
- Stachenfeld NS, & Taylor HS (2014). Challenges and methodology for testing young healthy women in physiological studies. American Journal of Physiology. Endocrinology and Metabolism, 306(8), E849–853. 10.1152/ajpendo.00038.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhre K, Shin S-Y, Petersen A-K, Mohney RP, Meredith D, Wägele B, … Gieger C (2011). Human metabolic individuality in biomedical and pharmaceutical research. Nature, 477(7362), 54–60. 10.1038/nature10354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundström I, Andersson A, Nyberg S, Ashbrook D, Purdy RH, & Bäckström T (1998). Patients with premenstrual syndrome have a different sensitivity to a neuroactive steroid during the menstrual cycle compared to control subjects. Neuroendocrinology, 67(2), 126–138. [DOI] [PubMed] [Google Scholar]
- Tekin I, Roskoski R, Carkaci-Salli N, & Vrana KE (2014). Complex molecular regulation of tyrosine hydroxylase. Journal of Neural Transmission, 121(12), 1451–1481. 10.1007/s00702-014-1238-7 [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Becker E, Lieb R, & Krause P (2002). Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychological Medicine, 32(1), 119–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


