Cotton leafroll dwarf disease (CLRDD), caused by the aphid-borne Cotton leafroll dwarf virus (CLRDV; genus, Polerovirus; family, Luteoviridae), has been recently reported from the major cotton-growing regions of the United States. Here, we present the nearly complete genome sequence of a CLRDV isolate from cotton in Georgia.
ABSTRACT
Cotton leafroll dwarf disease (CLRDD), caused by the aphid-borne Cotton leafroll dwarf virus (CLRDV; genus, Polerovirus; family, Luteoviridae), has been recently reported from the major cotton-growing regions of the United States. Here, we present the nearly complete genome sequence of a CLRDV isolate from cotton in Georgia.
ANNOUNCEMENT
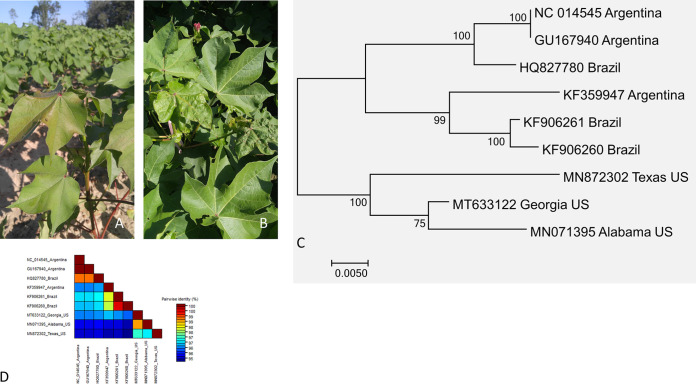
Cotton is the second most important agricultural commodity for the state of Georgia, with a farm gate value of $901.5 million (1). Cotton leafroll dwarf virus (CLRDV), a phloem-limited virus, is associated with the emerging cotton leafroll dwarf disease (CLRDD) in the United States. It was first reported from Alabama in 2019 (2) and subsequently from the major cotton-growing regions in the United States, including Florida (3), Georgia (4), Louisiana (5), Mississippi (6), South Carolina (7), and Texas (8). Symptoms of the disease include reddening of the leaves and petioles and drooling, crinkling, and deformation of the leaves (Fig. 1A and B), and it has the potential to cause significant yield and economic losses. The viral genome consists of a single-stranded positive-sense RNA approximately 5.8 kb long encoding seven different proteins (9, 10).
FIG 1.
Symptomatology and sequence analysis of Cotton leafroll dwarf virus (CLRDV). Cotton plant with symptoms of reddening and drooling (A) and crinkling and deformation (B) of leaves. Maximum likelihood phylogenetic tree (C) and pairwise identity matrix (D) of nearly complete CLRDV nucleotide sequence from Georgia with other reported sequences from GenBank.
In summer 2018, symptomatic plants (n = 20) showing reddening, drooling of leaves, and reddening of petioles, along with asymptomatic plants (n = 20), were collected from three counties, Early, Seminole, and Tift, in Georgia. CLRDV was detected from symptomatic samples but not from the asymptomatic tissue tested (4). To understand the genomic composition of CLRDV from Georgia, a nearly complete genome of an isolate from Seminole County was sequenced using Sanger’s method and analyzed.
Total RNA was extracted from a pooled sample of symptomatic leaves, petioles, and bark tissues using the modified cetyltrimethylammonium bromide method (11, 12). Complementary DNA (cDNA) was synthesized from 2.5 μg of total RNA using Superscript III reverse transcriptase (Invitrogen, USA) and specific reverse primers targeting different open reading frames (ORFs) (Table 1) of the virus genome following the manufacturer’s recommended conditions. The cDNA (2 μl) and specific primer combinations (Table 1) were used to amplify different ORFs of the CLRDV genome using Platinum Taq DNA polymerase (Invitrogen, USA). Products of predicted sizes were cloned into the pGEM-T Easy I cloning vector (Promega, USA), and both strands were sequenced using the SP6 to T7 sequencing primers (GenScript, USA). The nearly complete nucleotide sequence was assembled from the consensus sequence of three clones for the target region. The sequence was annotated with the help of BioEdit (13) and MEGA X (14) software and submitted to GenBank (accession number MT633122). The maximum likelihood phylogenetic tree of nearly full-length nucleotide sequences was constructed using the CLRDV sequences from GenBank and the isolate sequenced in this study with MEGA X (14) software. Pairwise comparisons of the nucleotide sequences were performed with SDT v.2.1 (15) software.
TABLE 1.
Sequences of oligonucleotide primer combinations, target regions, and annealing temperatures used to amplify the Cotton leafroll dwarf virus genome sequence in this study
| Strain name | Sequence (5′–3′) | Primer position | Amplicon length (bp) | Tma (°C) |
|---|---|---|---|---|
| SB19F | ACAAAAGAACGATAGAGGGGTTGTT | 1–25 | 1,352 | 60 |
| SB20R | ACCACCAACGTGGACTCCGAC | 1352–1332 | ||
| SB28F | TTCAGGTGGATACAGTGGGAC | 1286–1306 | 1,547 | 58 |
| SB19R | GTGGGAACCAGGTATTCCCGC | 2813–2833 | ||
| SB25F | CGCCTCATCATGTCTGTATCCC | 2609–2630 | 1,075 | 56 |
| SB15R | CCTACGTGGTCGTCTTCTTCCATTG | 3683–3659 | ||
| SB11F | AGGTTTTCTGGTAGCAGTACCAATATCAACGTTA | 3544–3569 | 775 | 60 |
| SB11R | TATCTTGCATTGTGGATTTCCCTCATAA | 4346–4319 | ||
| SB16F | ACGACGAAGACGAGGAGGTC | 3752–3771 | 1,307 | 64 |
| SB16R | AAAGTTGTGGCGTCTGGGGTT | 5059–5039 | ||
| SB3F | GCTGCACGCGCAGTGGAAGTG | 4729–4749 | 1,065 | 68 |
| SB3R | TGCCTATCCTTTCGGAGTCGTTCC | 5794–5771 |
Tm, annealing temperature.
The CLRDV genome from Georgia characterized in this study was 5,868 bp long and encoded seven ORFs, as reported earlier for isolates from North and South America (8–10). It was 95 to 98% identical to the genome of other CLRDV isolates from the United States (Alabama, GenBank accession number MN071395; Texas, MN872302) and South America (KF359947, KF906261, KF906260, NC_014545, GU167940, and HQ827780) (Fig. 1C). The US isolates formed a clade separate from that of the South American isolates in the phylogenetic analysis based on nearly full-length nucleotide sequences (Fig. 1D).
Data availability.
The nearly complete genome of CLRDV from Georgia described in this study was deposited in GenBank under accession number MT633122.
ACKNOWLEDGMENTS
We acknowledge support received in part from the Georgia Cotton Commission, Cotton, Inc., and the USDA National Institute of Food and Agriculture (Hatch Project 1020319) to accomplish the work done.
We are thankful for the comments and suggestions from two anonymous reviewers.
REFERENCES
- 1.The University of Georgia Center for Agribusiness and Economic Development. 2018. Georgia farm gate value report 2017. The University of Georgia Center for Agribusiness and Economic Development, Athens, GA. [Google Scholar]
- 2.Avelar S, Schrimsher DW, Lawrence K, Brown JK. 2019. First report of Cotton leafroll dwarf virus associated with cotton blue disease symptoms in Alabama. Plant Dis 103:592. doi: 10.1094/PDIS-09-18-1550-PDN. [DOI] [Google Scholar]
- 3.Iriarte FB, Dey KK, Small IM, Conner KN, O’Brien GK, Johnson L, Savery C, Carter E, Sprague D, Nichols RL, Wright DL, Mulvaney MJ, Paret M. 2020. First report of Cotton leafroll dwarf virus in Florida. Plant Dis doi: 10.1094/PDIS-10-19-2150-PDN. [DOI] [Google Scholar]
- 4.Tabassum A, Bag S, Roberts P, Suassuna N, Chee P, Whitaker JR, Conner KN, Brown J, Nichols RL, Kemerait RC. 2019. First report of Cotton leafroll dwarf virus infecting cotton in Georgia, U.S.A. Plant Dis 103:1803. doi: 10.1094/PDIS-12-18-2197-PDN. [DOI] [Google Scholar]
- 5.Price T, Valverde R, Singh R, Davis J, Brown S, Jones H. 2020. First report of Cotton leafroll dwarf virus in Louisiana. Plant Health Prog 21:142–143. doi: 10.1094/PHP-03-20-0019-BR. [DOI] [Google Scholar]
- 6.Aboughanem-Sabanadzovic N, Allen TW, Wilkerson TH, Conner KN, Sikora EJ, Nichols RL, Sabanadzovic S. 2019. First report of Cotton leafroll dwarf virus in upland cotton (Gossypium hirsutum) in Mississippi. Plant Dis 103:1798. doi: 10.1094/PDIS-01-19-0017-PDN. [DOI] [Google Scholar]
- 7.Wang H, Greene J, Muellar J, Conner K, Jacobson A. 2020. First report of Cotton leafroll dwarf virus in cotton fields of South Carolina. Plant Dis doi: 10.1094/PDIS-03-20-0635-PDN. [DOI] [Google Scholar]
- 8.Ali A, Mokhtari S. 2020. Complete genome sequence of Cotton leafroll dwarf virus isolated from cotton in Texas, USA. Microbiol Resour Announc 9:e01587-19. doi: 10.1128/MRA.01587-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Da Silva AKF, Romanel E, Da F Silva T, Castilhos Y, Schrago CG, Galbieri R, Bélot J-L, Vaslin MFS. 2015. Complete genome sequences of two new virus isolates associated with cotton blue disease resistance breaking in Brazil. Arch Virol 160:1371–1374. doi: 10.1007/s00705-015-2380-8. [DOI] [PubMed] [Google Scholar]
- 10.Distéfano AJ, Kresic IB, Hopp HE. 2010. The complete genome sequence of a virus associated with cotton blue disease, Cotton leafroll dwarf virus, confirms that it is a new member of the genus Polerovirus. Arch Virol 155:1849–1854. doi: 10.1007/s00705-010-0764-3. [DOI] [PubMed] [Google Scholar]
- 11.Cordova I, Jones P, Harrison NA, Oropeza C. 2003. In situ PCR detection of phytoplasma DNA in embryos from coconut palms with lethal yellowing disease. Mol Plant Pathol 4:99–108. doi: 10.1046/j.1364-3703.2003.00152.x. [DOI] [PubMed] [Google Scholar]
- 12.Dellaporta SL, Wood J, Hicks JB. 1983. A plant DNA minipreparation: version II. Plant Mol Biol Report 1:19–21. doi: 10.1007/BF02712670. [DOI] [Google Scholar]
- 13.Hall TA. 1999. BioEdit: a user friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98. [Google Scholar]
- 14.Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: Molecular Evolutionary Genetics Analysis across computing platforms. Mol Biol Evol 35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhire BM, Varsani A, Martin DP. 2014. SDT: a virus classification tool based on pairwise sequence alignment and identity calculation. PLoS One 9:e108277. doi: 10.1371/journal.pone.0108277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The nearly complete genome of CLRDV from Georgia described in this study was deposited in GenBank under accession number MT633122.