Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a beta coronavirus that causes infectious respiratory disease, named as coronavirus disease of 2019 (COVID-19). While extensive studies have provided basic information on clinical characteristics of COVID-19, the disease pathology is not fully known. The SARS-CoV-2 virus structural studies and biochemical experiments have also indicated that the virus receptor-binding domain (RBD) binds with a high affinity to angiotensin-converting enzyme-2 (ACE-2) receptor from humans; however, the mechanism remains unclear. Hereunder, a summary of relevant findings in the SARS-CoV-2 virus pathology and major pathogenicity mechanisms are discussed. This review of studies provides additional enlightenments on the way forward to prevent further spread or even cure for the SARS-CoV-2 virus-caused COVID-19 disease, either-or should a similar viral plague occur in the future.
Impact statement
The current survey of studies outlines the direct and indirect effects of SARS-CoV-2 on the specific body systems and summarizes the SARS-CoV-2 main pathogenicity mechanisms that require attention during patient hospitalization and for further research.
Keywords: SARS-CoV-2, Coronavirus, COVID-19, pathology, pathogenicity
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in Wuhan city of China’s Hubei Province in December 2019 and was then characterized as a pandemic in March 2020.1 The genomic characterization of SARS-CoV-2 shares 79.5% of the genetic sequence of the SARS-CoV coronavirus that caused the 2002–2003 epidemic.2,3 All coronaviruses that have caused diseases to humans (SARS-CoV, MERS-CoV, HKU1, HCoV-NL63, HCoV-OC43, and HCoV-229E) have had spillover from animals4; however, there is no evidence to support such a zoonotic theory for the origin of SARS-CoV-2 to-date. Of note, in addition to these viruses, studies indicate that other several known coronaviruses are circulating in animals that have not yet spillover to infect humans.5,6
These coronaviruses, named for the crown-like protein spikes on their surface, belong to a family of positive-sense, single-stranded RNA viruses called Coronaviridae.7,8 Coronaviridae are infectious viruses known to cause illnesses ranging from mild respiratory symptoms, even asymptomatic in immunocompetent cases, to more severe diseases such as the SARS-CoV-2 virus-caused 2019 novel coronavirus disease (abbreviated as COVID-19). The SARS-CoV-2 virus is an emerging respiratory beta coronavirus, like SARS-CoV (China, 2002) and MERS-CoV (Saudi Arabia, 2012).9 The SARS-CoV-2 virus-caused COVID-19 disease, with droplets and close contact as the main modes of transmission,10 is clinically characterized with the patient flu-like symptoms including fever, cough, and shortness of breath during the first 2 to 14 days of the viral incubation period.11 As the COVID-19 disease advances, hypoxic respiratory failure and even death are notable in older adults and people of any age who have severe underlying medical conditions such as chronic lung disease and diabetes.12–15 Noteworthy, some COVID-19 cases have also been observed to remain asymptomatic while they are likely contagious.16,17
Although extensive studies have provided basic information on clinical characteristics of COVID-19 disease, many relevant features of the pathogenicity mechanisms of the disease-causing SARS-CoV-2 virus are not fully known. This review of studies provides enlightenment on the SARS-CoV-2 virus pathology in reference to published literature findings on the effects of the virus in different human body systems. Moreover, this survey summarizes the SARS-CoV-2 virus major pathogenicity mechanisms that may play a role in the way forward to prevent further spread or even cure for the SARS-CoV-2 virus-caused COVID-19 disease, either-or should a similar viral plague occur in the future.
Pathology
The pathological lesions of SARS-CoV-2 in the body systems of COVID-19 cases are not fully known. The preliminary data acquired from the studies on clinical characteristics of COVID-19 suggest that the effect of the SARS-CoV-2 is not confined to the respiratory tract, rather may also invade multi-body systems that warrant a need for multidisciplinary attention on the way forward to compact the pandemic. This section of the study summarizes the human body system pathological features (Table 1) and results of examination procedures (Table 2) used to confirm the diagnosis for the SARS-CoV-2 virus-caused COVID-19 disease that have been reported incompletely.
Table 1.
Pathological features of the SARS-CoV-2 virus in the body systems of COVID-19 cases.
| Body system | Pathological features | References |
|---|---|---|
| Respiratory | A damage of pneumocytes with hyaline membrane formation, interstitial lymphocyte infiltration, and multinucleated syncytial cells in the lungs. | 18–20 |
| Cardiovascular | Myocardial and blood vessels injury. | 11,21–25 |
| Immune | Lymphopenia and exacerbated inflammation. | 12,24,26 |
| Digestive | Fecal sample positive for SARS-CoV-2 RNA. | 27–29 |
| Urinary | Proteinuria, elevated levels of serum creatinine and blood urea nitrogen, tubular necrosis, luminal brush border sloughing, and vacuole degeneration. | 30–33 |
| Reproductive | Decreased serum testosterone to luteinizing hormone ratio in male patients. | 34 |
| Skeletal | Joint problems such as arthritis. | 35 |
| Integumentary | Ischemic changes in the fingers and toes. | 36 |
| Nervous | Viral encephalitis, acute toxic encephalitis, and acute cerebrovascular disease. | 20,37–41 |
| Other | Abnormal levels of alanine aminotransferase and aspartate aminotransferase in serum bilirubin. | 12,25,42,43 |
Table 2.
Examination procedures used to confirm SARS-CoV-2 virus infection.
| Test | Positive test characteristics | References |
|---|---|---|
| RT-PCR | Detecting the SARS-CoV-2 virus unique sequences of RNA in sputum, throat swabs, and/or secretions of the lower respiratory tract samples. | 44,45 |
| Ab | Detecting IgM, IgA, IgG, or total antibodies against SARS-CoV-2 via serology. | 44,45 |
| ELISA | Detecting IgM and IgG antibodies based on recombinant SARS-CoV-2 nucleocapsid protein and spike protein via serology. | 46 |
| IHC | A damage of pneumocytes with hyaline membrane formation, interstitial lymphocyte infiltration, and multinucleated syncytial cells in the lungs. | 18,19 |
| CXR | Shows multiple small patchy shadows and interstitial changes in the lung periphery that gradually spread to the bronchi and then the whole lung with infrequent interlobar pleural thickening and pleural effusion. | 47 |
| CT Scan | Presentation of bilateral pulmonary parenchymal ground-glass opacity with pulmonary consolidation in a bilateral diffuse distribution, sometimes with a rounded morphology in a peripheral lung distribution. | 21,48,49 |
| FDG-PET Scan | Detect lung lesions characterized by increased FDG uptake in lymph node. | 50,51 |
RT-PCR: reverse transcriptase-polymerase chain reaction; Ab: antibody; ELISA: enzyme-linked immunosorbent assay; IHC: immunohistochemistry; CXR: chest X-ray; CT: computed tomography; FDG-PET: fluorodeoxyglucose-positron emission tomography.
Respiratory system
Several studies have reported that the SARS-CoV-2 virus infection causes a highly proportionate debilitation and pneumonia to those seen in SARS-CoV and MERS-CoV infections.52,53 This pathological lesion is particularly notable in older adults and people of any age with underlying medical conditions such as lung disease (asthma, chronic obstructive pulmonary disease, etc.). The COVID-19 clinical symptoms including fever, cough, and myalgias often resemble more of influenza than that of the common cold. Radiographic abnormalities on COVID-19 cases chest computed tomography (CT) scan are featured by bilateral pulmonary parenchymal ground-glass opacity with pulmonary consolidation in a bilateral diffuse distribution, sometimes with a rounded morphology in a peripheral lung distribution.54 Consistent with the pathology of SARS-CoV and MERS infections,52,53 histopathological studies in the lungs of patients who died from SARS-CoV-2 infection demonstrated damages of pneumocytes with hyaline membrane formation, interstitial lymphocyte infiltration, and multinucleated syncytial cells.18,19 Additionally, congestion of alveolar septal vessels with alveolar edema, infiltration of monocytes and lymphocytes in alveolar cavity and micro-thrombosis, alveolar exudate organization, pulmonary interstitial fibrosis, desquamation of mucosal epithelium, and mucus concealed bronchi have also been reported as prominent features of COVID-19.20,55 However, the pathogenicity mechanism of the SARS-CoV-2 to induce COVID-19 pneumonia and the pulmonary limitations in COVID-19 cases after their recovery from pneumonia remains to be determined.
Cardiovascular system
The SARS-CoV-2 infection-associated cardiovascular dysfunction in COVID-19 patients has been reported by several lines of clinical studies.11,21–25 The findings of these studies suggest the causality of the SARS-CoV-2 to the myocardium and blood vessels injury as the highest risk of mortality in COVID-19 patients, next to pulmonary injury. Analysis of SARS-CoV-2 infection-associated death cases, including an observational study of 52 COVID-19 patients whereby 19 (36.5%) of them showed acute myocardial injury,25 substantiate cardiovascular dysfunction as one of the major medical conditions in SARS-CoV-2 pathology. Moreover, other clinical studies have also reported coagulation aberrations in severe and critical COVID-19 patients11,21,24 that may lead to deep vein thrombosis and disseminated intravascular coagulation. However, whether the cardiac injury in COVID-19 patients is the direct or indirect pathogenic consequence of the SARS-CoV-2 infection remains largely unclear.
Immune system
In general, the entire human population remains susceptible to a novel virus and therefore lacks immunity to SARS-CoV-2. Only after the virus enters the host, the innate immune system of the host recognizes the virus through activation of pattern recognition receptors (PRRs) including the Toll-like receptor,56 nucleotide-binding oligomerization domain-like receptor,57 and retinoic acid-inducible gene-I-like receptor.58 The activation of the PRRs initiates a signaling cascade that induces the expression of type I interferons and other inflammatory cytokines to limit viral replication through different pathways. A delay in the expression of type I interferons has been reported to cause a loss to prevent viral replication and subsequently lead inflammatory cytokine storm in older adults and in aged experimental mouse models.59,60 Although yet unknown, it is plausible that SARS-CoV-2 follows similar escape mechanisms. Upon the virus entry, ordinarily, the host immune response recognizes and presents the virus to CD4+-T-helper cells, which subsequently stimulate the CD8+-T-killer cells and the B-cells to target any virally infected cells and to produce the virus-specific antibodies, respectively. However, it seems that the SARS-CoV-2 compromises the host immune system leading to lymphopenia, a significant reduction of lymphocytes, and exacerbated inflammation with a yet unknown etiology.
Digestive system
Gastrointestinal symptoms among COVID-19 patients has been reported by several cohort clinical studies.11,12,15,21,24,25,27,60–65 These studies detailed that COVID-19 gastrointestinal symptoms mainly include loss of appetite, nausea, vomiting, diarrhea, and abdominal discomfort. These gastrointestinal symptoms of COVID-19 were also common in SARS-CoV-166 and MERS-CoV67-infected patients but with more frequency than in SARS-CoV-2-infected COVID-19 patients, presumably due to the differences among the coronaviruses tropism to the gastrointestinal tract. Remarkably, a reverse transcriptase-polymerase chain reaction (RT-PCR) diagnostic test has revealed the presence of the SARS-CoV-2 RNA in the stool of confirmed COVID-19 patients,27–29 which sensitizes a new possibility of the fecal-oral route of SARS-CoV-2 transmission. Moreover, this presence of SARS-CoV-2 in the COVID-19 cases stool enlightens a need for considering rectal swab RT-PCR diagnostic test alongside the nasopharyngeal swab test to confirm the diagnosis of COVID-19 and before discharging hospitalized COVID-19 patients. There are several hypotheses on SARS-CoV-2 infection-induced gastrointestinal symptoms including the possible SARS-CoV-2 interaction with the angiotensin-converting enzyme-2 (ACE-2) receptor found in the human gastrointestinal tract (discussed in the next section), but the exact pathogenicity mechanism needs yet to be investigated.
Urinary system
Clinical studies of severe COVID-19 patients have shown evidence on the possible implications of SARS-CoV-2 infection that may induce urinary complications, including acute kidney impairment.11,21,24,30–33 These studies indicated that proteinuria, and elevated levels of serum creatinine and blood urea nitrogen, suggesting renal injury, have been observed in severe COVID-19 patients. Moreover, another report of histopathological examination on autopsy kidney from deceased COVID-19 patients33 determined acute renal tubular damage, including tubular necrosis, luminal brush border sloughing, and vacuole degeneration. Similar pathological features were also observed in autopsy kidney samples from SARS-CoV68 and MERS-CoV69-infected patients, suggesting that coronaviruses may have similar pathogenicity mechanisms. These pathological features provide insights that the urinary system is susceptible to damage by SARS-CoV-2 infection; however, the mechanisms remain unclear.
Reproductive system
Consistent with the findings of previous related studies,70,71 studies of COVID-19 patients on the vulnerability of human gonad to SARS-CoV-2 infection have determined the virus in the testis of male patients,34 but not in the females’ ovary or uterus.72 These studies suggest that the SARS-CoV-2 infection may cause impairment of sex hormones in males; however, the pathogenicity mechanism and also the virus contagions in the genital system remain unknown. Of note, an investigational study of women with severe COVID-19 illness to detect SARS-CoV-2 in their vaginal fluid reported negative results,72 suggesting low to no risk of the virus transmission to their sexual partner. Moreover, another published study has also reported that no SARS-CoV-2 was found in the amniotic fluid or umbilical cord blood of COVID-19 women patients and their neonatal throat swabs.73 Altogether, the studies suggest that the risk of SARS-CoV-2 transmission from mother to a newborn baby during pregnancy and childbirth is low.
Skeletal system
Skeletal joints problem such as arthritis associated with SARS-CoV-2 infection has been reported in severe COVID-19 patients.35 Of note, SARS-CoV-2 in people with rheumatoid arthritis (RA) has been observed to cause severe symptoms than in their counterparts who never experienced RA.35 The reason for the severity of the COVID-19 symptoms in people with RA is mostly because of their compromised immune system and thus increasing both the risk and the disease development further. However, whether the joint injury associated with the SARS-CoV-2 infection is caused by the direct effect of the virus or subjected to immune reaction is not yet known. Moreover, studies indicate that certain drugs such as corticosteroids used for the management of SARS-CoV-2-related infections, including SARS-CoV74 and MERS-CoV75 infection, have documented negative effects; therefore, drugs applied for the therapeutics of COVID-19 patients particularly those who have RA require extra caution.
Integumentary system
Specific integumentary change due to SARS-CoV-2 infection has not been reported aside from the intravascular coagulation-associated ischemic changes11,21,24 that may occur in the fingers and toes of severe COVID-19 patients.36 However, several skin injuries from long-term contact to personal protective equipment (PPE) and exaggerated personal hygiene (hand washing) have been mainly common among healthcare workers.76,77 These studies further underlined that prolonged use of PPE such as masks, gloves, and goggles, and the excessive hand washing may cause various degrees of cutaneous diseases, even dermatitis. Therefore, the commonly reported skin injuries among health professionals including burning, itching, and stinging are attributable to the prolonged use of PPE, not SARS-CoV-2 infection unless diagnosed. However, the potential effect of SARS-CoV-2 infection on the hydro-lipid mantle of the skin surface requires further elucidation.
Nervous system
Evidence on the neurological effects of SARS-CoV-2 is not yet studied appropriately. However, the neurological manifestations of neuroinvasion and neurotropism that have been reported as common characteristics for human coronaviruses78–80 may also be applicable for SARS-CoV-2. Reports on studies of SARS-CoV81,82 and MERS-CoV83 have shown a positive test of the SARS-CoV-1 in the SARS patients cerebrospinal fluid and an altered mental status in 26% of studied patients, respectively. Moreover, other studies using transgenic mice models, SARS-CoV84,85 or MERS-CoV,86 revealed that the virus could enter the brain through the olfactory nerves. Concerning SARS-CoV-2, a study on 214 patients with moderate to severe COVID-19 indicated that 36.4% of the patients revealed neuropathic-related clinical findings including headache, dizziness, ataxia, myopathy, acute cerebrovascular problems, depressed level of consciousness, and impairment of senses of smell and taste.87 Other studies on COVID-19 patients have also suggested possible pathological lesions of SARS-CoV-2 in the nervous system including viral encephalitis,40 acute toxic encephalitis,20,38 and acute cerebrovascular disease.37,39,41 Whether these neurological manifestations are subject to the direct effect of the SARS-CoV-2 virus infection or are consequences to COVID-19 disease is yet to be determined in future studies.
Other systems
Published data reporting clinical features of SARS-CoV-2 infected patients have delineated various degrees of liver injury during the course of the COVID-19 disease11,12,21,24,25,42,43,63,64,88 involving abnormal levels of alanine aminotransferase and aspartate aminotransferase alongside elevated serum bilirubin.12,25,42,43 Additionally, a histological study on a deceased COVID-19 patient, liver biopsy, reported microvesicular steatosis and lobular activity,70 which requires medical attention. The liver injury in COVID-19 patients is presumed to be caused by either SARS-CoV-2 direct infection of hepatocytes for the virus may directly bind to the angiotensin-converting enzyme-2 (ACE-2) receptor found in the cholangiocytes (discussed in the next section), or following to an immune-related inflammation, or drug-mediated hepatotoxicity. However, the pathogenicity mechanism of SARS-CoV-2 infection associated liver injury remains to be studied.
Pathogenicity
The to-date available reports describing clinical characteristics of COVID-19 disease have contributed to a better understanding of the disease pathology in the individual cases body systems. However, further study on the pathogenicity of the SARS-CoV-2 virus is required to obtain additional insights on the way forward to control COVID-19 pandemic or should a similar viral plague occur in the future. Pathogenicity characteristics of the SARS-CoV-2 virus (Figure 1) are highly complex, with multiple factors leading to severe injury in the respiratory tract and several other body systems (Figure 2).
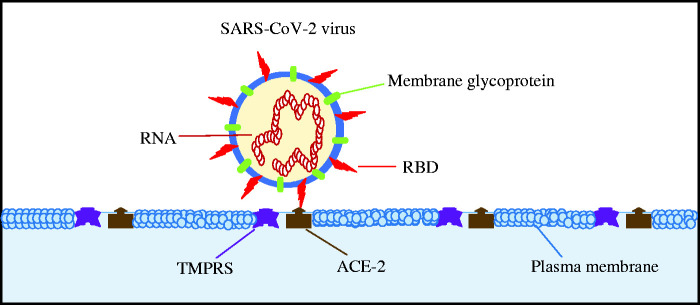
Figure 1.
Schematic representation of the SARS-CoV-2 virus pathogenicity characteristics. The entry of the virus into the target cell depends on the binding of the virus surface protein spikes, variable receptor-binding domain (RBD), to the angiotensin-converting enzyme-2 (ACE-2) receptor augmented by type 2 transmembrane serine protease (TMPRSS2). (A color version of this figure is available in the online journal.)
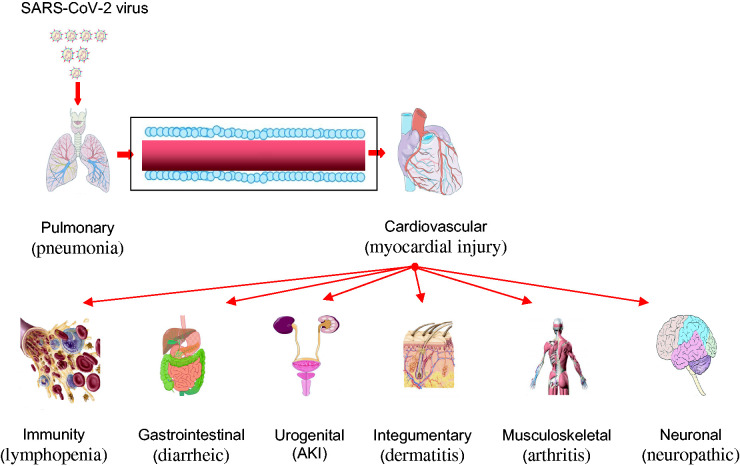
Figure 2.
Outline of the SARS-CoV-2 virus pathophysiology and pathogenicity. The virus has direct or indirect effects on multi-body systems that warrant a need for multidisciplinary attention during patient hospitalization and further research. The most common COVID-19 features to a body system include pneumonia (pulmonary), myocardial injury (cardiovascular), lymphopenia (immunity), diarrheic (gastrointestinal), acute kidney impairment, AKI, (urogenital), dermatitis (integumentary), arthritis (musculoskeletal), and neuropathic (neuronal). (A color version of this figure is available in the online journal.)
Coronaviruses are morphologically characterized by the crown-shaped protein spikes on their surface. According to structural studies89–91 and biochemical experiments,2,91,92 the SARS-CoV-2 virus surface protein spikes contain a variable receptor-binding domain (RBD) that allows the virus to bind with a high affinity to the angiotensin-converting enzyme-2 (ACE-2) receptor found in the human respiratory tract, lungs, gastrointestinal tract, kidneys, and the heart; however, the mechanism remains unclear. In addition to the ACE-2 receptor, the presence of type 2 transmembrane serine protease (TMPRSS2) in the target cells have been suggested to augment the SARS-CoV-2 viral entry as is the case in influenza and human metapneumovirus.93–96 Briefly, the TMPRSS2 proteolysis to the complex formed following the binding of the SARS-CoV-2 RBD with the host ACE-2 receptor, and thereby enhances cleavage of the host target cell receptor and activates the virus surface protein. It may be premature to reach into conclusion with the aforementioned concepts at this stage; however, it is worth noticing that the presence of both ACE-2 receptor and TMPRSS2 in the target cell determines the host susceptibility to the SARS-CoV-2 virus.
The SARS-CoV-2 virus entry into the target cell compromises the host immune system leading to lymphopenia, a significant reduction of lymphocytes and exacerbates inflammation as the COVID-19 disease advances12,24,26; however, the mechanism is not fully known. In brief, following the SARS-CoV-2 virus infection, mainly people in an immune-impaired state, such as the old aged and individuals of any age but who have severe underlying medical conditions, show significantly lower white blood cell count in their peripheral blood. While the T lymphocytes and B lymphocytes significantly reduce in the peripheral blood of the patient, the inflammatory factors such as IL-6 are significantly increased contributing to the aggravation of the COVID-19 disease. Ordinarily, in an infection process, the host immune response mediated by antigen-presenting cells (APCs) recognizes and presents the foreign antigen to CD4+-T-helper cells, which subsequently stimulate the CD8+-T-killer cells and the B-cells to target any that specific foreign antigen containing cell and to produce the antigen-specific antibodies, respectively. Interestingly, aside from the symptomatic COVID-19 cases, reports have indicated that there are some people with effective immune function, immunocompetent, capable of withstanding the SARS-CoV-2 virus infection and that even remain asymptomatic while they are likely contagious.16,17
Concluding remarks
In conclusion, there is no vaccine to prevent SARS-CoV-2 virus infection or specific medicine to treat COVID-19 disease to-date. The acquired basic information on clinical characteristics of COVID-19 has contributed to thus far adoption of symptomatic treatment and oxygen therapy to enhance the patient immune function. Worthy of note is that the SARS-CoV-2 virus virulence depends on the presence of ACE-2 receptor and TMPRSS2 in the host target cell. Advanced in vitro and in vivo experiments in the fields of pathogenicity of the SARS-CoV-2 virus is crucial to fully elucidate the pathogenesis of COVID-19 disease or should a similar viral plague occur in the future.
DECLARATION OF CONFLICTING INTERESTS
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Henok Kessete Afewerky https://orcid.org/0000-0001-7030-3224
References
- 1.World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19 – 11 March 2020.
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020; 579:270–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. SARS-CoV that caused the 2002. –2003 pandemic – 5 July 2003.
- 4.Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res 2018; 100:163–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ge X, Li Y, Yang X, Zhang H, Zhou P, Zhang Y, Shi Z. Metagenomic analysis of viruses from bat fecal samples reveals many novel viruses in insectivorous bats in China. J Virol 2012; 86:4620–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ge XY, Li JL, Yang XL, Chmura AA, Zhu G, Epstein JH, Mazet JK, Hu B, Zhang W, Peng C, Zhang YJ, Luo CM, Tan B, Wang N, Zhu Y, Crameri G, Zhang SY, Wang LF, Daszak P, Shi ZL. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature 2013; 503:535–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marra MA, Jones SJ, Astell CR, Holt RA, Brooks-Wilson A, Butterfield YS, Khattra J, Asano JK, Barber SA, Chan SY, Cloutier A, Coughlin SM, Freeman D, Girn N, Griffith OL, Leach SR, Mayo M, McDonald H, Montgomery SB, Pandoh PK, Petrescu AS, Robertson AG, Schein JE, Siddiqui A, Smailus DE, Stott JM, Yang GS, Plummer F, Andonov A, Artsob H, Bastien N, Bernard K, Booth TF, Bowness D, Czub M, Drebot M, Fernando L, Flick R, Garbutt M, Gray M, Grolla A, Jones S, Feldmann H, Meyers A, Kabani A, Li Y, Normand S, Stroher U, Tipples GA, Tyler S, Vogrig R, Ward D, Watson B, Brunham RC, Krajden M, Petric M, Skowronski DM, Upton C, Roper RL. The genome sequence of the SARS-associated coronavirus. Science 2003; 300:1399–404 [DOI] [PubMed] [Google Scholar]
- 8.International Committee on Taxonomy of Viruses. Naming the 2019 Coronavirus – 5 February 2020.
- 9.Yu F, Du L, Ojcius DM, Pan C, Jiang S. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect 2020; 22:74–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kakimoto K, Kamiya H, Yamagishi T, Matsui T, Suzuki M, Wakita T. Initial investigation of transmission of COVID-19 among crew members during quarantine of a cruise ship – Yokohama, Japan, February 2020. MMWR Morb Mortal Wkly Rep 2020; 69:312–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395:497–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, Liu L, Shan H, Lei CL, Hui DSC, Du B, Li LJ, Zeng G, Yuen KY, Chen RC, Tang CL, Wang T, Chen PY, Xiang J, Li SY, Wang JL, Liang ZJ, Peng YX, Wei L, Liu Y, Hu YH, Peng P, Wang JM, Liu JY, Chen Z, Li G, Zheng ZJ, Qiu SQ, Luo J, Ye CJ, Zhu SY, Zhong NS. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA 2020; [DOI] [PubMed] [Google Scholar]
- 14.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, JR, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: a systematic review and Meta-analysis. Int J Infect Dis 2020; 94:91–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bai Y, Yao L, Wei T, Tian F, Jin DY, Chen L, Wang M. Presumed asymptomatic carrier transmission of COVID-19. JAMA 2020; 323:1406–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, Zimmer T, Thiel V, Janke C, Guggemos W, Seilmaier M, Drosten C, Vollmar P, Zwirglmaier K, Zange S, Wolfel R, Hoelscher M. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020; 382:970–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikolich-Zugich J, Knox KS, Rios CT, Natt B, Bhattacharya D, Fain MJ. SARS-CoV-2 and COVID-19 in older adults: what we may expect regarding pathogenesis, immune responses, and outcomes. Geroscience 2020; 42:505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yi Y, Lagniton PNP, Ye S, Li E, Xu RH. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci 2020; 16:1753–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med 2020; 8:420–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020; 395:507–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Wang L, Yan S, Yang F, Xiang L, Zhu J, Shen B, Gong Z. Clinical characteristics of 25 death cases with COVID-19: a retrospective review of medical records in a single medical center, Wuhan, China. Int J Infect Dis 2020; 94:128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;e200950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel Coronavirus-infected pneumonia in Wuhan, China. Jama 2020; 323:1061–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suxin Wan QY, Fan S, Lv J, Zhang X, Guo L, Lang C, Xiao Q, Xiao K, Yi Z, Qiang M, Xiang J, Zhang B, Chen Y. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv 2020;
- 27.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology 2020; 158:1831–1833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK. Washington state-nCo VCIT. First case of 2019 novel coronavirus in the United States. N Engl J Med 2020; 382:929–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Guo C, Tang L, Hong Z, Zhou J, Dong X, Yin H, Xiao Q, Tang Y, Qu X, Kuang L, Fang X, Mishra N, Lu J, Shan H, Jiang G, Huang X. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5:434–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, Li J, Yao Y, Ge S, Xu G. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020; 97:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lushina N, Kuo JS, Shaikh HA. Pulmonary, cerebral, and renal thromboembolic disease associated with COVID-19 infection. Radiology 2020;201623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu L, Xu X, Ma K, Yang J, Guan H, Chen S, Chen Z, Chen G. Successful recovery of COVID-19 pneumonia in a renal transplant recipient with long-term immunosuppression. Am J Transplant 2020; 20:1859–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua Su MY, Wan C, Yi L-X, Tang F, Zhu H-Y, Yi F, Yang H-C, Fogo AB, Nie X, Zhang C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; https://doiorg/101016/jkint202004003 [DOI] [PMC free article] [PubMed]
- 34.Ling Ma WX, Li D, Shi L, Mao Y, Xiong Y, Zhang Y, Zhang M. Effect of SARS-CoV-2 infection upon male gonadal function: a single centerbased study. medRxiv https://doiorg/101101/2020032120037267 2020;
- 35.Favalli EG, Ingegnoli F, De Lucia O, Cincinelli G, Cimaz R, Caporali R. COVID-19 infection and rheumatoid arthritis: faraway, so close! Autoimmun Rev 2020; 19:102523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin L, Lu L, Cao W, Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection – a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 2020; 9:727–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C, Zhang XR, Ju ZY, He WF. Advances in the research of cytokine storm mechanism induced by corona virus disease 2019 and the corresponding immunotherapies. Zhonghua Shao Shang Za Zhi 2020; 36:E005. [DOI] [PubMed] [Google Scholar]
- 38.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak – an update on the status. Mil Med Res 2020; 7:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. Hlh across speciality collaboration UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moriguchi T, Harii N, Goto J, Harada D, Sugawara H, Takamino J, Ueno M, Sakata H, Kondo K, Myose N, Nakao A, Takeda M, Haro H, Inoue O, Suzuki-Inoue K, Kubokawa K, Ogihara S, Sasaki T, Kinouchi H, Kojin H, Ito M, Onishi H, Shimizu T, Sasaki Y, Enomoto N, Ishihara H, Furuya S, Yamamoto T, Shimada S. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis 2020; 94:55–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, Wang Y, Chen Y, Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID-19) implicate special control measures. J Med Virol 2020; 92:568–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fan Z, Chen L, Li J, Cheng X, Jingmao Y, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical features of COVID-19-related liver damage. Clin Gastroenterol Hepatol 2020; 18:1561–1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao D, Yao F, Wang L, Zheng L, Gao Y, Ye J, Guo F, Zhao H, Gao R. A comparative study on the clinical features of COVID-19 pneumonia to other pneumonias. Clin Infect Dis 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel R, Babady E, Theel ES, Storch GA, Pinsky BA, St George K, Smith TC, Bertuzzi S. Report from the American society for microbiology COVID-19 international summit. Value of diagnostic testing for SARS-CoV-2/COVID-19. mBio 2020; 11:e00722-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases – 19 March 2020. 2020;WHO/COVID-19/laboratory/2020.5
- 46.Liu W, Liu L, Kou G, Zheng Y, Ding Y, Ni W, Wang Q, Tan L, Wu W, Tang S, Xiong Z, Zheng S. Evaluation of nucleocapsid and spike protein-based ELISAs for detecting antibodies against SARS-CoV-2. J Clin Microbiol 2020; 58:e00461–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents 2020; 55:105951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology 2020; 295:202–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi H, Han X, Zheng C. Evolution of CT manifestations in a patient recovered from 2019 novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. Radiology 2020; 295:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin C, Liu F, Yen TC, Lan X. ( 18)F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging 2020; 47:1281–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, Chen B, Zhang Z, Guan W, Ling Z, Jiang R, Hu T, Ding Y, Lin L, Gan Q, Luo L, Tang X, Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging 2020; 47:1275–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, Cai J, Li X, Kang W, Weng D, Lu Y, Wu D, He L, Yao K. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 2003; 200:282–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, Mutei MA, Abdel-Wareth L, Uyeki TM, Swerdlow DL, Barakat M, Zaki SR. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle east respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol 2016; 186:652–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zu ZY, Jiang MD, Xu PP, Chen W, Ni QQ, Lu GM, Zhang LJ. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology 2020: 200490 [DOI] [PMC free article] [PubMed]
- 55.Luo WY, H, Gou J, Li X, Sun Y, Li J, Liu L. Clinical pathology of critical patient with novel coronavirus pneumonia (COVID-19). Preprints 2020, 2020020407 2020;
- 56.Sheahan T, Morrison TE, Funkhouser W, Uematsu S, Akira S, Baric RS, Heise MT. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog 2008; 4:e1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ben Addi A, Lefort A, Hua X, Libert F, Communi D, Ledent C, Macours P, Tilley SL, Boeynaems JM, Robaye B. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A(2B) receptor. Eur J Immunol 2008; 38:1610–20 [DOI] [PubMed] [Google Scholar]
- 58.Zust R, Cervantes-Barragan L, Habjan M, Maier R, Neuman BW, Ziebuhr J, Szretter KJ, Baker SC, Barchet W, Diamond MS, Siddell SG, Ludewig B, Thiel V. Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol 2011; 12:137–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Huang KJ, Su IJ, Theron M, Wu YC, Lai SK, Liu CC, Lei HY. An interferon-gamma-related cytokine storm in SARS patients. J Med Virol 2005; 75:185–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rockx B, Baas T, Zornetzer GA, Haagmans B, Sheahan T, Frieman M, Dyer MD, Teal TH, Proll S, van den Brand J, Baric R, Katze MG. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J Virol 2009; 83:7062–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu K, Fang YY, Deng Y, Liu W, Wang MF, Ma JP, Xiao W, Wang YN, Zhong MH, Li CH, Li GC, Liu HG. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei province. Chin Med J (Engl) 2020; 133:1025–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, Zhang W, Wang Y, Bao S, Li Y, Wu C, Liu H, Liu D, Shao J, Peng X, Yang Y, Liu Z, Xiang Y, Zhang F, Silva RM, Pinkerton KE, Shen K, Xiao H, Xu S, Wong GWK. Chinese pediatric novel coronavirus study T. SARS-CoV-2 infection in children. N Engl J Med 2020; 382:1663–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis 2020; 20:425–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, Li SB, Wang HY, Zhang S, Gao HN, Sheng JF, Cai HL, Qiu YQ, Li LJ. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020; 368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang JJ, Dong X, Cao YY, Yuan YD, Yang YB, Yan YQ, Akdis CA, Gao YD. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020; [DOI] [PubMed] [Google Scholar]
- 66.Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY; Group HUSS. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet 2003; 361:1767–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chan JF, Lau SK, To KK, Cheng VC, Woo PC, Yuen KY. Middle east respiratory syndrome coronavirus: another zoonotic betacoronavirus causing SARS-like disease. Clin Microbiol Rev 2015; 28:465–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, Fung KS, Tang HL, Yan WW, Chan HW, Lai TS, Tong KL, Lai KN. Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 2005; 67:698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alsaad KO, Hajeer AH, Al Balwi M, Al Moaiqel M, Al Oudah N, Al Ajlan A, AlJohani S, Alsolamy S, Gmati GE, Balkhy H, Al-Jahdali HH, Baharoon SA, Arabi YM. Histopathology of Middle east respiratory syndrome coronovirus (MERS-CoV) infection – clinicopathological and ultrastructural study. Histopathology 2018; 72:516–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ding Y, He L, Zhang Q, Huang Z, Che X, Hou J, Wang H, Shen H, Qiu L, Li Z, Geng J, Cai J, Han H, Li X, Kang W, Weng D, Liang P, Jiang S. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J Pathol 2004; 203:622–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu J, Qi L, Chi X, Yang J, Wei X, Gong E, Peh S, Gu J. Orchitis: a complication of severe acute respiratory syndrome (SARS. ). Biol Reprod 2006; 74:410–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Qiu L, Liu X, Xiao M, Xie J, Cao W, Liu Z, Morse A, Xie Y, Li T, Zhu L. SARS-CoV-2 is not detectable in the vaginal fluid of women with severe COVID-19 infection. Clin Infect Dis 2020;ciaa375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen H, Guo J, Wang C, Luo F, Yu X, Zhang W, Li J, Zhao D, Xu D, Gong Q, Liao J, Yang H, Hou W, Zhang Y. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet 2020; 395:809–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med 2006; 3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, Jose J, Pinto R, Al-Omari A, Kharaba A, Almotairi A, Al Khatib K, Alraddadi B, Shalhoub S, Abdulmomen A, Qushmaq I, Mady A, Solaiman O, Al-Aithan AM, Al-Raddadi R, Ragab A, Balkhy HH, Al Harthy A, Deeb AM, Al Mutairi H, Al-Dawood A, Merson L, Hayden FG, Fowler RA. Saudi Critical care trial G. Corticosteroid therapy for critically ill patients with Middle East respiratory syndrome. Am J Respir Crit Care Med 2018; 197:757–67 [DOI] [PubMed] [Google Scholar]
- 76.Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol 2020; 82:1085–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yan Y, Chen H, Chen L, Cheng B, Diao P, Dong L, Gao X, Gu H, He L, Ji C, Lai JH, Lei W, Li T, Li L, Li L, Liu R, Liu D, Lu W, Shi Q, Song Y, Tao J, Wang J, Wang B, Wu G, Xiang Y, Xie L, Xu J, Yao J, Zhang Z, Zhang F, Zhong J, Li S, Li H. H. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease. Dermatol Ther 2019; 2020:e13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dube M, Talbot PJ. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the Central nervous system? Viruses 2019; 12:14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hwang CS. Olfactory neuropathy in severe acute respiratory syndrome: report of a case. Acta Neurol Taiwan 2006; 15:26–8 [PubMed] [Google Scholar]
- 80.Tsai LK, Hsieh ST, Chao CC, Chen YC, Lin YH, Chang SC, Chang YC. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol 2004; 61:1669–73 [DOI] [PubMed] [Google Scholar]
- 81.Hung EC, Chim SS, Chan PK, Tong YK, Ng EK, Chiu RW, Leung CB, Sung JJ, Tam JS, Lo YM. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem 2003; 49:2108–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible Central nervous system infection by SARS coronavirus. Emerging Infect Dis 2004; 10:342–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, Selim MA, Al Mutairi M, Al Nakhli D, Al Aidaroos AY, Al Sherbeeni N, Al-Khashan HI, Memish ZA, Albarrak AM. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis 2014; 29:301–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McCray PB, Jr, Pewe L, Wohlford-Lenane C, Hickey M, Manzel L, Shi L, Netland J, Jia HP, Halabi C, Sigmund CD, Meyerholz DK, Kirby P, Look DC, Perlman S. Lethal infection of K18-hACE2 mice infected with severe acute respiratory syndrome coronavirus. J Virol 2007; 81:813–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 2008; 82:7264–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li K, Wohlford-Lenane C, Perlman S, Zhao J, Jewell AK, Reznikov LR, Gibson-Corley KN, Meyerholz DK, McCray PB., Jr. Middle East respiratory syndrome coronavirus causes multiple organ damage and lethal disease in mice transgenic for human dipeptidyl peptidase 4. J Infect Dis 2016; 213:712–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77:683–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cai Q, Huang D, Ou P, Yu H, Zhu Z, Xia Z, Su Y, Ma Z, Zhang Y, Li Z, He Q, Liu L, Fu Y, Chen J. COVID-19 in a designated infectious diseases hospital outside Hubei province, China. Allergy 2020; 395:497–506 [DOI] [PubMed] [Google Scholar]
- 89.Walls AC, Park Yj Tortorici Ma Wall A, McGuire At, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020; 181:281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on Decade-Long structural studies of SARS coronavirus. J Virol 2020; 94:e00127–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, Graham BS, McLellan JS. Cryo-EM structure of the 2019-nCoV spike in the perfusion conformation. Science 2020; 367:1260–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Letko M, Marzi A, Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol 2020; 5:562–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glowacka I, Bertram S, Muller MA, Allen P, Soilleux E, Pfefferle S, Steffen I, Tsegaye TS, He Y, Gnirss K, Niemeyer D, Schneider H, Drosten C, Pohlmann S. Evidence that TMPRSS2 activates the severe acute respiratory syndrome coronavirus spike protein for membrane fusion and reduces viral control by the humoral immune response. J Virol 2011; 85:4122–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pohlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 2014; 88:1293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shulla A, Heald-Sargent T, Subramanya G, Zhao J, Perlman S, Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J Virol 2011; 85:873–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Peng Zhou X-LY, Wang X-G, Hu B, Zhang L, Zhang W, Si H-R, Zhu Y, Li B, Huang C-L, Chen H-D, Chen J, Luo Y, Guo Jiang R-D, Liu M-Q, Chen Y, Shen X-R, Wang X, Zheng X-S, Zhao K, Chen Q-J, Deng F, Liu L-L, Yan B, Zhan F-X, Wang Y-Y, Xiao G, Shi Z-L. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. bioRxiv 2020;