Summary
Rare diseases (RD) of genetic origin are raising public health concern contributing to a massive economic burden in India. Establishing Specialty Centers to bridge the RD community with apex centers is felt as a need in developing countries. Hence a Comprehensive Rare Disease Care (CRDC) model was set up at the department of pediatrics under Center for Human Genomics and Counseling at a medical college hospital in South India. The patients suspected to have genetic disease were evaluated as per the work flow of the designed model. The utilization statistics depict the outcome of this model. In the face of limited resources, it was possible to establish a functional RD unit with meticulous planning, supportive administration and trained interdisciplinary staff. A scalable prototype that could be replicated in other Medical colleges and Hospitals of India is described.
Keywords: Rare/genetic diseases, medical genetics, model, planning, public health, genetic counseling, management, interdisciplinary team
1. Epidemiological scenario of rare diseases in India
Rare diseases (RD) are health conditions that affect a small percentage of the population. India contains a wide array of them due to cultural and genetic diversity (1). The majority of RD are genetic in origin. Besides the large population and high birth rate, consanguinity among many communities has contributed to increased occurrence (2).
There is no universal definition or prevalence data for RD and each country has adopted a definition based on its population, healthcare system and resources (3). Hence there is no figure on their burden and associated morbidity and mortality. If the international estimate of 6% to 8% prevalence is applied to India, we must have around 72 to 96 million people affected by RDs, which would be significant number (4). The "Rare Disease Terminology & Definitions Used in Outcomes Research Working Group" has concluded the average global prevalence of 40-50 cases per 100,000 people can be called a rare disease (5).
Almost all known reported genetic disorders are found in India. Diagnosis may be difficult, due to variable presentations and modest number of clinicians experienced in handling specific RD. They are often inherited, severe, chronic, untreatable and life-threatening. A vast majority of them overwhelmingly affect children, thus contributing to the disease burden of the country (6).
2. Current status of genetic outpatient and laboratory services for diagnosis and treatment of RD in India
Medical genetic services are presently available in 13 different premier cities as a separate department or unit under the pediatrics department. They cater to an average of 20-40 patients on a daily basis. In addition, most medical geneticists are also actively involved in diagnostic, counselling and research laboratory work due to lack of well-defined roles and sufficient manpower (7). The clinical evaluation, investigations, genetic counselling and referral to patient advocacy groups are performed in isolation leading to compartmentalization of health care delivery. Hence, the patients from several strata of society are deprived of timely and adequate genetic facilities.
3. Need to establish a Comprehensive Rare Disease Care (CRDC) model for RD
Though clinical diagnosis has gained momentum, genetic outpatient and laboratory services are available only in first-tier cities across the country. Referral to these centers has led to overloading of the premier centers and depriving timely access to patients from farther regions. Patients from the rural and suburban areas of the country need to travel long distances to reach them (6). Public health policy or national program addressing these concerns is not available. The cost of treatment/ management is high and causes considerable financial burden to the individuals and their families, hindering their treatment.
To address this concern, Comprehensive Rare Disease Care (CRDC) model for handling RD attainable at tertiary care teaching hospitals was planned. This model was developed at Centre for Human Genomics and Counselling (CHGC) services for screening and diagnosis of rare diseases at JSS Medical College and Hospital, using a project management cycle framework. The experience of establishment and significant outcomes of this model are delineated in this manuscript.
4. Purpose and distinctiveness of CRDC model in comparison with established genetic services of apex centers in India
In 2015, around 40 Clinical geneticists were practicing all over India in 13 different premier cities like New Delhi, Lucknow, and Vellore etc. as a separate department or units under the pediatrics department. They are functioning as per Medical Council of India Regulations (7). Once genetic causation is suspected, most of the cases are refereed to these centers, which are obviously a strain on the meager resources. With the existing immense caseload across the nation, these centers and geneticists would be inadequate.
This CRDC model is expected to bridge this gap by attending the patients/families suspected or affected with genetic etiology in specialty departments and connect them with apex centers when necessary, cost cutting the burden associated with unnecessary visits. It was built in line with guidelines and recommendations of successful working models in other specialties (8).
5. Experience in establishing CRDC model
Since many of the genetic diseases present immediately after birth of children, Department of Pediatrics was chosen to be the work setting to initiate the CRDC model at tertiary care hospitals (9). The center utilized the existing expertise of the institute by obtaining necessary training at apex centers. Meticulous planning and designing with a clear vision of operation, interdisciplinary framework and utilization of available resources were key highlights of the model. Needs assessment aided obtaining baseline data on prevalence and pattern of suspected genetic disorders in this region. This helped to prioritize the investigations to be initiated. Visits to apex centers and discussions with expertise facilitated this stage. The multidisciplinary planning team included representation from the departments of Hospital Administration, Anatomy, Biochemistry, Pathology, Radiology, Pediatrics, Obstetrics and Gynecology. The workflow and standard operating procedure were defined by a team of experts.
Prerequisites for establishing CHGC under CRDC model:
i) Pre-Conception and Pre-Natal Diagnostic Techniques (PCPNDT) act registration: provides regulatory guidelines for genetic counseling centers, genetic laboratories, clinics, regulations for prenatal diagnostic procedures and registration guidelines. Permission decisions must be taken keeping futuristic goals in mind.
ii) Manpower: a) Essential minimum requirement: An inhouse Pediatrician trained in clinical geneticsa genetic counselor b) Ideal situation: Department of Medical genetics with clinical geneticist and genetic counselor.
iii) Consent forms: for genetic tests and procedures, usage of familial information in clinical diagnosis, permission for getting clinical photography and video recording, including for research and publication purposes (10).
iv) Documentation and Record Maintenance: The outcome of genetics consultations are not just applicable to individuals, but also families, and have potential significance for future generations as well. Preferably records should be maintained indefinitely except in certain circumstances where the information will be of minimal importance to future interaction with the patient or other members of the family (11).
Before inception, communication was sent to all clinical departments and announced through mass media to sensitize the nature and scope of work, to ensure prompt referrals. A biweekly clinic, dedicated to the detailed assessment of new cases and follow - up of the registered cases was planned. Staffing comprised of a multidisciplinary team including genetic counselor, trained pediatrician, hospital administrator, staff nurse, medico social worker. The team was reinforced with experts from laboratory and researchers working for projects in genetics. One faculty in each speciality department was assigned to address the patients requiring referrals (to narrow down the clinical diagnosis & correlation with investigations). The upgradation and strengthening of existing lab facilities in biochemistry, pathology (fetal autopsy) microbiology, speech & hearing, occupational therapy, physiotherapy, physical & rehabilitation center were done.
The workflow of the CHGC under the CRDC model is depicted in Figure 1. The consultant/Proband with suspected or confirmed genetic etiology referred from specialty departments will be evaluated by a Genetic counselor. The clinical proforma records socio-demographic details, pedigree, occupational and exposure history. Detailed birth and clinical history, examination, primary investigations, and differential diagnosis are documented. The pathway of care also records the sequence of healthcare providers accessed prior to being evaluated in the clinic and source of referral to the center. The average assessment time for a single patient during pretest counseling was 45 min. After a detailed assessment, the case will be discussed with the consultant wherever necessary to verify the differential diagnosis, discuss if further investigation is required to obtain more phenotype details. Pretest counseling will be done explaining the probable diagnosis, need for testing and its details. The necessary genetic tests will be offered in accord with the clinician. The principles of genetic counseling i.e. autonomy, non-directiveness, and confidentiality are strictly adhered to at every level. The Turn Around Time (TAT) varies from 10 days to 8 weeks, depending on the type of investigation sent.
Figure 1.
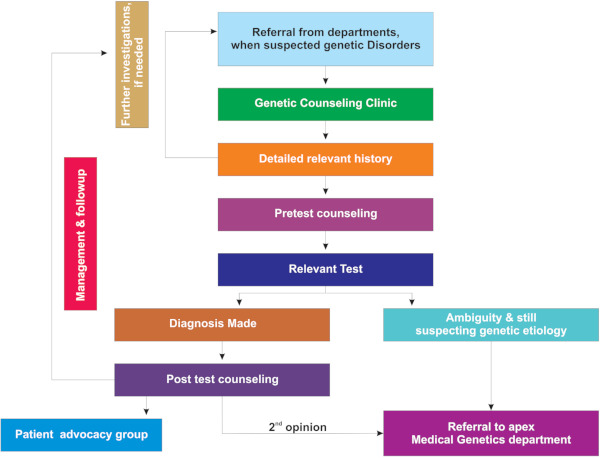
Process flow of the Center for Human Genomics and Counseling (CHGC) under Comprehensive Rare Disease Care (CRDC) model.
Once the reports are available, they are called for post-test counseling depending on the outcome. The number of sessions required varies from case to case. If the confirmatory diagnosis was established, a further management plan is drawn up and discussed. If the reports are inconclusive or ambiguous, further evaluation is suggested. Referrals are sent to apex centers wherever needed. The follow up and monitoring of confirmed cases are continued as required. The psychosocial interventions, support through advocacy groups, coping strategies, and quality of life were addressed. Extended family evaluation is done if necessary. Additionally they are directed to Patient Advocacy groups and Non- Government Organizations (ex: Organization for Rare Disease India) for assistance on moral support, research advances, clinical trials, and education with awareness.
The outcome of CHGC under the CRDC model is depicted by the case statistics in Figure 2. The patient inflow gradually picked up due to availability of accessible genetic services avoiding concerns associated with reaching apex centres.
Figure 2.
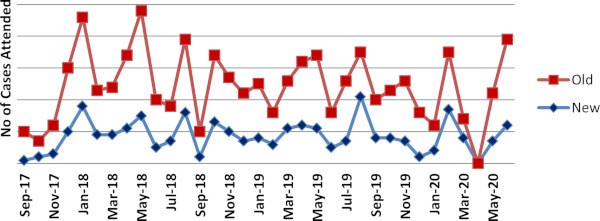
The outpatient statistics of New and Old cases at Human Genomics and Counseling (CHGC) since inception until date.
6. Challenge and perspectives
The CRDC model, though in its infancy, provides some opportunities and highlights key challenges. The center offers convenient one stop services to patients of surrounding districts with suspected or confirmed genetic causation. In the current Indian context of human resource supply constraints, it is scalable and can facilitate the development of locally acceptable and efficient models of care. Training the pediatricians to manage these patients in primary care and vertically integrate with such centers and first tier cities is advisable. This may pre-empt visits to specialists (especially lifelong follow-up and monitoring conditions) unless deemed necessary. The center provides opportunities for better training of healthcare personnel dealing with RD patients. The pediatric residents get extensive exposure to the varied clinical presentations, and management approaches. The training modules can be extended to other health professionals in the future. Last, such a dedicated establishment may yield better and diverse research opportunities focusing on medical genetics.
The challenges associated are a coordination of multiple stakeholders, space and infrastructure, cost-effective expansion, lack of national standardization, customization to local requirements, and shortage of manpower compared to case load. Finally, the education and training of health professionals to be medical geneticists/genetic counselors is a long way to accomplish adequate numbers.
Future Strategy is to establish a network and comprehensive system for coordination of referrals in the tiered healthcare system. Also a mechanism to raise awareness on RD among the medical fraternity and general public is to be planned.
To conclude, RDs are an essential public health concern and provide a challenge to healthcare worldwide. Provision of affordable services with preventive and palliative potential to several RDs should be a practical reality. This CRDC model could provide a cost-effective and geographically compatible model of care which vertically integrates apex centers and the primary health care system. This scalable prototype can be replicated in similar resource-constrained settings in a high prevalence geographical location.
Acknowledgements
To Departments of Paediatrics and Hospital Administration, JSS Medical College and Hospital, JSS Academy of Higher Education and Research for their exceptional support in this research. Ethical approval: The study was approved by the institutional Ethical committee.
References
- 1. GUaRDIAN Consortium, Sivasubbu S, Scaria V. Genomics of rare genetic diseases-experiences from India. Hum Genomics. 2019; 14:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verma IC. Burden of genetic disorders in India. Indian J Pediatr. 2000; 67:893-898. [DOI] [PubMed] [Google Scholar]
- 3. Choudhury MC, Saberwal G. The role of patient organizations in the rare disease ecosystem in India: an interview based study. Orphanet J Rare Dis. 2019; 14:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Šimerka P. Council Recommendation of 8 June 2009 on an action in the field of rare diseases (2009/C 151/02). Official Journal of the European Union. 2009; 171:7-10. [Google Scholar]
- 5. Richter T, Nestler-Parr S, Babela R, Khan ZM, Tesoro T, Molsen E, Hughes DA; International Society for Pharmacoeconomics and Outcomes Research Rare Disease Special Interest Group. Rare disease terminology and definitions-a systematic global review: report of the ISPOR Rare Disease Special Interest Group. Value Health. 2015; 18:906-914. [DOI] [PubMed] [Google Scholar]
- 6. Verma IC, Puri RD. Global burden of genetic disease and the role of genetic screening. Semin Fetal Neonatal Med. 2015; 20:354-363. [DOI] [PubMed] [Google Scholar]
- 7. Aggarwal S, Phadke SR. Medical genetics and genomic medicine in India: current status and opportunities ahead. Mol Genet Genomic Med. 2015; 3:160-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menon V, Sarkar S, Thomas S. Establishing a psychosomatic clinic in a low resource setting: Process, challenges, and opportunities. J Neurosci Rural Pract. 2016; 7:171-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar P, Radhakrishnan J, Chowdhary MA, Giampietro PF. Prevalence and patterns of presentation of genetic disorders in a pediatric emergency department. Mayo Clin Proc. 2001; 76:777-783. [DOI] [PubMed] [Google Scholar]
- 10. Mcguire A. Health Research Authority. https://www.hra.nhs.uk/planning-and-improving-research/application-summaries/research-summaries/comparing-breast-cervical-and-bowel-cancer-screening-participation (Accessed 21 November 2018). .
- 11. Shoenbill K, Fost N, Tachinardi U, Mendonca EA. Genetic data and electronic health records: A discussion of ethical, logistical and technological considerations. J Am Med Informatics Assoc. 2014; 21:171-180. [DOI] [PMC free article] [PubMed] [Google Scholar]


