Abstract
Mechanical ventilation (MV) is a tool used for the treatment of patients with acute or chronic respiratory failure. However, MV is a non-physiological resource, and it can cause metabolic disorders such as release of pro-inflammatory cytokines and production of reactive oxygen species. In clinical setting, maneuvers such as sigh, are used to protect the lungs. Thus, this study aimed to evaluate the effects of sigh on oxidative stress and lung inflammation in healthy adult Wistar rats submitted to MV. Male Wistar rats were divided into four groups: control (CG), mechanical ventilation (MVG), MV set at 20 sighs/h (MVG20), and MV set at 40 sighs/h (MVG40). The MVG, MVG20, and MVG40 were submitted to MV for 1 h. After the protocol, all animals were euthanized and the blood, bronchoalveolar lavage fluid, and lungs were collected for subsequent analysis. In the arterial blood, MVG40 presented higher partial pressure of oxygen and lower partial pressure of carbon dioxide compared to control. The levels of bicarbonate in MVG20 were lower compared to CG. The neutrophil influx in bronchoalveolar lavage fluid was higher in the MVG compared to CG and MVG40. In the lung parenchyma, the lipid peroxidation was higher in MVG compared to CG, MVG20, and MVG40. Superoxide dismutase and catalase activity were higher in MVG compared to CG, MVG20, and MVG40. The levels of IL-1, IL-6, and TNF in the lung homogenate were higher in MVG compared to CG, MVG20, and MVG40. The use of sigh plays a protective role as it reduced redox imbalance and pulmonary inflammation caused by MV.
Keywords: Mechanical ventilation, sigh, lung, inflammation, oxidation, rats
Impact statement
Mechanical ventilation (MV) is a widely used tool to restore and maintain alveolar ventilation. Despite its clinical importance, MV can promote or aggravate an existing lung injury, even at a tidal volume close to the physiological, thus promoting inflammation and oxidative stress. However, when mechanical ventilation was associated with a sigh, the inflammatory response and oxidative damage were minimized. The results of this study indicate a promising effect of using the sigh maneuver to prevent lung injury associated with ventilation.
Introduction
Mechanical ventilation (MV) is a tool used to restore or improve gas exchange in patients unable to maintain adequate alveolar ventilation.1,2 Annually, there are hundreds of thousands of people in intensive care units and operating rooms who are submitted to MV, and it is estimated that between 4 and 13% require mechanical ventilation for long periods.3–5 Indications for mechanical ventilation include respiratory failure secondary to lung disease, neuromuscular disease, and patients undergoing surgery under general anesthesia.2
Despite the clinical relevance of MV, studies show that its use can promote or aggravate an existing lung injury, which is called ventilator-induced lung injury (VILI).6 The mechanisms involved in lung injury triggered by MV are not entirely understood. Still, studies suggest that cyclic opening and closing of the distal airways and alveoli may cause cell damage increasing the production and secretion of inflammatory cytokines such as interleukin 1 (IL-1) and interleukin-6 (IL-6), thus promoting the recruitment and activation of inflammatory cells, especially neutrophils.6–8 In addition to the production of inflammatory markers, in response to stress caused by MV, there is an increase in the production of reactive oxygen species (ROS).8 The high production of reactive species, which is characterized in an imbalance between oxidants and antioxidants, where the concentration of reactive species is transiently or chronically increased, causing damage to cellular metabolism regulation and promoting macromolecule oxidation as lipids, proteins, and DNA.9,10
In order to prevent the onset or aggravation of lung injuries caused by MV, some maneuvers are known for their protective actions in the respiratory system, called recruitment maneuvers, such as sighing, gradual insufflation, and variable ventilation.11–13 Among these, sighing is a lung inflation maneuver that, when used during MV, it attempts to simulate the natural reflex that occurs during spontaneous ventilation.14,15 Studies report that sigh leads to maximum expansion of the lungs, which prevents the progressive collapse of the alveoli, and it is associated with improved gas exchange.16 In patients with acute respiratory distress syndrome, cyclic sighing is associated with improved lung function.17 Although there are beneficial effects already reported in the literature, the use of sigh may increase respiratory system stress and it may possibly lead to biological impacts.18 Moreover, the protective mechanisms of sigh in the face of the consequences of mechanical ventilation are still poorly understood, the study of the sighing effects becomes extremely relevant, since the understanding of the possible protective effect of this lung inflation maneuver aims to avoid or even minimize local and systemic aggravations induced by mechanical ventilation. It is believed that the present experimental study in healthy rats may contribute in clarifying the effects of sigh, thus avoiding possible initial changes as injuries caused by mechanical ventilation such as inflammation and oxidative stress. Therefore, this study aimed to analyze the effects of sigh on status redox and pulmonary inflammatory response in healthy adult Wistar rats submitted to mechanical ventilation.
Materials and methods
Animals
Thirty-one male Wistar rats were obtained from the Animal Science Center of the Federal University of Ouro Preto (UFOP). The animals were kept in cages in the experiment room under controlled conditions of light, temperature, humidity (12 h light/dark, 24 °C, 50 ± 10%, respectively), and had free access to water and food. The experimental procedures performed in this study followed the Ethical Principles of Animal Experimentation and were approved by the Animal Use Ethics Committee (CEUA) under protocol number 2018/13.
The animals were randomly divided into four groups: control group (CG) maintained on spontaneous ventilation (n = 6), mechanical ventilation group (MVG) submitted to mechanical ventilation (n = 9), mechanical ventilation group set at 20 sighs/h (MVG20) (n = 8), and mechanical ventilation group set at 40 sighs/h (MVG40) (n = 8).
Spontaneous ventilation
The control group was kept under spontaneous ventilation in ambient room air. All animals were initially kept in a hermetically sealed box, before pulmonary function analysis. Subsequently, each animal was placed individually in this box for 8 min, and a full-body plethysmography was performed to record the following ventilatory parameters: respiratory rate, tidal volume, and minute volume.
For data collection, a cylinder containing 8000 L (180 kgf/cm2, White Martins, Praxair Inc., São Paulo, Brazil) of medical grade air (O2 at 21%) was coupled to the Bourdon tube and the Thorpe tube (0–15 L/min). A silicone conduit was connected to the Thorpe tube into a hermetically sealed box, then the animals were exposed to medicinal air with known volume detected through a tube connected to the system. The flow of respiratory gases was captured by the table spirometer (ADInstruments, Dunedin, New Zealand), then the signal was amplified and analyzed by Power Lab software (ADInstruments, Dunedin, New Zealand).
Mechanical ventilation
The animals in the groups: MVG, MVG20, and MVG40 were anesthetized with an intraperitoneal injection of Midazolam (5 mg/kg) and Fentanyl (0.16 mg/kg). Subsequently, a neuromuscular blockade was performed by intravenous injection of suxamethonium chloride (1 mL/kg). The animals were placed on a surgical table in the supine position to make an incision in the anterior cervical region. The musculature was dissected with the aid of a hemostatic forceps. The trachea was exposed, and the animals were tracheostomized with the aid of a polyethylene cannula (16 G) and the stainless steel cannula (Harvard Apparatus, MA, USA) was inserted into the site of the tracheostomy and the animals were connected to the mechanical ventilator (Harvard Apparatus, MA, USA) according to the following parameters: tidal volume (VT) of 7 mL/kg, respiratory rate (RR) of 80 breaths/min, inspired fraction of oxygen (FiO2) at 21%, PEEP of 3 cm H2O, in volume-controlled mode. All animals were submitted to MV for 60 min.
The rats in the groups, MVG20 and MVG40, received identical volume-controlled ventilation, MVG (7 mL/kg) with a regular application of lung inflation maneuver (20 or 40 sighs/h; sigh volume 7 mL/kg above VT). The adjustment was performed based on the manual from the manufacturer (Harvard Apparatus, MA, USA) using the respiratory rate reference. The ventilator was programmed to automatically perform a sigh, specifically in the MVG20, it was programmed to trigger a sigh every 240 respiratory incursions, and for the MVG40, it triggered one sigh every 120 respiratory cycles. In the three groups submitted to MV (MVG, MVG20, and MVG40), the analysis of the peripheral saturation and body temperature was performed throughout the mechanical ventilation period.19
Evaluation of cardiovascular parameters
The animals from the four experimental groups (CG, MVG, MVG20, MVG40) were evaluated for cardiovascular parameters such as the mean arterial pressure (MAP) and heart rate (HR). A skin incision was made to separate the musculature and locate the femoral vascular-nerve bundle, and the right femoral artery was cannulated. The mean arterial pressure (MAP) was monitored by a Gould pressure transducer connected to an amplifier (ML221 Bridge Amp). The pulsable blood pressure (PAP) and heart rate (HR) were continuously sampled by a 16-bit analog/digital conversion system (PowerLab 4/30) (ADInstruments, Dunedin, New Zealand) at a sampling frequency of 100 Hz, after which the signal was processed by the Lab Char 7 software (ADInstruments, Dunedin, New Zealand). MAP and HR were derived in real-time from pulsatile blood pressure pulses using Chart 5 software. These variables were simultaneously displayed on different channels on the monitor and stored on the hard disk in the computer.19
Blood collection and euthanasia
At the end of mechanical ventilation, a 200 μL aliquot of blood was collected directly from the right femoral artery using a heparinized syringe (Monovette®, Sarstedt) for arterial blood gas analysis. Samples were analyzed by the PRIME +® VET gasometer (Nova Biomedical Corporation, Waltham, Mass).
After blood was collected for blood gas analysis, the animals had their thorax exposed, after which an incision was made in the third intercostal space, then the animals were euthanized by exsanguination.19,20
Collection and analysis of bronchoalveolar lavage fluid
Immediately after euthanasia, the thorax of each animal was opened, the left main bronchus was clamped, the trachea was cannulated, and the right lung was washed with saline (0.9%). The procedure was performed three times, totaling a final volume of BALF of 2.5 to 3.0 mL. The samples were stored in polypropylene tubes and kept on ice (4°C) to avoid cell lysis.21,22
For the evaluation of airway leukocytes, the BALF was centrifuged for 10 min at 4°C and 3000 r/min (Eppendorf 5415 R; Eppendorf®, Hamburg, Germany). The supernatant was stored at −80°C, and the pellet was resuspended in 0.1 mL saline. For the total count, 20 μL of the resuspended solution was placed in a tube containing 180 μL of the Turk solution. This solution was used for total leukocyte count in a Neubauer chamber. The differential count, and the samples were centrifuged in a cytocentrifuge (INBRAS Health Equipment, SP, BR). The slides were stained with rapid staining kits. There were 100 cells counted under an optical microscope at 100× magnification, protocol adapted from Campos et al. and Chirico et al.21,23
Lung collection
After BALF collection, the right ventricle was perfused with saline to remove blood from the lungs. The right lung was clamped, and the left lung was instilled with 4% buffered formalin (pH 7.2) at a pressure of 25 cm H2O for 2 min. The left lung was removed and immersed in a fixative solution for 48 h, then the samples were processed, and the slides were stained with hematoxylin and eosin (H&E) for stereological analysis. The right lung was collected, and 100 mg of tissue was homogenized with 1 mL phosphate buffer (pH 7.8), the samples were centrifuged for 10 min at 4°C and 13000 r/min, and the supernatant was collected and stored at −80°C.24
Analysis of antioxidant defense and oxidative stress biomarkers
Superoxide dismutase (SOD) activity was measured in tissue homogenate according to the method described by Marklund and Marklund,25 which is based on SOD ability to inhibit pyrogallol autoxidation. The catalase activity (CAT) was measured by decreasing hydrogen peroxide (H2O2) according to the Aebi method.26 Glutathione dosage was adapted from the commercial Sigma kit (# CS0260, Sigma, St. Louis, MO, USA), which uses a kinetic method to measure total glutathione (GSH + GSSG) by reducing 5.5'-Dithio Acid -bis- (2-nitrobenzoic) to thio-2-nitrobenzoic acid.27 Lipid peroxidation analysis was performed according to the method described by Buege and Aust,28 in which thiobarbituric acid reacts with oxidized lipids and generates malondialdehyde. Carbonylated protein analysis was performed according to protocol adapted from Reznick and Packer.29 Total protein content was determined according to the method described by Bradford.30
Stereological analysis
In order to evaluate the structure of the lung parenchyma, 20 random fields of H&E stained slides were photographed using an optical microscope Primo star equipped with a digital camera (Axiocam 105) (Carl Zeiss AG, Oberkochen, Germany) coupled with the ZEN lite image capture software using the 40× microscopic objective. Analysis of the volume density of the alveolar septum (Vv [sa]) and alveolar airspace (Vv [a]) was performed using a 16-point test system and a known test area where the forbidden line was taken as the limit for avoiding overestimation in the number of structures. The number of points (Pp) that reached the alveolar septa (Vv [sa]) and the alveolar spaces (Vv [a]) was evaluated according to the total number of points in the test system (Pt). Reference volume was estimated by point counting using the system test point (Pt). The total area of 1.94 mm2 was analyzed to determine the volumetric analysis of alveolar septa (Vv [sa]) and alveolar spaces (Vv [a]) in H&E-stained sections.24,31
Immunoenzymatic assay for inflammatory markers
Interleukin-1 (IL-1), interleukin-6 (IL-6), monocyte chemotactic protein 1 (MCP1 or CCL2), macrophage inflammatory protein 1-alpha (MIP-1α or CCL3), regulated on activation, normal T cell expressed and secreted (RANTES or CCL5), and tumor necrosis factor (TNF) were performed in lung homogenate by the ELISA method. The assays were performed using commercial kits (PeproTech, Ribeirão Preto, Brazil), and the antibodies and reagents were prepared according to the instructions from the manufacturer. The assay was performed in 96-well plates, and 100 μL of monoclonal antibody against the peptides (or proteins) reconstituted in phosphate-buffered saline (PBS) were added. After 12 h of incubation at room temperature, blocking was performed with a solution containing PBS and 1% fetal bovine serum for 1 h. Samples were added in a volume of 50 μL per well. Subsequently, diluted secondary antibodies in PBS and 1% fetal bovine serum were added. Staining intensity reading was performed on an ELISA reader at 450 nm. The quantification of chemokines and interleukins present in the samples was determined based on the optical density obtained with the peptide standard curve, analyzed by the SOFT Max PRO 4.0 software.19,32
Statistical analysis
Data were expressed as mean ± standard error of the mean. For the analysis of two experimental groups with normal distribution, the paired T-test was used. For the analysis of more than two groups with normal distribution, univariate analysis of variance (one-way ANOVA) was used, followed by the Bonferroni's post hoc test. For nonparametric data, we used the Kruskal–Wallis test, followed by the Dunn´s post hoc test. In both cases, a significant difference was considered when P <0.05. All analyses were performed using GraphPad Prism software version 5.00 for Windows 7, GraphPad Software (San Diego, CA, USA).
Results
Hemodynamic monitoring
The animals from the four experimental groups were evaluated for heart rate and mean arterial pressure throughout the experimental protocol. For the evaluated parameters, no significant difference was observed at the beginning and at the end of the experiment (P> 0.05) (Table 1).
Table 1.
Hemodynamics of animals submitted to mechanical ventilation.
| Initial CG | Final CG | P | Initial MVG | Final MVG | P | Initial MVG20 | Final MVG20 | P | Initial MVG40 | Final MVG40 | P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (bpm) | 441.4±23.5 | 441.0±16.4 | >0.05 | 421.5±16.1 | 415.8±17.04 | >0.05 | 394.5±19.1 | 414.6±13.3 | >0.05 | 424.0±9.04 | 433.3±7.03 | >0.05 |
| MAP (mmHg) | 125.8 ± 3.6 | 126.7 ± 2.3 | >0.05 | 116.0 ± 4.7 | 117.6 ± 6.2 | >0.05 | 124.5 ± 9.1 | 131.6 ± 5.8 | >0.05 | 123.4 ± 8.2 | 132.9 ± 3.1 | >0.05 |
Note: Data are expressed as mean ± standard error of the mean and were analyzed by paired T-test (P <0.05), n = 5–8.
CG: control group; MVG: mechanical ventilation group; MVG20: mechanical ventilation group + 20 sighs/h; MVG40: mechanical ventilation group + 40 sighs/h; HR: heart rate; MAP: mean arterial pressure.
Analysis of arterial blood gas and pulmonary function
Arterial blood gas analysis revealed higher partial oxygen pressure in MVG and MVG40 when compared with the control (P <0.05). This same result was also observed in the PaO2/FiO2 ratio, and the MVG and MVG40 groups presented higher ratio when compared to the control group (P <0.05), which may be justified by an increase in PaO2, since the inspired fraction of oxygen was a fixed variable in the experiment. MVG40 presented lower partial pressure of carbon dioxide compared to CG, besides MVG20 presented lower bicarbonate concentration (HCO3) compared to control group (P <0.05) (Table 2).
Table 2.
Arterial blood gas and pulmonary function of animals submitted to mechanical ventilation.
| CG | MVG | MVG20 | MVG40 | |
|---|---|---|---|---|
| pH | 7.38 ± 0.01 | 7.39 ± 0.01 | 7.37 ± 0.01 | 7.39 ± 0.01 |
| SO2 (%) | 95.75 ± 0.61 | 94.44 ± 0.92 | 93.38 ± 1.23 | 94.50 ± 0.92 |
| PaO2(mmHg) | 82.17 ± 4.15 | 99.20 ± 3.89a | 95.73 ± 2.63 | 100.20 ± 3.62a |
| PaCO2 (mmHg) | 35.37 ± 2.31 | 31.95 ± 1.29 | 30.20 ± 1.25 | 28.68 ± 0.80a |
| HCO3(mmol/L) | 21.65 ± 1.55 | 19.38 ± 0.55 | 18.10 ± 0.59a | 18.29 ± 0.71 |
| PaO2/FiO2 | 393.20 ± 19.87 | 474.40 ± 18.70a | 458.00 ± 12.61 | 479.20 ± 17.31a |
| RR (breaths/min) | 99.80 ± 6.16 | 80.00 ± 0.00 a | 80.00 ± 0.00 a | 80.00 ± 0.00a |
| VT (mL) | 2.89 ± 0.26 | 2.68 ± 0.07 | 2.60 ± 0.08 | 2.72 ± 0.05 |
| Vmin (mL/min) | 246.70 ± 13.39 | 214.60 ± 5.99 | 277.30 ± 9.14b | 363.20 ± 7.18a,b,c |
CG: control group; MVG: mechanical ventilation group; MVG20: mechanical ventilation group + 20 sighs/h; MVG40: mechanical ventilation group + 40 sighs/h; SO2: oxygen saturation; PaO2: partial pressure of oxygen; PaCO2: p partial pressure of carbon dioxide; HCO3: bicarbonate; FiO2: inspired fraction of oxygen; RR: respiratory rate; VT: tidal volume; Vmin: minute ventilation.
aRepresents a significant difference in relation to CG.
bRepresents a significant difference in relation to MVG.
cRepresents a significant difference in relation to MV20.
Note: Data are expressed as mean ± standard error of the mean. Analysis of variance one-way ANOVA followed by Bonferroni's post hoc test (P <0.05), arterial blood gas n = 6–9 and pulmonary function n = 5°.
Regarding pulmonary function, the groups submitted to mechanical ventilation had lower respiratory rate when compared to the control group (P < 0.05). For minute volume analysis, the ventilated group with 20 sighs/h presented higher volume compared to MVG, and MVG40 presented higher minute volume when compared to the other experimental groups (P < 0.05) (Table 2).
Cells recruitment to bronchoalveolar lavage fluid
After 1 h of ventilation, MVG presented higher leukocyte count in BALF compared to CG (P < 0.05). Regarding differential cell count, a higher neutrophil count was observed in the group submitted only to ventilation compared to CG, whereas the association of mechanical ventilation with the sigh maneuver at a rate of 40 sighs/h led to a lower neutrophil count compared to MVG (P < 0.05) (Table 3).
Table 3.
The effects of mechanical ventilation on recruitment of cells from BALF.
| CG | MVG | MVG20 | MVG40 | |
|---|---|---|---|---|
| Leukocytes (×105/mL) | 13.75 ± 1.34 | 20.94 ± 1.51a | 15.54 ± 1.61 | 16.00 ± 1.42 |
| Macrophages (×105/mL) | 13.18 ± 1.25 | 17.41 ± 1.11 | 14.07 ± 1.46 | 15.04 ± 1.39 |
| Lymphocytes (×105/mL) | 0.24 (0.16; 0.60) | 0.35 (0.16; 1.23) | 0.15 (0.11; 0.79) | 0.16 (0.0; 0.80) |
| Neutrophils (105/mL) | 0.15 (0.0; 0.72) | 2.46 (2.11; 5.22)a | 0.87 (0.61; 2.52) | 0.76 (0.34; 0.99)b |
CG: control group; MVG: mechanical ventilation group; MVG20: mechanical ventilation group + 20 sighs/h; MVG40: mechanical ventilation group + 40 sighs/h.
aRepresents a significant difference between groups when compared to CG.
bRepresents a significant difference between groups when compared to MVG.
Note: Data are expressed as mean ± standard error of the mean. One-way analysis of variance ANOVA followed by Bonferroni's post hoc test, for the analysis of the lymphocytes and neutrophils the Kruskal–Wallis test followed by the Dunn's post hoc test was used (P <0.05), n = 6–9.
Oxidative stress in the lung parenchyma
The activity of the antioxidant enzymes superoxide dismutase and catalase was higher in MVG compared to the control group, while the ventilated groups with 20 and 40 sighs/h presented the activity of both enzymes similar to the control group and lower than MVG (P < 0.05). For the glutathione system analysis, no differences were observed between the experimental groups (P > 0.05). The analysis of lipid peroxidation levels in MVG was higher compared to CG, while MVG20 and MV40 presented lower levels of lipid peroxidation compared to MVG, but higher than in the control group (P < 0.05). The protein cabonylation in the MVG40 group presented lower level of carbonylated protein compared to MVG (P < 0.05) (Table 4).
Table 4.
Biomarkers of oxidative stress in lung parenchyma of animals submitted to mechanical ventilation.
| CG | MVG | MVG20 | MVG40 | |
|---|---|---|---|---|
| SOD (U/mg protein) | 15.66 ± 2.59 | 30.04 ± 1.70a | 19.34 ± 1.47b | 16.42 ± 3.36b |
| CAT (U/mg protein) | 1.27 ± 0.19 | 2.27 ± 0.31a | 1.30 ± 0.17b | 1.17 ± 0.17b |
| GSH/GSSG ratio | 0.77 (0.60; 0.95) | 0.75 (0.64; 0.92) | 0.88 (0.80; 0.90) | 0.88 (0.82; 1.13) |
| TBARS (nmol/mg protein) | 0.54 ± 0.06 | 1.69 ± 0.02a | 1.08 ± 0.14a,b | 1.17 ± 0.15a,b |
| Protein carbonyl (nmol/mg protein) | 9.13 ± 1.24 | 16.70 ± 2.71 | 13.76 ± 2.25 | 8.42 ± 1.17b |
Note: Data are expressed as mean ± standard error of the mean and as median (minimum value and maximum value). One-way analysis of variance ANOVA followed by Bonferroni's post hoc test, (P <0.05), n = 5 for SOD and CAT, n = 5–6 for TBARS and protein carbonyl; for the analysis of the GSH/GSSG ratio, the Kruskal–Wallis test followed by the Dunn's post-hoc test was used (P <0.05), n = 5.
aRepresents a significant difference in relation to CG.
bRepresents a significant difference in relation to MVG.
CG: control group; MVG: mechanical ventilation group; MVG20: mechanical ventilation group + 20 sighs/h; MVG40: mechanical ventilation group + 40 sighs/h; SOD: superoxide dismutase; CAT: catalase; GSH: glutathione sulfide; GSSG: oxidized glutathione; TBARS: thiobarbituric acid reactive substances.
Evaluation of cytokine and chemokine levels in the lung parenchyma
Inflammatory markers CCL2, CCL3, CCL5, IL-1, IL-6, and TNF were measured to assess the inflammatory status of the lungs. Concerning IL-1, IL-6, and TNF cytokines, a higher level of the three markers in the MVG was observed compared to CG, and the ventilated groups with 20 and 40 sighs/h presented lower concentration of the three cytokines in the lung parenchyma compared to MVG (P < 0.05).
For the CCL2 and CLL3 chemokines, no significant differences were observed between the experimental groups, whereas, for the CCL5 analysis, the MVG40 presented a higher level of this chemokine in the lung parenchyma compared to the control group (P < 0.05) (Table 5).
Table 5.
Inflammatory markers in lung parenchyma of animals submitted to mechanical ventilation.
| CG | MVG | MVG20 | MVG40 | |
|---|---|---|---|---|
| CCL2 (pg/mL) | 1523.0 ± 38.2 | 1520.6 ± 63.4 | 1554.8 ± 79.6 | 1482.1 ± 40.4 |
| CCL3 (pg/mL) | 173.4 ± 13.7 | 185.4 ± 14.3 | 157.6 ± 9.6 | 157.7 ± 8.5 |
| CCL5 (pg/mL) | 744.4 ± 80.2 | 895.2 ± 64.5 | 1028.6 ± 74.2 | 1086.5 ± 88.1a |
| IL-1 (pg/mL) | 861.8 ± 126.6 | 1178.3 ± 36.2a | 741.7 ± 41.5b | 733.4 ± 52.1b |
| IL-6 (pg/mL) | 1317.4 ± 41.8 | 1605.9 ± 56.1a | 1344.8 ± 26.6b | 1337.4 ± 30.7b |
| TNF (pg/mL) | 1185.1 ± 27.3 | 1478.7 ± 41.9a | 1185.1 ± 40.1b | 1202.4 ± 21.5b |
Note: Data are expressed as mean ± standard error of the mean. Analysis of variance one-way ANOVA followed by Bonferroni's post hoc test (P <0.05) n = 6–9 to CCL2 and CCL5; n =5–8 to CCL2; n = 5 to IL-1, IL-6, and TNF.
aRepresents a significant difference in relation to CG.
bRepresents a significant difference in relation to MVG.
CG: control group; MVG: mechanical ventilation group; MVG20: mechanical ventilation group + 20 sighs/h; MVG40: mechanical ventilation group + 40 sighs/h; CCL2 or MCP1: monocyte chemotactic protein 1; CCL3 or MIP-1α: macrophage inflammatory protein 1-alpha; CCL5 or RANTES: regulated on activation, normal T cell expressed and secreted; IL-1: interleukin 1; IL-6: interleukin 6; TNF: tumor necrosis factor.
Stereological analysis of the lung parenchyma
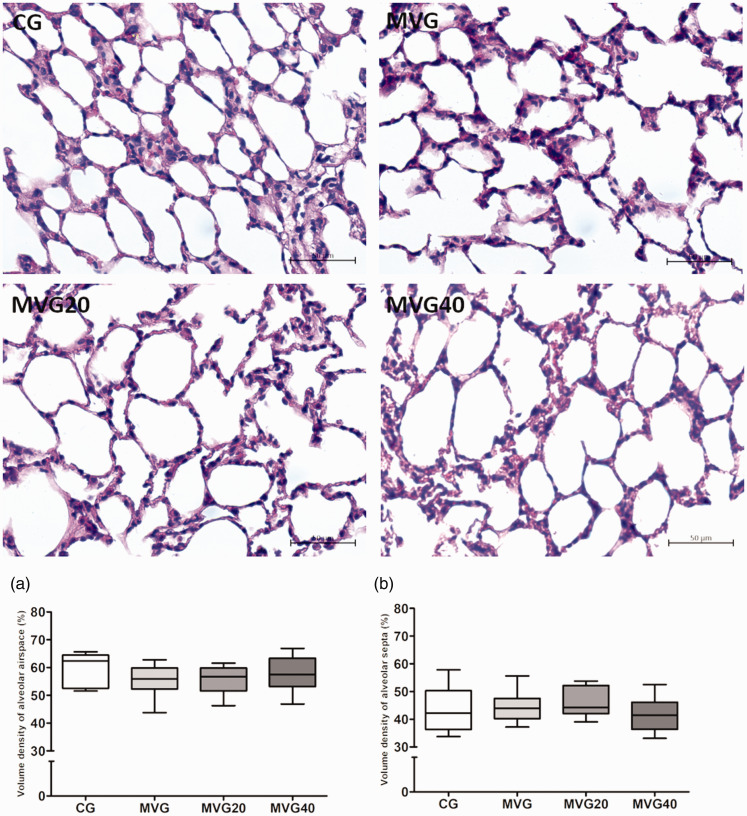
Stereological analysis of the parenchyma showed no significant differences between the experimental groups for both alveolar airspace volume density (Vv [a]) and alveolar septa volume density (Vv [sa]) (P > 0.05) (Figure 1).
Figure 1.
Stereological analyses of lung sections. Photomicrographs of lung sections stained with hematoxylin and eosin. Bar = 50 µm, 400× magnification. (a) Volume density of alveolar septa. (b) Volume density of alveolar airspace. The A and B data were expressed in median, minimum, and maximum value and were analyzed by Kruskal–Wallis followed by Dunn’s post-test, n = 6–8. (A color version of this figure is available in the online journal.)
Discussion
The aim of this study was to evaluate the effect of mechanical ventilation with and without the use of sigh on oxidative stress and lung inflammatory response in healthy Wistar rats. We observed that mechanical ventilation promoted the recruitment of inflammatory cells, increased inflammatory markers and oxidative stress, while the use of sigh at both frequencies promoted a reduction in the concentration of inflammatory markers and lower oxidative stress.
Although mechanical ventilation is indispensable in the case of respiratory failure, it is already described in the literature that MV is associated with complications, as hemodynamic changes, impaired lung function, and tissue damage.33 These complications are related to the mechanical effects of intrathoracic pressures generated by the ventilator, alveolar, and systemic inflammation.34 In the present study, we did not observe hemodynamic changes at the beginning and end of the experimental procedure in all experimental groups, the experimental time of 60 min did not appear to induce lung injury as evidenced by Figure 1, and thus may not be sufficient to induce hemodynamic changes. Recently, Walesa et al.35 did a comparison between neutrally assisted, controlled, and physiologically variable ventilation in healthy rabbits. The animals were ventilated for up to 7 h, where marked deteriorations in elastance were observed in group using pressure-controlled, while no changes were detected in ventilation with and without regular sighs, and others, therefore, suggesting protective effects on respiratory function as applying regular sighs during pressure-controlled ventilation. Furthermore, there was no difference in blood gas, hemodynamic, or inflammatory parameters between all groups studied.35
Currently, ventilatory strategies such as the use of smaller tidal volumes and individualized PEEP value have been used in order to limit alveolar distension pressure and prevent alveolar collapse.36,37 In rabbits with severe acute respiratory distress syndrome (ARDS) ventilated either with conventional monotonous or variable ventilation patterns, a moderate PEEP (6 cmH2O), both a variable ventilation based on pre-recorded physiological breathing pattern and the conventional pressure control mode prevented further lung function deterioration, that was otherwise observed in the animals ventilated with conventional pressure-controlled ventilation with regular sighs or mathematically derived variable ventilation patterns, suggesting that physiologically variable ventilation was protective in avoiding the progression of lung injury, including when increasing PEEP, which may jeopardize cardiac output.38 In this study with healthy Wistar rats, we adopted strategies, where no acid–base disorder was observed in the blood gas analysis of the experimental groups. Although lower tidal volume is used to limit alveolar distension injury, Tsuno et al.39 demonstrated that low tidal volume may promote progressive atelectasis and consequently hypoxia. On the other hand, Egbert et al.40 demonstrated in patients under general anesthesia that the use of recruitment maneuvers can improve compliance and prevent atelectasis.
Bronchoalveolar lavage fluid is used for the differential diagnosis of lung diseases,41 and studies have already shown significant changes in BALF in ventilated patients with lung injury.42 In this study, we demonstrated that healthy animals submitted only to mechanical ventilation had higher total leukocyte and neutrophil counts compared to the control group. Neutrophils are the main inflammatory cells involved in acute lung injury and ventilator-induced lung injury (VILI),43 and our findings corroborate previous studies published by the group showing that 1 h of mechanical ventilation promotes the recruitment of inflammatory cells to the airspace.19,22
Studies have shown that a sigh in humans happens spontaneously a few times an hour, while in rodents it happens dozens of times.44–46 In this study, we used two frequencies and evaluated its effects in animals submitted to mechanical ventilation. In the clinical study, Mauri et al.47 demonstrated that in acute respiratory failure, sigh improves oxygenation, decreases regional tension and the risk of ventilator-induced lung injury, therefore, limiting clinical sequelae. The ventilated group using the frequency of 40 sighs/h had a lower neutrophil count compared to the ventilated group. We believe that sigh acted by protecting the lungs of the animals, and possibly, the properties of sigh, already described in the literature, have minimized cell injury and neutrophil recruitment into the airspace.
Neutrophils may contribute to lung injury through different mechanisms including the production of reactive oxygen species.43 Excessive formation of reactive species during mechanical ventilation is also associated with the development of organ damage, acute lung injury, and ventilator-induced lung injury.48 Reactive oxygen species can attack cell membrane fatty acids and trigger lipid peroxidation and formation of new reactive oxygen species.49,50 In our study, we observed greater oxidative damage in the group submitted to mechanical ventilation only. Previous studies have shown that mechanical ventilation with high tidal volumes and for periods ranging from 2 to 4 h promotes increased levels of malondialdehyde.49–51 Our results differ from the literature since we demonstrate that the use of physiological tidal volume for a short period of time promotes increased production of reactive species and consequently increased oxidation of membrane lipids. In addition, our results show that sigh, at both frequencies, promoted less oxidative damage when compared to the group submitted to mechanical ventilation without the use of lung inflation maneuver. The sigh acted by minimizing the recruitment of inflammatory cells and possibly in these groups there was less damage compared to ventilation without the use of lung inflation maneuver.
In order to balance the reactive oxygen species produced during mechanical ventilation, the body has an antioxidant defense system that is carried out by different pathways. One of them is the elimination of reactive oxygen species through the action of antioxidant enzymes including superoxide dismutase, catalase, and glutathione peroxidase.50,52 Our results demonstrate that mechanical ventilation promoted increased activity of antioxidant enzymes superoxide dismutase and catalase. These findings differ from Chen et al. and Marín-Corral et al. who observed a decrease in the activity of antioxidant enzymes in the lungs of the rat.49,53 Cells subjected to oxidative stress may increase the synthesis of antioxidant enzymes, but in situations in which the oxidative process is more intense, endogenous enzymes cannot remove the reactive species formed and this generates disturbances in oxidant and antioxidant system, which impairs cellular metabolism regulation and promotes oxidation of biomolecules.49 Possibly, our results differ from those already published because in our study we promote an acute lung injury, and therefore there is an increase in the activity of enzymes in order to remove reactive oxygen species produced during ventilation. However, studies prior to ours used high tidal volumes, which generated intense oxidative stress and thus depletion of the enzymes involved in antioxidant defense.49,53 Furthermore, the results demonstrate that, regardless of frequency, sigh protected the lungs, since the activity of antioxidant enzymes is lower than in the group subjected to ventilation without sigh breath. The mechanisms by which sigh protects the lung are unclear, but we believe that by presenting fewer inflammatory cells in the airspace the production of reactive species is lower in animals ventilated with sigh and therefore there is no change in the antioxidant system enzyme level.
Mechanical ventilation with positive pressure leads to abnormal mechanical forces in the lungs. These mechanical forces are recognized and translated into biological signals by the lung cells, and are associated with induction of pro-inflammatory genes, release of cytokines that question the inflammatory response, changes in the vascular endothelium, including the expression of adhesion molecules, increased vascular permeability, and activation of inflammatory cells.54,55
We observed higher levels of IL-1 and TNF in the lungs of animals submitted to mechanical ventilation. Timmermans et al.56 used wild-type mice and IL-1 knockout animals; consequently, higher concentrations of IL-1β and neutrophil influx were observed in the lungs of wild-type ventilated animals, which was not observed for knockout animals. Wu et al.57 observed higher TNF expression in the lung of ventilated rats with high tidal volumes. Truflandier et al.58 observed higher TNF-α expression in the spinal cord of healthy rats submitted to mechanical ventilation for long periods.
In addition, we observed the highest IL-6 concentration in the ventilated animals without sigh. IL-6 secretion was stimulated by cell exposure to TNF and IL-1; however, the precise role of interleukin in ventilator-induced lung injury is still unclear.59,60 Ko et al. observed that mice ventilated with high tidal volume; there was an increase of IL-6 concentration in lung and bronchoalveolar lavage.60 Our results differ from the literature since we showed that in animals with healthy lungs and ventilated for a short period with physiological tidal volume higher IL-6 concentration in the lung. Although, in this study, it is not possible to elucidate the mechanisms by which ventilation without the use of sigh led to an increase in IL-1, TNF, and IL-6 according to the data already described in the literature, it is possible that in this experimental model, mechanical ventilation promoted cell damage and activation of pro-inflammatory genes and consequent increase in local production of inflammatory markers.54
Experimental groups ventilated with the use of sigh at both frequencies had lower concentrations of IL-1, IL-6, and TNF in the lung compared to the group submitted to mechanical ventilation only. Moraes et al. in an experimental model of acute pulmonary injury of pulmonary origin observed lower expression of IL-1 and IL-6 in animals controlled in the pressure-controlled mode associated with the use of sigh at a rate of 10 sighs/h.18 In our study, we evaluated the effects of sigh in the lungs of healthy animals; our results are unprecedented, since previous studies used sigh in models of lung injury. Possibly in this experimental model, the sigh acted to minimize cell damage and the recruitment of inflammatory cells, which promoted less production and release of cytokines.
We also evaluated the pulmonary parenchyma in order to evaluate changes in pulmonary histoarchitecture. According to Weibel, the stereological analysis of the lungs leads to discoveries about the whole pulmonary architecture and its structures.61,62 Lipid and protein oxidation, influx of inflammatory cells are involved in pulmonary parenchyma remodeling.22 In our study, although we observed influx of inflammatory cells, lipid oxidation and increased activity of antioxidant enzymes in the group submitted to mechanical ventilation only, and no changes in pulmonary histoarchitecture were observed. This can be explained by the short time of mechanical ventilation and the ventilatory strategies adopted in the study, and this short-term model of mechanical ventilation in healthy animals was chosen to answer a specific research question, which was to investigate the effects of sigh on status redox and pulmonary inflammatory response in healthy adult Wistar rats submitted to mechanical ventilation, and the initial goals were achieved, but all this needs to be acknowledged and could be addressed in future research.
The findings of this study have to be seen in light of some limitations. The first one, we showed the inflammatory and oxidative effects by mechanical ventilation and the protective effect of sigh. However, a more detailed mechanistic study may explain these results better. The second limitation was concerned with the importance to measure the respiratory mechanics of the animals studied to show the effect of sigh as inducing alveolar recruitment, such an investigation was outside the scope of the current study. The third limitation was that arterial blood gases were perfomed only at the end of the ventilation because of our methodology used; therefore with the data and samples collected, we were able to demonstrate only the hyperventilation caused during mechanical ventilation and the respiratory rate adjustment was performed based on previous studies carried out by our research group, that together also limits the analysis of our data.
In conclusion, this study showed that mechanical ventilation for a short period of time and the use of physiological tidal volume promoted acute inflammatory response and pulmonary oxidative stress. In addition, we demonstrated that sigh maneuver played a protective role in the lungs of animals during mechanical ventilation.
Authors’ contributions
ACLS, NAM and FSB conceived and designed research; ACLS, NAM, ABFS, TFC, LSC, MAGSO and GPC performed experiments; ACLS, NAM, SDC and FSB analyzed data; ACLS, NAM, ABFS and FSB interpreted results of experiments; ACLS, NAM and ABFS prepared tables; AT contributed equipment and laboratory; ACLS, NAM and ABFS drafted manuscript; ACLS, NAM, ABFS and FSB edited and revised manuscript; All approved final version of manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by: Coordenação de Aperfeiçoamento Pessoal de Ensino Superior (CAPES) (PNPD/CAPES-Process # 88882.316933/2019–01 and CAPES-PVEX-Process # 88881.172437/2018–01) at the Federal University of Ouro Preto (UFOP), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and it was supported in part by funds from the Nova Biomedical Corporation (Waltham, MA). FSB and AT are in credit with the CNPq for the fellowship of research productivity.
ORCID iDs
Sílvia Dantas Cangussú https://orcid.org/0000-0002-1061-6445
Frank Silva Bezerra https://orcid.org/0000-0002-0804-5196
References
- 1.Ahmed S, Daniel Martin A, Smith BK. Inspiratory muscle training in patients with prolonged mechanical ventilation: narrative review. Cardiopulm Phys Ther J 2019; 30:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powers SK, Wiggs MP, Sollanek KJ, Smuder AJ. Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol 2013; 305:R464–77 [DOI] [PubMed] [Google Scholar]
- 3.Goligher EC, Dres M, Fan E, Rubenfeld GD, Scales DC, Herridge MS, Vorona S, Sklar MC, Rittayamai N, Lanys A, Murray A, Brace D, Urrea C, Reid WD, Tomlinson G, Slutsky AS, Kavanagh BP, Brochard LJ, Ferguson ND. Mechanical ventilation-induced diaphragm atrophy strongly impacts clinical outcomes. Am J Respir Crit Care Med 2018; 197:204–13 [DOI] [PubMed] [Google Scholar]
- 4.Ball L, Dameri M, Pelosi P. Modes of mechanical ventilation for the operating room. Best Pract Res Clin Anaesthesiol 2015; 29:285–99 [DOI] [PubMed] [Google Scholar]
- 5.Howe KP, Clochesy JM, Goldstein LS, Owen H. Mechanical ventilation antioxidant trial. Am J Crit Care 2015; 24:440–5 [DOI] [PubMed] [Google Scholar]
- 6.Nickles HT, Sumkauskaite M, Wang X, Wegner I, Puderbach M, Kuebler WM. Mechanical ventilation causes airway distension with proinflammatory sequelae in mice. Am J Physiol Lung Cell Mol Physiol 2014; 307:L27–37 [DOI] [PubMed] [Google Scholar]
- 7.Faller S, Seiler R, Donus R, Engelstaedter H, Hoetzel A, Spassov SG. Pre- and posttreatment with hydrogen sulfide prevents ventilator-induced lung injury by limiting inflammation and oxidation. PLoS One 2017; 12:e0176649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen L, Xia HF, Shang Y, Yao SL. Molecular mechanisms of ventilator-induced lung injury. Chin Med J 2018; 131:1225–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holguin F. Oxidative stress in airway diseases. Ann Am Thorac Soc 2013; 10(Suppl):S150–7 [DOI] [PubMed] [Google Scholar]
- 10.Lushchak VI. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem Biol Interact 2014; 224:164–75 [DOI] [PubMed] [Google Scholar]
- 11.Rocco PR, Pelosi P, de Abreu MG. Pros and cons of recruitment maneuvers in acute lung injury and acute respiratory distress syndrome. Expert Rev Respir Med 2010; 4:479–89 [DOI] [PubMed] [Google Scholar]
- 12.Santos RS, Silva PL, Pelosi P, Rocco PR. Recruitment maneuvers in acute respiratory distress syndrome: the safe way is the best way. World J Crit Care Med 2015; 4:278–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantin JM, Godet T, Jabaudon M, Bazin JE, Futier E. Recruitment maneuvers in acute respiratory distress syndrome. Ann Transl Med 2017; 5:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vlemincx E, Meulders M, Abelson JL. Sigh rate during emotional transitions: more evidence for a sigh of relief. Biol Psychol 2017; 125:163–72 [DOI] [PubMed] [Google Scholar]
- 15.Vlemincx E, Meulders M, Luminet O. A sigh of relief or a sigh of expected relief: sigh rate in response to dyspnea relief. Psychophysiology 2018; 55. DOI: 10.1111/psyp.12979 [DOI] [PubMed] [Google Scholar]
- 16.Hess DR, Bigatello LM. Lung recruitment: the role of recruitment maneuvers. Respir Care 2002; 47:17; discussion 317–8 [PubMed] [Google Scholar]
- 17.Pelosi P, Bottino N, Panigada M, Eccher G, Gattinoni L. [ The sigh in ARDS (acute respiratory distress syndrome).]. Minerva Anestesiol 1999; 65:313–7 [PubMed] [Google Scholar]
- 18.Moraes L, Santos CL, Santos RS, Cruz FF, Saddy F, Morales MM, Capelozzi VL, Silva PL, de Abreu MG, Garcia CS, Pelosi P, Rocco PR. Effects of sigh during pressure control and pressure support ventilation in pulmonary and extrapulmonary mild acute lung injury. Crit Care 2014; 18:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andrade MC, Souza ABF, Horta JG, P, Costa G, Talvani A, Cangussu SD, Menezes RCA, Bezerra FS. Applying positive End-Expiratory pressure during mechanical ventilation causes pulmonary redox imbalance and inflammation in rats. Shock 2018; 50:572–8 [DOI] [PubMed] [Google Scholar]
- 20.Murta GL, Campos KK, Bandeira AC, Diniz MF, Costa Gde P, Costa DC, Talvani A, Lima WG, Bezerra FS. Oxidative effects on lung inflammatory response in rats exposed to different concentrations of formaldehyde. Environ Pollut 2016; 211:206–13 [DOI] [PubMed] [Google Scholar]
- 21.Chirico MTT, Bezerra FS, Guedes MR, Souza AB, Silva FC, Campos G, de Noronha SR, Mesquita LBT, Reis TO, Cangussu SD, Chianca DA, Jr., de Menezes RC. Tobacco-Free cigarette smoke exposure induces anxiety and panic-related behaviours in male wistar rats. Sci Rep 2018; 8:4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Souza ABF, Chirico MTT, Cartelle CT, de Paula Costa G, Talvani A, Cangussu SD, de Menezes RCA, Bezerra FS. High-fat diet increases HMGB1 expression and promotes lung inflammation in mice subjected to mechanical ventilation. Oxid Med Cell Longev 2018; 2018:7457054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campos KKD, de Oliveira Ramos C, Martins TL, Costa GP, Talvani A, Garcia CCM, Oliveira LAM, Cangussu SD, Costa DC, Bezerra FS. Lycopene mitigates pulmonary emphysema induced by cigarette smoke in a murine model. J Nutr Biochem 2018; 65:93–100 [DOI] [PubMed] [Google Scholar]
- 24.Ramos CO, Campos KKD, Costa GP, Cangussú SD, Talvani A, Bezerra SF. Taurine treatment decreases inflammation and oxidative stress in lungs of adult mice exposed to cigarette smoke. Regul Toxicol Pharmacol 2018; 98:50–7 [DOI] [PubMed] [Google Scholar]
- 25.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem 1974; 47:469–74 [DOI] [PubMed] [Google Scholar]
- 26.Aebi H. Catalase in vitro. Meth Enzymol 1984; 105:121–6 [DOI] [PubMed] [Google Scholar]
- 27.Griffith OW. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 1980; 106:207–12 [DOI] [PubMed] [Google Scholar]
- 28.Buege JA, Aust SD. Microsomal lipid peroxidation. Meth Enzymol 1978; 52:302–10 [DOI] [PubMed] [Google Scholar]
- 29.Reznick AZ, Packer L. Oxidative damage to proteins: spectrophotometric method for carbonyl assay. Meth Enzymol 1994; 233:357–63 [DOI] [PubMed] [Google Scholar]
- 30.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976; 72:248–54 [DOI] [PubMed] [Google Scholar]
- 31.Mandarim-de-Lacerda CA. Stereological tools in biomedical research. An Acad Bras Cienc 2003; 75:469–86 [DOI] [PubMed] [Google Scholar]
- 32.Froes SDP, Souza ABF, Matos NA, Philips NE, Costa GP, Talvani A, Cangussu SD, Bezerra FS. Intranasal instillation of distilled water, hypertonic saline and sodium bicarbonate promotes redox imbalance and acute lung inflammation in adult mice. Respir Physiol Neurobiol 2019; 266:27–32 [DOI] [PubMed] [Google Scholar]
- 33.Keszler M. Mechanical ventilation strategies. Semin Fetal Neonatal Med 2017; 22:267–74 [DOI] [PubMed] [Google Scholar]
- 34.Pham T, Brochard LJ, Slutsky AS. Mechanical ventilation: state of the art. Mayo Clin Proc 2017; 92:1382–400 [DOI] [PubMed] [Google Scholar]
- 35.Walesa M, Bayat S, Albu G, Baudat A, Petak F, Habre W. Comparison between neurally-assisted, controlled, and physiologically variable ventilation in healthy rabbits. Br J Anaesth 2018; 121:918–27 [DOI] [PubMed] [Google Scholar]
- 36.Sahetya SK, Brower RG. Lung recruitment and titrated PEEP in moderate to severe ARDS: is the door closing on the open lung? JAMA 2017; 318:1327–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bendixen HH, Hedley-Whyte J, Laver MB. Impaired oxygenation in surgical patients during general anesthesia with controlled ventilation. A concept of atelectasis. N Engl J Med 1963; 269:991–6 [DOI] [PubMed] [Google Scholar]
- 38.Fodor GH, Bayat S, Albu G, Lin N, Baudat A, Danis J, Peták F, Habre W. Variable ventilation is equally effective as conventional pressure control ventilation for optimizing lung function in a rabbit model of ARDS. Front Physiol 2019; 10:803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsuno K, Prato P, Kolobow T. Acute lung injury from mechanical ventilation at moderately high airway pressures. J Appl Physiol 1990; 69:956–61 [DOI] [PubMed] [Google Scholar]
- 40.Egbert LD, Laver MB, Bendixen HH. Intermittent deep breaths and compliance during anesthesia in man. Anesthesiology 1963; 24:57–60 [Google Scholar]
- 41.Gharsalli H, Mlika M, Sahnoun I, Maalej S, Douik El Gharbi L, Mezni FE. The utility of bronchoalveolar lavage in the evaluation of interstitial lung diseases: a clinicopathological perspective. Semin Diagn Pathol 2018; 35:280–7 [DOI] [PubMed] [Google Scholar]
- 42.Tsangaris I, Lekka ME, Kitsiouli E, Constantopoulos S, Nakos G. Bronchoalveolar lavage alterations during prolonged ventilation of patients without acute lung injury. Eur Respir J 2003; 21:495–501 [DOI] [PubMed] [Google Scholar]
- 43.Yildiz C, Palaniyar N, Otulakowski G, Khan MA, Post M, Kuebler WM, Tanswell K, Belcastro R, Masood A, Engelberts D, Kavanagh BP. Mechanical ventilation induces neutrophil extracellular trap formation. Anesthesiology 2015; 122:864–75 [DOI] [PubMed] [Google Scholar]
- 44.McCutcheon FH. Atmospheric respiration and the complex cycles in mammalian breathing mechanisms. J Cell Comp Physiol 1953; 41:291–303 [DOI] [PubMed] [Google Scholar]
- 45.Haldane JS, Meakins JC, Priestley JG. The effects of shallow breathing. J Physiol 1919; 52:433–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li P, Janczewski WA, Yackle K, Kam K, Pagliardini S, Krasnow MA, Feldman JL. The peptidergic control circuit for sighing. Nature 2016; 530:293–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mauri T, Eronia N, Abbruzzese C, Marcolin R, Coppadoro A, Spadaro S, Patroniti N, Bellani G, Pesenti A. Effects of sigh on regional lung strain and ventilation heterogeneity in acute respiratory failure patients undergoing assisted mechanical ventilation. Crit Care Med 2015; 43:1823–31 [DOI] [PubMed] [Google Scholar]
- 48.Wagner J, Strosing KM, Spassov SG, Lin Z, Engelstaedter H, Tacke S, Hoetzel A, Faller S. Sevoflurane posttreatment prevents oxidative and inflammatory injury in ventilator-induced lung injury. PLoS One 2018; 13:e0192896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen J, Lin J, Luo H, Li M. Effects of human interleukin-10 on Ventilator-Associated lung injury in rats. Inflammation 2019; 42:538–47 [DOI] [PubMed] [Google Scholar]
- 50.Wang X, An X, Wang X, Bao C, Li J, Yang D, Bai C. Curcumin ameliorated ventilator-induced lung injury in rats. Biomed Pharmacother 2018; 98:754–61 [DOI] [PubMed] [Google Scholar]
- 51.Huang CS, Kawamura T, Lee S, Tochigi N, Shigemura N, Buchholz BM, Kloke JD, Billiar TR, Toyoda Y, Nakao A. Hydrogen inhalation ameliorates ventilator-induced lung injury. Crit Care 2010; 14:R234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008; 4:89–96 [PMC free article] [PubMed] [Google Scholar]
- 53.Marin-Corral J, Martinez-Caro L, Lorente JA, de Paula M, Pijuan L, Nin N, Gea J, Andres E, Barreiro E. Redox balance and cellular inflammation in the diaphragm, limb muscles, and lungs of mechanically ventilated rats. Anesthesiology 2010; 112:384–94 [DOI] [PubMed] [Google Scholar]
- 54.Uhlig U, Uhlig S. Ventilation-induced lung injury. Compr Physiol 2011; 1:635–61 [DOI] [PubMed] [Google Scholar]
- 55.Spieth PM, Bluth T, Gama De Abreu M, Bacelis A, Goetz AE, Kiefmann R. Mechanotransduction in the lungs. Minerva Anestesiol 2014; 80:933–41 [PubMed] [Google Scholar]
- 56.Timmermans K, van der Wal SE, Vaneker M, van der Laak JA, Netea MG, Pickkers P, Scheffer GJ, Joosten LA, Kox M. IL-1beta processing in mechanical ventilation-induced inflammation is dependent on neutrophil factors rather than caspase-1. Intensive Care Med Exp 2013; 1:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wu NC, Liao FT, Cheng HM, Sung SH, Yang YC, Wang JJ. Intravenous superoxide dismutase as a protective agent to prevent impairment of lung function induced by high tidal volume ventilation. BMC Pulm Med 2017; 17:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truflandier K, Beaumont E, Charbonney E, Maghni K, de Marchie M, Spahija J. Mechanical ventilation modulates pro-inflammatory cytokine expression in spinal cord tissue after injury in rats. Neurosci Lett 2018; 671:13–8 [DOI] [PubMed] [Google Scholar]
- 59.Ghandadi M, Sahebkar A. Curcumin: an effective inhibitor of interleukin-6. Curr Pharm Des 2017; 23:921–31 [DOI] [PubMed] [Google Scholar]
- 60.Ko YA, Yang MC, Huang HT, Hsu CM, Chen LW. NF-kappaB activation in myeloid cells mediates ventilator-induced lung injury. Respir Res 2013; 14:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol 2007; 102:459–67 [DOI] [PubMed] [Google Scholar]
- 62.Weibel ER. Why measure lung structure? Am J Respir Crit Care Med 2001; 163:314–5 [DOI] [PubMed] [Google Scholar]