Abstract
Cardiovascular disease is the leading cause of mortality worldwide. Atherosclerosis constitutes most cardiovascular disease etiologies. Atherosclerosis is a chronic inflammatory and lipid-driven disease affecting the intima of blood vessels, resulting in an increase in its thickness and, therefore, narrowing of the arterial lumen. Many blood and immune cells have been shown to be implicated in atherosclerosis pathophysiology. Neutrophils are among those cells with their novel function of forming neutrophil extracellular traps. Neutrophil extracellular traps are mesh-like structures formed and released on activation of neutrophils. These structures consist of decondensed chromatin, histones, and other components, including nuclear and cellular proteins, cytoskeleton, proteases, and azurophilic granules. Neutrophil extracellular traps contain these elements and hold other circulating elements in the blood, such as tissue factor, fibrin, and other coagulation factors. Neutrophil extracellular traps are also implicated in the pathogenesis of atherothrombosis, which evolves as a consequence of atherosclerosis. In this review, we aim to demonstrate the process of neutrophil extracellular traps formation, release, and interaction with other blood cells, meaning it could be possible to use neutrophil extracellular traps as a therapeutic target in deceleration of atherosclerosis progression.
Impact statement
Fatal consequences of atherosclerosis and atherothrombosis give research in this field great importance. This review provides recent information about the implications of neutrophils in the pathophysiology of atherosclerosis and atherothrombosis via formation and release of neutrophil extracellular traps (NETs), thereby enhancing our understanding on how the process of atherosclerosis develops and how its consequences occur. Information provided in this review suggests NETs as a new therapeutic target and a rich point for research. This review gives answers to questions about the mechanisms of atherosclerosis and atherothrombosis progression through studying the implications of NETs in these processes.
Keywords: Atherosclerosis, pathophysiology, vascular, inflammation, cells, immunobiology
Introduction
Cardiovascular disease represents the leading cause of mortality and disability worldwide, especially in western countries.1 Etiologies of CVD include the following respectively: Coronary heart disease (CHD)—the major cause of death (42.6%); stroke (17.0%); high blood pressure (10.5%); Heart failure (9.4%); diseases of the arteries (2.9%); other CVD etiologies (17.6%).2 Atherosclerosis results directly in the above-mentioned CVD etiologies and is responsible for most cardiovascular events.3 It is a chronic inflammatory and lipid-driven disease that affects the intima of blood vessels resulting in increasing its thickness though plaque formation and development.4 Atherosclerosis is triggered and develops through endothelial damage, sub-endothelial accumulation of low-density lipoproteins and infiltration of different inflammatory cells, such as macrophages, dendritic cells, neutrophils, and T-lymphocytes. Recent studies show the presence of large amounts of neutrophils at the site of atherosclerotic plaques in coronary arteries resulting in its erosion, rupture, and intraplaque hemorrhage.3 While the role of these cells, which result in a chronic inflammation process, is well established, current studies are conducted to fully demonstrate the role of neutrophils and their extrusions, NETs, and the ability to use them as a promising biomarker and therapeutic target.5 NETs are web-like structures formed and released upon activation of neutrophils. They consist of decondensed chromatin, histones, and other components, such as nuclear and cellular proteins, cytoskeleton, proteases, and granzymes.6 NETs have been shown to be a part of innate immune response against bacterial, viral, and fungal infections. Moreover, NETs have been observed to be implicated in the etiology and progression of both atherosclerosis and atherothrombosis and their consequences.7 This review focuses on the role of NETs in atherogenesis and atherothrombosis and the possibility of using them as a therapeutic target through targeting their initiators, mediators, downstream events, and certain components in their structure in the prevention of the above diseases.
The pathophysiology of atherosclerosis and atherothrombosis
Increasing evidence has proved that atherosclerosis is an inflammatory disease and inflammation in atherosclerosis is not just an epiphenomenon.8 Recent studies suggested that immunity and infection are also implicated in the initiation and development of atherosclerosis and atherothrombosis.9 The hallmarks of atherosclerosis are the deposition of lipid in the arterial wall at certain areas, smooth muscle cells proliferation, abundant extracellular matrix and inflammatory cells recruitment resulting in atherosclerotic plaque formation. Endothelial cell injury is the first vascular event of atherogenesis on which the successive events of atherosclerotic lesion development are built.10 Endothelial activation and disturbance of endothelial normal functions, which follow endothelial injury, occur as an inflammatory response triggered by various stimuli, such as infection, abnormal lipid metabolism, low levels of nitric oxide, and flow disturbance. Reduction of nitric oxide, which is essential for normal vascular wall functions and maintenance of the quiescent state of vascular endothelium, produces a state of imbalance between the vascular vasodilators and vasoconstrictors resulting in production of ROS through the high activity of nitric oxide synthase leading to endothelial cell activation; this activation is considered reversible with a rapid withdrawal of the stimulus. However, persistence of stimuli produces a chronic state of endothelial activation in which endothelial cells express adhesion molecules, procoagulant molecules, and pro-inflammatory mediators.11 Repetitive cycles of endothelial injury and repair last for years stimulating the proliferation of vascular smooth muscle cells. Moreover, lipid and inflammatory cells accumulate, initiating atheromatous plaque formation that contains multiple layers of foam cells and lipid pools. These lesions are reversible at less susceptible areas in earlier stages; however, they progress rapidly to advanced types at more susceptible areas. Advanced types of plaque contain confluent extracellular lipid core and fibromuscular layers. Repeated cycles of plaque surface defects, hematoma, and thrombosis progressively cause lumen narrowing. Thereafter, plaques may show calcification and fibrous changes.11 Atherothrombosis is a sequela of atherosclerosis contributing to fatal vascular events. Two relatively distinct mechanisms explain the development of atherothrombosis as reported in the literature; rupture of the plaque fibrous cap that occurs in what’s called “vulnerable plaque,” which is covered with thin fibrous cap poor in collagen with less smooth muscle cells but contains a larger amount of lipid and abundant accumulation of inflammatory cells; and superficial erosion of the plaque that is characterized by a higher content of extracellular matrix, smooth muscle cells, and NETs but less content of lipid and inflammatory cells. While lipid-lowering therapies improve the “vulnerable plaque” and hinder the first mechanism, the erosion mechanism may still explain the high risk of atherosclerosis complications.12 The pivotal role of inflammation and immunity throughout the process of atherosclerosis initiation and development appears clearly through the intensive implication of various inflammatory cells, inflammatory mediators, and immune mechanisms in the pathophysiology of atherosclerosis. TLR4 signaling in vascular smooth muscle cells is involved in the inflammatory process and lipid accumulation through activation of NF-κB pathway and regulation of ATP-binding cassette sub-family γ member 1 (ABCG1), leading to atheromatous plaque progression. The JAK-STAT signaling pathway also plays a role in the modulation of atherogenesis and plaque development. Pro-inflammatory cytokines, including INF-γ, TNF-α, and interleukins, such as IL-1β, IL-6, act as mediators in the interplay between the elements of atherosclerosis inflammatory process.10 There have been some studies that revealed the presence of different immune cells in atherosclerotic lesions including T and B lymphocytes, natural killer cells, dendritic cells, monocytes, macrophage, and neutrophils.13 Recent studies reported the detection of microbial antigens and genetic materials within the atherosclerotic plaques. Acceleration of atherogenesis and deterioration of atherosclerotic lesions via inflammatory processes associated with infections provides a reliable explanation for the implications of infections in atherosclerosis pathophysiology. Endotoxins of the Gram-negative bacteria have been shown to stimulate inflammatory responses that could be further explained through “echo” effect; bacterial endotoxins and pathogen-associated molecular patterns (PAMPs) diffuse in the circulation and act as stimuli eliciting an immune response within the atherosclerotic lesions through interaction with TLRs on leukocytes and vascular endothelial cells. Chronic infections produce prolonged inflammatory state and immune response causing progression of atherosclerosis and its complications. Moreover, the acute infectious state is associated with a hypercoagulable state that may contribute to atherothrombosis progression.9 As NETs were originally described as an immune defense mechanism against bacteria, their implication in atherosclerosis and atherothrombosis may provide a link between infection and atherosclerosis and thrombosis.
NETs formation and release
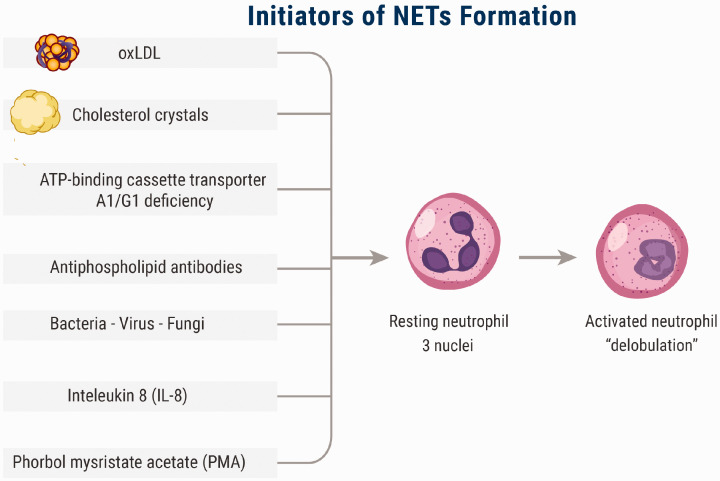
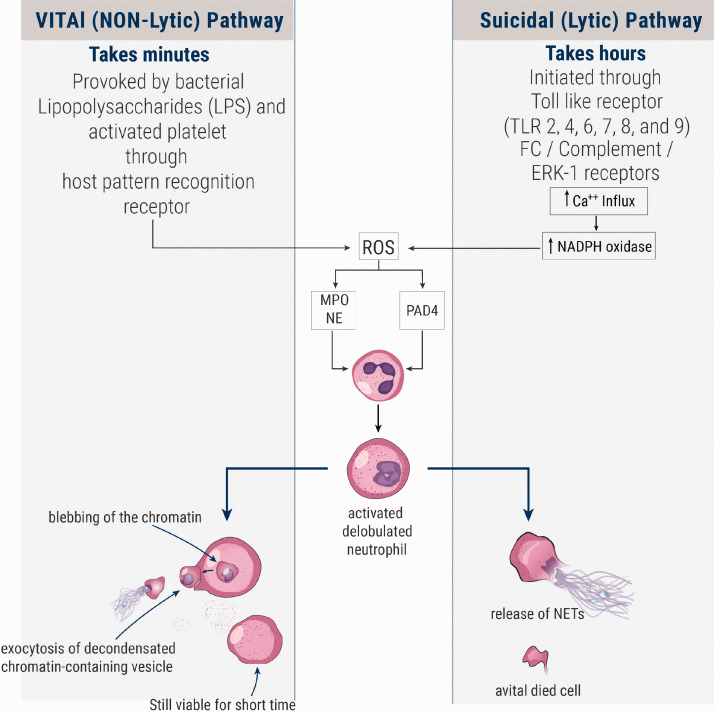
Brinkmann et al. described for the first time a powerful weapon used by neutrophils in their fight against bacteria and these were named neutrophils extracellular traps (NETs). They described them as “structures composed of granule and nuclear constituents that disarm and kill bacteria extracellularly” and stated that “NETs contained smooth stretches with a diameter of 15 to 17 nm and globular domains of approximately 25 nm which aggregated into larger threads with diameters of up to 50 nm; DNA is the main component in NETs with presence of histones (H1, H2A, H2B, H3, and H4), H2A-H2B-DNA and neutrophil granules proteins”.6 NETs are formed through a process named NETosis14 or NETotic cell death15 which is a specific type of cell death differs from necrosis and apoptosis.15,16 The process of NETs formation is initiated through stimulation of toll-like receptors (TLRs)—2, 4, 6, 7, 8, and 9, extracellular signal-regulated kinase (ERK), and Fc receptors by many factors, such as oxidized low-density lipoprotein (oxLDL), cholesterol crystals, infectious agents, activated platelets, and antiphospholipid antibodies (e.g. anti-β2-glycoprotein I)17–21 (Figure 1). This pathway is called the suicidal (lytic) pathway and takes hours to occur.17 On activation of neutrophils, NADPH oxidase activity increases as a result. Consequently, large amounts of reactive oxygen species (ROS), such as oxygen radicals and hydroxyl radical, are produced due to oxidation of NADPH by NADPH oxidase.17,22 ROS activate protein-arginine deiminase 4 (PAD4), which in turn is responsible for chromatin decondensation via citrullination of histones.23 Furthermore, ROS lead to release of azurophilic granule proteins such as neutrophil elastase (NE) and myeloperoxidase (MP), which further aid in the process of chromatin decondensation and nuclear membrane breaking down.24 There is another pathway of NETs formation called the vital (non-lytic) pathway; this pathway, which takes minutes to occur, is initiated through stimulation of host pattern recognition receptor on the surface of neutrophils by many factors such as bacterial lipopolysaccharide and activated platelets. It does not necessarily depend on NADPH oxidase; instead, it depends on ROS generated via the mitochondria. In the vital pathway, blebbing of the chromatin occurs shortly after neutrophil activation, and, consequently, NETs emerge from the cell via exocytosis of decondensed chromatin-containing vesicles25,26 (Figure 2). During the process of NETs formation, the following steps occur respectively: the cells flatten and multiple intracellular vacuoles are formed; the nuclear chromatin is decondensed; the space separating the outer and inner nuclear membrane increases; the nuclear membrane breaks down forming vesicles; the nuclei expand losing their lobular shape occupying most of the cell space in a process called "delobulation"; membranes of the granular and nuclear vesicles are lost, blending their contents together with the decondensed chromatin; until this moment the cell is still viable; and finally the cell membrane breaches releasing NETs and declaring cell death. After that, NETs emerge from the cell containing decondensed chromatin, histones, nuclear and cellular proteins, cytoskeleton, proteases, and tissue factor. It also forms a trap that captures the circulating elements in the blood.6,16
Figure 1.
The initiators of NETs. NETs formation and release are initiated by many factors, such as oxidized low-density lipoprotein (oxLDL), cholesterol crystals, infectious agents, IL-8, activated platelets, and antiphospholipid antibodies (e.g. anti-β2-glycoprotein I). (A color version of this figure is available in the online journal.)
Figure 2.
The two pathways of NETosis (NETs formation). The right-handed pathway is the lytic (suicidal) pathway, in which neutrophil shrivel up or die after the release of NETs. The left-handed pathway is the non-lytic (vital) pathway, in which neutrophils remain viable after the release of NETs via exocytosis in vesicles. (A color version of this figure is available in the online journal.)
NETS in atherosclerosis
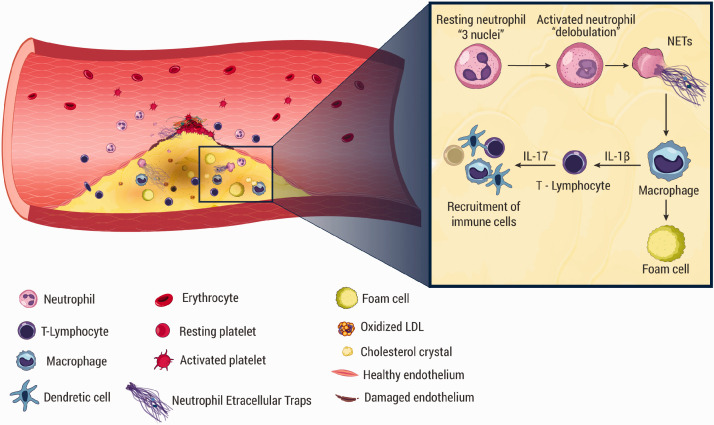
Being a part of neutrophil mechanisms in immunity and relying on the microbicidal effect of their components and augmentation of the microbicidal effect of other products through prevention of the spread of pathogens and their function as scaffolds, NETs were expected to be present in infectious diseases and this has been established.6 Moreover, NETs have been observed and suggested to be implicated in non-infectious diseases including autoimmune diseases,27 malignancies,28 and atherosclerosis.29 In atherosclerosis, implication of NETs has been strongly suggested in both the process of atherosclerotic lesion development and atherothrombosis. Megens et al.29 reported detection of NETs in both human atherosclerotic plaques, derived from endarterectomy, and in mice atherosclerosis model. Borissoff et al. concluded by a study on 282 participants that NETs formation was associated with coronary artery disease, prothrombotic state, and occurrence of adverse cardiac events; they observed elevated levels of cell death and NETosis markers (e.g. double-stranded DNA, nucleosomes, and MPO-DNA complexes) independently associated with severe coronary atherosclerosis and prothrombotic state. Moreover, they suggested that these observed markers could serve as biomarkers for prediction of coronary vascular events.30 Knight et al.31 reported that in murine models of atherosclerosis, inhibition of PAD4 by Cl-amidine blocked NETs formation and reduced the plaques size and delayed the occurrence of thrombosis, denoting the significant role of NETs in atherosclerosis. The exact mechanisms and roles performed by NETs in the pathogenesis of atherosclerosis are still to be investigated. Warnatsch et al. observed that cholesterol crystals stimulated NETosis and NETs production, which in turn enhanced macrophages to produce cytokines including IL-1β, which recruited more neutrophils to the site of lesion. NETs enhanced the release of cytokines resulting in activation of TH17 cells with more leukocyte recruitment.32 In a recent study , Tall and Westerterp33 demonstrated that activation of inflammasomes in macrophages or neutrophils resulted in membrane pore formation releasing IL-1β, IL-18, and NETs contributing in atherosclerotic plaque formation and thrombosis (Figure 3). Soehnlein et al.34 noted the importance of proteinase 3 (PR3), present in NETs, in neutrophil-macrophage interplay via protease-mediated cytokine processing, especially cleavage of pro-IL-1β.35 Wang et al.36 reported that in aged mice, myeloid cell mitochondrial oxidative stress (MitoOS) promoted atherosclerosis development and NETosis. Ionita et al. observed a positive correlation between neutrophils number and the levels of IL-8, which is known as an inducer of NETosis6 in the atherosclerotic plaques.3 Gupta et al.37 reported that activated endothelial cells, when they were Co-cultured with neutrophils, induced NETs formation through activation of neutrophils partially via IL-8; moreover, the produced NETs were susceptible to produce endothelial cells damage. Supporting these observations, Carmona-Rivera et al.38 reported that the matrix metalloproteinase-9 (MMP-9) present in NETs activated endothelial MMP-2 producing endothelial dysfunction in SLE. With regard to NETs roles in atherosclerosis pathogenesis, Döring et al.39 reported that extracellular DNA derived from NETs may take part in activation of plasmacytoid dendritic cells (pDCs) present in atherosclerotic plaques, resulting in aggravation of atherosclerotic lesions. Tillack et al.40 reported a T-cell direct priming mediated by NETs decreasing the activation threshold of T-cells promoting their immune response and extending a link between innate and adaptive immune responses. Cathelicidins, which are components in NETs,34 have been reported to mediate monocyte recruitment41 in atherosclerosis42 and enhance endothelial proliferation.43 Interestingly, in 2018, Yamamoto et al.44 observed that in ApoE–/– mice exposed to repeated social defeat (RSD) producing depressive-like behaviors, there was marked increase in atherosclerosis lesions that had been promoted through augmented NETs formation.
Figure 3.
The implications of different blood cells in atherosclerosis pathogenesis. NETs are released on activation of neutrophils by factors such as OX-LDL and cholesterol crystals. NETs stimulate macrophage to produce IL-1β, which stimulates T-helper 17 to secrete IL-17. IL-17 is responsible for recruitment of more immune cells, including dendritic cells, macrophages, and lymphocytes. (A color version of this figure is available in the online journal.)
NETs and atherothrombosis
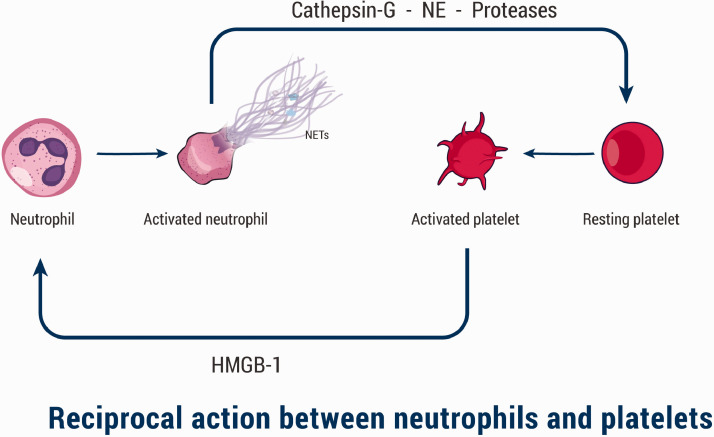
Thrombus formation may evolve on top of rupture or erosion of atheromatous plaque in arteries (i.e. atherothrombosis), or may result from other medical disorders, such as deep venous thrombosis, SLE, and antiphospholipid syndrome.19,38,45 Although NETS have been reported to be implicated in these conditions, we focus mainly in this review on atherothrombosis. Impaired collagen synthesis in addition to overexpression of collagenases mediated by inflammatory mediators disrupts the integrity of the plaque’s fibrous cap leading to plaque erosion. The eroded lesions contain high amounts of NETs and extracellular matrix.12 NETs trigger the coagulation cascade aiding in the formation of thrombi on top of the atheromatous plaque, leading to arterial occlusion. Circulating leukocytes, especially monocytes and neutrophils, have been found to play a crucial role in developing atherothrombosis; moreover, neutrophils are considered as predictors of acute coronary events.46–48 Noticeable amounts of neutrophils have been found in coronary thrombi occurring as sequelae for atherosclerosis.49,50 Neutrophils, NETs, IL17A, and IL17F have been detected in fresh and lytic but never in organized thrombi isolated from patients after acute myocardial infarction which suggested their role in the early stages of thrombus formation.51 Interestingly, Mangold et al. observed that in patients with ST-elevation acute coronary syndrome, NETs were not only implicated in thrombi formation but also NETs burden correlated positively with infarct size and negatively with ST-segment resolution.52 On a study on 26 thrombectomies from patients suffering from acute myocardial infarction, Maugeri et al. reported an interaction between activated platelets and neutrophils; they found that activated platelets induced neutrophils to form and release NETs via high-mobility group box 1 (HMGB-1).53 Moreover, they speculated that use of HMGB1 inhibitors may prevent thromboinflammatory complications. In another study, platelet-derived HMGB-1 has been shown to accelerate NETs formation and promote coagulation cascade.54 Activated neutrophils in turn activate more platelets in a reciprocal process through NE, proteases, and cathepsin-G embedded on NETs18,55–57 (Figure 4). As a result, thrombi are formed, leading to narrowing of the arterial lumen. In addition, in a recent study, Pertiwi et al.6 observed presence of neutrophils and NETs in large amounts in the ruptured atherosclerotic plaques; moreover, NETs have been observed in the interstitial adventitia, perivascular adipose tissue, and inside the lumen of micro-vessels. Semeraro et al.58 demonstrated that histones, present in NETs, enhanced thrombin generation in platelet-rich plasma (PRP) and produced platelet activation through TLR2 and TLR4. Alongside this result, Ammollo et al.59 demonstrated that histones of NETs enhanced thrombin generation by reducing activation of protein C through hindering the activity of protein C-thrombomodulin (TM). Moreover, Varjú et al. reported that DNA and histones of NETs produced an increase in the thickness of fibrin and decreased the permeability of its fibers in plasma clots. DNA alone inhibited plasmin-mediated lysis of clots; furthermore, NETs hindered tPA-induced resolution of plasma clots.60 Martinod et al.61 demonstrated that in PAD4–/–, mice thrombosis was highly suppressed, whereas Fuchs et al.62 reported that thrombi formation was preventable by disintegration of NETs by DNase or the anticoagulant heparin formation. De Meyer et al.63 reported that NETs have been implicated in cerebral ischemia/reperfusion injury in mice, and DNase 1 improved the outcome.
Figure 4.
The interplay between neutrophils and platelets. Activated platelets secrete high-mobility group box 1 (HMGB-1) that activate and induce neutrophils to form and release NETs. Consequently, neutrophils produce NE, proteases, and cathepsin-G embedded on NETs, which in turn activate more platelets in a reciprocal process. (A color version of this figure is available in the online journal.)
NETs as a possible therapeutic target
Besides therapies targeting the conventional risk factors of atherosclerosis, such as hypercholesterolemia and hypertension, targeting the inflammatory process that occurs in atherosclerosis is a promising trend to reduce the harmful complications of atherosclerosis. In addition to their lipid-lowering effect, statins also have an anti-inflammatory effect.8 Canakinumab anti-inflammatory thrombosis outcomes study (CANTOS) revealed promising results in atherosclerosis-related events prevention; Canakinumab, a monoclonal antibody targeting IL-1β, significantly reduced the risk of MI, stroke, or cardiovascular death occurrence (the primary endpoint of the study) by 15%.64 Prevention of infections (e.g. influenza vaccination), which are factors accelerating the development of atherosclerosis and its consequences, provided positive results in reducing atherosclerotic events.9 NETs are strong vital weapons for fighting bacteria. Targeting an essential part of our immunity carries possible hazards. However, targeting certain initiators and mediators implicated in the development of atherosclerosis, atherothrombosis, and their fatal complications is something worth studying. A trial like CANTOS is a good example for this concept. DNase 1 significantly reduced thrombi formation and improved MI fate.47,65 Inhibition of MMP-2 activation improved endothelial dysfunction and NET-induced vascular affection in systemic lupus erythematosus (SLE).40 Treatment with metformin decreased phorbol myristate acetate (PMA)-induced NET formation in SLE.66 Vitamin D 1,25(OH)2D3 reduced NETs formation and the consequent endothelial damage in SLE.67 Tofacitinib, a Janus kinase inhibitor, modulated NETs formation and improved murine lupus and its associated vascular damage.68 Celastrol, a triterpenoid compound, has been reported to inhibit inflammatory stimuli-induced NETosis in rheumatoid arthritis (RA) and SLE.69 Tocilizumab (TCZ) reduced NETosis and oxidative stress, and improved endothelial function in RA.70
Conclusions
It is well established now that atherosclerosis is an inflammatory disease characterized by lipid deposition in the vascular wall and plaque formation. Immunity and infections play a role in the initiation and development of atherosclerosis. NETs are a part of the pathophysiology of atherosclerosis and atherothrombosis. NETs have been shown to accelerate the progression of atherosclerosis and atherothrombosis. Targeting the components of NETs, NETs initiators, and NETs effectors might give positive results in decelerating the progression of atherosclerosis and atherothrombosis. The high mortality rate related to atherosclerosis complications requires more effort to clarify the underlying mechanisms and to develop possible strategies preventing the disease occurrence and development. Over decades, our knowledge about atherogenesis and atherothrombosis pathophysiology has developed. The exact pathways that links NETs to atherosclerosis and atherothrombosis are to be investigated. Understanding the possible mechanisms by which NETs contribute to atherogenesis and atherothrombosis development enables us to improve our vision for atherosclerosis-related risk. More Trials studying the possible therapeutic targets will provide future solutions that may reduce the current high atherosclerosis-related morbidity and mortality rates.
Authors’ contributions
All authors participated in conducting the literature search, screening, summarizing, and reviewing the manuscript. MO designed the included figures and illustrations. MNM managed and edited the references.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs
Mostafa N Mostafa https://orcid.org/0000-0002-9413-9235
Mahmoud Osama https://orcid.org/0000-0003-4919-5358
References
- 1.Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: a systematic analysis for the global burden of disease study 2016. Lancet 2017;390:1260–344 [DOI] [PMC free article] [PubMed]
- 2.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020; 141:e139–e596 [DOI] [PubMed] [Google Scholar]
- 3.Ionita MG, van den Borne P, Catanzariti LM, Moll FL, de Vries J-P, Pasterkamp G, Vink A, de Kleijn D. High neutrophil numbers in human carotid atherosclerotic plaques are associated with characteristics of rupture-prone lesions. Arterioscler Thromb Vasc Biol 2010; 30:1842–8 [DOI] [PubMed] [Google Scholar]
- 4.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med 1999; 340:115–26 [DOI] [PubMed] [Google Scholar]
- 5.Pertiwi KR, van der Wal AC, Pabittei DR, Mackaaij C, van Leeuwen MB, Li X., de Boer OJ. Neutrophil extracellular traps participate in all different types of thrombotic and haemorrhagic complications of coronary atherosclerosis. Thromb Haemost 2018; 118:1078–87 [DOI] [PubMed] [Google Scholar]
- 6.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science 2004; 303:1532–5 [DOI] [PubMed] [Google Scholar]
- 7.Franck G, Mawson TL, Folco EJ, Molinaro R, Ruvkun V, Engelbertsen D, Liu X, Tesmenitsky Y, Shvartz E, Sukhova GK, Michel JB, Nicoletti A, Lichtman A, Wagner D, Croce KJ, Libby P. Roles of PAD4 and NETosis in experimental atherosclerosis and arterial injury: implications for superficial erosion. Circ Res 2018; 123:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hansson GK. Inflammation and atherosclerosis: the end of a controversy. Circulation 2017; 136:1875–7 [DOI] [PubMed] [Google Scholar]
- 9.Libby P, Loscalzo J, Ridker PM, Farkouh ME, Hsue PY, Fuster V, Hasan AA, Amar S. Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol 2018; 72:2071–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Y, Xian X, Wang Z, Bi Y, Chen Q, Han X, Tang D, Chen R. Research progress on the relationship between atherosclerosis and inflammation. Biomolecules 2018; 8:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown RA, Shantsila E, Varma C, Lip GY. Current understanding of atherogenesis. Am J Med 2017; 130:268–82 [DOI] [PubMed] [Google Scholar]
- 12.Quillard T, Franck G, Mawson T, Folco E, Libby P. Mechanisms of erosion of atherosclerotic plaques. Curr Opin Lipidol 2017; 28:434–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winkels H, Ehinger E, Ghosheh Y, Wolf D, Ley K. Atherosclerosis in the single-cell era. Curr Opin Lipidol 2018; 29:389–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mesa MA, Vasquez G. NETosis. Autoimmune Dis 2013; 2013:651497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJM, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D'Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Munoz-Pinedo C, Nagata S, Nunez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi Y, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang D, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Vander Heiden MG, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan J, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 2018; 25:486–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176:231–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Awasthi D, Nagarkoti S, Kumar A, Dubey M, Singh AK, Pathak P, Chandra T, Barthwal MK, Dikshit M. Oxidized LDL induced extracellular trap formation in human neutrophils via TLR-PKC-IRAK-MAPK and NADPH-oxidase activation. Free Radic Biol Med 2016; 93:190–203 [DOI] [PubMed] [Google Scholar]
- 18.Moschonas IC, Tselepis AD. The pathway of neutrophil extracellular traps towards atherosclerosis and thrombosis. Atherosclerosis 2019; 288:9–16 [DOI] [PubMed] [Google Scholar]
- 19.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Núñez-Álvarez C, Hernández-Ramírez D, Bockenstedt PL, Liaw PC, Cabral AR, Knight JS. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol 2015; 67:2990–3003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saitoh T, Komano J, Saitoh Y, Misawa T, Takahama M, Kozaki T, Uehata T, Iwasaki H, Omori H, Yamaoka S, Yamamoto N, Akira S. Neutrophil extracellular traps mediate a host defense response to human immunodeficiency virus-1. Cell Host Microbe 2012; 12:109–16 [DOI] [PubMed] [Google Scholar]
- 21.Liu L, Mao Y, Xu B, Zhang X, Fang C, Ma Y, Men K, Qi X, Yi T, Wei Y, Wei X. Induction of neutrophil extracellular traps during tissue injury: involvement of STING and toll-like receptor 9 pathways. Cell Prolif 2019; 52:e12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal 2002; 4:49–60 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, Hayama R, Leonelli L, Han H, Grigoryev SA, Allis CD, Coonrod SA. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol 2009; 184:205–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papayannopoulos V, Metzler KD, Hakkim A, Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J Cell Biol 2010; 191:677–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilsczek FH, Salina D, Poon KKH, Fahey C, Yipp BG, Sibley CD, Robbins SM, Green FHY, Surette MG, Sugai M, Bowden MG, Hussain M, Zhang K, Kubes P. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 2010; 185:7413–25 [DOI] [PubMed] [Google Scholar]
- 26.Yipp BG, Kubes P. NETosis: how vital is it? Blood 2013; 122:2784–94 [DOI] [PubMed] [Google Scholar]
- 27.Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 2016; 12:402–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Wysocki RW, Amoozgar Z, Maiorino L, Fein MR, Jorns J, Schott AF, Kinugasa-Katayama Y, Lee Y, Won NH, Nakasone ES, Hearn SA, Küttner V, Qiu J, Almeida AS, Perurena N, Kessenbrock K, Goldberg MS, Egeblad M. Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci Transl Med 2016; 8:361ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Megens RTA, Vijayan S, Lievens D, Döring Y, van Zandvoort M, Grommes J, Weber C, Soehnlein O. Presence of luminal neutrophil extracellular traps in atherosclerosis. Thromb Haemost 2012; 107:597–98 [DOI] [PubMed] [Google Scholar]
- 30.Borissoff JI, Joosen IA, Versteylen MO, Brill A, Fuchs TA, Savchenko AS, Gallant M, Martinod K, Ten Cate H, Hofstra L, Crijns HJ, Wagner DD, Kietselaer B. Elevated levels of circulating DNA and chromatin are independently associated with severe coronary atherosclerosis and a prothrombotic state. Arterioscler Thromb Vasc Biol 2013; 33:2032–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Knight JS, Luo W, O'Dell AA, Yalavarthi S, Zhao W, Subramanian V, Guo C, Grenn RC, Thompson PR, Eitzman DT, Kaplan MJ. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res 2014; 114:947–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V. Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015; 349:316–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tall AR, Westerterp M. Inflammasomes, neutrophil extracellular traps, and cholesterol. J Lipid Res 2019; 60:721–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A. Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans. PLoS Pathog 2009; 5:e1000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soehnlein O, Ortega-Gómez A, Döring Y, Weber C. Neutrophil-macrophage interplay in atherosclerosis: protease-mediated cytokine processing versus NET release. Thromb Haemost 2015; 114:866–67 [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Wang W, Wang N, Tall AR, Tabas I. Mitochondrial oxidative stress promotes atherosclerosis and neutrophil extracellular traps in aged mice. Arterioscler Thromb Vasc Biol 2017; 37:e99–e107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S, Resink TJ. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett 2010; 584:3193–97 [DOI] [PubMed] [Google Scholar]
- 38.Carmona-Rivera C, Zhao W, Yalavarthi S, Kaplan MJ. Neutrophil extracellular traps induce endothelial dysfunction in systemic lupus erythematosus through the activation of matrix metalloproteinase-2. Ann Rheum Dis 2015; 74:1417–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Döring Y, Manthey HD, Drechsler M, Lievens D, Megens RTA, Soehnlein O, Busch M, Manca M, Koenen RR, Pelisek J, Daemen MJ, Lutgens E, Zenke M, Binder CJ, Weber C, Zernecke A. Auto-antigenic protein-DNA complexes stimulate plasmacytoid dendritic cells to promote atherosclerosis. Circulation 2012; 125:1673–83 [DOI] [PubMed] [Google Scholar]
- 40.Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol 2012; 188:3150. [DOI] [PubMed] [Google Scholar]
- 41.Wantha S, Alard J-E, Megens RTA, van der Does AM, Döring Y, Drechsler M, Pham CTN, Wang M-W, Wang J-M, Gallo RL, von Hundelshausen P, Lindbom L, Hackeng T, Weber C, Soehnlein O. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res 2013; 112:792–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doring Y, Drechsler M, Wantha S, Kemmerich K, Lievens D, Vijayan S, Gallo RL, Weber C, Soehnlein O. Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circ Res 2012; 110:1052–6 [DOI] [PubMed] [Google Scholar]
- 43.Kougias P, Chai H, Lin PH, Yao Q, Lumsden AB, Chen C. Defensins and cathelicidins: neutrophil peptides with roles in inflammation, hyperlipidemia and atherosclerosis. J Cell Mol Med 2005; 9:3–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamamoto K, Yamada H, Wakana N, Kikai M, Terada K, Wada N, Motoyama S, Saburi M, Sugimoto T, Kami D, Ogata T, Ibi M, Yabe-Nishimura C, Matoba S. Augmented neutrophil extracellular traps formation promotes atherosclerosis development in socially defeated apoE(-/-) mice. Biochem Biophys Res Commun 2018; 500:490–96 [DOI] [PubMed] [Google Scholar]
- 45.Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 2012; 10:136–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyss CA, Neidhart M, Altwegg L, Spanaus KS, Yonekawa K, Wischnewsky MB, Corti R, Kucher N, Roffi M, Eberli FR, Amann-Vesti B, Gay S, von Eckardstein A, Lüscher TF, Maier W. Cellular actors, toll-like receptors, and local cytokine profile in acute coronary syndromes. Eur Heart J 2010; 31:1457–69 [DOI] [PubMed] [Google Scholar]
- 47.Horne BD, Anderson JL, John JM, Weaver A, Bair TL, Jensen KR, Renlund DG, Muhlestein JB. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005; 45:1638–43 [DOI] [PubMed] [Google Scholar]
- 48.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature 2011; 473:317–25 [DOI] [PubMed] [Google Scholar]
- 49.Distelmaier K, Adlbrecht C, Jakowitsch J, Winkler S, Dunkler D, Gerner C, Wagner O, Lang IM, Kubicek M. Local complement activation triggers neutrophil recruitment to the site of thrombus formation in acute myocardial infarction. Thromb Haemost 2009; 102:564–72 [DOI] [PubMed] [Google Scholar]
- 50.Riegger J, Byrne RA, Joner M, Chandraratne S, Gershlick AH, Ten Berg JM, Adriaenssens T, Guagliumi G, Godschalk TC, Neumann FJ, Trenk D, Feldman LJ, Steg PG, Desmet W, Alfonso F, Goodall AH, Wojdyla R, Dudek D, Philippi V, Opinaldo S, Titova A, Malik N, Cotton J, Jhagroe DA, Heestermans AA, Sinnaeve P, Vermeersch P, Valina C, Schulz C, Kastrati A, Massberg S. Histopathological evaluation of thrombus in patients presenting with stent thrombosis. A multicenter European study: a report of the prevention of late stent thrombosis by an interdisciplinary global European effort consortium. Eur Heart J 2016; 37:1538–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Boer OJ, Li X, Teeling P, Mackaay C, Ploegmakers HJ, van der Loos CM, Daemen MJ, de Winter RJ, van der Wal AC. Neutrophils, neutrophil extracellular traps and interleukin-17 associate with the organisation of thrombi in acute myocardial infarction. Thromb Haemost 2013; 109:290–97 [DOI] [PubMed] [Google Scholar]
- 52.Mangold A, Alias S, Scherz T, Hofbauer T, Jakowitsch J, Panzenböck A, Simon D, Laimer D, Bangert C, Kammerlander A, Mascherbauer J, Winter M-P, Distelmaier K, Adlbrecht C, Preissner KT, Lang IM. Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ Res 2015; 116:1182–92 [DOI] [PubMed] [Google Scholar]
- 53.Maugeri N, Campana L, Gavina M, Covino C, De Metrio M, Panciroli C, Maiuri L, Maseri A, D'Angelo A, Bianchi ME, Rovere-Querini P, Manfredi AA. Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J Thromb Haemost 2014; 12:2074–88 [DOI] [PubMed] [Google Scholar]
- 54.Stark K, Philippi V, Stockhausen S, Busse J, Antonelli A, Miller M, Schubert I, Hoseinpour P, Chandraratne S, von Brühl ML, Gaertner F, Lorenz M, Agresti A, Coletti R, Antoine DJ, Heermann R, Jung K, Reese S, Laitinen I, Schwaiger M, Walch A, Sperandio M, Nawroth PP, Reinhardt C, Jäckel S, Bianchi ME, Massberg S. Disulfide HMGB1 derived from platelets coordinates venous thrombosis in mice. Blood 2016; 128:2435–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C, Brinkmann V, Lorenz M, Bidzhekov K, Khandagale AB, Konrad I, Kennerknecht E, Reges K, Holdenrieder S, Braun S, Reinhardt C, Spannagl M, Preissner KT, Engelmann B. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010; 16:887–96 [DOI] [PubMed] [Google Scholar]
- 56.Gale AJ, Rozenshteyn D. Cathepsin G, a leukocyte protease, activates coagulation factor VIII. Thromb Haemost 2008; 99:44–51 [DOI] [PubMed] [Google Scholar]
- 57.Elaskalani O, Abdol Razak NB, Metharom P. Neutrophil extracellular traps induce aggregation of washed human platelets independently of extracellular DNA and histones. Cell Commun Signal 2018; 16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL, Esmon CT. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011; 118:1952–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost 2011; 9:1795–803 [DOI] [PubMed] [Google Scholar]
- 60.Varjú I, Longstaff C, Szabó L, Farkas ÁZ, Varga-Szabó VJ, Tanka-Salamon A, Machovich R, Kolev K. DNA, histones and neutrophil extracellular traps exert anti-fibrinolytic effects in a plasma environment. Thromb Haemost 2015; 113:1289–98 [DOI] [PubMed] [Google Scholar]
- 61.Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M, Hu J, Wang Y, Wagner DD. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci U S A 2013; 110:8674–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr., Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA 2010; 107:15880–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Meyer SF, Suidan GL, Fuchs TA, Monestier M, Wagner DD. Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler Thromb Vasc Biol 2012; 32:1884–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017; 377:1119–31 [DOI] [PubMed] [Google Scholar]
- 65.Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L, Brill A, Wang Y, Wagner DD. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 2014; 123:141–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang H, Li T, Chen S, Gu Y, Ye S. Neutrophil extracellular trap mitochondrial DNA and its autoantibody in systemic lupus erythematosus and a proof-of-Concept trial of metformin. Arthritis Rheumatol 2015; 67:3190–200 [DOI] [PubMed] [Google Scholar]
- 67.Handono K, Sidarta YO, Pradana BA, Nugroho RA, Hartono IA, Kalim H, Endharti AT. Vitamin D prevents endothelial damage induced by increased neutrophil extracellular traps formation in patients with systemic lupus erythematosus. Acta Med Indones 2014; 46:189–98 [PubMed] [Google Scholar]
- 68.Furumoto Y, Smith CK, Blanco L, Zhao W, Brooks SR, Thacker SG, Abdalrahman Z, Sciumè G, Tsai WL, Trier AM, Nunez L, Mast L, Hoffmann V, Remaley AT, O'Shea JJ, Kaplan MJ, Gadina M. Tofacitinib ameliorates murine lupus and its associated vascular dysfunction. Arthritis Rheumatol 2017; 69:148–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu Y, Koehn CD, Yue Y, Li S, Thiele GM, Hearth-Holmes MP, Mikuls TR, O'Dell JR, Klassen LW, Zhang Z, Su K. Celastrol inhibits inflammatory stimuli-induced neutrophil extracellular trap formation. Curr Mol Med 2015; 15:401–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ruiz-Limón P, Ortega R, Arias de la Rosa I, Abalos-Aguilera MDC, Perez-Sanchez C, Jimenez-Gomez Y, Peralbo-Santaella E, Font P, Ruiz-Vilches D, Ferrin G, Collantes-Estevez E, Escudero-Contreras A, López-Pedrera C, Barbarroja N. Tocilizumab improves the proatherothrombotic profile of rheumatoid arthritis patients modulating endothelial dysfunction, NETosis, and inflammation. Transl Res 2017; 183:87–103 [DOI] [PubMed] [Google Scholar]