Abstract
Objective
Bupivacaine is an amide local anesthetic with possible side effects that include an irregular heart rate. However, the mechanism of bupivacaine-induced cardiotoxicity has not been fully elucidated, thus we aimed to examine this mechanism.
Methods
We performed electrocardiogram recordings to detect action potential waveforms in Sprague Dawley rats after application of bupivacaine, while calcium (Ca2+) currents in neonatal rat ventricular cells were examined by patch clamp recording. Western blot and quantitative real-time polymerase chain reaction assays were used to detect the expression levels of targets of interest.
Results
In the present study, after application of bupivacaine, abnormal action potential waveforms were detected in Sprague Dawley rats by electrocardiogram recordings, while decreased Ca2+ currents were confirmed in neonatal rat ventricular cells by patch clamp recording. These alterations may be attributed to a deficiency of CaV1.3 (L-type) Ca2+ channels, which may be regulated by the multifunctional protein calreticulin.
Conclusions
The present study identifies a possible role of the calreticulin–CaV1.3 axis in bupivacaine-induced abnormal action potentials and Ca2+ currents, which may lead to a better understanding anesthetic drug-induced cardiotoxicity.
Keywords: Bupivacaine, cardiotoxicity, CaV1.3, calreticulin, L-type Ca2+ channel, ventricular cells
Introduction
Bupivacaine is an anesthetic drug with possible side effects that include low blood pressure and an irregular heart rate (HR).1,2 The underlying mechanism of bupivacaine-induced cardiotoxicity has not yet been clearly reported. Given the key roles that ion channels play in regulating cardiac electrophysiological activities,3,4 it is thus necessary to focus on the roles of ion channels in bupivacaine-induced arrhythmias.
L-type calcium (Ca2+) channels are widely expressed in cardiac cells, are responsible for inward Ca2+ current, and trigger Ca2+ release from the sarcoplasmic reticulum to promote excitation-contraction coupling.5,6 CaV1.3 channels belong to the CaV1 Ca2+ channel family, which produces L-type Ca2+ currents and is essential for intracellular Ca2+ homeostasis.7,8 It is widely accepted that an abnormal Ca2+ level in cardiomyocytes is an important cause of arrhythmia.9,10 Therefore, alterations in the action potential waveform caused by changes in Ca2+ currents are useful for understanding drug-induced arrhythmia.
Calreticulin is a multifunctional protein in the endoplasmic reticulum. It is widely recognized as a resident endoplasmic reticulum protein that participates in Ca2+ binding and storage. Recent evidence has also shown that calreticulin can be translocated to the cell surface in response to oxidative stress or other pathological or physiological stimuli, where it can regulate diverse biological processes including Ca2+ channel currents.11–13
In the present study, we aim to investigate the underlying mechanism of abnormal action potential waveforms induced by bupivacaine in Sprague Dawley (SD) rats. We provide evidence that bupivacaine inhibits CaV1.3 (L-type) Ca2+ channels likely by promoting calreticulin expression in rat cardiomyocytes, resulting in a decrease in Ca2+ current and eventually leading to arrhythmia.
Methods
Animal studies
We established rat models by injection of bupivacaine (1 µg/g, 2 µg/g, or 4 µg/g). All animal experiments were approved by the Experimental Animal Ethics Committee of Daqing Longnan Hospital and were conducted in compliance with animal use guidelines (SYXK (Hei) 2006-033). Eight-week-old male or female SD rats were supplied by the Medical Experimental Animal Center of Harbin Medical University (Harbin, China). Rats were housed under standard animal room conditions (temperature 20°C; humidity 55% to 60%). Food and water were freely available throughout the experiments.
Electrocardiogram recordings
Electrocardiogram (ECG) recordings were performed on SD rats purchased from the Second Affiliated Hospital of Harbin Medical University (Harbin, China). After intraperitoneal injection of bupivacaine (1 µg/g, 2 µg/g, or 4 µg/g) or saline, SD rats (300±20 g) were intraperitoneally anesthetized with 10% chloral hydrate (3 µl/kg) (Merck, Kenilworth, NJ, USA), and then ECG recordings were performed using the BL-420S biosignal acquisition and processing system (Techman Soft, Sichuan Province, China). The left ventricular end diastolic pressure (LVEDP), left ventricular systolic pressure (LVSP), and HR were recorded using a pressure-digital converter. The maximal rates of pressure increase (+dp/dt) and decrease (-dp/dt) were calculated using the digitized left ventricular pressure.
Primary culture of rat ventricular cardiomyocytes
Neonatal rat ventricular cells (NRVCs) were isolated from the hearts of 1- to 3-day-old newborn rats. In brief, the newborn rats were euthanized by cervical dislocation, and after all vital characteristics, such as muscle tone, disappeared, the ventricles were excised, cut into small pieces, and digested with a 0.25% trypsin solution. After the cells were isolated, NRVCs were enriched by the differential preplating method. Then, purified NRVCs were plated on 12-well plates and maintained for 48 hours in Dulbecco’s Modified Eagle Medium supplemented with 10% fetal bovine serum.
Transfection of siRNA
Calreticulin siRNA or siNC (100 nM) (RiboBio, Guangzhou, China) was transfected into neonatal rat cardiomyocytes for 48 hours using Lipofectamine 2000 (Invitrogen Corp., Carlsbad, CA, USA) before stimulation with bupivacaine for 24 hours.
Electrophysiology
Ca2+ channel currents were measured using the whole-cell patch clamp technique with an Axopatch 200 amplifier (Molecular Devices, San Jose, CA, USA) in the voltage clamp mode at room temperature. Recording pipettes with resistances between 2 and 3 MΩ were used and filled with an internal solution containing (mM) CsCl 120, MgCl2 1, HEPES 10, Mg-ATP 4, EGTA 10, and Na2-GTP 0.3, pH 7.2; the extracellular solution contained (mM): TEA-Cl 140, MgCl2 2, CaCl2 10, HEPES 10, and glucose 5, pH 7.4.
Western blot assay
Whole-cell proteins were isolated from neonatal rat ventricular myocytes. Samples containing 100 μg of protein in 10 µL of loading buffer (Beyotime Biotechnology, Shanghai, China) were loaded and separated by electrophoresis on 8% sodium-dodecyl sulfate polyacrylamide gels. Then, proteins in the gels were transferred to polyvinylidene fluoride membranes and blocked for 1 hour using 5% nonfat milk, followed by incubation with primary antibodies to calreticulin (ab22683, Abcam, Cambridge, UK, observed band size 65 kDa) and CaV1.3 (ab84811, Abcam, observed band size 245 kDa) overnight at 4°C on a shaker. The membranes were washed three times with 0.05% phosphate buffered saline plus 0.05% Tween and incubated with corresponding fluorescent secondary antibodies (926-32211 and 926-32210, LI-COR Biosciences, Lincoln, NE, USA) for 1 hour in the dark at room temperature. Finally, the bands on the membranes were detected with the Odyssey instrument (LI-COR Biosciences), and Odyssey software v1.2 was used to analyze and quantify the bands. The intensities of proteins were normalized to the respective β-actin intensity of each gel.
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was performed to determine the relative expression levels of genes. Total RNA was extracted from neonatal rat cardiomyocytes with the Trizol reagent (Invitrogen, USA) according to the manufacturer’s instructions. The concentration and purity of total RNA were detected by a NanoDrop Spectrophotometer (NanoDrop Technolo-gies, Wilmington, DE, USA). Afterward, a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA) was used to synthesize cDNA. qRT-PCR was performed using the SYBR Green PCR Master Mix Kit (Applied Biosystems, USA). GAPDH was used as an internal control. Finally, the results were calculated using the 2−ΔΔCT method. The primer sequences were as follows: GAPDH: forward 5′-AACGACCCCTTCATTGAC CTC-3′ and reverse 5′-CCTTGACTGTGC CGTTGAACT-3′; calreticulin: forward 5′-ATCATGTTTGGTCCCGACATC-3′ and reverse 5ʹ-TCATCCTTGCAACGGATGTC-3′; and CaV1.3: forward 5ʹ-AGCCAACA GTGACAACAAGG-3′ and reverse 5ʹ-TTCAACTCCGAGATCCTTCG-3′.
Statistical analysis
Statistical analyses were performed using GraphPad 6 software (GraphPad Software Inc., San Diego, CA, USA). Statistical comparisons among experimental groups were evaluated using one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc test. Data are expressed as the mean±SEM. P values less than 0.05 were considered significant.
Ethics approval
All animal experiments performed in this study were approved by the Experimental Animal Ethics Committee of Daqing Longnan Hospital and were conducted in compliance with the animal use guidelines (SYXK (Hei) 2006-033).
Results
The effect of bupivacaine on rat electrocardiogram recordings
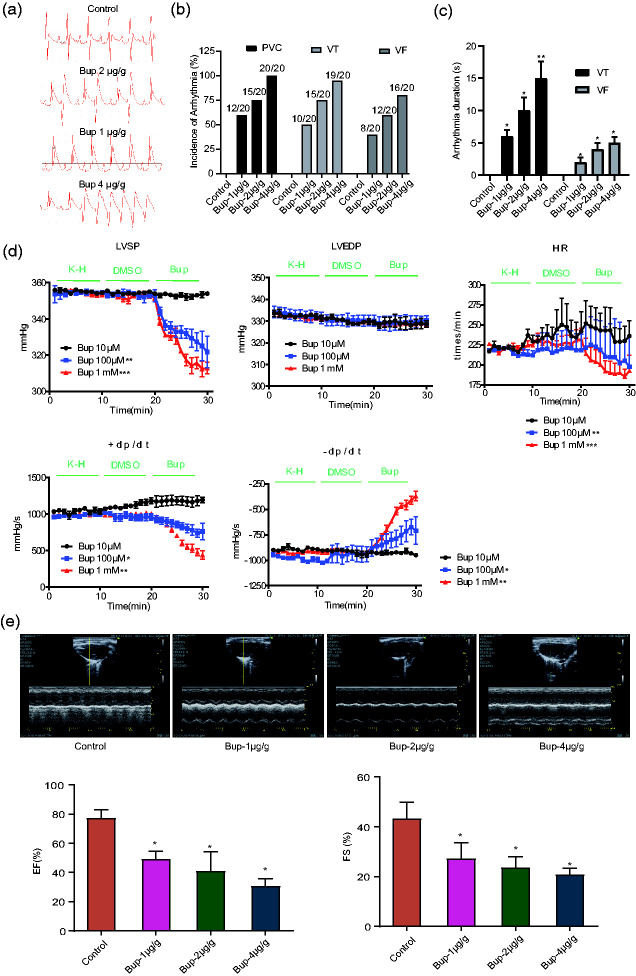
To determine the possible effect of bupivacaine on the action potential waveform, we examined a series of bupivacaine concentrations (1 µg/g, 2 µg/g, and 4 µg/g) in SD rats. The ECG results showed that chronic treatment with bupivacaine (1 µg/g, 2 µg/g, or 4 µg/g) significantly affected the action potential waveform of SD rats (Figure 1a). Meanwhile, three typical types of ventricular arrhythmia occurred in rats treated with bupivacaine: premature ventricular contraction, ventricular tachycardia, and ventricular fibrillation (Figure 1b). The arrhythmic duration was significantly longer in rats treated with bupivacaine than in the control animals (Figure 1c). Therefore, the cardiac function of the SD rats was examined to evaluate the effect of bupivacaine. The results in Figure 1d show that bupivacaine induced a significant decrease in HR, LVSP, and ± dt/dp but not in LVEDP, which suggests that bupivacaine has a significant influence on the action potential waveform and heart rhythm of SD rats. Additionally, the effect of bupivacaine on heart function in rats was determined by ultrasound. As depicted in Figure 1e, different concentrations of bupivacaine could inhibit the heart function of rats, including the ejection fraction and fractional shortening.
Figure 1.
Effect of bupivacaine on rat electrocardiograms. (a) Representative action potential waveforms of Sprague Dawley rats with or without chronic treatment with bupivacaine (1 µg/g, 2 µg/g, or 4 µg/g). (b) Statistical analysis of the incidence of arrhythmias. (c) Duration (in seconds) of arrhythmias. (d) Bupivacaine induces a significant decrease in the heart rate, left ventricular systolic pressure, and maximal rate of pressure increase or decrease but does not affect left ventricular end diastolic pressure (n=20). **P<0.01 or ***P<0.001 vs. control group. (e) The effect of bupivacaine on rat heart function determined by ultrasound. (n=20). *P<0.05 vs. the control group. Bup, bupivacaine; PVC, premature ventricular contraction; VT, ventricular tachycardia; VF, ventricular fibrillation; LVSP, left ventricular systolic pressure; LVEDP, left ventricular end diastolic pressure; HR, heart rate; +/– dp/dt, maximal rates of pressure increase and decrease; EF, ejection fraction; FS, fractional shortening.
Bupivacaine inhibits the calcium current of neonatal rat ventricular cells
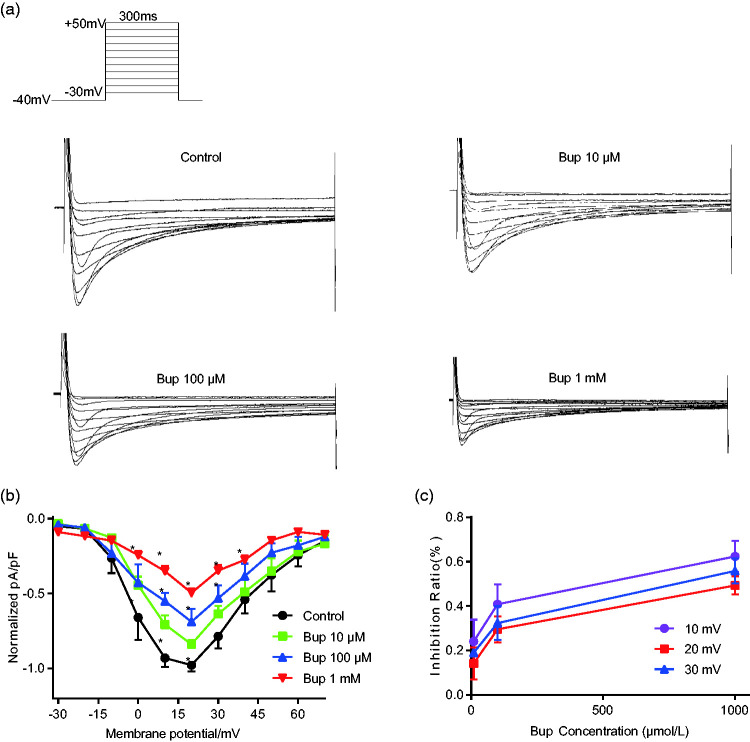
To further explore the causes of arrhythmia induced by bupivacaine, patch-clamp recordings were used to detect the effects of different concentrations of bupivacaine on L-type Ca2+ channel currents. Figure 2a shows representative L-type Ca2+ channel currents, which indicate that bupivacaine significantly inhibited L-type Ca2+ currents in a concentration-dependent manner. The normalized current–voltage (I-V) relationship in Figure 2b was constructed after normalizing the currents at various test potentials to the maximum current and dividing by cell membrane capacitance. The results show that bupivacaine obviously inhibited the L-type Ca2+ channel I-V relationship, and the inhibition ratio is shown in Figure 2c.
Figure 2.
Bupivacaine inhibits calcium (Ca2+) currents in neonatal rat ventricular cells. (a) Representative Ca2+ currents of neonatal rat ventricular cells with or without chronic bupivacaine treatment (10 µM, 100 µM, or 1 mM). (b) A statistical analysis of Ca2+ currents shows the normalized current–voltage relationships in the absence and presence of bupivacaine. (c) The inhibition of Ca2+ currents by bupivacaine (n=11). *P<0.05 vs. the control group. Bup, bupivacaine.
The effect of bupivacaine on CaV1.3 and calreticulin
CaV1.3 channels are responsible for inward Ca2+ current, thus we focused on the expression level of CaV1.3 in neonatal rat cardiomyocytes after administration of bupivacaine (10 µM, 100 µM, or 1 mM). Figure 3a–b show that bupivacaine inhibited the mRNA and protein expression levels of CaV1.3 in a concentration-dependent manner. To further explore the potential mechanism of bupivacaine-induced CaV1.3 deficiency, we detected the expression levels of calreticulin. The results showed that the mRNA and protein expression levels of calreticulin were negatively correlated with the bupivacaine concentration in CaV1.3 channels. Therefore, bupivacaine promotes the mRNA and protein expression in a concentration-dependent manner (Figure 3c–d).
Figure 3.
Effect of bupivacaine on CaV1.3 and calreticulin expression. (a) Bupivacaine inhibits CaV1.3 mRNA expression in a concentration-dependent manner. (b) Bupivacaine inhibits CaV1.3 protein expression in a concentration-dependent manner. (c) Bupivacaine increases calreticulin mRNA expression in a concentration-dependent manner. (d) Bupivacaine increases calreticulin protein expression in a concentration-dependent manner (n=6). *P<0.05 or **P<0.01 vs. the control group. Bup, bupivacaine.
Knockdown of calreticulin partially reversed CaV1.3 deficiency
The alteration of calreticulin expression led us to investigate the possible relationship between calreticulin and CaV1.3 channels. First, we knocked down calreticulin expression by siRNA. Figure 4a–b show the efficiency of siRNA knockdown. Transfection with si-calreticulin significantly reduced the mRNA (Figure 4a) and protein expression levels (Figure 4b) of calreticulin. We found that calreticulin knockdown could partially reverse the bupivacaine-induced CaV1.3 deficiency of both mRNA and protein expression (Figure 4c–d). Moreover, calreticulin knockdown partially reversed the decreased Ca2+ current caused by bupivacaine, as shown in Figure 4e. Therefore, the above evidence suggests that a strong relationship exists between calreticulin and CaV1.3 channels regarding bupivacaine-induced cardiotoxicity.
Figure 4.
Knockdown of calreticulin partially reverses CaV1.3 deficiency. (a) The efficiency of calreticulin siRNA knockdown was determined by qRT-PCR and (b) western blotting. (c) Calreticulin knockdown partially reversed the bupivacaine-induced decrease in CaV1.3 mRNA. (d) and protein expression (n=6). *P<0.05 or ***P<0.001 vs. the control group, #P<0.05 vs. 100 µM bupivacaine. (e) Calreticulin knockdown partially reversed the bupivacaine-induced decrease in Ca2+ current induced by bupivacaine (n=11). *P<0.05 vs. the control group, #P<0.05 vs. 100 µM bupivacaine. Bup, bupivacaine.
Discussion
Heart arrhythmia may predispose individuals to complications such as stroke or heart failure and can eventually lead to sudden death.14,15 The side effects of clinical drugs include cardiotoxicity, and the most common symptom of cardiotoxicity is arrhythmia.16–18 Drug inhibition or blockade of ion channels is the most common treatment for heart arrhythmia.19,20 Therefore, changes in action potential waveforms because of alterations in ion currents are useful for understanding drug-induced arrhythmia.
Bupivacaine, an anesthetic drug, has been reported to induce an irregular HR,1 but the underlying mechanism is unknown. Given the key roles that ion channels play in regulating cardiac electrophysiological activities, it is thus necessary to focus on the roles of ion channels in bupivacaine-induced arrhythmias.
In this study, we characterized the role of the calreticulin–CaV1.3 axis in bupivacaine-induced abnormal action potentials in SD rats and Ca2+ currents in neonatal rat ventricular cells. We observed that the mRNA and protein expression levels of CaV1.3 were dramatically decreased in the presence of bupivacaine in neonatal rat ventricular cells, which could be attributed to the increased expression levels of calreticulin. However, knockdown of calreticulin reversed these effects. To the best of our knowledge, the current study is the first to present new insights into the mechanism of bupivacaine-induced arrhythmias. Additionally, the results suggest that the use of bupivacaine as an anesthetic should be avoided when studying phenomena such as arrhythmia; however, if it is used as an anesthetic, its interference with the experimental results should be excluded.
Conclusion
This study revealed that the calreticulin–CaV1.3 axis plays a crucial role in bupivacaine-induced cardiotoxicity in cellular and animal models. Thus, drugs that block or reduce Ca2+ current may offer novel approaches for the prevention of cardiac arrhythmias caused by bupivacaine.
Authors' contributions
GYN was mainly responsible for designing the research and performing real-time PCR assays and data analysis. CB and ZX mainly performed the western blotting assays and patch clamp experiments. YR and HQL were mainly responsible for the animal studies and electrocardiogram recordings. LBD was responsible for the writing and revision of the manuscript. All authors have read and approved the manuscript for submission.
Availability of data and materials
The data and graphs used in the present study are available from the corresponding authors on reasonable request.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
BaiDong Li https://orcid.org/0000-0001-5628-6958
References
- 1.Partownavid P, Umar S, Li J, et al. Fatty-acid oxidation and calcium homeostasis are involved in the rescue of bupivacaine-induced cardiotoxicity by lipid emulsion in rats. Crit Care Med 2012; 40: 2431–2437. DOI: 10.1097/CCM.0b013e3182544f48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu L, Jin Z, Cai X, et al. Comparative regimens of lipid rescue from bupivacaine-induced asystole in a rat model. Anesth Analg 2019; 128: 256–263. DOI: 10.1213/ANE.0000000000003711. [DOI] [PubMed] [Google Scholar]
- 3.Camerino DC, Tricarico D, Desaphy JF. Ion channel pharmacology. Neurotherapeutics 2007; 4: 184–198. DOI: 10.1016/j.nurt.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YY, Li G, Che H, et al. Characterization of functional ion channels in human cardiac c-kit+ progenitor cells. Basic Res Cardiol 2014; 109: 407. DOI: 10.1007/s00395-014-0407-z. [DOI] [PubMed] [Google Scholar]
- 5.Yamakage M, Namiki A. Calcium channels–basic aspects of their structure, function and gene encoding; anesthetic action on the channels–a review. Can J Anaesth 2002; 49: 151–164. DOI: 10.1007/BF03020488. [DOI] [PubMed] [Google Scholar]
- 6.Eisner DA, Caldwell JL, Kistamas K, et al. Calcium and excitation-contraction coupling in the heart. Circ Res 2017; 121: 181–195. DOI: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh A, Gebhart M, Fritsch R, et al. Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem 2008; 283: 20733–20744. DOI: 10.1074/jbc.M802254200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fourbon Y, Gueguinou M, Felix R, et al. Ca(2+) protein alpha 1D of CaV1.3 regulates intracellular calcium concentration and migration of colon cancer cells through a non-canonical activity. Sci Rep 2017; 7: 14199. DOI: 10.1038/s41598-017-14230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souza DS, Menezes-Filho JER, Santos-Miranda A, et al. Calcium overload-induced arrhythmia is suppressed by farnesol in rat heart. Eur J Pharmacol 2019; 859: 172488. DOI: 10.1016/j.ejphar.2019.172488. [DOI] [PubMed] [Google Scholar]
- 10.Mohamed BA, Hartmann N, Tirilomis P, et al. Sarcoplasmic reticulum calcium leak contributes to arrhythmia but not to heart failure progression. Sci Transl Med 2018; 10: eaan0724. DOI: 10.1126/scitranslmed.aan0724. [DOI] [PubMed] [Google Scholar]
- 11.Wang WA, Groenendyk J, Michalak M. Calreticulin signaling in health and disease. Int J Biochem Cell Biol 2012; 44: 842–846. DOI: 10.1016/j.biocel.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Gold LI, Eggleton P, Sweetwyne MT, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. FASEB J 2010; 24: 665–683. DOI: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karnabi E, Qu Y, Yue Y, et al. Calreticulin negatively regulates the surface expression of Cav1.3 L-type calcium channel. Biochem Biophys Res Commun 2013; 437: 497–501. DOI: 10.1016/j.bbrc.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin CA, Matthews GD, Huang CL. Sudden cardiac death and inherited channelopathy: the basic electrophysiology of the myocyte and myocardium in ion channel disease. Heart 2012; 98: 536–543. DOI: 10.1136/heartjnl-2011-300953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moccia F, Lodola F, Stadiotti I, et al. Calcium as a key player in arrhythmogenic cardiomyopathy: adhesion disorder or intracellular alteration? Int J Mol Sci 2019; 20: 3986. DOI: 10.3390/ijms20163986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauckneht M, Ferrarazzo G, Fiz F, et al. Doxorubicin effect on myocardial metabolism as a prerequisite for subsequent development of cardiac toxicity: a translational (18)F-FDG PET/CT observation. J Nucl Med 2017; 58: 1638–1645. DOI: 10.2967/jnumed.117.191122. [DOI] [PubMed] [Google Scholar]
- 17.Kuok KI, In Ng PC, Ji X, et al. Supramolecular strategy for reducing the cardiotoxicity of bedaquiline without compromising its antimycobacterial efficacy. Food Chem Toxicol 2018; 119: 425–429. DOI: 10.1016/j.fct.2017.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Zhi D, Feng PF, Sun JL, et al. The enhancement of cardiac toxicity by concomitant administration of Berberine and macrolides. Eur J Pharm Sci 2015; 76: 149–155. DOI: 10.1016/j.ejps.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Tang L, Gamal El-Din TM, Lenaeus MJ, et al. Structural basis for diltiazem block of a voltage-gated Ca2+ channel. Mol Pharmacol 2019; 96: 485–492. DOI: 10.1124/mol.119.117531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen IS, Liu C, Tateyama M, et al. Non-sedating antihistamines block G-protein-gated inwardly rectifying K(+) channels. Br J Pharmacol 2019; 176: 3161–3179. DOI: 10.1111/bph.14717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data and graphs used in the present study are available from the corresponding authors on reasonable request.