Abstract
The anterior abdominal wall, which is composed of three layers (skin and adipose tissues; the myofascial layer; and the deep layer, consisting of the transversalis fascia, preperitoneal fat, and the parietal peritoneum), has many functions: containment, support and protection for the intraperitoneal contents, and involvement in movement and breathing. While hernias are often encountered and well reviewed in the literature, the other abdominal wall pathologies are less commonly described. In this pictorial review, we briefly discuss the normal anatomy of the anterior abdominal wall, describe the normal ultrasonographic anatomy, and present a wide range of pathologic abnormalities beyond hernias. Sonography emerges as the diagnostic imaging of first choice for assessing abdominal wall disorders, thus representing a valuable tool for ensuring appropriate management and limiting functional impairment.
Keywords: Abdominal wall, Sonography, Abdominal wall sonography
Introduction
The anterior abdominal wall is composed of three layers: skin and adipose tissues; the myofascial layer, consisting of muscles and their fascial envelopes; and a deep layer, consisting of the transversalis fascia, preperitoneal fat, and the parietal peritoneum.
The anterior abdominal wall has many functions, such as containment, support, and protection for the intraperitoneal contents and involvement in movement and breathing.
While hernias are often encountered and are well reviewed in the literature, other abdominal wall pathologies are less commonly described.
In this pictorial review, we briefly discuss the normal anatomy of the anterior abdominal wall, describe the normal ultrasonographic anatomy, and present a wide range of pathologic abnormalities beyond hernias.
The pictorial review is based on the combined experience of our centers, as indicated by the references in the text, with a thorough analysis of the literature from the past 18 years (2001–2019). A systematic search of the literature was performed in PubMed and included original studies and review articles in English dealing with sonographic descriptions of the abdominal wall and related disorders. Case reports and case series were selected according to clinical relevance.
Normal anatomy and sonographic appearance
The anterior abdominal wall has three layers [1]. The most superficial layer consists of the skin and adipose tissues. The middle layer is the myofascial layer, which consists of muscles and their fascial envelopes. The deep layer is formed by the transversalis fascia, preperitoneal fat, and the parietal peritoneum [2, 3].
The most superficial layer provides coverage for the underlying tissues.
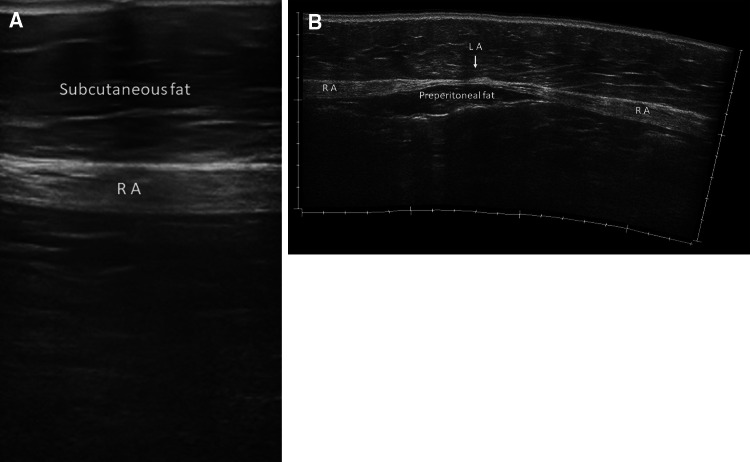
The middle myofascial layer has two vertical paramedian muscles, the rectus abdominis (Figs. 1, 2) and its accessory pyramidal muscle, and three lateral muscles, the obliquus externus, the obliquus internus, and the transversus abdominis (Fig. 3).
Fig. 1.

Ultrasound panoramic image of the anterior abdominal wall. RA rectus abdominis, EO external oblique, IO internal oblique, TA transversus abdominis, LA linea alba, LS semilunar line
Fig. 2.
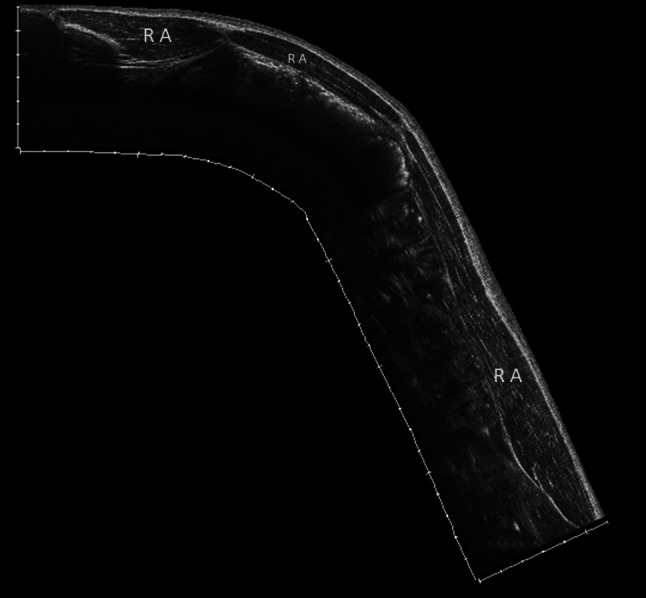
Ultrasound panoramic image of the rectus abdominis. The rectus abdominis muscle extends from the last costal cartilage to the upper edge of the pubis. It is composed of several muscular bodies separated by 3 or 4 tendinous intersections
Fig. 3.

Ultrasound panoramic image of the lateral abdominal wall. Laterally, the external oblique (EO), internal oblique (IO), and transversus abdominis (TA) muscles extend from the lateral edge of the rectus to the flanks with three overlapping layers
The rectus abdominis muscles extend from the last costal cartilage to the upper edge of the pubis (Fig. 2). They are composed of several muscular bodies separated by three or four tendinous intersections.
The pyramidal muscles are small and variable triangular muscles, localized between the pubis and the linea alba.
Laterally, the external oblique, the internal oblique, and the transversus abdominis muscles extend from the lateral edge of the rectus to the flanks with three overlapping layers (Fig. 3).
The aponeuroses of these muscles form the rectus sheaths, enveloping the right and left rectus muscles and forming the linea alba [4, 5]. In the superficial part, the sheath extends over the entire abdominal wall, at the back, and it stops at its lower part and forms a fibrous arch: the arcuate line. It has a variable position but is often localized in the upper second quarter of the umbilical–pubic distance. The linea alba opposes the diastasis of the rectus abdominis muscles.
At the lateral edge of the rectus abdominis, the semilunar line marks the interface with the oblique muscles (Fig. 1).
The deep layer of the anterior abdominal wall, formed by the transversalis fascia, preperitoneal fat, and the parietal peritoneum, is quite elastic and covers and protects the underlying viscera.
The skin of the anterior abdominal wall is vascularized by arteries (the perforating arteries) that arise from the superior and inferior epigastric arteries, pass through the body of the rectus abdominis muscles, perforate the aponeurosis of the sheath, and with an oblique course go to vascularize the subcutaneous fatty tissue.
Additionally, small tributaries of the lower six internal intercostal arteries contribute to the blood supply of the rectus abdominis muscle and rectus sheath [6].
The arteries run with the superior and inferior epigastric veins. Deep lymphatics travel with the epigastric veins.
The ventral rami of the spinal nerves supply the rectus abdominis muscles and sheath. The thoracoabdominal nerves, arising from the T7–T11 spinal segments and the subcostal nerve (T12), innervate the rectus abdominis muscle. The subcostal nerve innervates the pyramidalis muscle [7].
Abdominal wall hernias
Muscles and tissues provide strength to the abdominal wall to hold all the contents of the abdominal cavity. Sometimes, there is an opening in the abdominal wall, allowing what is inside to push through to the outside; this is called a hernia.
Some hernias occur in natural openings in the abdominal wall (umbilical, paraumbilical, and Spigelian hernias) (Table 1) that allow body structures to go from the inside to the outside of the abdomen.
Table 1.
Abdominal hernia anatomy
| Umbilical | Herniation through the umbilicus |
|---|---|
| Paraumbilical | Herniation through the linea alba |
| Spigelian | Herniation in the Spigelian aponeurosis between the linea semilunaris and the lateral border of the rectus muscle |
| Incisional parastomal | Herniation through an incisional site or adjacent to a stoma |
Umbilical hernias occur in the abdominal wall near the umbilicus. True umbilical hernias are common in otherwise healthy infants. The sac protrudes through the remnant of the vitellointestinal duct that has failed to cicatrize, usually as a result of neonatal sepsis. True umbilical hernias can also be seen in adults with chronically elevated intra-abdominal pressure, such as ascites caused by liver failure (Fig. 4), chronic ambulatory peritoneal dialysis, or slowly enlarging intraperitoneal masses.
Fig. 4.

Umbilical hernia. Ultrasonography shows ascites (arrows) caused by liver failure through a defect (caliper) in adults with chronically elevated intra-abdominal pressure
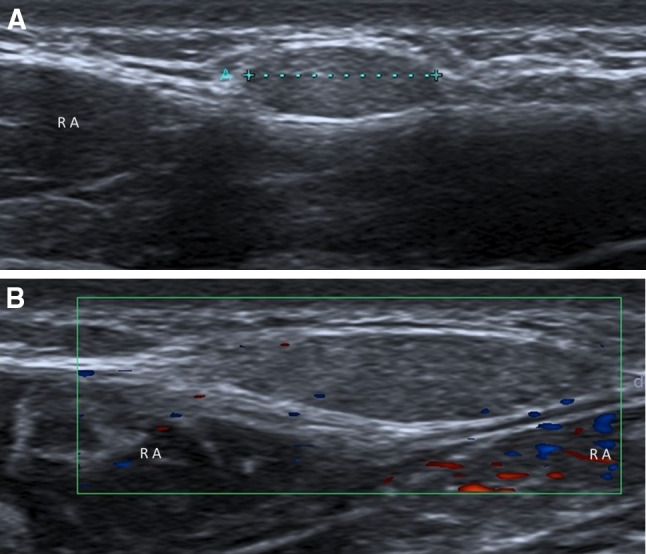
Paraumbilical hernias (Fig. 5) typically occur in corpulent women between the ages of 35 and 50. They usually occur through a defect in the linea alba just cranial to the umbilical cicatrix, from where they extend anteroinferiorly into the umbilicus [8, 9].
Fig. 5.
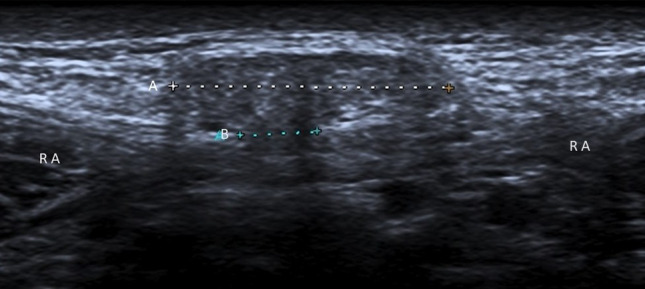
Paraumbilical hernia. Ultrasonography shows preperitoneal fat (A: caliper) through a defect (B: caliper) in the linea alba, just cranial to the umbilical cicatrix. These hernias typically occur between 35 and 50 years of age. RA rectus abdominis
A Spigelian hernia is a protrusion through the Spigelian fascia. The hernia is often interparietal and frequently has no mass at physical examination.
When people have undergone abdominal surgery, sometimes the incisions where the abdominal cavity was entered do not heal well, and a hernia can form in this location; this is known as an incisional hernia. Incisional hernias occur in approximately 10% of all anterior abdominal wall closures. They are more common in obese and elderly patients and are associated with persistent postoperative coughing and abdominal distension. The abdominal wall closure is particularly prone to dehiscence after peritonitis. Hernias occur more commonly through incisions in the hypovascular linea alba than through transverse muscle-splitting approaches [10, 11].
A parastomal hernia is an incisional hernia in which the bowel loops herniate through the abdominal wall defect that was created for the stoma.
Mesentery (Fig. 6), bowel (Fig. 7), and fat are the most common hernia contents, but solid organs may also herniate through abdominal wall defects.
Fig. 6.
Umbilical hernia. Ultrasonography shows omental fat (A: caliper) through a defect (B: caliper) in the linea alba
Fig. 7.

Incisional hernia. Ultrasonography shows bowel loops (A: caliper) through a defect (B: caliper) of the abdominal wall
Abdominal wall hernias are common surgical problems, usually managed without radiologic evaluation, so most imaging of hernias is probably incidental to the investigation of an unrelated clinical problem. However, physical examination alone can be difficult and unrewarding if the patient is obese, if the hernia is small, or if the hernia arises in a rare anatomic location. The use of computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI) for the investigation of abdominal wall hernias is well documented. Each of these imaging techniques has been shown to be more sensitive and specific than physical examination in the detection of difficult hernias in difficult patients [12–19].
The advent of noninvasive cross-sectional imaging techniques has lowered the clinician's threshold for investigating diagnostically challenging patients.
Ultrasound has a high sensitivity (85–92.7%) and specificity (81.5–93.8%) for detecting abdominal wall hernias. It is also relatively cheap, widely available, and safe and is therefore becoming the first-line imaging tool in many centers. Real-time imaging with ultrasound allows assessment of the patient in several positions and maximizes the chance of showing the hernia while the patient performs a Valsalva maneuver [20, 21].
CT has a reported sensitivity of 83% (and a specificity of 67–83%) and is also being more widely used to investigate hernias. It is limited by the necessary supine position of the patient and the lack of real-time imaging, which is prohibited by the large doses of ionizing radiation involved. It is, however, particularly useful when ultrasound is limited by the patient’s obesity or when the size of the hernia precludes accurate sonographic assessment of the relevant anatomy [22].
MRI is relatively expensive and less available in most centers, but it is capable of showing exquisite anatomic detail without the need for ionizing radiation. Direct comparison of MRI and ultrasound has shown that they are comparably sensitive techniques (94.5% and 92.7%, respectively) [23].
The most common complications of abdominal wall hernias are bowel obstruction secondary to the hernia, incarceration, and strangulation. Presenting symptoms may include abdominal pain, vomiting, and distention. Physical examination may reveal a firm, tender abdominal wall mass. Abdominal distention, dehydration, or peritoneal signs eventually become manifest [24].
In the United States, complications related to external hernias represent one of the most common reasons for emergent surgery performed in patients over 50 years old [25, 26, 27].
Over one million hernia repairs are performed annually. Surgical correction of hernias is currently the major operation most frequently performed by general surgeons in the United States and the second most common abdominopelvic surgery after cesarean section [28].
To prevent acute complications, external hernias are usually repaired electively [29].
Repair of abdominal wall hernias with synthetic patches was first described in 1962. Since then, these patches have been widely used, and the various procedures using mesh in abdominal wall repair have become a commonplace treatment. Several reports have shown that compared with simple sutures, mesh is superior, with significantly reduced recurrence rates [30–32].
Materials from which the mesh is manufactured are usually derived from polypropylene or polytetrafluoroethylene and typically function by providing a bridge across deficient tissues. The mesh is incorporated into the adjacent tissues and should restore the structure and function of the abdominal wall [33].
Diastasis of the rectus abdominis muscles
Traction of the rectus abdominis muscles can lead to a widening of the linea alba, causing a clinical condition known as diastasis of the rectus abdominis muscles (DRAM).
DRAM is characterized by thinning and widening of the linea alba, combined with laxity of the ventral abdominal musculature. This causes the midline to “bulge’’ when intra-abdominal pressure is increased. This clinical condition is often seen in pregnancy [34, 35].
According to the Beer classification, DRAM is defined as an interrectus distance (IRD) of 22 mm or more, assessed three centimeters above the umbilicus. This measure must be taken in a relaxed state of the patient, avoiding traction that can make muscles to get closer to each other. DRAM is frequently misclassified as a primary ventral hernia, although the musculofascial continuity of the midline and the subsequent absence of a true hernia sac is what sets DRAM apart from a ventral hernia [36].
Although the association is not definitive, the presence, size, and duration of DRAM have been linked to pelvic and low back pain [37, 38].
DRAM has been found to weaken abdominal muscles [39] and disturb their functions in lumbopelvic stability; it has also been associated with pelvic floor dysfunction [40].
In clinical practice, measurement of DRAM width is often performed to screen for the presence of clinically important DRAM, determining whether a widening is over or under an accepted cut-off value [39, 41, 42].
Ultrasound and calipers are satisfactory tools for DRAM measurement. This has been supported by empirical results [43] and further calculations of diagnostic accuracy values based on these results [44].
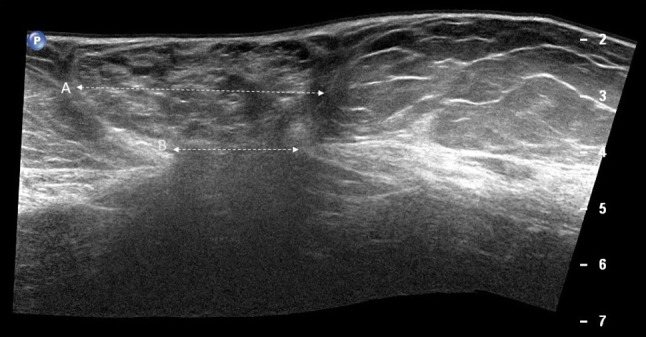
Ultrasound has been most widely researched with regard to its reliability and has shown to be a reliable method when images were taken by experienced sonographers. Calipers also seem a reliable method for measuring DRAM width (Fig. 8) [45].
Fig. 8.
Diastasis of the rectus abdominis muscles (a, b). Diastasis of the rectus abdominis muscles is defined as an interrectus distance of 22 mm or more (a calipers), assessed three centimeters above the umbilicus. This measure must be taken in a relaxed state of the patient, avoiding traction that can make muscles to get closer to each other. b Ultrasound panoramic image. RA rectus abdominis, LA linea alba
For clinical out- and in-patient services and many research purposes, CT and MRI scans are not feasible methods for measuring DRAM width. Also, insufficient evidence was found for them to be considered “gold standard” as often claimed [46, 47].
DRAM is mostly treated conservatively, with or without the help of a physiotherapist [48].
In case of severe functional or cosmetic impairment, the patient can be referred to a plastic or general surgeon. If surgical treatment is considered, several techniques, ranging from laparoscopic, endoscopic, hybrid, and open repairs, are available. Currently, there is no consensus on the preferred surgical management of DRAM [49].
Atrophy of the abdominal wall muscles
Muscle atrophy is the loss of muscle mass, which can be caused by aging, malnutrition, immobility, medications, and a wide range of injuries or diseases that impact the musculoskeletal or the nervous system.
The most frequent cause is advancing age, which brings progressive loss of muscle strength, muscle mass, and muscle quality, resulting in a condition known as sarcopenia. Age-related muscle atrophy has been associated with a decrease in motor function. Atrophy in particular was associated with chronic bed rest and is more marked in the antigravity muscles, such as back and abdominal muscles [50–52].
Furthermore, atrophy of the abdominal wall muscles is a significant side effect of laparotomy, which, despite the widespread use of laparoscopy, is still frequently required and performed. In a study, chevron incisions were found to result in more atrophy compared with midline incisions [53].
The etiology is twofold: physical transection and remodeling of muscle fibers and, more importantly, direct transection of the intercostal nerves. Management of patients with symptomatic abdominal wall atrophy is supportive only.
In our experience, atrophy of the muscles of the abdominal wall is also frequent in obese persons, and it represents a problem for bariatric surgery.
Regardless of the cause, muscle atrophy is characterized by the replacement of muscle tissue with fat and fibrous tissue.
Atrophy of the abdominal wall muscles can be objectified by measuring muscle thickness. On ultrasound images, infiltration of fat and fibrous tissue increases muscle echo intensity and increases interface and attenuation, and the muscles become whiter (Fig. 9) [54, 55].
Fig. 9.
Muscular atrophy in an obese patient (a, b). On an ultrasound image, the infiltration of fat and fibrous tissue increases muscle echo intensity (a) and increases interface, and the muscles become whiter (b ultrasound panoramic image). There is marked hypertrophy of the subcutaneous adipose tissue. RA rectus abdominis, LA linea alba
Sarcopenia, myosteatosis, and impaired aerobic fitness (objectively measured by reduced oxygen uptake) have been associated with poor postoperative outcomes and survival [56].
Hematomas and injuries of the abdominal wall intrinsic muscles
Hematomas of the abdominal wall muscles are frequent in patients under anticoagulant treatment. The muscular sheath prevents their extension, but the pressure causes acute pain. They are more or less hypoechoic masses (Fig. 10), sometimes pure liquid. Simple monitoring is the rule in the absence of hemodynamic repercussions.
Fig. 10.
Hematoma of the rectus abdominis muscle. a Transverse ultrasound scan. b Longitudinal ultrasound scan. Hematoma: arrows. RA rectus abdominis
Intrinsic muscle injuries of the abdominal wall are rather rare but have been described in several studies. They occur due to the contraction and simultaneous elongation of the muscles, leading to the destruction of myofibers [57].
Athletes of some sports are most frequently affected by intrinsic muscle injuries of the abdominal wall. These players often have asymmetrical hypertrophy of the muscle on the side opposite to the dominant arm, and the tears are almost always on the contralateral side of the dominant arm [58]. Usually, tennis players complain of sudden-onset pain and point tenderness that generally occur during service and smash in the side opposite to the dominant arm, and the injuries often affect the rectus abdominis muscle [59]. Volleyball and handball players make similar biomechanical movements, and the injuries usually affect the rectus abdominis muscle on the nondominant side. The injury mechanism is indirect at the time of the flexion/extension transition when players serve or attack in the case of volleyball or throw in the case of handball [60, 61]. In baseball players, the abdominal muscles (the internal and external oblique, rectus, and transversus abdominis muscles) play an important role in pitching and hitting. Proper abdominal muscle activation during throwing and swinging is crucial for generating optimal ball velocity and bat speed.
Injuries contralateral to the dominant arm are more common and usually affect the internal/external oblique muscle [62]. For similar reasons, hockey players’ tears frequently affect the oblique muscles [63]. In these sports, the abdominal muscle injuries involve similar sequences and are produced during brusque flexoextension movements and rotations of the trunk during eccentric contraction or concentric–eccentric transitions in the serve or attack in volleyball, the serve in tennis, the batting in baseball, the throwing of the ball in handball, or the hitting of the hockey puck; the injuries start in the legs and transfer to the trunk, the shoulder, and the arm [61].
Sports that involve hitting or throwing a ball with the use of the upper limbs can lead to injuries to the abdominal wall muscles. Ultrasound examination of a patient with suspected muscle injury should always be preceded by an accurate reporting of the patient’s medical history, including the mechanism of injury, symptoms, possible pain, and loss of strength.
Careful clinical evaluation may reveal the presence of morphological alterations, such as a subcutaneous hematoma.
Ultrasound scanning of the abdominal muscles should be transverse and longitudinal from the proximal attachment to the distal attachment, including the myotendinous junction and enthesis. On sonography (Fig. 11), the muscle tear appears as a disruption of the normal echogenic fibrillar pattern and the presence of anechoic clefts and irregular linear bands with or without fluid collections [64].
Fig. 11.

Intrinsic muscle injuries. Tears appear as a disruption of the normal fibrillar pattern (arrows). a Longitudinal ultrasound scan, b transverse ultrasound scan and presence of fluid collections
Abdominal wall endometriosis
Endometriosis is the presence of endometrial glands and stroma outside the endometrial cavity. The ectopic tissue is responsive to ovarian hormonal stimulation and proliferates when stimulated by cyclic estrogens, thus seeming to “menstruate.”
Endometriosis was first described by Rokitansky [65]. It generally occurs in pelvic sites, such as the ovaries, bowel, or pelvic peritoneum, but it rarely arises in extrapelvic sites, such as surgical scars [66].
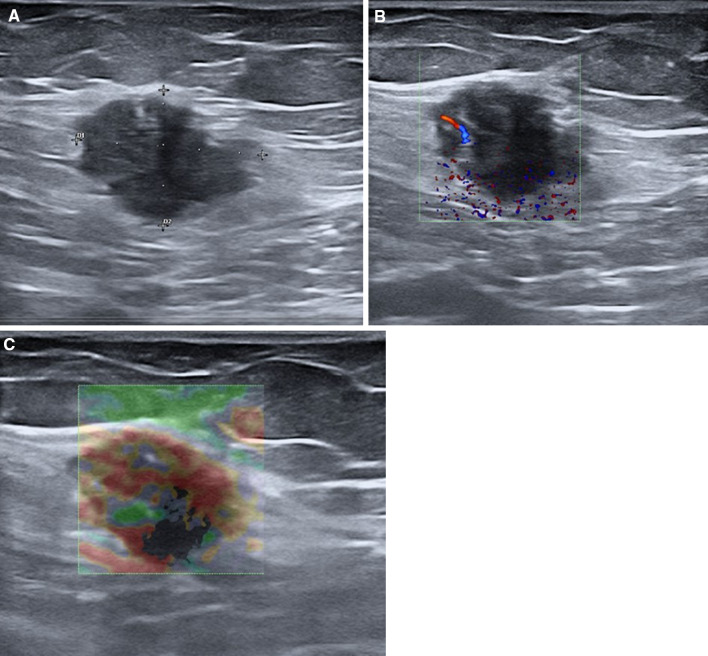
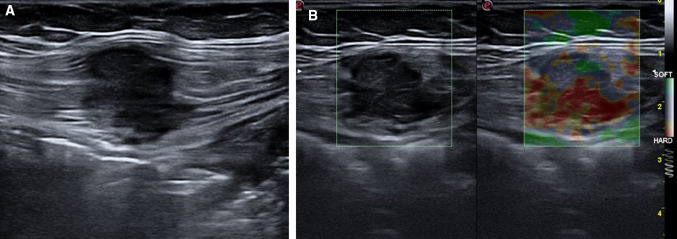
Nowadays, surgical scar endometriosis following obstetric and gynecological procedures is more frequent due to an increasing number of caesarian sections worldwide [67]. Endometriosis of the abdominal wall (AWE) can affect the cutaneous–subcutaneous (Fig. 12) or intramuscular tissues (Fig. 13).
Fig. 12.
Endometriosis of subcutaneous tissues. B-mode ultrasound shows a hypoechoic mass inside the subcutaneous tissues (a) with intralesional vascular signals with color Doppler ultrasound (b) and a hard pattern with strain elastosonography (c)
Fig. 13.
Endometriosis of muscular tissues. B-mode ultrasound shows a hypoechoic mass inside the muscular tissues (a), with a hard pattern with strain elastosonography (b)
The occurrence of symptoms and the growth of the endometriosis depend on estrogen stimulation, so a periodic increase in pain intensity associated with menstruation can occur [66].
Abdominal wall endometriosis (AWE) is often misdiagnosed with several other pathological conditions, such as desmoid tumors, lymphomas, hernias, metastatic carcinomas, sarcomas, and hematomas [68].
Several studies found the time interval between surgery and clinical presentation to be between 3 months and 10 years [69].
The physiopathological processes underlying endometriosis are unclear, and three theories (tubal regurgitation, celomic metaplasia, and vascular spread) have been postulated to explain it [70].
A widely accepted explanation for the presence of endometriosis in unusual sites (e.g., the lungs, the brain, and incisional scars) is that endometrial cells are transported through hematogenous, lymphatic, or iatrogenic routes [70]. Some authors suggested that natural killer activity and/or altered peritoneal macrophage maturation may play a role in its pathogenesis [71].
Health care providers should suspect cutaneous endometriosis in any women with pain and a lump in the incisional scar after pelvic surgery [72]. Cesarean section (CS) is the most common surgery performed around the world; the World Health Organization (WHO) suggests a cesarean rate of 5–15%, but the worldwide percentage is higher [73]. Generally, abdominal wall endometriosis is confined to the peritoneal surface, and it is mainly associated with cesarean section (incidence 1–2%), but it may also result from a previous surgical procedure [69].
The pathogenesis of endometriosis is complex, and AWE is believed to be the result of mechanical iatrogenic implantation, through the direct inoculation of the abdominal fascia and/or subcutaneous tissue with endometrial cells during the surgical intervention, which, under estrogen stimulation, become active and expand [70].
Some authors have examined factors contributing to CSE and defined possible causes, including the easy separation and transport of endometrial cells by the amniotic fluid flow into the pelvic cavity after hysterotomy; the large amount of endometrial cells spreading into the pelvis before hysterotomy closure, which can become trapped in the wound; and the nurturing role of blood and hormones after inoculation of the cells, allowing them to grow and develop into subcutaneous masses [74].
It is important to highlight that a higher incidence is reported after early hysterotomy (end of the second or beginning of the third trimester), as the early decidua seems to have more pluripotential capabilities, potentially resulting in enhanced cellular replication producing endometriosis [73]. Endometriosis guidelines report that only histological examination can provide the definitive confirmation of the diagnosis [75]. However, medical history together with a gynecological examination has a combined sensitivity of around 80% for diagnosing endometriosis. As evidenced in our cases, patients are referred to medical examination due to the presence of abdominal/pelvic pain that often has no clear and immediate anatomical and pathological explanations; therefore, a diagnosis of irritable bowel or functional disorder is often wrongly reached.
As previously mentioned, endometriosis location can be variable and widespread; the qualitative assessment of pain often shows a close relationship with the menstrual cycle, and this represents the main clue for the diagnosis of endometriosis [75].
B-mode ultrasound images with a high-frequency linear probe can identify the presence of endometriosis foci inside the superficial tissues of the abdominal wall showing the hypoechoic nodule located inside the tissues (cutaneous, subcutaneous, or muscular) (Figs. 12a, 13a). Fine intralesional vascular spots are depicted with color-power Doppler (Fig. 12b), and a hard pattern of the mass is identified with the elastosonography strain modality (Figs. 12c, 13b) [76].
Vascular malformation of the abdominal wall
Vascular anomalies may be isolated or multiple and rarely affect the abdominal wall.
In 1982, Mulliken and Glowacki created the first classification based on the normal development, cellular kinetics, and histopathology of vascular abnormalities. Endothelial malformations are biologically classified into two major groups: hemangiomas and vascular malformations [77].
In 1996, in Rome, the International Society for the Study of Vascular Anomalies (ISSVA) also classifies vascular anomalies into tumors and malformations (Table 2).
Table 2.
Vascular anomalies
| Vascular tumors | Hemangiomas, hemangioendotheliomas, angiosarcomas |
|---|---|
| Vascular malformations | Slow flow (capillary, venous, and lymphatic) and fast flow (arterial and arteriovenous) anomalies |
Vascular tumors are hemangiomas, hemangioendotheliomas, and angiosarcomas, while vascular malformations are classified based on blood flow rate as slow-flow (capillary, venous, and lymphatic) and fast-flow (arterial and arteriovenous) anomalies [78].
This classification was broadened and detailed during the 2014 ISSCA workshop in Melbourne, which further categorized vascular tumors and malformations into subdivisions of these two groups. Tumors are classified according to their clinical behavior, and malformations are classified according to their flow characteristics, histopathologic features, and associations with other anomalies [79].
The classification system was recently updated during the May 2018 ISSCA meeting in Amsterdam [80].
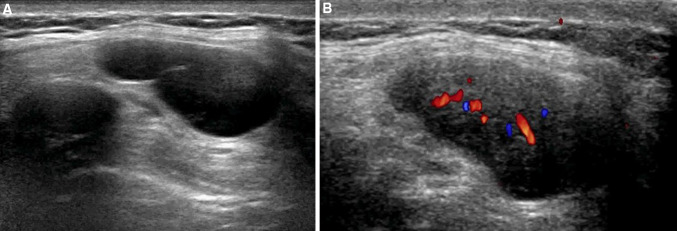
Vascular malformations are commonly seen in the head, neck, and extremities and rarely affect the trunk [81]. There are only a few case reports regarding AVM and vascular tumors of the anterior abdominal wall [82–84]. Ultrasound and MRI, along with magnetic resonance angiography (MRA), are the most used imaging modalities for the evaluation of vascular anomalies of the abdominal wall [85].
Whereas ultrasound can assess the vascularity of the anomaly (Fig. 14), the type of vessels feeding the lesion, and the presence of a high-flow component, MRI and MRA are excellent methods for depicting the anatomical extent, multicompartmental involvement, and vascular angioarchitecture. Ultrasound is great for follow-up and helps guide interventional therapies. CT is rarely used due to radiation concerns. Digital subtraction angiography (DSA) is usually performed for therapeutic intervention [86].
Fig. 14.

Vascular malformation of the abdominal wall. Ultrasound shows dilated vessels in the subcutaneous tissues (a, b panoramic view). Color Doppler ultrasound confirms the vascular nature of the lesions. RA rectus abdominis, EO external oblique, IO internal oblique, TA transversus abdominis, LA linea alba, LS semilunar line
Abdominal wall tumors
All the soft tissue tumors can be found in the abdominal wall, but the most frequent ones are lipomas (Fig. 15), myxoid tumors (Fig. 16), and neurofibromas (Fig. 17).
Fig. 15.
Lipoma. Ultrasound shows an oval, isoechoic mass (a) with its greatest diameter parallel to the skin; it is not hypervascularized (b) on color Doppler imaging
Fig. 16.
Desmoid tumor. Ultrasound shows an oval, poorly defined, solid, nonencapsulated mass (a) with internal vascularity (b, c)
Fig. 17.
Neurofibroma. Ultrasound shows an oval, hypoechoic mass with well-defined margins (a) and internal vascularity (b)
The most common benign tumor of the abdominal wall is a lipoma [87]. Lipomas are mesenchymal tumors made up of mature adipose tissue [88].
They can affect any region of the body and can occur at any age, but they are more common in the fifth decade and are multiple in 5% of patients. Often associated with a period of weight gain, lipomas are painless and can vary in size, from very small to giant.
Lipomas can be located in superficial tissues (subcutaneous) or less frequently in deep tissues (subfascial tissues) [89]. The superficial lipomas generally can be clinically detected and appear as compressible, palpable soft tissue masses that do not adhere to the surrounding tissue.
Lipomas may be located in subcutaneous tissues (anterior to the muscle fascia), above muscles (supramuscular), between muscles (intermuscular), within muscles (intramuscular), or below muscles (submuscular) [90].
The deep-seated lipomas are hard to detect clinically, and not infrequently, the clinical presentation of such a lipoma is similar to that of a sarcoma [91].
Ultrasound is generally performed to confirm the clinical diagnosis. Lipomas may have a variable echotexture (hypoechoic, isoecogenic, or hyperechoic) relative to adjacent fat or muscular tissues, can be homogeneous or inhomogeneous, and can be encapsulated or nonencapsulated, and sometimes their differentiation from other masses is difficult [92].
Usually, they present as an oval ipoechoic compressible mass (Fig. 15a) with its greatest diameter parallel to the skin, containing short linear reflective striations that run parallel to the skin. Lipomas are usually not hypervascularized at color (Fig. 15b) or power Doppler imaging [92].
Desmoid tumors (also known as aggressive fibromatosis) are rare benign tumors derived from mesenchymal progenitor cells with a locally aggressive tendency for recurrence but not with metastatic potential [93].
They make up 0.03% of all neoplasms and less than 3% of all soft tissue tumors [94]. Typically, they occur in women, particularly in young women (25–40 years of age), and are usually associated with previous surgical scars, trauma, estrogen therapy, pregnancy, familial adenomatosis polyposis, and Gardner syndrome [93].
Desmoid tumors can develop in any part of the body, such as musculoaponeurotic structures and connective tissues, but especially in the abdominal wall (the rectus and internal oblique muscles and their fascias) [95].
The course of desmoid tumor development is unpredictable, as spontaneous regression, a long-lasting stable state, and disease progression can occur, and reliable and validated predictive factors are lacking.
Desmoid tumors comprise at least two different clinicopathological entities: sporadic desmoid tumors and desmoid tumors associated with germline mutations of APC [96].
On ultrasound, desmoids frequently appear as oval, poorly or well-defined solid soft tissue masses with variable echogenicity (Fig. 16a) that are nonencapsulated and can infiltrate the surrounding tissue, generally abdominal wall muscles. They may present internal vascularity (Fig. 16b, c) and, characteristically, do not cross the midline [97].
Peripheral nerve tumors are mostly benign and include two histotypes: schwannomas and neurofibromas [98].
Neurofibromas infiltrate nerve trunks, and many neurofibromas require nerve resection and graft for removal.
Neurofibromas are round, oval or fusiform, and hypoechoic with well-defined margins, and internal cystic changes may be observed (Fig. 17). The most important criterion for the ultrasound diagnosis is the relation of the mass to a nerve [99].
Conclusions
A variety of disorders affecting the abdominal wall is commonly encountered in daily clinical practice. Knowledge of the medical history, as well as the symptoms and signs, is basic for a correct diagnostic approach. However, the clinical presentations are often similar, and imaging is required to narrow down the list of different diagnoses. Sonography is effective in assessing abdominal wall disorders, thus representing a valuable tool for ensuring appropriate management and limiting functional impairment.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Grevious MA, Cohen M, Shah SR, Rodriguez P. Structural and functional anatomy of the abdominal wall. Clin Plast Surg. 2006;33(2):169–179. doi: 10.1016/j.cps.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Parikh KR, Al-Hawary M, Millet JD, Burney R, Finks J, Maturen K. Incisional hernia repair: what the radiologist needs to know. Am J Roentgenol. 2017;209(6):1239–1246. doi: 10.2214/AJR.17.18137. [DOI] [PubMed] [Google Scholar]
- 3.Hellinger A, Roth I, Biber FC, Frenken M, Witzleb S, Lammers BJ. Surgical anatomy of the abdominal wall. Chirurg. 2016;87(9):724–730. doi: 10.1007/s00104-016-0257-3. [DOI] [PubMed] [Google Scholar]
- 4.Punekar IRA, Khouri JS, Catanzaro M, Shaikh AL, Langstein HN. Redefining the rectus sheath: implications for abdominal wall repair. Plast Reconstr Surg. 2018;141(2):473–479. doi: 10.1097/PRS.0000000000004043. [DOI] [PubMed] [Google Scholar]
- 5.Naraynsingh V, Maharaj R, Dan D, Hariharan S. Strong linea alba: myth or reality? Med Hypotheses. 2012;78(2):291–292. doi: 10.1016/j.mehy.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 6.El-Mrakby HH, Milner RH. The vascular anatomy of the lower anterior abdominal wall: a microdissection study on the deep inferior epigastric vessels and the perforator branches. Plast Reconstr Surg. 2002;109(2):539–543. doi: 10.1097/00006534-200202000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Stecco C, Azzena GP, Macchi V, Porzionato A, Behr A, Rambaldo A, Tiengo C, De Caro R. Rectus abdominis muscle innervation: an anatomical study with surgical implications in DIEP flap harvesting. Surg Radiol Anat. 2018;40(8):865–872. doi: 10.1007/s00276-017-1944-6. [DOI] [PubMed] [Google Scholar]
- 8.Smith DC. Umbilical, epigastric and rarer abdominal wall hernias. In: Morris PJ, Wood WC, editors. Oxford textbook of surgery. 2. Oxford: Oxford University Press; 2000. pp. 1877–1881. [Google Scholar]
- 9.Khati NJ, Enquist EG, Javitt MC. Imaging of the umbilicus and peri-umbilical region. Radiographics. 1998;18:413–431. doi: 10.1148/radiographics.18.2.9536487. [DOI] [PubMed] [Google Scholar]
- 10.Bucknall TE, Cox PJ, Ellis H. Burst abdomen and incisional hernia: a prospective study of 1129 major laparotomies. Br Med J. 1982;284:931–933. doi: 10.1136/bmj.284.6320.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenall MJ, Evans M, Pollock AV. Midline or trans- verse laparotomy? A random controlled clinical trial. Part 1: influence on healing. Br J Surg. 1980;67:188–190. doi: 10.1002/bjs.1800670308. [DOI] [PubMed] [Google Scholar]
- 12.Yeh H-C, Lehr-Janus C, Cohen BA, et al. Ultrasonography and CT of abdominal and inguinal hernias. J Clin Ultrasound. 1984;12:479–486. doi: 10.1002/jcu.1870120805. [DOI] [PubMed] [Google Scholar]
- 13.Chou T-Y, Chu C-C, Diau G-Y, et al. Inguinal hernia in children: US versus exploratory surgery and intraoperative contralateral laparoscopy. Radiology. 1996;201:385–388. doi: 10.1148/radiology.201.2.8888228. [DOI] [PubMed] [Google Scholar]
- 14.Ekberg O. Inguinal herniography in adults: technique, normal anatomy, and diagnostic criteria for hernias. Radiology. 1981;138:31–36. doi: 10.1148/radiology.138.1.7455093. [DOI] [PubMed] [Google Scholar]
- 15.Zarvan NP, Lee FT, Yandow DR, et al. Abdominal hernias: CT findings. Am J Roentgenol. 1995;164:1391–1395. doi: 10.2214/ajr.164.6.7754880. [DOI] [PubMed] [Google Scholar]
- 16.Wechsler RJ, Kurtz AB, Needieman L, et al. Cross- sectional imaging of abdominal wall hernias. Am J Roentgenol. 1989;153:517–521. doi: 10.2214/ajr.153.3.517. [DOI] [PubMed] [Google Scholar]
- 17.Middlebrook MR, Eftekhari F. Sonographic findings in Richter's hernia. Gastrointest Radiol. 1992;17:229–230. doi: 10.1007/BF01888555. [DOI] [PubMed] [Google Scholar]
- 18.Toms AP, Dixon AK, Murphy JMP, et al. Illustrated review of new imaging techniques in the diagnosis of abdominal wall hernias. Br J Surg. 1999;86:1243–1250. doi: 10.1046/j.1365-2168.1999.01211.x. [DOI] [PubMed] [Google Scholar]
- 19.Ianora AA, Midiri M, Vinci R, et al. Abdominal wall hernias: imaging with spiral CT. Eur Radiol. 2000;10:914–919. doi: 10.1007/s003300051036. [DOI] [PubMed] [Google Scholar]
- 20.Van den Berg JC, de Valois JC, Go PM, et al. Detection of groin hernia with physical examination, ultrasound, and MRI compared with laparoscopic findings. Invest Radiol. 1999;34:739–743. doi: 10.1097/00004424-199912000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Truong S, Pfingsten FP, Dreuw B, et al. Value of sonography in diagnosis of uncertain lesions of the abdominal wall and inguinal region. Chirurg. 1993;64:468–475. [PubMed] [Google Scholar]
- 22.Hojer A-M, Rygaard H, Jess P. CT in the diagnosis of abdominal wall hernias: a preliminary study. Eur Radiol. 1997;7:1416–1418. doi: 10.1007/s003300050309. [DOI] [PubMed] [Google Scholar]
- 23.Van den Berg JC, de Valois JC, Go PM, et al. Groin hernia: can dynamic magnetic resonance imaging be of help? Eur Radiol. 1998;8:270–273. doi: 10.1007/s003300050377. [DOI] [PubMed] [Google Scholar]
- 24.Neblett WW, 3rd, Pietsch JB, Holcomb GW., Jr Acute abdominal conditions in children and adolescents. Surg Clin N Am. 1988;68:415–430. doi: 10.1016/s0039-6109(16)44486-5. [DOI] [PubMed] [Google Scholar]
- 25.Rutkow IM. Demographic and socioeconomic aspects of hernia repair in the United States in 2003. Surg Clin N Am. 2003;83:1045–1051. doi: 10.1016/S0039-6109(03)00132-4. [DOI] [PubMed] [Google Scholar]
- 26.Rutkow IM. Epidemiologic, economic, and sociologic aspects of hernia surgery in the United States in the 1990s. Surg Clin N Am. 1998;78:941–951. doi: 10.1016/S0039-6109(05)70363-7. [DOI] [PubMed] [Google Scholar]
- 27.Aguirre DA, Santosa AC, Casola G, Sirlin CB. Abdominal wall hernias: imaging features, complications, and diagnostic pitfalls at multi–detector row CT. Radiographics. 2005;25(6):1501–1520. doi: 10.1148/rg.256055018. [DOI] [PubMed] [Google Scholar]
- 28.Rutkow IM. Surgical operations in the United States: then (1983) and now (1994) Arch Surg. 1997;132:983–990. doi: 10.1001/archsurg.1997.01430330049007. [DOI] [PubMed] [Google Scholar]
- 29.Courtney CA, Lee AC, Wilson C, O’Dwyer PJ. Ventral hernia repair: a study of current practice. Hernia. 2003;7:44–46. doi: 10.1007/s10029-002-0102-0. [DOI] [PubMed] [Google Scholar]
- 30.Usher FC. Hernia repair with Marlex mesh: an analysis of 541 cases. Arch Surg. 1962;84:325–328. doi: 10.1001/archsurg.1962.01300210059012. [DOI] [PubMed] [Google Scholar]
- 31.Burger JW, Luijendikj RW, Hop WC, Halm JA, Verdaasdonk EG, Jeekel J. Long-term follow-up of a randomized controlled trial of suture versus mesh repair of incisional hernia. Ann Surg. 2004;240:578–585. doi: 10.1097/01.sla.0000141193.08524.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamadar DA, Jacobson JA, Girish G, et al. Abdominal wall hernia mesh repair sonography of mesh and common complications. J Ultrasound Med. 2008;27:907–917. doi: 10.7863/jum.2008.27.6.907. [DOI] [PubMed] [Google Scholar]
- 33.Novitsky YW, Harrell AG, Hope WW, Kercher KW, Heniford BT. Meshes in hernia repair. Surg Technol Int. 2007;16:123–127. [PubMed] [Google Scholar]
- 34.Brauman D. Diastasis recti: clinical anatomy. Plast Reconstr Surg. 2008;122(5):1564–1569. doi: 10.1097/PRS.0b013e3181882493. [DOI] [PubMed] [Google Scholar]
- 35.Mommers EHH, Ponten JEH, Al Omar AK, de Reilingh TSV, Bouvy ND, Nienhuijs SW. The general surgeon's perspective of rectus diastasis. A systematic review of treatment options. Surg Endosc. 2017;31(12):4934–4949. doi: 10.1007/s00464-017-5607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beer GM, et al. The normal width of the linea alba in nulliparous women. Clin Anat. 2009;22(6):706–711. doi: 10.1002/ca.20836. [DOI] [PubMed] [Google Scholar]
- 37.Taranto I. The relief of low back pain with WARP adominoplasty: a preliminary report. Plast Reconstr Surg. 1990;85:545e55. doi: 10.1097/00006534-199004000-00009. [DOI] [PubMed] [Google Scholar]
- 38.Khushboo D, Amrit K, Mahesh M. Correlation between diastasis rectus abdominis and lumbopelvic pain and dysfunction. Indian J Physiother Occup Ther. 2014;8:210e4. [Google Scholar]
- 39.Liaw LJ, Hsu MJ, Liao CF, Liu MF, Hsu AT. The relationships between inter-recti distance measured by ultrasound imaging and abdominal muscle function in postpartum women: a 6-month follow-up study. J Orthop Sports Phys Ther. 2011;41:435e43. doi: 10.2519/jospt.2011.3507. [DOI] [PubMed] [Google Scholar]
- 40.Spitznagle TM, Leon FC, Dillen LR. Prevalence of diastasis recti abdominals in uro-gynecological population. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:321e8. doi: 10.1007/s00192-006-0143-5. [DOI] [PubMed] [Google Scholar]
- 41.Rath AM, Attali P, Dumas JL, Goldlust D, Zhang J, Chevrel JP. The abdominal linea alba: an anatomo-radiologic and biomechanical study. Surg Radiol Anat. 1996;18:281e8. doi: 10.1007/BF01627606. [DOI] [PubMed] [Google Scholar]
- 42.Coldron Y, Stokes M, Newham D, Cook K. Postpartum characteristics of rectus abdominis on ultrasound imaging. Man Ther. 2008;13:112e21. doi: 10.1016/j.math.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Barbosa S, de Sa RA, Coca Velarde LG. Diastasis of rectus abdominis in the immediate puerperium: correlation between imaging diagnosis and clinical examination. Arch Gynecol Obst. 2013;288(2):299e303. doi: 10.1007/s00404-013-2725-z. [DOI] [PubMed] [Google Scholar]
- 44.van de Water AT, Benjamin DR. Measure DRAM with a purpose: diagnose or evaluate. Arch Gynecol Obst. 2014;289(1):3e4. doi: 10.1007/s00404-013-2964-z. [DOI] [PubMed] [Google Scholar]
- 45.van de Water AT, Benjamin DR. A systematic review of their measurement properties and meta-analytic reliability generalisation. Man Ther. 2015;21:41–53. doi: 10.1016/j.math.2015.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Mendes DDA, Nahas FX, Veiga DF, Mendes FV, Figueiras RG, Gomes HC, et al. Ultrasonography for measuring rectus abdominis muscles diastasis. Acta Circ Bras. 2007;22(3):182e6. doi: 10.1590/s0102-86502007000300005. [DOI] [PubMed] [Google Scholar]
- 47.Mota P, Pascoal AG, Sancho F, Bo K. Test-retest and intrarater reliability of 2-dimensional ultrasound measurements of distance between rectus abdominis in women. J Orthop Sports Phys Ther. 2012;42:940e6. doi: 10.2519/jospt.2012.4115. [DOI] [PubMed] [Google Scholar]
- 48.Benjamin DR, van de Water AT, Peiris CL. Effects of exercise on diastasis of the rectus abdominis muscle in the antenatal and postnatal periods: a systematic review. Physiotherapy. 2014;100(1):1–8. doi: 10.1016/j.physio.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Akram J, Matzen SH. Rectus abdominis diastasis. J Plast Surg Hand Surg. 2014;48(3):163–169. doi: 10.3109/2000656X.2013.859145. [DOI] [PubMed] [Google Scholar]
- 50.Janssen I. Influence of sarcopenia on the development of physical disability: the cardiovascular health study. J Am Geriatr Soc. 2006;54:56–62. doi: 10.1111/j.1532-5415.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- 51.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 year. J Appl Physiol. 2000;89:81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 52.Ikezoe T, Mori N, Nakamura M, Ichihashi N. Effects of age and inactivity due to prolonged bed rest on atrophy of trunk muscles. Eur J Appl Physiol. 2012;112(1):43–48. doi: 10.1007/s00421-011-1952-x. [DOI] [PubMed] [Google Scholar]
- 53.Vigneswaran Y, Poli E, Talamonti MS, Haggerty SP, Linn JG, Ujiki MB. Rectus abdominis atrophy after ventral abdominal incisions: midline versus chevron. Hernia. 2017;21(4):619–622. doi: 10.1007/s10029-017-1593-z. [DOI] [PubMed] [Google Scholar]
- 54.Heckmatt JZ, Dubowitz V, Leeman S. Detection of pathological change in dystrophic muscle with B-scan ultrasound imaging. Lancet. 1980;1:1389–1390. doi: 10.1016/s0140-6736(80)92656-2. [DOI] [PubMed] [Google Scholar]
- 55.Pillen S, van Alfen N. Skeletal muscle ultrasound. Neurol Res. 2011;33(10):1016–1024. doi: 10.1179/1743132811Y.0000000010. [DOI] [PubMed] [Google Scholar]
- 56.West MA, van Dijk DPJ, Gleadowe F, Reeves T, Primrose JN, Hilal MA, Edwards MR, Jack S, Rensen SSS, Grocott MPW, Levett DZH, Damink SWMO. Myosteatosis is associated with poor physical fitness in patients undergoing hepatopancreatobiliary surgery. J Cachexia Sarcopenia Muscle. 2019;10(4):860–871. doi: 10.1002/jcsm.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee JC, Mitchell AW, Healy JC. Imaging of muscle injury in the elite athlete. Br J Radiol. 2012;85(1016):1173–1185. doi: 10.1259/bjr/84622172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanchis-Moysi J, Idoate F, Dorado C, Alayón S, Calbet JAL. Large asymmetric hypertrophy of rectus abdominis muscle in professional tennis players. Plos One. 2010;5:1–8. doi: 10.1371/journal.pone.0015858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Connell D, Ali K, Javid M, Bell P, Batt M, Kemp S. Sonography and MRI of rectus abdominis muscle strain in elite tennis players. Am J Roentgenol. 2006;187(6):1457–1461. doi: 10.2214/AJR.04.1929. [DOI] [PubMed] [Google Scholar]
- 60.Cruz AR, Mautner K. Rectus abdominis injury in elite volleyball players. Int J Athl Ther Train. 2016 doi: 10.1123/ijatt.2014-0120. [DOI] [Google Scholar]
- 61.Balius R, Pedret C, Pacheco L, Gutierrez J, Vives J, Escoda J. Rectus abdominis muscle injuries in elite handball players: management and rehabilitation. Open Access J Sports Med. 2011;2:69–73. doi: 10.2147/OAJSM.S17504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Conte SA, Thompson MM, Marks MA, Dines JS. Abdominal muscle strains in professional baseball: 1991–2010. Am J Sports Med. 2012;40(3):650–656. doi: 10.1177/0363546511433030. [DOI] [PubMed] [Google Scholar]
- 63.Lacroix VJ, Kinnear DG, Mulder DS, Brown RA. Lower abdominal pain syndrome in national hockey league players: a report of 11 cases. Clin J Sport Med. 1998;8:5–9. doi: 10.1097/00042752-199801000-00002. [DOI] [PubMed] [Google Scholar]
- 64.Draghi F, Zacchino M, Canepari M, Nucci P, Alessandrino F. Muscle injuries: ultrasound evaluation in the acute phase. J Ultrasound. 2013;16(4):209–214. doi: 10.1007/s40477-013-0019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rokitansky K. U ¨ ber Uterusdru ¨sen-Neubildung. Z Gesellschaft Aerzte (Wien) 1860;16:577–581. [Google Scholar]
- 66.Davis AC, Goldberg JM. Extrapelvic endometriosis. Semin Reprod Med. 2017;35:98–101. doi: 10.1055/s-0036-1597122. [DOI] [PubMed] [Google Scholar]
- 67.Betrán AP, Ye J, Moller AB, et al. The increasing trend in caesarean section rates: global, regional and national estimates: 1990–2014. PLoS One. 2016;11:e0148343. doi: 10.1371/journal.pone.0148343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bozkurt M, Çil AS, Bozkurt DK. Intramuscular abdominal wall endometriosis treated by ultrasound-guided ethanol injection. Clin Med Res. 2014;12:160–165. doi: 10.3121/cmr.2013.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wicherek L, Klimek M, Skret-Magierlo J, et al. The obstetrical history in patients with Pfannenstiel scar endometriomas—an analysis of 81 patients. Gynecol Obstet Invest. 2007;63:107–113. doi: 10.1159/000096083. [DOI] [PubMed] [Google Scholar]
- 70.Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98:511–519. doi: 10.1016/j.fertnstert.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang PH, Juang CM, Chao HT, et al. Wound endometriosis: risk factor evaluation and treatment. J Chin Med Assoc. 2003;66:113–119. [PubMed] [Google Scholar]
- 72.Mistrangelo M, Gilbo N, Cassoni P, et al. Surgical scar endometriosis. Surg Today. 2014;44:767–772. doi: 10.1007/s00595-012-0459-3. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Y, Zhang J, Zamora J, Vogel JP, Souza JP, Jayaratne K, et al. Increases in caesarean delivery rates and change of perinatal outcomes in low- and middle-income countries: a hospital-level analysis of two WHO surveys. Paediatr Perinat Epidemiol. 2017;31:251–262. doi: 10.1111/ppe.12363. [DOI] [PubMed] [Google Scholar]
- 74.Hufnagel D, Li F, Cosar E, et al. The role of stem cells in the etiology and pathophysiology of endometriosis. Semin Reprod Med. 2015;33:333–340. doi: 10.1055/s-0035-1564609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirsch M, Begum MR, Paniz É, et al. Diagnosis and management of endometriosis: a systematic review of international and national guidelines. BJOG. 2018;125:556–564. doi: 10.1111/1471-0528.14838. [DOI] [PubMed] [Google Scholar]
- 76.Cocco G, Ricci V, Boccatonda A, Schiavone C. Focused ultrasound for the diagnosis of non-palpable endometriotic lesions of the abdominal wall: a not-uncommon surgical complication. J Ultrasound. 2020 doi: 10.1007/s40477-019-00425-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mulliken JB, Glowacki J. Hemangiomas and vascular malformations in infants and children: a classification based on endothelial characteristics. Plast Reconsr Surg. 1982;69:412–422. doi: 10.1097/00006534-198203000-00002. [DOI] [PubMed] [Google Scholar]
- 78.Enjolras O, Mulliken JB. Vascular tumors and vascular malformations (new issues) Adv Dermatol. 1998;12:375. [PubMed] [Google Scholar]
- 79.Dasgupta R, Fishman SJ. ISSVA classification. Semin Pediatr Surg. 2014;23:158–161. doi: 10.1053/j.sempedsurg.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 80.ISSVA (2018) ISSVA classification for vascular anomalies. Amsterdam: International Society for the Study of Vascular Anomalies. https://www.issva.org/classification. Accessed 20 Dec 2018
- 81.Ernemann U, Kramer U, Miller S, Bisdas S, Rebmann H, Breuninger H, et al. Current concepts in the classification, diagnosis and treatment of vascular anomalies. Eur J Radiol. 2010;75(1):1–2. doi: 10.1016/j.ejrad.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 82.Aravinda PS, Nag HH, Betigeri M, Sakhuja VK, Sharma P. An unusual case of giant arterio-venous malformation of anterior abdominal wall. J Clin Diagn Res. 2017;11(8):PD07–PD08. doi: 10.7860/JCDR/2017/27706.10388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saad DF, et al. Intramuscular hemangioma of the abdominal wall. J Pediatr Surg. 2005;41(3):601–602. doi: 10.1016/j.jpedsurg.11.086. [DOI] [PubMed] [Google Scholar]
- 84.Krause KI, et al. Anterior abdominal wall angiosarcoma in a morbidly obese woman. J Am Acad Dermatol. 1986;15(2):327–330. doi: 10.1016/S0190-9622(86)70170-9. [DOI] [PubMed] [Google Scholar]
- 85.Alessandrino F, Maira A, Tarantino CC. US and MRI features in venous vascular malformation of the abdominal wall. A case report. J Ultrasound. 2012;15(3):171–173. doi: 10.1016/j.jus.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masand P. Radiographic findings associated with vascular anomalies. Semin Plast Surg. 2014;28(2):69–78. doi: 10.1055/s-0034-1376266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Gudinchet JY. Sonography of the thoracic and abdominal walls. J Clin Ultrasound. 2004;32:500–510. doi: 10.1002/jcu.20070. [DOI] [PubMed] [Google Scholar]
- 88.Bancroft LW, Kransdorf MJ, Peterson JJ, O’Connor MI. Benign fatty tumors: classification, clinical course, imaging appearance, and treatment. Skeletal Radiol. 2006;35(10):719–733. doi: 10.1007/s00256-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 89.Kransdorf MJ, Bancroft LW, Peterson JJ, Murphey MD, FosterTemple WCHT. Imaging of fatty tumors: distinction of lipoma and well-differentiated liposarcoma. Radiology. 2002;224(1):99–104. doi: 10.1148/radiol.2241011113. [DOI] [PubMed] [Google Scholar]
- 90.Paunipagar BK, Griffith JF, Rasalkar DD, Chow LTC, Kumta SM, Ahuja A. Ultrasound features of deep-seated lipomas. Insights Imaging. 2010;1(3):149–153. doi: 10.1007/s13244-010-0019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Al-Qattan MM, Weinberg M, Clarke HM. Two rapidly growing fatty tumors of the upper limb in children: lipoblastoma and infiltrating lipoma. J Hand Surg Am. 1995;20(1):20–23. doi: 10.1016/S0363-5023(05)80051-6. [DOI] [PubMed] [Google Scholar]
- 92.Inampudi P, Jacobson JA, Fessell DP, et al. Soft-tissue lipomas: accuracy of sonography in diagnosis with pathologic correlation. Radiology. 2004;233:763–767. doi: 10.1148/radiol.2333031410. [DOI] [PubMed] [Google Scholar]
- 93.Teo HEL, Peh WCG, Shek TWH. Case 84: desmoid tumor of the abdominal wall. Radiology. 2005;236:81–84. doi: 10.1148/radiol.2361031038. [DOI] [PubMed] [Google Scholar]
- 94.AlShammari A, AlSumai TS, Alhudaib AA, Khalifa H, Aburahmah M. Ultrasound guided sparing resection of locally recurrent abdominal wall desmoid tumor. J Surg Case Rep. 2019;2019(6):rjz165. doi: 10.1093/jscr/rjz165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Crago AM, Denton B, Salas S, Dufresne A, Mezhir AJ, Hameed M, et al. A prognostic nomogram for prediction of recurrence in desmoid fibromatosis. Ann Surg Oncol. 2013;258:347–353. doi: 10.1097/SLA.0b013e31828c8a30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Penel N, Chibon FDR, Salas SB. Adult desmoid tumors. Curr Opin Oncol. 2017;29(4):268–274. doi: 10.1097/CCO.0000000000000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baz AAM, El-Azizi HMS, Mohamed MSQ, et al. Role of high-resolution ultrasound in the assessment of abdominal wall masses and mass-like lesions. Egypt J Radiol Nucl Med. 2019;50:33. doi: 10.1186/s43055-019-0027-6. [DOI] [Google Scholar]
- 98.Beaman FD, Kransdorf MJ, Menke DM. Schwannoma: radiologic pathologic correlation. Radiographics. 2004;24:1477–1481. doi: 10.1148/rg.245045001. [DOI] [PubMed] [Google Scholar]
- 99.Bianchi S, Draghi F, Beggs I. Ultrasound of the peripheral nerves. In: Allan P, Baxter GM, Weston MJ, editors. Clinical ultrasound. Philadelphia: Elsevier; 2011. pp. 1158–1167. [Google Scholar]