Abstract
目的
研究脉冲电磁场(pulse electromagnetic fields, PEMF)促进大鼠成骨细胞成熟分化是否与初级纤毛和PI3K/AKT途径相关,探讨PEMF促进骨形成的作用机制。
方法
用酶解法获取新生SD大鼠颅骨成骨细胞(rat calvarial osteoblasts, ROB), 50 Hz、0.6 mT PEMF处理0、0.5、1、1.5和2 h后检测磷脂酰肌醇-3-激酶(phosphatidylinositol-3-kinase, PI3K)和蛋白激酶B(protein kinase B, AKT)蛋白表达量及初级纤毛长度和发生率的变化情况;用LY294002阻断PI3K/AKT信号途径,观察PEMF促进ROB成骨性分化是否受到影响;以RNAi法干扰IFT88的基因表达以抑制初级纤毛发生,观察PEMF激活的PI3K/AKT信号途径及ROB的成骨性分化是否受到影响。成骨性分化指标包括碱性磷酸酶(alkaline phosphatase, ALP)活性,real-time PCR法和Western blot法检测的成骨性相关基因BMP-2、COL-1和OSX的基因表达量,以及钙化结节数量等。
结果
经PEMF处理0、0.5、1、1.5、2 h后,ROB的PI3K、AKT蛋白表达量升高(P<0.01),初级纤毛变长;其中PI3K在0.5 h时蛋白表达量达到最高,随着PEMF处理时间增加,蛋白表达量降低; AKT在0.5 h和1.5 h时蛋白表达量较高。用PI3K阻断剂LY294002阻断PI3K/AKT信号途径后,PEMF不再能提高成骨细胞中ALP 活性和成骨性相关基因BMP-2、COL-1、OSX的基因表达量,但在阻断前PEMF能增加ROB中ALP活性和成骨性相关基因的表达;RNAi干扰初级纤毛发生后,PEMF不再能增加PI3K的蛋白表达量,说明初级纤毛干扰后,PEMF不能激活PI3K/AKT信号途径;其次,PEMF提高ALP活性的作用消失,也不能提高BMP-2、COL-1和OSX的基因表达量,其增加ROB钙化结节形成能力的作用也消失,说明初级纤毛干扰后,PEMF促进成骨细胞成熟矿化的能力消失。
结论
PEMF通过成骨细胞表面的初级纤毛激活了PI3K/AKT信号途径,进而发挥了骨形成活性的促进作用。
Keywords: 脉冲电磁场, 成骨细胞, RNA干扰, PI3K
Abstract
Objective
To study whether the pulsed electromagnetic fields (PEMF) promoting rat osteoblasts differentiation and maturation is related to the primary cilia and PI3K/AKT pathway, and to explore the mechanism of PEMF in promoting bone differentiation.
Methods
Enzyme solution was used to obtain newborn SD rats calvarial osteoblasts (ROB), which were processed by 50 Hz 0.6 mT PEMF for 0, 0.5, 1, 1.5 and 2 h, detecting PI3K and AKT protein expression and changes in primary cilia length and incidence; with LY294002 blocking PI3K/AKT signaling pathways we observed whether PEMF promoted osteogenic differentiation of ROB was affected; by interfering IFT88 gene expression by RNAi to inhibit primary cilia we observed whether PI3K/AKT signaling pathway and osteogenic differentiation of ROB was affected. Osteogenic differentiation indexes included alkaline phosphatase (ALP) activity, Real-time PCR and Western blot detection of osteogenic related genes of BMP-2, COL-1 and OSX and calcified nodules number, etc.
Results
After exposure to PEMF for 0, 0.5, 1, 1.5, and 2 h, the protein expression of PI3K and AKT in ROB were increased significantly (P<0.01) and the primary cilia became longer; and the protein expression of PI3K reached the highest level at 0.5 h, as the treatment time of PEMF increased, the PI3K protein expression decreased. AKT showed higher protein expression at 0.5 h and 1.5 h. After blocking the PI3K/AKT signaling pathway with the PI3K blocker LY294002, PEMF could no longer increase ALP activity and the gene expressions of BMP-2, COL-1, OSX which were osteogenically related. However, PEMF could increase the ALP activity and the osteogenically related gene expression in ROB before blocking. After RNAi interfered the primary cilia, PEMF could no longer increase the protein expression of PI3K, which indicated that PEMF could not activate the PI3K/AKT signaling pathway after primary cilia interfering; secondly, the effect of PEMF on enhancing ALP activity disappeared, it also decrease the gene expressions of BMP-2, COL-1, and OSX, and the ability of increasing the calcification nodule formation also disappeared, indicating that the ability of PEMF to promote osteoblast maturation and mineralization disappeared after primary cilia interference.
Conclusion
PEMF activated the PI3K/AKT signaling pathway through primary cilia on the surface of osteoblasts, then promoted bone formation activity and differentiation.
Keywords: Pulse electromagnetic fields (PEMF), Osteoblast, RNA interference, PI3K
初级纤毛是一种存在于大多数哺乳动物细胞表面的特殊细胞器,在细胞分裂间期和G0 期通过中心粒锚定于细胞表面[1,2]。近年研究表明,初级纤毛内含有多种受体、激酶及信号蛋白[如PDGFRαα、PKA、Hh(Hedgehog)和Wnt途径相关的信号蛋白]等,有可能是药物或其他疗法的作用靶点之一[3,4,5,6]。PI3K-AKT信号途径是处于G蛋白偶联受体(G protein-coupled receptors,GPCR)和酪氨酸激酶活性受体下游,但又在eNOS、mTOR等上游的一段非常重要的中间信号途径,其中磷脂酰肌醇-3-激酶(phosphatidylinositol-3-kinase,PI3K)可以磷酸化肌醇磷脂肌醇环上的3'-OH,蛋白激酶B(protein kinase B, AKT)是原癌基因c-akt的表达产物,参与PI3K介导的信号过程,为神经元存活、神经和血管新生、突触结构形成和突触间信息传递所必须[7,8,9]。PI3K/AKT信号转导途径具有调控物质代谢、基因表达、细胞迁移、增殖等多种生物学功能,成骨细胞的矿化成熟过程也离不开PI3K/AKT信号途径的参与[10,11,12,13]。本课题组在前期研究中已经发现低频脉冲电磁场(pulse electromagnetic fields, PEMF)可以促进成骨细胞的矿化成熟,提高实验动物峰值骨密度,具有良好的防治骨质疏松和促进骨折愈合的临床应用前景[14,15,16,17],但低频电磁场促进骨形成活性是否与初级纤毛和PI3K-AKT信号途径有关,目前尚未见报道,本研究旨在探讨这一问题。
1. 材料与方法
1.1. 实验动物
出生48 h以内的SPF级SD 大鼠10只,雄性9~10 g,购自甘肃省中医药大学 SPF级动物实验中心,合格证号SCXK(甘)2015-0002。
1.2. 试剂与仪器
1.2.1 试剂 α-MEM 培养基和胎牛血清(fetal bovine serum,FBS)购自美国 Gibco 公司; β-actin 一抗购自美国 Cell signaling 公司,PI3K、 AKT和乙酰化α-Tubulin抗体一抗购自美国Abcam公司,p-AKT一抗购自美国Cell signaling公司,二抗购自北京中杉金桥生物公司;Total RNA提取试剂盒、Prime ScriptTM reagent逆转录试剂盒、Prime Ex TapTM Ⅱ PCR 试剂盒均购自大连Takara公司;FITC标记的免疫荧光二抗购自美国KPL公司;碱性磷酸酶(alkaline phosphatase, ALP)活性测定试剂盒购自南京建成生物公司。
1.2.2 仪器 CO2 细胞培养箱购自美国Thermo Revco公司,荧光显微镜购自日本Olympus公司,紫外分光光度计购自美国Thermo 公司,实时荧光定量PCR仪购自美国ABI公司,台式高速冷冻离心机购自德国Heraeus公司,电泳仪电转移仪购自美国Bio-Rad公司,酶标仪购自美国Bio-Rad公司。
1.3. 新生大鼠颅骨成骨细胞(rat calvarial osteoblasts, ROB)分离培养
将大鼠乳鼠置于75%(体积分数)乙醇溶液中浸泡处死,无菌条件下取其颅骨,剔除骨膜及结缔组织,将骨片剪成 1 mm × 1 mm 大小,用 0.25%(质量分数)胰蛋白酶于 37 ℃水浴 10 min × 2 次,弃消化液。0.1%(质量分数)Ⅱ型胶原酶于 37 ℃水浴连续消化5次,每次20 min,收集消化液,1 000 r/min 离心10 min,弃上清液,用含 10%(体积分数)胎牛血清的 α-MEM 培养液悬浮细胞沉淀。以2×104 个 /mL 的浓度接种于大培养皿(直径100 mm)中,37 ℃ 5%(体积分数) CO2在饱和湿度条件下培养,每3天换液1次,待细胞培养至80%皿底时传代。
1.4. 初级纤毛免疫荧光染色
将P1代细胞进行爬片培养,用50 Hz 0.6 mT PEMF处理1.5 h后,取出爬片并用PBS漂洗3次,4%(质量分数)多聚甲醛固定10 min,PBS漂洗3次,用0.5% (质量分数)Triton-X100透膜20 min,PBS漂洗3次,滴加山羊血清工作液并37 ℃孵育15~20 min,倾去工作液,滴加α-Tubulin抗体(体积比 1 ∶300)并4 ℃过夜,PBS漂洗3 次,每次3 min,滴加FITC标记的免疫荧光二抗(体积比 1 ∶500)并37 ℃避光孵育45 min,PBS漂洗3 次每次3 min,封片后置于荧光显微镜下观察。
1.5. Western blot印迹分析
将培养液吸出,用 4 ℃预冷的 PBS 漂洗 2 遍后,加入300 μL 的细胞裂解液[50 mmol/L Tris-HCl,pH 8.0、150 mmol/L NaCl、100 mg/L PMSF、1 mg/L抑蛋白酶肽、1%(质量分数)Tween-20、0.5%(质量分数)去氧胆酸钠、1%(质量分数) SDS],于冰上静置 30 min,使细胞充分裂解。4 ℃、12 000 r/min 离心 15 min,取上清液,用 BCA 法进行总蛋白质浓度的测定。95 ℃变性6 min,各组取10 μg 蛋白质样品,经 15% (质量分数)SDS-PAGE分离后,将蛋白质转移至PVDF 膜上,5%(质量分数)脱脂奶粉室温下摇床振荡封闭2 h,加入 β-actin、PI3K、P-AKT和AKT一抗(均为体积比 1 ∶1 000稀释), 4 ℃过夜。次日加入辣根过氧化物酶标记的二抗(体积比1 ∶10 000 稀释), 摇床震荡2 h。每进行下一步实验前 PVDF 膜均用 4 ℃预冷的 PBST 摇床漂洗3次,每次10 min。用增强化学发光法检测目的蛋白,X线片曝光后,用 Image-Pro plus6.0软件扫描,测定条带光密度值,重复3次。
1.6. RNA法干扰初级纤毛发生
以IFT88基因全序列为模板,设计特异性抑制IFT88的siRNA序列(siRNA:5'-GGAUAUGGGUCCAAGACAUCC-3')。siRNA 序列与pENTRTM/U6 载体相连后利用脂质体转入成骨细胞,所用的对照组转入相应的阴性对照载体,转染 24 h 后,RT-PCR 和 Western blot 检测 IFT88 的表达量,检测 RNA 干扰效率。以干扰对照siRNA同时转染对照组ROB,用作干扰对照(scrambled control, SC)组。
1.7. 碱性磷酸酶活性的测定
成骨细胞用电磁场处理1.5 h后继续培养12 h,测碱性磷酸酶活性,测定方法按试剂盒说明书进行,弃培养液,用PBS洗2次,加入基质液和缓冲液250 μL,轻微震荡混匀,37 ℃孵育15 min,加入显色液750 μL,在520 nm 处测光密度。
1.8. real time RT-PCR分析
磁场处理3 d(每天1.5 h)后,提取每组总RNA。提取过程:加Trizol 裂解液1 mL,裂解5 min,转移至Ep管中,加入原裂解液1/5体积的三氯甲烷(200 μL), 剧烈震荡,静置5 min,于12 000 r/min 4 ℃ 离心20 min,吸取上清,加入等体积异丙醇,4 ℃ 静置10 min,12 000 r/min 4 ℃ 离心20 min,弃上清液,用75% (体积分数)乙醇清洗沉淀,30 μL 的DEPC水溶解沉淀,在260和280 nm 处测光密度,计算浓度,并用1%(质量分数)琼脂糖电泳检测其完整性。
根据试剂盒说明书进行逆转录,使用Prime ScriptTM RT 试剂盒反应液20 μL,逆转录条件:37 ℃ 15 min,85 ℃ 5 s。使用 SYBR Premix Ex TaqTM real time PCR 试剂盒配制 20 μL PCR反应体系,反应条件为:95 ℃ 预变性 30 s,95 ℃ 变性 5 s,60 ℃ 退火 31 s,共进行40个循环。RT-PCR 引物由TaKaRa 公司设计合成。
1.9. 钙化结节染色
将传代后的成骨细胞接种在35 mm培养皿中,脉冲电磁场处理12 d,每天磁场照射1.5 h待细胞形成钙化结节时进行钙化结节染色,具体方法为PBS漂洗2次,加入4%(体积分数)多聚甲醛固定10 min,弃固定液,加入 0.1%(质量分数)茜素红Tris-HCl染色液(pH 8.3),37 ℃水浴60 min;PBS漂洗干燥后照相记录结果。
1.10. 统计学分析
用SPSS16.0统计软件进行分析,计量资料以 x±s 表示,多组均数比较采用单因素方差分析(ONE-WAY ANOVA)方法,组间两两比较选用 LSD 法,P<0.05 认为差异有统计学意义。
2. 结果
2.1. PEMF处理导致初级纤毛长度增加
经对乙酰化α-tubulin进行免疫荧光染色,在荧光正置显微镜下可清晰观察到ROB初级纤毛(图1A)。用50 Hz 0.6 mT PEMF 处理1.5 h后(图1B), 发现初级纤毛经PEMF处理后,纤毛长度显著大于照射前(P<0.01), 但发生率变化不明显(图1C、1D)。
1.
电磁场对初级纤毛的影响
Effect of pulse electromagnetic fieldsl (PEMF) on primary cilia
A, immunostaining for primary cilia; B, after exposure at the 50 Hz 0.6 mT PEMF for 1.5 h, then put the cells in CO2 cell incubator for 2 h, and the primary cilia get longer; C, cell with primary cilia; D, the length of primary cilia; Con, control; *P<0.05, vs. control.
2.2. PEMF激活PI3K/AKT信号途径
2.2.1 PEMF处理显著提高PI3K和AKT表达水平 PI3K经PEMF处理0.5 h后表达量升高,1 h后达到最高(P<0.01),随后开始下降,1.5 h时仍显著高于对照组(P<0.01,图2)。AKT在PEMF处理的各个时间点均无明显变化,但其磷酸化活性形式(p-AKT)的表达量在 0.5 h即达到最高值(P<0.01), 1 h后下降,到 1.5 h又明显升高(P<0.01), 2 h时再次下降(图3)。
2.
50Hz 0.6mT 电磁场能够增强PI3K蛋白表达量
Treatment with 50 Hz 0.6 mT pulse electromagnetic fields (PEMF) enhances the expression of PI3K
The protein level was examined by Western blotting. Prorein in lysates were separated by 12% SDS-PAGE. After transferring onto the membrane the blots were probed with anti-PI3K. Actin was used as a loading control. Band intensities were analyzed by densitometry scanning using image. The data point represent x±s of relative optical density (n=3). # P<0.01 vs. control.
3.
50 Hz 0.6 mT 电磁场增强p-AKT蛋白表达量
Treatment with 50 Hz 0.6 mT pulse electromagnetic fields (PEMF) enhances the expression of p-AKT
The protein level was examined by Western blotting. Protein in lysates were separated by 12% SDS-PAGE. After transferring onto the membrane the blots were probed with anti-p-AKT. Band intensities were analyzed by densitometry scanning using image. The data points represent x±s of relative optical density (n=3). # P<0.01 vs. control.
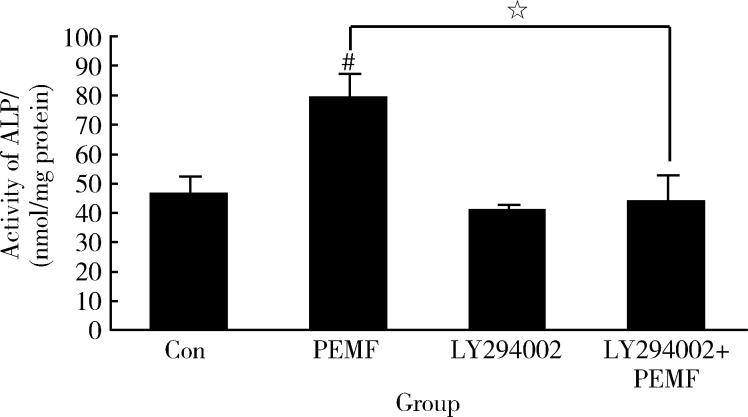
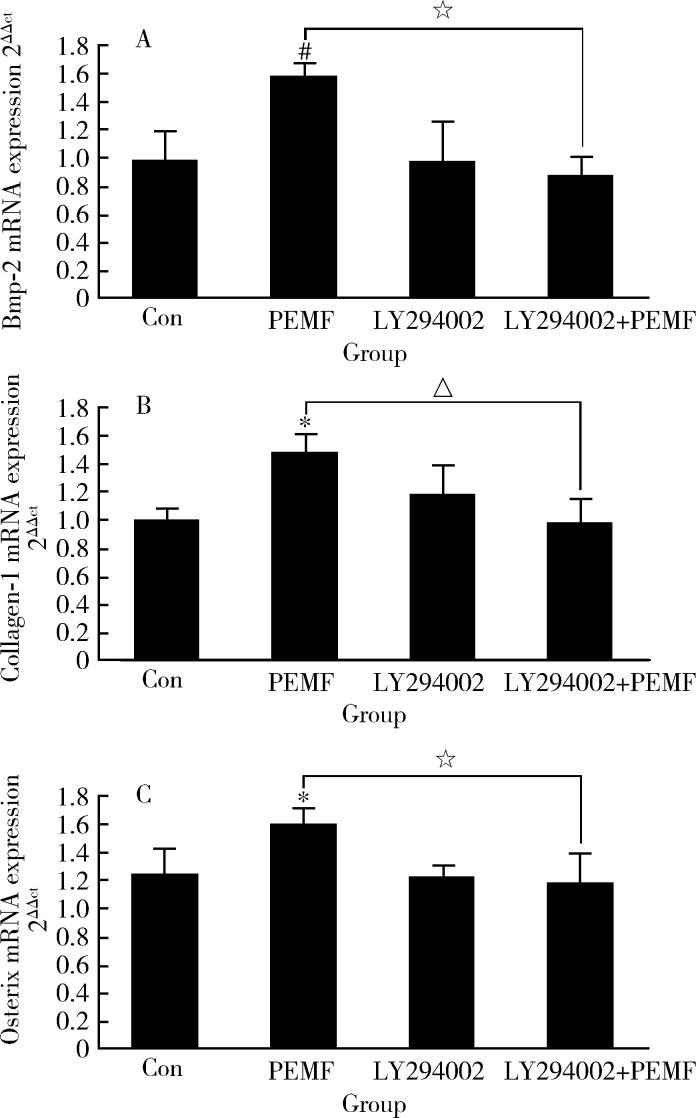
2.2.2 PI3K/AKT信号途径被阻断后成骨细胞的成骨性分化受阻 用50 μmol/L LY294002(PI3K特异性抑制剂)预处理ROB,再用PEMF照射细胞,以观察PI3K/AKT信号途径在PEMF促进ROB成骨性分化中的作用。PEMF本可以显著提高ALP活性(P组),但用抑制剂预处理后,这种提高作用消失(LY294002+P组,图4)。PEMF也可以增加ROB中Bmp-2、Collagen-1和 Osterix 的表达水平,但在抑制剂存在的情况下,提高作用也消失(图5)。
4.
电磁场和LY294002对大鼠成骨细胞ALP活性的影响
Effect of pulse electromagnetic fields (PEMF) and LY294002 on ALP activity in rat osteoblast cells
The ALP activity of osteoblast cultures was measured using an ALP assay kit at one day (1.5 h/d) after PEMF treatment. Osteoblasts were seeded at 1×105 cells/mL in 96-well plate. They were sub-cultures and randomly divided into four group: Control group, PEMF group (treated by 50 Hz 0.6 mT PEMF), LY294002 group (treated with 50 μmol/L LY294002), and LY294002+PEMF group (treated with 50 μmol/L LY294002 and 50 Hz 0.6 mT PEMF). The data represent x±s (n=3). # P<0.01 vs. control (Con);☆ P<0.01 vs. PEMF.
5.
电磁场和LY294002对大鼠成骨细胞成骨性基因表达的影响
Effect of pulse electromagnetic fields (PEMF) and LY294002 on gene expression in rat osteoblast cells
After treated by PEMF for 3 days (1.5 h/d), osteoblasts were washed with PBS and total RNA was isolated using RNAisoTM Plus reagent, mRNA expression of Bmp-2 (A), Collagen-1 (B) and Osterix (C) was quantitated by RT-PCR. The GAPDH was used as internal control. The data represent x±s of mRNA expression level (n=3). *P<0.05, # P<0.01, vs. control (Con); △P<0.05, ☆P<0.01, vs. PEMF.
2.3. PEMF促进骨形成活性需要初级纤毛的参与
2.3.1 干扰IFT88基因表达后初级纤毛发生被抑制 IFT88蛋白是初级纤毛进行“鞭毛内运输”(intraflagellar transport,IFT)的关键组分,如果干扰IFT88的基因表达则可以达到抑制初级纤毛发生的效果。本实验干扰组IFT88的mRNA和蛋白的表达水平明显下降(P<0.01,图6)。同时,可看到干扰组ROB的初级纤毛明显变短,大部分呈斑点状,拥有初级纤毛的比例也从干扰前的65.2% 降到干扰后的15.9%(图7)。
6.
siRNA转染干扰IFT88基因及蛋白的表达
siRNA transfection interferes IFT88 gene and protein expression
The inhibition of IFT88 gene expression as revealed by RT-PCR (A), and the protein expression inhibition as revealed by Western blot (B and C) in siRNA group compared to negative control. SC, scrambled control. #P<0.01, vs. SC.
7.
干扰IFT88基因后对大鼠成骨细胞初级纤毛的影响
Effects of IFT88 siRNA transfection on primary cilia of rat osteoblasts
A, normal primary cilia as immunofluorescence stained by acetylated-tubulin (green); B, primary cilia became dotted and short after 24 h of IFT88 siRNA transfection; C, percentages of cells with primary cilia in negative control and siRNA groups. SC, scrambled control. # P<0.01, vs. SC.
2.3.2 PEMF不能激活初级纤毛被抑制的成骨细胞的PI3K/AKT信号途径 PEMF可以显著提高干扰对照组(SC组)的PI3K蛋白表达量(P<0.05),但初级纤毛被干扰后(siRNA), 提高效应消失(siRNA+PEMF,图8), 表明除去初级纤毛后PEMF不能激活PI3K/AKT信号途径。
8.
电磁场和RNAi对PI3K蛋白表达的影响
Effect of PEMF and RNAi on the protein expression of PI3K
The protein level was examined by Western blot. Protein in lysates were separated by 12% SDS-PAGE. After transferring onto the membrane the blots were probed with anti-PI3K. Actin was used as a loading control. Band intensities were analyzed by densitometry scanning using image. The data point represent x±s of relative optical density (n=3). SC, scrambled control. *P<0.05, vs. SC; ☆P<0.01, vs. SC+PEMF.
2.3.3 PEMF不能促进初级纤毛被抑制的ROB的成骨性分化
用50 Hz 0.6 mT PEMF 同时处理各组细胞1.5 h,12 h后测定ALP活性(图9), NC+PEMF组的ALP活性高于NC组,但siRNA+PEMF组却低于SC+PEMF组(P<0.01), 表明PEMF不能提高初级纤毛被抑制的ROB的ALP活性。
9.
电磁场和RNAi对ALP活性的影响
Effect of PEMF and RNAi on ALP activity in rat osteoblast cells
The ALP activity of osteoblast cultures was measured using an ALP assay kit at one day (1.5 h/d) after PEMF treatment. Osteoblasts were seeded at 1×105cells/mL in 96-well plate. They were sub-cultures and randomly divided into four group: SC group (transfected with scrambled control siRNA), SC+PEMF group (transfected with scrambled control siRNA and treated by 50 Hz 0.6 mT PEMF), siRNA group(transfected with IFT88 siRNA), and siRNA+PEMF group (transfected with IFT88 siRNA and treated by 50 Hz 0.6 mT PEMF). The data represent x±s of absorbance value±standard deviation (n=3). SC, scrambled control. #P<0.01, vs. SC;☆P<0.01, vs. SC+PEMF.
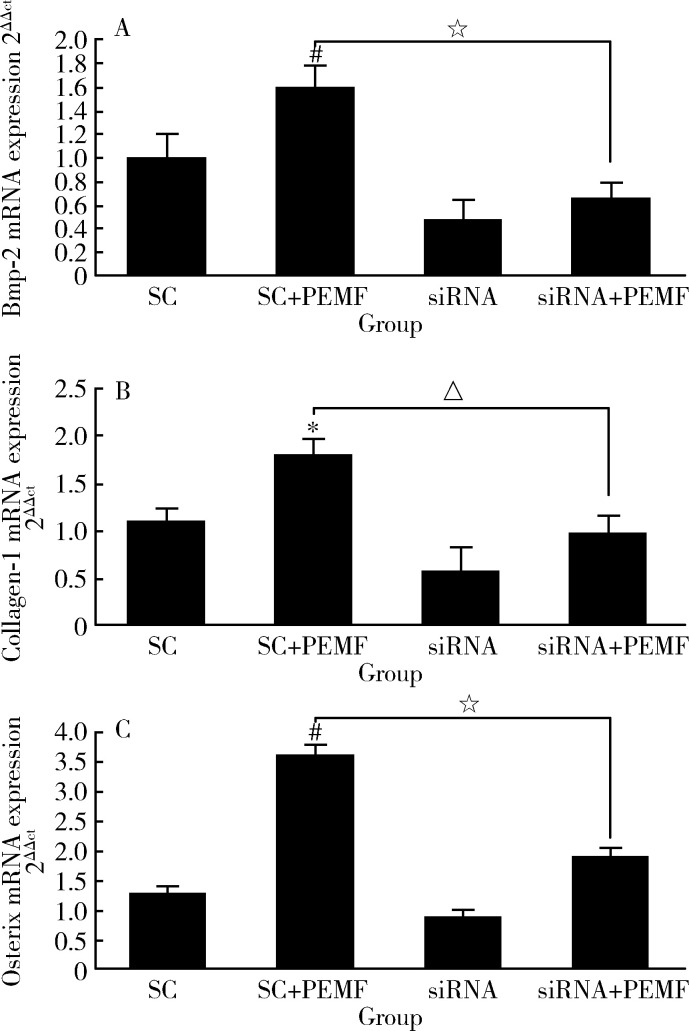
PEMF对成骨相关基因的表达的影响呈相同趋势,PEMF可以显著提高SC组细胞的BMP-2、Collagen-Ⅰ和Osterix的基因表达水平,但在siRNA+PEMF组,这种提高作用消失(图10)。
10.
电磁场和RNAi对成骨性相关基因的影响
Effect of PEMF and RNAi on osteogenic gene expression in rat osteoblast cells
After treated by PEMF for 3 days (1.5 h/d), osteoblasts were washed with PBS and total RNA was isolated using RNAisoTM Plus reagent, mRNA expression of Bmp-2 (A), Collagen-1 (B) and Osterix (C) was quantitated by RT-PCR. The GAPDH was used as internal control. The data represent x±s of mRNA expression level±standard deviation (n=3). SC, scrambled control. *P<0.05, # P<0.01 vs. SC; △P<0.05, ☆ P<0.01 vs. SC+PEMF.
茜素红染色的钙化结节数能直观显示成骨细胞的成熟矿化情况(图11), SC+PEMF组的钙化结节面积和数量都显著大于SC组(P<0.01), 但siRNA+PEMF组的面积和数量与SC组和siRNA组处于同一水平,显著低于SC+PEMF组(P<0.01)。
11.
IFT88 siRNA介导的初级纤毛抑制对PEMF诱导大鼠颅骨成骨细胞矿化成熟的影响
Effects of IFT88 siRNA-mediated primary cilium inhibition on PEMF-induces promotion on mineralization of rat calvarial osteoblasts
A, the Alizarin red staining results after 13 d; B, quantification of mineral matrix deposition using Image-Pro Plus 6.0 program. Results shown are the average of three independent tests. Each red dot represents a calcified nodule unit. Large numbers of red dots indicate more active osteogenic differentiation. C, the number of calcified nodules quantified by Image-Pro Plus 6.0. The data points represent x±s of calcified tubercle area level (n=3). SC, scrambled control. # P<0.01, vs. SC, ☆P<0.01, vs. SC+PEMF.
3. 讨论
骨质疏松症是一种骨量减少、骨组织显微结构受损,从而导致骨折危险度升高的全身型骨代谢障碍疾病,严重威胁着中老年人的身体健康和生活质量[18,19]。目前主要以药物治疗为主,但药物疗法尚存在毒副作用和价格昂贵等问题[20,21,22]。因此,寻找毒副作用更小、使用方便、价格低廉的物理疗法一直是人们努力的方向之一。1977年,Bassett[23]提出用电磁场刺激治疗骨不连,从此开创了用电磁场促进骨折愈合和治疗骨质疏松的历史,但其作用机制至今不明,严重制约了其临床应用及最佳疗效的发挥。
由于初级纤毛是凸起于细胞表面的杆状结构,就像是一根细胞“天线”,具备最早接触电磁场的结构优势,加之近年来越来越多的研究结果表明,其具有感受外界信息并将其转化为胞内化学信号的功能[24,25,26],本课题组提出假说,认为初级纤毛是成骨细胞表面的电磁信号接收装置,具有将电磁物理信号转化为胞内化学信号的功能,为证明这一假说,本研究观察了PEMF对初级纤毛形态的影响,结果发现,随着处理时间的延长,初级纤毛长度显著增加,但纤毛发生率并无明显改变。进一步研究表明,PEMF激活了PI3K/AKT信号途径,并且这种激活作用为PEMF促进骨形成所必需。当本课题组用RNA干扰法抑制初级纤毛发生后,PEMF既不能激活PI3K/AKT信号途径,又不能促进成骨细胞的成骨性分化,说明PEMF促进骨形成必须激活PI3K/AKT信号途径,但这种激活必须在初级纤毛存在的情况下才能发生。
综上所述,本研究发现PEMF处理可以改变成骨细胞表面的初级纤毛长度,而且PEMF促进骨形成活性离不开初级纤毛的参与,很可能是由初级纤毛首先感知到低频电磁场信号,再通过一定途径将其转为胞内化学信号,其中PI3K/AKT信号途径被激活就是这样的化学途径之一,但具体作用机制尚待进一步深入研究。
(本文编辑:王 蕾)
Funding Statement
国家自然科学基金(81270963); 国家自然科学基金(81471090); 甘肃省自然科学基金(1506RJZA306)
the National Natural Science Foundation of China(81270963); the National Natural Science Foundation of China(81471090); Gansu Natural Science Foundation(1506RJZA306)
References
- 1.石 文贵, 马 小妮, 陈 克明. 初级纤毛在细胞信号转导中的作用与机制. 浙江大学学报(医学版) 2014;43(3):359–365. doi: 10.3785/j.issn.1008-9292.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Shi WG, Xie YF, He JP, et al. Microgravity induces inhibition of osteoblastic differentiation and mineralization through abrogating primary cilia. Sci Rep. 2017;7(1):1866–1877. doi: 10.1038/s41598-017-02049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wanka H, Lutze P, Staar D, et al. (Pro)renin receptor (ATP6AP2) depletion arrests As4.1 cells in the G0/G1 phase thereby increasing formation of primary cilia. J Cell Mol Med. 2017;21(7):13–19. doi: 10.1111/jcmm.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhai YK, Guo XY, Ge BF, et al. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone. 2014;66(9):189–198. doi: 10.1016/j.bone.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 5.Ehnert S, Sreekumar V, Asperawerz RH, et al. TGF-β1 impairs mechanosensation of human osteoblasts via HDAC6-mediated shortening and distortion of primary cilia. J Mol Med. 2017;95(6):1–11. doi: 10.1007/s00109-017-1526-4. [DOI] [PubMed] [Google Scholar]
- 6.Kirschen GW, Liu H, Lang T, et al. The radial organization of neuronal primary cilia is acutely disrupted by seizure and ischemic brain injury. Front Biol. 2017;12(2):1–15. doi: 10.1007/s11515-017-1447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akama KT. McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synjournal via the Akt /protein kinase B pathway. J Neurosci. 2003;23(6):2333–2339. doi: 10.1523/JNEUROSCI.23-06-02333.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markus A, Zhong J, Snider WD. Raf and akt mediate distinct aspects of sensory axon growth. Neuron. 2002;35(1):65–76. doi: 10.1016/s0896-6273(02)00752-3. [DOI] [PubMed] [Google Scholar]
- 9.孟 金兰, 陈 雅嘉, 陈 红, et al. PI3K/Akt 信号通路在硫化氢保护PC12细胞对抗化学性缺氧损伤的作用. 中国药理学通报. 2013;29(2):257–261. [Google Scholar]
- 10.Chen Y, Hu Y, Yang L, et al. Runx2 alleviates high glucose-suppressed osteogenic differentiation VIA PI3K/AKT/GSK3β/β-catenin pathway. Cell Biol Int. 2017;41(8):822–832. doi: 10.1002/cbin.10779. [DOI] [PubMed] [Google Scholar]
- 11.Ma Y, Fu S, Lu L, et al. Role of androgen receptor on cyclic mechanical stretch-regulated proliferation of C2C12 myoblasts and its upstream signals: IGF-1-mediated PI3K/Akt and MAPKs pathways. Mol Cell Endocrinol. 2017;45(8):83–93. doi: 10.1016/j.mce.2017.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Wang J, Ma XY, Feng YF, et al. Magnesium lons promote the biological behaviour of rat calvarial osteoblasts by activating the PI3K/Akt signalling pathway. Biol Trace Elem Res. 2017;179(2):1–10. doi: 10.1007/s12011-017-0948-8. [DOI] [PubMed] [Google Scholar]
- 13.Song F, Wang Y, Jiang D, et al. Cyclic compressive stress regulates apoptosis in rat osteoblasts: involvement of PI3K/Akt and JNK MAPK signaling pathways. PLoS One. 2016;11(11):165–176. doi: 10.1371/journal.pone.0165845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou J, Ming LG, Ge BF, et al. Effects of 50 Hz sinusoidal electromagnetic fields of different intensities on proliferation differentiation and mineralization potentials of rat osteoblasts. Bone. 2011;49(4):753–761. doi: 10.1016/j.bone.2011.06.026. [DOI] [PubMed] [Google Scholar]
- 15.高 玉海, 成 魁, 葛 宝丰, et al. 50Hz不同强度正弦交变电磁场对大鼠峰值骨量的影响. 生物医学工程学杂志. 2015;13(1):116–119. [Google Scholar]
- 16.任 茜, 王 鸣刚, 陈 克明, et al. 脉冲电磁场促进大鼠成骨细胞成熟与矿化依赖于NO/cGMP信号途径. 中国生物化学与分子生物学报. 2016;32(11):1242–1248. [Google Scholar]
- 17.方 清清, 李 志忠, 陈 克明, et al. 50Hz 1.8mT正弦交变电磁场通过调节成骨细胞PGE2分泌影响Opg/Rankl的基因表达. 中国生物化学与分子生物学报. 2015;18(9):983–988. [Google Scholar]
- 18.Liu JM, Ning G, Chen JL. Osteoporotic fractures in Asia: risk factors and strategies for prevention. J Bone Miner Metab. 2007;25(1):1–5. doi: 10.1007/s00774-006-0720-1. [DOI] [PubMed] [Google Scholar]
- 19.张 辉, 李 亮, 王 茜, et al. 骨形成蛋白-7对多孔钽/软骨细胞复合物分泌功能以及 Col-Ⅱ、AGG 和 Sox9基因表达的影响. 北京大学学报(医学版) 2015;47(2):219–225. [Google Scholar]
- 20.Paula FJ, Rosen CJ. Back to the future: revisiting parathyroid hormone and calcitonin control of bone remodeling. Horm Metab Res. 2010;42(5):299–306. doi: 10.1055/s-0030-1248255. [DOI] [PubMed] [Google Scholar]
- 21.Bonnelye E, Chabadel A, Saltel F, et al. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro. Bone. 2008;42(1):129–138. doi: 10.1016/j.bone.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 22.何 伟, 李 自力, 崔 元璐, et al. 淫羊藿苷对大鼠成骨细胞核结合因子α1、骨形成蛋白-2、骨形成蛋白-4 mRNA表达的影响. 北京大学学报(医学版) 2009;41(6):669–673. [Google Scholar]
- 23.Bassett CA. The development and application of pulsed electromagnetic fields (PEMFs) for ununited fractures and arthrodeses. Orthop Clin North Am. 1984;15(1):61–87. [PubMed] [Google Scholar]
- 24.Spasic M, Jacobs CR. Lengthening primary cilia enhances cellular mechanosensitivity. Eur Cells Master. 2017;33(2):158–169. doi: 10.22203/eCM.v033a12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan JL, Zhou J, Ma HP, et al. Pulsed electromagnetic fields promote osteoblast mineralization and maturation needing the existence of primary cilia. Mol Cell Endocrinol. 2015;404(3):132–140. doi: 10.1016/j.mce.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 26.Xie YF, Shi WG, Zhou J, et al. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRⅡ localized at the base of primary cilium. Bone. 2016;93(2):22–32. doi: 10.1016/j.bone.2016.09.008. [DOI] [PubMed] [Google Scholar]