Abstract
The highly polyphagous and invasive brown marmorated stink bug, Halyomorphahalys (Stål) (Hemiptera: Pentatomidae), has become a significant insect pest in North America since its detection in 1996. It was first documented in northern Utah in 2012 and reports of urban nuisance problems and plant damage have since increased. Biological control is the preferred solution to managing H.halys in North America and other invaded regions due to its alignment with integrated pest management and sustainable practices. Native and non-native biological control agents, namely parasitoid wasps, have been assessed for efficacy. Trissolcusjaponicus (Ashmead) (Hymenoptera: Scelionidae) is an effective egg parasitoid of H.halys in its native range of southeast Asia and has recently been documented parasitising H.halys eggs in North America and Europe. Field surveys for native and exotic egg parasitoids using wild (in situ) and lab-reared H.halys egg masses were conducted in suburban and agricultural sites in northern Utah from June to September 2017–2019. Seven native wasp species in the families Eupelmidae and Scelionidae were discovered guarding H.halys eggs and adult wasps from five of these species completed emergence. Native species had low mean rates of adult emergence from wild (0.5–3.7%) and lab-reared (0–0.4%) egg masses. In 2019, an adventive population of T.japonicus was discovered for the first time in Utah, emerging from 21 of the 106 wild H.halys egg masses found that year, and none from lab-reared eggs. All T.japonicus emerged from egg masses collected on Catalpaspeciosa (Warder). Our results support other studies that have observed biological control of H.halys from T.japonicus and improved parasitoid wasp detection with wild as compared to lab-reared H.halys egg masses.
Keywords: parasitoid wasp, stink bug, egg mass, biological control
Introduction
The brown marmorated stink bug, Halyomorphahalys (Stål) (Hemiptera: Pentatomidae), is a severe agricultural and urban nuisance pest that originates from southeast Asia (Hoebeke and Carter 2003) and has invaded numerous countries worldwide (Cesari et al. 2015, Gariepy et al. 2014, Haye et al. 2015b, Macavei et al. 2015, Milonas and Partsinevelos 2014, Vétek et al. 2014). As of 2020, it has been detected in 46 U.S. States and four Canadian Provinces, with 11 States reporting severe agricultural damage (StopBMSB.org). Halyomorphahalys was first detected in Utah in 2012 and has been considered a pest to fruit and vegetable crops since 2017. With the threat of increasing economic agricultural damage, development of proactive management tactics is imperative. In the U.S. Mid-Atlantic region, where H.halys has been a severe pest, effective control has relied on broad spectrum insecticides, leading to increased application frequency and disruption of integrated pest managment, including secondary pest outbreak (Lee et al. 2014, Leskey et al. 2014, Leskey et al. 2012a, Leskey et al. 2012b). Physical or cultural control (e.g. trap cropping and mass trapping) can offer some mitigation of plant damage, but may not be economically viable (Mathews et al. 2017). The most effective management tactics have paired cultural and chemical tactics (e.g. orchard perimeter insecticide applications and treatment of trap trees) (Blaauw et al. 2014).
Biological control by egg parasitoids has proven effective in suppressing H.halys populations in its native range (Yang et al. 2009, Zhang et al. 2017). Halyomorphahalys sentinel egg mass surveys in North America have identified parasitism by native parasitoids in the families Scelionidae, Encyrtidae and Eupelmidae (Abram et al. 2017, Cornelius et al. 2016a, Cornelius et al. 2016b, Balusu et al. 2019, Talamas et al. 2015a, Talamas et al. 2015b). However, parasitism rates are low, likely due to inability of native species to overcome healthy H.halys egg defences (Abram et al. 2017, Abram et al. 2014, Dieckhoff et al. 2017,Herlihy et al. 2016). Measuring native parasitoid effectiveness against H.halys eggs solely by wasp emergence may underestimate their impact, as partial development of a native wasp inside H.halys eggs can cause egg mortality (Abram et al. 2019a, Abram et al. 2014, Cornelius et al. 2016a, Schumm 2020). Therefore, evaluating native wasp parasitism rates, especially in novel landscapes where new behaviour or species may be observed, deserves critical analysis.
Trissolcusjaponicus (Ashmead) (Hymenoptera: Scelionidae) is an egg parasitoid native to the home range of H.halys (Yang et al. 2009). Adventive T.japonicus have been discovered emerging from H.halys egg masses in North America (Milnes et al. 2016, Talamas et al. 2015b) and Europe (Stahl et al. 2019, Sabbatini Peverieri et al. 2018). Research has assessed the effectiveness of these adventive populations against H.halys and a recent study in Washington State revealed parasitoid emergence rates reaching 77% (Milnes and Beers 2019). Conversely, initial parasitism of H.halys eggs by T.japonicus in Europe has been as low as 2%. (Stahl et al. 2019).
Though adult wasp emergence has been documented on eggs of some native pentatomidae species, Trissolcusjaponicus has shown superior adult emergence rates on H.halys eggs (Milnes and Beers 2019). Laboratory paired-host tests demonstrated significantly higher T.japonicus parasitism rates of H.halys over other stink bug species. However, no-choice tests documented T.japonicus readily parasitising Banasadimidiata (Say) and Holcostethusabbreviatus Uhler (Hedstrom et al. 2017). Recent field tests in the Pacific Northwest found significantly lower T.japonicus parasitism rates of native stink bug egg masses (0.4–8%) compared to H.halys (77%) (Milnes and Beers 2019). These findings suggest that, although non-target effects occur, natural settings may support more targeted control of H.halys by T.japonicus.
The primary objective of this study was to utilise H.halys egg mass surveys to identify potential parasitoid species for suppression of this invasive insect pest in northern Utah. Northern Utah provides novel geographic and environmental conditions for detection of H.halys parasitoids, most notably high elevation (>1200 m) and arid sites with a hot summer and cold winter climate (Utah Climate Center 2020), as compared to other regions where H.halys occurs (Herlihy et al. 2016, Jarrett et al. 2019, Milnes et al. 2016, Stahl et al. 2019). Secondly, we compared parasitism rates of wild (in situ) versus lab-reared egg masses to better understand effective survey approaches and projection of natural parasitism rates in the field (Abram et al. 2017).
Materials and Methods
Survey Sites
Surveys for native and exotic parasitoid wasps of H.halys eggs in northern Utah were conducted from June through September in each of 2017, 2018 and 2019. The surveys included a total of 17 field sites. Sites 1, 4, 5, 8 and 10–17 were located in suburban landscapes containing mixed woody ornamental trees and shrubs. Sites 2, 3, 6, 7 and 9 were in conventionally-managed agricultural row crops and orchards (Fig. 1). Survey sites were chosen, based on areas of established H.halys populations and preferred host plant availability (Tables 1, 2).
Figure 1.
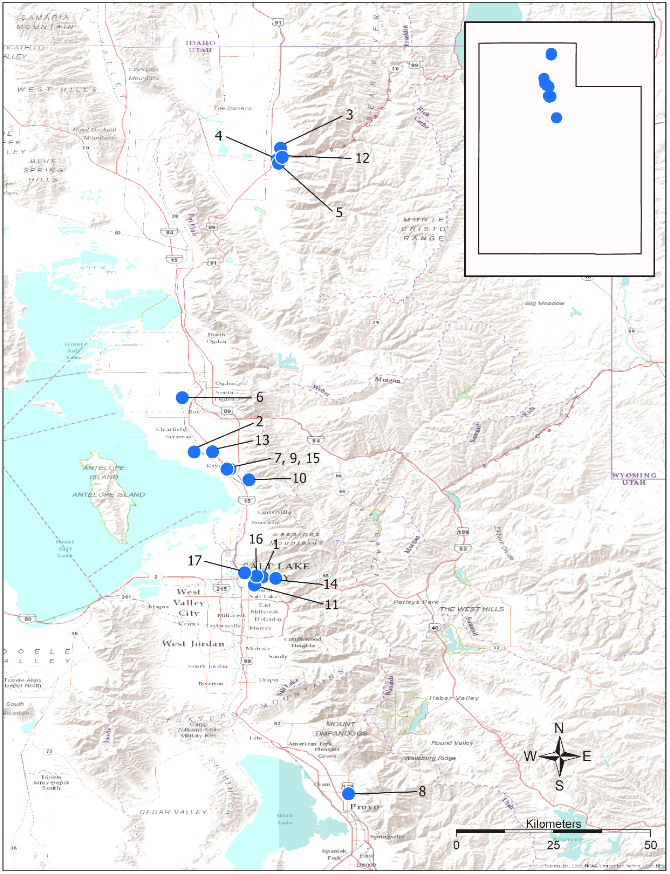
Blue dots indicate deployment and collection sites of lab-reared and wild egg masses in northern Utah, 2017-2019. Trissolcusjaponicus was discovered at Sites 1 and 17. Geographical coordinates are as follows: Site 1: 40°46'14.8"N, 111°51'18.6"W; Site 2: 41°3'36.899"N, 112°0'46.944"W; Site 3: 41°45'48.39"N, 111°48'46.148"W; Site 4: 41°44'11.685"N, 111°49'14.446"W; Site 5: 41°43'41.968"N, 111°49'4.005"W; Site 6: 41°11'9.164"N, 112°2'25.368"W; Site 7: 41°1'18.787"N, 111°55'59.663"W; Site 8 40°16'07.6"N, 111°39'20.7"W; Site 9: 41°01'12.2"N, 111°55'49.4"W; Site 10: 40°59'44.2"N, 111°53'09.8"W; Site 11: 40°45'08.2"N, 111°52'25.2"W; Site 12: 41°44'32.9"N, 111°48'32.8"W; Site 13: 41°03'37.0"N, 111°58'13.8"W; Site 14: 40°46'03.3"N, 111°49'27.5"W; Site 15: 41°01'13.1"N, 111°56'12.9"W; Site 16: 40°46'23.0"N, 111°52'07.3"W; and Site 17: 40°46'49.3"N, 111°53'46.5"W.
Table 1.
Number of deployed and parasitised fresh lab-reared H.halys egg masses by native wasps on multiple plant species in northern Utah from June through September, 2017 – 2019. Parasitism denotes adult wasp emergence.
| Plant Species | Total Egg Masses (Eggs) | Parasitised Egg Masses (Eggs) |
| Acernegundo | 13 (311) | 0 (0) |
| Ailanthusaltissima | 7 (187) | 0 (0) |
| Catalpaspeciosa | 62 (1503) | 1 (5) |
| Cerciscanadensis | 19 (474) | 0 (0) |
| Elaeagnusangustifolia | 5 (137) | 0 (0) |
| Helianthusannuus | 1 (25) | 0 (0) |
| Malusdomestica | 48 (1099) | 1 (1) |
| Malus sp. | 6 (122) | 0 (0) |
| Prunusarmeniaca | 11 (277) | 0 (0) |
| Prunuscerasus | 12 (298) | 0 (0) |
| Prunusdomestica | 3 (83) | 0 (0) |
| Prunuspersica | 17 (453) | 3 (7) |
| Robiniapseudoacacia | 3 (73) | 0 (0) |
| Sambucus sp. | 6 (168) | 0 (0) |
| Zeamays | 22 (559) | 0 (0) |
Table 2.
Number of deployed and parasitised wild H.halys egg masses by all wasps, native and exotic, on multiple plant species in northern Utah from June through September, 2017–2019. Parasitism denotes adult wasp emergence.
| Plant Species | Year | Total Egg Masses (Eggs) | All Parasitised Egg Masses (Eggs) | Egg Masses Parasitized by T.japonicus (Eggs)* |
| Acergrandidentatum | 2018 | 1 (22) | 1 (1) | 0 (0) |
| Catalpaspeciosa | 2017 | 4 (108) | 1 (4) | 0 (0) |
| 2018 | 6 (164) | 1 (7) | 0 (0) | |
| 2019 | 105 (2791) | 43 (796) | 21 (452) | |
| Prunuscerasus | 2018 | 1 (28) | 0 (0) | 0 (0) |
| 2019 | 1 (28) | 0 (0) | 0 (0) | |
| Zeamays | 2017 | 1 (28) | 0 (0) | 0 (0) |
* Wasp identity confirmed upon adult emergence from H.halys eggs.
Stink Bug Colony
Halyomoprhahalys egg masses were reared in the Department of Biology at Utah State University, Logan, Utah. The colony was initiated and continuously supplemented from wild H.halys collections in northern Utah beginning in 2016 and further supplemented in 2019 by egg masses from a colony at the New Jersey Department of Agriculture in Trenton, New Jersey. The lab colony was maintained at 25–28°C, 40–60% RH, with a 16:8 hr photoperiod.
Survey Methods
Fresh lab-reared egg masses were deployed at field sites within 24–48 hr post-oviposition. All lab-reared egg masses were oviposited on to paper towels, assessed for the number of eggs they contained and attached to wax-covered cardstock (4 cm x 4 cm), using double-sided sticky tape with sand to cover excess adhesive before field deployment. Lab-reared egg masses mounted on cardstock were attached to the underside of plant leaves (Table 1) 2–3 m above the ground using metal safety pins and collected approximately 48 hr after deployment The number of lab-reared egg masses deployed each season was dependent on the lab colony fecundity: 114, 93 and 28 in 2017, 2018 and 2019, respectively. Wild H.halys egg masses were identified through 30-min bouts of physical inspection of preferred host plants (Table 2). Each branch was inspected up to a height of 3 m using a step ladder. The number of wild egg masses identified in the survey was 5, 8 and 106 in 2017, 2018 and 2019, respectively. Wild egg masses were collected at the time of detection.
Upon collection, all egg masses were inspected for the presence of guarding parasitoid wasps. If present, wasps were collected with an aspirator (Carolina Scientific Supply Co. Burlington, NC) and placed into a 47 mm plastic Petri dish (Fisher Scientific Co. L.L.C. Pittsburgh, PA) with the associated egg mass to allow for further oviposition during transport to the lab in a cooler at ambient temperature, 15.5–24°C.
In the lab, egg masses were stored under the same conditions as the H.halys colony described above. Guarding female wasps were removed upon arrival at the lab, preserved in ethanol and later pinned for identification. Collected egg masses were inspected for the number of hatched (H.halys emergence), parasitised (parasitoid wasp emergence), missing (number of lab-reared eggs not present after field collection), unhatched or predated eggs (e.g. chewing or sucking damage) present approximately one week after collection, following procedures established by Ogburn et al. (2016). Egg masses were observed again six weeks after collection to identify late-emerging wasps or those with partially-developed wasps within eggs (Stahl et al. 2019). Wasp species were identified using the keys to Nearctic Trissolcus (Talamas et al. 2015a), Nearctic Telenomus (Johnson 1984) and Nearctic Anastatus (Burks 1967).
Statistical Methods
Parasitism (defined as the proportion of egg masses in which one or more eggs produced adult wasps) was compared amongst years and egg types (wild and lab-reared) using a generalised linear model with a quasi-binomial distribution to account for over-dispersion due to small sample sizes in some years and zero-inflation. We report means and intervals that have been inverse-linked from the logit scale of the statistical model to the original proportion scale. Computations used the glm function in the stats package and various functions in the car (Fox and Weisberg 2019) and emmeans (Lenth 2019) packages in R software (R version 3.6.1; R Core Team 2019).
Voucher Specimens
Three voucher specimens of Trissolcusjaponicus from this study have been deposited in the Florida State Collection of Arthropods, Gainesville, Florida (FSCA 00090589, FSCA 00090661, FSCA 00090662). A Darwin Core Archive of the data associated with these specimens is provided in Suppl. material 1.
Results
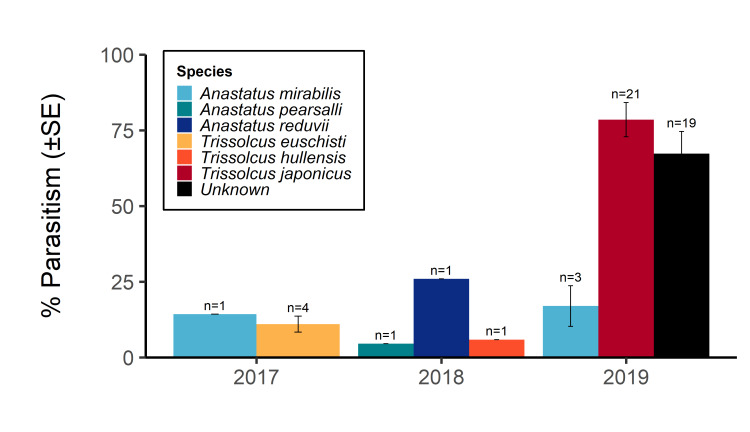
Over the three year survey period, a total of 39 parasitoids from five native wasp species emerged from six wild and five lab-reared H.halys egg masses. Anastatusmirabilis (Walsh & Riley), A.pearsalli Ashmead, A.reduvii Ashmead, Trissolcuseuschisti (Ashmead) and T.hullensis (Harrington) were documented from both guarding females and successful emergence from H.halys egg masses (Fig. 2). Trissolcusutahensis (Ashmead) and Telenomuspodisi Ashmead were observed guarding H.halys eggs, but did not successfully emerge as adults. Catalpaspeciosa (Warder), Malusdomestica Borkh and Prunuspersica (L.) Batsch were the only plant species on which lab-reared egg masses were parasitised and this parasitism was by native wasp species exclusively (Table 1). In June 2019, the Asian parasitoid T.japonicus was first discovered in Utah emerging from two wild H.halys egg masses at Site 1 in Salt Lake City (Figs 1, 2, 3) and was detected consistently from June through September. Further, a single egg mass with emergent T.japonicus was found at Site 17 in August 2019. Trissolcusjaponicus was only detected at these two suburban landscape sites. A total of 452 T.japonicus emerged from 21 of the 106 wild egg masses found in 2019 (Fig. 2). Parasitised wild egg masses were collected on two tree species, C.speciosa and Acergrandidentatum Nutt, with attack by T.japonicus occurring only on C.speciosa (Table 2).
Figure 2.
Percent parasitism (± SE) of eggs in wild and lab-reared egg masses with adult wasp emergence in northern Utah, 2017–2019. Sample size (n) represents the number of egg masses parasitised by the indicated wasp species in each year. Bars without standard error lines represent single egg masses. The Unknown category represents egg masses in which parasitoid wasp emergence was confirmed, but no wasp specimens remained to confirm species identification.
Figure 3.
Photo of female Trissolcusjaponicus (FSCA 00090662), found in Salt Lake City, Site 1, on 17 June 2019. Key identifying characters include the episternal foveae occurring in a continuous line from the postacetabular sulcus to the mesopleural pit.
When native wasp species successfully emerged from H.halys eggs, the mean number of parasitised eggs per affected egg mass was low, 4–25%. When considering only those egg masses giving rise to adult T.japonicus in 2019, the mean egg parasitism rate per mass was 78.5%. Additionally in 2019, a group of 19 wild egg masses experienced a similarly high mean parasitism rate of 67.3%, though these egg masses did not have adult wasps present at the time of collection, only signs of chewing and emergence (Fig. 2).
Mean parasitism of lab-reared egg masses was 0.42% and 0.05% in 2017 and 2018, with no wasps emerging in 2019. Mean parasitism rates of wild-collected egg masses in 2017, 2018 and 2019 were 2.9%, 3.7% and 28.2%, respectively (Table 3). The generalised linear model did not reveal a significant two-way interaction between year and egg type (P = 0.196, F2,348 = 1.63). Significantly more wasps emerged from wild than lab-reared egg masses (P = 0.002, F1,348 = 9.50). There were no significant differences in mean wasp emergence amongst years (P > 0.797, F2,348 = 0.23).
Table 3.
Table 3. Mean parasitism of lab-reared and wild egg masses collected in northern Utah, 2017–2019. LCL and UCL refer to the lower and upper limit of a 68% confidence interval, respectively and approximately depict the mean +/- 1 SE. See Suppl. materials 2, 3.
| Egg Type | Year | Mean Parasitism (%) | LCL | UCL |
| Lab-reared | 2017 | 0.42 | 0.19 | 0.94 |
| Wild | 2017 | 2.94 | 0.74 | 11.00 |
| Lab-reared | 2018 | 0.05 | 0.00 | 0.73 |
| Wild | 2018 | 3.74 | 1.41 | 9.53 |
| Lab-reared | 2019 | 0.00 | 0.00 | 100.00 |
| Wild | 2019 | 28.20 | 25.90 | 30.64 |
Discussion
Surveys of wild and lab-reared H.halys eggs in northern Utah demonstrated relatively high diversity of native parasitoid wasps, but these native species all exhibited low rates of parasitism. These findings are congruent with other North American surveys of H.halys egg parasitoids (Dieckhoff et al. 2017, Abram et al. 2019b). Low native parasitism rates could be caused by deterrence from natural chemical defences on and within H.halys eggs or a lack of effective venom at the time of female oviposition needed for successful wasp development in the exotic host egg (Haye et al. 2015a, Tognon et al. 2016). Other research suggests that the use of parasitism emergence as a metric of parasitoid effectiveness underestimates native wasp effects on H.halys eggs since partially-developed wasps that do not complete emergence often kill the stink bug host (Abram et al. 2019a, Cornelius et al. 2016b, Schumm 2020). Although our egg dissections revealed many unhatched H.halys eggs with undifferentiated contents (Milnes and Beers 2019), the ultimate cause of egg death could not be ascertained.
Our results support those of Jones et al. (2014) and Abram et al. (2017) who found that wild (in situ) egg masses more accurately detect the presence and ability of parasitoid wasps to emerge from H.halys eggs than do field-deployed lab-reared egg masses. This difference may be due to a variety of factors, including the age of the egg mass upon deployment, length of egg mass exposure to field conditions and deployment height of egg masses in host trees. Hedstrom et al. (2017) noted the importance of semiochemical cues associated with the success of T.japonicus in finding and stinging H.halys egg masses. Research by Boyle et al. (2019) has also shown that kairomones, left by H.halys on host plant leaves, are detectable by T.japonicus and the wasp resides on these leaves longer than those lacking such kairomones. Therefore, lower parasitism rates of lab-reared egg masses could be due to reduced chemical cues.
Although current parasitism by the exotic T.japonicus in northern Utah is modest, relative to those in its native range (Yang et al. 2009, Zhang et al. 2017), our results indicate that T.japonicus has the potential to provide biological control of H.halys in the Intermountain West. Parasitism rates were not shown to be different amongst years, but our data clearly show higher parasitism in 2019, when T.japonicus was discovered, as compared to previous years. The dissonance of biological and statistical conclusions in our results is likely due to the variable and low sample size of egg masses. Trissolcusjaponicus may have killed more H.halys eggs than we were able to document, based on identification of the causal wasp. Indeed, many egg masses were attacked by parasitoids that had already emerged from eggs before collection in 2019, with higher mean parasitism rates in affected egg masses than those observed for native wasp species, suggesting that at least some of the unidentified parasitoids were T.japonicus. It is also of interest to point out that T.japonicus was detected in two suburban landscape sites in Salt Lake County in 2019 and not in agricultural sites.
The northern Utah region differs in its climate and topography from most locations in which T.japonicus has been documented or predicted to become established in North America (Avila and Charles 2018). Given the arid, high elevation conditions of northern Utah that include cold winters and hot summers, detection of an adventive T.japonicus population implies potential for range expansion into other locations within the greater Intermountain West region. These results support the possibility of an eventual intersection of eastern and western T.japonicus populations in North America (Jarrett et al. 2019, Talamas et al. 2015b, Milnes et al. 2016). Further research should focus on the capacity of T.japonicus to persist in the Intermountain West, specifically focusing on overwintering behaviour where heavy snowfall accumulation and consistent sub-zero temperatures occur (Lowenstein et al. 2019, Nystrom Santacruz et al. 2017). In fact, follow-up surveys in 2020 have documented continued detection of T.japonicus in Salt Lake and expansion into Utah counties (K. Richardson, personal communication). Laboratory rearing and releases, in conjunction with conservation efforts, are critical next steps in supporting the future establishment of T.japonicus populations in Utah.
Conclusions
Our findings show that an adventive population of T.japonicus in northern Utah is causing higher levels of reproductive parasitism of H.halys eggs compared to native wasp species and wild (in situ) egg masses provide a more accurate measure of parasitoid activity compared to those deployed from lab colonies. This study reports the first detection of T.japonicus in the Intermountain West, a novel geographic location for this parasitoid in North America.
Supplementary Material
Darwin Core Archive of Trissolcusjaponicus voucher specimens
Elijah Talamas
Data type
Occurrences
Brief description
Three female specimes of Trissolcusjaponicus are deposited in the Florida State Collection of Arthropods. The attached file provides their occurrence data in Darwin Core format.
File: oo_423049.xls
Comprehensive data file for all lab-reared (H) and wild (N) egg mass parasitism in Utah 2017–2019
Mark Cody Holthouse and Zachary R. Schumm
Data type
Counts and Occurrences
Brief description
Archive of all lab-reared (H) and wild collected (N) Halyomorphahalys egg masses inspected in northern Utah 2017–2019.
File: oo_429562.csv
Jupypter Notebook of GLM Model
Mark Cody Holthouse
Data type
Jupyter Notebook (R code)
Brief description
This file can be opened on Jupyter Notebook. The file contains R code displaying the generalised linear model used to compare mean parasitism by egg type and year in northern Utah.
File: oo_429561.html
Acknowledgements
We thank Kate Richardson, Hanna Kirkland, Chelise Dever, Stephanie Hall, Erin Berdahl, Lily Bourett, James Withers, Loren Linford, Ben Steadman and Ryan West for their assistance with field research. In addition, we thank Susan Durham for statistical support and pre-submission review of the manuscript. Funding was provided by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Specialty Crop Research Initiative under award number 2016-51181-25409; USDA Specialty Crop Block Grant; Utah Department of Agriculture and Food; Western Sustainable Agriculture Research and Education program under award number 2017-38640-26913 and subaward number [GW18-106]; USDA APHIS PPQ under cooperative agreements AP18PPQFO000C099 and AP19PPQFO000C343; and Utah State University Extension. This research was supported by the Utah Agricultural Experiment Station, Utah State University and approved as journal paper number 9313. Elijah Talamas was supported by the Florida Department of Agriculture and Consumer Services-Division of Plant Industry and the USDA Farm Bill: Identification, monitoring and redistribution of Trissolcusjaponicus – Biological Control of Brown Marmorated Stink Bug (BMSB). USDA is an equal opportunity employer and service provider. Maps throughout this study were created using ArcGIS® software by Esri. ArcGIS® and ArcMap™ are the intellectual property of Esri and are used herein under licence. Copyright © Esri. All rights reserved. For more information about Esri® software, please visit www.esri.com.
References
- Abram Paul K., Hoelmer Kim A., Acebes-Doria Angelita, Andrews Heather, Beers Elizabeth H., Bergh J. Christopher, Bessin Ric, Biddinger David, Botch Paul, Buffington Matthew L., Cornelius Mary L., Costi Elena, Delfosse Ernest S., Dieckhoff Christine, Dobson Rachelyn, Donais Zachary, Grieshop Matthew, Hamilton George, Haye Tim, Hedstrom Christopher, Herlihy Megan V., Hoddle Mark S., Hooks Cerruti R. R., Jentsch Peter, Joshi Neelendra K., Kuhar Thomas P., Lara Jesus, Lee Jana C., Legrand Ana, Leskey Tracy C., Lowenstein David, Maistrello Lara, Mathews Clarissa R., Milnes Joshua M., Morrison William R., Nielsen Anne L., Ogburn Emily C., Pickett Charles H., Poley Kristin, Pote John, Radl James, Shrewsbury Paula M., Talamas Elijah, Tavella Luciana, Walgenbach James F., Waterworth Rebeccah, Weber Donald C., Welty Celeste, Wiman Nik G. Indigenous arthropod natural enemies of the invasive brown marmorated stink bug in North America and Europe. Journal of Pest Science. 2017;90(4):1009–1020. doi: 10.1007/s10340-017-0891-7. [DOI] [Google Scholar]
- Abram Paul K., Brodeur Jacques, Urbaneja Alberto, Tena Alejandro. Nonreproductive effects of insect parasitoids on their hosts. Annual Review of Entomology. 2019;64(1):259–276. doi: 10.1146/annurev-ento-011118-111753. [DOI] [PubMed] [Google Scholar]
- Abram Paul K., Talamas Elijah J., Acheampong Susanna, Mason Peter G., Gariepy Tara D. First detection of the samurai wasp, Trissolcusjaponicus (Ashmead) (Hymenoptera, Scelionidae), in Canada. Journal of Hymenoptera Research. 2019;68:29–36. doi: 10.3897/jhr.68.32203. [DOI] [Google Scholar]
- Abram P. K., Gariepy T. D., Boivin G., Brodeur J. An invasive stink bug as an evolutionary trap for an indigenous egg parasitoid. Biological Invasions. 2014;16(7):1387–1395. doi: 10.1007/s10530-013-0576-y. [DOI] [Google Scholar]
- Avila G. A., Charles J. G. Modelling the potential geographic distribution of Trissolcusjaponicus: a biological control agent of the brown marmorated stink bug, Halyomorphahalys. BioControl. 2018;63(4):505–518. doi: 10.1007/s10526-018-9866-8. [DOI] [Google Scholar]
- Balusu Rammohan R, Cottrell Ted E, Talamas Elijah J, Toews Michael D, Blaauw Brett R, Sial Ashfaq A, Buntin David G, Vinson Edgar L, Fadamiro Henry Y, Tillman Glynn P. New record of Trissolcussolocis (Hymenoptera: Scelionidae) parasitising Halyomorphahalys (Hemiptera: Pentatomidae) in the United States of America. Biodiversity Data Journal. 2019;7:e30124. doi: 10.3897/BDJ.7.e30124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaauw Brett R, Polk Dean, Nielsen Anne L. IPM-CPR for peaches: incorporating behaviorally-based methods to manage Halyomorphahalys and key pests in peach. Pest Management Science. 2014;71(11):1513–1522. doi: 10.1002/ps.3955. [DOI] [PubMed] [Google Scholar]
- Boyle Sean M, Weber Donald C, Hough-Goldstein Judith, Hoelmer Kim A. Host kairomones influence searching behavior of Trissolcusjaponicus (Hymenoptera: Scelionidae), a parasitoid of Halyomorphahalys (Heteroptera: Pentatomidae) Environmental Entomology. 2019;49(1):15–20. doi: 10.1093/ee/nvz155. [DOI] [PubMed] [Google Scholar]
- Burks B. D. The North American species of Anastatus Motschulsky (Hymenoptera, Eupelmidae) Transactions of the American Entomological Society. 1967;93:423–431. [Google Scholar]
- Cesari Michele, Maistrello Lara, Ganzerli Francesco, Dioli Paride, Rebecchi Lorena, Guidetti Roberto. A pest alien invasion in progress: potential pathways of origin of the brown marmorated stink bug Halyomorphahalys populations in Italy. Journal of Pest Science. 2015;88(1):1–7. doi: 10.1007/s10340-014-0634-y. [DOI] [Google Scholar]
- Cornelius Mary L., Dieckhoff Christine, Vinyard Bryan T., Hoelmer Kim A. Parasitism and predation on sentinel egg masses of the Brown Marmorated Stink Bug (Hemiptera: Pentatomidae) in three vegetable crops: Importance of dissections for evaluating the impact of native parasitoids on an exotic pest. Environmental Entomology. 2016;45(6):1536–1542. doi: 10.1093/ee/nvw134. [DOI] [PubMed] [Google Scholar]
- Cornelius Mary L., Dieckhoff Christine, Hoelmer Kim A., Olsen Richard T., Weber Donald C., Herlihy Megan V., Talamas Elijah J., Vinyard Bryan T., Greenstone Matthew H. Biological control of sentinel egg masses of the exotic invasive stink bug Halyomorphahalys (Stål) in Mid-Atlantic USA ornamental landscapes. Biological Control. 2016;103:11–20. doi: 10.1016/j.biocontrol.2016.07.011. [DOI] [Google Scholar]
- Dieckhoff Christine, Tatman Kathleen M., Hoelmer Kim A. Natural biological control of Halyomorphahalys by native egg parasitoids: a multi-year survey in northern Delaware. Journal of Pest Science. 2017;90:1143–1158. doi: 10.1007/s10340-017-0868-6. [DOI] [Google Scholar]
- Fox J, Weisberg S. Thousand Oaks CA: Sage; 2019. An R companion to applied regression. Third edition. [Google Scholar]
- Gariepy T. D., Fraser H., Scott-Dupree C. D. Brown marmorated stink bug (Hemiptera: Pentatomidae) in Canada: recent establishment, occurrence, and pest status in southern Ontario. The Canadian Entomologist. 2014;146(5):579–582. doi: 10.4039/tce.2014.4. [DOI] [Google Scholar]
- Haye T., Fischer S., Zhang J., Gariepy T. Can native egg parasitoids adopt the invasive brown marmorated stink bug, Halyomorphahalys (Heteroptera: Pentatomidae), in Europe? Journal of Pest Science. 2015;88(4):693–705. doi: 10.1007/s10340-015-0671-1. [DOI] [Google Scholar]
- Haye Tim, Gariepy Tara, Hoelmer Kim, Rossi Jean-Pierre, Streito Jean-Claude, Tassus Xavier, Desneux Nicolas. Range expansion of the invasive brown marmorated stinkbug, Halyomorphahalys: an increasing threat to field, fruit and vegetable crops worldwide. Journal of Pest Science. 2015;88(4):665–673. doi: 10.1007/s10340-015-0670-2. [DOI] [Google Scholar]
- Hedstrom Christopher, Lowenstein David, Andrews Heather, Bai Barry, Wiman Nik. Pentatomid host suitability and the discovery of introduced populations of Trissolcusjaponicus in Oregon. Journal of Pest Science. 2017;90(4):1169–1179. doi: 10.1007/s10340-017-0892-6. [DOI] [Google Scholar]
- Herlihy Megan V., Talamas Elijah J., Weber Donald C. Attack and success of native and exotic parasitoids on eggs of Halyomorphahalys in three Maryland habitats. PLOS One. 2016;11(3) doi: 10.1371/journal.pone.0150275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebeke E. R, Carter M. E. Halyomorphahalys (Stǻl) (Heteroptera: Pentatomidae): a polyphagous plant pest from Asia newly detected in North America. Proceedings of the Entomological Society of Washington. 2003;105(1):225–237. [Google Scholar]
- Jarrett Benjamin J M, Pote John, Talamas Elijah, Gut Larry, Szucs Marianna. The discovery of Trissolcusjaponicus (Hymenoptera: Scelionidae) in Michigan. The Great Lakes Entomologist . 2019;52(8) [Google Scholar]
- Johnson N. F. Systematics of Nearctic Telenomus: classification and revisions of the Podisi and Phymatae species groups (Hymenoptera: Scelionidae) Bulletin of the Ohio Biological Survey. 1984;6(3):1–113. [Google Scholar]
- Jones Ashley L., Jennings David E., Hooks Cerruti R. R., Shrewsbury Paula M. Sentinel eggs underestimate rates of parasitism of the exotic brown marmorated stink bug, Halyomorphahalys. Biological Control. 2014;78:61–66. doi: 10.1016/j.biocontrol.2014.07.011. [DOI] [Google Scholar]
- Lee Doo-Hyung, Short Brent D., Nielsen Anne L., Leskey Tracy C. Impact of organic insecticides on the survivorship and mobility of Halyomorphahalys (Stål) (Hemiptera: Pentatomidae) in the Laboratory. Florida Entomologist. 2014;97(2):414–421. doi: 10.1653/024.097.0211. [DOI] [Google Scholar]
- Lenth R. emmeans: Estimated Marginal Means, aka Least-Squares Means. https://CRAN.R-project.org/package=emmeans. 2019 R package version 1.4.1.
- Leskey Tracy C., Lee Doo-Hyung, Short Brent D., Wright Starker E. Impact of insecticides on the invasive Halyomorphahalys (Hemiptera: Pentatomidae): Analysis of insecticide lethality. Journal of Economic Entomology. 2012;105(5):1726–1735. doi: 10.1603/EC12096. [DOI] [PubMed] [Google Scholar]
- Leskey Tracy C., Short Brent D., Butler Bryan R., Wright Starker E. Impact of the invasive Brown Marmorated Stink Bug, Halyomorphahalys (Stål), in Mid-Atlantic tree fruit orchards in the United States: Case studies of commercial management. Psyche: A Journal of Entomology. 2012;2012:1–14. doi: 10.1155/2012/535062. [DOI] [Google Scholar]
- Leskey Tracy C., Short Brent D., Lee Doo-Hyung. Efficacy of insecticide residues on adult Halyomorphahalys (Stål) (Hemiptera: Pentatomidae) mortality and injury in apple and peach orchards. Pest Management Science. 2014;70(7):1097–1104. doi: 10.1002/ps.3653. [DOI] [PubMed] [Google Scholar]
- Lowenstein D. M, Andrews H., Hilton R. J., Kaiser C, Wiman N. G. Establishment in an introduced range: dispersal capacity and winter survival of Trissolcusjaponicus, an adventive egg parasitoid. Insects. 2019;10(12):443. doi: 10.3390/insects10120443.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macavei Laura Ioana, Bâe Raul, Oltean Ion, Florian Teodora, Varga Mircea, Costi Elena, Maistrello Lara. First detection of Halyomorphahalys (Stål), a new invasive species with a high potential of damage on agricultural crops in Romania. Lucrări Ştiinţifice. 2015;58(5):105–108. [Google Scholar]
- Mathews Clarissa R., Blaauw Brett, Dively Galen, Kotcon James, Moore Jennifer, Ogburn Emily, Pfeiffer Douglas G., Trope Taliaferro, Walgenbach James F., Welty Celeste, Zinati Gladis, Nielsen Anne L. Evaluating a polyculture trap crop for organic management of Halyomorphahalys and native stink bugs in peppers. Journal of Pest Science. 2017;90(4):1245–1255. doi: 10.1007/s10340-017-0838-z. [DOI] [Google Scholar]
- Milnes Joshua M., Wiman Nik G., Talamas Elijah J., Brunner Jay F., Hoelmer Kim A., Buffington Matthew L., Beers Elizabeth H. Discovery of an exotic egg parasitoid of the Brown Marmorated Stink Bug, Halyomorphahalys (Stål) in the Pacific Northwest. Proceedings of the Entomological Society of Washington. 2016;118(3):466–470. doi: 10.4289/0013-8797.118.3.466. [DOI] [Google Scholar]
- Milnes Joshua M., Beers Elizabeth H. Trissolcusjaponicus (Hymenoptera: Scelionidae) causes low levels of parasitism in three North American Pentatomids under field conditions. Journal of Insect Science. 2019;19(4) doi: 10.1093/jisesa/iez074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milonas P. G., Partsinevelos G. K. First report of brown marmorated stink bug Halyomorphahalys Stål (Hemiptera: Pentatomidae) in Greece. EPPO Bulletin. 2014;44(2):183–186. doi: 10.1111/epp.12129. [DOI] [Google Scholar]
- Nystrom Santacruz Erica, Venette Robert, Dieckhoff Christine, Hoelmer Kim, Koch Robert L. Cold tolerance of Trissolcusjaponicus and T.cultratus, potential biological control agents of Halyomorphahalys, the brown marmorated stink bug. Biological Control. 2017;107:11–20. doi: 10.1016/j.biocontrol.2017.01.004. [DOI] [Google Scholar]
- Ogburn Emily C., Bessin Ricardo, Dieckhoff Christine, Dobson Rachelyn, Grieshop Matthew, Hoelmer Kim A., Mathews Clarissa, Moore Jennifer, Nielsen Anne L., Poley Kristin, Pote John M., Rogers Mary, Welty Celeste, Walgenbach James F. Natural enemy impact on eggs of the invasive brown marmorated stink bug, Halyomorphahalys (Stål) (Hemiptera: Pentatomidae), in organic agroecosystems: A regional assessment. Biological Control. 2016;101:39–51. doi: 10.1016/j.biocontrol.2016.06.002. [DOI] [Google Scholar]
- Team R Core. Statistical Computing, Vienna, Austria; 2019. R: A language and environment for statistical computing. R version 3.6.1. [Google Scholar]
- Sabbatini Peverieri Giuseppino, Talamas Elijah, Bon Marie Claude, Marianelli Leonardo, Bernardinelli Iris, Malossini Giorgio, Benvenuto Luca, Roversi Pio Federico, Hoelmer Kim. Two Asian egg parasitoids of Halyomorphahalys (Stål) (Hemiptera, Pentatomidae) emerge in northern Italy: Trissolcusmitsukurii (Ashmead) and Trissolcusjaponicus (Ashmead) (Hymenoptera, Scelionidae) Journal of Hymenoptera Research. 2018;67:37–53. doi: 10.3897/jhr.67.30883. [DOI] [Google Scholar]
- Schumm Zachary Ryan. Ecology and economic impact of the invasive brown marmorated stink bug (Hemiptera: Pentatomidae; Halyomorphahalys) in the Utah agricultural landscape. All Graduate Theses and Dissertations; Logan: 2020. 186. [Google Scholar]
- Stahl Judith, Tortorici Francesco, Pontini Marianna, Bon Marie-Claude, Hoelmer Kim, Marazzi Cristina, Tavella Luciana, Haye Tim. First discovery of adventive populations of Trissolcusjaponicus in Europe. Journal of Pest Science. 2019;92(2):371–379. doi: 10.1007/s10340-018-1061-2. [DOI] [Google Scholar]
- Talamas Elijah J., Johnson Norman F., Buffington Matthew. Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae) Journal of Hymenoptera Research. 2015;43:45–110. doi: 10.3897/JHR.43.8560. [DOI] [Google Scholar]
- Talamas Elijah J., Herlihy Megan V., Dieckhoff Christine, Hoelmer Kim A., Buffington Matthew, Bon Marie-Claude, Weber Donald C. Trissolcusjaponicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. Journal of Hymenoptera Research. 2015;43:119–128. doi: 10.3897/JHR.43.4661. [DOI] [Google Scholar]
- Tognon Roberta, Sant’Ana Josué, Zhang Qing-He, Millar Jocelyn G., Aldrich Jeffrey R., Zalom Frank G. Volatiles mediating parasitism of Euschistusconspersus and Halyomorphahalys eggs by Telenomuspodisi and Trissolcus erugatus. Journal of Chemical Ecology. 2016;42(10):1016–1027. doi: 10.1007/s10886-016-0754-3. [DOI] [PubMed] [Google Scholar]
- Center Utah Climate. Climate Data. https://climate.usu.edu/mapGUI/mapGUI.php
- Vétek Gábor, Papp Veronika, Haltrich Attila, Rédei Dávid. First record of the brown marmorated stink bug, Halyomorphahalys (Hemiptera: Heteroptera: Pentatomidae), in Hungary, with description of the genitalia of both sexes. Zootaxa. 2014;3780(1) doi: 10.11646/zootaxa.3780.1.8. [DOI] [PubMed] [Google Scholar]
- Yang Zhong-Qi, Yao Yan-Xia, Qiu Lan-Fen, Li Zhong-Xin. A new species of Trissolcus (Hymenoptera: Scelionidae) parasitizing eggs of Halyomorphahalys (Heteroptera: Pentatomidae) in China with comments on its biology. Annals of the Entomological Society of America. 2009;102(1):39–47. doi: 10.1603/008.102.0104. [DOI] [Google Scholar]
- Zhang Jinping, Zhang Feng, Gariepy Tara, Mason Peter, Gillespie Dave, Talamas Elijah, Haye Tim. Seasonal parasitism and host specificity of Trissolcusjaponicus in northern China. Journal of Pest Science. 2017;90(4):1127–1141. doi: 10.1007/s10340-017-0863-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Darwin Core Archive of Trissolcusjaponicus voucher specimens
Elijah Talamas
Data type
Occurrences
Brief description
Three female specimes of Trissolcusjaponicus are deposited in the Florida State Collection of Arthropods. The attached file provides their occurrence data in Darwin Core format.
File: oo_423049.xls
Comprehensive data file for all lab-reared (H) and wild (N) egg mass parasitism in Utah 2017–2019
Mark Cody Holthouse and Zachary R. Schumm
Data type
Counts and Occurrences
Brief description
Archive of all lab-reared (H) and wild collected (N) Halyomorphahalys egg masses inspected in northern Utah 2017–2019.
File: oo_429562.csv
Jupypter Notebook of GLM Model
Mark Cody Holthouse
Data type
Jupyter Notebook (R code)
Brief description
This file can be opened on Jupyter Notebook. The file contains R code displaying the generalised linear model used to compare mean parasitism by egg type and year in northern Utah.
File: oo_429561.html