Abstract
Objective
Peritoneal metastases (PM) are a challenge in gynecological cancers, but its appearance has never been described in a population-based study. Therefore, we describe the incidence of PM and identify predictors that increase the probability of peritoneal spread.
Methods
All ovarian, endometrial and cervical cancer patients diagnosed in the Netherlands between 1989 and 2015 were identified from the Netherlands Cancer Registry and stratified for PM. Crude and age-adjusted incidence over time was calculated. Independent predictors for PM were identified using uni- and multivariable analyses.
Results
The 94,981 patients were diagnosed with ovarian, endometrial or cervical cancer and respectively 61%, 2% and 1% presented with PM. Predictors for PM in ovarian cancer were: age between 50 and 74 years (odds ratio [OR]=1.19; 95% confidence interval [CI]=1.08–1.32), other distant metastases (OR=1.25; 95% CI=1.10–1.41), poor differentiation grade (OR=2.00; 95% CI=1.73–2.32) and serous histology. Predictors in endometrial cancer were lymph node metastases (OR=2.32; 95% CI=1.65–3.26), other distant metastases (OR=1.38; 95% CI=1.08–1.77), high-grade tumors (OR=1.95; 95% CI=1.38–2.76) and clear cell (OR=1.49; 95% CI=1.04–2.13) or serous histology (OR=2.71; 95% CI=2.15–3.42). In cervical cancer, the risk is higher in adenocarcinoma than in squamous cell carcinoma (OR=4.92; 95% CI=3.11–7.79).
Conclusion
PM are frequently seen in patients with ovarian cancer. In endometrial and cervical cancer PM are rare. Histological subtype was the strongest predictive factor for PM in all 3 cancers. Better understanding of predictive factors for PM and thus the biological behavior is of paramount importance.
Keywords: Peritoneal Neoplasms, Ovarian Neoplasms, Endometrial Neoplasms, Uterine Cervical Neoplasms, Incidence, Epidemiology
INTRODUCTION
In 2015, over 4,000 women in the Netherlands were diagnosed with ovarian, endometrial or cervical cancer [1]. Ovarian cancer is known to present with peritoneal metastases (PM) already at the time of diagnosis in a considerable number of patients. Due to the advanced stage at diagnosis it is associated with an impaired clinical outcome [2,3,4]: the overall 5-year survival rate is less than 30% [3,4,5,6]. In cervical and endometrial cancer, PM are less often reported [7,8].
A better understanding of the biological behavior of gynecological cancers is of paramount importance to improve treatment strategies. Analyzing the pattern of metastasis in gynecological cancers and the predictive factors for having PM can contribute to the clarification of this behavior. Until recently, gynecological cancer patients were treated according to the origin of the gynecological tumor, independent of other factors like histological type. Nowadays more attention is given to the differences between the histological subtypes of gynecological cancer and the pattern of metastases [9,10,11]. The histological subtype is expected to be a predictor for the occurrence of PM [12]. Nevertheless, more knowledge about predictive factors on PM is necessary. This is especially the case since promising therapeutic modalities are upcoming.
To the best of our knowledge, no population-based studies on the incidence of PM in gynecological cancers have been conducted. In addition, predictive patient- and tumor characteristics for the development of PM of gynecological origin are not yet studied on a large scale. Therefore, we describe the incidence of PM in gynecological cancers and identify predictors that increase the probability of peritoneal spread, so we can contribute to an increasing clinical guidance into a more targeted treatment strategy for patients with gynecological cancers.
MATERIALS AND METHODS
1. Data collection
We conducted a retrospective study. All consecutive patients with primary ovarian (C56, C57, C48), endometrial (C54, C55) and cervical (C53) cancer diagnosed between 1989 and 2015 were identified from the Netherlands Cancer Registry (NCR) [13]. Registration in the NCR takes place based on notification of newly diagnosed malignancies in the Netherlands by the automated nationwide pathology archive (Pathological Anatomical National Automated Archive [PALGA]). Specially trained administrators of the NCR routinely extract information on patients and tumor characteristics from the medical records. Completeness of the NCR is estimated to be over 95% [14].
The data contained patient characteristics, such as date of diagnosis and age at diagnosis. Socio-economic status was based on a patient's postal area according to the Netherlands Institute for Social Research [15]. A medical history of other malignant (non-) gynecological tumors was derived from the NCR.
The International Federation of Gynecology and Obstetrics (FIGO) stage was derived from the tumor, node, and metastasis (TNM) staging system and was based on postoperative findings for ovarian cancer and endometrial cancer. In cervical cancer patients, FIGO was based on clinical staging, using all available medical information of the patients as accurately as possible including imaging techniques, scopies and biopsies. Moreover, if patients underwent neoadjuvant chemotherapy, stage was based on clinical findings again including imaging, scopies and biopsies in order to avoid downstaging in case of good response. Lymph node involvement and distant metastases were identified. In case patients did not undergo surgical treatment, FIGO stage, lymph node involvement and distant metastases were based on clinical information (such as imaging techniques). As such, this was not in all cases histologically confirmed. Type of histology was classified into groups based on the primary site of the tumor.
2. Statistical analyses
Patients were distributed among period groups according to the year of diagnosis (1989–1994, 1995–2000, 2001–2005, 2005–2010, 2011–2015) and into age groups (18–49 years, 50–74 years, >75 years). Descriptive statistics were used to provide an overview of all patients with gynecological cancer. Early stage ovarian (FIGO stage I–IIa), endometrial (FIGO stage I–II) and cervical (FIGO stage I–II) cancer patients were excluded from further analyses, as by definition, they could not develop PM. Ovarian cancer patients with an unknown FIGO stage were included, as TNM was not registered within the NCR for primary peritoneal cancers until 2010. Endometrial and cervical cancer patients with an unknown FIGO stage were excluded. Crude and European standardized (European standardized rate) incidence rates of ovarian, endometrial and cervical cancer patients with PM were calculated. Since registration in the NCR expanded in 2005 for the localization of metastases, a subgroup analysis regarding age-adjusted incidence rates between 2005 and 2015 was conducted. Independent predictors for the occurrence of PM were identified using the univariable and multivariable logistic regression analyses. Only characteristics that were significant in univariable analyses were included in the multivariable analyses. A p-value <0.05 was considered statistically significant for all analyses. Statistical analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc., Cary, NC, USA).
3. Ethical approval
Local ethical approval was not applicable, since this study concerns retrospective population-based data, provided by the NCR. The medical ethical committee of the NCR assessed, approved and provided the required dataset for this study.
RESULTS
Between 1989 and 2015, in total 94,981 patients were diagnosed with ovarian (n=33,366), endometrial (n=42,333) and cervical cancer (n=19,282) in the Netherlands (Fig. 1, Table 1). The vast majority of PM (96%) was found in patients with ovarian cancer.
Fig. 1. Flowchart of patients diagnosed with gynecological cancer between 1995 and 2015 in the Netherlands, according to NCR data.
FIGO, International Federation of Gynecology and Obstetrics; NCR, Netherlands Cancer Registry.
*Patients with an unknown FIGO stage are excluded.
Table 1. Patient and tumor characteristics of all patients diagnosed with gynecological cancer between 1989 and 2015 in the Netherlands (n=94,981).
| Characteristics | Ovarian cancer (n=33,366) | Endometrial cancer (n=42,333) | Cervical cancer (n=19,282) | |
|---|---|---|---|---|
| Age at diagnosis | 64.4 (18–100) | 67.0 (24–102) | 51.4 (18–103) | |
| Age | ||||
| 18–49 | 4,721 (14) | 2,109 (5) | 10,346 (54) | |
| 50–74 | 20,232 (61) | 28,858 (68) | 6,394 (33) | |
| >75 | 8,413 (25) | 6,394 (33) | 2,542 (13) | |
| Period of diagnosis | ||||
| 1989–1994 | 6,831 (20) | 7,537 (18) | 4,430 (23) | |
| 1995–2000 | 7,536 (23) | 8,419 (20) | 4,348 (23) | |
| 2001–2005 | 6,047 (18) | 8,113 (19) | 3,278 (17) | |
| 2006–2010 | 6,503 (19) | 8,847 (21) | 3,621 (19) | |
| 2011–2015 | 6,449 (19) | 9,417 (22) | 3,605 (19) | |
| Vital status* | 8,068 (24) | 21,826 (52) | 10,970 (57) | |
| Previous gynecological tumor | 1,119 (3) | 1,080 (3) | 226 (1) | |
| Previous non-gynecological tumors | 5,240 (16) | 9,436 (22) | 2,480 (13) | |
| Socio-economic status | ||||
| High | 10,296 (31) | 12,548 (30) | 5,651 (29) | |
| Middle | 13,663 (41) | 17,344 (41) | 6,987 (36) | |
| Low | 9,407 (28) | 12,441 (29) | 6,644 (35) | |
| FIGO stage | ||||
| I | 6,958 (21) | 31,208 (74) | 10,374 (54) | |
| II | 2,663 (8) | 3,273 (8) | 3,254 (17) | |
| III | 14,640 (44) | 3,566 (8) | 3,241 (17) | |
| IV | 5,809 (17) | 2,389 (6) | 1,857 (10) | |
| Unknown | 3,296 (10) | 1,897 (5) | 556 (3) | |
| Differentiation grade | ||||
| I | 3,249 (9) | 16,607 (39) | 1,134 (6) | |
| II | 5,527 (17) | 13,331 (31) | 5,391 (28) | |
| III | 11,996 (36) | 7,601 (18) | 4,961 (26) | |
| Unknown | 12,594 (38) | 4,794 (11) | 7,796 (31) | |
| Histology | ||||
| Serous | 13,603 (41) | 1,493 (5) | - | |
| Mucinous | 3,378 (10) | - | - | |
| Clear cell | 1,520 (5) | 824 (2) | - | |
| Endometrioid | 3,107 (9) | 22,248 (53) | - | |
| Squamous carcinoma | - | - | 14,325 (74) | |
| Adenocarcinoma NOS | 9,243 (28) | 13,574 (32) | 3,410 (18) | |
| Other | 2,515 (8) | 4,194 (10) | 1,547 (8) | |
| Peritoneal metastases | 20,402 (61) | 711 (2) | 98 (1) | |
Values are presented as mean (range) or number (%).
*Vital status of all patients was assessed on February 1, 2017.
1. Ovarian cancer with PM
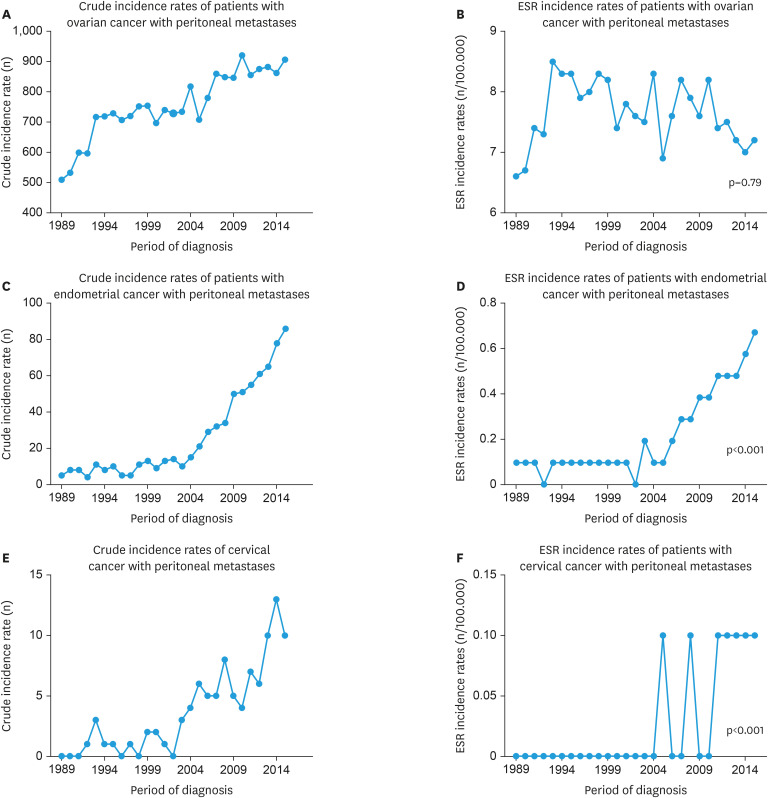
Sixty-one percent (n=20,401) of all ovarian cancer patients presented with PM. The mean age at diagnosis was 65.7 years (Table 2). The 89% of advanced stage ovarian cancer patients had PM without other metastases. The histology in the majority of patients was serous adenocarcinoma (51%) or adenocarcinoma not otherwise specified (NOS) (28%). An increase in crude incidence of PM was seen over the time (Fig. 2A). The age-adjusted incidence between 1989 and 2015 however was stable (p=0.79, Fig. 2B). The conducted subgroup analysis of incidence rates between 2005 and 2015 that was performed because of a change in registration from 2005 onwards showed a non-significant decrease in the age-adjusted incidence of PM (p=0.33). An age at diagnosis between 50 and 74 years was associated with an increased risk of PM (odds ratio [OR]=1.19; 95% confidence interval [CI]=1.08–1.32) compared to an age between 18 and 49 years (Table 3). A reduced risk was seen in patients with an age at diagnosis above 75 years (OR=0.85; 95% CI=0.76–0.95) and a medical history of one or more non-gynecological tumors (OR=0.65; 95% CI=0.60–0.71). Serous histology showed to have the highest risk of PM, compared to other histological subtypes. The risk of PM was higher in patients with a moderate to poor differentiation grade (grade II and III) compared to good differentiation grade (grade I) and with the presence of distant metastases (OR=1.25; 95% CI=1.10–1.41). The presence of positive lymph nodes reduced the risk of PM (OR=0.76; 95% CI=0.63–0.93).
Table 2. Patient and tumor characteristics of all patients with peritoneal metastases of ovarian, endometrial or cervical origin between 1989 and 2015 in the Netherlands (n=21,211).
| Characteristics | Ovarian cancer (n=20,402) | Endometrial cancer (n=711) | Cervical cancer (n=98) | |
|---|---|---|---|---|
| Age at diagnosis | 65.7 (18–100) | 69.1 (25–95) | 61.5 (23–93) | |
| Age | ||||
| 18–49 | 2,181 (11) | 28 (4) | 23 (23) | |
| 50–74 | 12,885 (63) | 462 (65) | 50 (51) | |
| >75 | 5,336 (26) | 220 (31) | 25 (26) | |
| Period of diagnosis | ||||
| 1989–1994 | 3,672 (18) | 44 (6) | 5 (5) | |
| 1995–2000 | 4,359 (21) | 53 (7) | 6 (6) | |
| 2001–2005 | 3,729 (18) | 73 (10) | 14 (14) | |
| 2006–2010 | 4,257 (21) | 196 (28) | 27 (28) | |
| 2011–2015 | 4,384 (21) | 345 (49) | 46 (47) | |
| Previous gynecological tumor | 350 (2) | 17 (2) | 1 (1) | |
| Previous non-gynecological tumors | 2,450 (12) | 129 (18) | 12 (12) | |
| Socio-economic status | ||||
| High | 6,276 (31) | 194 (27) | 17 (17) | |
| Middle | 8,444 (41) | 315 (44) | 43 (44) | |
| Low | 5,682 (28) | 202 (28) | 38 (39) | |
| Metastatic pattern | ||||
| PM alone | 18,083 (89) | 554 (78) | 54 (55) | |
| PM + positive lymph nodes | 531 (3) | 53 (7) | 18 (18) | |
| PM + (other) distant metastases | 1,620 (8) | 93 (13) | 16 (16) | |
| PM + lymph nodes and distant metastases | 168 (1) | 9 (1) | 10 (10) | |
| FIGO stage | ||||
| I | 1 (0) | 2 (0.3) | 0 (0) | |
| II | 29 (0.1) | 0 (0) | 0 (0) | |
| III | 13,960 (68) | 2 (0.3) | 1 (1) | |
| IV | 4,759 (23) | 684 (96) | 93 (95) | |
| Unknown | 1,653 (8) | 23 (3) | 4 (4) | |
| Differentiation grade | ||||
| I | 847 (4) | 41 (6) | 2 (2) | |
| II | 2,994 (15) | 97 (14) | 15 (15) | |
| III | 8,701 (43) | 326 (46) | 33 (34) | |
| Unknown | 7,860 (38) | 247 (34) | 48 (49) | |
| Histology | ||||
| Serous | 10,390 (51) | 196 (28) | - | |
| Mucinous | 1,064 (5) | - | - | |
| Clear cell | 424 (2) | 44 (6) | - | |
| Endometrioid | 989 (5) | 221 (31) | - | |
| Squamous carcinoma | - | - | 43 (44) | |
| Adenocarcinoma NOS | 6,587 (32) | 110 (15) | 37 (38) | |
| Other | 948 (5) | 140 (20) | 18 (18) | |
Values are presented as mean (range) or number (%).
FIGO, International Federation of Gynecology and Obstetrics; NOS, not otherwise specified; PM, peritoneal metastases.
Fig. 2. Crude and European standardized incidence rates for peritoneal metastases in patients with ovarian (A, B), endometrial (C, D) and cervical cancer (E, F) between 1989 and 2015 in the Netherlands.
Table 3. Multivariable logistic regression analyses: predictors of having peritoneal metastases in patients diagnosed with advanced stage gynecological cancer between 1995 and 2015 in the Netherlands.
| Characteristics | Ovarian cancer | Endometrial cancer | Cervical cancer | |
|---|---|---|---|---|
| Age | ||||
| 18–49 | Ref. | - | - | |
| 50–74 | 1.19 (1.08–1.32) | - | - | |
| >75 | 0.85 (0.76–0.95) | - | - | |
| Socio-economic status | ||||
| High | Ref. | - | - | |
| Middle | 0.97 (0.90–1.05) | - | - | |
| Low | 0.91 (0.84–0.99) | - | - | |
| More non-gyn tumors | ||||
| Yes | 0.65 (0.60–0.71) | - | - | |
| No | Ref. | - | - | |
| Metastatic pattern | ||||
| No lymphatic or distant metastases | Ref. | Ref. | Ref. | |
| Positive lymph nodes | 0.76 (0.63–0.93) | 2.32 (1.65–3.26) | 4.16 (2.38–7.28) | |
| Distant metastases | 1.25 (1.10–1.41) | 1.38 (1.08–1.77) | 3.46 (1.90–6.30) | |
| Lymph nodes and distant metastases | 0.64 (0.48–0.88) | 0.75 (0.37–1.51) | 5.19 (2.53–10.66) | |
| Differentiation grade | ||||
| I | Ref. | Ref. | Ref. | |
| II | 1.78 (1.51–2.08) | 1.13 (0.77–1.65) | 1.16 (0.26–5.19) | |
| III | 2.00 (1.73–2.32) | 1.95 (1.38–2.76) | 1.52 (0.35–6.51) | |
| Unknown | 1.70 (1.47–1.97) | 2.56 (1.77–3.70) | 2.24 (0.53–9.49) | |
| Histology | ||||
| Adenocarcinoma NOS | 0.54 (0.50–0.58) | 0.61 (0.48–0.77) | 4.92 (3.11–7.79) | |
| Serous | Ref. | 2.71 (2.15–3.42) | - | |
| Mucinous | 0.51 (0.44–0.58) | - | - | |
| Clear cell | 0.22 (0.18–0.26) | 1.49 (1.04–2.13) | - | |
| Endometrioid | 0.25 (0.22–0.28) | Ref. | - | |
| Squamous carcinoma | - | - | Ref. | |
| Other | 0.11 (0.10–0.12) | 1.04 (0.81–1.34) | 2.72 (1.46–5.05) | |
Values are presented as odds ratio (confidence interval). All odds ratios are adjusted for the listed variables.
2. Endometrial cancer with PM
In all endometrial cancer patients, the vast majority was diagnosed in FIGO stage I disease (74%). Only 2% (n=709) presented with PM. The mean age at diagnosis for advanced stage patients was 69.1 years. The most common histological subtypes were endometrioid (31%) and serous (28%) and 78% had no other metastases than PM. An increase in crude and age-adjusted (p<0.001) incidence of PM was seen (Fig. 2C and D), as well as in a subgroup analysis of age-adjusted incidence rates between 2005 and 2015 (p<0.001). The risk of PM was increased in serous (OR=2.71; 95% CI=2.15–3.42) and clear cell (OR=1.48; 95% CI=1.04–2.13) subtypes compared to the endometrioid subtype. A moderate to poor differentiation grade (grade II and III) increased the risk as well. Other distant metastases than PM (OR=1.38; 95% CI=1.08–1.77) and affected lymph nodes (OR=2.32; 95% CI=1.65–3.26) were associated with a higher risk on PM.
3. Cervical cancer with PM
One percent (n=98) of all cervical cancer patients presented with PM. The mean age at diagnosis of only advanced stage patients was 51.4 years. Thirty-eight percent had an adenocarcinoma and 44% had a squamous cell carcinoma. Fifty-five percent of patients had PM without other distant metastases. An increase in crude and age-adjusted (p<0.001) incidence of PM is seen (Fig. 2E and F); a subgroup analysis of age-adjusted incidence rates between 2005 and 2015 did underline this increase (p=0.019). The risk of PM was higher in adenocarcinoma compared to squamous cell carcinoma (OR=4.92; 95% CI=3.11–7.79). For cervical cancer, every type of metastatic pattern was significantly associated with the occurrence of PM.
DISCUSSION
This study showed that PM are most common in ovarian cancer when studying gynecological cancers. Moreover, more than half of all ovarian cancer patients presented with PM, negatively influencing overall survival rates. In addition to serous ovarian cancer patients, serous and clear cell endometrial cancer patients and patients with adenocarcinoma of the cervix are at highest risk for the occurrence of PM.
Although the majority of PM were found in ovarian cancer patients, our data show that PM also occur in endometrial and cervical cancer. In case of endometrial cancer this is in line with 2 previous review articles [16,17].
The age-adjusted incidence of PM in ovarian cancer was stable between 1989 and 2015. In endometrial cancer a significant increase in incidence was observed and might be explained by the increase of surgical staging in high-risk endometrial cancer over time, ever since national guidelines recommend to conduct a surgical exploration and extensive staging in case of serous and clear cell endometrial cancer [18]. This, together with the improvement of radiological diagnostics such as computed tomography-scans, can explain the significant increase in the incidence of PM of endometrial cancer. In cervical cancer, incidence rates are based on a small number of patients.
As stated previously, our results show that histological subtype is the strongest predictor for the occurrence of PM. For example, PM occur more often in serous ovarian and endometrial cancers and in adenocarcinomas of the cervix than in squamous cell carcinomas, which is in line with a previous study [19]. In addition, a moderate to poor differentiation grade is an independent predictor for the occurrence of PM in ovarian and endometrial cancer. Previous studies in colorectal and gastric cancer patients show similar predictive factors like histological type and differentiation grade [20,21]. In those studies, PM were also more often found in younger patients (below 60 years), which is in line with our findings in ovarian cancer patients. Probably this is due to the fact that older patients less often underwent surgical exploration of the peritoneal cavity. This effect was eliminated as much as possible by extracting data on PM from surgical, pathological and radiological reports. A small percentage of the, mainly older, patients will still not have gone through a full diagnostic workup and some data may be lacking [22]. Our data also suggest that the risk of PM in ovarian cancer is reduced in case of a previous non-gynecological cancer. We cannot explain this in a biological way but we suspect that these patients (and cancer patients in generally) are more often exposed to thorough examinations, leading to diagnosis in an earlier stage.
In ovarian cancer patients with affected lymph nodes, a lower risk of PM was found. This is consistent with the revised FIGO staging system, in which the presence of affected lymph nodes is now considered to be FIGO stage IIIa disease instead of FIGO stage IIIc disease [23]. However, in endometrial and cervical cancer patients the risk of PM was significantly increased in case of affected lymph nodes. This pattern was seen in colorectal and gastric cancer as well [20,21]. This difference may well be explained by the biological behavior of ovarian cancer versus endometrial and cervical cancer. In ovarian cancer, an increasing amount of evidence for the ‘tubal-hypothesis’ exists, which suggests that ovarian cancer originates in the epithelium of the fallopian tube and spread throughout the abdomen [24]. In endometrial and cervical cancer, like in colorectal cancer, PM are caused by serosal infiltration of the primary tumor and subsequent shedding of malignant cells into the peritoneal cavity [25]. One can imagine that this difference in biological behavior and aggressiveness can also influence the occurrence of affected lymph nodes.
A lack of lymph node sampling in ovarian cancer patients may play a role as well. In patients with gross intra-abdominal disease, and macroscopically normal lymph nodes, standard lymphadenectomy does not contribute to prolonged overall nor progression-free survival in these patients [26,27]. This might influence the correlation between affected lymph nodes and the risk on PM. It also may be a consequence of the retrospective design of our study.
This study adds to the literature on the biological behavior of gynecological tumors and this may contribute to the development of more effective therapeutic strategies. As our study and other population-based studies pointed out, the peritoneum is a preferred localization for metastases of various kinds of cancer [3,20,21]. Therefore, the most important clinical consequence of identifying predictors for the occurrence of PM is the development of a more targeted treatment strategy, like intraperitoneal (IP) chemotherapy. Upcoming treatment strategies like hyperthermic intraperitoneal chemotherapy (HIPEC) are promising. In IP chemotherapy and HIPEC, the drugs are administered directly into the peritoneal cavity, increasing the drug's dose delivered to the tumor site [28,29,30]. Several studies show the beneficial effect of IP chemotherapy after cytoreductive surgery for ovarian cancer patients, resulting in an increase of median survival [31,32,33,34]. Other studies, including a recent Dutch study, demonstrated the beneficial effect of HIPEC on recurrence free and overall survival in ovarian cancer patients [35,36,37,38,39]. In our study, we have shown that serous and clear cell histology in endometrial cancer is an independent predictor for the occurrence of PM. Therefore, treatment strategies used for ovarian cancer could be considered for these types of cancers as well, i.e., extensive cytoreductive surgery in combination with chemotherapy. The indication for IP chemotherapy or even HIPEC in serous and clear cell endometrial cancer could be subject for future studies, taking into account the quality of life after such treatment strategies [40,41,42].
Our study provides an overview of population-based data over more than twenty-five years. The retrospective design however has its limitations. One is the extraction of data from medical files; some details on background information were missing. Also, because PM are best diagnosed during an operative procedure, one might speculate that this study could underestimate the true incidence of PM. This might specifically be the case in older patients or in patients who for other reasons did not undergo a surgical procedure. There might be some bias because of increasing adequacy of pathological methods influencing the diagnosed histological type of a cancer, especially in case of adenocarcinoma NOS subtypes.
In conclusion, PM are frequently seen in patients with ovarian cancer. Ovarian cancers, serous and clear cell endometrial cancers and adenocarcinoma of the cervix have the highest risk for the occurrence of PM. Therefore, we suggest a therapeutic approach in which a cancer is treated in accordance with its histological subtype and its pattern of metastasis. Considering the possible beneficial effect of IP chemotherapy and HIPEC on the survival of advanced stage ovarian cancer patients, this might be a starting point for new research into those treatment strategies in patients with serous or clear cell endometrial cancer and adenocarcinoma of the cervix.
ACKNOWLEDGMENTS
The authors thank the Netherlands Cancer Registry (NCR) for providing the data and the registration clerks for the dedicated data collection.
Footnotes
Presentation: Poster presentation at the 17th Biennial Meeting of the International Gynecologic Cancer Society (IGCS 2018). Kyoto, Japan, September 14–16, 2018.
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: V.A.A., B.D., B.L.
- Data curation: T.M., V.D.A.M.
- Formal analysis: T.M., R.K., B.L.
- Methodology: T.M., R.K., B.L.
- Software: T.M., R.K., B.L.
- Validation: V.D.A.M.
- Visualization: B.L.
- Writing - original draft: B.L.
- Writing - review & editing: T.M., V.D.A.M., B.D., R.K., D.H.I., V.A.A.
References
- 1.Wittekind CG, Hutten RP, Klimpfinger M, Sobin LH. Tumour lymph node metastasis classification. TNM atlas. 5th ed. Berlin: Springer-Verlag; 2005. [Google Scholar]
- 2.Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–6994. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomassen I, Verhoeven RH, van Gestel YR, van de Wouw AJ, Lemmens VE, de Hingh IH. Population-based incidence, treatment and survival of patients with peritoneal metastases of unknown origin. Eur J Cancer. 2014;50:50–56. doi: 10.1016/j.ejca.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Fagotti A, Gallotta V, Romano F, Fanfani F, Rossitto C, Naldini A, et al. Peritoneal carcinosis of ovarian origin. World J Gastrointest Oncol. 2010;2:102–108. doi: 10.4251/wjgo.v2.i2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Altena AM, Karim-Kos HE, de Vries E, Kruitwagen RF, Massuger LF, Kiemeney LA. Trends in therapy and survival of advanced stage epithelial ovarian cancer patients in the Netherlands. Gynecol Oncol. 2012;125:649–654. doi: 10.1016/j.ygyno.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 6.Coccolini F, Campanati L, Catena F, Ceni V, Ceresoli M, Jimenez Cruz J, et al. Hyperthermic intraperitoneal chemotherapy with cisplatin and paclitaxel in advanced ovarian cancer: a multicenter prospective observational study. J Gynecol Oncol. 2015;26:54–61. doi: 10.3802/jgo.2015.26.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Belval CC, Barranger E, Dubernard G, Touboul E, Houry S, Daraï E. Peritoneal carcinomatosis after laparoscopic radical hysterectomy for early-stage cervical adenocarcinoma. Gynecol Oncol. 2006;102:580–582. doi: 10.1016/j.ygyno.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Delotte J, Desantis M, Frigenza M, Quaranta D, Bongain A, Benchimol D, et al. Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of endometrial cancer with peritoneal carcinomatosis. Eur J Obstet Gynecol Reprod Biol. 2014;172:111–114. doi: 10.1016/j.ejogrb.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 9.Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. doi: 10.2353/ajpath.2010.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Halkia E, Spiliotis J, Sugarbaker P. Diagnosis and management of peritoneal metastases from ovarian cancer. Gastroenterol Res Pract. 2012;2012:541842. doi: 10.1155/2012/541842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alexandre J, Ray-Coquard I, Selle F, Floquet A, Cottu P, Weber B, et al. Mucinous advanced epithelial ovarian carcinoma: clinical presentation and sensitivity to platinum-paclitaxel-based chemotherapy, the GINECO experience. Ann Oncol. 2010;21:2377–2381. doi: 10.1093/annonc/mdq257. [DOI] [PubMed] [Google Scholar]
- 12.Greggi S, Mangili G, Scaffa C, Scala F, Losito S, Iodice F, et al. Uterine papillary serous, clear cell, and poorly differentiated endometrioid carcinomas: a comparative study. Int J Gynecol Cancer. 2011;21:661–667. doi: 10.1097/IGC.0b013e3182150c89. [DOI] [PubMed] [Google Scholar]
- 13.Fritz A, Percy C, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, et al. International classification of diseases for oncology. 3rd ed. Geneva: World Health Organization; 2000. [Google Scholar]
- 14.Schouten LJ, Höppener P, van den Brandt PA, Knottnerus JA, Jager JJ. Completeness of cancer registration in Limburg, The Netherlands. Int J Epidemiol. 1993;22:369–376. doi: 10.1093/ije/22.3.369. [DOI] [PubMed] [Google Scholar]
- 15.Tesser PT, Van Praag CS, van Dugteren FA. Rapportage minderheden 1995: concentratie en segregatie. Rijswijk: Sociaal en Cultureel Planbureau; 1995. [Google Scholar]
- 16.Boruta DM, 2nd, Gehrig PA, Fader AN, Olawaiye AB. Management of women with uterine papillary serous cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;115:142–153. doi: 10.1016/j.ygyno.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Olawaiye AB, Boruta DM., 2nd Management of women with clear cell endometrial cancer: a Society of Gynecologic Oncology (SGO) review. Gynecol Oncol. 2009;113:277–283. doi: 10.1016/j.ygyno.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, et al. Carcinoma of the ovary. Int J Gynaecol Obstet. 2006;95:S161–92. doi: 10.1016/S0020-7292(06)60033-7. [DOI] [PubMed] [Google Scholar]
- 19.Greggi S, Benedetti-Panici P, Amoroso M, Scambia G, Paratore M, Salerno M, et al. Intraperitoneal tumor spread in locally advanced cervical carcinoma undergoing neoadjuvant chemotherapy. Int J Gynecol Cancer. 1998;8:207–214. [Google Scholar]
- 20.Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128:2717–2725. doi: 10.1002/ijc.25596. [DOI] [PubMed] [Google Scholar]
- 21.Thomassen I, van Gestel YR, van Ramshorst B, Luyer MD, Bosscha K, Nienhuijs SW, et al. Peritoneal carcinomatosis of gastric origin: a population-based study on incidence, survival and risk factors. Int J Cancer. 2014;134:622–628. doi: 10.1002/ijc.28373. [DOI] [PubMed] [Google Scholar]
- 22.Maas HA, Kruitwagen RF, Lemmens VE, Goey SH, Janssen-Heijnen ML. The influence of age and co-morbidity on treatment and prognosis of ovarian cancer: a population-based study. Gynecol Oncol. 2005;97:104–109. doi: 10.1016/j.ygyno.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 23.Society of Gynecologic Oncology. New FIGO ovarian cancer staging guidelines [Internet] Chicago, IL: Society of Gynecologic Oncology; c2014. Available from: https://www.sgo.org/clinical-practice/guidelines/new-figo-ovarian-cancer-staging-guidelines/ [Google Scholar]
- 24.Corzo C, Iniesta MD, Patrono MG, Lu KH, Ramirez PT. Role of fallopian tubes in the development of ovarian cancer. J Minim Invasive Gynecol. 2017;24:230–234. doi: 10.1016/j.jmig.2016.12.007. [DOI] [PubMed] [Google Scholar]
- 25.van Baal JO, Van de Vijver KK, Nieuwland R, van Noorden CJ, van Driel WJ, Sturk A, et al. The histophysiology and pathophysiology of the peritoneum. Tissue Cell. 2017;49:95–105. doi: 10.1016/j.tice.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Harter P, Sehouli J, Lorusso D, Reuss A, Vergote I, Marth C, et al. LION: Lymphadenectomy in ovarian neoplasms—A prospective randomized AGO study group led gynecologic cancer intergroup trial. J Clin Oncol. 2017;35:5500. [Google Scholar]
- 27.Yamazaki H, Todo Y, Shimada C, Takeshita S, Minobe S, Okamoto K, et al. Therapeutic significance of full lymphadenectomy in early-stage ovarian clear cell carcinoma. J Gynecol Oncol. 2018;29:e19. doi: 10.3802/jgo.2018.29.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ansaloni L, Coccolini F, Morosi L, Ballerini A, Ceresoli M, Grosso G, et al. Pharmacokinetics of concomitant cisplatin and paclitaxel administered by hyperthermic intraperitoneal chemotherapy to patients with peritoneal carcinomatosis from epithelial ovarian cancer. Br J Cancer. 2015;112:306–312. doi: 10.1038/bjc.2014.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bakrin N, Classe JM, Pomel C, Gouy S, Chene G, Glehen O. Hyperthermic intraperitoneal chemotherapy (HIPEC) in ovarian cancer. J Visc Surg. 2014;151:347–353. doi: 10.1016/j.jviscsurg.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Hasovits C, Clarke S. Pharmacokinetics and pharmacodynamics of intraperitoneal cancer chemotherapeutics. Clin Pharmacokinet. 2012;51:203–224. doi: 10.2165/11598890-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 31.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 32.Alberts DS, Liu PY, Hannigan EV, O'Toole R, Williams SD, Young JA, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med. 1996;335:1950–1955. doi: 10.1056/NEJM199612263352603. [DOI] [PubMed] [Google Scholar]
- 33.Markman M, Bundy BN, Alberts DS, Fowler JM, Clark-Pearson DL, Carson LF, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol. 2001;19:1001–1007. doi: 10.1200/JCO.2001.19.4.1001. [DOI] [PubMed] [Google Scholar]
- 34.Classe JM, Muller M, Frenel JS, Berton Rigaud D, Ferron G, Jaffré I, et al. Intraperitoneal chemotherapy in the treatment of advanced ovarian cancer. J Gynecol Obstet Biol Reprod (Paris) 2010;39:183–190. doi: 10.1016/j.jgyn.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Van Driel WJ, Sikorska K, van Leeuwen JS, Schreuder H, Hermans R, De Hingh IH, et al. A phase 3 trial of hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer. J Clin Oncol. 2017;35:5519. [Google Scholar]
- 36.Ansaloni L, Agnoletti V, Amadori A, Catena F, Cavaliere D, Coccolini F, et al. Evaluation of extensive cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with advanced epithelial ovarian cancer. Int J Gynecol Cancer. 2012;22:778–785. doi: 10.1097/IGC.0b013e31824d836c. [DOI] [PubMed] [Google Scholar]
- 37.Bakrin N, Cotte E, Golfier F, Gilly FN, Freyer G, Helm W, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for persistent and recurrent advanced ovarian carcinoma: a multicenter, prospective study of 246 patients. Ann Surg Oncol. 2012;19:4052–4058. doi: 10.1245/s10434-012-2510-4. [DOI] [PubMed] [Google Scholar]
- 38.Chua TC, Robertson G, Liauw W, Farrell R, Yan TD, Morris DL. Intraoperative hyperthermic intraperitoneal chemotherapy after cytoreductive surgery in ovarian cancer peritoneal carcinomatosis: systematic review of current results. J Cancer Res Clin Oncol. 2009;135:1637–1645. doi: 10.1007/s00432-009-0667-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang XJ, Li Y, al-shammaa Hassan AH, Yang GL, Liu SY, Lu YL, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy improves survival in selected patients with peritoneal carcinomatosis from abdominal and pelvic malignancies: results of 21 cases. Ann Surg Oncol. 2009;16:345–351. doi: 10.1245/s10434-008-0226-2. [DOI] [PubMed] [Google Scholar]
- 40.Tsilimparis N, Bockelmann C, Raue W, Menenakos C, Perez S, Rau B, et al. Quality of life in patients after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: is it worth the risk? Ann Surg Oncol. 2013;20:226–232. doi: 10.1245/s10434-012-2579-9. [DOI] [PubMed] [Google Scholar]
- 41.Passot G, Bakrin N, Roux AS, Vaudoyer D, Gilly FN, Glehen O, et al. Quality of life after cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy: a prospective study of 216 patients. Eur J Surg Oncol. 2014;40:529–535. doi: 10.1016/j.ejso.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 42.Chia CS, Tan WJ, Wong JF, Tan GH, Lim C, Wang W, et al. Quality of life in patients with peritoneal surface malignancies after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol. 2014;40:909–916. doi: 10.1016/j.ejso.2013.12.028. [DOI] [PubMed] [Google Scholar]