Abstract
Objective
To assess the effectiveness of lymphadenectomy at primary debulking surgery (PDS) on the survival of patients with epithelial ovarian cancer (EOC).
Methods
We searched PubMed, Ichushi, and the Cochrane Library. Randomized controlled trials (RCTs) and retrospective cohort studies comparing survival of women with EOC undergoing lymphadenectomy at PDS with that of women without lymphadenectomy were included. We performed a meta-analysis of overall survival (OS), progression-free survival (PFS), and adverse events.
Results
For advanced-stage EOC, 2 RCTs including 1,074 women and 7 cohort studies comprising 3,161 women were evaluated. Meta-analysis revealed that lymphadenectomy was associated with improved OS (hazard ratio [HR]=0.80; 95% confidence interval [CI]=0.70–0.90). However, meta-analysis of 2 RCTs revealed no significant difference in OS between the lymphadenectomy and no-lymphadenectomy groups (OS: HR=1.02; 95% CI=0.85–1.22). For early-stage EOC, 1 RCT comprising 268 women and 4 cohort studies comprising 14,228 women were evaluated. Meta-analysis showed that lymphadenectomy was associated with improved OS (HR=0.75; 95% CI=0.68–0.82). A RCT of early-stage EOC reported that lymphadenectomy was not associated with improved OS (HR=0.85; 95% CI=0.49–1.47). Surgery-related deaths were similar in both groups (risk ratio [RR]=1.00; 95% CI=0.99–1.01); however, blood transfusion was required less frequently in the no-lymphadenectomy group (RR=0.74; 95% CI=0.63–0.86).
Conclusions
Meta-analysis of RCTs and observational studies suggest that lymphadenectomy was associated with improved OS in advanced- and early-stage EOC. However, results from RCTs demonstrate that lymphadenectomy was not associated with improved OS in advanced- and early-stage EOC.
Keywords: Ovarian Neoplasms, Lymph Node Excision, Meta-Analysis, Systematic Review
INTRODUCTION
Epithelial ovarian, fallopian tubal, and peritoneal cancer is one of the most common cancers in women, with over 295,000 new cases diagnosed worldwide each year [1]. About 70%–80% of epithelial ovarian cancers (EOCs) are of a serous histologic type. The less common types include endometrioid (10%), clear cell (10%), mucinous (3%–4%), transitional (Brenner) (<1%), and undifferentiated carcinomas (2%) [2]. The primary treatment includes staging laparotomy with maximal cytoreduction and adjuvant platinum-based chemotherapy. In advanced-stage EOC, no visible disease achieved by maximal cytoreduction is the most favorable prognostic factor, and thus is the main goal of primary debulking surgery (PDS) [3,4,5]. Although pelvic or para-aortic lymph node metastasis is reported in approximately 14% of clinical stage I or II EOC [6], the efficacy of lymphadenectomy on improved overall survival (OS) has not been established. Observational retrospective studies suggested that lymphadenectomy may be a favorable prognostic factor for OS in advanced- and early-stage EOCs. Therefore, the aim of this current study was to evaluate the role of lymphadenectomy in advanced- and early-stage EOCs.
MATERIALS AND METHODS
1. Search strategy
We searched the following databases from January 1967 to September 2018: PubMed, Ichushi, and the Cochrane Library. We identified all relevant articles found on PubMed and used the “related articles” feature to conduct a further search for newly published articles. We sought articles in all languages. The search strategy is described in Supplementary Table 1.
2. Study selection
RCTs and retrospective studies were included. The study participants comprised women with advanced- or early-stage EOC who had a confirmed pathological diagnosis from surgery. The primary surgical procedures included standard surgical staging with or without lymphadenectomy. PDS, including the pelvic region only, or pelvic and para-aortic lymphadenectomy was defined as the treatment group, whereas PDS without systematic lymphadenectomy was defined as the control group. PDS with lymph node biopsy was also included in the control group. The primary outcome was OS, survival until death from any cause. The secondary outcomes included progression-free survival (PFS) and adverse events (AEs).
3. Data extraction
To select the studies, we downloaded all titles and abstracts retrieved by electronic searching to a reference management database, BunKan, where 2 review authors (C.T. and S.M.) independently examined the references. We excluded those studies which clearly did not meet the inclusion criteria and obtained full text copies of potentially relevant references. The 2 authors independently assessed the eligibility of all retrieved papers, resolving disagreements by discussion, and we collected the following data: authors, year of publication, journal citation, country, setting, inclusion and exclusion criteria, study design and methodology, study population (total number enrolled, participant characteristics, age, size of residual tumors after primary surgery, stage, histology, and number of lymph nodes removed), interventions (expertise of surgeons and type of chemotherapy), risk of bias, duration of follow-up, and outcomes (OS, PFS, and AEs).
We extracted outcome data as follows:
• For OS and PFS data, we extracted the hazard ratio (HR) and its standard error from trial reports.
• For dichotomous outcomes (AEs), we extracted the number of participants in each group who experienced the outcome of interest and the number assessed at the end point, to estimate a risk ratio (RR).
Where possible, all extracted data were relevant to an intention-to-treat analysis, in which participants were analyzed in the groups to which they were originally assigned.
4. Assessment of risk of bias in included studies
We assessed the risk of bias in the included RCTs using the Cochrane Collaboration's tool and the criteria specified in chapter 8 of the Cochrane Handbook [7]. This includes the following assessments: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases [7]. For observational studies, the sources of bias included selection bias, performance bias, detection bias, attrition bias, and others. Two review authors (C.T. and S.M.) independently applied the risk of bias tool, resolving differences by discussion. We interpreted the results of our meta-analysis in the light of the findings of the risk of bias assessments. We did not impute missing outcome data.
5. Assessment of heterogeneity and publication bias
We assessed heterogeneity between studies by visual inspection of forest plots and by estimation of the I2 statistic. To evaluate the publication bias, we performed a funnel plot analysis. All studies were distributed evenly across the graph, indicating that no publication bias existed in the meta-analysis (Supplementary Fig. 1).
6. Data synthesis and analysis
We pooled the findings of the included studies into meta-analyses, using adjusted summary statistics when available and unadjusted results, otherwise. For time-to-event data, we produced and pooled HRs using the generic inverse variance facility of Review Manager 5. For dichotomous outcomes, we calculated the RR for each study and then pooled them. We used random-effects models for all meta-analyses.
RESULTS
1. Overview of the clinical trials included in the systematic review
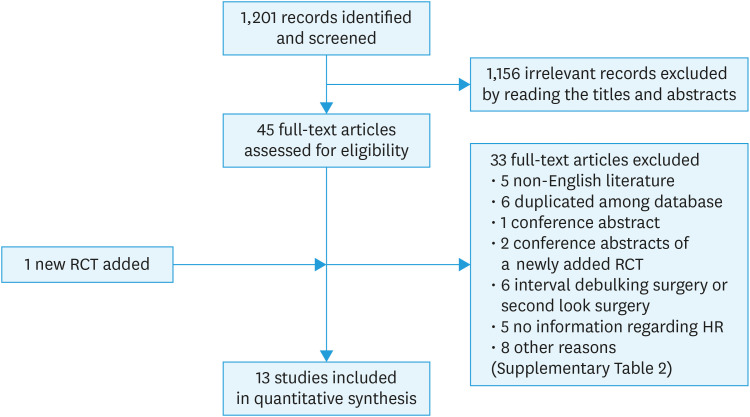
We examined the titles and abstracts of 1,201 references identified by the original search (depicted in yellow cells, Supplementary Table 1) and determined that 45 studies were potentially relevant to this review. We obtained full text copies of the 45 studies, and 2 authors (C.T. and S.M.) assessed them independently for eligibility. Of the 45 studies, 33 were excluded during this process. The reasons for exclusion are presented in the characteristics of excluded studies table (Supplementary Table 2). We added an article from March 2019 which was already included in the Cochrane database [8] (Fig. 1). Ultimately, 3 randomized controlled trials (RCTs) and ten retrospective studies met all inclusion criteria (Fig. 1, Table 1).
Fig. 1. Flow diagram of study selection.
HR, hazard ratio; RCT, randomized controlled trial.
Table 1. Clinical characteristics of RCTs and observational studies included in the systematic review.
| Stages | Author | Design of study | Clinical stage | Histology | Debulking status | No. of patients | Definition of SL and control | Quality | |
|---|---|---|---|---|---|---|---|---|---|
| SL | Control | ||||||||
| Advanced-stage | Panici et al. [9] | RCT | IIIB–IV | Various | Optimal or residual tumors <2 cm | 216 | 211 | SL: pelvic and para-aortic SL | 62.1% had residual postoperative intraabdominal tumor (R0 SL: 37.0%, Control: 37.4%) |
| Control: removal of ≥1 cm LN | Resection of bulky lymph nodes allowed in control | ||||||||
| Surgical quality not assessed | |||||||||
| Harter et al. [8] | RCT | IIB–IV | Various | Complete | 323 | 324 | SL: pelvic and para-aortic SL | All data available to study question (R0 SL: 99.4%, Control: 99.4%) | |
| Control: not performed | |||||||||
| du Bois et al. [10] | Observational | IIB–IV | Various | Optimal | 610 | 894 | SL: pelvic and para-aortic SL | Exploratory analysis of 3 RCTs | |
| Control: not performed | Surgical quality not clear | ||||||||
| SL by discretion of surgeon | |||||||||
| Abe et al. [11] | Observational | III–IV | Various | Optimal or residual tumors <2 cm | 28 | 28 | SL: pelvic and/or para-aortic SL | Small sample size | |
| Control: not performed | Surgical quality unclear | ||||||||
| Chang et al. [12] | Observational | IIIC (node metastasis only excluded) | Various | Optimal or suboptimal | 135 | 54 | SL: pelvic and/or para-aortic SL | Single center | |
| Control: not performed | 22% of SL were pelvic only | ||||||||
| Sakai et al. [13] | Observational | III–IV | Various | Optimal | 87 | 93 | SL: pelvic and para-aortic SL | Patient characteristics was balanced | |
| Control: removal of ≥1 cm LNs | Surgical quality unclear | ||||||||
| Pereira et al. [14] | Observational | IIIC–IV (peritoneal implants >2 cm with positive nodes) | Various | Optimal or suboptimal | 30 | 53 | SL: >40 resected pelvic and para-aortic LNs | Single center | |
| Control: ≤40 resected pelvic and para-aortic LNs | Selection bias | ||||||||
| Control underwent lymphadenectomy | |||||||||
| Paik et al. [15] | Observational | III (node metastasis only excluded)–IV | Various | Optimal or suboptimal | 135 | 126 | SL: pelvic and/or para-aortic SL | Single center | |
| Control: not performed | Selection bias | ||||||||
| SL group younger than control | |||||||||
| Only 8 removed LNs in SL group exists | |||||||||
| Zhou et al. [16] | Observational | IIIC–IV | Various | Optimal or suboptimal | 367 | 521 | SL: >20 resected LNs | SEER study | |
| Control: not performed | Age and residual tumor different between SL and control | ||||||||
| Early-stage | Maggioni et al. [17] | RCT | I–II | Various | Optimal | 138 | 130 | SL: pelvic and para-aortic SL (unilateral pelvic lymphadenectomy allowed in unilateral tumors) | Surgical quality not assessed |
| Control: random sampling | Unilateral lymphadenectomy allowed | ||||||||
| Abe et al. [11] | Observational | I–II | Various | Optimal or residual tumors <2 cm | 40 | 22 | SL: pelvic and/or para-aortic SL | Small sample size | |
| Control: not performed | Residual tumor different between SL and control | ||||||||
| Oshita et al. [18] | Observational | I–II | Various | Unknown | 284 | 138 | SL: pelvic and para-aortic SL | Selection bias | |
| Control: not performed | Surgical quality unclear | ||||||||
| Svolgaard et al. [19] | Observational | I | Various | Unknown | 216 | 411 | SL: pelvic SL or para-aortic SL or both | Selection bias | |
| Control: not performed | No background information of each group | ||||||||
| Pelvic SL 44%, para-aortic SL 7%, both 48% | |||||||||
| Matsuo et al. [20] | Observational | I–II | Various | Unknown | 8,489 | 4,628 | SL: ≥12 resected pelvic LNs | SEER study | |
| Control: <12 resected pelvic LNs | Details of lymphadenectomy not clear | ||||||||
LN, lymph node; R0, no residual disease; RCT, randomized controlled trial; SL, systematic lymphadenectomy.
2. Lymphadenectomy for advanced-stage ovarian cancer
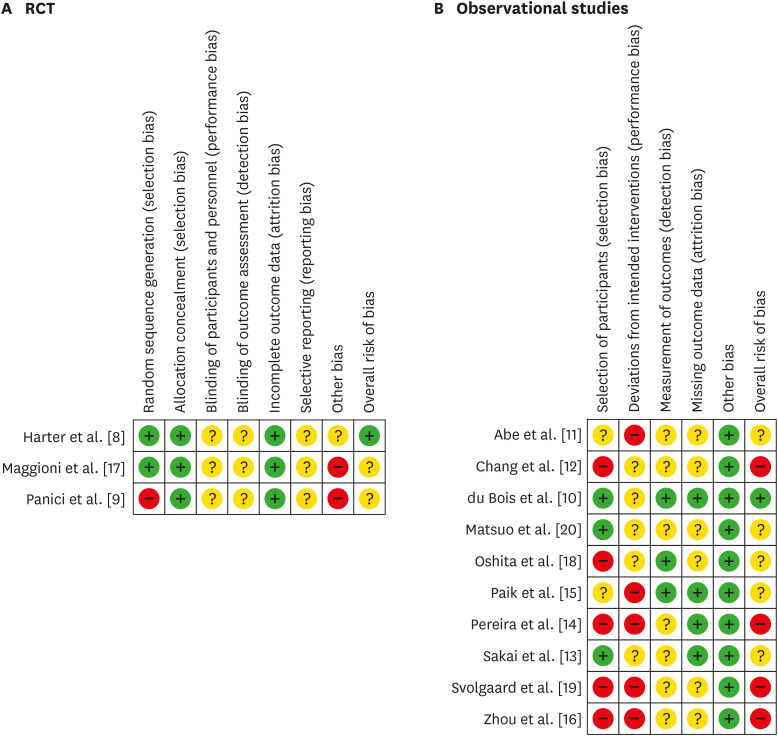
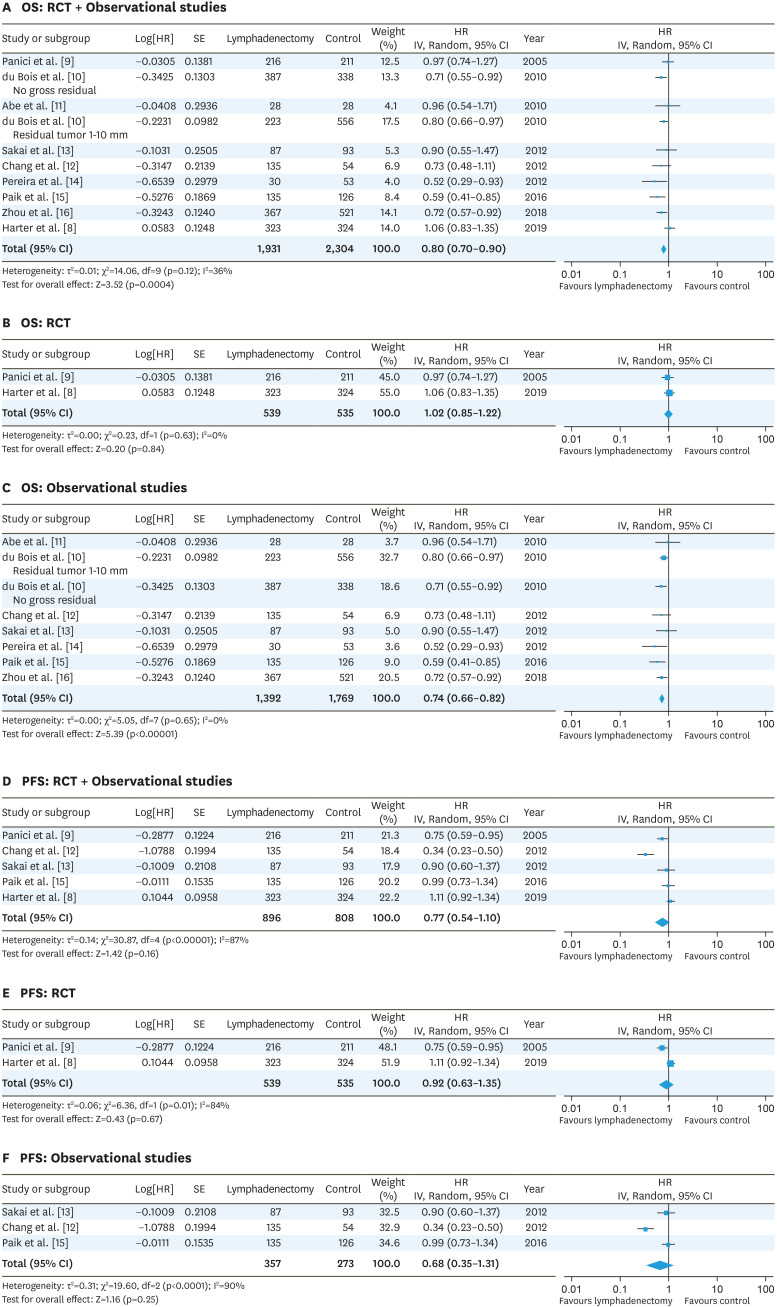
For advanced-stage EOC, we identified 2 RCTs [8,9] and 7 observational studies, including one SEER database study [10,11,12,13,14,15,16] (Table 1). Two cohorts from the observational study of du Bois et al. [10] were selected: 1) no gross residual and 2) a residual tumor 1–10 mm. Regarding Panici et al.'s risk of bias [9], the selection bias of random sequence generation is high because more than two thirds of the included patients (61.8%) had residual postoperative intraabdominal tumor which may have affected the prognosis, and resection of bulky lymph nodes was allowed in the control group. Another source of bias was that participating centers were not assessed for surgical quality (Fig. 2, Supplementary Fig. 2). The LION trial [8] has a low risk of bias since patients could undergo randomization only if macroscopically complete resection had been achieved, which is an appropriate design to compare the effectiveness of lymphadenectomy on survival. In addition, only patients with clinically negative lymph nodes were included in the study, and surgical quality was assured [8]. For observational studies, the overall risk of bias was generally considered unclear to high (Fig. 2, Supplementary Fig. 2). The meta-analysis of RCTs and observational studies using a random-effects model revealed that lymphadenectomy improved OS (HR=0.80; 95% CI=0.70–0.90) (Fig. 3A); however, the meta-analysis of RCTs alone showed that lymphadenectomy did not improve OS (HR=1.02; 95% CI=0.85–1.22) (Fig. 3B). The meta-analysis of observational studies showed that lymphadenectomy improved OS (HR=0.74; 95% CI=0.66–0.82) (Fig. 3C). Furthermore, the meta-analysis revealed that lymphadenectomy did not improve PFS (both RCT and observational studies: HR=0.77; 95% CI=0.54–1.10; RCTs: HR=0.92; 95% CI=0.63–1.35; and observational studies: HR=0.68; 95% CI=0.35–1.31) (Fig. 3D-F). In the meta-analysis of PFS including observational studies, heterogeneity was high (I2=87%–90%) due to the results of Chang et al. [12]. It is not clear why the HR of lymphadenectomy on PFS was very low (HR=0.34), but lymphadenectomy was not a significant predictor of OS in their study [12].
Fig. 2. Methodological quality summary: Review authors' judgements about each methodological quality item for each RCT (A) and observational studies (B). Green: low risk of bias; yellow: unclear risk of bias; red: high risk of bias.
RCT, randomized controlled trial.
Fig. 3. Forest plots for the lymphadenectomy vs. control studies of the OS (A-C) and PFS (D-F) in advanced-stage ovarian cancer. The test for heterogeneity is indicated with the I2 value.
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; RCT, randomized controlled trial; SE, standard error.
3. Lymphadenectomy for early-stage ovarian cancer
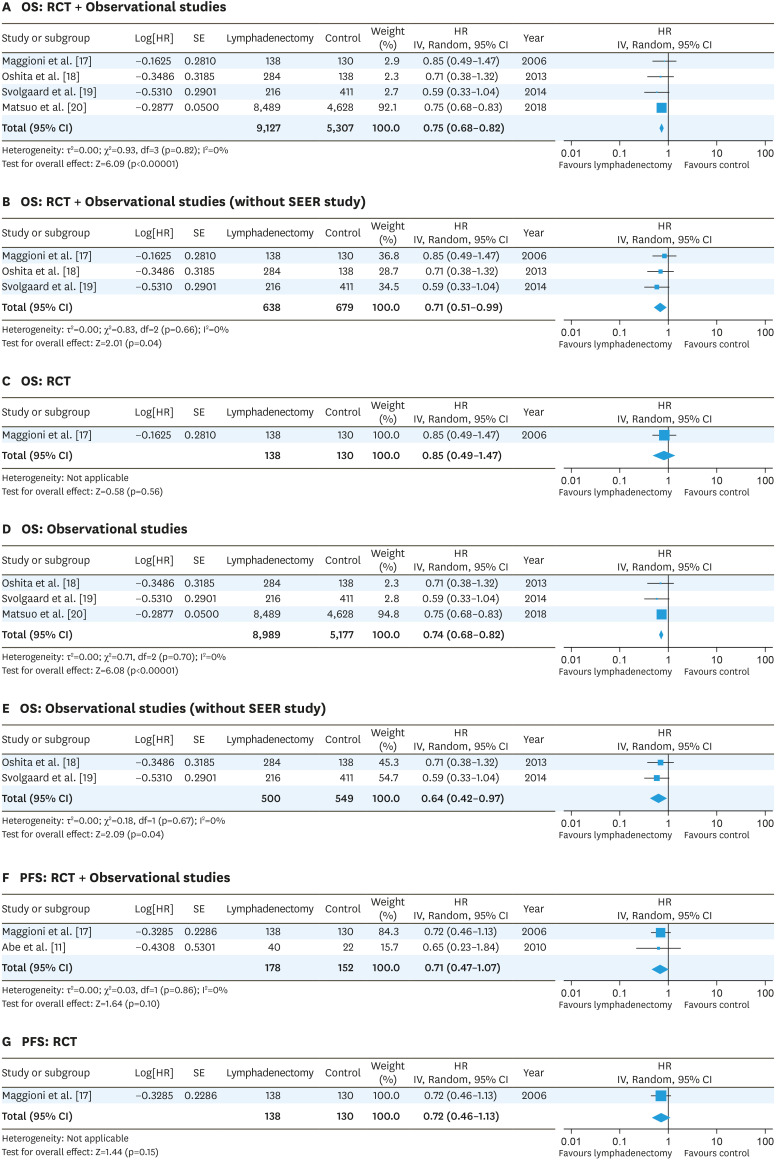
For early-stage EOC, one RCT [17] and 4 observational studies, including one SEER database study [11,18,19,20], were included in the systematic review (Table 1). In the RCT [17], biases resulted because participating centers were not assessed for surgical quality, unilateral lymphadenectomy was allowed in unilateral tumors, and the primary endpoint of the study was the prevalence of patients with positive retroperitoneal nodes (Fig. 2A). Overall risk of bias in all 4 observational studies was not considered low (Fig. 2B). A meta-analysis of the RCT and retrospective early-stage EOC studies revealed that lymphadenectomy was associated with favorable OS (HR=0.75; 95% CI=0.68–0.82, without SEER study; HR=0.71; 95% CI=0.51–0.99) (Fig. 4A and B). The RCT of early-stage EOC reported no significant effects of lymphadenectomy on OS (HR=0.85; 95% CI=0.49–1.47) [17] (Fig. 4C). A meta-analysis of the observational studies showed that lymphadenectomy was associated with favorable OS (HR=0.74; 95% CI=0.68–0.82, without SEER study; HR=0.64; 95% CI=0.42–0.97) (Fig. 4D and E). Meta-analysis of the RCT and retrospective early-stage EOC studies revealed that lymphadenectomy was not associated with favorable PFS (HR=0.71; 95% CI=0.47–1.07) (Fig. 4F). The RCT reported no significant effects of lymphadenectomy on PFS (HR=0.72; 95% CI=0.46–1.13) [17] (Fig. 4G).
Fig. 4. Forest plots for the lymphadenectomy vs. control studies of the OS (A-E) and PFS (F, G) in early-stage ovarian cancer. The test for heterogeneity is indicated with the I2 value.
CI, confidence interval; HR, hazard ratio; OS, overall survival; PFS, progression-free survival; RCT, randomized controlled trial; SE, standard error.
4. Risk of AEs
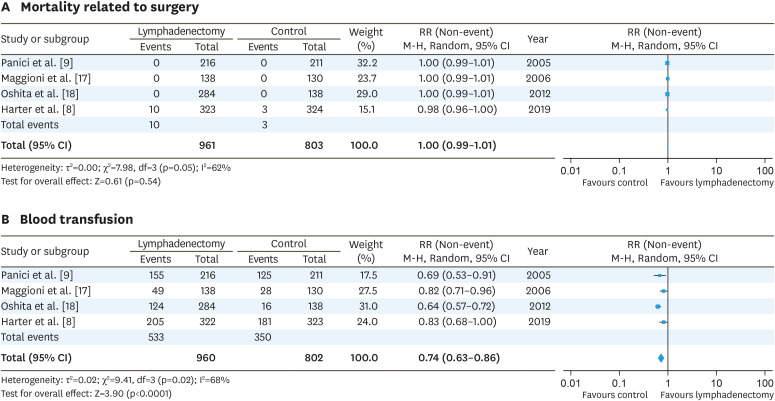
Using 3 RCTs and one observational study, AEs associated with lymphadenectomy were analyzed [8,9,17,18]. Lymphadenectomy was not associated with mortality related to surgery (RR=1.00; 95% CI=0.99–1.01) (Fig. 5A), although the LION trial reported that lymphadenectomy was significantly associated with mortality within 60 days following surgery [8]. Patients without lymphadenectomy required blood transfusion less frequently than the lymphadenectomy group (RR=0.74; 95% CI=0.63–0.86) (Fig. 5B).
Fig. 5. RR of adverse events. Mortality related to surgery (A) and blood transfusion (B).
CI, confidence interval; RR, risk ratio.
DISCUSSION
Pelvic and para-aortic lymphadenectomy has been routinely performed at PDS in both advanced- and early-stage EOCs relying on data from retrospective studies. In advanced-stage EOC, pelvic and para-aortic lymphadenectomy, which can contribute to maximal cytoreduction, has been performed as an important surgical procedure [3,4,5]. One RCT [9], which did not exhibit an advantage to lymphadenectomy, has been criticized on several points: surgical quality was not assessed, bulky lymph node dissection was allowed in the control group, and approximately 60% of the cases with lymphadenectomy had residual intraabdominal tumor. The LION study is considered to be a well-designed trial to measure the benefit of lymphadenectomy, because randomization was performed only after complete surgical resection of intraabdominal lesions has been achieved, surgical quality was assured, and cases with only clinically negative lymph node metastasis were included in the study. The trial revealed that 55.7% of the cases in the lymphadenectomy group had lymph node metastasis pathologically, and lymphadenectomy did not provide any survival benefit [8]. This may indicate that adjuvant chemotherapy can eliminate the effect of micro-metastases in the lymph nodes on survival. Meta-analysis of advanced-stage EOC including observational studies showed that lymphadenectomy improved OS (RCT and observational studies: HR=0.80; observational studies: HR=0.74) (Fig. 3A and C). However, a meta-analysis of 2 RCTs revealed that lymphadenectomy did not improve OS (HR=1.02) (Fig. 3B). The heterogeneity of the retrospective studies was low (I2=0%) (Fig. 3C). The difference between the result of retrospective studies and RCTs may be attributed to several biases, including selection bias for patients with older age, low performance status, or preexisting disorders who did not undergo lymphadenectomy. A meta-analysis of RCTs and observational studies did not reveal a benefit of lymphadenectomy on PFS in advanced-stage EOC (Fig. 3D-F). It is not known whether chemotherapy would be effective in the case of grossly apparent lymph node metastasis. At present, removing apparently clinically metastatic lymph nodes may be the most realistic approach; however, whether it is necessary to remove grossly apparent metastatic lymph nodes has not been elucidated yet.
In early-stage EOC, lymphadenectomy is believed to be an important procedure to remove micrometastases which may contribute to improved survival and to find cases in which adjuvant chemotherapy can be omitted. In fact, a review of 14 retrospective studies of pT1 or pT2 EOC showed lymph node metastases were found in an average of 14.2% (range 6.1%–29.6%) of cases, 2.9% in only a pelvic lymph node, 7.1% in only a para-aortic lymph node, and 4.3% in both pelvic and para-aortic lymph nodes [6]. One RCT of early-stage EOC, which did not exhibit any survival benefit to lymphadenectomy, has been criticized for its small number of cases and the allowance of lymph node biopsy in the control group [17]. In that study, 18% of the lymphadenectomy group and 4% of the control group with stage I disease at randomization had lymph node metastasis. Moreover, 31% of the lymphadenectomy group and 20% of the control group with stage II disease had lymph node metastasis. Although many more lymph nodes with metastasis had been removed in the lymphadenectomy group, lymphadenectomy did not exhibit a benefit in either OS or PFS [17]. A meta-analysis of RCTs and observational studies showed that lymphadenectomy improved OS but did not improve PFS in early-stage EOC (Fig. 4). The risk of bias of the RCT and observational studies of early-stage EOC ranged from unclear to high (Fig. 2). Thus, as suggested by the former meta-analysis [21], the efficacy of lymphadenectomy on survival is still unknown because of the lack of a well-designed RCT in early-stage ovarian cancer. Based on the LION study of advanced-stage EOC [8], the effects of occult lymph node metastasis can be reversed by adjuvant chemotherapy. Thus, it can be said that the main purpose of systematic lymphadenectomy in early-stage EOC is to identify the patients who can avoid adjuvant chemotherapy. Lymphadenectomy can be possibly omitted with improvements in diagnostic imaging or with sentinel lymph node biopsy.
Regarding the histologic subtypes, clear cell ovarian cancer is more chemoresistant than serous histologic-type cancer [22]. The study of Panici et al. [9] includes only 16 cases (3.7%), and the LION trial only includes 14 cases (2.2%) of clear cell histologic type. The SEER database study of early-stage EOC showed that an effective lymphadenectomy was associated with a survival benefit for serous, endometrioid, and clear cell but not for mucinous tumors [20]. In a retrospective cohort study of 240 patients with clear cell ovarian cancer, lymphadenectomy was a strong prognostic factor [23]. Therefore, it may be too early to conclude that lymphadenectomy has no impact on survival for clear cell ovarian cancer. A RCT of adequate clear cell EOC cases is necessary to provide evidence that lymphadenectomy improves the survival of clear cell ovarian cancer patients.
In conclusion, this systematic review and meta-analysis suggest that pelvic and para-aortic lymphadenectomy at PDS has no additional effect on survival and appears to increase the rate of AEs.
ACKNOWLEDGMENTS
This systematic review is conducted as a project of the “Ovarian Cancer Treatment Guideline 2020” edited by the Japan Society of Gynecologic Oncology. The authors thank Sho Sasaki and Toshio Morizane at Minds; Japan Council for Quality Health Care for guidance and assistance, and Shinichi Abe at Jikei University for the literature survey.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: C.T., S.M., S.T., N.S., M.M.
- Data curation: C.T., S.M.
- Formal analysis: C.T., S.M.
- Funding acquisition: M.M., K.H., A.D.
- Investigation: C.T., S.M., S.T.
- Methodology: C.T., S.M.
- Project administration: C.T., S.M., S.T., N.S., M.M.
- Supervision: S.T., N.S., M.M., K.H., A.D.
- Writing - original draft: C.T., S.M.
- Writing - review & editing: C.T., S.M., S.T.
SUPPLEMENTARY MATERIALS
Search strategies for the PubMed, Ichushi, and Cochrane Library
Characteristics of excluded studies
The funnel plot for 10 cohorts (9 studies) of advanced-stage ovarian cancer (A) and 5 studies of early-stage ovarian cancer (B).
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included RCTs (A) and observational studies (B).
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Gilks CB, Prat J. Ovarian carcinoma pathology and genetics: recent advances. Hum Pathol. 2009;40:1213–1223. doi: 10.1016/j.humpath.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Winter WE, 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26:83–89. doi: 10.1200/JCO.2007.13.1953. [DOI] [PubMed] [Google Scholar]
- 4.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Aghajanian C, Barakat RR, Chi DS. The effect of maximal surgical cytoreduction on sensitivity to platinum-taxane chemotherapy and subsequent survival in patients with advanced ovarian cancer. Gynecol Oncol. 2008;108:276–281. doi: 10.1016/j.ygyno.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Kleppe M, Wang T, Van Gorp T, Slangen BF, Kruse AJ, Kruitwagen RF. Lymph node metastasis in stages I and II ovarian cancer: a review. Gynecol Oncol. 2011;123:610–614. doi: 10.1016/j.ygyno.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Higgins JP, Green GS. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] [Internet] London: The Cochrane Collaboration; 2011. [cited 2019 May 11]. Available from: http://handbook.cochrane.org. [Google Scholar]
- 8.Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N Engl J Med. 2019;380:822–832. doi: 10.1056/NEJMoa1808424. [DOI] [PubMed] [Google Scholar]
- 9.Panici PB, Maggioni A, Hacker N, Landoni F, Ackermann S, Campagnutta E, et al. Systematic aortic and pelvic lymphadenectomy versus resection of bulky nodes only in optimally debulked advanced ovarian cancer: a randomized clinical trial. J Natl Cancer Inst. 2005;97:560–566. doi: 10.1093/jnci/dji102. [DOI] [PubMed] [Google Scholar]
- 10.du Bois A, Reuss A, Harter P, Pujade-Lauraine E, Ray-Coquard I, Pfisterer J, et al. Potential role of lymphadenectomy in advanced ovarian cancer: a combined exploratory analysis of three prospectively randomized phase III multicenter trials. J Clin Oncol. 2010;28:1733–1739. doi: 10.1200/JCO.2009.25.3617. [DOI] [PubMed] [Google Scholar]
- 11.Abe A, Furumoto H, Irahara M, Ino H, Kamada M, Naka O, et al. The impact of systematic para-aortic and pelvic lymphadenectomy on survival in patients with optimally debulked ovarian cancer. J Obstet Gynaecol Res. 2010;36:1023–1030. doi: 10.1111/j.1447-0756.2010.01274.x. [DOI] [PubMed] [Google Scholar]
- 12.Chang SJ, Bristow RE, Ryu HS. Prognostic significance of systematic lymphadenectomy as part of primary debulking surgery in patients with advanced ovarian cancer. Gynecol Oncol. 2012;126:381–386. doi: 10.1016/j.ygyno.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Sakai K, Kajiyama H, Umezu T, Shibata K, Mizuno M, Suzuki S, et al. Is there any association between retroperitoneal lymphadenectomy and survival benefit in advanced stage epithelial ovarian carcinoma patients? J Obstet Gynaecol Res. 2012;38:1018–1023. doi: 10.1111/j.1447-0756.2011.01826.x. [DOI] [PubMed] [Google Scholar]
- 14.Pereira A, Pérez-Medina T, Magrina JF, Magtibay PM, Millan I, Iglesias E. The role of lymphadenectomy in node-positive epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22:987–992. doi: 10.1097/IGC.0b013e318257b958. [DOI] [PubMed] [Google Scholar]
- 15.Paik ES, Shim M, Choi HJ, Lee YY, Kim TJ, Lee JW, et al. Impact of lymphadenectomy on survival after recurrence in patients with advanced ovarian cancer without suspected lymph node metastasis. Gynecol Oncol. 2016;143:252–257. doi: 10.1016/j.ygyno.2016.08.321. [DOI] [PubMed] [Google Scholar]
- 16.Zhou J, Zhang WW, Zhang QH, He ZY, Sun JY, Chen QH, et al. The effect of lymphadenectomy in advanced ovarian cancer according to residual tumor status: a population-based study. Int J Surg. 2018;52:11–15. doi: 10.1016/j.ijsu.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Maggioni A, Benedetti Panici P, Dell'Anna T, Landoni F, Lissoni A, Pellegrino A, et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br J Cancer. 2006;95:699–704. doi: 10.1038/sj.bjc.6603323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oshita T, Itamochi H, Nishimura R, Numa F, Takehara K, Hiura M, et al. Clinical impact of systematic pelvic and para-aortic lymphadenectomy for pT1 and pT2 ovarian cancer: a retrospective survey by the Sankai Gynecology Study Group. Int J Clin Oncol. 2013;18:1107–1113. doi: 10.1007/s10147-012-0483-8. [DOI] [PubMed] [Google Scholar]
- 19.Svolgaard O, Lidegaard O, Nielsen ML, Nedergaard L, Mosgaard BJ, Lidang M, et al. Lymphadenectomy in surgical stage I epithelial ovarian cancer. Acta Obstet Gynecol Scand. 2014;93:256–260. doi: 10.1111/aogs.12322. [DOI] [PubMed] [Google Scholar]
- 20.Matsuo K, Machida H, Mariani A, Mandelbaum RS, Glaser GE, Gostout BS, et al. Adequate pelvic lymphadenectomy and survival of women with early-stage epithelial ovarian cancer. J Gynecol Oncol. 2018;29:e69. doi: 10.3802/jgo.2018.29.e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim HS, Ju W, Jee BC, Kim YB, Park NH, Song YS, et al. Systematic lymphadenectomy for survival in epithelial ovarian cancer: a meta-analysis. Int J Gynecol Cancer. 2010;20:520–528. doi: 10.1111/IGC.0b013e3181d6de1d. [DOI] [PubMed] [Google Scholar]
- 22.Sugiyama T, Kamura T, Kigawa J, Terakawa N, Kikuchi Y, Kita T, et al. Clinical characteristics of clear cell carcinoma of the ovary: a distinct histologic type with poor prognosis and resistance to platinum-based chemotherapy. Cancer. 2000;88:2584–2589. [PubMed] [Google Scholar]
- 23.Magazzino F, Katsaros D, Ottaiano A, Gadducci A, Pisano C, Sorio R, et al. Surgical and medical treatment of clear cell ovarian cancer: results from the multicenter Italian Trials in Ovarian Cancer (MITO) 9 retrospective study. Int J Gynecol Cancer. 2011;21:1063–1070. doi: 10.1097/IGC.0b013e318218f270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search strategies for the PubMed, Ichushi, and Cochrane Library
Characteristics of excluded studies
The funnel plot for 10 cohorts (9 studies) of advanced-stage ovarian cancer (A) and 5 studies of early-stage ovarian cancer (B).
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included RCTs (A) and observational studies (B).