Abstract
Background
Two randomized phase III trials (EORTC55971 and CHORUS) showed similar progression-free and overall survival in primary or interval debulking surgery in ovarian cancer, however both studies had limitations with lower rate of complete resection and lack of surgical qualifications for participating centers. There is no consensus on whether neoadjuvant chemotherapy followed by interval debulking surgery (NACT-IDS) could be a preferred approach in the management of advanced epithelial ovarian cancer (EOC) in the clinical practice.
Methods
The Asian SUNNY study is an open-label, multicenter, randomized controlled, phase III trial to compare the effect of primary debulking surgery (PDS) to NACT-IDS in stages IIIC and IV EOC, fallopian tube cancer (FTC) or primary peritoneal carcinoma (PPC). The hypothesis is that PDS enhances the survivorship when compared with NACT-IDS in advanced ovarian cancer. The primary objective is to clarify the role of PDS and NACT-IDS in the treatment of advanced ovarian cancer. Surgical quality assures include at least 50% of no gross residual (NGR) in PDS group in all centers and participating centers should be national cancer centers or designed ovarian cancer section or those with the experience participating surgical trials of ovarian cancer. Any participating center should be monitored evaluating the proportions of NGR by a training set. The aim of the surgery in both arms is maximal cytoreduction. Tumor burden of the disease is evaluated by diagnostic laparoscopy or positron emission tomography/computed tomography scan. Patients assigned to PDS group will undergo upfront maximal cytoreductive surgery within 3 weeks after biopsy, followed by 6 cycles of standard adjuvant chemotherapy. Patients assigned to NACT group will undergo 3 cycles of NACT-IDS, and subsequently 3 cycles of adjuvant chemotherapy. The maximal time interval between IDS and the initiation of adjuvant chemotherapy is 8 weeks. Major inclusion criteria are pathologic confirmed stage IIIC and IV EOC, FTC or PPC; ECOG performance status of 0 to 2; ASA score of 1 to 2. Major exclusion criteria are non-epithelial tumors as well as borderline tumors; low-grade carcinoma; mucinous ovarian cancer. The sample size is 456 subjects. Primary endpoint is overall survival.
Trial Registration
ClinicalTrials.gov Identifier: NCT02859038
Keywords: Ovarian Cancer, Cytoreduction Surgical Procedures, Neoadjuvant Therapy
INTRODUCTION
Epithelial ovarian cancer (EOC) is the most lethal of all gynecological cancers [1]. Optimal surgical cytoreduction especially with no residual disease followed by adjuvant platinum-based chemotherapy is associated with longer progression-free survival (PFS) and overall survival (OS) [2,3,4,5,6]. Neoadjuvant chemotherapy (NACT) can decrease the tumor burden such that complete cytoreduction is more feasible. And NACT may improve patient performance status, shorten the operation time, and reduce the perioperative morbidity and mortality [7].
The study of European Organisation for Research and Treatment of Cancer (EORTC) 55971 demonstrated that NACT followed by interval debulking surgery (NACT-IDS) was not inferior to primary debulking surgery (PDS) in the management of patients with stage IIIC or IV EOC. And lower toxicity was observed in the NACT group [7]. However, in the EORTC study, the rate of complete debulking surgery in the PDS group was 19.4% and it obviously varies in each study centers (range, 3.9%–62.9%). The median OS was 29 months in the PDS group and 30 months in the NACT-IDS group, respectively. The survival was comparatively lower than reported data in regard to advanced EOC in some retrospective studies or prospective clinical trials [3,5,8]. Similar results from another European randomized multicenter clinical trial (CHemotherapy OR Upfront Surgery [CHORUS]) comparing PDS to NACT-IDS had been reported later [9]. The median OS was 22.6 months in the PDS group versus 24.1 months in the NACT group, which was also poorer than other studies. Japan Clinical Oncology Group (JCOG) 0602 is a third phase III clinical trial which compare the survival of PDS to NACT-IDS [10,11]. The survival data of JCOG0602 from Japan had been reported in the 2018's annual American Society of Clinical Oncology conference. Non-inferiority of NACT compared with PDS was not confirmed in OS in this study. Further researches are needed to determine the role of NACT in selected patients.
Chi analyzed the outcomes of patients with stage IIIC or IV ovarian cancer from Memorial Sloan-Kettering Cancer Center (MSKCC) during the same time period in which the EORTC 55971 trial was conducted. The results showed that median PFS and OS of the 285 patients who underwent PDS were 17 and 50 months, respectively. Patients who received PDS from MSKCC had a prolonged survival when compared with patients in EORTC 55971 study [12].
In summary, there exist debatable issues in both EORTC 55971 and CHORUS studies comparing upfront surgery to NACT-IDS, but gynecologic oncologists have not yet reached a consensus on whether NACT-IDS could be a preferred approach in the management of advanced EOC. In order to further investigate the role of NACT-IDS and PDS in treatment of advanced EOC, well-designed trials with surgical quality assurance are needed. In the SUNNY study, patients with stages IIIC and IV ovarian cancer of any tumor burden are enrolled and randomized. The goal of our study is to evaluate whether upfront surgery with quality guarantee can improve OS as compared to NACT-IDS.
METHODS
1. Trial design and surgical quality assurance
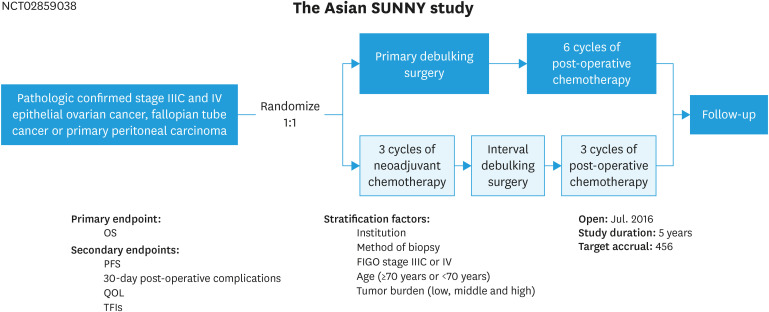
The SUNNY study (Shanghai Gynecologic Oncology Group SOC-2, NCT02859038) is a randomized, open-label, multicenter, phase III clinical trial in Asian countries. Patients with pathologically confirmed stages IIIC and IV EOC, fallopian tube cancer (FTC) or primary peritoneal carcinoma (PPC) will be randomly assigned in a 1:1 ratio to PDS group or NACT-IDS group) (Fig. 1). Patients assigned to PDS group will undergo upfront maximal cytoreductive surgery within 3 weeks after biopsy, followed by 6 cycles of standard adjuvant chemotherapy. Patients assigned to NACT-IDS group will undergo 3 cycles of chemotherapy followed by IDS, and subsequently 3 cycles of adjuvant chemotherapy. The maximal time interval between IDS and the initiation of adjuvant chemotherapy is 8 weeks. The hypothesis is that upfront radical surgery enhances the survivorship when compared with NACT-IDS in patients with advanced ovarian cancer.
Fig. 1. Study schema of the SUNNY study.
FIGO, International Federation of Gynecology and Obstetrics; OS, overall survival; PFS, progression-free survival; QOL, quality of life; TFI, treatment-free interval.
Maximal cytoreduction should be carried out in each patient either in the PDS group or in the NACT group. Surgical quality assures include at least 50% no gross residual (NGR) in PDS group in all centers and participating centers should be national cancer centers or designed ovarian cancer section or those with experience participating surgical trials of ovarian cancer. It is required that the number of surgeries for ovarian cancer should be 200 or more per year for the center and 20 per year for the surgeon in China. Any participating center should be monitored evaluating the proportions of NGR by a training set. The tumor size and location through the whole abdominal cavity both before and after surgery should be documented exactly, as well as the procedures performed in surgery. It is recommended, but not a mandatory to take pictures before and post operation. The goal of the surgery is maximal cytoreductive surgery. If an en bloc resection of the disease is impossible either in PDS or NACT-IDS group, particularly for miliary carcinomatosis around the mesentery, or on liver surface, the electronic surgical devices are recommended to use achieving optimal cytoreduction.
When IDS is performed, 1) if there is no visible lesion in the peritoneum of the pelvic, paracolic sulcus or diaphragm, there is no need to resect the peritoneum; however, if there is any suspected visible lesion after NACT, according to the imaging at diagnosis, the involved peritoneum before NACT should be resected; 2) if there is no visible disease on the surface of small intestine or mesenterium, none further procedures are required; resection or coagulation is recommended if there are any visible lesions; 3) bowel resection or splenectomy is not mandatory except when complete resection possible. Training set is recommended for each trial center. In this study, tumor burden was an important stratification factor. At the beginning, laparoscopic biopsy was recommended. Then, the investigators found that the diagnostic laparoscopy might be not safe for the patients in disease progression or recurrence. So, the combined peritoneal carcinoma index (cPCI) scoring system based on positron emission tomography/computed tomography (PET/CT) scan was developed (Table 1), and it can also be used as CT evaluation based on local practice.
Table 1. PET/CT based cPCI scoring system of tumor burden in ovarian cancer for the SUNNY study (version 2)*.
| Regions of the diseases | The cPCI scoring criteria | Scores | Assessment power |
|---|---|---|---|
| Diaphragmatic peritoneum (regions 1 and 3) | Solitary or localized nodules or no disease in these regions. | □ Score=0 | □ Low |
| Diffused and confluent carcinomatosis of right or left side diaphragmatic peritoneum or hepatorenal recess. | □ Score=2 | □ High | |
| □ Middle | |||
| Liver lesions (regions 1) | No surface lesions. | □ Score=0 | □ Low |
| Any surface lesions (include lesions on the porta hepatis and hepatic fissure). | □ Score=2 | □ High | |
| □ Middle | |||
| Lesions in the hepatogastric space or the space between stomach and spleen (regions 2 and 3) | No such manifestations. | □ Score=0 | □ Low |
| Lesions observed on the surface of stomach, and/or lesser omentum or hepatogastric space, and/or the space between stomach and spleen (splenic vascular pedicle, include the splenic hilum), and/or the surface of spleen. | □ Score=2 | □ High | |
| □ Middle | |||
| Omental lesions extend to the hepatic flexure or splenic flexure (regions 4 and 8) | No such manifestations. | □ Score=0 | □ Low |
| Omental lesion extends to the hepatic flexure and/or splenic flexure of the colon. | □ Score=2 | □ High | |
| □ Middle | |||
| Small bowel mesentery involvement (region 0) | Solitary or localized nodules or no disease of the small bowel mesentery. | □ Score=0 | □ Low |
| Diffused diseases of the small bowel and/or the mesentery. | □ Score=2 | □ High | |
| □ Middle | |||
| Peritoneal disease (regions 4 to 8) | Localized diseases involving the peritoneum of the middle abdomen. | □ Score=0 | □ Low |
| Diffused thickening of left and right paracolic gutter peritoneum, combined with the thickening pelvic peritoneum, and/or involving the colon. | □ Score=2 | □ High | |
| □ Middle | |||
| Bowel infiltration (regions 5 to 8) | Tumor not involving the bowel wall. | □ Score=0 | □ Low |
| Tumor involved the bowel wall (CT assists to evaluate the disease on the rectum, sigmoid colon and the right colon, including the distortion of the sigmoid colon or right colon, or the involved bowel and/or the involved mesenteric lesions with a continuous length ≥50 mm). | □ Score=2 | □ High | |
| □ Middle | |||
| Suprarenal lymph node metastases | Suprarenal lymph nodes <1 cm. Resectable cardiophrenic lymph nodes and/or supraclavicular lymph nodes, no matter the size. | □ Score=0 | NA |
| Suprarenal lymph nodes ≥1 cm. | □ Score=2 | ||
| Pulmonary embolism | None | □ Score=0 | NA |
| Yes | □ Score=2 |
Other notes: 1) Regions 0–8 see reference [13]. 2) The power of each item is classified as low, middle, and high. Every 2 items with the middle power are given a score of 2, otherwise it is 0. 3) Middle tumor burden was defined as carcinomatosis or the number of lesions ≥3 found by diagnostic laparoscopy or PET/CT scan in the upper abdomen, and the cPCI <10. High tumor burden was defined as the cPCI ≥10. 4) Any imaging showing small bowel mesenteric retraction will be defined as high tumor burden directly. 5) PET/CT based cPCI scoring system discussants: Rongyu Zang, Hongcheng Shi, Rong Jiang, Tingyan Shi, Guobing Liu, Songqi Cai, Jianqing Zhu, Jae-Weon Kim for Shanghai Gynecologic Oncology Group, Korean Gynecologic Oncology Group, and Japanese Gynecologic Oncology Group.
cPCI, combined peritoneal carcinoma index; NA, not applicable; PET/CT, positron emission tomography/computed tomography.
*Pulmonary embolism was assessed by computed tomographic pulmonary angiography, and other items are evaluated by PET/CT.
2. Eligibility criteria
Major inclusion criteria are pathologic confirmed stage IIIC and IV EOC, FTC, or PPC (diagnosis by laparoscopic biopsy, core needle biopsy or fine needle aspiration); aged ≥18 years; ECOG performance status of 0 to 2; American Society of Anesthesiologists score of 1 to 2.
Major exclusion criteria are non-epithelial tumors as well as borderline tumors; low-grade carcinoma; mucinous ovarian cancer; synchronous or metachronous (within 5 years) malignancy other than carcinoma in situ or breast cancer (without any signs of relapse or activity).
3. Outcomes
The primary endpoint is OS. Secondary endpoints are PFS; 30-day post-operative complications; quality of life assessments; accumulating treatment-free intervals (TFIs); the rates of TFI1=0 month and TFI1<6 months, and the rates of TFI2=0 month and TFI2<6 months in patients who underwent laparoscopic biopsy compared with those in patients without laparoscopic biopsy.
4. Randomization and stratification
The centralized randomization system was performed to screening subjects, generating the random allocation sequence, enrolling individuals, and assigning them to treatment. Participants were stratified at screening by institution, method of biopsy (laparoscopy or non-laparoscopy), International Federation of Gynecology and Obstetrics (FIGO) stage (IIIC or IV), age (≥70 years or <70 years), tumor burden (low, middle, or high). Low tumor burden was defined as the number of lesions in the upper abdomen <3 found by diagnostic laparoscopy or PET/CT scan, and without any of them, 1) extracapsular spread or merged retroperitoneal lymph nodes; 2) suprarenal vein lymph node metastases, excluding supraclavicular lymph node metastasis only; 3) mediastinal and internal mammary lymph node metastases. Middle tumor burden was defined as carcinomatosis or the number of lesions ≥3 found by diagnostic laparoscopy or PET/CT scan in the upper abdomen, and the cPCI <10. High tumor burden was defined as the cPCI ≥10.
5. Sample size
In the randomized clinical trial of EORTC 55791, patients with stages IIIC and IV EOC who received NACT had a median survival of 30 months [7]. However, the retrospective data from MSKCC showed a longer median OS of 50 months in 285 patients with stages IIIC and IV who received PDS during the same time period [11]; in 2009 Arbeitsgemeinschaft Gynäkologische Onkologie (AGO) data from a combined exploratory analysis of 3 prospective randomized trials (AGO-OVAR 3, 5, and 7) reported that 3126 patients with FIGO stage IIB–IV, of which there is 73.9% diagnosed of stage IIIC–IV, had a median survival of 44.1 months [5]. Based on these results, assuming the median OS of 44.1 months in upfront surgery arm and a hazard ratio of 0.6803, with an accrual time of 5 years, approximately 456 subjects will be randomized to PDS group and NACT group at a 1:1 ratio (228 in the control group and 228 in the treatment group) to ensure 90.0% power using a 2-sided log-rank test with a type I error rate of 0.05 to reject the null hypothesis. The sample size calculation was done using the PASS software (version 11.0).
6. Statistical methods
The hypothesis H0: “no difference in the survival probability between treatment groups at any time” will be tested against the alternative H1: “difference in survival probability between groups”. For the analysis of the study, 3 patient populations are formed: the intention-to-treat (ITT) population will consist of all randomized patients with signed consent; the per protocol population, being a subset of the ITT-population, will exclude those patients from the efficacy analysis who showed major protocol violations; the safety set population will focus on all randomized patients who at least initiated study therapy.
This study will be considered sufficiently mature for an analysis of the survival hypothesis, H0, when there are at least 284 deaths reported among those enrolled (and randomized) subjects of this study. This target size provides 90% power, if upfront surgery truly decreases the death rate 32%. This treatment effect size is comparable to increasing the percent surviving 14.1 months (30–44.1 months).
Interim analyses are planned when there are at least 142 deaths reported among all those patients randomly allocated. The last patient in will be Q4, 2020 and estimated results are reached at Q4, 2023. But the actual time for the interim analyses and final analyses will coincide with the nearest scheduled Data Monitoring Committee meeting for which the required number of events has occurred.
DISCUSSION
Till now, there is no consensus on the optimal timing of cytoreductive surgery in the management of patients with advanced EOC. Part of the patients with advanced disease do benefit from NACT-IDS. The poor survival outcomes and limited rates of NGR in PDS might invalidate their findings in the studies of EORTC and CHORUS. Residual disease after cytoreductive surgery is the most prominent prognostic factor in patients with advanced ovarian cancer. The rates of NGR depend on surgical techniques and efforts of surgeons, as well as tumor extent and distribution. So, it is crucial to both qualify and train the surgeons participating these studies, and stratify patients based on the tumor burden. In the SUNNY study, maximal cytoreduction even in those with high tumor burden should be carried out for each patient in either upfront or interval surgery. It is required that the percentage of patients with NGR should be at least 50% of NGR in PDS group. It is recommended that the participating centers should be specialist ovarian cancer centers with multidisciplinary team. And disease distribution inside abdomen and outside abdomen were carefully assessed and addressed in stratification. We look forward to the survival data from the SUNNY study a few years later.
Unlike the drug trials, surgical trials for ovarian cancer are always facing difficulties because the quality of the trials are not only associated with trials' design and conducting, but also associated with the surgery itself. And the biases come from either randomization or the surgeons/patients' choice. However, “more is different”, we need more trials to minimize those biases.
Footnotes
Funding: The study is supported by Clinical Trial Project of Zhongshan Hospital, Fudan University (No. 2016ZSLC21).
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
- Conceptualization: J.R., Z.J., K.J.W., L.J., K.K., K.H.S., Z.Y., Z.P., Z.T., A.D., Y.A., C.X., W.X., Z.D., Z.W., J.H., S.T., G.W., Y.S., F.Y., X.L., O.A., Z.R.
- Supervision: X.L.
- Visualization: Z.R.
- Writing - original draft: J.R., X.L., Z.R.
- Writing - review & editing: J.R., Z.J., K.J.W., L.J., K.K., K.H.S., Z.Y., Z.P., Z.T., A.D., Y.A., C.X., W.X., Z.D., Z.W., J.H., S.T., G.W., Y.S., F.Y., X.L., O.A., Z.R.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Ozols RF, Bundy BN, Greer BE, Fowler JM, Clarke-Pearson D, Burger RA, et al. Phase III trial of carboplatin and paclitaxel compared with cisplatin and paclitaxel in patients with optimally resected stage III ovarian cancer: a Gynecologic Oncology Group study. J Clin Oncol. 2003;21:3194–3200. doi: 10.1200/JCO.2003.02.153. [DOI] [PubMed] [Google Scholar]
- 3.Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103:559–564. doi: 10.1016/j.ygyno.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 4.Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25:3621–3627. doi: 10.1200/JCO.2006.10.2517. [DOI] [PubMed] [Google Scholar]
- 5.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire (GINECO) Cancer. 2009;115:1234–1244. doi: 10.1002/cncr.24149. [DOI] [PubMed] [Google Scholar]
- 6.Ren Y, Jiang R, Yin S, You C, Liu D, Cheng X, et al. Radical surgery versus standard surgery for primary cytoreduction of bulky stage IIIC and IV ovarian cancer: an observational study. BMC Cancer. 2015;15:583. doi: 10.1186/s12885-015-1525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vergote I, Tropé CG, Amant F, Kristensen GB, Ehlen T, Johnson N, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong DK, Bundy B, Wenzel L, Huang HQ, Baergen R, Lele S, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354:34–43. doi: 10.1056/NEJMoa052985. [DOI] [PubMed] [Google Scholar]
- 9.Kehoe S, Hook J, Nankivell M, Jayson GC, Kitchener H, Lopes T, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 10.Onda T, Satoh T, Saito T, Kasamatsu T, Nakanishi T, Nakamura K, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. Eur J Cancer. 2016;64:22–31. doi: 10.1016/j.ejca.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Onda T, Satoh T, Ogawa G, Saito T, Kasamatsu T, Nakanishi T, et al. Comparison of survival between primary debulking surgery and neoadjuvant chemotherapy for stage III/IV ovarian, tubal and peritoneal cancers in phase III randomised trial. Eur J Cancer. 2020;130:114–125. doi: 10.1016/j.ejca.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 12.Chi DS, Musa F, Dao F, Zivanovic O, Sonoda Y, Leitao MM, et al. An analysis of patients with bulky advanced stage ovarian, tubal, and peritoneal carcinoma treated with primary debulking surgery (PDS) during an identical time period as the randomized EORTC-NCIC trial of PDS vs neoadjuvant chemotherapy (NACT) Gynecol Oncol. 2012;124:10–14. doi: 10.1016/j.ygyno.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 13.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]