Abstract
Carpal tunnel syndrome (CTS) is the most frequent entrapment neuropathy of peripheral nerves, with an incidence of 1–3 patients in 1000. CTS typically occurs between 45 and 60 years of age, and it is more frequent in women than in men. The main cause of CTS is chronic compression of the median nerve and ischemic suffering secondary to increased pressure in the carpal tunnel. There are many possible causes of CTS, which can be differentiated into idiopathic causes, which include most cases, and secondary causes. Classical CTS diagnosis is based on the patient’s clinical examination and electrophysiological tests, such as electromyography and nerve conduction studies. The latter are helpful for determining the site of nerve compression, assessing its severity, monitoring the course of the disease after therapy, and excluding other causes of median nerve pain, such as cervical radiculopathies, brachial plexopathies, polyneuropathy, or other forms of mononeuropathies. However, clinical examination and electrophysiological tests are not able to differentiate idiopathic forms from secondary forms of CTS, and discrepancies are possible between clinical examination and electrophysiological tests (false negatives). Ultrasound examination is able to recognize most of the secondary forms of CTS. It can evaluate the morphological alterations of the nerve and correlate them with the severity of nerve suffering in all cases, even idiopathic ones, with a sensitivity and specificity equal to those of electrophysiological tests. It can also highlight some anatomical predisposing variants or conditions that may represent contraindications to minimally invasive treatments. Ultrasound examination also plays a fundamental role in evaluating patients with an unfavorable outcome after surgical treatment.
Keywords: Carpal tunnel, Carpal tunnel syndrome, Entrapment neuropathy, Median nerve, Sonography
Introduction
Thanks to technological progress, ultrasound is now one of the main diagnostic methods for imaging peripheral nerves and the carpal tunnel. Knowledge of the anatomy of the carpal tunnel and the semiotics of pathological changes in compressed nerves allow ultrasound examination to be complementary to electrophysiological tests in the diagnosis of carpal tunnel syndrome (CTS).
Normal anatomy and sonography of the carpal tunnel
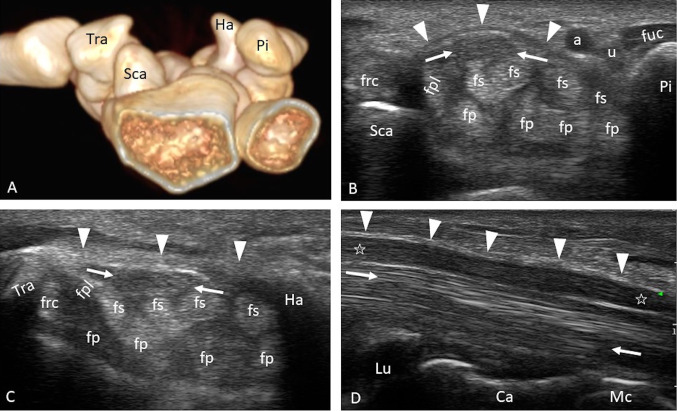
The carpal tunnel is an osteofibrous tunnel made up of the carpal bones and the flexor retinaculum [1–3]. The lunate and the capitate represent the floor of the carpal tunnel, which on the sides is delimited by four bony protuberances that represent the anatomical and ultrasound findings of the proximal (the pisiform and scaphoid tubercle) and distal portions of the carpal tunnel (the hook of the hamate and trapezius tubercle) (Fig. 1a). The flexor retinaculum, a direct continuation of the antibrachial fascia, represents the roof of the carpal tunnel, and it extends from a plane passing through the radiocarpal joint to a plane passing through the proximal metaphysis of the third metacarpal (Fig. 1d). It is located below the long palmar tendon and the palmar fascia, adhering to the pisiform and the hamate’s hook medially and to the tubercle of the scaphoid and the trapezius laterally (Fig. 1b, c). The flexor retinaculum represents the floor of the Guyon canal, which is delimited by the pisiform and the hamate’s hook medially, surmounted by the palmar ligament of the carpus, which acts as a roof; the nerve and the ulnar artery run inside the canal (Fig. 1b). The minimum width of the carpal tunnel is at the level of a plane passing through the hamate’s hook and the tubercle of the trapezius (distal tunnel), where the average width is reduced from 25 to 20 mm and the thickness of the retinaculum is greater than in the proximal tunnel (1–1.6 mm vs. 0.6–1 mm); the average length of the retinaculum is about 26 mm [1, 3, 4]. The flexor retinaculum on the radial side is divided into two fascicles that surround the flexor radialis carpi tendon (FRC), which runs in an osteofibrous tunnel separate from the carpal tunnel proper, delimited by the tubercle of the scaphoid and by the crest of the trapezius that surmounts it (Fig. 1c); it is surrounded by its own synovial sheath, and it is inserted at the base of metacarpals II and III. On the ulnar side, a second tendon is recognizable, which runs outside the carpal tunnel represented by the flexor ulnaris carpi (FUC); it has no synovial sheath, and it inserts partly on the pisiform and partly on the hamate’s hook (the pisohamate ligament) (Fig. 1b). The carpal tunnel is crossed by the median nerve and by nine tendons represented by the flexor pollicis longus (FPL) covered by its own synovial sheath, by the four tendons of the flexor digitorum superficialis (FDS), and by the four tendons of the flexor digitorum profundus (FDP) of the fingers, covered by a common synovial sheath (Fig. 1b–d). About 5 cm upstream of the carpal tunnel, the sensitive palmar cutaneous branch originates from the median nerve, occasionally visible under normal conditions on ultrasound examination. The nerve runs parallel to the median on the radial side for about 2 cm, from which it then separates, reaching the surface below the antibrachial fascia, between the tendons of the palmaris longus and the FRC. Distally the nerve crosses the antibrachial fascia and superficially runs to the retinaculum of the flexors, where it supplies the radial half of the palm. In the carpal tunnel, the median nerve is located medially to the FPL tendon and superficially to the flexor tendons of fingers II and III; it has oval morphology and widens and flattens while progressing through the carpal tunnel (with widths and thicknesses of 6.1 × 2.1 mm in the proximal tunnel and 7.7 × 1.9 mm in the distal tunnel) (Fig. 1b–d) [1–4]. After passing the distal margin of the flexor retinaculum, the median nerve is divided into three common digital branches, which provide sensory supply to the palmar aspect of the first three fingers and the radial half of the fourth, as well as a recurrent motor branch for the muscles of the thenar eminence and the first two lumbricals. The ultrasound aspect of the median nerve is typical of all nerves and depends on the organization of the nerve fascicles, each consisting of several axons and surrounded by loose connective tissue called the endoneurium; each fascicle is wrapped by a common connective sheath called the perineurium (Fig. 2) [5, 6]. Each nerve fasciculus with its surrounding perineurium is immersed in a richly vascularized connective tissue called the epineurium, which is internally looser and externally more tenacious because it separates the nerve from the surrounding environment and protects it from compression, traction, and friction trauma, to which it is constantly subjected by movements of the joints [1, 3]. The nerve is vascularized by perineural vascular plexuses located in the endoneurium, perineurium, and epineurium; the plexuses are mutually connected by oblique and horizontal anastomoses [1, 3]. The smallest nerve structure that can be resolved by ultrasound is the nerve fascicle, which has a hypoechoic echo structure compared with the peri- and epineural connectives, which have a hyperechoic appearance and which are confused with the fibro-adipose connective that surrounds the nerve and the adjacent soft tissues (Fig. 2a–c) [4–6]. In the axial scan, the nerve has a typical “hive” appearance, whereas in the longitudinal scan, it has a fascicular aspect (Fig. 2b–d).
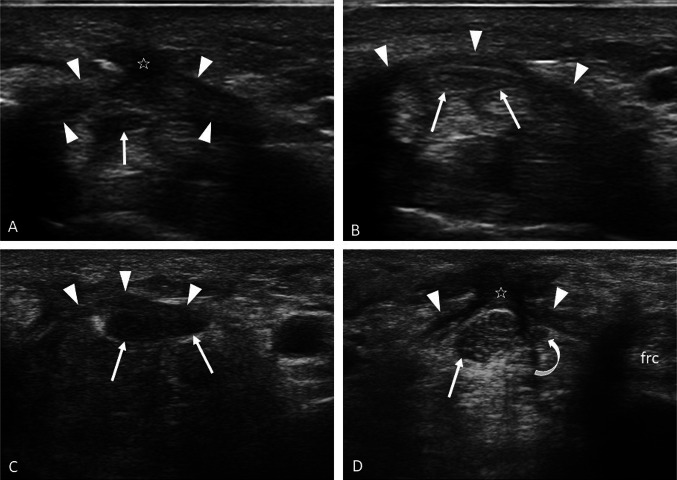
Fig. 1.
a–d Carpal tunnel anatomy. a The bone structures that delimit the carpal tunnel are represented. Axial scans of the proximal carpal tunnel (b) and the distal carpal tunnel (c). The flexor’s retinaculum (arrowheads) is recognizable, which in the proximal tunnel has a convex course towards the palmar surface and in the distal tunnel has a rectilinear course. The median nerve (arrows) is located below the retinaculum, superficially to the flexor tendons of fingers II and III and medially to the FPL; it has an oval morphology in the proximal tunnel and moderately flattens progressing in the distal tunnel. d Longitudinal scan of the carpal tunnel. The median nerve (stars) has a uniform thickness except for minimal flattening at the level of the distal tunnel, and it is located between the retinaculum (arrowheads) and the flexor tendons (arrows); the lunate and the capitate represent the carpal tunnel floor. Sca scaphoid, Pi pisiform, Ha hamate, Tra trapezium, Lu lunate, Ca capitate, Mc metacarpal III, fpl flexor pollicis longus tendon, fs flexor digitorum superficialis tendon, fp flexor digitorum profundus tendon, frc flexor radialis carpi tendon, fuc flexor ulnaris carpi tendon, a ulnar artery, u ulnar nerve
Fig. 2.
a–d Morphology of the normal median nerve. a Axial scan of the median nerve that presents a typical architecture of hypoechoic nerve fascicles delimited by the hyperechoic connective tissue of the perineurium and epineurium, comparable to that of a hive. b, c Longitudinal scan and representative diagram (d) of the nerve, presenting a fascicular architecture because of the alternation between hypoechoic nerve fascicles (black arrows) and hyperechoic connective tissue of the perineurium and epineurium (white arrow)
Anatomical variants
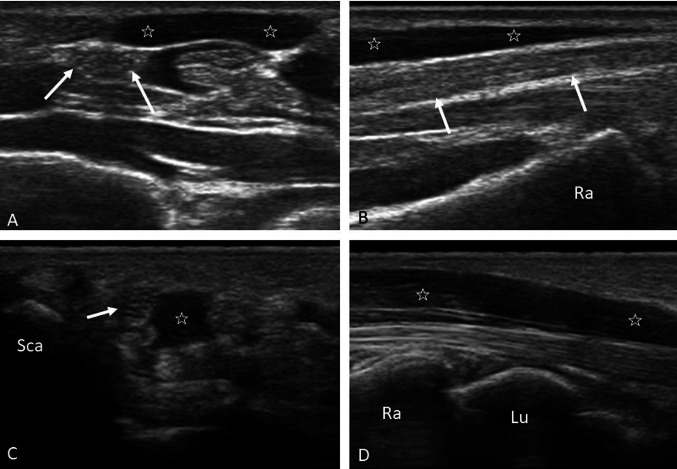
Some anatomical variants that are easily recognizable by ultrasound are of clinical interest because they can favor or cause CTS or because they could be damaged during surgery if not recognized (Figs. 3, 4) [7]. The most common anatomical variant found on ultrasound examination is the early subdivision of the nerve in the forearm into two main fascicles (the median bifid nerve) that run parallel in the carpal tunnel and that may have similar dimensions or in some cases are differently sized, with one fascicle being prevalent over the other (Fig. 3a). They are frequently accompanied by the persistence of the median artery of the forearm, a branch of the ulnar artery that is normally obliterated after birth and that is located between the two branches of the nerve (Fig. 3b); occasionally, the median artery can flank a nonbifid nerve. The two nerves and the median artery can be mutually separated or wrapped by a common epineurium [7, 8]. Muscle bellies can develop inside the carpal tunnel, such as the accessory muscle belly of the flexor superficialis of the index finger (Fig. 4c, d); present an origin inside the carpal tunnel, such as the lumbrical muscles; or insert on the flexor retinaculum, such as the reverse palmar muscle (Fig. 4a, b). These various muscle bellies can cause CTS if hypertrophic [7, 9, 10]. Among the anatomical variants of clinical interest, recall that the synovial sheath of the FPL tendon can be in continuity at the carpal tunnel with the sheath of the flexor tendons of the fingers, which is why septic tenosynovitis of the FPL can easily extend to the common sheath of the finger flexors—for example, after surgery of the trigger finger.
Fig. 3.
a–d Median bifid nerve and patent median artery. B-mode axial scan of the proximal carpal tunnel (a) and color Doppler (b), which highlight two nerve bundles (arrows) separated from each other but surrounded by a common epineurium that includes the median artery (arrowhead). Axial b-mode (c) and color Doppler scanning (d) of a case of acute CTS because of thrombosis of the median artery (arrowhead); the median nerve is bifid (arrows)
Fig. 4.
a–d Anatomical muscle variants. Axial (a) and longitudinal (b) scans of the reverse palmar muscle (stars); note the relationship with the median nerve (arrows), which runs immediately below the muscle. Axial (c) and longitudinal (d) scans of the accessory muscle belly of the superficial flexor of the index finger (stars), which is located within the carpal tunnel in close proximity to the median nerve (arrow). Sca scaphoid, Ra radius, Lu lunate
Carpal tunnel syndrome
Carpal tunnel syndrome (CTS) is the most frequent entrapment neuropathy of peripheral nerves (with an incidence of 1–3 patients in 1000). CTS typically occurs between 45 and 60 years of age, and it is more frequent in women than in men [1].
CTS’s main cause is chronic compression of the median nerve, which is secondary to increased pressure in the carpal tunnel. The carpal tunnel is an osteofibrous canal with a fixed volume, and it is not adaptable to pressure variation. The increase in pressure in the carpal tunnel interferes with venous drainage, causing stasis, hypoxic damage of the capillary endothelium, and an increase in permeability and edema in the carpal tunnel, which leads to a progressive thickening of the flexor tendons’ synovial sheaths. The increased interstitial pressure, the edema, and the sheaths’ thickening in the carpal tunnel are the causes of nerve compression both in idiopathic and in some secondary forms of CTS [1].
The compression is responsible for the alteration of the microcirculation of the nerve, which causes venous stasis, edema, and ischemic suffering involving mainly the epineurium; these events interfere with the axonal flow in the nerve bundles, which manifests itself by a slowing of the conduction speed in structurally intact nerve fibers. In the early stages, the damage could be reversible if the intraneural edema is reabsorbed through the restoration of normal microcirculation and correct electrolyte composition, on which the functionality of the axon depends. If the microcirculation’s damage persists, the edema worsens the hypoxic damage through a reduced oxygen diffusion to axons, Schwan cells, and the endothelium. Consequently, the edema is progressively replaced by intraneural fibrotic tissue, which thickens the epineurium, distorts the architecture of the nerve, and worsens the compression of nerve fibers. Secondary to the damage to myelin and axons, the axons may degenerate, resulting in progressive loss of nerve function and atrophy of the innervated muscles [1].
The carpal tunnel may be narrower on a congenital basis or due to developmental anomalies, which may predispose to a greater increase in pressure. These anomalies may concern bones, such as Madelung deformity or osteopetrosis, or muscles (e.g., abnormal muscle development) (Fig. 4) [1].
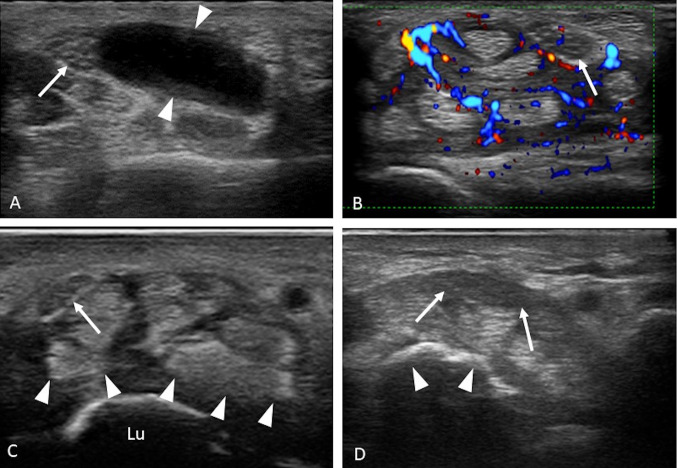
An increase in carpal tunnel content causes increased pressure inside and consequently increased pressure on the nerve. The main causes are edema and inflammatory tissue in simple, immune-mediated or septic tenosynovitis (Fig. 5b); hemorrhage, such as that in fractures, carpal sprains, dislocations (Fig. 5d), or treatment with anticoagulants; crystalline material deposits, such as uric acid crystals in gout (Fig. 5c) and calcium pyrophosphate crystals in pseudogout, or amorphous material deposits (amyloid); and space-occupying lesions, such as ganglion cysts (Fig. 5a), giant-cell tumors of the tendon sheath, lipomas, and vascular malformations [1].
Fig. 5.
a–d Secondary causes of CTS. a Axial scan showing a ganglion cyst (arrowheads) tightly attached to the median nerve (arrows). b Axial color Doppler scan showing hyperplasia and hyperemia of the synovial sheath of the flexors in a case of rheumatoid arthritis; note the recognizable intraneural vascular spots of the median nerve (arrow). c Axial scan of the proximal carpal tunnel showing periarticular hyperechoic crystalline deposits (arrowheads) in a case of gout; the median nerve is displaced and flattened (arrow); lu lunate. d Axial scan of the proximal carpal tunnel, which highlights the volar dislocation of the semilunar (arrowheads); the median nerve is pushed to the surface, thickened, and hypoechoic (arrows)
Systemic endocrine diseases could be CTS’s secondary causes: diabetes, hypo- or hyperthyroidism, and acromegaly. In women, during pregnancy and breastfeeding, hormonal influences can account for the higher incidence of CTS (the balance between estrogen and progesterone). In most cases, CTS is idiopathic—that is, it does not have a cause attributable to one of those mentioned above (Fig. 6) [1].
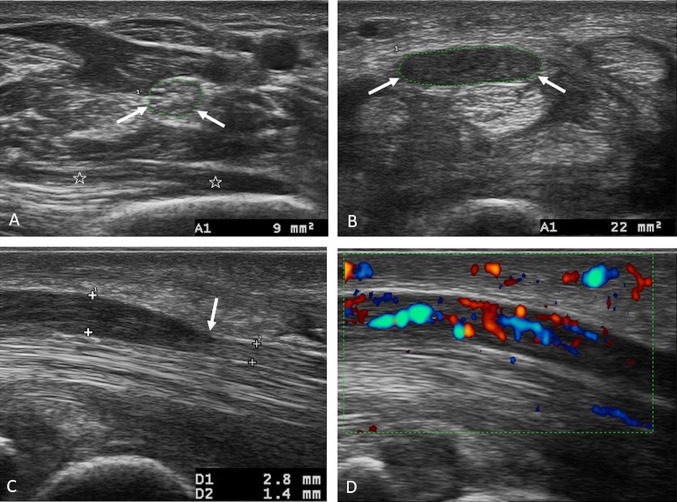
Fig. 6.
a–d Morphological alterations of the median nerve in a case of idiopathic CTS. a Axial scan of the median nerve in the forearm passing through the proximal third of the pronator quadratus muscle (stars); the median nerve has a transverse area within the normal limits (9 mm2). b Axial scan passing through the entrance into the proximal carpal tunnel, showing a marked volumetric increase (a transverse area of 22 mm2) of the median nerve, which is hypoechoic due to edema. Nerve suffering is severe both in absolute value and in the differential calculation between the area in the forearm and the area in the carpal tunnel (13 mm2). c Longitudinal scan of the median nerve in the carpal tunnel, which highlights the compression site at the distal tunnel (arrow); the nerve is thickened and hypoechoic upstream (calipers). d Longitudinal color Doppler scan of the median nerve showing hypervascularization of the nerve upstream of the compression; note the recognizable intraneural vascular plexuses dilated by vascular congestion
The patient complains of pain and nocturnal paresthesia (numbness, tingling, burning or cooling sensation) in the first three fingers and the ulnar side of the ring finger. The patient’s solution is a change in hand position; he or she massages or shakes the hand and sometimes dangles it from the bed. The pain can radiate to the forearm and shoulder. During the day, the typical symptoms occur during activities that require forced postures of the wrist in flexion or extension: easy activities such as tightening the steering wheel, holding the phone, reading a book, sewing, or grasping work tools with force, all of which cause a pressure increase in the carpal tunnel. In severe cases, the patient loses manual dexterity (difficulty in handling small objects, which often fall from the hand) because of loss of sensitivity and atrophy of the intrinsic muscles of the hand innervated by the median nerve [1].
Carpal tunnel syndrome: sonography
Classical CTS diagnosis is based on the patient’s clinical examination and electrophysiological tests, such as electromyography (EMG) and nerve conduction studies (NCS). The latter are helpful for determining the site of nerve compression, assessing its severity, monitoring the course of the disease after therapy, and excluding other causes of median pain, such as cervical radiculopathies, brachial plexopathies, polyneuropathy, or other forms of mononeuropathies. However, the clinical examination and the electrophysiological tests are not able to differentiate idiopathic forms from secondary forms of CTS, and discrepancies are possible between clinical examination and electrophysiological tests (false negatives) [11–14].
Ultrasound examination is able to recognize most of the secondary forms of CTS. It can evaluate the morphological alterations of the nerve and correlate them with the severity of nerve suffering in all cases, even idiopathic ones, with a sensitivity and specificity equal to those of electrophysiological tests [11, 14–16]. It can also highlight some anatomical predisposing variants or conditions that may represent contraindications to minimally invasive treatments. Ultrasound examination also plays a fundamental role in evaluating patients with an unfavorable outcome after surgical treatment [15, 17, 18].
We share a three-step approach in evaluating STC cases:
-Recognition of anatomical variants: the median bifid nerve (Fig. 3a, b) and the patent median artery, whose thrombosis is a rare cause of acute CTS (Fig. 3c, d); and muscle abnormalities such as the reverse palmaris muscle, the superficial flexor muscle accessory belly of the index finger, or the origin of the lumbrical muscles within the carpal tunnel (Fig. 4).
-Recognition of secondary causes of CTS: serous or hyperplastic tenosynovitis; space-occupying lesions (ganglion cysts, giant-cell tumors of the tendon sheaths, lipomas, vascular malformations); crystalline periarticular or articular deposits such as those in gout or pseudogout; hematomas as in cases of carpal fracture, sprains, or dislocations and in patients being treated with anticoagulants (Fig. 5).
-Recognition of morphological alterations of the nerve secondary to compression within the carpal tunnel (Fig. 6).
With longitudinal scans, the compression site is easily recognizable (Fig. 6c), generally at the distal tunnel level, as the nerve flattens where it is compressed and thickens upstream (more rarely downstream of the compression site) because of vascular congestion and edema of the epineurium and nervous fascicles (Fig. 6b, c). Edema causes loss of the fascicular structure of the nerve due to the hypo-echogenicity of the epineurium, which is confused with the swollen nervous fascicles [15, 16, 19, 20]. On power Doppler examination, hyperemia of the swollen nerve is appreciated due to vascular congestion of the peri- and intraneural plexuses (Fig. 6d) [21].
It is possible to quantify the degree of swelling of the nerve by calculating the transverse area of the nerve (n.v. < 10 mm2) in the axial scan, which is maximum upstream of the compression site [14, 19]. It is also possible to evaluate the degree of nerve suffering, which grows as the area grows and which correlates well with the results of electrophysiological tests: the suffering is mild between 10 and 12.9 mm2, moderate between 13 and 14.9 mm2, and severe if > 15 mm2 (Fig. 6b) [12, 22].
In cases of mild suffering, it is possible to improve the diagnostic accuracy of the ultrasound examination—that is, reducing the risk of false positives depending on the physiological variability of the nerve in the individual patient compared with the cut-off considered normal in the general population. This is possible by measuring the difference between the maximum transverse area of the nerve in the carpal tunnel and the area of the nerve evaluated in the forearm at the level of the proximal third of the pronator quadratus muscle; a difference > 2 mm2 has a sensitivity and specificity close to 100% (Fig. 6a, b) [23].
In the case of a bifid median nerve, the threshold values increase because the nerve has larger overall dimensions than in cases with a single fascicle; the absolute threshold value is > 12 mm2, relative to the differential of the area measured in the carpal tunnel and that in the forearm at the level of the pronator quadratus muscle, which is > 4 mm2 [24].
In advanced cases and elderly patients, the nerve may be less swollen than expected based on the severity of the damage due to a loss of the number of axons and a reduction of the edema replaced by fibrotic tissue. In these cases, the neural bundles present a greater echogenicity, and the external epineurium is thickened and hyperechoic (Fig. 8a) [17].
Fig. 8.
a–d Case of partial iatrogenic lesion of the bifid median nerve. a Axial scanning of the proximal tunnel. The two fascicles of the bifid median nerve surrounded by a common epineurium, which is thickened and hyperechoic because of fibrosis, are recognizable; the ulnar fascicle is thickened more than the radial one (arrows). b Axial scan of the distal tunnel. The radial fascicle (arrow) is recognizable, while the ulnar fascicle (star) of the nerve, which is totally encased by scar tissue (arrowheads), is not recognizable. c Longitudinal scan of the median nerve in the carpal tunnel, which highlights the proximal (arrows) and distal (arrowheads) stumps of the amputated nerve (ulnar fascicle) and the gap between the two stumps occupied by scar tissue (stars). d Axial scan of the thenar eminence, which highlights hypotrophy and hyperechoic fat involution of the thenar muscles because of advanced denervation (split screen for comparison with the healthy side); fpl flexor pollicis longus tendon
In addition, signs of denervation of the intrinsic musculature of the hand (the thenar eminence, the first two lumbricals) represented by a reduction in the trophism and hyperechogenicity of the muscles by infiltration of fibro-adipose tissue are also documented (Fig. 8d). Another sign that can be evaluated in CTS cases is the greater protrusion of the retinaculum of the flexors at the level of the distal tunnel, where it assumes a convex course toward the surface instead of the normal rectilinear course.
Recurrent carpal tunnel syndrome after surgery: sonography
The surgical treatment of CTS has the purpose of reducing the pressure inside the carpal tunnel and the consequent compression of the nerve by affecting the flexor retinaculum in all its anatomical extensions up to the antibrachial fascia. A period of correct immobilization will follow to allow adequate scarring of the retinaculum in a more elongated and convex position toward the surface, which increases the anteroposterior diameter of the canal but ensures that the flexor tendons and the median nerve are contained in the carpal tunnel in a slightly more superficial position than normal, without losing its role of a pulley during the flexion of the fingers [18]. After surgery and relief of the symptoms, there is an incomplete decrease in the cross-sectional area of the median nerve compared with clinical and electrodiagnostic tests [25, 26].
After surgical treatment for CTS, the patient may not recover and complain of the same symptoms, worsening symptoms, or new symptoms.
In the event of an immediate worsening of the symptoms or the acute appearance of new symptoms not present before surgery, iatrogenic damage to the nerve, the digital division branches, the palmar skin branch (Fig. 7d), or the recurrent motor branch is to be considered (Fig. 8a–d) [18].
Fig. 7.
a–d Cases of persistent and recurrent CTS after surgery. Axial scans of the distal (a) and proximal (b) carpal tunnel in a case of persistence of symptoms due to failure of incision of the retinaculum at the level of the proximal tunnel (arrowheads) and consequent failure of expansion of the carpal canal; note that in A the stumps of the retinaculum are thickened (arrowheads) and incorporated by the hypertrophic scar (star). c Axial scan of the median nerve in a case of recurrent CTS for adhesions between the nerve (arrows), which is thickened and hypoechoic, and the radial stump of the flexor retinaculum (arrowheads), which is tightly attached to the nerve. d Axial scan at the entrance of the carpal tunnel in a case of recurrent CTS with signs of involvement of the cutaneous sensory branch of the median nerve. The two stumps of the retinaculum (arrowheads) are recognizable and embedded on the surface by the hypertrophic scar (star), which contacts the median nerve (arrows); laterally to the nerve, the cutaneous sensory branch of the median nerve (curved arrow) is recognizable and thickened by cicatricial encasement downstream of the selected scan; frc flexor radialis carpi tendon
In the event that the patient complains of the same symptoms (persistent CTS), the most probable hypothesis is that the flexor retinaculum has not been completely but only partially incised or that there has been scarring with restitutio ad integrum of the retinaculum with no expansion of the carpal tunnel (Fig. 7a, b). The most common site of incomplete incision of the retinaculum is at the distal tunnel level, and more rarely at the proximal tunnel level (Fig. 7b) [18, 25]. Other events to consider in these cases are an incorrect preoperative diagnosis and the presence of tenosynovitis or amyloidosis [18].
In the event that the symptoms improve in the weeks and months following the surgery (by convention a 6-month interval) but subsequently recur within 1 year after the intervention (early recurrent CTS), the main culprit is perineural fibrosis (Fig. 7c, d). Perineural fibrosis can cause traction nerve damage due to focal adhesions or direct compression secondary to encasement [18]. Adhesions are more pronounced in cases of bleeding or extensive synovectomy and in cases of prolonged postoperative immobilization. They can settle between the nerve and the radial stump of the incised retinaculum (Fig. 7c), with the lateral wall of the carpal tunnel and with the sheath of the flexor tendons (fingers I, II, and III). In the case of adhesions with flexor tendons, the symptoms complained of by the patient become more acute with the complete extension of the fingers because of traction damage [18, 27, 28].
In the event that the patient complains of symptoms after years of surgery (late recurrent CTS), the cause is not attributable to scarring or postsurgical perineural fibrosis but to a new increase in pressure in the carpal tunnel linked to its volumetric reduction—for example, in degenerative diseases such as osteoarthritis [18].
Among the possible rare complications that should be remembered after a surgical procedure are the dislocation of the median nerve on the surface beyond the stumps of the retinaculum where it adheres to the skin scar, which is extremely painful even when just touching or extending the wrist, possibly in association with dislocation of the flexor tendons; these complications are the consequence of inadequate healing or removal of a part of the retinaculum. Other complications are infections and injury to the ulnar nerve, flexor tendons, superficial palmar arterial arch, and radial and ulnar arteries [18].
All the abovementioned complications after surgery can be evaluated by ultrasound.
Conclusions
Ultrasound examination is as useful as electrophysiological tests in the evaluation of idiopathic forms of CTS. It is superior to any diagnostic method in the evaluation of most secondary forms of CTS and postsurgical complications. Knowledge of the anatomy of the carpal tunnel and the semiotics of pathological changes of the compressed nerve is essential to allow ultrasound examination to be complementary to electrophysiological tests in the diagnosis of carpal tunnel syndrome.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Luchetti R, Amadio P. Carpal tunnel syndrome. Berlin: Springer-Verlag; 2007. pp. 32–59. [Google Scholar]
- 2.Lee JC, Healy JC. Normal sonographic anatomy of the wrist and hand. Radiographics. 2005;25:1577–1590. doi: 10.1148/rg.256055028. [DOI] [PubMed] [Google Scholar]
- 3.Presazzi A, Bortolotto C, Zacchino M, Madonia L, Draghi F. Carpal tunnel: normal anatomy, anatomical variants and ultrasound technique. J Ultrasound. 2011;14(1):40–46. doi: 10.1016/j.jus.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gitto S, Draghi F. Normal sonographic anatomy of the wrist with emphasis on assessment of tendons, nerves, and ligaments. J Ultrasound Med. 2016;35(5):1081–1094. doi: 10.7863/ultra.15.06105. [DOI] [PubMed] [Google Scholar]
- 5.Silvestri E, Martinoli C, Derchi LE, et al. Echotexture of peripheral nerves: correlation bewteen US and histologic findings and criteria to differentiate tendons. Radiology. 1995;197:291–296. doi: 10.1148/radiology.197.1.7568840. [DOI] [PubMed] [Google Scholar]
- 6.Barbuto L, Di Serafino M, Della Vecchia N, Rea G, Esposito F, Vezzali N, Ferro F, Caprio MG, Vola EA, Romeo V, Vallone G. Pediatric musculoskeletal ultrasound: a pictorial essay. J Ultrasound. 2019;22(4):491–502. doi: 10.1007/s40477-018-0337-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell I, Chesney A, Seal S, et al. Anatomical variations of the carpal tunnel structures. Can J Plast Surg. 2009;17:3–7. doi: 10.1177/229255030901700302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Chen J, Hu B, Jiang LX. Sonographic findings of the bifid median nerve and persistent median artery in carpal tunnel: a preliminary study in Chinese individuals. Clinics. 2017;72(6):358–362. doi: 10.6061/clinics/2017(06)05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sookur PA, Naraghi AM, Bleakney RR, et al. Accessory muscles: anatomy, symptoms and radiologic evaluation. Radiographics. 2008;28:481–499. doi: 10.1148/rg.282075064. [DOI] [PubMed] [Google Scholar]
- 10.Rodrigues J, Santos-Faria D, Silva J, Azevedo S, Tavares-Costa J, Teixeira F. Sonoanatomy of anterior forearm muscles. J Ultrasound. 2019;22(3):401–405. doi: 10.1007/s40477-019-00388-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gitto S, Draghi AG, Draghi F. Sonography of non-neoplastic disorders of the hand and wrist tendons. J Ultrasound Med. 2018;37(1):51–68. doi: 10.1002/jum.14313. [DOI] [PubMed] [Google Scholar]
- 12.El Miedany YM, Aty SA, Ashour S. Ultrasonography versus nerve conduction study in patients with carpal tunnel syndrome: substantive or complementary tests? Rheumatology. 2004;43:887–895. doi: 10.1093/rheumatology/keh190. [DOI] [PubMed] [Google Scholar]
- 13.Rahmani M, Ghasemi Esfe AR, Vaziri-Bozorg SM, et al. The ultrasonographic correlates of carpal tunnel syndrome in patients with normal electrodiagnostic tests. Radiol Med. 2011;116:489–496. doi: 10.1007/s11547-011-0632-6. [DOI] [PubMed] [Google Scholar]
- 14.Fowler JR, Munsch M, Tosti R, et al. Comparison of ultrasound and electrodiagnostic testing for diagnosis of carpal tunnel syndrome: study using a validated clinical tool as the reference standard. J Bone Joint Surg Am. 2014;3:96–113. doi: 10.2106/JBJS.M.01250. [DOI] [PubMed] [Google Scholar]
- 15.Martinoli C, Bianchi S, Gandolfo N, et al. US of nerve entrapments in osteofibrous tunnels of the upper and lower limbs. Radiographics. 2000;20:199–217. doi: 10.1148/radiographics.20.suppl_1.g00oc08s199. [DOI] [PubMed] [Google Scholar]
- 16.Nakamichi KI, Tachibana S. Enlarged median nerve in idiopathic carpal tunnel syndrome. Muscle Nerve. 2000;23:1713–1718. doi: 10.1002/1097-4598(200011)23:11<1713::AID-MUS7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Miwa M, Miwa H. Ultrasonography of carpal tunnel syndrome: clinical significance and limitations in elderly patients. Intern Med. 2011;50:2157–2161. doi: 10.2169/internalmedicine.50.5771. [DOI] [PubMed] [Google Scholar]
- 18.Mosier BA, Hughes TB. Recurrent carpal tunnel syndrome. Hand Clin. 2013;29:427–434. doi: 10.1016/j.hcl.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 19.Wong SM, Griffith JF, Hui ACF, Tang A, Wong KS. Discriminatory sonographic criteria for the diagnosis of carpal tunnel syndrome. Arthritis Rheum. 2002;46:1914–1921. doi: 10.1002/art.10385. [DOI] [PubMed] [Google Scholar]
- 20.Hammer HB, Hovden IAH, Haavardsholm EA, Kvien TK. Ultrasonography shows increased cross-sectional area of the median nerve in patients with arthritis and carpal tunnel syndrome. Rheumatology. 2006;45:584–588. doi: 10.1093/rheumatology/kei218. [DOI] [PubMed] [Google Scholar]
- 21.Ghasemi-Esfe AR, Khalilzadeh O, Vaziri-Bozorg SM, et al. Color and power Doppler US for diagnosing carpal tunnel syndrome and determining its severity: a quantitative image processing method. Radiology. 2011;261:499–506. doi: 10.1148/radiol.11110150. [DOI] [PubMed] [Google Scholar]
- 22.Karadag YS, Karadag Ö, Cicekli E, et al. Severity of carpal tunnel syndrome assessed with high frequency ultrasonography. Rheumatol Int. 2010;30:761–765. doi: 10.1007/s00296-009-1061-x. [DOI] [PubMed] [Google Scholar]
- 23.Klauser A, Halpern EJ, De Zordo T, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250:171–177. doi: 10.1148/radiol.2501080397. [DOI] [PubMed] [Google Scholar]
- 24.Klauser A, Halpern EJ, Faschingbauer R, et al. Bifid median nerve in carpal tunnel syndrome: assessment with US cross-sectional area measurement. Radiology. 2011;250:808–815. doi: 10.1148/radiol.11101644. [DOI] [PubMed] [Google Scholar]
- 25.Abicalaf CA, De Barros N, Sernik RA, et al. Ultrasound evaluation of patients with carpal tunnel syndrome before and after endoscopic release of the transverse carpal ligament. Clin Radiol. 2007;62:891–896. doi: 10.1016/j.crad.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 26.Sueleyman T, Staub F, Dombert T, et al. Sonographic short-term follow-up after surgical decompression of the median nerve at the carpal tunnel: a single-center prospective observational study. Neurosurg Focus. 2015;39:1–5. doi: 10.3171/2015.6.FOCUS15216. [DOI] [PubMed] [Google Scholar]
- 27.Vidoni A, Shrivastava M, Botchu R. Intrasynovial spindle cell lipoma of the deep flexor of the middle finger causing intermittent carpal tunnel syndrome-case report and review of the literature. J Ultrasound. 2018 doi: 10.1007/s40477-018-0281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan TC, Yeo CJ, Smith EW. High definition ultrasound as diagnostic adjunct for incomplete carpal tunnel release. Hand Surg. 2011;16:289–294. doi: 10.1142/S0218810411005564. [DOI] [PubMed] [Google Scholar]