Abstract
The Achilles tendon is one of the most commonly ruptured tendons in the human body. Minimally invasive and open surgical repairs are commonly undertaken to manage acute Achilles ruptures. This article describes the postoperative imaging findings and their evolution after surgery. Ultrasound and magnetic resonance imaging provide crucial information regarding the morphology, structure, vascularization and mobility of the Achilles tendon on the surrounding planes. Morphologically, a repaired tendon is physiologically larger and wider than an intact one, with a loss of its fibrillary structure; the presence of surgical material in the context of the tendon is normal after the rupture has been repaired. After surgery, the tendon is more vascularized in power-Doppler imaging. Elastography and diffusion tensor Imaging are innovative tools which allow for the visualization of microstructural abnormalities not apprehensible using conventional imaging techniques. A treated Achilles tendon is unlikely to regain a normal imaging appearance, and the health care professional must distinguish between postoperative findings and actual pathological features. In this context, clinical examination still reigns supreme.
Keywords: Achilles tendon, Ultrasound, MRI, Postoperative
Introduction
The Achilles tendon (AT) is the conjoint terminal structure of the gastrocnemius and soleus muscles, and plays an important role in the foot’s plantar flexion and in hindfoot inversion [1]. Ruptures of the AT are more frequent than ruptures in any other tendon, accounting for about 50% of all operative tendon repairs. The injury mostly plagues active men in early middle age, with imaging and histological features of intratendinous abnormalities, which are considered to be essential conditions for AT rupture [2].
The intratendinous abnormalities are, from a histological viewpoint, an expression of the failed healing response typical of tendinopathy, with a multifactorial pathogenesis from intrinsic and extrinsic factors. Intrinsic patient characteristics such as increasing age, male sex, and obesity demonstrate a positive association with AT pathology [3], while the use of fluoroquinolones and corticosteroids represents the main extrinsic factor associated with the weakening of the AT structure, resulting in an increased risk of rupture [4].
Treatment options for patients with acute AT rupture include conservative therapy, open surgical repair and percutaneous surgical repair [5]. The treatment decisions for acute AT ruptures are still debated [6, 7]. In many countries, conservative management has become standard, but several surgeons still choose to repair AT ruptures because nonsurgical treatment is associated with a lower rate of return to sport and a greater rate of tendon elongation [6, 8].
Etiology
The etiology of AT rupture is not still clear, with two major theories having been proposed. Arner et al. found degenerative changes in all 74 of their patients with acute AT ruptures [9], and this study was the basis for the “degenerative theory,” according to which chronic degeneration of the AT may lead to a rupture without the need for excessive loads to be applied [10]. These degenerative changes have been seen in several studies, including the assessment of AT within 24 h of the rupture or in operated AT indicating preceding chronic changes [11, 12]. The postulated mechanism is that impaired blood flow to the tendon could play a major role, with resultant hypoxia and altered metabolism [13].
The “mechanical theory” hypothesized that different movements and forces exerted on the tendon can lead to rupture. Barfred et al. [14] noted that a tendon was at greatest risk of rupture when obliquely loaded at a short initial length with massive muscle contraction. This risk is increased when there is a dysfunction in the body’s ability to limit excessive and uncoordinated muscle contractions [4].
Surgical procedures
Several surgical approaches (open or percutaneous) have been described to repair AT ruptures. In addition to direct end-to-end AT repair, various means of augmentation of the repaired tendon have been described, including the use of the gastrocnemius turn down the flap and plantaris [15].
Surgical treatment aims to restore both the function and strength of the gastrocnemius-soleus complex by maintaining the optimal length-tension relationship [10]. Some techniques allow for bridging the defect with biological tissue, synthetic material or allogeneic tissues that provide satisfactory strength for the repair [16–18]. However, the use of such augmentation techniques has no advantages over primary AT repairs.
Open repair can be performed under general or spinal anesthesia; some authors’ routines identify the sural nerve during open repair procedures [19].
Percutaneous techniques carry a lower rate of complications than traditional open repair techniques [20]. In 1977, Ma and Griffith published the first article about percutaneous repair of acute AT ruptures [21]. Percutaneous techniques produce a lower risk of wound soft tissue complications, including a lower incidence of infections and hematoma in the zone of injury [19]. In 2008, Metz et al. [22] reported similar risks of complications in patients who underwent minimally invasive surgery and those treated with non-operative treatment and immediate full weight-bearing. Other studies [23, 24] reported favorable cosmetic appearance, fewer wound complications, high patient satisfaction, and better imaging results using percutaneous techniques. Mini-open technique AT repair techniques can be used successfully in higher-demand patients, with acceptable rates of adverse outcomes [20–22, 25].
Imaging
The follow-up of an operated AT is primarily clinical. Postoperative imaging has improved thanks to the recent technological advances in magnetic resonance imaging (MRI) and in ultrasound (US) that allow for better representation of tendon specimens.
The postoperative imaging appearance of AT repair depends on the surgical technique used. The evaluation of AT via such imaging allows to obtain important information regarding general morphology, tendon structure, grade of vascularity and tissue mobility.
Ultrasound
Given its capability to perform a dynamic evaluation of the structure examined, ultrasound plays a crucial role in the follow-up of operated tendons [26–28].
The operated AT is thicker and wider than a normal AT; its mean thickness is about 10 mm, and it ranges from 7 to 16 mm, whereas the average thickness of a healthy tendon is 5.4 mm (4–8 mm) [29, 30]. This increase in size occurs during the first 3–6 months after surgery and the AT can gradually decrease in thickness 1 year after surgery [31] or remain thickened [27].
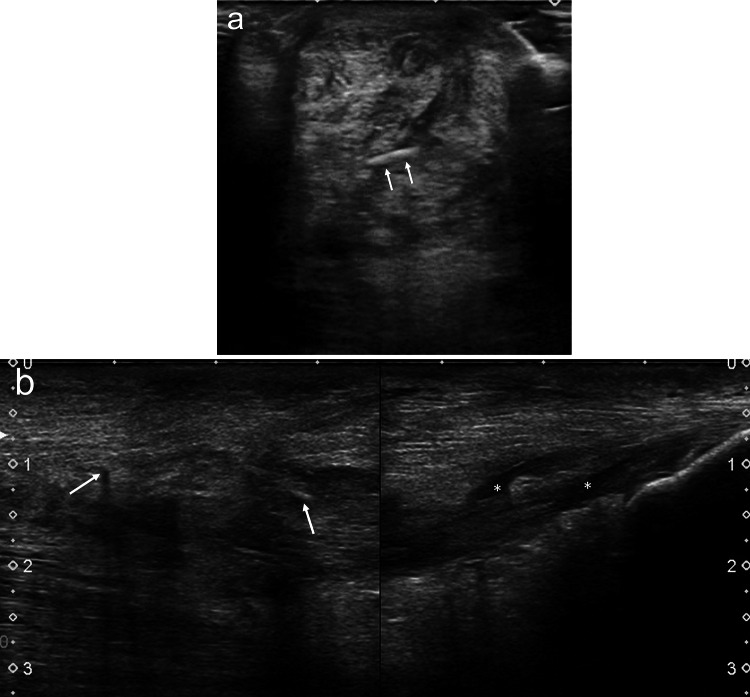
Fluid collections are suggestive of a poor prognosis, and if occurring in more than 50% of the affected tendon, should be considered pathological [32]. The contours of the tendon may be irregular, with hypoechoic peritendinous areas, which may persist for up to 3 months [27] and small hypoechoic areas surrounding the stitches up to 6 months after surgery [33] (Fig. 1a, b).
Fig. 1.
a, b Transverse (a) and longitudinal (b) US scans of Achilles tendon 4 months after surgery. The tendon appears thickened, with hypoechoic areas around the stitches (arrows) and small fluid collections (asterisks)
The incidence of postoperative tendon calcifications after percutaneous Achilles tendon repair is 11.1%. They have no negative impact on the postoperative clinical outcome [34] (Fig. 2).
Fig. 2.
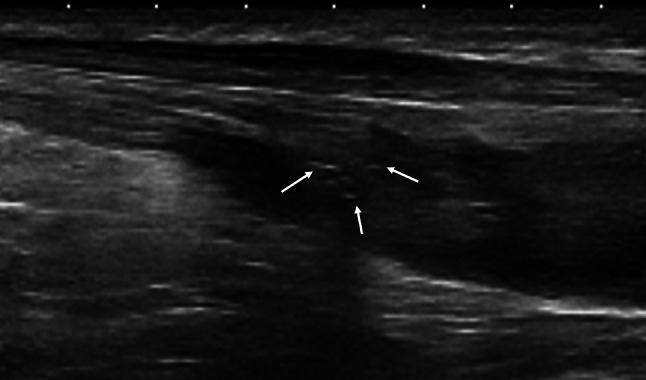
Longitudinal US scan of Achilles tendon 1 year after surgery shows several little calcifications (arrows)
The micro vascularity assessment with power Doppler shows newer vessels with higher flow rates during the healing process [21, 22] (Fig. 3); the vascular response may indicate the process of tendon healing, with initial high flow vascularity within and around repaired tendons, and the total blood flow amount consistently and predictably decreases with time [35]. The increased vascularity shown by power Doppler indicates the healing progress of repaired AT and persists until avascular scar formation occurs. If there is increased vascularity 2 years after surgery, and the tendon is symptomatic, this should be investigated [35]. As in the native Achilles tendon, the plantar flexion position of the foot should be maintained for power Doppler evaluation of the tendon, so as not to compress the new vessels [36, 37].
Fig. 3.

Longitudinal power Doppler US scan of Achilles tendon 3 months after reconstruction with plantaris tendon shows diffuse neo-vascularization of the tendon
One year after surgery, adhesions between the tendon and the skin may be evident in up to 40% of patients [39]. Dynamic ultrasound examination is definitely a good method of highlighting focal peritendinous adhesions, indistinct tendon borders and limited tendon gliding function [32, 35] (Fig. 4).
Fig. 4.

Longitudinal US scans of Achilles tendon 3 months after open surgery shows focal adhesion. The superficial border of the tendon is indistinct, and the superficial tendon fibers (arrows) appears to be attracted by the subcutaneous scar
In the assessment of postoperative infection, the US is particularly sensitive to evaluate soft-tissue edema, fistula and fluid collections. Power or color-flow Doppler is an added feature that can identify the hyperemia of the surrounding soft tissues indicative of inflammation, thus favoring a collection as being infected [26] (Fig. 5).
Fig. 5.

Longitudinal power Doppler US scan of an infection 2 months after Achilles tendon calcaneal re-attachment. The screw (white arrows) is extruded from the calcaneal tuberosity (Ct). The fistula (black arrows) runs from the calcaneal cortex to the cutaneous wound. Note the neo-vascularization of the enthesis of the Achilles tendon (AT)
Sonoelastography is an US technique that aims to assess tissue elasticity, and its usefulness in the musculoskeletal field has recently increased [38–41]. Over the last few years, ultrasound elastography has increased in diagnostic utility with the introduction of the shear wave method, which has the advantage of being operator-independent, reproducible and quantitative [42]. After surgical treatment of a complete tear, the tendon stiffness pattern gradually increases at 12, 24 and 48 weeks as the wound-healing process continues. A hard and heterogeneous pattern of surgically repaired tendon structure at elastography may be a physiological feature of tendon healing. [42, 43] (Fig. 6).
Fig. 6.
a, b Axial US scan (elastography on the left; grey-scale on the right) of Achilles tendon 4 months after surgery. The operated tendon shows heterogeneous elastography patter, predominantly hard in the deep portion
If a partial re-rupture is suspected, sonographic diagnosis is harder because of the structural characteristics of the tendon following surgery, particularly if large fluid collections are present; dynamic evaluation during ankle flexion and extension is helpful to evidence the gap of tendon discontinuity [44, 45].
Intraoperative ultrasound examination with high-frequency probes can be of assistance during percutaneous repair of Achilles tendon ruptures, with no complications related to the sonography [46]. The possibility of performing intraoperative ultrasound is helpful in detecting anatomical variants in the course of the sural nerve and the relationship between the nerve and the AT since this nerve can run separately or in close contact with the tendon [47]. Post-surgical evaluation of the sural nerve can be useful to exclude iatrogenic nerve entrapments in patients operated on with a minimally invasive technique [46].
Magnetic resonance imaging
MRI is useful in evaluating the healing process of surgically treated AT. In almost all surgically repaired AT, a high signal intensity area (on fluid sensitive sequences) at the rejoined tendon ends is identified (Fig. 7). This finding is generally evident between 6 weeks and 3 months after surgery; after 6 months, this area tends to greatly decrease in size. The high-signal-intensity findings on MRI seem to be correlated with the healing response and the actual tendon tissue composition with respect to morphology and biochemistry [48, 49]. Fujikawa et al. explored the MRI features of normal healing of the expected residual gap in surgically repaired AT, reporting visible gaps 4 weeks after surgery on T1- and T2-weighted images, after both percutaneous repair and open surgery. At 8 weeks, a gap was visible on T1-weighted images in 80% of cases after percutaneous repair and in 10% after open surgical repair; T2-weighted images showed a tendon gap in 63% of ATs repaired with the percutaneous technique but in none of the tendons in the open surgical repair group. After 12 weeks, neither T1-weighted nor T2-weighted images showed a tendon gap in either group of patients [50].
Fig. 7.
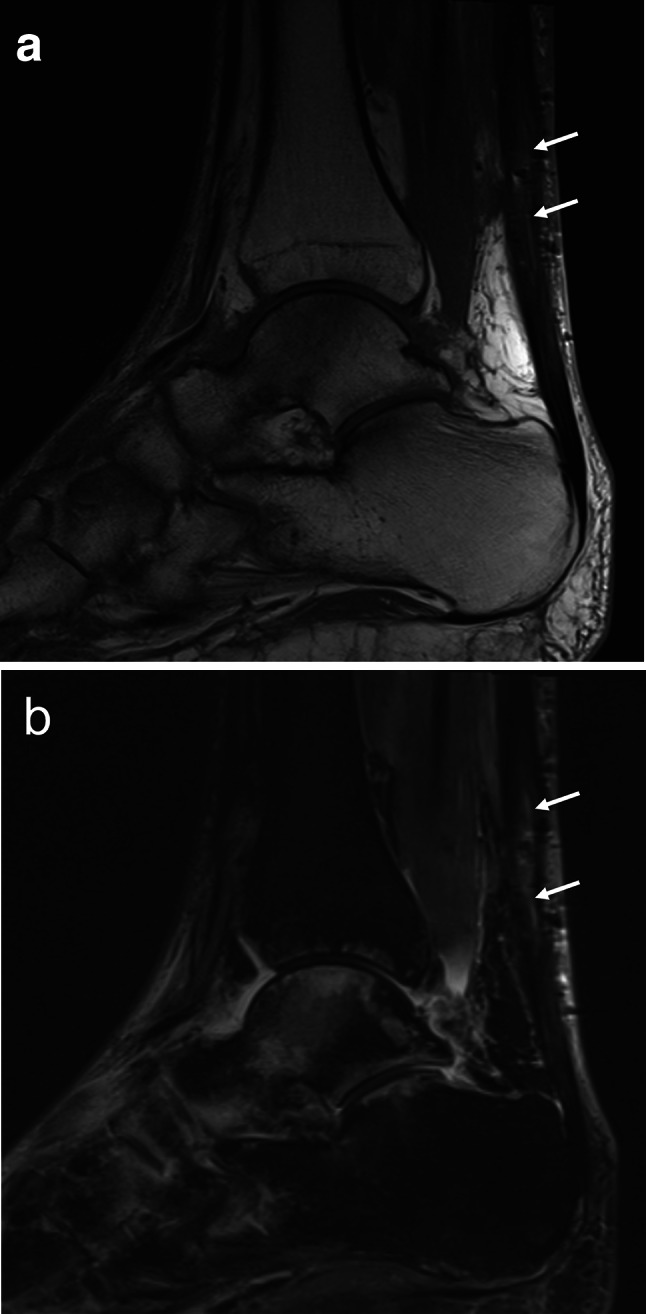
a, b Sagittal T1w (a) and STIR (b) MR images of Achilles tendon 8 months after surgery. The tendon appears thickened, with hyperintense areas (arrows). Note the bone marrow edema syndrome of tarsal structures
Karjalainen et al. analyzed 21 surgically repaired AT ruptures with MRI at 3 weeks, 6 weeks, 3 and 6 months after surgery, and found intratendinous areas of high-intensity signals in almost all surgically repaired AT (19/21) at 3 months after surgery on both proton-density- and T2-weighted images [51].
Hahn et al. demonstrated the postoperative MRI course after flexor hallucis longus tendon transfer and noted that full tendon integration can be expected in only half the patients, with fatty muscle degeneration in the gastrocnemius and soleus muscles being common after this procedure [52].
The analysis of MRI images acquired after gadolinium-based contrast agent injection shows larger high signal intensity changes; these changes slowly decrease with time and it is reported that no significant intratendinous signal enhancement should be encountered at the 2-year MRI follow-up. This supports the theory that the gadolinium-contrast agent interacts with the pathological intratendinous tendon healing process [48]. Nevertheless, the use of gadolinium-based contrast agents in this setting is not completely justified, especially in the light of the controversial accumulation of these media in human tissues, although its clinical impact is still unclear [53].
After tendon augmentation, the graft could be well detected on MRI, especially in T1w images (Fig. 8).
Fig. 8.
Sagittal T1w (a) and STIR (b) MR images of Achilles tendon 10 months after augmentation. The graft can be detected (arrows)
In MRI examinations, the surgical wound scar appears as a low-signal-intensity area in the subcutaneous fat tissue, and the AT seems to be attached to the skin at the site of the scar, thereby preventing the physiological glide of the tendon [54].
The AT re-rupture may be clearly detected on MRI examinations to the same degree as in the native tendon [49] (Fig. 9).
Fig. 9.

STIR MR image of Achilles tendon 16 months after surgery shows a re-rupture of its middle third. (asterisks)
Advanced MRI application
Over the last few years, the use of diffusion tensor imaging (DTI) in musculoskeletal field has been growing, not only in experimental settings but also in clinical practice [55]. DTI is based on the concept that molecular mobility in human tissues is usually non-isotropic, which implies that diffusion does not occur equally in all directions; protein fibers and cell membranes tend to hinder water diffusion. Thus, the diffusion of water molecules in healthy tissues is lower than that of free water. The subsequent anisotropy is related to the presence of an organized structure that restricts molecular movement in some directions; thus, the measurement of fractional anisotropy (FA), an important DTI parameter, reflects information about the architectural organization of tissues [2]. After surgical procedures, the use of DTI may assist in ascertaining the microstructural properties and integrity restoration of the ruptured tendon during the healing process. Sarman et al. analyzed pre and postoperative DTI examinations of ATs of 16 patients with a median follow-up duration of 21 (range 6–80) months; they found that tendon FA values of the ruptured AT were significantly lower than those of the normal side (p = 0.001) [2]. FA and fiber density index measurements could facilitate a description of the pathologic changes in tendon fibers and provide quantitative measurements in both healthy and injured skeletal tendons, even though large DTI studies are still needed in this setting (Fig. 10).
Fig. 10.
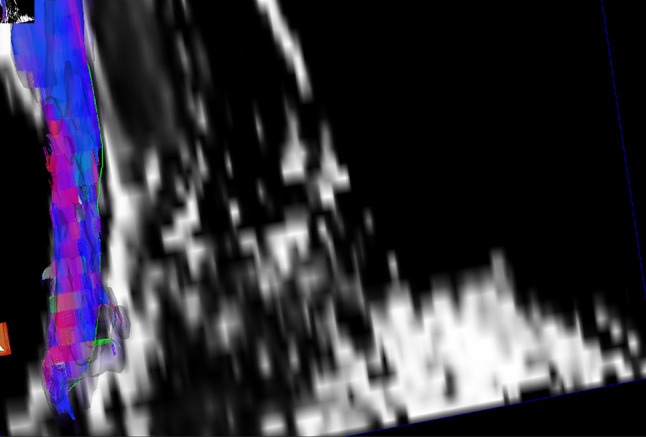
Tractography of Achilles tendon 6 months after surgery shows a fiber thickening with a moderate structural distortion at the suture at the level of its middle third
Conclusion
The MRI and US evaluation of the operated Achilles tendon requires knowledge of normal and pathological changes to identify post-operative complications.
Funding
No funding was received for this review.
Availability of data and material
The authors are able to provide complete data transparency.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Doral MN, Alam M, Bozkurt M, et al. Functional anatomy of the Achilles tendon. Knee Surgery, Sport Traumatol Arthrosc. 2010;18:638–643. doi: 10.1007/s00167-010-1083-7. [DOI] [PubMed] [Google Scholar]
- 2.Sarman H, Atmaca H, Cakir O, et al. Assessment of postoperative tendon quality in patients with achilles tendon rupture using diffusion tensor imaging and tendon fiber tracking. J Foot Ankle Surg. 2015;54:782–786. doi: 10.1053/j.jfas.2014.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Franceschi F, Papalia R, Paciotti M, et al. Obesity as a risk factor for tendinopathy: a systematic review. Int J Endocrinol. 2014;2014:670262. doi: 10.1155/2014/670262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egger AC, Berkowitz MJ. Achilles tendon injuries. Curr Rev Musculoskelet Med. 2017;10:72–80. doi: 10.1007/s12178-017-9386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maffulli N. Rupture of the Achilles tendon. J Bone Jt Surg Am. 1999;81:1019–1036. doi: 10.2106/00004623-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Maffulli N, Peretti GM. Surgery or conservative management for Achilles tendon rupture? BMJ. 2019;364:k5344. doi: 10.1136/bmj.k5344. [DOI] [PubMed] [Google Scholar]
- 7.Maffulli N, Peretti GM. Treatment decisions for acute Achilles tendon ruptures. Lancet (London, England) 2020;395:397. doi: 10.1016/S0140-6736(19)33133-2. [DOI] [PubMed] [Google Scholar]
- 8.Soroceanu A, Sidhwa F, Aarabi S, et al. Surgical versus nonsurgical treatment of acute Achilles tendon rupture: a meta-analysis of randomized trials. J Bone Jt Surg Am. 2012;94:2136–2143. doi: 10.2106/JBJS.K.00917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arner O, Lindholm A, Orell SR. Histologic changes in subcutaneous rupture of the Achilles tendon; a study of 74 cases. Acta Chir Scand. 1959;116:484–490. [PubMed] [Google Scholar]
- 10.Longo UG, Petrillo S, Maffulli N, Denaro V. Acute Achilles tendon rupture in athletes. Foot Ankle Clin. 2013;18:319–338. doi: 10.1016/j.fcl.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Davidsson L, Salo M. Pathogenesis of subcutaneous tendon ruptures. Acta Chir Scand. 1969;135:209–212. [PubMed] [Google Scholar]
- 12.Józsa L, Kannus P. Histopathological findings in spontaneous tendon ruptures. Scand J Med Sci Sports. 1997;7:113–118. doi: 10.1111/j.1600-0838.1997.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 13.Kannus P, Józsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Jt Surg Am. 1991;73:1507–1525. doi: 10.2106/00004623-199173100-00009. [DOI] [PubMed] [Google Scholar]
- 14.Barfred T (1973) Achilles tendon rupture. Aetiology and pathogenesis of subcutaneous rupture assessed on the basis of the literature and rupture experiments on rats. Acta Orthop Scand Suppl, pp 3–126 [PubMed]
- 15.Gulati V, Jaggard M, Al-Nammari SS, et al. Management of achilles tendon injury: a current concepts systematic review. World J Orthop. 2015;6:380–386. doi: 10.5312/wjo.v6.i4.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadek AF, Fouly EH, Laklok MA, Amin MF. Functional and MRI follow-up after reconstruction of chronic ruptures of the Achilles tendon Myerson type III using the triple-loop plantaris tendon wrapped with central turndown flap: a case series. J Orthop Surg Res. 2015;10:109. doi: 10.1186/s13018-015-0256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razik Ibrahim SA. Surgical Treatment of chronic Achilles tendon rupture. J Foot Ankle Surg. 2009;48:340–346. doi: 10.1053/j.jfas.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Lee Y-S, Lin C-C, Chen C-N, et al. Reconstruction for neglected Achilles tendon rupture: the modified Bosworth technique. Orthopedics. 2005;28:647–650. doi: 10.3928/0147-7447-20050701-10. [DOI] [PubMed] [Google Scholar]
- 19.Lim J, Dalai R, Waseem M. Percutaneous vs. open repair of the ruptured Achilles tendon—a prospective randomized controlled study. Foot Ankle Int. 2001;22:559–568. doi: 10.1177/107110070102200705. [DOI] [PubMed] [Google Scholar]
- 20.Pedowitz D, Kirwan G. Achilles tendon ruptures. Curr Rev Musculoskelet Med. 2013;6:285–293. doi: 10.1007/s12178-013-9185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma GW, Griffith TG (1977) Percutaneous repair of acute closed ruptured achilles tendon: a new technique. Clin Orthop Relat Res, pp 247–55 [PubMed]
- 22.Metz R, Verleisdonk E-JMM, van der Heijden GJ-M-G, et al. Acute achilles tendon rupture. Am J Sports Med. 2008;36:1688–1694. doi: 10.1177/0363546508319312. [DOI] [PubMed] [Google Scholar]
- 23.Diao Z-B, Chu H-K, Li N, et al. Short-term clinical effects of Achillon in repair of acute Achilles tendon rupture. Zhongguo Gu Shang. 2012;25:959–961. [PubMed] [Google Scholar]
- 24.Klein EE, Weil L, Baker JR, et al. Retrospective analysis of mini-open repair versus open repair for acute Achilles tendon ruptures. Foot Ankle Spec. 2013;6:15–20. doi: 10.1177/1938640012463052. [DOI] [PubMed] [Google Scholar]
- 25.Henríquez H, Muñoz R, Carcuro G, Bastías C. Is percutaneous repair better than open repair in acute Achilles tendon rupture? Clin Orthop Relat Res. 2012;470:998–1003. doi: 10.1007/s11999-011-1830-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan VO, Morrison WB, Kavanagh EC. Postoperative infection in the foot and ankle. Semin Musculoskelet Radiol. 2012;6:241–253. doi: 10.1055/s-0032-1320124. [DOI] [PubMed] [Google Scholar]
- 27.Gitto S, Draghi AG, Bortolotto C, Draghi F. Sonography of the Achilles tendon after complete rupture repair: what the radiologist should know. J Ultrasound Med. 2016;35:2529–2536. doi: 10.7863/ultra.16.01092. [DOI] [PubMed] [Google Scholar]
- 28.Barbuto L, Di Serafino M, Della Vecchia N, et al. Pediatric musculoskeletal ultrasound: a pictorial essay. J Ultrasound. 2019;22:491–502. doi: 10.1007/s40477-018-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fornage BD. Achilles tendon: us examination. Radiology. 1986;159:759–764. doi: 10.1148/radiology.159.3.3517959. [DOI] [PubMed] [Google Scholar]
- 30.Maffulli N, Dymond NP, Regine R. Surgical repair of ruptured Achilles tendon in sportsmen and sedentary patients: a longitudinal ultrasound assessment. Int J Sports Med. 1990;11:78–84. doi: 10.1055/s-2007-1024767. [DOI] [PubMed] [Google Scholar]
- 31.Blei CL, Nirschl RP, Grant EG. Achilles tendon: us diagnosis of pathologic conditions. Work in progress. Radiology. 1986;159:765–767. doi: 10.1148/radiology.159.3.3517960. [DOI] [PubMed] [Google Scholar]
- 32.Möller M, Kälebo P, Tidebrant G, et al. The ultrasonographic appearance of the ruptured Achilles tendon during healing: a longitudinal evaluation of surgical and nonsurgical treatment, with comparisons to MRI appearance. Knee Surg Sports Traumatol Arthrosc. 2002;10:49–56. doi: 10.1007/s001670100245. [DOI] [PubMed] [Google Scholar]
- 33.Cohen M. US imaging in operated tendons. J Ultrasound. 2012;15:69–75. doi: 10.1016/j.jus.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ateschrang A, Körner D, Joisten K, et al. Incidence and risk factors for postoperative Achilles tendon calcifications after percutaneous repair. Arch Orthop Trauma Surg. 2018;138:203–210. doi: 10.1007/s00402-017-2829-1. [DOI] [PubMed] [Google Scholar]
- 35.Chun KA, Cho K-H. Postoperative ultrasonography of the musculoskeletal system. Ultrason (Seoul, Korea) 2015;34:195–205. doi: 10.14366/usg.15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zappia M, Maggialetti N, Natella R, et al. Diagnostic imaging: pitfalls in rheumatology. Radiol Med. 2019;124(11):1167–1174. doi: 10.1007/s11547-019-01017-9. [DOI] [PubMed] [Google Scholar]
- 37.Zappia M, Cuomo G, Martino MT, et al. The effect of foot position on power doppler ultrasound grading of Achilles enthesitis. Rheumatol Int. 2016;36:871–874. doi: 10.1007/s00296-016-3461-z. [DOI] [PubMed] [Google Scholar]
- 38.Klauser AS, Faschingbauer R, Jaschke WR. Is sonoelastography of value in assessing tendons? Semin Musculoskeletal Radiol. 2010;14(3):323–333. doi: 10.1055/s-0030-1254521. [DOI] [PubMed] [Google Scholar]
- 39.Sconfienza LM, Silvestri E, Cimmino MA. Sonoelastography in the evaluation of painful Achilles tendon in amateur athletes. Clin Exp Rheumatol. 2010;28:373–378. [PubMed] [Google Scholar]
- 40.Sconfienza LM, Albano D, Allen G, et al. Clinical indications for musculoskeletal ultrasound updated in 2017 by European Society of Musculoskeletal Radiology (ESSR) consensus. Eur Radiol. 2018;28:5338–5351. doi: 10.1007/s00330-018-5474-3. [DOI] [PubMed] [Google Scholar]
- 41.Robotti G, Draghi F, Bortolotto C, Canepa MG. Ultrasound of sports injuries of the musculoskeletal system: gender differences. J Ultrasound. 2020 doi: 10.1007/s40477-020-00438-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang L, Wan W, Wang Y, et al. Evaluation of elastic stiffness in healing Achilles tendon after surgical repair of a tendon rupture using in vivo ultrasound shear wave elastography. Med Sci Monit. 2016;22:1186–1191. doi: 10.12659/MSM.895674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tan S, Kudaş S, Özcan AŞ, et al. Real-time sonoelastography of the Achilles tendon: pattern description in healthy subjects and patients with surgically repaired complete ruptures. Skelet Radiol. 2012;41:1067–1072. doi: 10.1007/s00256-011-1339-4. [DOI] [PubMed] [Google Scholar]
- 44.Rupp S, Tempelhof S, Fritsch E. Ultrasound of the Achilles tendon after surgical repair: morphology and function. Br J Radiol. 1995;68:454–458. doi: 10.1259/0007-1285-68-809-454. [DOI] [PubMed] [Google Scholar]
- 45.Shih K-S, Huang Y-P, Wang T-G, et al (2008) Sonographic appearance of surgically repaired Achilles tendons. 台灣復健醫學雜誌 36:23–30
- 46.Zappia M, Berritto D, Oliva F, et al. High resolution real time ultrasonography of the sural nerve after percutaneous repair of the Achilles tendon. Foot Ankle Surg. 2017;37:636–643. doi: 10.1016/j.fas.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 47.Kammar H, Carmont MR, Kots E, et al. Anatomy of the sural nerve and its relation to the achilles tendon by ultrasound examination. Orthopedics. 2014;37:e298–e301. doi: 10.3928/01477447-20140225-64. [DOI] [PubMed] [Google Scholar]
- 48.Shalabi A, Kristoffersen-Wiberg M, Aspelin P, Movin T. MR evaluation of chronic Achilles tendinosis. A longitudinal study of 15 patients preoperatively and two years postoperatively. Acta Radiol. 2001;42:269–276. doi: 10.1080/028418501127346819. [DOI] [PubMed] [Google Scholar]
- 49.Barile A, Bruno F, Mariani S, et al. Follow-up of surgical and minimally invasive treatment of Achilles tendon pathology: a brief diagnostic imaging review. Musculoskelet Surg. 2017;101:51–61. doi: 10.1007/s12306-017-0456-1. [DOI] [PubMed] [Google Scholar]
- 50.Fujikawa A, Kyoto Y, Kawaguchi M, et al. Achilles tendon after percutaneous surgical repair: serial MRI observation of uncomplicated healing. AJR Am J Roentgenol. 2007;189:1169–1174. doi: 10.2214/AJR.07.2260. [DOI] [PubMed] [Google Scholar]
- 51.Karjalainen PT, Aronen HJ, Pihlajamäki HK, et al. Magnetic resonance imaging during healing of surgically repaired achilles tendon ruptures. Am J Sports Med. 1997;25:164–171. doi: 10.1177/036354659702500204. [DOI] [PubMed] [Google Scholar]
- 52.Hahn F, Meyer P, Maiwald C, et al. Treatment of chronic achilles tendinopathy and ruptures with flexor hallucis tendon transfer: clinical outcome and MRI Findings. Foot Ankle Int. 2008;29:794–802. doi: 10.3113/FAI.2008.0794. [DOI] [PubMed] [Google Scholar]
- 53.Doniselli FM, Albano D, Chianca V, et al. Gadolinium accumulation after contrast-enhanced magnetic resonance imaging: what rheumatologists should know. Clin Rheumatol. 2017;36(5):977–980. doi: 10.1007/s10067-017-3604-y. [DOI] [PubMed] [Google Scholar]
- 54.Karjalainen PT, Ahovuo J, Pihlajamäki HK, et al. Postoperative MR imaging and ultrasonography of surgically repaired achilles tendon ruptures. Acta Radiol. 1996;37:639–646. doi: 10.1177/02841851960373P244. [DOI] [PubMed] [Google Scholar]
- 55.Chianca V, Albano D, Messina C, et al. Diffusion tensor imaging in the musculoskeletal and peripheral nerve systems: from experimental to clinical applications. Eur Radiol Exp. 2017;1:1–8. doi: 10.1186/s41747-017-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors are able to provide complete data transparency.