Abstract
Introduction May–Thurner syndrome (MTS) is a vascular anatomic variant resulting in compression of the left common iliac vein by the right common iliac artery, affecting approximately 22% of the population. In adults, following acute deep vein thrombosis (DVT) of the iliofemoral veins, the incidence of postthrombotic syndrome (PTS) and recurrent DVT are high if treated with anticoagulation alone, warranting adjunctive treatment with thrombolysis and stent placement. However, there is paucity of literature documenting the course of treatment and associated outcomes in pediatric patients with MTS.
Methods A retrospective chart review of pediatric patients (≤ 18 years of age) with radiologic confirmation of MTS with or without DVT evaluated and/or treated at our institution from January 1, 2005 through December 31, 2015 was conducted.
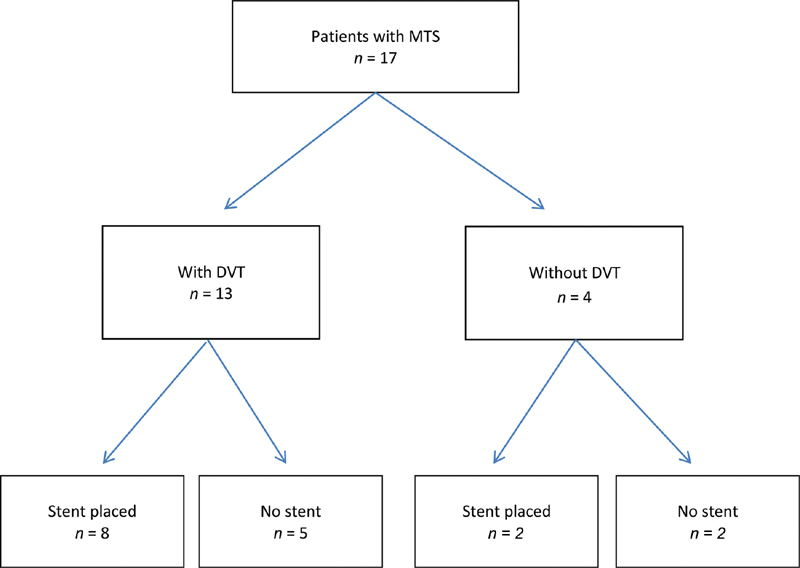
Results Seventeen patients (4 male; 13 female) were identified. Median age was 15.4 years (range 8.8–17.1 years) with a median follow-up of 1.2 years (range 0.4–7.5 years). Thirteen (76.5%) patients presented with left lower extremity DVT. Management included catheter-directed thrombolysis ( n = 5), systemic thrombolysis ( n = 1), and mechanical thrombectomy ( n = 1). Fifteen patients were treated with anticoagulation including two patients with endovascular stents without DVT. Median duration of anticoagulation was 6.3 months (range 3.2–18.7 months). Ten patients (59%) underwent stent placements.
Complete and partial thrombus resolution was noted in six patients each and no resolution in one patient. Four patients had recurrence/progression of thrombus ( n = 3 with stents) at a median time of 29 days (range 12–495 days). No bleeding complications were observed. Clinically documented or self-reported PTS was noted in 8 patients (62%).
Conclusion There are no clear guidelines for MTS management in children and adolescents. In our cohort, thrombolysis, anticoagulation, or stent placements were not associated with bleeding risks, with recurrence/progression of DVT and signs and symptoms of PTS noted in 30 and 62%, respectively. Further studies are needed to determine a standardized treatment approach of the pediatric patient with MTS with or without thrombosis.
Keywords: May–Thurner syndrome, deep vein thrombosis, pediatric, children
Introduction
May–Thurner syndrome (MTS) also known as Cockett syndrome is an anatomic variant resulting in compression of the left iliac vein by the overlying right common iliac artery against the lumbar vertebrae. As the vessel is compressed, changes of chronic endothelial irritation including collagen deposition can develop over time with partial or complete occlusion of the vein. This anatomic variant associated with MTS is known to increase the incidence of left-sided lower extremity deep vein thrombosis (DVT). 1 2 3 4 The true prevalence of MTS is unknown, but it is estimated to be associated in 18 to 49% of left leg DVT. 1 2 Clinically, most patients present with acute DVT in the left lower extremity or progressively unilateral leg swelling and pain without an identifiable thrombosis; however, many patients may remain asymptomatic throughout their lifetime. 3 The clinical association of DVT and MTS is relatively low with a reported range of 2 to 3%. 5
The main treatment goal of MTS associated with iliofemoral DVT is directed toward prevention of postthrombotic syndrome (PTS) (e.g., swelling secondary to venous insufficiency, pain, skin discoloration, and venous stasis skin ulceration). Anticoagulant therapy with unfractionated heparin (UFH), low molecular weight heparin (LMWH), with or without transition to warfarin, or direct-acting anticoagulants (e.g., rivaroxaban, apixaban) is the main treatment modality for patients presenting with acute DVT. Anticoagulation prevents thrombus propagation and/or pulmonary embolism (PE) but does not resolve the chronic venous occlusion secondary to thrombus or anatomic occlusion of the iliac vein secondary to compression by the overlying right iliac artery. Acute thrombosis associated with MTS has been historically treated with catheter-directed thrombolysis (CDT), mechanical thrombectomy (CDT in combination with mechanical thrombectomy is often referred to as pharmacomechanical thrombectomy [PMT]), percutaneous balloon angioplasty, and stenting. 5 6 7 8 In adults with MTS and DVT, recurrence of thrombosis is more common in those patients treated with anticoagulation alone 9 than those treated with PMT. Most adults with DVT in the setting of MTS undergo stent placement 10 11 whether in combination with PMT or later in association with venous recanalization of the chronic postthrombotic obstruction, angioplasty, and stent placement. However, few case reports and studies have reported the course of treatment and outcomes in pediatric patients with MTS. 12 13 14 There are currently no established standard treatment approaches in pediatric patients with MTS.
The purpose of our study was to retrospectively review our institutional management of pediatric MTS with or without DVT, and outcomes including thrombus response, recurrence/progression of thrombus, stent failure, and development of PTS.
Methods
This study was approved by the Mayo Clinic Institutional Review Board. Medical record data was abstracted for all pediatric patients (≤ 18 years of age) who presented for evaluation and/or treatment of MTS with or without DVT. The study period comprised from January 1, 2005 to December 31, 2015. Patient demographic data, baseline thrombosis risk factors (e.g., body mass index [BMI]), congenital or acquired thrombophilia, immobility, and oral contraceptive pill (OCP) use, were reviewed. Overweight was defined as a BMI ≥ 25 kg/m 2 and obesity as a BMI ≥ 30 kg/m 2 . 15 Thrombophilia testing included antiphospholipid antibodies (i.e., lupus anticoagulant immunoglobulin [Ig] G, IgM, and anticardiolipin antibodies), activated protein C (APC) resistance with reflex gene sequencing for factor V Leiden if APC resistance abnormal, prothrombin G20210A mutation, antithrombin activity and antigen, protein C activity, free protein S, fibrinogen level, and fibrin D-dimer. Radiological techniques used for diagnosis of MTS and DVT, modality of treatment (e.g., catheter or systemic thrombolysis, thrombectomy, balloon angioplasty, stent placement), duration, and type of anticoagulation were also collected. Thrombosis outcomes were defined as complete resolution (no residual thrombus identified by ultrasound Doppler, magnetic resonance venography [MRV], or computed tomography venography [CTV], venography), partial resolution (residual thrombosis), and no resolution (complete occlusion of one or more affected left lower extremity veins). Recurrence and progression of DVT were defined as per guidelines developed by the Perinatal and Pediatric Haemostasis Subcommittee of the Scientific and Standardization Committee of International Society of Thrombosis and Haemostasis. 15
Stent failure was defined as no demonstrable blood flow secondary to thrombosis or mechanical failure if no thrombosis. Signs and symptoms of PTS were reviewed from medical-clinical notes (e.g., pain, swelling, dilated blood vessels, varicosities, and skin ulceration). A previously developed and validated PTS survey instrument was mailed to patients with thrombosis. 16 17
Statistical Analyses
Standard statistical methods were used to summarize collected data: frequency and percent for categorical variables and mean, median, and range for ordinal or continuous variables. Analysis was performed using JMP software (JMP version 13, SAS Institute Inc, Cary, North Carolina, United States; 1989–2016). Comparative analysis among subgroups and significance of difference (two-tailed p -value < 0.05) were reported if sample size allowed analysis. Contingency analysis by Fisher's exact test was used for categorical comparisons and survival analysis was performed by Kaplan–Meier test.
Results
Demographics
Seventeen patients (13 female) were identified. Median follow-up was 1.2 years (range 0.4–7.5 years) ( Table 1 ). Fifteen patients identified as Caucasian, one patient each identified as African American and Hispanic. Median age at diagnosis of MTS was 15.4 years (range 8.8–17.1 years).
Table 1. Patient characteristics, management, and outcomes.
| Case ID | Gender/Age in years | Risk factors | Location extent thrombosis | Thrombolysis | Stenting | Anticoagulation | Recurrence/Progression (time) | Thrombosis outcomes (CR/PR/NR) | PTS/duration follow-up (y) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F/16.7 | OCP | Inferior IVC/external and internal left iliac veins/common femoral extending popliteal and tibial veins | Catheter-directed Alteplase (0.5 mg/h, 1 mg/h) | Yes | UFH/LMWH/Warfarin/Clopidogrel | No | PR | No (5.6) |
| 2 | F/16.2 | OCP | Left common iliac/common femoral, upper deep femoral to popliteal/PE | Catheter-directed Alteplase (0.5 mg/h) | Yes | UFH/Warfarin LMWH recurrent occlusion stent |
Yes (6 d) | PR | No (1.2) |
| 3 | F/15.5 | Heterozygous factor V Leiden/immobility and OCP | Left iliofemoral to popliteal | None | Yes | UFH/Warfarin/Clopidogrel | No | PR | No (0.4) |
| 4 | F/15.2 | OCP/ overweight (BMI 25.6) | Mid to distal left common iliac vein and external iliac vein | None | No | Warfarin/Clopidogrel | No | CR | Yes (0.9) |
| 5 | F/14.6 | OCP | NA | None | No | None | No | NA | NA |
| 6 | M/14.5 | None | Left common iliac vein through common femoral to popliteal | Catheter-directed Alteplase (0.5 mg/h, 0.75 mg/h) | No | LMWH/Warfarin | Yes (11 d) | PR | No (3.0) |
| 7 | M/17.1 | Heterozygous factor V Leiden Overweight (BMI 29.34) |
Common femoral, superficial femoral to popliteal and proximal calf veins/PE | Systemic t-PA (0.03 mg/kg/h, 0.06 mg/kg/h) | No | UFH/LMWH/Rivaroxaban | No | PR | Yes (1.2) |
| 8 | F/11.6 | None | Left iliac vein | None | Yes | LMWH | No | CR | Yes (7.5) |
| 9 | F/16.4 | Heterozygous factor V Leiden/Immobility/OCP | Left common iliac, femoral, deep femoral, and popliteal | Catheter-directed thrombolysis outside institution | Yes | LMWH/Warfarin | Yes (104 d) | NR | Yes (0.4) |
| 10 | M/8.8 | None | NA | None | No | None | No | NA | NA |
| 11 | F/13.6 | None | NA | None | Yes | Rivaroxaban | No | NA | NA |
| 12 | M/14.8 | None | Left common and external iliac and femoral | None | No | ASA initially then LMWH/Warfarin after balloon venoplasty | No | PR | Yes (0.7) |
| 13 | F/14.6 | Heterozygous factor V Leiden, protein C deficiency, and OCP | Left common and external iliac and common femoral | Mechanical thrombectomy-outside institution | Yes | LMWH/Warfarin | Yes (20 d) | CR | Yes (3.2) |
| 14 | F/15.4 | Immobility and OCP Obese (BMI 30.66) |
Left external iliac/common femoral to popliteal/PE | Catheter-directed Alteplase (0.5 mg/h) | Yes | LMWH/Warfarin | No | CR | Yes (1.1) |
| 15 | F/16.5 | None | NA | None | Yes | Heparin/LMWH/ Warfarin/Rivaroxaban and clopidogrel after Nutcracker repair |
No | NA | NA |
| 16 | F/16.2 | Immobility and OCP Overweight (BMI 29.4) |
Left external iliac and common femoral | None | Yes | LMWH/Warfarin/Rivaroxaban | No | CR | Yes (1.0) |
| 17 | F/16.8 | Homozygous factor V Leiden/OCP Obese (BMI 33.01) |
Left common femoral to posterior tibial/PE | None | No | LMWH/Warfarin | No | CR | No (0.8) |
Abbreviations: ASA, acetylsalicylic acid; BMI, body mass index; CR, complete resolution; DVT, deep vein thrombosis; LMWH, low molecular weight heparin; NA, not applicable; NR, no resolution; OCP, oral contraceptive pills; PE, pulmonary embolism; PR, partial resolution; PTS, postthrombotic syndrome; UFH, unfractionated heparin.
Clinical Presentation
Pain and swelling were the most common presenting symptoms ( n = 13, 76%). Thirteen patients presented with acute DVT of the left lower extremity (76%) while four were diagnosed with MTS without DVT (asymptomatic sibling of MTS patient [ n = 1] and pain and/or swelling [ n = 3]) ( Fig. 1 ). Four patients were noted to have PE at the time of DVT diagnosis ( Table 1 ).
Fig. 1.
Summary of patients with May–Thurner syndrome.
Thrombotic Risk Factors
Comorbidities associated with risk for thrombosis such as increased BMI (≥ 25 kg/m 2 ), immobility, OCP use, and thrombophilia were analyzed among patients with or without DVT ( Table 1 ). The median BMI was 23.63 kg/m 2 (range 18.32–33.01 kg/m 2 ). The median BMI was similar at 23.81 kg/m 2 when four patients without DVT were excluded (range 18.44–33.01 kg/m 2 ). Thrombophilia testing was performed in only six patients. Five patients tested positive for factor V Leiden mutation (heterozygous = 4; homozygous = 1). Ten out of the 13 (76%) patients with DVT had one or more thrombosis risk factors such as inherited thrombophilia, immobility, increased BMI, and/or use of OCP. Increased BMI ( n = 5), immobility ( n = 4), and OCP use ( n = 10) as risk factors for thrombosis were found to be nonsignificant ( p -value 0.26, 0.52, and 0.25, respectively).
Diagnostics
Radiological modalities used to establish the diagnosis of MTS with or without DVT included: ultrasound Doppler and venography ( n = 7), ultrasound Doppler with MRV ( n = 5), ultrasound Doppler and CTV ( n = 4), and MRV only ( n = 1).
Management
Management in patients with or without DVT is summarized in Table 1 .
Initial treatment: Anticoagulation and Thrombolysis
Out of the 13 patients that presented with acute DVT, 6 (35%) were treated with thrombolysis in addition to systemic anticoagulation (catheter-directed = 5; systemic = 1) at a median of 1 day from DVT diagnosis (range 0–8 days). The median treatment duration was 2 days (range 1–4 days). One patient underwent PMT upon diagnosis of DVT at an outside institution 3 years prior to presentation. Overall, choice of anticoagulation therapy was variable and consisted of initial UFH or LMWH that were later transitioned to or continued on LMWH, warfarin, or direct oral anticoagulants such as rivaroxaban. Median duration of anticoagulation was 6.3 months (range 3.2–18.7 months). Antiplatelet agents such as clopidogrel and aspirin were used in some patients ( Table 1 ). Anticoagulation with or without antiplatelet agents were used in patients without DVT who underwent placement of a stent or balloon angioplasty.
Endovascular Stenting
Ten patients (59%) had stent placement at a median of 3.5 days (range 0–376 days) in those following DVT diagnosis ( n = 8) ( Table 2 ). In two patients without DVT, the stents were placed at 767 and 1,117 days (2.1 and 3.1 years) from the diagnosis of MTS, respectively. Stent failure occurred in 5 patients (50%) at a median of 1.7 months (range 0.3–72 months): 1 due to growth-related stent migration from compression site, 1 due to stent narrowing, and 3 due to thrombosis leading to stent replacement. All 5 patients underwent interventional stent recanalization procedure, of which 4 remained patent while 1 remained occluded. Proportion of stent survival at last follow-up was 90% (9 of 10 stents) with a median follow-up of 1.1 years (range 0.2–6.5 years). There was no association between stent size (diameter of 14 mm vs. > 14 mm) and stent failure ( p = 0.52)
Table 2. Summary of endovascular stenting and patency rates.
| Case ID | Thrombosis | Time to stent placement from MTS diagnosis in days (in years) | Stent location | Type of stent (number) | Stent size | Time to stent failure in days (in years) | Additional stent (location) |
Stent patency at last follow-up | Duration from MTS diagnosis in months (in years) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Yes | 4 | CIV | Protégé TM1 | 14 mm × 80 mm | − | NA | Patent | 67 (5.6) |
| EIV extending into CFV | Protégé TM2 | 14 mm × 80 mm, 14 mm × 40 mm | |||||||
| 2 | Yes | 0 | CIV | Wallstent TM1 | 14 mm a | 9 | Protégé™ (CIV) | Patent | 14.3 (1.2) |
| EIV extending into CFV | Wallstent TM2 | 14 mm a , 12 mm a | |||||||
| 3 | Yes | 0 | CIV | Wallstent TM1 | 18 mm × 60 mm | − | NA | Patent | 4.5 |
| 8 | Yes | 337 | CIV | Protégé TM1 | 14 mm × 60 mm | 2190 (6 y) | Wallstent™ (IVC) | Patent | 89.6 (7.5) |
| 9 | Yes | 1 | CIV | Wallstent TM2 | 14 mm × 60 mm, 14 mm × 40 mm | 112 | Not placed | Occluded (CIV, EIV) | 5.3 |
| EIV extending into CFV | Protégé TM2 | 14 mm × 80 mm, 14 mm × 80 mm | |||||||
| 11 | No | 1117 (3.1 y) | CIV | Wallstent TM1 | 16 mm × 60 mm | − | NA | Patent | 36.7 (3.1) |
| EIV | Wallstent TM1 | 16 mm × 60 mm | |||||||
| 13 | Yes | 0 | CIV, EIV, and CFV | Unknown | Unknown | 31 | Type unknown | Patent | 38.1 (3.2) |
| 14 | Yes | 1 | CIV | Wallstent TM1 | 16 mm × 60 mm | − | NA | Patent | 13.4 (1.1) |
| 15 | No | 767 (2.1 years) | CIV | Wallstent TM1 | 16 mm × 40 mm | 51 | Not placed | Patent | 26.9 (2.2) |
| 16 | Yes | 287 | CIV | Wallstent TM2 | 14 mm × 60 mm, 14 mm × 40 mm | − | NA | Patent | 12 |
| EIV | Protégé TM2 | 14 mm × 80 mm, 14 mm × 80 mm |
Abbreviations: CIV, left common iliac vein; CFV, left common femoral vein; EIV, left external iliac vein; IVC, inferior vena cava; MTS, May–Thurner syndrome; NA, not applicable.
Note: Protégé™ [Medtronic, Minneapolis, Minnesota, United States]; Wallstent™ [Boston Scientific, Marlborough, Massachusetts, United States].
Stent length information not available.
Outcomes
Complete resolution of thrombus was noted in 6 (46%) patients, partial resolution in 6 (46%), and no response in 1 (8%). Four patients (31%) had recurrence/progression of thrombus ( n = 3 with stents) at a median time of 29 days (range 12–495 days). No bleeding complications were observed. One patient had progression of DVT 11 days from diagnosis; CDT was initiated with partial resolution of the thrombosis; no stent was placed due to multiple collateral vessels. The role of thrombolysis therapy and its potential role for the prevention of recurrent thrombosis was analyzed and found to be nonsignificant ( p = 1.0). Neither degree of thrombosis clearance (complete vs. partial or no resolution) nor stent implantation (stent vs. no stent) were found to be associated with thrombosis recurrence ( p = 0.26 and p = 1.0, respectively).
On reviewing patients charts for signs and symptoms of PTS such as swelling, pain, skin discoloration, varicosities, skin ulceration, and claudication, five patients were noted to have had signs and symptoms consistent with PTS at the time of last follow-up. Only 4 patients responded to the mailed PTS survey that confirmed PTS documentation in 1 patient and an additional 3 patients reported PTS symptoms (mild PTS = 1; moderate PTS = 3) resulting in a total of 8 (62%) patients with PTS following DVT at a median follow-up of 16 months (range 9.6–89.8 months). Of the 8 patients with DVT that had stent placements, 5 (63%) patients had clinically documented and/or self-reported PTS at a median follow-up of 17 months (range 4.9–89.8 months). The degree of DVT clearance (complete vs. partial or no resolution), use of thrombolysis, and stent placement were not found to be significantly associated with development of PTS ( p = 1.0, 0.10, and 0.62, respectively).
Discussion
Compression of the left iliac vein by the overriding right iliac artery, also known as MTS, can result in left iliofemoral thrombosis in approximately 50 to 60% of patients as a result of intraluminal spurs from compression. 4 18 Similar to other pediatric series, a female preponderance was noted (M:F ratio 1:3.3). Other precipitating factors such as immobility, inherited thrombophilia, surgery, hormonal therapy, and pregnancy were also noted. 4 19 20 21 In our review, 77% of patients with MTS and DVT had one or more thrombosis risk factors such as inherited thrombophilia, immobility, and/or use of OCPs.
Several radiological imaging modalities are useful in establishing the diagnosis of MTS. Ultrasound Doppler is usually the first and fastest method used to diagnose DVT. 22 However, this radiological technique cannot identify the anatomic compression of the left iliac vein reliably and cannot demonstrate the venous spurs caused by chronic compression. To accurately diagnose MTS, other cross-sectional imaging modalities such as CTV or angiography, magnetic resonance angiography, and/or venography are used for detailed visualization of the pelvic vasculature and anatomy. 23 24 25
Anticoagulation is the first-line treatment used to prevent clot extension and occurrence of PE. However, anticoagulation alone neither corrects the anatomic compression of the left iliac vein nor results in clot dissolution. Surgical thrombectomy was considered a treatment option before PMT and stent placements became more commonly used. 26 27 Despite the different surgical techniques, similar primary and secondary patency have been reported at 5 years (42 and 59%, respectively). 28 Due to advances in endovascular techniques, MTS patients rarely have to undergo open vascular surgical procedures. 4 8 10 19 29 In adults, catheter-directed PMT with or without stent placement in addition to anticoagulation are considered the standard treatment for MTS with acute thrombosis with patency rates reported at 79 to 100% at 2 years. 11 29 30 31 32 To date, pediatric (≤ 18 years of age) literature consist primarily of case reports and limited case series, of which the largest series include Goldenberg et al, 12 Murphy et al, 33 and Goldman et al 14 that studied 6 (prospective), 5 (prospective), and 10 (retrospective) cases, respectively. In these reports, all patients presenting with thrombotic MTS received anticoagulation with catheter-directed mechanical or pharmacomechanical thrombolysis followed by endovascular stent placement. Murphy et al and Goldman et al 14 reported primary patency rates of 100% at the end of procedure and 79% at 12 months, respectively, while Goldman et al 14 reported secondary patency rate of 100% at 12 months and 89% at 36 months, as defined by the Society of Interventional Radiology. 34 Primary and secondary patency rates in the 6 patients in Goldenberg study were 40 and 20% at 12 and 24 months, respectively. 12 14 In our series, only 59% patients (10/17) had stent placements. Five stent failures were noted due to thrombosis or mechanical failure. Patency was restored in 3 patients with additional stent placements ( n = 3) and CDT ( n = 1). A primary patency rate of 62% at 12 months ( Fig. 2A ), with secondary patency rate of 88% at last follow-up ( Fig. 2B ) were observed. The limited sample size precluded definitive comparisons of outcomes based on thrombolytic therapy.
Fig. 2.
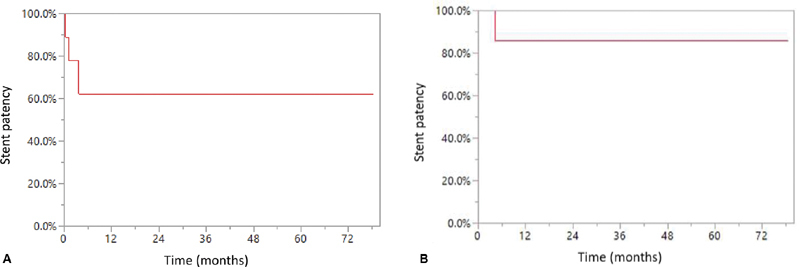
Stent patency. ( A ) Primary patency. ( B ) Secondary patency.
The role of congenital or acquired thrombophilia as a risk factor for thrombosis recurrence in MTS is inconsistent in the pediatric and adult literature. 12 14 35 Goldman et al reported a single patient in their cohort of patients with MTS, with loss of secondary stent patency due to congenital thrombophilia and poor adherence to anticoagulation. 14 Goldenberg et al reported 2 recurrent thromboses out of 6 patients with MTS to have associated hypercoagulability. 12 In contrast to the pediatric literature, a large retrospective analysis by Neglén et al in 870 adult patients did not show an increased risk for stent thrombosis in association with thrombophilia. 35 In our series, of the 5 patients with congenital thrombophilic risk factor, only 2 experienced recurrence/progression of thrombosis ( Table 1 ) at a median follow-up of 16.8 months (range 4.9–41.4 months); however, the generalizability of these findings is limited to the lack of uniform testing for hypercoagulability disorders in these patients (only 6 patients were tested).
Regardless for treatment modalities, these patients are at risk of developing PTS that is usually associated with swelling, pain, signs of venous insufficiency with dilated blood vessels, varicosities, skin changes, and potential for stasis ulceration in the long term, especially with recurrence of DVT or chronic venous insufficiency. 36 In one study, among adult patients with iliofemoral DVT who were managed with anticoagulation alone, 71% had leg swelling and 18% skin ulcerations at 10 years of follow-up. 37 In our study, signs and symptoms of PTS were clinically documented in 8 of the 13 patients who sustained a DVT; however, the grading severity could not be analyzed due to nonstandardized screening practices and documentation. A previously validated PTS survey tool was sent to all patients who experienced a DVT in the setting of PTS; however, this was met with a poor survey response rate (30.7%). Among patients with stent placement, 63% had clinically documented and/or self-reported PTS. With the exception of Murphy et al reporting 0% PTS symptoms in their cohort at last follow-up, our results are comparable to PTS rates reported by Goldman et al at 60% and Goldenberg et al at 75% of evaluable patients at last follow-up.
Although our study reports the largest pediatric cohort with MTS and real-life experience, we acknowledge several limitations that include retrospective study design, small sample size, heterogeneous treatment strategies, nonstandardized management and documentation, and recall bias. The clinical decision for CDT versus systemic thrombolysis, angioplasty, or stent placement, was at the discretion of the treating physician that incorporated patients' preferences as well. Due to nonstandardized screening methods during clinical documentation of PTS and poor response rate of the mailed-out survey, this study may underestimate the prevalence and severity of PTS.
In summary, MTS should be considered in adolescents with left iliofemoral thrombosis. CDT should be considered early on following diagnosis. For those patients who underwent anticoagulation with or without thrombolysis, no bleeding or procedural complications were observed. The decision algorithm for placement of endovascular stents in adolescents that have achieved full growth is not well established. Our institutional practice for stent placement takes into account the degree of vascular occlusion, resolution with anticoagulation and/or thrombolysis or thrombectomy, and vascular evidence of chronicity (e.g., presence of surrounding collateral vessels), to maximize the chances of successful endovascular stenting and prevent recurrent thrombosis or mechanical failure. Our study emphasizes the need of prospective multi-institutional collaborative efforts to study MTS in children and adolescents to establish standardized guidelines for diagnosis, management including anticoagulation, endovascular approach, and monitoring DVT-related sequelae to optimize long-term outcomes.
Conflict of Interest None declared.
Authors' Contributions
All authors have contributed significantly to this study and preparation of the manuscript. All authors have reviewed and approved the manuscript.
References
- 1.May R, Thurner J. The cause of the predominantly sinistral occurrence of thrombosis of the pelvic veins. Angiology. 1957;8(05):419–427. doi: 10.1177/000331975700800505. [DOI] [PubMed] [Google Scholar]
- 2.Cockett F B, Thomas M L. The iliac compression syndrome. Br J Surg. 1965;52(10):816–821. doi: 10.1002/bjs.1800521028. [DOI] [PubMed] [Google Scholar]
- 3.Kibbe M R, Ujiki M, Goodwin A L, Eskandari M, Yao J, Matsumura J. Iliac vein compression in an asymptomatic patient population. J Vasc Surg. 2004;39(05):937–943. doi: 10.1016/j.jvs.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 4.Mickley V, Schwagierek R, Rilinger N, Görich J, Sunder-Plassmann L. Left iliac venous thrombosis caused by venous spur: treatment with thrombectomy and stent implantation. J Vasc Surg. 1998;28(03):492–497. doi: 10.1016/s0741-5214(98)70135-1. [DOI] [PubMed] [Google Scholar]
- 5.Rajachandran M, Schainfeld R M. Diagnosis and treatment of May-Thurner syndrome. Vasc Dis Manag. 2014;11(11):E265–E273. [Google Scholar]
- 6.Bozkaya H, Cinar C, Ertugay S. Endovascular treatment of iliac vein compression (May-Thurner) syndrome: angioplasty and stenting with or without manual aspiration thrombectomy and catheter-directed thrombolysis. Ann Vasc Dis. 2015;8(01):21–28. doi: 10.3400/avd.oa.14-00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mousa A Y, AbuRahma A F. May-Thurner syndrome: update and review. Ann Vasc Surg. 2013;27(07):984–995. doi: 10.1016/j.avsg.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Hager E S, Yuo T, Tahara R. Outcomes of endovascular intervention for May-Thurner syndrome. J Vasc Surg Venous Lymphat Disord. 2013;1(03):270–275. doi: 10.1016/j.jvsv.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Lamont J P, Pearl G J, Patetsios P. Prospective evaluation of endoluminal venous stents in the treatment of the May-Thurner syndrome. Ann Vasc Surg. 2002;16(01):61–64. doi: 10.1007/s10016-001-0143-3. [DOI] [PubMed] [Google Scholar]
- 10.O'Sullivan G J, Semba C P, Bittner C A. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000;11(07):823–836. doi: 10.1016/s1051-0443(07)61796-5. [DOI] [PubMed] [Google Scholar]
- 11.Patel N H, Stookey K R, Ketcham D B, Cragg A H. Endovascular management of acute extensive iliofemoral deep venous thrombosis caused by May-Thurner syndrome. J Vasc Interv Radiol. 2000;11(10):1297–1302. doi: 10.1016/s1051-0443(07)61304-9. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg N A, Branchford B, Wang M, Ray C, Jr, Durham J D, Manco-Johnson M J. Percutaneous mechanical and pharmacomechanical thrombolysis for occlusive deep vein thrombosis of the proximal limb in adolescent subjects: findings from an institution-based prospective inception cohort study of pediatric venous thromboembolism. J Vasc Interv Radiol. 2011;22(02):121–132. doi: 10.1016/j.jvir.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raffini L, Raybagkar D, Cahill A M, Kaye R, Blumenstein M, Manno C. May-Thurner syndrome (iliac vein compression) and thrombosis in adolescents. Pediatr Blood Cancer. 2006;47(06):834–838. doi: 10.1002/pbc.20728. [DOI] [PubMed] [Google Scholar]
- 14.Goldman R E, Arendt V A, Kothary N. Endovascular management of May-Thurner syndrome in adolescents: a single-center experience. J Vasc Interv Radiol. 2017;28(01):71–77. doi: 10.1016/j.jvir.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell L G, Goldenberg N A, Male C, Kenet G, Monagle P, Nowak-Göttl U. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost. 2011;9(09):1856–1858. doi: 10.1111/j.1538-7836.2011.04433.x. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Rodriguez V, Matsumoto J M. Development and initial validation of a questionnaire to diagnose the presence and severity of post-thrombotic syndrome in children. Pediatr Blood Cancer. 2012;58(04):643–644. doi: 10.1002/pbc.24027. [DOI] [PubMed] [Google Scholar]
- 17.Kumar R, Rodriguez V, Matsumoto J M. Health-related quality of life in children and young adults with post-thrombotic syndrome: results from a cross-sectional study. Pediatr Blood Cancer. 2014;61(03):546–551. doi: 10.1002/pbc.24840. [DOI] [PubMed] [Google Scholar]
- 18.Steinberg J B, Jacocks M A. May-Thurner syndrome: a previously unreported variant. Ann Vasc Surg. 1993;7(06):577–581. doi: 10.1007/BF02000154. [DOI] [PubMed] [Google Scholar]
- 19.Oguzkurt L, Tercan F, Ozkan U, Gulcan O. Iliac vein compression syndrome: outcome of endovascular treatment with long-term follow-up. Eur J Radiol. 2008;68(03):487–492. doi: 10.1016/j.ejrad.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 20.Hassell D R, Reifsteck J E, Harshfield D L, Ferris E J. Unilateral left leg edema: a variation of the May-Thurner syndrome. Cardiovasc Intervent Radiol. 1987;10(02):89–91. doi: 10.1007/BF02577974. [DOI] [PubMed] [Google Scholar]
- 21.Abboud G, Midulla M, Lions C. “Right-sided” May-Thurner syndrome. Cardiovasc Intervent Radiol. 2010;33(05):1056–1059. doi: 10.1007/s00270-009-9654-z. [DOI] [PubMed] [Google Scholar]
- 22.Ahmed H K, Hagspiel K D. Intravascular ultrasonographic findings in May-Thurner syndrome (iliac vein compression syndrome) J Ultrasound Med. 2001;20(03):251–256. doi: 10.7863/jum.2001.20.3.251. [DOI] [PubMed] [Google Scholar]
- 23.Chung J W, Yoon C J, Jung S I. Acute iliofemoral deep vein thrombosis: evaluation of underlying anatomic abnormalities by spiral CT venography. J Vasc Interv Radiol. 2004;15(03):249–256. doi: 10.1097/01.rvi.0000109402.52762.8d. [DOI] [PubMed] [Google Scholar]
- 24.Oguzkurt L, Tercan F, Pourbagher M A, Kizilkilic O, Turkoz R, Boyvat F. Computed tomography findings in 10 cases of iliac vein compression (May-Thurner) syndrome. Eur J Radiol. 2005;55(03):421–425. doi: 10.1016/j.ejrad.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 25.Gurel K, Gurel S, Karavas E, Buharalıoglu Y, Daglar B. Direct contrast-enhanced MR venography in the diagnosis of May-Thurner syndrome. Eur J Radiol. 2011;80(02):533–536. doi: 10.1016/j.ejrad.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 26.Jost C J, Gloviczki P, Cherry K J., JrSurgical reconstruction of iliofemoral veins and the inferior vena cava for nonmalignant occlusive disease J Vasc Surg 20013302320–327., discussion 327–328 [DOI] [PubMed] [Google Scholar]
- 27.Plate G, Einarsson E, Ohlin P, Jensen R, Qvarfordt P, Eklöf B. Thrombectomy with temporary arteriovenous fistula: the treatment of choice in acute iliofemoral venous thrombosis. J Vasc Surg. 1984;1(06):867–876. doi: 10.1067/mva.1984.avs0010867. [DOI] [PubMed] [Google Scholar]
- 28.Garg N, Gloviczki P, Karimi K M. Factors affecting outcome of open and hybrid reconstructions for nonmalignant obstruction of iliofemoral veins and inferior vena cava. J Vasc Surg. 2011;53(02):383–393. doi: 10.1016/j.jvs.2010.08.086. [DOI] [PubMed] [Google Scholar]
- 29.Heijmen R H, Bollen T L, Duyndam D AC, Overtoom T T, Van Den Berg J C, Moll F L. Endovascular venous stenting in May-Thurner syndrome. J Cardiovasc Surg (Torino) 2001;42(01):83–87. [PubMed] [Google Scholar]
- 30.Bjarnason H, Kruse J R, Asinger D A. Iliofemoral deep venous thrombosis: safety and efficacy outcome during 5 years of catheter-directed thrombolytic therapy. J Vasc Interv Radiol. 1997;8(03):405–418. doi: 10.1016/s1051-0443(97)70581-5. [DOI] [PubMed] [Google Scholar]
- 31.Verhaeghe R, Stockx L, Lacroix H, Vermylen J, Baert A L. Catheter-directed lysis of iliofemoral vein thrombosis with use of rt-PA. Eur Radiol. 1997;7(07):996–1001. doi: 10.1007/s003300050239. [DOI] [PubMed] [Google Scholar]
- 32.Hurst D R, Forauer A R, Bloom J R, Greenfield L J, Wakefield T W, Williams D M. Diagnosis and endovascular treatment of iliocaval compression syndrome. J Vasc Surg. 2001;34(01):106–113. doi: 10.1067/mva.2001.114213. [DOI] [PubMed] [Google Scholar]
- 33.Murphy E H, Davis C M, Journeycake J M, DeMuth R P, Arko F R. Symptomatic ileofemoral DVT after onset of oral contraceptive use in women with previously undiagnosed May-Thurner Syndrome. J Vasc Surg. 2009;49(03):697–703. doi: 10.1016/j.jvs.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 34.Vedantham S, Grassi C J, Ferral H. Reporting standards for endovascular treatment of lower extremity deep vein thrombosis. J Vasc Interv Radiol. 2009;20(07):S391–S408. doi: 10.1016/j.jvir.2009.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Neglén P, Hollis K C, Olivier J, Raju S. Stenting of the venous outflow in chronic venous disease: long-term stent-related outcome, clinical, and hemodynamic result. J Vasc Surg. 2007;46(05):979–990. doi: 10.1016/j.jvs.2007.06.046. [DOI] [PubMed] [Google Scholar]
- 36.Manco-Johnson M J.Postthrombotic syndrome in children Acta Haematol 2006115(3-4):207–213. [DOI] [PubMed] [Google Scholar]
- 37.Plate G, Eklöf B, Norgren L, Ohlin P, Dahlström J A. Venous thrombectomy for iliofemoral vein thrombosis--10-year results of a prospective randomised study. Eur J Vasc Endovasc Surg. 1997;14(05):367–374. doi: 10.1016/s1078-5884(97)80286-9. [DOI] [PubMed] [Google Scholar]


