Abstract
Aims
The aim of this study was to evaluate the possible value of dobutamine stress cardiac magnetic resonance imaging (CMR) to predict adverse outcome in Tetralogy of Fallot (TOF) patients.
Methods and results
In previous prospective multicentre studies, TOF patients underwent low-dose dobutamine stress CMR (7.5 µg/kg/min). Subsequently, during regular-care patient follow-up, patients were assessed for reaching the composite endpoint (cardiac death, arrhythmia-related hospitalization, or cardioversion/ablation, VO2 max ≤65% of predicted). A normal stress response was defined as a decrease in end-systolic volume (ESV) and increase in ejection fraction. The relative parameter change during stress was calculated as relative parameter change = [(parameterstress − parameterrest)/parameterrest] * 100. The predictive value of dobutamine stress CMR for the composite endpoint was determined using time-to-event analyses (Kaplan–Meier) and Cox proportional hazard analysis. We studied 100 patients [67 (67%) male, median age at baseline CMR 17.8 years (interquartile range 13.5–34.0), age at TOF repair 0.9 years (0.6–2.1)]. After a median follow-up of 8.6 years (6.7–14.1), 10 patients reached the composite endpoint. An abnormal stress response (30% vs. 4.4%, P = 0.021) was more frequently observed in composite endpoint patients. Also in endpoint patients, the relative decrease in right ventricular ESV decreased less during stress compared with the patients without an endpoint (−17 ± 15 vs. −26 ± 13 %, P = 0.045). Multivariable analyses identified an abnormal stress response (hazard ratio 10.4; 95% confidence interval 2.5–43.7; P = 0.001) as predictor for the composite endpoint.
Conclusion
An abnormal ventricular response to dobutamine stress is associated with adverse outcome in patients with repaired TOF.
Keywords: stress imaging, dobutamine, cardiac magnetic resonance imaging, Tetralogy of Fallot, congenital heart disease, outcome
Introduction
Survival after surgical repair of Tetralogy of Fallot (TOF) in infancy is good, long-term survival of 95% at 10 years and >90% at 25 years has been reported.1–3 However, TOF patients frequently develop long-term problems such as pulmonary regurgitation (PR), right ventricular (RV) dilatation, ventricular dysfunction, arrhythmias, and even mortality.1,2,4,5
In TOF patients, peak VO2, N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, and QRS duration have been related to ventricular volumes, function, and outcome.6–10 RV end-diastolic volume (EDV), left ventricular (LV) end-systolic volume (ESV), LV ejection fraction (EF), and myocardial deformation parameters are associated with sudden cardiac death, severe arrhythmias, and heart failure.8,11–13 Nonetheless, additional parameters are needed for better risk stratification.5 This search for additional outcome determinants is hampered by long symptom-free intervals and therefore surrogate outcome markers are often used.14
A potential additional parameter is dobutamine stress cardiac magnetic resonance imaging (CMR). This technique combines the gold standard for volumetric cardiac parameters with functional information during (dobutamine) stress.14 Compared with healthy volunteers, TOF patients often have an abnormal decrease in RVEDV, an impaired decrease in RVESV, and an impaired increase RVEF following the use of dobutamine stress.15–18 Luijnenburg et al.17 showed that this poorer RVEF stress response is associated with a larger decrease in peak VO2 at 5-year follow-up. Ventricular response to exercise or dobutamine stress CMR might help identifying patients at risk for developing an event.19,20
Our aim was to evaluate the possible additional value of stress CMR, compared with the existing parameters, to predict adverse outcome in TOF patients.
Methods
Patients
We included TOF patients that underwent successful dobutamine stress CMR in four prospective studies in tertiary referral centres between 2002 and 2012.17,21–23 The institutional review boards approved the studies. All participants, and if necessary their parents, gave written informed consent before inclusion. The inclusion and exclusion criteria, study protocol, methods, adverse event rate, and results have been published previously.17,21–23
Patients underwent the baseline CMR study at rest and during low-dose dobutamine stress. NT-proBNP was measured with the Elecsys electrochemiluminescence immunoassay (Roche Diagnostics) and cardiopulmonary exercise testing (CPET) was performed according to previously described standardized protocols within 1 year from the dobutamine CMR.17,21–23 From that CPET, the baseline peak VO2 was assessed and expressed as percentage of predicted values. Exercise tests with a peak respiratory exchange rate of ≥1.0 were included in the analysis.
Composite study endpoint
After the study examination patients received regular patient-specific care in the outpatient clinic. Since information on how to interpret stress CMR in TOF patients was not available at that time, stress CMR parameters were not used for clinical decision-making.
Before data acquisition, we defined our study endpoint as a composite of cardiac death, hospitalization for arrhythmias, or cardioversion/ablation for arrhythmias and reaching a CPET VO2 max below 65% of predicted (due to cardiac reasons) during follow-up after the study CPET.9,10 The medical records of all patients were reviewed up until January 2019.
CMR acquisition and analysis
All participants underwent CMR on the locally available scanners with dedicated phased-array cardiac surface coils. All images were obtained during breath-hold. CMR imaging was performed at rest and repeated during continuous infusion of low-dose (7.5 μg/kg/min) dobutamine hydrochloride (Centrafarm Services, Etten-Leur, The Netherlands). The dobutamine infusion was decreased (or stopped if necessary) when the heartrate increased >50%, if the systolic and/or diastolic blood pressure increased >50% or decreased >20%, if serious arrhythmias occurred, or if a patient did not tolerate the dobutamine effect. Technical details on our rest and dobutamine stress protocol have been published previously.17,21–23
Analysis was performed with the software packages MASS and FLOW (Medis Medical Imaging Systems, Leiden, The Netherlands). Contours were manually drawn, under supervision of an experienced observer (W.A.H.), in end-diastole and end-systole. Papillary muscle and large trabeculae were excluded from the blood pool. Biventricular EDV and ESV were obtained and used to calculate the EF. All ventricular volumes were indexed (i) for body surface area.
A normal stress response to low-dose dobutamine in healthy individuals consist of a decrease in ESV and a subsequent increase in EF.15 Therefore, an abnormal response to stress was defined as the inability to decrease ESV during stress and/or the inability to increase EF. Relative changes in CMR parameters during stress were calculated as follows: relative parameter change = [(parameterstress − parameterrest)/parameterrest] * 100.
Statistical methods
Continuous variables with a normal distribution were summarized as mean (standard deviation). Differences between groups were analysed by Student’s t-tests. Variables with a non-normal distribution were presented as median (interquartile range), and between-group differences were analysed by Mann–Whitney U tests. Categorical variables were presented as numbers and percentages, whereas between-group differences were evaluated by χ2 tests or the Fisher’s exact test. Differences between rest and stress CMR measurements were analysed with paired Student’s t-tests.
The correlation between relative change in RVESV and NT-proBNP was evaluated with the Spearman’s rank correlation. The cumulative endpoint-free survival was estimated with Kaplan–Meier curves and differences between patients with and without the cumulative endpoint were evaluated by the log-rank test. We applied Cox proportional hazard regression analyses to relate CMR parameters, age at CMR, NT-proBNP levels, and QRS-duration with endpoint-free survival. In a multivariable cox regression model, we included NT-proBNP and a CMR stress parameter. We performed two separate multivariable analyses with two parameters instead of a multivariable model with more parameters due to the limited cumulative endpoints and therefore limited statistical power. All analyses were performed using the SPSS statistical software package version 24.0 (IBM Corp., Armonk, NY, USA), two-sided P-values <0.05 were considered statistically significant.
Results
In total, 104 patients underwent dobutamine stress CMR. Of these 104, four patients were not included in the final analysis because the dobutamine infusion had to be terminated or decreased because of adverse effects such as ventricular bigeminy and >50% increase of heartrate. These adverse effects recovered spontaneously directly after termination of the dobutamine infusion.17,21–23
Dobutamine stress CMR was well-tolerated and successfully completed in 100 patients, these patients were included in this analysis. The median age at dobutamine CMR was 17.8 (13.5–34.0) years, median time between the TOF repair and dobutamine CMR was 16.7 (12.6–33.2) years. Patients characteristics are shown in Table 1. At dobutamine CMR, 15 (15%) patients had undergone a first pulmonary valve replacement (PVR) and seven (7%) patients had a residual pulmonary stenosis with a peak gradient >30 mmHg (but <60 mmHg) measured by ultrasound.
Table 1.
Patient characteristics at dobutamine CMR
| Total (n = 100) | Composite endpoint (n = 10) | No composite endpoint (n = 90) | P-valuea | |
|---|---|---|---|---|
| Male, n (%) | 67 (67.0) | 7 (70.0) | 60 (66.7) | 0.83 |
| 22q11 syndrome, n (%) | 3 (3.0) | 1 (10.0) | 2 (2.2) | 0.27 |
| Age at CMR (years) | 17.8 (13.5–34.0) | 15.2 (10.4–34.6) | 18.2 (14.0–34.6) | 0.35 |
| Time after TOFr (years) | 16.7 (12.6–33.2) | 15.6 (13.2–17.5) | 17.0 (12.1–33.6) | 0.30 |
| Length (cm) | 166.8 ± 15.7 | 166.5 ± 22.4 | 166.9 ± 14.9 | 0.96 |
| Weight (kg) | 61.5 ± 18.7 | 61.8 ± 23.7 | 61.5 ± 18.2 | 0.97 |
| BSA (m2) | 61.5 ± 18.7 | 1.7 ± 0.44 | 1.68 ± 0.32 | 0.99 |
| Palliative shunt, n (%) | 18 (18.0) | 1 (10.0) | 17 (18.9) | 0.69 |
| Age at TOFr (years) | 0.9 (0.6–2.1) | 0.6 (0.2–1.6) | 1.0 (0.6–2.1) | 0.076 |
| Transannular patch, n (%) | 80 (80.0) | 8 (80.0) | 72 (80.0) | 1.00 |
| PVR at baseline, n (%) | 15 (15.0) | 3 (30.0) | 12 (13.3) | 0.17 |
| Pulmonary stenosis at baseline, n (%)b | 7 (7.0) | 1 (1.0) | 6 (6.0) | 0.53 |
| QRS duration (ms) | 129 ± 24 (n = 98) | 134 ± 23 (n = 10) | 129 ± 25 (n = 85) | 0.50 |
| NT-proBNP (μmol/L) | 12.9 (6.8–21.2) (n = 96) | 16.4 (10.7–41.2) (n = 10) | 12.4 (6.6–20.3) (n = 86) | 0.13 |
| Peak VO2 (mL/min/kg) | 35.7 ± 9.5 (n = 92) | 35.6 ± 9.6 (n = 7) | 37.6 ± 8.2 (n = 85) | 0.60 |
| Peak VO2 (% of predicted) | 89.9 ± 19.3 (n = 92) | 89.8 ± 19.3 (n = 7) | 92.1 ± 21.1 (n = 85) | 0.76 |
Results are given as mean (standard deviation), as median (interquartile range), or as counts (percentages).
CMR, cardiovascular magnetic resonance imaging; PVR, pulmonary valve replacement; TOFr, Tetralogy of Fallot repair; VO2, oxygen uptake.
P-value between patients with and without a composite endpoint.
>30 mmHg (but <60 mmHg) measured by ultrasound.
After the stress CMR, the median follow-up was 8.6 (6.7–14.1) years. At the latest follow-up, 10 patients (10%) had reached the composite endpoint, median 6.2 (3.1–13.3) years after stress CMR (Table 2). All patients were alive at their latest follow-up.
Table 2.
Clinical state at latest follow-up
| Patients (n = 100) | |
|---|---|
| Median age at latest follow-up (years) | 29.4 (24.3–39.7) |
| Median time after dobutamine CMR (years) | 8.6 (6.7–14.1) |
| Composite endpoint, n (%) | 10 (10.0) |
| Median time after dobutamine CMR (years) | 6.2 (3.1–13.3) |
| Median time after TOFr (years) | 26.0 (17.1–37.2) |
| Ablation/cardioversion of arrhythmias, n (%) | 5 (5.0) |
| Atrial arrhythmia, n (%) | 3 (3.0) |
| Ventricular arrhythmia, n (%) | 2 (2.0) |
| ICD after VT, n (%) | 1 (1.0) |
| VO2 max ≤65% of predicted, n (%) | 4 (4.0) |
Results are given as median (interquartile range) or as counts (percentages).
CMR, cardiac magnetic resonance imaging; ICD, implantable cardioverter defibrillator; TOFr, Tetralogy of Fallot repair; VT, ventricular tachycardia; VO2, oxygen uptake.
Ventricular response to stress
The results of the CMR studies are shown in Table 3. For the entire group, during dobutamine stress, there was a significant decrease in LVESV (33 ± 9 vs. 22 ± 8 mL/m2, P < 0.001) and RVESV (67 ± 26 vs. 51 ± 23 mL/m2, P < 0.001). Both LVEF (59 ± 7 vs. 72 ± 7%, P < 0.001) and RVEF (49 ± 7 vs. 61 ± 8%, P < 0.001) increased significantly during stress. In seven patients (7%), an abnormal stress response (i.e. inability to decrease ESV and/or increase EF during stress) was observed; three patients could not increase EF, two patients could not decrease ESV during stress, and in two patients no change in these parameters was noted.
Table 3.
CMR parameters for patients with and without a composite endpoint
| All patients (n = 100) |
Composite endpoint (n = 10) |
No composite endpoint (n = 90) |
P-value event vs. no event in stress | ||||
|---|---|---|---|---|---|---|---|
| Rest | Stress | Rest | Stress | Rest | Stress | ||
| Rest CMR | |||||||
| LV EDV (mL/m2) | 80 ± 13 | 78 ± 14a | 84 ± 15 | 84 ± 14 | 79 ± 12 | 77 ± 14a | 0.15 |
| ESV (mL/m2) | 33 ± 9 | 22 ± 8a | 35 ± 10 | 26 ± 12a | 33 ± 8 | 22 ± 8 | 0.12 |
| SV (mL/m2) | 47 ± 8 | 55 ± 9a | 49 ± 10 | 58 ± 8a | 46 ± 8 | 55 ± 9a | 0.34 |
| EF (%) | 59 ± 7 | 72 ± 7a | 58 ± 8 | 70 ± 9a | 59 ± 7 | 72 ± 7a | 0.34 |
| RV EDV (mL/m2) | 130 ± 39 | 125 ± 38a | 123 ± 43 | 121 ± 35 | 130 ± 39 | 126 ± 38a | 0.70 |
| ESV (mL/m2) | 67 ± 26 | 51 ± 23a | 58 ± 26 | 47 ± 22a | 68 ± 26 | 51 ± 23a | 0.64 |
| SV (mL/m2) | 63 ± 17 | 75 ± 20a | 65 ± 22 | 74 ± 20a | 62 ± 17 | 75 ± 20a | 0.86 |
| EF (%) | 49 ± 7 | 61 ± 8a | 51 ± 5 | 62 ± 8a | 49 ± 8 | 61 ± 9a | 0.60 |
| Mass volume ratio (g/mL) | 0.18 ± 0.05 | 0.18 ± 0.04 | 0.19 ± 0.05 | 0.86 | |||
| PR (%) | 29 (10–44) (n = 95) | 30 (10–44) (n = 80) | 17 (9–27) (n = 10) | 17 (6–32) (n = 9) | 32 (9–45) (n = 85) | 33 (11–46) (n = 71) | 0.14 |
| Relative change during stress | |||||||
| LV EDV (%) | −3 ± 9 | 0 ± 6 | −3 ± 9 | 0.22 | |||
| ESV (%) | −35 ± 14 | −27 ± 16 | −36 ± 14 | 0.062 | |||
| SV (%) | 20 ± 15 | 22 ± 17 | 20 ± 15 | 0.74 | |||
| EF (%) | 24 ± 12 | 21 ± 15 | 24 ± 11 | 0.43 | |||
| RV EDV (%) | −3 ± 9 | 2 ± 11 | −3 ± 9 | 0.13 | |||
| ESV (%) | −25 ± 13 | −17 ± 15 | −26 ± 13 | 0.045 | |||
| SV (%) | 21 ± 17 | 18 ± 18 | 21 ± 17 | 0.61 | |||
| EF (%) | 24 ± 13 | 17 ± 12 | 25 ± 13 | 0.052 | |||
| Abnormal stress response, n (%) | 7 (7.0) | 3 (30.0) | 4 (4.4) | 0.021 | |||
Results are given as mean (standard deviation) or as median (interquartile range) or as counts (percentages).
No statistical significant differences at rest between the group with and without a composite endpoint, no significant differences were found.
CMR, cardiovascular magnetic resonance; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricle; PR, pulmonary regurgitation; RV, right ventricle; SV, stroke volume.
A statistical significant difference between rest vs. stress within the subgroup.
Study endpoints
There was no difference in median age at dobutamine CMR between the patients who did and did not reach the composite endpoint. NT-proBNP levels, predicted-peak VO2, and rest CMR parameters did not differ between both groups (Tables 1and3).
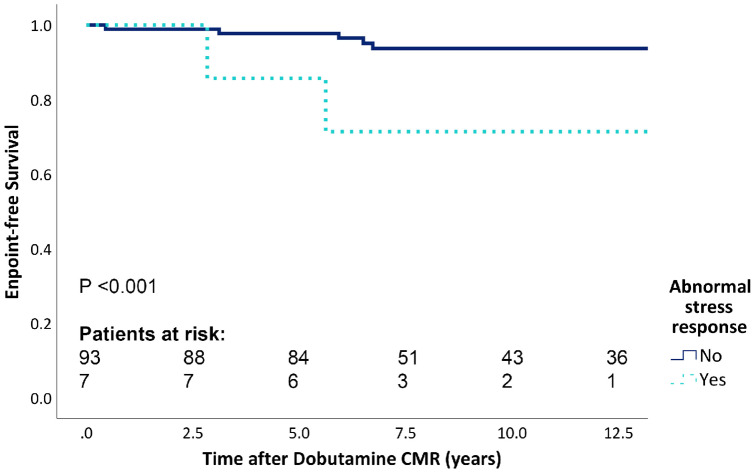
Patients with an abnormal stress response had a poorer endpoint-free survival (Figure 1, P < 0.001). A significantly lower relative decrease in RVESV during stress was observed in endpoint patients compared with patients who did not reach the study endpoint (−17 ± 15 vs. −26 ± 13%, P = 0.045).
Figure 1.
Endpoint-free survival for normal vs. abnormal dobutamine stress response. CMR, cardiac magnetic resonance imaging.
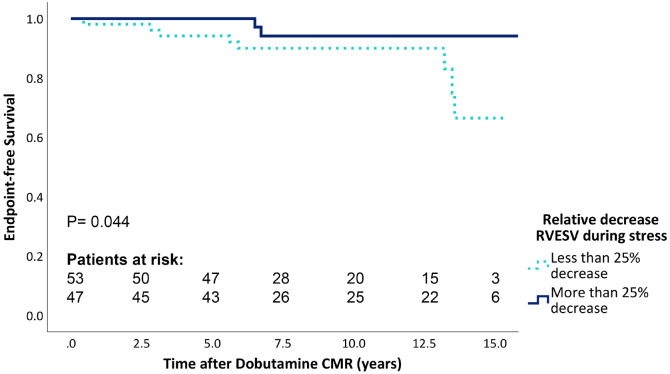
In Figure 2, the endpoint-free survival for the patients with the largest (≥−25%) and lowest (<−25%) relative decrease in RVESV during stress is shown. Patients with a larger relative decrease during stress experienced a better endpoint-free survival (P = 0.044).
Figure 2.
Endpoint-free survival for the relative decrease of RVESV during stress. CMR, cardiac magnetic resonance imaging; RVESV, right ventricular end systolic volume.
Determinants of the composite endpoint
Univariable analysis showed that patients were significantly more likely to experience the composite endpoint when they had an abnormal stress response [hazard ratio (HR) 10.5; 95% confidence interval (CI) 248–44.78], higher NT-proBNP levels (HR 1.03; 95% CI 1.00–1.05), or a higher relative decrease in RVESV during stress (HR 1.06; 95% CI 1.01–1.12) (see Table 4). There was no statistical significant correlation between NT-proBNP levels and relative change in RVESV during stress, r = 0.06, P = 0.54.
Table 4.
Univariable cox-regression analyses for the composite endpoint
| Univariable analysis |
|||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Age at CMR (years) | 1.01 | 0.95–1.07 | 0.74 |
| NT-proBNP (μmol/L) | 1.03 | 1.00–1.05 | 0.030 |
| QRS duration (ms) | 1.01 | 0.99–1.04 | 0.41 |
| Rest CMR | |||
| LVEDV (mL/m2) | 1.02 | 0.97–1.08 | 0.43 |
| LVESV (mL/m2) | 0.86 | 0.93–1.09 | 0.86 |
| LVEF (%) | 1.01 | 0.92–1.11 | 0.82 |
| RVEDV (mL/m2) | 0.99 | 0.97–1.01 | 0.22 |
| RVESV (mL/m2) | 0.98 | 0.95–1.01 | 0.094 |
| RVEF (%) | 1.04 | 0.95–1.04 | 0.83 |
| RV mass volume ratio (g/mL) (↑0.1) | 1.40 | 0.32–6.02 | 0.65 |
| Relative change during stress | |||
| LVESV (%) | 1.04 | 0.99–1.09 | 0.086 |
| LVEF (%) | 0.98 | 0.92–1.04 | 0.42 |
| RVEDV (%) | 1.07 | 1.01–1.15 | 0.036 |
| RVESV (%) | 1.06 | 1.01–1.12 | 0.016 |
| RVEF (%) | 0.94 | 0.89–0.99 | 0.043 |
| Abnormal stress response | 10.5 | 2.48–44.78 | 0.001 |
CMR, cardiac magnetic resonance imaging; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricle; RV, right ventricle; SV, stroke volume.
In a multivariable model with both NT-proBNP and relative change in RVESV, both parameters remained predictive for the composite endpoint. In a multivariable model with NT-proBNP and abnormal stress response, NT-proBNP lost its predictive value for the composite endpoint however abnormal stress response remained a strong independent predictor for the composite endpoint (see Table 5).
Table 5.
Multivariable cox-regression analyses for the composite endpoint
| Multivariable analysis |
|||
|---|---|---|---|
| HR | 95% CI | P-value | |
| Multivariable Model 1 | |||
| NT-proBNP (μmol/L) | 1.0 | 1.0–1.1 | 0.055 |
| Abnormal stress response | 10.4 | 2.5–43.7 | 0.001 |
| Multivariable Model 2 | |||
| NT-proBNP (μmol/L) | 1.0 | 1.0–1.1 | 0.009 |
| Relative change in RVESV (%) | 1.1 | 1.0–1.1 | 0.004 |
CMR, cardiac magnetic resonance imaging; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; LV, left ventricle; RV, right ventricle; SV, stroke volume.
Discussion
In this study, we demonstrated, despite a relatively low number of endpoints, a clear relation between ventricular response to low-dose dobutamine stress CMR and a composite endpoint in TOF patients mid-term after TOF-repair. An abnormal response to dobutamine stress and a diminished relative decrease in RVESV during stress were associated with adverse outcome.
Patients with TOF are at increased risk of mortality and morbidity.1,2,4,5 Many of the studies that have looked at predictors in TOF patients have been performed in older patients cohorts with an older age at initial repair.4,7–9,12,13,24 What our current study adds, in a cohort of adolescents and young adults (median age 17.8 years), is that stress CMR is more predictive for poor outcome after 8.6 years follow-up than conventional CMR imaging parameters. Also, the median age at TOF-repair in our cohort was 0.9 years, which is highly comparable to the clinical practice for the past two decades.25,26
In other studies, several predictors for outcome have been described.4,8,9,12,24,27
Clinical and non-imaging parameters
Of the non-imaging derived parameter, NT-proBNP, QRS duration, and peak VO2 are related to outcome in TOF.4,8,9 In an adult congenital heart disease (ConHD) cohort, a NT-proBNP level of >33 μmol/L identified patients with increased risk for poor outcome.28 Also in adult TOF patients elevated NT-proBNP levels are related to adverse clinical outcome.8 Although the median NT-proBNP levels in our study were relatively low (12.9 μmol/L). We observed that higher NT-proBNP levels were associated with the composite endpoint in univariable analysis and in a multivariable model with relative change in RVESV. In a multivariable model with abnormal stress response NT-proBNP lost its predictive value for the composite endpoint, however, a strong trend (P = 0.055) remained.
QRS duration ≥180 ms in adults and ≥170 ms in children are known predictors for ventricular tachycardia and sudden death.4,29 In our relatively young TOF cohort, we observed no differences in QRS duration between the patients with and without a composite endpoint. Only three patients had a QRS duration >170 ms.
Peak VO2 is an established prognostic marker in ConHD patients and TOF patients and guidelines recommend cardiopulmonary exercise testing during routine follow-up of TOF patients.9,10,14,26,27 Diller et al.10 reported in 2005 that older (31.8 years) TOF patients with a peak VO2 of ≤15.5 mL/kg/min have an increased risk of hospitalization and death during follow-up. Giardini et al.30 observed that a lower peak VO2 is associated with death and hospitalization during follow-up. In another larger and relatively young (25.5 years) TOF cohort, a peak VO2 ≤65% of predicted was associated with an increased risk for death, sustained ventricular tachycardia, and cardiac-related hospitalizations.9
As mentioned, research in ConHD patients is complicated by the relatively low incidence of hard endpoints, resulting in the use of surrogate endpoints.14 Based on the relations between exercise performance, particularly VO2 max and subsequent outcome, peak VO2 ≤65% was included in the composite endpoint used in our study.
RV size and function
Alongside clinical parameters, RV size is a key factor in outcome in TOF and the decision for PVR. If PVR is performed on time, i.e. before irreversible remodelling has occurred, it might help to normalize volumes and improve function. However, survival benefit of PVR using the current criteria has not been demonstrated.5,14,31,32 International guidelines indicate that several factors such as the presence of symptoms, degree of PR, RVEDV, RVESV, and objective exercise performance can be used to determine if PVR is justified.14,26 However, often no quantitative limits are given and the criteria differ between guidelines.14,26 Also, RV size parameters fail to take into consideration the underlying RV mechanics, such as contractility, diastolic function, energy loss, ventriculo-arterial coupling, or ventricular function during stress.21,33–35 Contractility can be maintained in severe RV dilatation.34 Diastolic RV function is impaired in many TOF patients and relates to clinical state but has not been shown to predict poor outcome.36 Latus et al.34 showed that RV-pulmonary arterial (PA) coupling is impaired in TOF patients at rest and did not improve with dobutamine stress. In patients without a transannular patch, RV-PA coupling did improve with dobutamine stress.34 Whether these findings relate to our and observations of others of impaired reduction of RVESV with dobutamine stress,18 is not certain at present.
It is important to identify parameters for early RV dysfunction that have prognostic impact. The cardiac response to dobutamine stress could be an additional parameter in this decision process, combining volumetric and functional parameters with a state of stress, unmasking abnormalities only visible during stress.21
Stress CMR
Previous studies using low-dose dobutamine stress CMR, demonstrated that TOF patients are able to decrease ESV and increase EF during stress. Some studies also describe an abnormal decrease in RVEDV.16–18,21 In a 5-year follow-up study of TOF patients, our group previously showed that patients with a small relative increase in RVEF during dobutamine stress were more likely to have a large decrease in peak VO2 5 years later.17 However, the relationship between stress response and cardiac events has not been described yet in TOF patients.
In systemic RV patients with a biventricular circulation, a previous study identified that an abnormal stress response (present in 17 of the 39 patients) was predictive for cardiac events during follow-up.20 We observed an abnormal stress response in only seven patients, most likely explained by the differences in age and type of ConHD. However, these seven patients experienced more often an adverse outcome during follow-up and had a worse endpoint-free survival.
In young Fontan patients, without other known parameters predictive for cardiac events, the relationship between single ventricle functional reserve (EFstress−EFrest) and events has recently been described by our group.19 In our current TOF study, the relative decrease of RVESV during stress was also associated with the composite endpoint. All these studies mentioned above indicate that dobutamine stress imaging may be helpful in risk stratification and predicting future events.
Limitations
Although the absolute number of TOF patients receiving a dobutamine CMR is the largest reported cohort so far, the number of hard endpoints during follow-up was limited, which is a known limitation in ConHD research.14 We therefore used a composite endpoint of cardiac death, arrhythmias, and diminished exercise tolerance to assess the possible association between the stress CMR and subsequent adverse outcome. Relatively few patients reached this composite endpoint, which is a limitation of our study. However, despite the small number of endpoints obtained, we observed a clear relationship between the ventricular stress response and subsequent outcomes during follow-up. Information on how to interpret stress CMR in TOF patients was not available at the time of stress CMR, and therefore, stress CMR parameters were not used for clinical decision-making.
Secondly, it is not certain whether dobutamine stress CMR gives the same results as exercise stress CMR. Because our CMR scanner is not suitable for supine leg exercise, the effects of exercise on cardiac function had to be simulated with pharmacological stress. Thirdly, late gadolinium enhancement or T1 mapping could not be performed because of time constraints during the extensive scan protocol. Both factors are associated with outcome in TOF patients.37,38
Conclusion
An abnormal ventricular response to dobutamine stress is associated with adverse outcome in young TOF patients.
Funding
This work was supported by the Dutch Heart Foundation (2013T091, 2008B026, and 2006B095 to E.v.d.B., N.D., and S.E.L.).
Conflict of interest: none declared.
References
- 1. Geva T. Tetralogy of Fallot repair: ready for a new paradigm. J Thorac Cardiovasc Surg 2012;143:1305–6. [DOI] [PubMed] [Google Scholar]
- 2. Luijten LW, van den Bosch E, Duppen N, Tanke R, Roos-Hesselink J, Nijveld A. et al. Long-term outcomes of transatrial-transpulmonary repair of tetralogy of Fallot. Eur J Cardiothorac Surg 2015;47:527–34. [DOI] [PubMed] [Google Scholar]
- 3. Hickey EJ, Veldtman G, Bradley TJ, Gengsakul A, Manlhiot C, Williams WG. et al. Late risk of outcomes for adults with repaired tetralogy of Fallot from an inception cohort spanning four decades. Eur J Cardiothorac Surg 2009;35:156–64; discussion 164. [DOI] [PubMed] [Google Scholar]
- 4. Gatzoulis MA, Balaji S, Webber SA, Siu SC, Hokanson JS, Poile C. et al. Risk factors for arrhythmia and sudden cardiac death late after repair of tetralogy of Fallot: a multicentre study. Lancet 2000;356:975–81. [DOI] [PubMed] [Google Scholar]
- 5. Frigiola A, Tsang V, Bull C, Coats L, Khambadkone S, Derrick G. et al. Biventricular response after pulmonary valve replacement for right ventricular outflow tract dysfunction: is age a predictor of outcome? Circulation 2008;118:S182–90. [DOI] [PubMed] [Google Scholar]
- 6. Abd El Rahman MY, Abdul-Khaliq H, Vogel M, Alexi-Meskishvili V, Gutberlet M, Lange PE.. Relation between right ventricular enlargement, QRS duration, and right ventricular function in patients with tetralogy of Fallot and pulmonary regurgitation after surgical repair. Heart 2000;84:416–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eindhoven JA, Menting ME, van den Bosch AE, Cuypers JA, Ruys TP, Witsenburg M. et al. Associations between N-terminal pro-B-type natriuretic peptide and cardiac function in adults with corrected tetralogy of Fallot. Int J Cardiol 2014;174:550–6. [DOI] [PubMed] [Google Scholar]
- 8. Westhoff-Bleck M, Kornau F, Haghikia A, Horke A, Bertram H, Treptau J. et al. NT-proBNP indicates left ventricular impairment and adverse clinical outcome in patients with tetralogy of Fallot and pulmonary regurgitation. Can J Cardiol 2016;32:1247.e29–e36. [DOI] [PubMed] [Google Scholar]
- 9. Muller J, Hager A, Diller GP, Derrick G, Buys R, Dubowy KO. et al. Peak oxygen uptake, ventilatory efficiency and QRS-duration predict event free survival in patients late after surgical repair of tetralogy of Fallot. Int J Cardiol 2015;196:158–64. [DOI] [PubMed] [Google Scholar]
- 10. Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS. et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation 2005;112:828–35. [DOI] [PubMed] [Google Scholar]
- 11. Orwat S, Diller GP, Kempny A, Radke R, Peters B, Kuhne T. et al. Myocardial deformation parameters predict outcome in patients with repaired tetralogy of Fallot. Heart 2016;102:209–15. [DOI] [PubMed] [Google Scholar]
- 12. Knauth AL, Gauvreau K, Powell AJ, Landzberg MJ, Walsh EP, Lock JE. et al. Ventricular size and function assessed by cardiac MRI predict major adverse clinical outcomes late after tetralogy of Fallot repair. Heart 2008;94:211–6. [DOI] [PubMed] [Google Scholar]
- 13. Diller GP, Kempny A, Liodakis E, Alonso-Gonzalez R, Inuzuka R, Uebing A. et al. Left ventricular longitudinal function predicts life-threatening ventricular arrhythmia and death in adults with repaired tetralogy of Fallot. Circulation 2012;125:2440–6. [DOI] [PubMed] [Google Scholar]
- 14. Stout KK, Daniels CJ, Aboulhosn JA, Bozkurt B, Broberg CS, Colman JM. et al. 2018 AHA/ACC guideline for the management of adults with congenital heart disease. Circulation 2019;139:e637–97. [DOI] [PubMed] [Google Scholar]
- 15. De Wolf D, Suys B, Verhaaren H, Matthys D, Taeymans Y.. Low-dose dobutamine stress echocardiography in children and young adults. Am J Cardiol 1998;81:895–901. [DOI] [PubMed] [Google Scholar]
- 16. Uebing A, Fischer G, Schlangen J, Apitz C, Steendijk P, Kramer HH.. Can we use the end systolic volume index to monitor intrinsic right ventricular function after repair of tetralogy of Fallot? Int J Cardiol 2011;147:52–7. [DOI] [PubMed] [Google Scholar]
- 17. Luijnenburg SE, Mekic S, van den Berg J, van der Geest RJ, Moelker A, Roos-Hesselink JW. et al. Ventricular response to dobutamine stress relates to the change in peak oxygen uptake during the 5-year follow-up in young patients with repaired tetralogy of Fallot. Eur Heart J Cardiovasc Imaging 2014;15:189–94. [DOI] [PubMed] [Google Scholar]
- 18. Parish V, Valverde I, Kutty S, Head C, Qureshi SA, Sarikouch S. et al. Dobutamine stress MRI in repaired tetralogy of Fallot with chronic pulmonary regurgitation: a comparison with healthy volunteers. Int J Cardiol 2013;166:96–105. [DOI] [PubMed] [Google Scholar]
- 19. van den Bosch E, Bossers SSM, Robbers-Visser D, Boersma E, Roos-Hesselink JW, Breur H. et al. Ventricular response to dobutamine stress CMR is a predictor for outcome in Fontan patients. JACC Cardiovasc Imaging 2019;12:368–70. [DOI] [PubMed] [Google Scholar]
- 20. Winter MM, Scherptong RW, Kumar S, Bouma BJ, Tulevski II, Tops LF. et al. Ventricular response to stress predicts outcome in adult patients with a systemic right ventricle. Am Heart J 2010;160:870–6. [DOI] [PubMed] [Google Scholar]
- 21. van den Berg J, Wielopolski PA, Meijboom FJ, Witsenburg M, Bogers AJ, Pattynama PM. et al. Diastolic function in repaired tetralogy of Fallot at rest and during stress: assessment with MR imaging. Radiology 2007;243:212–9. [DOI] [PubMed] [Google Scholar]
- 22. Cuypers JA, Menting ME, Konings EE, Opic P, Utens EM, Helbing WA. et al. Unnatural history of tetralogy of Fallot: prospective follow-up of 40 years after surgical correction. Circulation 2014;130:1944–53. [DOI] [PubMed] [Google Scholar]
- 23. Duppen N, Etnel JR, Spaans L, Takken T, van den Berg-Emons RJ, Boersma E. et al. Does exercise training improve cardiopulmonary fitness and daily physical activity in children and young adults with corrected tetralogy of Fallot or Fontan circulation? A randomized controlled trial. Am Heart J 2015;170:606–14. [DOI] [PubMed] [Google Scholar]
- 24. Bokma JP, de Wilde KC, Vliegen HW, van Dijk AP, van Melle JP, Meijboom FJ. et al. Value of cardiovascular magnetic resonance imaging in noninvasive risk stratification in tetralogy of Fallot. JAMA Cardiol 2017;2:678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steiner MB, Tang X, Gossett JM, Malik S, Prodhan P.. Timing of complete repair of non-ductal-dependent tetralogy of Fallot and short-term postoperative outcomes, a multicenter analysis. J Thorac Cardiovasc Surg 2014;147:1299–305. [DOI] [PubMed] [Google Scholar]
- 26. Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N. et al. ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 2010;31:2915–57. [DOI] [PubMed] [Google Scholar]
- 27. Valente AM, Gauvreau K, Assenza GE, Babu-Narayan SV, Schreier J, Gatzoulis MA. et al. Contemporary predictors of death and sustained ventricular tachycardia in patients with repaired tetralogy of Fallot enrolled in the INDICATOR cohort. Heart 2014;100:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baggen VJ, van den Bosch AE, Eindhoven JA, Schut AW, Cuypers JA, Witsenburg M. et al. Prognostic value of N-terminal pro-B-type natriuretic peptide, troponin-T, and growth-differentiation factor 15 in adult congenital heart disease. Circulation 2017;135:264–79. [DOI] [PubMed] [Google Scholar]
- 29. Berul CI, Hill SL, Geggel RL, Hijazi ZM, Marx GR, Rhodes J. et al. Electrocardiographic markers of late sudden death risk in postoperative tetralogy of Fallot children. J Cardiovasc Electrophysiol 1997;8:1349–56. [DOI] [PubMed] [Google Scholar]
- 30. Giardini A, Specchia S, Tacy TA, Coutsoumbas G, Gargiulo G, Donti A. et al. Usefulness of cardiopulmonary exercise to predict long-term prognosis in adults with repaired tetralogy of Fallot. Am J Cardiol 2007;99:1462–7. [DOI] [PubMed] [Google Scholar]
- 31. Lee C, Kim YM, Lee CH, Kwak JG, Park CS, Song JY. et al. Outcomes of pulmonary valve replacement in 170 patients with chronic pulmonary regurgitation after relief of right ventricular outflow tract obstruction: implications for optimal timing of pulmonary valve replacement. J Am Coll Cardiol 2012;60:1005–14. [DOI] [PubMed] [Google Scholar]
- 32. Therrien J, Provost Y, Merchant N, Williams W, Colman J, Webb G.. Optimal timing for pulmonary valve replacement in adults after tetralogy of Fallot repair. Am J Cardiol 2005;95:779–82. [DOI] [PubMed] [Google Scholar]
- 33. Mercer-Rosa L, Fogel MA, Paridon SM, Rychik J, Yang W, Goldmuntz E.. Revisiting the end-diastolic forward flow (restrictive physiology) in tetralogy of Fallot: an exercise, echocardiographic, and magnetic resonance study. JACC Cardiovasc Imaging 2018;11:1547–8. [DOI] [PubMed] [Google Scholar]
- 34. Latus H, Binder W, Kerst G, Hofbeck M, Sieverding L, Apitz C.. Right ventricular-pulmonary arterial coupling in patients after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg 2013;146:1366–72. [DOI] [PubMed] [Google Scholar]
- 35. Kutty S, Valente AM, White MT, Hickey K, Danford DA, Powell AJ. et al. Usefulness of pulmonary arterial end-diastolic forward flow late after tetralogy of Fallot repair to predict a “restrictive” right ventricle. Am J Cardiol 2018;121:1380–6. [DOI] [PubMed] [Google Scholar]
- 36. Luijnenburg SE, Peters RE, van der Geest RJ, Moelker A, Roos-Hesselink JW, de Rijke YB. et al. Abnormal right atrial and right ventricular diastolic function relate to impaired clinical condition in patients operated for tetralogy of Fallot. Int J Cardiol 2013;167:833–9. [DOI] [PubMed] [Google Scholar]
- 37. Dobson RJ, Mordi I, Danton MH, Walker NL, Walker HA, Tzemos N.. Late gadolinium enhancement and adverse outcomes in a contemporary cohort of adult survivors of tetralogy of Fallot. Congenit Heart Dis 2017;12:58–66. [DOI] [PubMed] [Google Scholar]
- 38. Babu-Narayan SV, Kilner PJ, Li W, Moon JC, Goktekin O, Davlouros PA. et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of Fallot and its relationship to adverse markers of clinical outcome. Circulation 2006;113:405–13. [DOI] [PubMed] [Google Scholar]