Abstract
Cardiovascular disease is a major cause of mortality and morbidity worldwide. Exosome therapies are promising for cardiac repair. Exosomes transfer cargo between cells, have high uptake by native cells and are ideal natural carriers for proteins and nucleic acids. Despite their proreparative potential, exosome production is dependent on parent cell state with typically low yields and cargo variability. Therefore, there is potential value in engineering exosomes to maximize their benefits by delivering customized, potent cargo for cardiovascular disease. Here, we outline several methods of exosome engineering focusing on three important aspects: optimizing cargo, homing to target tissue and minimizing clearance. Finally, we put these methods in context of the cardiac field and discuss the future potential of vesicle design.
Keywords: : cardiac, cargo modulation, clearance, design, engineering, exosome, extracellular vesicle, homing, nanovesicle
Cardiovascular disease (CVD) continues to claim more lives globally than any other disease, with approximately 17.8 million deaths worldwide in 2017 alone [1]. CVD progressively weakens the cardiac myocardium and can result in unfavorable cardiac remodeling. Oftentimes, surgery is only palliative and long-term solutions for CVD remain few [2]. In the past decade, stem cell-based therapies have been extensively studied for cardiac repair. Both autologous and allogeneic stem cells have been administered to the diseased heart in attempts to induce local tissue regeneration and repair [3–5].
More recently, studies have shown that stem cell therapy-based reparative effects can be attributed to paracrine signaling [5–8]. Paracrine signaling is a part of intercellular communication wherein cell-free components are trafficked between nearby cells. In addition to soluble growth factors and cytokines, these signals include extracellular vesicles (EVs), which are lipid bilayer vesicles containing protein and/or nuclear cargo [9]. Being a relatively new field, the International Society for Extracellular Vesicles has not yet reached a consensus on specific markers for EV subtypes, but classification based on physical characteristics including size, density and origin condition are accepted [10]. For this review, we classify EVs grossly based on their size and parent cell condition, maintaining the naming used by the referenced papers (Figure 1). Vesicles derived from viable cells are grouped into exosomes and microvesicles. In contrast, vesicles derived from apoptotic bodies are referred to as apoptotic vesicles. Despite such classification, all subpopulations have some heterogeneity, highlighting the importance of the consistent International Society for Extracellular Vesicles nomenclature moving forward.
Figure 1. . Schematic of subpopulations of extracellular vesicles.
Exosomes and microvesicles are derived from viable cells. Apoptotic vesicles are derived from apoptotic cells. Exosomes are the smallest vesicles surrounded by a lipid bilayer membrane with lipid rafts, tetraspanins, immunoregulatory proteins, membrane trafficking proteins, integrins and flotillins embedded in it. Microvesicles are slightly larger than exosomes and apoptotic bodies can have the largest vesicles. Of the three types of extracellular vesicles, exosomes are known to be cardio protective and reparative.
Small EVs (historically referenced as exosomes) are less than 150-nm vesicles containing protein and nuclear cargo and are secreted by nearly all cell types. These vesicles form in the cytoplasm from the inward budding of endosomes. They were initially discovered in sheep reticulocytes in 1985 and were labeled as nothing more than carriers for waste export [11]. After several decades of exosome-related research, they have now been accepted as major players in paracrine signaling and potential biomarkers for several diseases [8]. Exosomes traffic mRNA, miRNA, DNA and proteins between cells. As cell-free components, these vesicles are often enriched in cargo compared with their parent cells, making exosome-based therapies a potential alternative to cell therapies for various diseases [12].
Medium/large EVs (historically termed as microvesicles) are slightly larger in size ranging from approximately 100 to 1000 nm. They were originally thought to be ‘dust material’ derived from platelets and their universal role in cellular interaction was only uncovered recently. These vesicles form from the outward budding of the cell’s plasma membrane [13]. Like exosomes, they also play a role in cell–cell communication with the transport of mRNA, miRNA, DNA and proteins between cells [14]. The precise differences between exosome and microvesicle-mediated transport are still unclear but being larger in size, microvesicles have been shown to successfully carry plasmid DNA [15]. Despite this, microvesicles remain poorly explored as a therapeutic for tissue repair and regeneration.
Medium/large EVs produced during cellular apoptosis (historically called apoptotic vesicles) can range from approximately 50 nm to 10 μm depending on their parent cell type. They are formed by indiscriminate blebbing of a cell’s membrane during apoptosis [16]. Consequently, their cargo usually consists of remnants of the parent cell such as cytoplasm, organelles and nuclear contents. As the frequency of cell apoptosis is higher in disease conditions, these vesicles could play an important role in regulating local disease microenvironments. For example, apoptotic vesicles from mature endothelial cells induced differentiation in endothelial progenitor cells [17]. However, the detailed role of apoptotic vesicles and their cargo in tissue homeostasis and repair still remains unclear.
In this review, we focus on exosomes. These natural vesicles have repeatedly been shown to be proreparative in cardiac conditions [18]. However, exosome production and potency are highly variable and dependent on the parent cell’s conditions. The reparative effects of exosomes have been associated with several factors including parent cell age and cell milieu [19,20]. Importantly, this cell dependence can also result in variability in the loaded cargo. While this variability has been studied for disease biomarker purposes, these variations also make developing standardized therapies a significant challenge. To address this, several attempts are being made to engineer the exosomes for specific disease outcomes. Here, we discuss exosome biology and outline different exosome/nanovesicle engineering methods to highlight recent advances in the field.
Exosome structure & function
A thorough understanding of exosome structure and function is important for designing exosomes and nanovesicles as efficient therapeutics. The exosome can be split into two main regions: the membrane and the internal core. Here, we will briefly outline the components of these two regions and our current understanding of their functionality.
Exosome lipids
Exosomes contain a bilayer membrane composed predominantly of amphiphilic molecules, lipid rafts and membrane proteins. The lipid classes found in exosome membranes typically include cholesterol (CHOL), sphingomyelin (SM), phosphatidylcholine (PC), phosphatidylserine (PS) and phosphatidylethanolamine (PE) [21]. The lipid distribution in the bilayer membrane is asymmetric with most lipid classes found on the inner leaflet and a few classes, such as SM and PC, typically found on the outer leaflet. This orientation of the lipids to one leaflet is associated with their packing parameter, and depends on the shape of the fatty acid tails of each molecule [22].
It is well known that membrane lipids play an important role in vesicle trafficking [23,24]. Interestingly, the ratios of lipid classes in exosomes is different to that of parent cells, suggesting active packing of lipids into the exosomes. A great summary of the variations in lipid profiles between parent cells and exosomes is outlined by Skotland et al. wherein exosomes were found to be enriched for CHOL, SM, PS and glycosphingolipids [25]. In addition, the membrane lipid composition was found to vary depending on the parent cell type and the local microenvironmental conditions, such as serum levels [12]. The exact rationale and advantage of such lipid enrichment in exosomes is unclear. However, the enriched lipids in exosomes correlated to the lipid headgroup charge, tail length and tail saturation across certain cell types [26]. Although there is limited comprehensive research on the lipid profiles of exosomes derived from cardiac cell types, these findings highlight the importance of lipid composition when engineering nanovesicles.
Exosome proteins
The proteins present in exosomes can be largely subdivided into two groups: those which are present in the membrane (including tetraspanins, immunoregulatory proteins, membrane trafficking proteins, integrins and flotillins) and those encapsulated within the exosome (including chaperones, cytoskeletal proteins, enzymes, signal transduction proteins and exosome biogenesis proteins) [27]. The proteins can also be divided into those inherent to exosomes (exosome markers) and those which relate to the parent cells and are in turn biomarkers for different physiological effects (origin markers). Common exosome markers include tetraspanins (CD9, CD63, CD81), ALIX, TSG101 and HSP70. In particular, ALIX and TSG101 are members of the endosomal sorting complex required for transport, the four-complex mechanism involved with cargo sorting and exosome biogenesis [28]. Exosome origin markers tend to be lineage and disease-specific and are often influenced by the proteins expressed on their parent cell membranes. For example, exosomes derived from stressed (hypoxic) cardiomyocytes (CMs) often include HSP60 in them [29,30]. Despite this, there are few confirmed cell-specific biomarker to develop a repertoire of exosome origin markers.
Exosome protein content varies based on parent cell type and, similar to exosomal lipids, is present in different ratios from that of the parent cell [26]. This further indicates selective protein loading into exosomes. These exosome proteins serve several important roles in the budding of exosomes from multivesicular bodies (MVBs), their trafficking and targeting to recipient cells, and their uptake. Therefore, when engineering vesicles it is important to recapitulate the relevant proteins in both the membrane and cargo.
Exosome nucleic cargo
A large part of exosome cargo consists of nucleic acids such as miRNA, mRNA, long noncoding RNA (lncRNA) and DNA. These are loaded into the vesicles from the cell cytoplasm during the formation of intraluminal vesicles (ILVs). Of these, miRNAs typically play an important role in cardiac repair [31]. One such cardioprotective miRNA from mesenchymal stem cells (MSCs) is miR-21-5p that was found to reduce procell death target genes [32]. Similarly, MSC-exosomes containing miR-125b-5p provided protection against ischemia-reperfusion-based injury [33]. As for mRNA, endothelial progenitor cell-derived exosomes increased endothelial cell angiogenesis by delivering PI3K/AKT pathway-associated mRNA [34]. More recently, groups have investigated the importance of lncRNA in cardiac-specific diseases. Evidence indicates that lncRNAs are more tissue specific than miRNA, however, the role of lncRNA in exosomes has not been fully explored. Zhu et al. demonstrated that umbilical cord-MSCs release MALAT1, an lncRNA previously associated with lung adenocarcinoma, and minimize age-induced cardiac dysfunction [35]. Another study showed the role of an lncRNA, NEAT1, in cardiac repair, which inhibited miR-142-3p and subsequently activated FOXO1 [36].
Exosome nucleic cargo composition is highly dependent on parent cell state and milieu [19,20]. These studies highlight the importance of exosomal cargo composition for inducing cardiac repair and targeting several different cell types. This indicates the value of exosomes as naturally derived and reliable avenues for cargo transport. Therefore, when designing vesicles, having control over cargo loading and copy number is crucial for tailored therapies.
Life of an exosome (biogenesis, release, uptake, effect)
Exosome biogenesis and release occurs through a series of endocytic steps: inward budding of the plasma membrane and formation of the early endosome; transformation of the early endosome into the late endosome; inward budding of the late endosome or MVB; and fusion of the MVB with the plasma membrane for release of exosomes into the extracellular space (Figure 2). While this process results in exosome release and subsequent signaling, the MVB may also fuse with the lysosome, instead of the plasma membrane, resulting in vesicle degradation [37,38].
Figure 2. . Exosome biogenesis and uptake.

Exosome biogenesis occurs in the parent cell (orange) through a series of endocytic steps, which conclude with exosome release from the MVB. Several protein complexes (not shown) are involved at each stage of biogenesis (e.g., ESCRT proteins). During inward budding, some membrane proteins and lipids are also incorporated (not shown). Once exosomes are released into the extracellular fluid, they get trafficked to the recipient cell (blue). Here, exosome uptake occurs through one of many methods: macropinocytosis, receptor–ligand mediated uptake, lipid raft mediated uptake (including clathrin-coated pits and caveolin) or direct fusion. In the recipient cell, the exosome is either degraded in the lysosome or activates intracellular signaling.
ESCRT: Endosomal sorting complex required for transport; ILV: Intraluminal vesicle; MVB: Multivesicular body.
Recently, several strategies to increase exosome production have been reported. For example, the overexpression of tetraspanin CD9 on HEK293 cells enhance exosome production by 2.4-fold [39]. Another study showed that small-molecule treatment of MSCs boosted exosome secretion by enhancing gene expression related to the endosomal sorting complex required for transport-independent pathway [40]. Exposing MSCs to norepinephrine codosed with N-methyldopamine enhanced exosome secretion (threefold) without dramatically changing the exosome protein composition. Recent studies have also suggested that hypoxic conditions increase production of MSC-derived exosomes, although the exact mechanisms of action are still unclear [20,41,42].
Each step in the biosynthesis of exosomes allows the vesicles to acquire certain cargo and membrane components. Exosomes contain membrane proteins, including tetraspanins and integrins, that originate from the cell’s plasma membrane. When the initial endocytosis of this membrane occurs, these proteins become incorporated into the early endosome and are carried along during the formation of exosomes [43]. Additionally, during the process of inward budding of the late endosomal membrane, cytosolic proteins, RNAs and other molecules are encapsulated, forming cargo-loaded ILVs inside the MVB. These ILVs are eventually released as exosomes when the MVB fuses to the cell’s plasma membrane [44].
Once released into the extracellular space, exosomes may be taken up by recipient cells via endocytic processes including lipid-raft-based uptake (including clathrin-coated pits, and caveolin), direct membrane fusion, macropinocytosis or through receptor–ligand interactions (Figure 2) [45–48]. Furthermore, there are known membrane proteins that play a role in the uptake process. Tetraspanins are highly enriched in exosomal membranes and are involved in cell fusion and penetration events [46]. Additionally, tetraspanin-rich domains are implicated in sorting receptors and intracellular molecules into exosomes. Heat-shock proteins (including HSP70 and HSP90) are implicated in antigen binding and presentation in exosomes, and annexins and Rab play a part in membrane fusion [49,50].
Finally, once internalized by recipient cells, exosomes either remain functional and transfer materials into recipient cells or are shuttled to the lysosome for degradation [38,51]. In the former case, exosomes fuse to endosomes, allowing for horizontal genetic transfer of the cargo to the recipient cell’s cytoplasm [52]. Here, the delivered intravesicular molecules can be involved in epigenetic reprogramming of recipient cells through the delivery of proteins, lipids and RNAs. Nonetheless, the effects of exosomes are not limited to their cargo; exosomes may also affect recipient cells via receptor–ligand-mediated signaling and the transfer of receptors to the cell surface [51]. It is evident that exosomes are highly tuned to be efficient messengers between cells and regulating such nanovesicles could be impactful for cell therapies.
Role of stem cell-derived exosomes in cardiac repair
Recently, emerging evidence has suggested that a primary role of exosomes in CVD is through shuttling proteins and noncoding RNAs, specifically miRNAs, between cells. In detail, exosomes activate signaling pathways by transferring functional proteins and/or miRNAs, substantially contributing to the beneficial effects of stem cell therapies for diseases such as cardiac hypertrophy [53,54] and myocardial infarction (MI) [55,56] among others. C-kit+ progenitor cells (CPCs) [19,57], cardiosphere-derived cells (CDCs) [9,58] and MSCs [59,60] are the most studied sources of exosomes implicated in cardiac repair by cellular therapy. The cargo of these exosomes has been shown to increase cardioprotective and regenerative signals [61].
One such reparative cargo molecule carried by CPC exosomes was miR-210, which was shown to enhance endothelial migration and capillary density in vitro [20]. In addition, the same study implicated CPC exosome miR-17 delivery to cardiac tissue as a means to reduce fibrotic scar tissue formation in an MI animal model. Another study using CPC sphere-derived exosomes suggested that exosome-mediated miRNA delivery could effectively increase angiogenesis and ablate fibrosis in vitro, especially through miR-320a and miR-423-5p [57]. Moreover, CDC- or CPC exosomes had cardioprotective effects (inhibition of apoptosis and stimulation of angiogenesis) via miR-132 delivery to the injured heart [62].
Other studies have shown that preconditioning MSCs further improved the treatment of cardiac diseases. Under hypoxic conditions, MSCs produced miR-22-enriched exosomes, which targeted apoptotic MECP2 and resulted in a reduction of the ischemic myocardium [63]. In addition, GATA-4-overexpressing MSCs released miR-19a enriched exosomes, which targeted PTEN in CMs [64]. The downregulation of MECP2 and PTEN levels was directly correlated to anti-apoptosis of CMs. Therefore, there are significant benefits to using MSC exosomes for the treatment of various cardiac diseases. Likewise, functional proteins on the exosome surface have a significant role in CVD. One study showed the cardioprotective role of rat plasma exosomes in CMs through HSP70-mediated TLR4 signaling [65]. Specifically, HSP70 activated TLR4 on CMs, leading to the activation of ERK/p38MAPK and subsequent HSP27 phosphorylation in the cells. In another study, pregnancy-associated plasma protein-A expressed on CPC exosome surfaces was shown to reduce CM apoptosis by enhancing IGF-1 proteolytic cleavage and subsequent IGF-1 receptor activation [66].
These studies show that, in the cardiac field, stem cell-derived exosomes are a primary vehicle for proreparative miRNA and protein transfer. This underscores the importance of such stem-cell-based nanovesicles and their cargo for cardiac repair and regeneration.
Limitations of exosomes
Despite their crucial role in paracrine signaling for cardiac regeneration and repair, stem/progenitor cell-derived exosomes have several limitations hindering their success as a therapeutic. One major drawback involves a supply and demand discrepancy. Cell-derived exosomes have variable, low yield that makes it challenging to meet demand for in vivo applications [67]. Furthermore, although several techniques exist for isolating exosomes from cells, efficient and reproducible exosome isolation is often inconsistent or costly [68]. Additionally, as exosomes are isolated from the conditioned media of parent cells, there is limited control over their composition and cargo encapsulation varies based on the physiologic state of the parent cell [69]. Also, as there are multiple types of cargo present in each exosome, the copy number of specific miRNA/RNA/DNA tends to be very low [70]. Therefore, even if certain nucleic acids are known to be favorable for cardiac repair, the potency of each exosome is limited, requiring higher dosing of vesicles to meet demand. Such higher doses pose their own risks including accumulation in the lungs and potential asphyxiation [71]. Moreover, when delivering exosome therapies, the targeting of the vesicles is likely dependent on membrane proteins for which there is limited control over. Finally, to be clinically relevant, it is important that exosome therapies avoid lysosomal degradation and minimize unfavorable immune responses [38].
Due to the multitude of challenges present in developing translational exosome therapies, there is value in studying methods to engineer exosomes specifically for cardiac repair. When designing vesicles, it is important to tailor the cargo to increase potency, home the exosomes to the cardiac site and minimize unfavorable vesicle loss. Below, we discuss three critical aspects for engineering effective, customized exosome therapies: optimizing cargo, targeting delivery and reducing clearance.
Nanovesicle engineering: cargo optimization
Numerous methods have been devised to modify exosomes and create synthetic exosome mimics to control or alter their cargo. Several of these methods have been explored, more in relation to noncardiac fields such as cancer and neurology. Here, we outline some of the key techniques that have been used for exosome cargo customization and highlight applications in cardiac therapies; subdividing cargo engineering into three sections: modifying exosomes, modifying parent cells and generating synthetic nanovesicles (Figure 3).
Figure 3. . Overview of cargo optimization techniques.
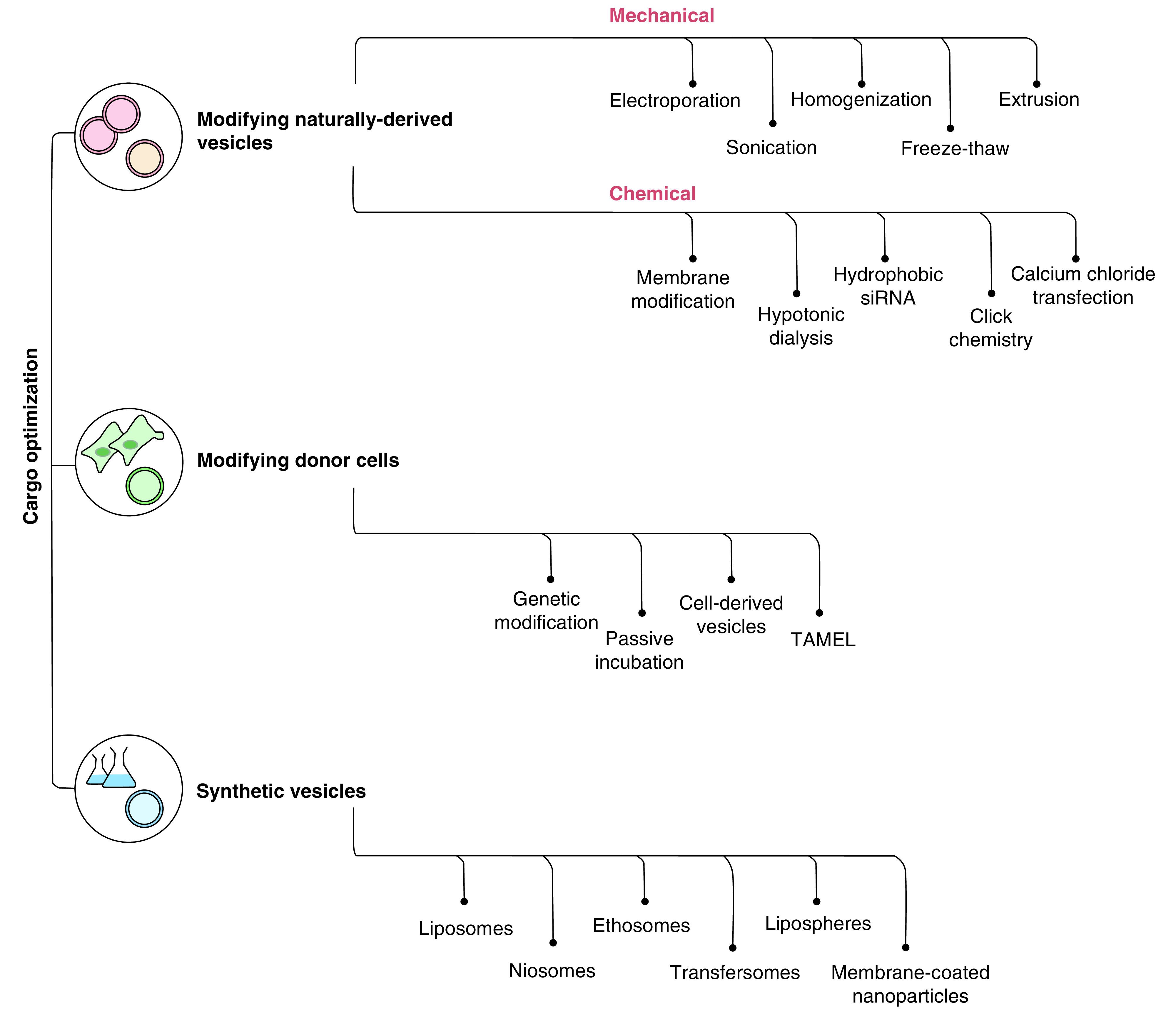
Numerous techniques have been devised to load cargo into exosomes and exosome mimetics. This flowchart provides an outline of naturally derived vesicle modification (red); donor cell modification (green) and synthetic vesicle generation (blue).
TAMEL: Targeted and modular extracellular vesicle loading.
Modifying exosomes
As discussed earlier, exosomes have complex membrane structures with a composition selectively distinguished from that of their parent cells. Therefore, there is merit to maintaining the complexity of exosome membranes while still modifying the cargo carried. Moreover, loading of desired cargo into exosomes can increase cargo uptake by recipient cells up to 60% more than unencapsulated or liposome-encapsulated cargo [72]. Several methods specific to modifying exosomes can be grossly classified into mechanical and chemical techniques. Here, we outline several of the current methods to alter exosome cargo, and briefly mention a few other unique methods to engineer exosomes. Prior reviews have also discussed exosome engineering methods, so here we aim to briefly discuss those and outline newer methods too [28,73–76].
Mechanical modification
Electroporation
Electroporation involves the creation of small pores in the exosome membrane to encapsulate cargo. A transmembrane voltage is applied to the membrane to develop small pores, which later self-seal. This method has commonly been used in several fields for exosome encapsulation of small interfering RNA (siRNA) and miRNA [77]. Lunavet et al. provided evidence of this concept by encapsulating fluorescently tagged siRNA into NIH3T3-cell membrane-derived nanovesicles via electroporation [78]. Loading efficiency of the siRNA was approximately 2%, with morphological integrity of the vesicle membrane maintained. When compared with the gold-standard, lipofectamine, the siRNA nanovesicles resulted in comparable gene knockdown, highlighting the potential of electroporation. Beyond RNA, electroporation was also used to encapsulate porphyrins into exosomes derived from human MSCs, human umbilical vein endothelial cells (HUVEC), human embryonic stem cells and MDA-MB231 breast cancer cells. Encapsulation of the porphyrins, despite their hydrophobicity, was significantly increased after electroporation [72]. In cardiac studies, miR-132 was encapsulated into MSC-derived exosomes through electroporation and in-turn significantly increased angiogenesis in a murine MI model [79]. Despite these findings, electroporation is not universally favorable for cargo encapsulation as it can lead to cargo aggregation, which reduces RNA functionality [80]. However, aggregation can be minimized by using trehalose pulse media during electroporation. Trehalose is a disaccharide more commonly used to protect cells from dehydration. When added during electroporation, aggregates reduced, and the colloidal stability of the exosomes increased [81].
Sonication
Sonication uses high-frequency sound energy to create microcavitation in water and disrupt the exosome membrane, so that the target therapeutics can be entrapped into the nanovesicles. Several types of nucleic acids (e.g., siRNA, miRNA or single-stranded DNA) have been encapsulated in such a manner [82]. Sonication is often paired with electroporation or passive incubation with the cargo to create desired nanovesicles [82,83]. For example, sonication was used to encapsulate catalase enzyme, with high encapsulation efficiency, prolonged release and maintained catalase activity [84]. In addition, sonication-based siRNA vesicles have knocked down corresponding RNA and successfully lead to the reduction of HER2 expression in MCF-7 breast cancer cells [82]. Moreover, the incorporation of paclitaxel (PTX) into exosomes via sonication reduced U-87 cancer cell viability by almost 50% [83]. Similarly, triptolide was also delivered through sonicated exosomes to human ovarian cancer cells, SKOV3 and in vivo in tumor-bearing mice [85]. Although triptolide was encapsulated with high efficiency and induced minimal cytotoxic and apoptotic effects compared with the naked drug, the triptolide vesicles had toxic effects on the liver and spleen. This underlines the potential challenge with homing the vesicles to the target site and minimizing clearance and uptake by other organs. Depending on the type of cargo being encapsulated, this could lead to toxic off-target effects, overriding the exosome’s inherent minimally toxic properties.
Homogenization
Similar to sonication, homogenization disrupts the vesicle membrane but instead of sound energy it uses mechanical shear force and cavitation. A special type of homogenization known as microfluidization uses flow through multiple microchannels to impart shear force and cavitation on vesicles and in turn rupture them. Noyes et al. show KRAS gene knockdown from KRAS-siRNA-loaded exosomes formed through this technique [86]. Furthermore, the size of these cargo-laden exosomes can be reduced to roughly 120-nm vesicles by performing multiple rounds of microcavitation. This method has not been tested on several different vesicles with different types of cargo though, so more robust study is required to understand its translatability.
Freeze–thaw cycles
This process involves incubating exosomes with the desired cargo, and repeating cycles of rapid freezing at -80°C and thawing. The formation of disordered ice crystals during rapid cooling ruptures the vesicle membrane, enabling cargo insertion. This technique has been utilized to develop ‘hybrid exosomes’ with modified phospholipid membrane content [87]. For example, a mixture of cell-derived exosomes and liposomes underwent repeated freeze–thaw cycles, generating hybrid exosomes with increased cellular uptake efficiency, compared with unmodified exosomes. Specifically, exosomes modified in this way with polyethylene glycol (PEG) had higher cellular uptake. Nevertheless, freeze–thaw cycles can form vesicle aggregates, produce greater exosome size-variability and reduce encapsulation efficiency, compared with sonication and extrusion [84]. Similar to electroporation, vesicle aggregation can be minimized by performing freeze–thaw cycles in the presence of trehalose [88].
Freeze-thawing has also been used to encapsulate hydrophobic drugs into exosomes. Typically, hydrophobic drugs are embedded into the exosome membrane. However, the membrane bilayer only accounts for a small fraction of total exosome volume, so hydrophobic drug encapsulation in this space is limited. To overcome this, Tran et al. combined hydrophobic aspirin with a polymer and used a modified freeze–thaw method (with prolonged incubation and ultrasonication steps) to encapsulate the aspirin into exosomes [89]. Interestingly, the combination of the polymer and modified freeze-thawing increased encapsulation efficiencies to up to 80% in some cases. This suggests that a combination of cargo modification and prolonged freeze–thaw could be a fruitful method to increase hydrophobic cargo encapsulation.
Extrusion
In extrusion-based methods, mechanical shear force is utilized to rupture the bilayer membrane, so that small molecules of interest, incubated with the exosomes, can enter the vesicle. This process involves repeatedly passing vesicles through a nanoporous membrane, forcing larger vesicles to break open and reform as they transfer across the pores. During extrusion, the vesicles are suspended in a solution containing the small molecules to be loaded, so as the vesicles are ruptured and reformed, these molecules enter and are encapsulated into the vesicles. When used to embed catalase, extrusion had reasonable encapsulation efficiency and maintained catalase’s enzymatic activity, comparable with outcomes when using sonication [84]. This technique is very commonly combined with other processes such as freeze–thaw or sonication to develop nanovesicles of uniform size. Extrusion has also been used to fine-tune the membrane properties of EVs by incorporating synthetic lipids into the membrane [77]. These hybrid vesicles had a uniform size around 100 nm. Interestingly, the particle number increased 6- to 40-fold higher than the original exosome number after extrusion, depending on the type of lipids embedded into the exosome membrane. Furthermore, the species of lipids embedded into the vesicle membrane impacted the RNA interference efficiency of the loaded siRNA cargo. This underscores the importance of membrane lipid components in EV cargo functionality.
Chemical modification
Membrane modification
Exosome membranes can be altered chemically to allow the loading of desired genetic material or drugs. Specifically, surfactants interact with membrane CHOL and permeabilize the membranes, so that coincubated cargo can be encapsulated. One such surfactant, saponin, was used to load hydrophobic porphyrins into nanovesicles [72]. This technique allowed for higher loading efficiency compared with passive incubation or electroporation without altering the size or ζ potential of the EVs. Furthermore, cellular uptake of the porphyrins increased approximately 8% using saponin-based EV loading. In another study by Haney et al., different methods of exosome loading were studied. Saponin-based encapsulation of the antioxidant, catalase, had the highest loading efficiency without altering the exosome size, but the cargo was also rapidly released from the exosomes. When added to neuronal PC12 cells that were pretreated to mimic neurodegeneration, exosomes with saponin-based catalase loading has significantly increased neuroprotective effects. Furthermore, when administered to mouse brains, these exosomes resulted in the highest neuroprotection. Such surfactant-based exosome loading seems to be a highly effective way to load cargo. However, saponin’s ability to remove membrane-bound CHOL makes it cytotoxic to cells, as it can destabilize the cell membrane. Therefore, limited concentrations should be utilized and postmodification purification of exosomes may be required [90].
Hypotonic dialysis
Hypotonic dialysis involves the transfer of cargo into exosomes in the presence of an osmotic gradient. Exosomes are dispersed in a hypotonic solution that causes the vesicles to swell and form pores, allowing cargo to be loaded into the exosomes. The exosomes are then transferred into an isotonic solution, so the pores can reseal, entrapping the cargo inside the vesicles. Fuhrmann et al. used hypotonic dialysis to load porphyrins into EVs and studied the loading efficiency [72]. Interestingly, MDA-MB231l derived EVs had more than 11-fold loading efficiency of cargo with hypotonic dialysis and saponin treatment, compared with extrusion. However, the uptake of dialyzed vesicles was much lower than those formed from the other two methods. Therefore, despite the efficiency of cargo capture, this technique may not be as translatable.
Hydrophobic siRNA
Another approach to exosome loading is to chemically modify the cargo prior to incorporation into the exosomes. One method is to lipid conjugate siRNA (e.g., with CHOL), so that it contains a hydrophobic region and can be embedded into the vesicle membrane. This technique was performed with exosomes loaded with HuR-hydrophobic siRNA (hsiRNA) and successfully silenced HuR in HEK293 cells in a dose-dependent manner [91]. Similarly, delivery of Htt-hsiRNA exosomes to primary neurons induced Htt-mRNA and protein silencing [92]. Interestingly, there appears to be a saturation limit for the potency of such hsiRNA-loaded vesicles. Haraszti et al. found that loading more than 5000 hsiRNA per vesicle may actually inhibit the effects of gene silencing [93]. It is thought that such high packing of cargo might alter membrane curvature and in turn vesicle composition. However, as these two factors are highly dependent on the parent cell of the exosomes and the lipid conjugate used, the exact loading threshold may vary for different vesicles.
Click chemistry
Click chemistry involves the bioconjugation of small molecules to specific biological molecules and has been used for nanoparticle (NP) surface modifications. In relation to cargo loading, click chemistry has been used to bind desired small molecules onto vesicle surfaces. Such bio-orthogonal click chemistry has been used to modify vesicle composition, specifically using dibenzobicycloctyne (DBCO) [94]. With this method, exosome size distribution and structural integrity (presence of membrane CD63) were maintained. Moreover, these DBCO-carrying exosomes were taken up by skin melanoma cells more efficiently than free DBCO. This technique has wider applications as it could be used to include several other substrates into the exosome membranes too.
An important consideration with click chemistry is the impact of the modification on exosome structure and functionality. Smyth et al. investigated this by conjugating a model azide onto breast cancer cell-derived exosomes [95]. They found that click chemistry did not alter the size of alkyne-cross-linked exosomes or affect their internalization by parent breast cancer cells. Furthermore, to assess if overmodification of exosome proteins with alkyne groups would impair function, a phenomenon seen with antibody modification, they quantified the number the alkyne modifications present with their click chemistry technique. They found that the number of modifications were minimal (<5), suggesting this would not affect vesicle function. This provides proof-of-concept that such techniques can be used to conjugate specific molecules to the membrane of the exosomes. Despite this, further progress with this technique has been limited.
Calcium chloride transfection
Another novel approach involved transfecting isolated exosomes with a calcium chloride-based transfection [96]. Macrophage-derived exosomes were coincubated with miR-15a or miR-15a inhibitor followed by addition of the calcium chloride. These transfected exosomes induced functional alterations in miR15a target genes and downstream proteins when delivered to THP-1 macrophages. Furthermore, when these exosomes were delivered to murine lungs, they were successfully taken up by alveolar macrophages, highlighting the translational potential of this technique. This direct technique may have more promise as it minimizes the traditional cell-based dependency of genetically modifying exosome cargo.
Modifying parent cells
Aside from directly re-engineering the exosomes to modify their cargo, another approach involves modifying the parent cells, which consequently alters the cargo encapsulated into the exosomes. One of the primary methods to accomplish this involves genetic modification of parent cells but other unique methods have been developed too.
Genetic modification
Genetic modification allows for the alteration of exosome cargo by capitalizing on the parent cell’s inherent ability to load exosome cargo. Thus, one can genetically engineer the parent cells to selectively load exosomes with target RNA cargo [97]. In one study, tumor cells were transfected with wild-type p53 and/or miR-125b altering the overall global miRNA profile of the released exosomes [98]. Favorably, the exosome miRNAs were more associated with apoptosis and p53 signaling, suggesting beneficial therapeutic response for tumor reduction. In the cardiac field, human CD34+ stem cell exosomes containing Cy3-labeled pre-miR miRNA precursors were synthesized and were specifically taken up by HUVEC [97]. As vascular repair is important for diseases such as MI, this shows promise for delivering selective miRNA for cardiac repair. However, a remaining challenge for all transfection-based modifications is remnants of contaminating transfection reagents in the vesicles.
Passive incubation
Aside from the active methods to load cargo into vesicles, a passive approach of incubating the cargo with the parent cells/exosomes can also be used. Human bone marrow MSCs were incubated with PTX for 24 h following which EVs isolated from their conditioned media were found to contain PTX [99]. When tested on pancreatic cancer cells, the PTX exosomes induced a dose-dependent inhibition of cell proliferation with approximately 50% growth inhibition when delivering 2.5 ng/μl of PTX. This shows that isolating exosomes from certain cells simply incubated with some drugs can allow for passive cargo control. However, the versatility of this method using other cell types and cargo compounds is unclear. Aside from cells, exosomes can also be directly loaded with cargo through passive incubation at room temperature for a few hours. This method was used to deliver the anti-inflammatory drug, curcumin, to myeloid cells [100]. Interestingly, using exosomes increased the anti-inflammatory effect of the curcumin compared with using liposomes. This suggests that exosomes are acting as more than transport vehicles and instead have a therapeutic role. In another study, brain endothelial cell-derived exosomes were passively loaded with anticancer drugs (PTX or doxorubicin) and delivered to the blood–brain barrier [101]. The cargo-laden exosomes had significant therapeutic efficacy compared with controls and reduced the cancer cells detected. Despite the ease of passive incubation and the functionality of the exosomes produced, active techniques for encapsulating cargo into exosomes have higher efficiency [72].
Cell-derived vesicles
To avoid the dependence on cell-based exosome production and the constraint of limited exosome yield, nanovesicles have also been formed from the cell’s plasma membrane [102]. NIH3T3 cells were transduced with c-Myc shRNA and extruded to produce 180–200 nm vesicles with approximately a hundred-times higher vesicle yield [78]. These exosome-mimetics were taken up by mouse lymphoma (λ820) cells and significantly reduced c-Myc expression. This suggests that such cell membrane-derived nanovesicles could be used for cardiac cells that have poor exosome yield. Another study used spin cups with sequentially smaller membrane pores to form less than 200-nm cell-derived nanovesicles (CDNs) from U937 cells [103]. The CDNs were compared with U937 exosomes and although they had similar physical characterization, the CDNs had almost double the PC lipid content. Moreover, when injected in vivo in mice, more than twice the amount of CDNs accumulated in the liver and kidneys compared with U937 exosomes. This further suggests that the protein and lipid enrichment in exosomes are valuable for targeted delivery – a challenge with using cell membranes for vesicle synthesis.
Targeted & modular EV loading
Hung et al. developed a new platform for loading cargo into vesicles by fusing a bacteriophage MS2 protein coat with proteins associated with the vesicle [104]. This technique increased RNA loading up to sixfold, although there was size dependency with smaller RNA (∼0.5 kb) loading being more efficient that larger RNA loading (mRNA >1.5 kb). Despite observing high loading and uptake efficiency of these vesicles, most cargo underwent rapid degradation in recipient cells. Although it is not clear, it is hypothesized that this loss is attributed to escape of EVs through the endosomal/lysosomal pathway. Thus, further study is still required to validate this method for cardiac cargo delivery. In addition, the effect of the bacteriophage coat on in vivo responses needs to be assessed.
Generating synthetic nanovesicles
The above section focused on modifying cargo in exosomes directly or indirectly. Nonetheless, significant research has focused on developing completely synthetic nanovesicles using phospholipids to emulate the function of EVs [28,105,106]. Still, these vesicles lack the varied and diverse lipid/protein membrane of exosomes. They cannot match exosomes and modified exosomes in terms of vesicle stability, tissue targeting and immune evasion. Despite these challenges, these nanovesicles are easier to scale up as they do not depend on cellular exosome production and can be designed to have controlled cargo release under specific physiological conditions. In addition, although challenging, their membranes can be specifically designed to reflect some of the functionally important components of exosome membranes.
Several types of synthetic nanovesicles have been designed depending on the cargo to be carried. The first of these are liposomes, which mimic the lipid bilayer of cells and EVs, and consist primarily of PC and PE [107,108]. Liposomes can range from nano- to micro-scales and can contain several lamellar membranes [109]. The smallest of these liposomes, small unilamellar vesicles, are the closest match to exosomes. A modification of liposomes are niosomes, named as such because their bilayer contains a nonionic surfactant typically composed of CHOL derivatives [110,111]. Their nonionic surface increases their biocompatibility and lowers their toxicity. Another modification involves adding ethanol to the membrane. Two examples include ethosomes, which are composed of phospholipids with water and ethanol, and transfersomes, which are composed of PC, surfactants and ethanol [112,113]. In these vesicles, the presence of ethanol helps solubilize the loaded cargo making the cargo easier to embed. Furthermore, the presence of the surfactant makes the structure of transfersomes ultra-flexible, so they can easily be delivered through spaces much smaller than their own size [114].
Another group of synthetic nanovesicles are NPs coated with membranes. Lipospheres consists of a lipid vesicle with a solid lipid core that remains solid at room and body temperature [115]. They have better higher stability and easier preparation methods compared with traditional liposomes [116]. However, as they contain a lipid core and have an amphiphilic membrane, they are poor carriers of hydrophilic cargo. Some other membrane-coated NPs capitalize on cell membranes to coat synthetic particles. Specifically, stem cell membranes are known to activate reparative and regenerative intracellular pathways in recipient cells, therefore, using their membranes as coats can be a clever way to mask the foreign NPs [117]. In Table 1 we summarize these synthetic nanovesicles and discuss their benefits and drawbacks for use as cargo-laden nanocarriers.
Table 1. . Types of synthetic nanovesicles.
| Type | Description | Benefits | Drawbacks | Ref. |
|---|---|---|---|---|
| Liposomes | Bilayer vesicle of synthetic amphiphilic lipids | • Similar structure and scale • Robust methods for synthesis • Therapies in preclinical and clinical trials • Enhanced stability in vivo |
• More immunogenic than exosomes • Poorly recapitulates complex exosome membrane – important for trafficking • Unmodified liposomes have quick degradation in vivo |
[107,170–172] |
| Niosomes | Vesicles with nonionic surfactant bilayer | • High biocompatibility • Low toxicity due to nonionic membrane |
• Poor stability • Potential to aggregate • Cargo leakage • Temperature-based size alteration |
[110,111,173] |
| Ethosomes | Vesicles with ethanol and water combined with the phospholipid bilayer | • High penetration • Easy size modulation • Useful for transdermal therapies |
• Requires high amounts of alcohol | [112,174] |
| Transfersomes | Vesicles with ethanol and surfactants combined with a phospholipid bilayer | • Ultra-flexible vesicles • Useful for transdermal therapies |
• High levels of surfactant can damage cell membranes • Poor chemical stability • Impure phospholipid membrane |
[113,114] |
| Lipospheres | Vesicles with a solid lipid core that encapsulate hydrophobic cargo | • High stability • Low-cost synthesis |
• Not suitable for hydrophilic protein/nucleic acid cargo • Inconsistent cargo release |
[115,116,175] |
| Membrane-coated NPs | NPs encased in cell membrane | • Immunocompatibility • Tissue homing • Prolonged retention and circulation in vivo |
• Potentially larger particle size because of extra membrane • Off-target effects with systemic delivery unknown |
[120,176–178] |
Outline of key synthetic nanovesicles and their benefits and drawbacks compared with naturally derived exosomes.
NP: Nanoparticle.
In the cardiac field, several groups have studied synthetic NPs as therapeutic carriers for MI. For example, liposomes loaded with erythropoietin and CD15s were delivered to rabbit MI models [118]. These synthetic vesicles reduced infarct size, improved LV function and induced favorable cardiac remodeling through proangiogenic and antifibrotic signaling. Liu et al. investigated the role of ligustrazine-loaded ethosome patches in cardiac repair after acute MI [119]. The ethosome-based drug delivery prolonged drug concentrations to the blood compared with controls and reduced MI in a long-term ischemia model. In another study, Tang et al. developed synthetic NPs cloaked in cardiosphere-derived stem cell membranes as therapeutics for MI [120]. The delivery of these particles to a mouse model of MI helped preserve the viable myocardium and also reduced T-cell infiltration. These studies highlight the potential of such synthetic nanovesicles as carriers for cargo. Although they cannot completely recapitulate the benefits of exosomes, they remain a promising avenue for cardiac repair.
In summary, numerous different types of exosome engineering have been attempted and created to optimize cargo encapsulation. However, each technique comes with its own benefits and inherent limitations, with several of these methods not yet explored for cardiac applications. As the field grows, the scope and relevance of developing customized cargo therapies for cardiac applications will likely increase. Therefore, awareness of such tailored therapeutics is valuable, and it is important to select the method most favorable for each specific cardiac application.
Nanovesicle engineering: targeted delivery
Tissue-specific cell-derived exosomes exhibit natural tropism, a major advantage for targeted delivery, in which vesicles display tissue-specific homing and preferential uptake based on their tissue of origin. A recent paper from Sancho-Albero et al. suggests that exosomes have selective tropism based on their source cells [121]. This study showed that MSC-derived exosomes loaded with gold NPs were selectively taken up by MSCs while recipient MSCs were cocultured with monocytes. In contrast, monocyte-derived exosomes were mostly internalized by monocytes, not by cocultured MSCs or melanoma cells.
Despite their natural homing properties, the majority of exosomes delivered intravenously rapidly accumulate in the liver, spleen and lung following delivery [122,123]. Rapid clearance thus remains a big hurdle in the use of exosomes. Tissue targeting/homing or local delivery by exosome-embedded hydrogel patch implantation could overcome the drawback of nonspecific exosome delivery. In this section, we will discuss exosomal surface peptides and hydrogel-based homing strategies for increasing cardiac-specific delivery.
Surface peptide engineering
Modifying exosome membranes by anchoring homing peptides is one way to address nonspecific delivery. Tissue-specific homing peptides are typically identified by in vivo screening of peptide libraries formed through phage display. Homing peptide design specifically depends on the vascular heterogeneity and pathophysiological conditions. These peptides have been used for targeted delivery of cells, oligonucleotides, imaging agents and inorganic NPs with high efficiency [124–127]. When homing peptides are applied to exosomes, they can alter the exosome cargo and specificity due to the alteration of surface composition. Thus, researchers have investigated various homing peptide conjugation methods that do not alter exosomal surface by altering specific native exosome proteins or lipids. For example, gene cloning to produce fusion proteins of homing peptides with exosome proteins is widely used due to high yield and universality. To make fusion proteins, homing peptide sequences are inserted into genes encoding LAMP2b, MHC or tetraspanin proteins. Additionally, anchoring homing peptides to the exosomal surface is another approach. For example, neutral phospholipid, dimyristoyl phosphatidylethanolamine (DMPE)-conjugated homing peptides are known to rapidly fuse with the exosome lipid bilayer [128].
Various cardiac homing peptides (CHP) have been designed for specifically targeting exosomes to cardiac tissue. As mentioned previously, homing peptide sequences vary depending on pathological condition or cell source. For example, CSTMLKAC oligopeptides target ischemic myocardium [129], whereas APWHLSSQYSRT peptides target cardiomyoblasts. In particular, Vandergriff et al. tagged CDC-derived exosomes by using dioleoylphosphatidylethanolamine (DOPE)-conjugated CSTSMLKAC [130]. This DOPE-conjugated CHP can be incorporated into the CDC exosomes by simply mixing two components. Furthermore, CHP functionalization improves exosome retention within the cardiac tissue, leading to the induction of CM survival and proliferation, vascular growth and inhibition of fibrosis in rat myocardium after ischemia/reperfusion injury. In another study, Xu et al. generated MSC exosomes expressing CHP peptide fused with LAMP2b [131]. CSTSMLKAC-LAMP2b expressing MSC exosomes significantly increased cargo delivery to injured CMs in vitro and in vivo, attenuated inflammation and cell death, increased angiogenesis and decreased fibrosis in the ischemic heart. Another study used cardiomyoblast-targeting peptide, APWHLSSQYSRT, to produce LAMP2b-CHP [132]. Here, the researchers produced engineered exosomes using HEK293 cells, and the CHP-expressing exosomes were preferentially delivered to rat CMs in vitro and mice myocardium in vivo with no observable toxicity. Moreover, they modified the N-terminus of the CHP sequence by adding a glycosylated motif, which protects the CHP peptide from degradation, leading to enhanced in vivo half-life and cardiac-specific exosome delivery. Moreover, Mentkowski et al. engineered CDCs to express CM-specific peptide, WLSEAGPVVTVRALRGTGSW, which was also fused to LAMP2b [133]. The engineered CDC exosomes increased CM preferential uptake that lead to decreased CM apoptosis and enhanced cardiac retention.
Finally, conjugating an antibody to the exosomal surface is another way to increase tissue specificity. Antes et al. used streptavidin–biotin conjugation to generate antibody-anchored exosomes. For this, they directly embeded DMPE-PEG-conjugated streptavidin to CDC exosomes and then anchored a biotinylated antibody [128]. The uptake of these engineered exosomes in cardiac cells was significantly enhanced in vitro and in vivo. This antibody-cloaking method is easy to implement and as effective as conjugating homing peptides to exosome membrane proteins. Moreover, given the robust nature of streptavidin-biotin chemistry, it can be conjugated with an antibody, a homing peptide or an imaging agent.
Exosome-embedded hydrogel patches
Retaining exosomes at the tissue defect site is crucial for exosomes to effectively exert their reparative effect. However, when injected alone, exosomes are rapidly cleared from the circulatory system in the body and their beneficial effects are quickly diminished. Alternative approaches of utilizing hydrogels or patches for exosome delivery enable sustained release of exosomes without cell injection and minimize loss. Moreover, these implanted scaffolds provide spatial control compared with systemic injection, which enhances target specific cargo delivery. One study showed the potential of using a photoreactive hydrogel patch for delivering MSC-derived exosomes, in which long-term treatment and repair in defect sites is possible through sustained exosome release for 2 weeks [134]. Another study showed that a collagen-based hydrogel significantly sustained exosome release, which led to ejection-fraction recovery and cardiac repair in acute MI [135]. In addition, they showed that induced pluripotent stem cell-derived CM exosomes express more cardioprotective miRNAs compared with induced pluripotent stem cell exosomes. Another study utilized gelatin-based hydrogels consisting of peptides that protect against oxidative stress to encapsulate human umbilical cord-MSC-derived exosomes [136]. This gelatin-based hydrogel gels at 37°C, thus this system does not require any additional procedure beyond physiological injection. The injected exosome/hydrogel mixture improved myocardial function by reducing inflammation, fibrosis and apoptosis, and promoting angiogenesis.
Nanovesicle engineering: avoiding clearance
Unlike traditional small-molecule drugs, size and physical properties of nanovesicles make these therapies difficult for translation. Notably, a critical problem for nanovesicle-based therapies is clearance. In particular, previous studies have shown that 95–99% of artificial nanovesicles systemically delivered are cleared and eliminated, resulting in low-circulating concentrations [137,138]. The system responsible for this clearance problem, the mononuclear phagocyte system (MPS), has been a major obstacle and focus of research dedicated to nanovesicle and NP translation. Functioning as a branch of the immune system, the MPS houses phagocytic cells, primarily monocytes and macrophages, residing throughout the body. The MPS is especially important in the lymphatic system and filtration organs like the liver, spleen and kidney where macrophages, histiocytes, Kupffer cells and mesangial cells clear foreign substances (Figure 4).
Figure 4. . Overview of exosome/nanoparticle clearance mechanisms.
Clearance of exosomes and nanoparticles by the body occurs through different paths after the activation of mononuclear phagocyte system. The key organs involved in the elimination of these particles are the liver (through Kupffer cells), the kidneys (through mesangial cells) and the spleen (through red pulp macrophages). Artificial nanoparticles get cleared by these organs more so than natural exosomes.
NP: Nanoparticle.
A significant cause of clearance of artificial nanovesicles and NPs arises from the adsorption of proteins onto the particle surface, and subsequent detection by the MPS. Dubbed the ‘protein corona,’ plasma proteins rapidly conjugate to the surface of systemically delivered artificial NPs to form a cloak [139–141]. While in some cases, NPs can be engineered to intentionally form protein coronas for targeted delivery, in most cases, this bioactive shell induces clearance, often accompanied by an inflammatory response [142]. On the other hand, exosomes and other naturally derived exosome mimics may avoid these adsorbed protein-induced clearance problems. While it is unclear whether a protein corona is formed on exosomes, studies have clearly shown that exosomes and exosome-coated NPs have longer circulation time and lower rates of uptake by phagocytes [143,144]. In comparing artificial lipid, cellular plasma membrane and exosome membrane-coated poly(lactic-co-glycolic acid) NPs, Liu et al. showed that exosome-coated NPs had the longest circulation half-life in vivo and the lowest uptake by macrophages and circulating monocytes [144]. Nevertheless, despite having improved circulation time compared with artificial NPs, systemic administration and introduction of naturally derived exosomes to plasma and other biofluids can still lead to MPS recognition and clearance. Surface modification, targeted delivery strategies (outlined above) and further immune evasion strategies (discussed below) can prolong exosome circulation.
Once systemically delivered, exosomes follow a two-phase exponential decay model with two half-lives: an initial distribution phase (t1/2 in minutes) and an elimination phase (t1/2 in hours) [122,145]. The pharmacokinetics, or distribution and elimination, of exosomes has been studied through various tracking methods including, radiolabeling, fluorescent lipophilic dyes (PKH67, DiD and DiR), fluorescent markers for exosome sequences/membrane proteins/endogenous RNA/DNA and Cre-recombinase systems [146]. For example, a group led by Takakura showed that exosomes are primarily cleared by macrophages in the liver and spleen by tracking exosomes labeled with a fusion protein, including luciferase and exosome-tropic lactadherin proteins [122,147]. Moreover, Zomer et al. employed a Cre-LoxP system to track exosomes. The group demonstrated the transfer of Cre mRNA-carrying exosomes from Cre+ parental cells to reporter+ recipient cells [146]. While there is some variability in exosome clearance based on parental cell type and route of administration (e.g., intravenous, intraperitoneal, subcutaneous), macrophages are the primary source of vesicle uptake and the liver, spleen, GI tract and lung represent the major organ sites of eliminated exosomes [122,148].
Furthermore, a few mechanisms of macrophage-induced clearance of nanovesicles have been reported. Canonically, macrophages recognize the PS amphiphiles on apoptotic cell membranes and engulf these cells [149]. Similarly, macrophages are also able to clear exosomes via PS recognition through class A macrophage scavenger receptor (SR-A) [150,151]. Additionally, recognition of sialic acid on the surface of exosomes by CD169 on macrophages has also been implicated as a means of vesicle clearance [152]. Clearly, elimination of exosomes by the MPS remains an obstacle for the translation of these therapies. To combat this clearance problem, several approaches, including tailoring NP properties and altering the MPS, have been explored. Here, we will discuss some of these efforts.
Size modification
A primary method for immune evasion and increased circulation time is controlling NP size. For example, vesicles sized 50–200 nm are subject to the enhanced permeation and retention (EPR) effect [153,154]. This passive phenomenon is mainly exploited in the context of cancer research, where leaky tumor vasculature allows for permeation of NPs. However, EPR is still relevant to the design of nanovesicles for cardiac applications, as these sizes exceed the limit of renal excretion threshold, thus increasing its circulating half-life. The EPR effect is also exploited in other inflammation-based diseases and infections with notable permeable endothelium. Furthermore, NP size, while not deterministic, plays a large role in determining the NP uptake mechanism. Small NPs, 10–250 nm, mostly enter cells via pinocytosis or macropinocytosis; large NPs, 250 nm to 3 μm, enter cells via phagocytosis; and medium NPs 120–200 nm may also enter cells via clathrin- or caveolin-dependent endocytosis [155,156]. Based on these studies and others, the optimal NP size has been identified for particles around 50–100 nm as they are subject to the EPR effect and reduced clearance, and have enhanced uptake when compared with smaller or larger particles [157,158]. When considering various EV types for cardiac disease, exosomes are ideal candidates for exploiting the EPR effect and evading clearance.
Polymer modifications
In addition to controlling size, NPs are often designed with a polymer shell to become biocompatible, acquire ‘stealth behavior’, evade the immune system and avoid clearance. Notably, the most common polymer-modifier, PEG, is the US FDA approved in the liposome-based cancer therapeutic Doxil. PEG is conjugated to combat the MPS problem: the flexible and hydrophilic properties of the polymer create a thick coat that hinders protein adsorption and NP nonspecific interactions in vivo, both significant contributors to clearance [159]. While the rationale for grafting other polymers to NP shells is generally the same, the properties of PEG and other polymers can be tailored for specific uses. Broadly, to avoid renal clearance, small-molecule drugs are often conjugated to larger PEGs, while larger drugs, like NPs and antibodies, are conjugated to smaller PEGs to avoid opsonization and elimination. Additionally, PEG may be attached to NP surfaces in different densities, and conformations, like high density brush-like and low-density mushroom-like conformations. Often, NPs are designed with PEG densities in between brush-like and mushroom-like conformations where the NP core can be sufficiently covered and protected from protein adsorption [160].
While PEG remains a widely popular modification for NP biomedical applications, it is not without its drawbacks. Most notably, PEG can trigger complement C activation and hypersensitivity reactions, and repeat injections can paradoxically lead to accelerated blood clearance [161,162]. In the case of hypersensitivity, when complement component 3 hydrolyzes, its fragments can adhere and decorate the surface of NPs, consequently attracting leukocytes and macrophages and triggering a proinflammatory response. In fact, complement activation and hypersensitivity reactions can occur in up to 25% of patients taking Doxil [163]. Furthermore, in the case of repeated injections of PEGylated NPs, the production of anti-PEG antibodies by the body significantly increases clearance and decreases drug bioavailability. While the exact mechanism responsible for this accelerated blood clearance phenomenon is still not fully understood, the working hypothesis suggests that PEGylated NPs induce splenic B cells to produce anti-PEG IgM antibodies that are able to bind to the second NP dose, thereby inducing the MPS to uptake the opsonized NPs [164].
In the context of EVs, a few groups have explored polymer modification of exosomes. For example, Sato et al. engineered PEGylated exosome-liposome hybrids to increase vesicle half-life and decrease vesicle immunogenicity. The group demonstrated enhanced cellular uptake of hybrid vesicles compared with unmodified exosomes, and hypothesized that PEG induces membrane fusion or reduces anionic–anionic repulsion of exosome and cellular membranes [87]. Furthermore, Kooijmans et al. engineered EVs decorated with PEG-conjugated ligands and demonstrated that the PEGylated EVs had longer circulation times than unmodified EVs in mice after intravenous injection [165]. Nonetheless, PEGylation of EVs and exosomes has not been studied as extensively as PEGylation of artificial NPs. Polymer modification of naturally derived nanovesicles and exosomes may represent a relatively unexplored, yet promising strategy for avoiding clearance, improving vesicle circulation time and enhancing drug efficacy.
Deactivating the MPS
Instead of modifying NP properties, an alternative approach for mitigating the clearance problem is deactivating the MPS, thus increasing circulating NP concentrations. A few different methods have investigated designing NPs with MPS-deactivating properties, or coadministering NPs with MPS-deactivating agents. For the former case, a recent study by Wan et al. investigated blocking MPS clearance as a means of improving exosome delivery to the myocardium [166]. The study showed that clathrin-dependent endocytosis was a key player in exosome clearance in the liver and spleen, and improved accumulation in the heart was achieved by loading exosomes with siRNA against clathrin heavy chain 1. Furthermore, as mentioned previously, SR-A plays a major role in macrophage recognition of PS on the surface of exosomes. A handful of studies have demonstrated reductions in exosome clearance through the depletion of PS exosome membrane content and the blocking of SR-A with dextran treatment [150,151].
Pretreatment or coadministration of MPS-deactivating molecules have also been explored as a means of reducing clearance. In a proof-of-principle approach, delivery of clodronate liposomes depleted resident macrophages and increased the circulating concentration of NPs. However, robust depletion of macrophages is not a tolerable or translatable approach and more nuanced means of deactivating the innate immune system have been explored. For example, pretreatment with the macrophage-deactivating antimalarial agent, chloroquine or the Kupffer cell phagocytosis inhibitor, gadolinium chloride, caused temporary MPS dysfunction and allowed for reduced NP clearance [167]. Additionally, overwhelming the MPS with a pretreatment of ‘decoy’ empty liposomes was shown to enhance tissue accumulation of NPs. Furthermore, a recent study by Qiu et al. demonstrated that pretreatment with breast cancer cell derived exosome-like NPs can temporarily repress Kupffer cell phagocytosis such that a following dose of chemotherapeutic-loaded liposomes can be preferentially delivered to the lungs and escape clearance [168]. To enhance the efficacy of systemically delivered nanovesicles for cardiac disease, these vesicles must reach the heart before being detected and cleared by the MPS. Designing nanovesicles or coadministering agents that deactivate the MPS will enhance circulation time of vesicles, improving the chances for each vesicle to reach its target: the heart.
Conclusion & future perspective
Exosomes are a highly translatable cell-free therapy for tissue repair. Since their discovery 35 years ago, many groups have investigated the role of exosomes and have elucidated the processes of biosynthesis, trafficking and uptake by recipient cells. Despite their role in almost all intercellular communication, there are limitations in exosome therapeutic application. Primarily, low-yield and nonspecific cargo hinder the full potential of exosome therapy. Considering exosomes have some intrinsic targeting and are relatively nonimmunogenic compared with artificial NPs, several methods to engineer exosomes to maintain their external membrane but alter their cargo have been explored. These include modifying the exosomes through mechanical and chemical processes; modifying the parent cells from which the exosomes are released; and developing synthetic mimics for exosomes. However, despite exosomes being naturally tropic and less immunogenic than their synthetic counterparts, to enhance their translational potential, exosomes must also be designed to better home to the desired tissue and avoid the MPS and clearance.
Several studies have explored the role of different miRNA and other nucleic acids in cardiac tissue repair and regeneration. RNA that are important for different CVD repair have been investigated and our understanding of RNA in intracellular pathways and outcomes is growing. As this field burgeons, researchers can utilize knowledge of relevant RNA combinations to develop more potent and cardiac-specific therapies. Moreover, systems biology and computational modeling tools can be used to understand interactions between different types of cargo (miRNA, mRNA, protein) and their respective cardiac outcomes [17,57,169]. Unsupervised and supervised learning approaches such as principal component analysis and partial least-squares regression allow us to filter through thousands of cargo molecules to determine which components are most related to important cardiac responses such as migration, proliferation, fibrosis and angiogenesis. Computational-based approaches can be extremely powerful when combined with exosome-engineering methods to tailor-make exosome cargo with different levels of desired, potent RNA/protein to elicit specific cardiac outcomes.
Even beyond cardiac diseases, designing customized exosomes can be used for several other diseases where intercellular communication is involved, such as cancer, neuropathies and other tissue regeneration applications. Such vesicles would also be beneficial for drug delivery as they can capitalize on the uptake efficiency of exosomes while providing control of the inherent cargo. Thus, the scope of this field is promising and stretches far beyond cardiac dysfunction.
Executive summary.
Background
Exosomes are important biomarkers and regulators of stem cell therapy.
Exosomes enriched in proteins, mRNAs and miRNAs characteristic of parental stem cells represent a promising alternative for treating cardiac diseases.
Exosome production and reparative effects are highly dependent on the parent cell’s conditions.
Variation in exosome composition makes developing standardized, cardiovascular therapies with exosomes a significant challenge.
Exosome engineering
Exosome cargo content can be optimized by modifying the exosome itself, the parent cell or designing synthetic vesicles.
Exosome tropism can be augmented by anchoring homing peptides or antibodies to the vesicle surface.
Exosome clearance can be mitigated by abating the mononuclear phagocyte system, or tailoring vesicle size/coating.
Footnotes
Author contributions
S Bheri was responsble for the conception of topic, manuscript writing and editing, figure creation and final approval of manuscript. J Hoffman and H-J Park were responsible for the conception of topic, manuscript writing and editing, and final approval of manuscript. ME Davis helped in manuscript writing and editing, and final approval of manuscript.
Financial & competing interests disclosure
Funding was received from the NIH (HL145644). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Benjamin EJ, Muntner P, Alonso A. et al. Heart disease and stroke statistics – 2019 update: a report from the American Heart Association. Circulation 139(10), e56–e528 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 129(9), 1033–1044 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Mayourian J, Ceholski DK, Gorski PA. et al. Exosomal microRNA-21-5p mediates mesenchymal stem cell paracrine effects on human cardiac tissue contractility. Circ. Res. 122(7), 933–944 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolli R, Hare JM, March KL. et al. Rationale and design of the CONCERT-HF trial (combination of mesenchymal and c-kit(+) cardiac stem cells as regenerative therapy for heart failure). Circ. Res. 122(12), 1703–1715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bao L, Meng Q, Li Y. et al. C-kit positive cardiac stem cells and bone marrow-derived mesenchymal stem cells synergistically enhance angiogenesis and improve cardiac function after myocardial infarction in a paracrine manner. J. Card. Fail. 23(5), 403–415 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Lee C, Mitsialis SA, Aslam M. et al. Exosomes mediate the cytoprotective action of mesenchymal stromal cells on hypoxia-induced pulmonary hypertension. Circulation 126(22), 2601–2611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong SG, Huber BC, Lee WH. et al. Microfluidic single cell analysis of transplanted human induced pluripotent stem cell-derived cardiomyocytes following acute myocardial infarction. Circulation 132(8), 762–771 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barile L, Milano G, Vassalli G. Beneficial effects of exosomes secreted by cardiac-derived progenitor cells and other cell types in myocardial ischemia. Stem Cell Investig. (1), 93–93 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saha P, Sharma S, Korutla L. et al. Circulating exosomes derived from transplanted progenitor cells aid the functional recovery of ischemic myocardium. Sci. Transl. Med. 11(493), eaau1168 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Théry C, Witwer KW, Aikawa E. et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7(1), 1535750 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 262(19), 9412–9420 (1987). [PubMed] [Google Scholar]
- 12.Haraszti RA, Miller R, Dubuke ML. et al. Serum deprivation of mesenchymal stem cells improves exosome activity and alters lipid and protein composition. iScience 16, 230–241 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams JB, Jauch EC, Lindsell CJ, Campos B. Endothelial microparticle levels are similar in acute ischemic stroke and stroke mimics due to activation and not apoptosis/necrosis. Acad. Emerg. Med. 14(8), 685–690 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Camussi G, Deregibus MC, Tetta C. Paracrine/endocrine mechanism of stem cells on kidney repair: role of microvesicle-mediated transfer of genetic information. Curr. Opin. Nephrol. Hypertens. 19(1), 7–12 (2010). [DOI] [PubMed] [Google Scholar]
- 15.Lamichhane TN, Raiker RS, Jay SM. Exogenous DNA loading into extracellular vesicles via electroporation is size-dependent and enables limited gene delivery. Mol. Pharm. 12(10), 3650–3657 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 45(3), 528–537 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Hristov M, Erl W, Linder S, Weber PC. Apoptotic bodies from endothelial cells enhance the number and initiate the differentiation of human endothelial progenitor cells in vitro. Blood 104(9), 2761–2766 (2004). [DOI] [PubMed] [Google Scholar]
- 18.Kaushal S, Wehman B, Pietris N. et al. Study design and rationale for ELPIS: a Phase I/IIb randomized pilot study of allogeneic human mesenchymal stem cell injection in patients with hypoplastic left heart syndrome. Am. Heart J. 192, 48–56 (2017). [DOI] [PubMed] [Google Scholar]
- 19.Agarwal U, George A, Bhutani S. et al. Experimental, systems, and computational approaches to understanding the microRNA-mediated reparative potential of cardiac progenitor cell-derived exosomes from pediatric patients. Circ. Res. 120(4), 701–712 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gray WD, French KM, Ghosh-Choudhary S. et al. Identification of therapeutic covariant microRNA clusters in hypoxia-treated cardiac progenitor cell exosomes using systems biology. Circ. Res. 116(2), 255–263 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Llorente A, Skotland T, Sylvanne T. et al. Molecular lipidomics of exosomes released by PC-3 prostate cancer cells. Biochim. Biophys. Acta 1831(7), 1302–1309 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Guida V. Thermodynamics and kinetics of vesicles formation processes. Adv. Colloid Interface Sci. 161(1–2), 77–88 (2010). [DOI] [PubMed] [Google Scholar]
- 23.Ikonen E. Roles of lipid rafts in membrane transport. Curr. Opin. Cell Biol. 13(4), 470–477 (2001). [DOI] [PubMed] [Google Scholar]
- 24.Huijbregts RP, Topalof L, Bankaitis VA. Lipid metabolism and regulation of membrane trafficking. Traffic 1(3), 195–202 (2000). [DOI] [PubMed] [Google Scholar]
- 25.Skotland T, Sandvig K, Llorente A. Lipids in exosomes: current knowledge and the way forward. Prog. Lipid Res. 66, 30–41 (2017). [DOI] [PubMed] [Google Scholar]
- 26.Haraszti RA, Didiot M-C, Sapp E. et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J. Extracell. Vesicles 5, 32570 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conigliaro A, Fontana S, Raimondo S, Alessandro R. Exosomes: nanocarriers of biological messages. Adv. Exp. Med. Biol. 998, 23–43 (2017). [DOI] [PubMed] [Google Scholar]
- 28.Li SP, Lin ZX, Jiang XY, Yu XY. Exosomal cargo-loading and synthetic exosome-mimics as potential therapeutic tools. Acta Pharmacol. Sin. 39(4), 542–551 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Knowlton AA. HSP60 trafficking in adult cardiac myocytes: role of the exosomal pathway. Am. J. Physiol. Circ. Physiol. 292(6), H3052–H3056 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Malik ZA, Kott KS, Poe AJ. et al. Cardiac myocyte exosomes: stability, HSP60, and proteomics. Am. J. Physiol. Heart Circ. Physiol. 304(7), H954–H965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Emanueli C, Shearn AIU, Angelini GD, Sahoo S. Exosomes and exosomal miRNAs in cardiovascular protection and repair. Vascul. Pharmacol. 71, 24–30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luther KM, Haar L, McGuinness M. et al. Exosomal miR-21a-5p mediates cardioprotection by mesenchymal stem cells. J. Mol. Cell. Cardiol. 119, 125–137 (2018). [DOI] [PubMed] [Google Scholar]
- 33.Varga ZV, Zvara A, Faragó N. et al. MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. Am. J. Physiol. Heart Circ. Physiol. 307(2), H216–H227 (2014). [DOI] [PubMed] [Google Scholar]
- 34.Deregibus MC, Cantaluppi V, Calogero R. et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood 110(7), 2440–2448 (2007). [DOI] [PubMed] [Google Scholar]
- 35.Zhu B, Zhang L, Liang C. et al. Stem cell-derived exosomes prevent aging-induced cardiac dysfunction through a novel exosome/lncRNA MALAT1/NF-κb/TNF-α signaling pathway. Oxid. Med. Cell. Longev. 2019, 9739258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen H, Xia W, Hou M. LncRNA-NEAT1 from the competing endogenous RNA network promotes cardioprotective efficacy of mesenchymal stem cell-derived exosomes induced by macrophage migration inhibitory factor via the miR-142-3p/FOXO1 signaling pathway. Stem Cell Res. Ther. 11(1), 31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pan BT, Teng K, Wu C, Adam M, Johnstone RM. Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J. Cell Biol. 101(3), 942–948 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian T, Zhu Y-L, Hu F-H, Wang Y-Y, Huang N-P, Xiao Z-D. Dynamics of exosome internalization and trafficking. J. Cell. Physiol. 228(7), 1487–1495 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Böker KO, Lemus-Diaz N, Rinaldi Ferreira R, Schiller L, Schneider S, Gruber J. The impact of the CD9 tetraspanin on lentivirus infectivity and exosome secretion. Mol. Ther. 26(2), 634–647 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J, Bonacquisti EE, Brown AD, Nguyen J. Boosting the biogenesis and secretion of mesenchymal stem cell-derived exosomes. Cells 9(3), 660–676 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-King H, García NA, Ontoria-Oviedo I, Ciria M, Montero JA, Sepúlveda P. Hypoxia inducible factor-1α potentiates Jagged 1-mediated angiogenesis by mesenchymal stem cell-derived exosomes. Stem Cells 35(7), 1747–1759 (2017). [DOI] [PubMed] [Google Scholar]
- 42.Zhu J, Lu K, Zhang N. et al. Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif. Cells Nanomedicine Biotechnol. 46(8), 1659–1670 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stoorvogel W, Strous GJ, Geuze HJ, Oorschot V, Schwartz AL. Late endosomes derive from early endosomes by maturation. Cell 65(3), 417–427 (1991). [DOI] [PubMed] [Google Scholar]
- 44.Jaiswal JK, Andrews NW, Simon SM. Membrane proximal lysosomes are the major vesicles responsible for calcium-dependent exocytosis in nonsecretory cells. J. Cell Biol. 159(4), 625–635 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Svensson KJ, Christianson HC, Wittrup A. et al. Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid raft-mediated endocytosis negatively regulated by caveolin-1. J. Biol. Chem. 288(24), 17713–17724 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 19, 397–422 (2003). [DOI] [PubMed] [Google Scholar]
- 47.Costa Verdera H, Gitz-Francois JJ, Schiffelers RM, Vader P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Rel. 266(July), 100–108 (2017). [DOI] [PubMed] [Google Scholar]
- 48.Viñas JL, Spence M, Gutsol A. et al. Receptor–ligand interaction mediates targeting of endothelial colony forming cell-derived exosomes to the kidney after ischemic injury. Sci. Rep. 8(1), 16320 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gastpar R, Gehrmann M, Bausero MA. et al. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 65(12), 5238–5247 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeppesen DK, Fenix AM, Franklin JL. et al. Reassessment of exosome composition. Cell 177(2), 428.e18–445.e18 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Al-Nedawi K, Meehan B, Micallef J. et al. Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat. Cell Biol. 10(5), 619–624 (2008). [DOI] [PubMed] [Google Scholar]
- 52.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA. et al. Functional delivery of viral miRNAs via exosomes. Proc. Natl Acad. Sci. USA 107(14), 6328–6333 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiao L, Hu S, Liu S. et al. MicroRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Invest. 129(6), 2237–2250 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bang C, Batkai S, Dangwal S. et al. Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Invest. 124(5), 2136–2146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu J, Jiang M, Deng S. et al. MiR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol. Ther. Nucleic Acids 11, 103–115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu L-P, Tian T, Wang J-Y. et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics 8(22), 6163–6177 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trac D, Hoffman JR, Bheri S, Maxwell JT, Platt MO, Davis ME. Predicting functional responses of progenitor cell exosome potential with computational modeling. Stem Cells Transl. Med. 8(11), 1212–1221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gallet R, Dawkins J, Valle J. et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38(3), 201–211 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lai RC, Arslan F, Lee MM. et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 4(3), 214–222 (2010). [DOI] [PubMed] [Google Scholar]
- 60.Arslan F, Lai RC, Smeets MB. et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 10(3), 301–312 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Exosomes in Cardiovascular Diseases. Xiao J, Cretoiu S (). Springer, Singapore: (2017). [Google Scholar]; • This is a useful reference for the introduction to cardiovascular disease exosomes and the important cargo for various cardiac disorders.
- 62.Barile L, Lionetti V, Cervio E. et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 103(4), 530–541 (2014). [DOI] [PubMed] [Google Scholar]
- 63.Feng Y, Huang W, Wani M, Yu X, Ashraf M. Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One 9(2), e88685 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu B, Kim HW, Gong M. et al. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 182, 349–360 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vicencio JM, Yellon DM, Sivaraman V. et al. Plasma exosomes protect the myocardium from ischemia-reperfusion injury. J. Am. Coll. Cardiol. 65(15), 1525 LP – 1536 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Barile L, Cervio E, Lionetti V. et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc. Res. 114(7), 992–1005 (2018). [DOI] [PubMed] [Google Scholar]
- 67.Cosme J, Guo H, Hadipour-Lakmehsari S, Emili A, Gramolini AO. Hypoxia-induced changes in the fibroblast secretome, exosome, and whole-cell proteome using cultured, cardiac-derived cells isolated from neonatal mice. J. Proteome Res. 16(8), 2836–2847 (2017). [DOI] [PubMed] [Google Scholar]
- 68.Helwa I, Cai J, Drewry MD. et al. A comparative study of serum exosome isolation using differential ultracentrifugation and three commercial reagents. PLoS One 12(1), e0170628–e0170628 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aatonen MT, Ohman T, Nyman TA, Laitinen S, Grönholm M, Siljander PR-M. Isolation and characterization of platelet-derived extracellular vesicles. J. Extracell. Vesicles 3, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chevillet JR, Kang Q, Ruf IK. et al. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc. Natl Acad. Sci. USA 111(41), 14888–14893 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Smyth T, Kullberg M, Malik N, Smith-Jones P, Graner MW, Anchordoquy TJ. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Rel. 199, 145–155 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Rel. 205, 35–44 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38(6), 754–763 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kao CY, Papoutsakis ET. Extracellular vesicles: exosomes, microparticles, their parts, and their targets to enable their biomanufacturing and clinical applications. Curr. Opin. Biotechnol. 60, 89–98 (2019). [DOI] [PubMed] [Google Scholar]
- 75.Zhang M, Zang X, Wang M. et al. Exosome-based nanocarriers as bio-inspired and versatile vehicles for drug delivery: recent advances and challenges. J. Mater. Chem. B 7, 2421–2433 (2019). [DOI] [PubMed] [Google Scholar]
- 76.Hood JL. Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 11, 1745–1756 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jhan Y-Y, Prasca-Chamorro D, Palou Zuniga G. et al. Engineered extracellular vesicles with synthetic lipids via membrane fusion to establish efficient gene delivery. Int. J. Pharm. 573, 118802 (2020). [DOI] [PubMed] [Google Scholar]
- 78.Lunavat TR, Jang SC, Nilsson L. et al. RNAi delivery by exosome-mimetic nanovesicles - implications for targeting c-Myc in cancer. Biomaterials 102, 231–238 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Ma T, Chen Y, Chen Y. et al. MicroRNA-132, delivered by mesenchymal stem cell-derived exosomes, promote angiogenesis in myocardial infarction. Stem Cells Int. 2018, 3290372 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kooijmans SAA, Stremersch S, Braeckmans K. et al. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Rel. 172(1), 229–238 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Hood JL, Scott MJ, Wickline SA. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 448, 41–49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamichhane TN, Jeyaram A, Patel DB. et al. Oncogene knockdown via active loading of small RNAs into extracellular vesicles by sonication. Cell. Mol. Bioeng. 9(3), 315–324 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salarpour S, Forootanfar H, Pournamdari M, Ahmadi-Zeidabadi M, Esmaeeli M, Pardakhty A. Paclitaxel incorporated exosomes derived from glioblastoma cells: comparative study of two loading techniques. Daru 27(2), 533–539 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Haney MJ, Klyachko NL, Zhao Y. et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Rel. 207, 18–30 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; • It highlights the potential of different exosome mimetics as a mode of delivery for small molecules and drugs.
- 85.Liu H, Shen M, Zhao D. et al. The effect of triptolide-loaded exosomes on the proliferation and apoptosis of human ovarian cancer SKOV3 cells. Biomed. Res. Int. 2019, 2595801 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Noyes AR, Mercaldi MP, Golden KE. et al. US20190175506: loading of extracellular vesicles through imparting of mechanical shear. (2019). [Google Scholar]
- 87.Sato YT, Umezaki K, Sawada S. et al. Engineering hybrid exosomes by membrane fusion with liposomes. Sci. Rep. 6, 1–11 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bosch S, de Beaurepaire L, Allard M. et al. Trehalose prevents aggregation of exosomes and cryodamage. Sci. Rep. 6, 36162 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tran PHL, Wang T, Yin W. et al. Development of a nanoamorphous exosomal delivery system as an effective biological platform for improved encapsulation of hydrophobic drugs. Int. J. Pharm. 566, 697–707 (2019). [DOI] [PubMed] [Google Scholar]
- 90.Podolak I, Galanty A, Sobolewska D. Saponins as cytotoxic agents: a review. Phytochem. Rev. 9(3), 425–474 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Loughlin AJ, Mäger I, de Jong OG. et al. Functional delivery of lipid-conjugated siRNA by extracellular vesicles. Mol. Ther. 25(7), 1580–1587 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Didiot M-C, Hall LM, Coles AH. et al. Exosome-mediated delivery of hydrophobically modified siRNA for huntingtin mRNA silencing. Mol. Ther. 24(10), 1836–1847 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haraszti RA, Miller R, Didiot M-C. et al. Optimized cholesterol-siRNA chemistry improves productive loading onto extracellular vesicles. Mol. Ther. 26(8), 1973–1982 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang M, Altinoglu S, Takeda YS, Xu Q. Integrating protein engineering and bioorthogonal click conjugation for extracellular vesicle modulation and intracellular delivery. PLoS One 10(11), e0141860–e0141860 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Smyth T, Petrova K, Payton NM. et al. Surface functionalization of exosomes using click chemistry. Bioconjug. Chem. 25(10), 1777–1784 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang D, Lee H, Zhu Z, Minhas JK, Jin Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Lung Cell. Mol. Physiol. 312(1), L110–L121 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mathiyalagan P, Sahoo S. Exosomes-based gene therapy for microRNA delivery. Methods Mol. Biol. 1521, 139–152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Trivedi M, Talekar M, Shah P, Ouyang Q, Amiji M. Modification of tumor cell exosome content by transfection with wt-p53 and microRNA-125b expressing plasmid DNA and its effect on macrophage polarization. Oncogenesis 5(8), e250 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pascucci L, Coccè V, Bonomi A. et al. Paclitaxel is incorporated by mesenchymal stromal cells and released in exosomes that inhibit in vitro tumor growth: a new approach for drug delivery. J. Control. Rel. 192, 262–270 (2014). [DOI] [PubMed] [Google Scholar]
- 100.Sun D, Zhuang X, Xiang X. et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 18(9), 1606–1614 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yang T, Martin P, Fogarty B. et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 32(6), 2003–2014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalimuthu S, Gangadaran P, Rajendran RL. et al. A new approach for loading anticancer drugs into mesenchymal stem cell-derived exosome mimetics for cancer therapy. Front. Pharmacol. 9, 1116 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goh WJ, Zou S, Ong WY. et al. Bioinspired cell-derived nanovesicles versus exosomes as drug delivery systems: a cost-effective alternative. Sci. Rep. 7(1), 14322 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hung ME, Leonard JN. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell. Vesicles 5(1), 1–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sokolov AV, Kostin NN, Ovchinnikova LA, Lomakin YA, Kudriaeva AA. Targeted drug delivery in lipid-like nanocages and extracellular vesicles. Acta Naturae 11(2), 28–41 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grimaldi N, Andrade F, Segovia N. et al. Lipid-based nanovesicles for nanomedicine. Chem. Soc. Rev. 45(23), 6520–6545 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Bangham AD. Liposomes: the Babraham connection. Chem. Phys. Lipids 64(1–3), 275–285 (1993). [DOI] [PubMed] [Google Scholar]
- 108.Monteiro N, Martins A, Reis RL, Neves NM. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 11(101), 20140459 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lin CM, Li CS, Sheng YJ, Wu DT, Tsao HK. Size-dependent properties of small unilamellar vesicles formed by model lipids. Langmuir 28(1), 689–700 (2012). [DOI] [PubMed] [Google Scholar]
- 110.Bartelds R, Nematollahi MH, Pols T. et al. Niosomes, an alternative for liposomal delivery. PLoS One 13(4), e0194179–e0194179 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rajera R, Nagpal K, Singh SK, Mishra DN. Niosomes: a controlled and novel drug delivery system. Biol. Pharm. Bull. 34(7), 945–953 (2011). [DOI] [PubMed] [Google Scholar]
- 112.Yang L, Wu L, Wu D, Shi D, Wang T, Zhu X. Mechanism of transdermal permeation promotion of lipophilic drugs by ethosomes. Int. J. Nanomedicine 12, 3357–3364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cevc G, Gebauer D, Stieber J, Schätzlein A, Blume G. Ultraflexible vesicles, transfersomes, have an extremely low pore penetration resistance and transport therapeutic amounts of insulin across the intact mammalian skin. Biochim. Biophys. Acta 1368(2), 201–215 (1998). [DOI] [PubMed] [Google Scholar]
- 114.Duangjit S, Opanasopit P, Rojanarata T, Ngawhirunpat T. Characterization and in vitro skin permeation of meloxicam-loaded liposomes versus transfersomes. J. Drug Deliv. 2011, 418316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 56(9), 1257–1272 (2004). [DOI] [PubMed] [Google Scholar]
- 116.Campbell RB, Ying B, Kuesters GM, Hemphill R. Fighting cancer: from the bench to bedside using second generation cationic liposomal therapeutics. J. Pharm. Sci. 98(2), 411–429 (2009). [DOI] [PubMed] [Google Scholar]
- 117.Xie Y, Ibrahim A, Cheng K. et al. Importance of cell-cell contact in the therapeutic benefits of cardiosphere-derived cells. Stem Cells 32(9), 2397–2406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yamada Y, Kobayashi H, Iwasa M. et al. Postinfarct active cardiac-targeted delivery of erythropoietin by liposomes with sialyl Lewis X repairs infarcted myocardium in rabbits. Am. J. Physiol. Heart Circ. Physiol. 304(8), H1124–1133 (2013). [DOI] [PubMed] [Google Scholar]
- 119.Liu X, Liu H, Zeng Z, Zhou W, Liu J, He Z. Pharmacokinetics of ligustrazine ethosome patch in rats and anti-myocardial ischemia and anti-ischemic reperfusion injury effect. Int. J. Nanomedicine 6, 1391–1398 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang J, Shen D, Caranasos TG. et al. Therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome. Nat. Commun. 8, 13724 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sancho-Albero M, Navascués N, Mendoza G. et al. Exosome origin determines cell targeting and the transfer of therapeutic nanoparticles towards target cells. J. Nanobiotechnol. 17(1), 16 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Takahashi Y, Nishikawa M, Shinotsuka H. et al. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 165(2), 77–84 (2013). [DOI] [PubMed] [Google Scholar]
- 123.Bala S, Csak T, Momen-Heravi F. et al. Biodistribution and function of extracellular miRNA-155 in mice. Sci. Rep. 5(1), 10721 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kean TJ, Duesler L, Young RG. et al. Development of a peptide-targeted, myocardial ischemia-homing, mesenchymal stem cell. J. Drug Target. 20(1), 23–32 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Won Y-W, McGinn AN, Lee M, Bull DA, Kim SW. Targeted gene delivery to ischemic myocardium by homing peptide-guided polymeric carrier. Mol. Pharm. 10(1), 378–385 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Buehler A, van Zandvoort MAMJ, Stelt BJ. et al. CNGR: a novel homing sequence for CD13/APN targeted molecular imaging of murine cardiac angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 26(12), 2681–2687 (2006). [DOI] [PubMed] [Google Scholar]
- 127.Song Y, Huang Z, Xu J. et al. Multimodal SPION-CREKA peptide based agents for molecular imaging of microthrombus in a rat myocardial ischemia-reperfusion model. Biomaterials 35(9), 2961–2970 (2014). [DOI] [PubMed] [Google Scholar]
- 128.Antes TJ, Middleton RC, Luther KM. et al. Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display. J. Nanobiotechnol. 16(1), 61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • This proof-of-concept study of cloaking fluorescent molecules, targeting antibodies and homing peptides across diverse cell types highlights the ability to craft extracellular vesicles for specific purposes such as tissue localization, targeting and imaging.
- 129.Kanki S, Jaalouk DE, Lee S, Yu AYC, Gannon J, Lee RT. Identification of targeting peptides for ischemic myocardium by in vivo phage display. J. Mol. Cell. Cardiol. 50(5), 841–848 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Vandergriff A, Huang K, Shen D. et al. Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 8(7), 1869–1878 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xu W, Yihuan C, Zhenao Z. et al. Engineered exosomes with ischemic myocardium-targeting peptide for targeted therapy in myocardial infarction. J. Am. Heart Assoc. 7(15), e008737 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; • It highlights exosome engineering with a myocardium-targeting peptide as a mode of delivery for small molecules and drugs with enhanced cardiac function in the ischemic heart.
- 132.Kim H, Yun N, Mun D. et al. Cardiac-specific delivery by cardiac tissue-targeting peptide-expressing exosomes. Biochem. Biophys. Res. Commun. 499(4), 803–808 (2018). [DOI] [PubMed] [Google Scholar]
- 133.Mentkowski KI, Lang JK. Exosomes engineered to express a cardiomyocyte binding peptide demonstrate improved cardiac retention in vivo. Sci. Rep. 9(1), 10041 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Liu X, Yang Y, Li Y. et al. Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale 9(13), 4430–4438 (2017). [DOI] [PubMed] [Google Scholar]
- 135.Liu B, Lee BW, Nakanishi K. et al. Cardiac recovery via extended cell-free delivery of extracellular vesicles secreted by cardiomyocytes derived from induced pluripotent stem cells. Nat. Biomed. Eng. 2(5), 293–303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Han C, Zhou J, Liang C. et al. Human umbilical cord mesenchymal stem cell derived exosomes encapsulated in functional peptide hydrogels promote cardiac repair. Biomater. Sci. 7(7), 2920–2933 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y-N, Poon W, Tavares AJ, McGilvray ID, Chan WCW. Nanoparticle–liver interactions: cellular uptake and hepatobiliary elimination. J. Control. Rel. 240, 332–348 (2016). [DOI] [PubMed] [Google Scholar]
- 138.Gustafson HH, Holt-Casper D, Grainger DW, Ghandehari H. Nanoparticle uptake: the phagocyte problem. Nano Today 10(4), 487–510 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lundqvist M, Stigler J, Elia G, Lynch I, Cedervall T, Dawson KA. Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc. Natl Acad. Sci. USA 105(38), 14265–14270 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tenzer S, Docter D, Kuharev J. et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 8(10), 772–781 (2013). [DOI] [PubMed] [Google Scholar]
- 141.Gunawan C, Lim M, Marquis CP, Amal R. Nanoparticle–protein corona complexes govern the biological fates and functions of nanoparticles. J. Mater. Chem. B 2(15), 2060–2083 (2014). [DOI] [PubMed] [Google Scholar]
- 142.Lu X, Xu P, Ding H-M, Yu Y-S, Huo D, Ma Y-Q. Tailoring the component of protein corona via simple chemistry. Nat. Commun. 10(1), 4520 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol. Pharm. Bull. 41(6), 835–842 (2018). [DOI] [PubMed] [Google Scholar]
- 144.Liu C, Zhang W, Li Y. et al. Microfluidic sonication to assemble exosome membrane-coated nanoparticles for immune evasion-mediated targeting. Nano Lett. 19(11), 7836–7844 (2019). [DOI] [PubMed] [Google Scholar]; •• This research article directly demonstrates the inherent immune-evading properties of exosome membranes, as compared with artificial membranes.
- 145.Lai CP, Mardini O, Ericsson M. et al. Dynamic biodistribution of extracellular vesicles in vivo using a multimodal imaging reporter. ACS Nano 8(1), 483–494 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zomer A, Maynard C, Verweij FJ. et al. In vivo imaging reveals extracellular vesicle-mediated phenocopying of metastatic behavior. Cell 161(5), 1046–1057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Imai T, Takahashi Y, Nishikawa M. et al. Macrophage-dependent clearance of systemically administered B16BL6-derived exosomes from the blood circulation in mice. J. Extracell. Vesicles 4, 26238 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]; • It uses a unique tracking method: fusion protein of luciferase and exosome-tropic lactadherin proteins, to demonstrate that macrophages significantly contribute to exosome clearance after systemic delivery.
- 148.Wiklander OPB, Nordin JZ, O’Loughlin A. et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL, Henson PM. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 148(7), 2207–2216 (1992). [PubMed] [Google Scholar]
- 150.Watson DC, Bayik D, Srivatsan A. et al. Efficient production and enhanced tumor delivery of engineered extracellular vesicles. Biomaterials 105, 195–205 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Matsumoto A, Takahashi Y, Nishikawa M. et al. Role of phosphatidylserine-derived negative surface charges in the recognition and uptake of intravenously injected B16BL6-derived exosomes by macrophages. J. Pharm. Sci. 106(1), 168–175 (2017). [DOI] [PubMed] [Google Scholar]
- 152.Saunderson SC, Dunn AC, Crocker PR, McLellan AD. CD169 mediates the capture of exosomes in spleen and lymph node. Blood 123(2), 208–216 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Greish K. Enhanced permeability and retention (EPR) effect for anticancer nanomedicine drug targeting. Methods Mol. Biol. 624, 25–37 (2010). [DOI] [PubMed] [Google Scholar]
- 154.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J. Control. Rel. 65(1–2), 271–284 (2000). [DOI] [PubMed] [Google Scholar]
- 155.Foroozandeh P, Aziz AA. Insight into cellular uptake and intracellular trafficking of nanoparticles. Nanoscale Res. Lett. 13(1), 339 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Behzadi S, Serpooshan V, Tao W. et al. Cellular uptake of nanoparticles: journey inside the cell. Chem. Soc. Rev. 46(14), 4218–4244 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Geiser M, Rothen-Rutishauser B, Kapp N. et al. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 113(11), 1555–1560 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu M, Guo H, Liu L, Liu Y, Xie L. Size-dependent cellular uptake and localization profiles of silver nanoparticles. Int. J. Nanomedicine 14, 4247–4259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Settanni G, Zhou J, Suo T. et al. Protein corona composition of poly(ethylene glycol)- and poly(phosphoester)-coated nanoparticles correlates strongly with the amino acid composition of the protein surface. Nanoscale 9(6), 2138–2144 (2017). [DOI] [PubMed] [Google Scholar]
- 160.Yang Q, Jones SW, Parker CL, Zamboni WC, Bear JE, Lai SK. Evading immune cell uptake and clearance requires PEG grafting at densities substantially exceeding the minimum for brush conformation. Mol. Pharm. 11(4), 1250–1258 (2014). [DOI] [PubMed] [Google Scholar]
- 161.Moghimi SM, Andersen AJ, Hashemi SH. et al. Complement activation cascade triggered by PEG–PL engineered nanomedicines and carbon nanotubes: the challenges ahead. J. Control. Rel. 146(2), 175–181 (2010). [DOI] [PubMed] [Google Scholar]
- 162.Szebeni J, Baranyi L, Savay S. et al. Role of complement activation in hypersensitivity reactions to Doxil and Hynic PEG liposomes: experimental and clinical studies. J. Liposome Res. 12(1–2), 165–172 (2002). [DOI] [PubMed] [Google Scholar]
- 163.Chanan-Khan A, Szebeni J, Savay S. et al. Complement activation following first exposure to PEGylated liposomal doxorubicin (Doxil®): possible role in hypersensitivity reactions. Ann. Oncol. 14(9), 1430–1437 (2003). [DOI] [PubMed] [Google Scholar]
- 164.Su Y, Liu M, Liang K, Liu X, Song Y, Deng Y. Evaluating the accelerated blood clearance phenomenon of PEGylated nanoemulsions in rats by intraperitoneal administration. AAPS PharmSciTech 19(7), 3210–3218 (2018). [DOI] [PubMed] [Google Scholar]
- 165.Kooijmans SAA, Fliervoet LAL, van der Meel R. et al. PEGylated and targeted extracellular vesicles display enhanced cell specificity and circulation time. J. Control. Rel. 224, 77–85 (2016). [DOI] [PubMed] [Google Scholar]
- 166.Wan Z, Zhao L, Lu F. et al. Mononuclear phagocyte system blockade improves therapeutic exosome delivery to the myocardium. Theranostics 10(1), 218–230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Wolfram J, Nizzero S, Liu H. et al. A chloroquine-induced macrophage-preconditioning strategy for improved nanodelivery. Sci. Rep. 7(1), 13738 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Qiu X, Li Z, Han X. et al. Tumor-derived nanovesicles promote lung distribution of the therapeutic nanovector through repression of Kupffer cell-mediated phagocytosis. Theranostics 9(9), 2618–2636 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Shoja-Taheri F, George A, Agarwal U, Platt MO, Gibson G, Davis ME. Using statistical modeling to understand and predict pediatric stem cell function. Circ. Genomic Precis. Med. 12(6), e002403 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Abu Lila AS, Ishida T. Liposomal delivery systems: design optimization and current applications. Biol. Pharm. Bull. 40(1), 1–10 (2017). [DOI] [PubMed] [Google Scholar]
- 171.Guo J, Ping Q, Jiang G, Huang L, Tong Y. Chitosan-coated liposomes: characterization and interaction with leuprolide. Int. J. Pharm. 260(2), 167–173 (2003). [DOI] [PubMed] [Google Scholar]
- 172.Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int. J. Nanomedicine 1(3), 297–315 (2006). [PMC free article] [PubMed] [Google Scholar]
- 173.Moghassemi S, Hadjizadeh A, Omidfar K. Formulation and characterization of bovine serum albumin-loaded niosome. AAPS PharmSciTech 18(1), 27–33 (2017). [DOI] [PubMed] [Google Scholar]
- 174.Touitou E, Godin B, Dayan N, Weiss C, Piliponsky A, Levi-Schaffer F. Intracellular delivery mediated by an ethosomal carrier. Biomaterials 22(22), 3053–3059 (2001). [DOI] [PubMed] [Google Scholar]
- 175.Wong HL, Bendayan R, Rauth AM, Li Y, Wu XY. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 59(6), 491–504 (2007). [DOI] [PubMed] [Google Scholar]
- 176.Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L. Erythrocyte membrane-camouflaged polymeric nanoparticles as a biomimetic delivery platform. Proc. Natl Acad. Sci. USA 108(27), 10980–10985 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Cao H, Dan Z, He X. et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano 10(8), 7738–7748 (2016). [DOI] [PubMed] [Google Scholar]
- 178.Luo L, Tang J, Nishi K. et al. Fabrication of synthetic mesenchymal stem cells for the treatment of acute myocardial infarction in mice. Circ. Res. 120(11), 1768–1775 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]




