Abstract
Background
Identifying the extent of environmental contamination of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is essential for infection control and prevention. The extent of environmental contamination has not been fully investigated in the context of severe coronavirus disease (COVID-19) patients.
Aim
To investigate environmental SARS-CoV-2 contamination in the isolation rooms of severe COVID-19 patients requiring mechanical ventilation or high-flow oxygen therapy.
Methods
Environmental swab samples and air samples were collected from the isolation rooms of three COVID-19 patients with severe pneumonia. Patients 1 and 2 received mechanical ventilation with a closed suction system, while patient 3 received high-flow oxygen therapy and non-invasive ventilation. Real-time reverse transcription–polymerase chain reaction (rRT–PCR) was used to detect SARS-CoV-2; viral cultures were performed for samples not negative on rRT–PCR.
Findings
Of the 48 swab samples collected in the rooms of patients 1 and 2, only samples from the outside surfaces of the endotracheal tubes tested positive for SARS-CoV-2 by rRT–PCR. However, in patient 3's room, 13 of the 28 environmental samples (fomites, fixed structures, and ventilation exit on the ceiling) showed positive results. Air samples were negative for SARS-CoV-2. Viable viruses were identified on the surface of the endotracheal tube of patient 1 and seven sites in patient 3's room.
Conclusion
Environmental contamination of SARS-CoV-2 may be a route of viral transmission. However, it might be minimized when patients receive mechanical ventilation with a closed suction system. These findings can provide evidence for guidelines for the safe use of personal protective equipment.
Keywords: COVID-19, SARS-CoV-2, Environmental contamination, Severe pneumonia
Introduction
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, which began in Wuhan, China, has become a global concern. The World Health Organization (WHO) announced the risk assessment of coronavirus disease (COVID-19) as very high at the global level, and currently, COVID-19 is labelled as a pandemic. In addition to community transmission, SARS-CoV-2 has also caused healthcare-associated outbreaks in hospitals, leading to concerns that it is transmitted not only through direct contact with droplets but also via environmental contamination or airborne transmission in specific circumstances, such as during aerosol-generating procedures (AGPs) [1]. A study on the Middle East respiratory syndrome coronavirus (MERS-CoV) reported extensive viable virus contamination of the air and environment in a MERS outbreak unit [2]. A recent study on SARS-CoV-2 also suggested the contaminated environment as a potential medium of transmission [3].
Identifying the exact extent of environmental contamination and associated potential risk of viral transmission is essential for infection prevention and control in hospitals and for the protection of healthcare workers. Whereas recent guidelines recommend the use of extended personal protective equipment (PPE), the strength of recommendation is weak, and its safety has not been fully studied [4]. Furthermore, since SARS-CoV-2 infection has clinical manifestations ranging from asymptomatic infection to acute respiratory distress syndrome (ARDS) requiring mechanical ventilation, the degree of air and environmental contamination may vary depending on the disease severity and subsequent treatment.
There are several reports on environmental contamination in the isolation rooms of COVID-19 patients [3,[5], [6], [7], [8], [9], [10]]. The patients in these studies exhibited varying disease severities, ranging from mild symptoms to severe disease requiring intensive care. Additionally, some reports showed a higher risk of environmental contamination in the intensive care unit (ICU) [7,9]. However, a study on the extent and risk factors of environmental contamination in critically ill patients who require intensive care and various AGPs is still lacking.
In this study, we investigated virus contamination by collecting environmental swab samples and air samples from negative pressure isolation rooms of patients with COVID-19 manifesting as severe pneumonia or ARDS.
Methods
Patients and rooms
Three laboratory-confirmed COVID-19 patients who required high-flow oxygen therapy or mechanical ventilation and were hospitalized in a tertiary care hospital were enrolled in this study. Environmental samples were collected from the negative-pressure isolation rooms of these patients between March 6th and 31st, 2020. The isolation rooms had 12 air changes per hour, and the average pressure gradient between the patient room and the anteroom was 2.5 hPa. All patients received intensive care in these isolation rooms without being moved to a separate ICU. Each negative pressure room had an anteroom and a restroom, but the restrooms were not being used at the time of the investigation since the enrolled patients were critically ill and could not move. Nurses performed daily routine cleaning, but disinfection was performed only after the patients were discharged. All patients were symptomatic, and their respiratory specimens persistently tested positive for SARS-CoV-2 by real-time reverse transcription polymerase chain reaction (rRT–PCR) up to the time of environmental sampling. Clinical and microbiological data of the patients were also obtained from the medical records.
Sample collection
SKC BioSampler (225–9595, SKC, Inc., Covington, GA, USA) and Swab sampler were used for sampling the air in each patient's negative pressure isolation room. The SKC BioSampler captures bioaerosols in 20 mL of phosphate-buffered saline (PBS) by an inertia impaction mechanism [[11], [12], [13]]. The collection efficiency of the SKC BioSampler for 100 nm sized particles, which is the known size of SARS-CoV-2, has been reported as 30–40% [14]. The Swab sampler, a useful air sampler for capturing airborne viruses, uses a cotton swab that acts as a filter for capturing airborne particles with 99% efficacy for airborne viruses, previously reported [15]. Air sampling was carried out at 1.2 m above floor level and at a distance of 1.0 m from the patient in the negative pressure rooms. Air samplers were operated for 20 min with airflow rates of 12.5 and 10 L/min for the SKC BioSampler and Swab sampler, respectively. The airborne particles collected on the cotton swab were recovered by vortexing the cotton part of the swab in 1 mL of PBS. The samples were stored at −80°C shortly after air sampling till further analysis.
Environmental surface samples from the patients' isolation rooms were obtained using sterile swabs, which were premoistened with a viral transport medium. All the rooms had the same size, structure, and facilities. Bedside tables, blood pressure cuffs, pillows, bedsheets, nasal prongs, outside surface of the ventilator circuit, tubing, masks, telephones, thermometers, keyboards, and fixed structures in the room (such as the doorknob, bed rails, floor, walls, window, and faucet handles), and grills of the ventilation exits in the ceiling were swabbed. All the environmental swabs were obtained on the same day as the air sampling procedure in each patient's room.
Laboratory procedure
Environmental samples were tested with specific rRT–PCR methods using PowerCheck 2019-nCoV (Kogene Biotech Inc. Seoul, South Korea) which targets the SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) and E genes [16]. Quantification and trend of the SARS-CoV-2 load were estimated by the value of the cycle threshold (C T) of rRT–PCR. A positive test result was defined as C T ≤35 for the RdRp and E genes. Viral culture was performed with samples that were not negative on rRT–PCR to identify the viable virus. Confluent monolayers of Vero E6 cells in 96-well plates were infected by 10-fold dilutions of the SARS-CoV-2 supernatants from the environmental samples. The inoculated cultures were grown in a humidified 37°C incubator with 5% CO2. After 72 h, areas of cell clearance with Crystal Violet staining were used to demonstrate the cytopathic effect. When the cytopathic effect was observed, detection of SARS-CoV-2 nucleic acid by rRT–PCR in the supernatant was performed to confirm a successful culture.
Ethics statements
This study was conducted in compliance with the Institutional Review Board of Severance Hospital (4-2020-0076) regarding the collection of environmental samples from the patients' rooms and the clinical data. Written informed consent was obtained from the participants. All the laboratory experiments were conducted in biosafety level 3 facilities in Severance Hospital permitted by the Korea Center for Disease Control and Prevention.
Results
Clinical and microbiological characteristics of the patients
Sample time-points in relation to the patients' clinical courses and C T values of rRT–PCR are summarized in Table I . Patient 1 was a 71-year-old man who presented with severe pneumonia. He was started on mechanical ventilation on hospital admission, 15 days after the onset of symptoms. Environmental sampling was undertaken on hospital day (HD) 7 when he was febrile with poor oxygenation, and on that day, chest imaging demonstrated severe ARDS. Using the patient's lower respiratory tract specimen, C T value was 23.28 for E gene PCR and 24.98 for RdRp gene PCR on that day. He was receiving regular and frequent endotracheal suctioning through a closed suction system connected to the ventilator.
Table I.
Sample time-points in relation to the patients' clinical course and clinical CT values
| Case | Contents | Hospital day |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 7 | 10 | 13 | 14 | |||
| 1 | CT value of PCR using sputum samples | E gene | 20.24 | 24.05 | 26.93 | 23.28 | 23.26 | 27.49 | |||
| RdRp | 22.31 | 25.73 | 28.86 | 24.98 | 25.05 | 29.65 | |||||
| Environmental sampling | Air/surface | Done | |||||||||
| Treatment | Mechanical ventilation | + | + | + | + | + | + | + | + | + | |
| Closed circuit suction | + | + | + | + | + | + | + | + | + | ||
| Symptoms | Sputum | + | + | + | + | + | + | + | + | + | |
| 2 | CT value of PCR using sputum samples | E gene | 22.53 | 18.18 | 23.99 | 34.35 | 27.96 | 40 | |||
| RdRp | 24.35 | 20.51 | 26.19 | 36.33 | 30.49 | 40 | |||||
| Environmental sampling | Air/surface | Done | |||||||||
| Treatment | Mechanical ventilation | – | + | + | + | + | + | + | – | – | |
| Closed circuit suction | – | + | + | + | + | + | + | – | – | ||
| Prone positioning | – | + | + | + | – | – | – | – | – | ||
| Symptoms | Sputum | + | + | + | + | + | + | + | – | – | |
| 3 | CT value of PCR using sputum samples | E gene | 13.32 | 13.97 | 20.11 | 16.53 | 24.70 | ||||
| RdRp | 15.76 | 16.32 | 22.29 | 15.60 | 24.72 | ||||||
| Environmental sampling | Air/surface | Done | |||||||||
| Treatment | HFNC | – | – | + | + | + | + | + | + | + | |
| Non-invasive ventilation | – | – | – | – | + | + | + | + | + | ||
| Manual ventilation | – | – | – | – | – | – | + | – | – | ||
| Symptoms | Cough, sputum | + | + | + | + | + | + | + | + | + | |
CT, cycle threshold; PCR, polymerase chain reaction; RdRp, RNA-dependent RNA polymerase; HFNC, high-flow oxygen therapy via nasal cannula; +, either a mentioned symptom existed or the mentioned treatment was performed; –, either a mentioned symptom did not exist or the mentioned treatment was not performed.
Patient 2 was a 67-year-old woman with rapidly progressing pneumonia who was started on mechanical ventilation on HD 2, five days after the onset of symptoms. Environmental sampling was performed on HD 4 when she had a sustained fever with rapid deterioration to severe ARDS. Using the patient's lower respiratory tract specimens, the C T values were as follows: 18.18 for E gene PCR and 20.51 for RdRp PCR on HD 3; and 23.99 for E gene PCR and 26.19 for RdRp PCR on HD 5. Prone positioning in accordance with the ARDS management guidelines was followed from HD 2 to 4, and regular and frequent endotracheal suction was performed through a closed suction system.
Patient 3 was a 44-year-old man with underlying terminal lung cancer and progressive pneumonia caused by SARS-CoV-2. He was hospitalized two days after the start of symptoms. Since he had given advance directives not to be administered mechanical ventilation through intubation, high-flow oxygen therapy via nasal cannula (HFNC) at 60 L/min was started from HD 3 (five days from the onset of symptoms). Because of weak breathing during sleep, he received non-invasive ventilation (NIV) using a facial mask at night, starting from HD 5. Environmental sampling was done on HD 13 when he had a persistent cough with sputum and shortness of breath and spat out sputum frequently. Using the patient's lower respiratory tract specimens, the C T values were as follows: 16.53 for E gene PCR and 15.32 for RdRp PCR on HD 10; and 24.70 for E gene PCR and 24.72 for RdRp PCR on HD 14. He could not move out of bed due to oxygen therapy; however, he was alert and using bedside tables, cups, telephone, and remote control for television. There was an event during which manual ventilation was performed once daily for 20 min between HD 10 and 12 because of loss of consciousness caused by sudden hypoxaemia as the nasal prong had fallen out from his nose.
Environmental contamination of the patient rooms
A summary of the environmental test results from the three patients' isolation rooms is given in Table II . Of the total 48 swab samples collected from the isolation rooms of patients 1 and 2, only the outside surface of the endotracheal tubes in the area connected to the ventilator circuit tested positive for SARS-CoV-2 by rRT–PCR in both cases. SARS-CoV-2 was not detected by rRT–PCR in other samples obtained from fomites, fixed structures, and ventilation exits in these rooms. C T values of positive samples were as follows: room of patient 1, 30.95 for E gene PCR and 31.36 for RdRp gene PCR; and room of patient 2, 32.33 for E gene PCR and 33.02 for RdRp gene PCR. Air samples were negative in both cases. Viable viruses were detected by viral culture on the outside surface of the endotracheal tube of patient 1.
Table II.
List of air and environmental swab samples and corresponding rRT–PCR and culture results for SARS-CoV-2
| Sample | Patient 1 |
Patient 2 |
Patient 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PCR |
CT value |
Culture | PCR |
CT value |
Culture | PCR |
CT value |
Culture | ||||
| E gene | RdRp | E gene | RdRp | E gene | RdRp | |||||||
| Air | – | ND | – | ND | – | ND | ||||||
| Air outlet fan | – | ND | – | ND | + | 33.93 | 34.99 | – | ||||
| Air inlet fan | – | ND | – | ND | – | ND | ||||||
| Nasal prong/endotracheal tube | + | 30.95 | 31.36 | + | + | 32.33 | 33.02 | – | + | 31.78 | 34.28 | + |
| Intravenous pole | – | ND | – | ND | – | ND | ||||||
| Computer | – | ND | – | ND | – | ND | ||||||
| Medication cart | – | ND | – | ND | – | ND | ||||||
| Window | – | ND | – | ND | U | U | U | – | ||||
| Window frame | – | ND | – | ND | – | 34.23 | 36.04 | – | ||||
| Blind curtain | – | ND | – | ND | – | ND | ||||||
| Wall 1 | – | ND | – | ND | – | ND | ||||||
| Wall 2 | – | ND | – | ND | – | ND | ||||||
| Floor near the patienta | – | ND | – | ND | + | 30.38 | 33.07 | + | ||||
| Floor far from the patientb | – | ND | – | ND | + | 31.97 | 34.28 | – | ||||
| Bed rails | – | ND | – | ND | + | 30.22 | 30.13 | + | ||||
| Bedsheet | – | ND | – | ND | + | 31.54 | 31.99 | + | ||||
| Pillows | – | ND | – | ND | ND | ND | ||||||
| Faucet handle | – | ND | – | ND | ND | ND | ||||||
| Door knob | – | ND | – | ND | – | ND | ||||||
| Call button | – | ND | – | ND | – | ND | ||||||
| Restraint | – | ND | – | ND | + | 34.08 | 35.18 | – | ||||
| Blood pressure cuff | – | ND | – | ND | – | ND | ||||||
| Ambu mask/NIV mask | – | ND | – | ND | + | 28.85 | 28.94 | + | ||||
| Ventilator | – | ND | – | ND | – | ND | ||||||
| Patient monitor | – | ND | – | ND | – | ND | ||||||
| Bedside table | ND | ND | ND | ND | U | 33.09 | U | + | ||||
| High-flow oxygen generator | ND | ND | ND | ND | + | 30.56 | 33.12 | – | ||||
| Telephone | ND | ND | ND | ND | + | 31.39 | 33.42 | – | ||||
| Remote controller | ND | ND | ND | ND | + | 29.48 | 29.66 | + | ||||
| Thermometer | ND | ND | ND | ND | + | 31.56 | 32.13 | – | ||||
| Cup | ND | ND | ND | ND | + | 32.32 | 33.55 | – | ||||
rRT–PCR, reverse transcription polymerase chain reaction; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; PCR, polymerase chain reaction; CT, cycle threshold; RdRp, RNA-dependent RNA polymerase; ND, not done; U; undetermined; NIV, non-invasive ventilator; –, negative; +, positive.
The floor within 1 m from the patient.
The floor at a distance of >2 m from the patient.
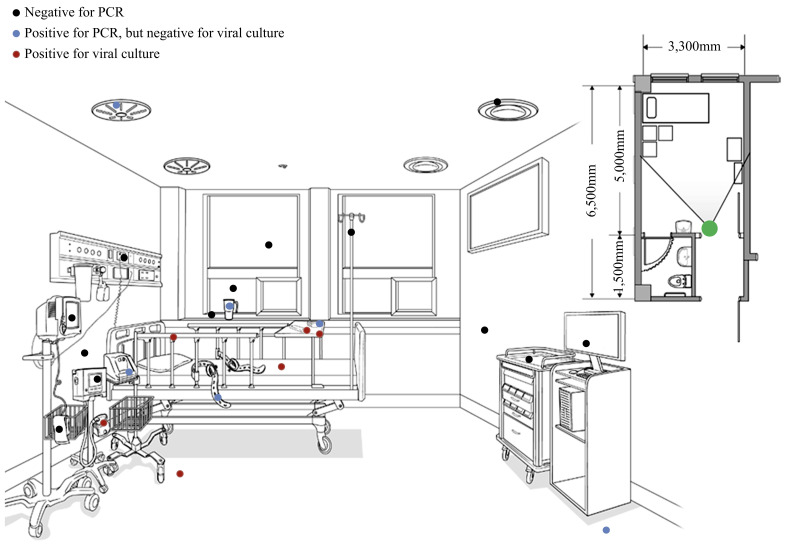
Twenty-eight swab samples were obtained from the isolation room of patient 3. Of these, 13 samples tested positive on rRT–PCR obtained from a thermometer, restraints, bedsheets, cup, nasal prongs, NIV mask, high-flow oxygen generator, telephone, remote control, and fixed structures including bed rails, floor, and the grill of an air outlet fan in the ceiling. The result of rRT–PCR for the air sample was negative. C T values of all PCR-positive samples from the room of patient 3 were >30, except for those from an NIV mask (28.85 for E gene PCR, 28.94 for RdRp PCR) and a remote controller (29.48 for E gene PCR, 29.66 for RdRp PCR). Viable viruses were detected in samples from a nasal prong, bedside table, floor near the patient, remote control, bed rails, bedsheets, and NIV mask. The results of, and locations from which, the environmental samples were taken in the room of patient 3 are shown in Figure 1 .
Figure 1.
Results of, and locations from which, the environmental samples were collected in the room of patient 3. Twenty-eight environmental swab samples were obtained from patient 3's room. Nasal prongs of high-flow oxygen generator, door knob, and telephone are not shown. Swabs from the following tested negative on polymerase chain reaction (PCR): air inlet fan, intravenous pole, computer, medication cart, blind curtain, wall 1, wall 2, window, window frame, door knob, call button, blood pressure cuff, ventilator, and patient monitor. Swabs from the following tested positive on PCR but negative on viral culture: air outlet fan, floor far from the patient, restraint, high-flow oxygen generator, telephone, thermometer, and cup. Swabs from the following tested positive on culture: nasal prong, floor near the patient, bed rails, bedsheets, non-invasive ventilator masks, bedside table, and remote controller. The solid lines radiating from the large green circle indicate the angle of observation used for illustration of the patient's room.
Discussion
The main finding of this study was the increased extent of environmental contamination of SARS-CoV-2 in the room of a patient receiving HFNC or NIV compared to that in the rooms of patients receiving mechanical ventilation with a closed suction system. Further, we found viable viruses on the contaminated surfaces. To the best of our knowledge, this is the first report on detection of viable viruses on environmental surfaces, providing evidence that indirect transmission via environmental contamination is possible.
The primary mechanism of SARS-CoV-2 transmission involves droplet spread [1]. International guidelines warn that airborne transmission of SARS-CoV-2 is possible, predominantly when AGPs are performed [1,4]. Indirect spread might occur when surfaces contaminated with viable viruses are touched, usually by hands, followed by contact with mucosal surfaces, although it is not clear if this happens with SARS-CoV-2 [17].
Recent studies on environmental contamination of SARS-CoV-2 reported the presence of SARS-CoV-2 nucleic acids on various surfaces or fomites from patients' isolation rooms [3,[5], [6], [7], [8], [9],18]. Some of these studies also suggested the possibility of airborne transmission due to detection of viral RNA in air samples. However, none of the studies could detect viable viruses in any sample, which might be important to substantiate the presence of a transmissible virus in the environment [2,17]. In our study, not only viral RNA but also viable SARS-CoV-2 was isolated by viral culture from samples from the patients' isolation rooms.
Among the PCR-positive samples from patient 3's room, viable viruses were detected only on surfaces within a distance at which the virus could be transmitted by droplets while the patient remained symptomatic. Although the culture was negative, viral RNA was also detected on the floor at a distance of >2 m from the patient, where direct droplet contamination was unlikely. This indicates the possibility of secondary contamination by PPE of healthcare workers or by aerosols in specific situations. Guo et al. showed the high risk of virus contamination on the floors of isolation rooms and shoe soles of PPE [7]. In this context, secondary contamination via shoe soles of PPE from the floor near the patient to the distant floor may have occurred in patient 3's room. Viral RNA detected in the air outlet fan on the ceiling cannot be ascribed to direct droplet contamination or secondary contamination by healthcare workers. Rather, it raises the possibility of airborne contamination by the aerosols generated.
SARS-CoV-2 is stable in the aerosolized state, and previous studies have detected viral RNA in air samples [5,7,18,19]. International guidelines have specified AGPs as suctioning, intubation, bronchoscopy, cardiopulmonary resuscitation, prone positioning, manual ventilation, and so on, and recommended the wearing of PPE including N95 masks or equivalent or higher level of respiratory protection for airborne transmission due to AGPs [4,20]. In this study, NIV was used intermittently, and manual ventilation using an Ambu-bag was performed in the isolation room of patient 3. Both procedures are listed as AGPs. Therefore, it is possible that contamination of distant environmental surfaces was caused by viral particles emitted in the air during AGPs. Moreover, patient 3 was treated with HFNC. Guidelines differ regarding HFNC use in COVID-19, with an evidence gap pertaining to the probability of aerosol dispersion and the associated infection risk for healthcare workers. WHO does not consider HFNC as an AGP, although some studies have mentioned that HFNC can lead to the emission of a concentrated jet of aerosol, which can spread considerably. Since HFNC has no sealed portion or filter, it can generate turbulence of the droplets emitted from the oropharynx due to the high flow rate [[21], [22], [23]]. However, it should be noted that recent studies have suggested that symptoms such as coughing and sneezing have a substantial effect on aerosol generation even without AGPs [22,24]. Patient 3 had persistent and active symptoms of severe cough and produced a large amount of sputum. Consequently, it might be possible that these symptoms, along with AGPs and the application of HFNC, caused aerosol production and contaminated distant environmental surfaces. This finding supports the current recommendation of PPE use in the presence of patients with active symptoms or during AGPs.
Despite the possibility of airborne transmission, all air samples tested negative for SARS-CoV-2 in the current study. In two studies reporting on the presence SARS-CoV-2 in the air, the authors collected 5040 and 9000 L of air from each site. Chia et al. have suggested that the presence of SARS-CoV-2 in the air may be highest during the first week of illness [5]. Additionally, Guo et al. showed that the rate of positivity was the highest near the air outlets, indicating that the concentration of virus-laden aerosols differed by the air sampling site [7]. In our study, only 200–250 L of air was collected during a period of 20 min from each isolation room, and the sampling site in this study was not near the air outlet. Besides, air sampling was performed in patient 3's room on the 15th day after the onset of symptoms. These reasons could have contributed to the absence of SARS-COV-2 in the air in our study.
In patients 1 and 2, only the outside surface of the endotracheal tube was positive for SARS-CoV-2 despite severe pneumonia, and the C T values were similar to those of clinical samples from patient 3. The surface of the endotracheal tube was likely contaminated by oropharyngeal secretions. However, no evidence of other adjacent or distant environmental contamination or evidence of airborne transmission was found. The varying degree of environmental contamination among these patients could be explained by the difference in the treatment or procedures performed. Patients 1 and 2 were started on mechanical ventilation through endotracheal intubation, and a closed suction system was applied from HD 1 and 2, respectively, whereas patient 3 underwent HFNC, NIV, and manual ventilation. A plausible explanation for this difference in environmental contamination and the presence of viable viruses is the difference in treatment. Previous studies have demonstrated that a closed suction system reduced environmental contamination by respiratory pathogens. In patients intubated using a closed suction system, cross-contamination by other bacteria and glove contamination of healthcare workers were significantly reduced [25]. Symptoms can be minimized by sedation, and respiration can be limited within the ventilator circuit. The closed system is maintained even during suction, which can generate aerosols, and is also recommended in the airway management guidelines for COVID-19 patients [26]. Current guidelines for critically ill patients with COVID-19 propose surgical or medical masks for respiratory protection during the management of patients on mechanical ventilation using a closed suction system if there are no additional aerosol-generating events [4]. The results of our study support this recommendation.
There are several limitations of this study. First, since a small number of patients were included in this study, the results should be cautiously interpreted. Second, the time-point of environmental and air sampling was far from the time of symptom onset; therefore, we could not investigate the environmental contamination during the acute phase infection. However, the patients were all symptomatic with severe pneumonia, and the C T values of their clinical samples were similar to those of the samples from patients in the study by Chia et al. [5]. Third, we performed the sampling of each room at a single time-point during the disease. Therefore, we did not track the degree of environmental contamination longitudinally with the changes in the treatment. Fourth, since the duration of air sampling was short, and no AGPs were performed at the time of air sampling, no evidence for airborne transmission was found in this study.
Despite these limitations, we isolated viable SARS-CoV-2 from environmental samples; this heightens concerns regarding transmission from environmental surfaces and aerosolization during NIV and HFNC. Furthermore, this is the first report to show that the degree of environmental contamination may be variable depending on the clinical procedures and treatment, regardless of similar disease severity and viral load. These findings may contribute to the establishment of guidelines for proper PPE use during the management of patients with COVID-19 in the context of the pandemic and shortage of medical resources.
Acknowledgements
The authors thank Medical Illustration & Design, part of the Medical Research Support Services of Yonsei University College of Medicine, for all artistic support related to this work.
Conflicts of interest statement
None declared.
Funding sources
This work was supported by the Research Program funded by the Korea Centers for Disease Control and Prevention (2019-ER5408-00), research grants for deriving the major clinical and epidemiological indicators of people with HIV (Korea HIV/AIDS Cohort Study, 2019-ER5101-00), and a grant from the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1324). D.S. was supported by the John and Mary Tu Foundation.
References
- 1.World Health Organization . WHO; Geneva: Mar 29th, 2020. Modes of transmission of virus causing COVID-19: implications for IPC precaution recommendations. [Google Scholar]
- 2.Kim S.H., Chang S.Y., Sung M., Park J.H., Bin Kim H., Lee H. Extensive viable Middle East Respiratory Syndrome (MERS) coronavirus contamination in air and surrounding environment in MERS isolation wards. Clin Infect Dis. 2016;63:363–369. doi: 10.1093/cid/ciw239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ong S.W.X., Tan Y.K., Chia P.Y., Lee T.H., Ng O.T., Wong M.S.Y. Air, surface environmental, and personal protective equipment contamination by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from a symptomatic patient. JAMA. 2020;323:1610–1612. doi: 10.1001/jama.2020.3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alhazzani W., Møller M.H., Arabi Y.M., Loeb M., Gong M.N., Fan E. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chia P.Y., Coleman K.K., Tan Y.K., Ong S.W.X., Gum M., Lau S.K. Detection of air and surface contamination by SARS-CoV-2 in hospital rooms of infected patients. Nat Commun. 2020;11:2800. doi: 10.1038/s41467-020-16670-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye G., Lin H., Chen S., Wang S., Zeng Z., Wang W. Environmental contamination of SARS-CoV-2 in healthcare premises. J Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo Z.D., Wang Z.Y., Zhang S.F., Li X., Li L., Li C. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583–1591. doi: 10.3201/eid2607.200885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colaneri M., Seminari E., Novati S., Asperges E., Biscarini S., Piralla A. Severe acute respiratory syndrome coronavirus 2 RNA contamination of inanimate surfaces and virus viability in a health care emergency unit. Clin Microbiol Infect. 2020;26 doi: 10.1016/j.cmi.2020.05.009. 1094.e1–1094.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu S., Wang Y., Jin X., Tian J., Liu J., Mao Y. Environmental contamination by SARS-CoV-2 in a designated hospital for coronavirus disease 2019. Am J Infect Control. 2020;48:910–914. doi: 10.1016/j.ajic.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J., Feng H., Zhang S., Ni Z., Ni L., Chen Y. SARS-CoV-2 RNA detection of hospital isolation wards hygiene monitoring during the Coronavirus Disease 2019 outbreak in a Chinese hospital. Int J Infect Dis. 2020;94:103–106. doi: 10.1016/j.ijid.2020.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen T.T., Poh M.K., Low J., Kalimuddin S., Thoon K.C., Ng W.C. Bioaerosol sampling in clinical settings: a promising, noninvasive approach for detecting respiratory viruses. Open Forum Infect Dis. 2017;4 doi: 10.1093/ofid/ofw259. ofw259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan M., Bonny T.S., Loeb J., Jiang X., Lednicky J.A., Eiguren-Fernandez A. Collection of viable aerosolized influenza virus and other respiratory viruses in a student health care center through water-based condensation growth. mSphere. 2017;2 doi: 10.1128/mSphere.00251-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Neil C.A., Li J., Leavey A., Wang Y., Hink M., Wallace M. Characterization of aerosols generated during patient care activities. Clin Infect Dis. 2017;65:1335–1341. doi: 10.1093/cid/cix535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J., Leavey A., Wang Y., O’Neil C., Wallace M.A., Burnham C.D. Comparing the performance of 3 bioaerosol samplers for influenza virus. J Aerosol Sci. 2018;115:133–145. doi: 10.1016/j.jaerosci.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H.R., An S., Hwang J., Park J.H., Byeon J.H. In situ lysis droplet supply to efficiently extract ATP from dust particles for near-real-time bioaerosol monitoring. J Hazard Mater. 2019;369:684–690. doi: 10.1016/j.jhazmat.2019.02.088. [DOI] [PubMed] [Google Scholar]
- 16.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT–PCR. Euro Surveill. 2020:25. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otter J.A., Donskey C., Yezli S., Douthwaite S., Goldenberg S.D., Weber D.J. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235–250. doi: 10.1016/j.jhin.2015.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santarpia J.L., Rivera D.N., Herrera V., Morwitzer M.J., Creager H., Santarpia G.W. Transmission potential of SARS-CoV-2 in viral shedding observed at the University of Nebraska Medical Center. medRxiv. 2020 Mar 26 [Google Scholar]
- 19.van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . WHO; Geneva: 2020. Rational use of personal protective equipment for coronavirus disease (COVID-19) and considerations during severe shortages.https://www.who.int/publications/i/item/rational-use-of-personal-protective-equipment-for-coronavirus-disease-(covid-19)-and-considerations-during-severe-shortages Available at: [last accessed August 2020] [Google Scholar]
- 21.Lyons C., Callaghan M. The use of high-flow nasal oxygen in COVID-19. Anaesthesia. 2020;75:843–847. doi: 10.1111/anae.15073. [DOI] [PubMed] [Google Scholar]
- 22.Wilson N.M., Norton A., Young F.P., Collins D.W. Airborne transmission of severe acute respiratory syndrome coronavirus-2 to healthcare workers: a narrative review. Anaesthesia. 2020;75:1086–1095. doi: 10.1111/anae.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tran K., Cimon K., Severn M., Pessoa-Silva C.L., Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One. 2012;7 doi: 10.1371/journal.pone.0035797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brown J., Pope C. Personal protective equipment and possible routes of airborne spread during the COVID-19 pandemic. Anaesthesia. 2020;75:1116–1117. doi: 10.1111/anae.15097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ricard J.D., Eveillard M., Martin Y., Barnaud G., Branger C., Dreyfuss D. Influence of tracheal suctioning systems on health care workers’ gloves and equipment contamination: a comparison of closed and open systems. Am J Infect Control. 2011;39:605–607. doi: 10.1016/j.ajic.2010.10.031. [DOI] [PubMed] [Google Scholar]
- 26.Cook T.M., El-Boghdadly K., McGuire B., McNarry A.F., Patel A., Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia. 2020;75:785–799. doi: 10.1111/anae.15054. [DOI] [PMC free article] [PubMed] [Google Scholar]