Abstract
Hyperlipidemia represents a major risk factor for cardiovascular diseases leading to myocardial injury. The present study aimed to illustrate the myocardial injury induced in a diabetic hyperlipidemic rat model and the effect of vitamin D3, 10-DHGD intake either individually or in combination form. Male albino rats were selected for the study, received alloxan, hypercholesterolemic diet, and categorized into four groups. The first one (DHC), received hypercholesterolemic diet only and referred to as control. The remaining groups (2, 3, 4) received vitamin D3, 10-DHGD, and combination of both, respectively. Certain biomarkers that were selected for MI evaluation included blood glucose, lipogram pattern, Copeptin, C-reactive protein, myeloperoxidase, heart fatty acid-binding protein, and histopathological changes in myocardium and aorta. Vitamin D3 and 10-DHGD intake induced significant hypoglycemic, hypolipidemic effects, decreased inflammation, and MI biomarkers. Decreased myocardial vacuoles, foam cells, and intimal lesions were also observed compared to DHC. Their combination intake induced more marked reduction in all biomarkers and showed a histopathological pattern similar to normal features of myocardium and aorta. Our findings suggest the therapeutic roles of vitamin D3, 10-DHGD, and their combination against myocardial injury in diabetic hyperlipidemic rats.
Impact statement
Hyperlipidemia represents a major risk factor for cardiovascular diseases leading to myocardial injury (MI). The present study aimed to illustrate the pattern of myocardial injury induced in diabetic hyperlipidemic rat model and the effect of vitamin D3, 10-dehydrogingerdione (10-DHGD) intake either individually or in combination form.
Keywords: 10-Dehydrogingerdione, vitamin D3, diabetic hyperlipidemia, myocardial infarction, CETP, copeptin
Introduction
Hyperlipidemia is a metabolic disorder, major risk factor for cardiovascular disease (CVD) and mostly characterized by significant increase of total, low-density lipoprotein cholesterol (LDL-c), triacylglycerol (TG) along with decreased high-density lipoprotein cholesterol (HDL-C) levels.1 The development of such disease is relatively high in diabetic people.2 Other risk factors contributing to CVD are many such as obesity, hypertension, smoking, and lack of exercise.3
Cardiovascular diseases (CVDs) are mainly attributed to atherosclerosis and subsequent development of coronary artery disease, cerebrovascular disease, venous thromboembolism, and peripheral vascular disease, leading finally to myocardial infarction (MI) and cardiac arrhythmia.4 MI usually developed in response to decreased oxygen and blood supply due to partial or complete blockage of cardiac arteries leading to heart damage or death to cardiac cells. This blockage may be caused by a ruptured atherosclerotic plaque.5,6 MI can lead to myocardial necrosis and subsequent complications; therefore, its early treatment may represent an important key.7,8 For the treatment to be of optimal benefit, it is vital that an accurate diagnosis is made as soon as possible. This can only be achieved with the help of biomarkers, which can also provide prognostic information that guides clinicians in treating the disease.7
Certain biomarkers are usually used for MI diagnosis like cardiac troponin (cTn), heart-type fatty acid binding protein (H-FABP), C-terminal-pro vasopressin (Copeptin), C-reactive protein (CRP), and myeloperoxidase (MPO).9,10
After an ischemic episode, myocardial cells release HFABP.11,12 HFABP is an emerging biomarker used for early diagnosis of MI due to its high sensitivity.13 It has been shown that combining HFABP with cardiac troponin (cTn) gives the best performance for early diagnosis of acute MI.11,14 Arginine vasopressin is usually released in response to stress within the circulatory system and Copeptin represents the c-terminal fragment of vasopressin and is mostly preferred than arginine vasopressin as an MI biomarker. This is due to its longer half-life in plasma15–17 and its early appearance after the onset of MI.18 Therefore, Copeptin can provide amendatory information for early diagnosis of acute MI especially if done along with cardiac troponin marker.15,19 Myeloperoxidase (MPO) and C-reactive protein (CRP) are two inflammatory markers that are usually increased in cardiovascular inflammatory processes and can predict recurrent atherothrombosis.20,21 Myeloperoxidase is an inflammatory and oxidative stress biomarker for MI. Its plasma level is elevated in people with acute MI and can be used as an early potential biomarker in the diagnosis of MI and patients with chest pain.20,22 CRP is a marker for systemic inflammation which can also predict cardiovascular events.23,24 High CRP levels have been shown to predict the long-term risk of MI.25 Moreover, many studies indicated to positive correlation with IL-6 in acute MI stage and negative one with HDL-C levels during the acute phase of atherosclerosis.26,27
10-Dehydrogingerdione (10-DHGD) is one of the active ginger constituents, and recent studies have shown 10-DHGD to be effective as hypolipidemic, anti-inflammatory, and anti-oxidant agent.28 10-DHGD acts as cholesteryl ester transfer protein (CEPT) inhibitor conferring protection against lipid-mediated atherosclerosis.29 In addition, 10-DHGD has therapeutic effects against aortic calcification and atherosclerotic lesions in aortas.30 Moreover, it has been shown to improve the atherogenic lipid profile by modifying the release of PCSK-9.31 Therefore, 10-DHGD may represent a potent therapeutic agent for the treatment of hyperlipidemia and inflammatory conditions and indeed may be protective against cardiovascular disease.30,32
Many studies indicated that vitamin D3 plays a key role in regulating skeletal and calcium homeostasis; additionally, it has important extra skeletal effects, particularly on the cardiovascular system.33 Recent studies indicated that it also regulates the inflammation and immune response;34,35 its deficiency is closely related to coronary heart disease and subsequent mortality.34,36,37 Moreover, its supplementation also induced hypoglycemic effect in diabetic patients.38
The present study aimed mainly to demonstrate the effects of vitamin D3 and 10-DHGD treatment either individually or in combination on glucose, lipid profile and biomarkers of MI in a model of diabetic hyperlipidemic rats. Experimental induction of diabetes in laboratory animals are usually done by diabetogenic agents, and alloxan is a characteristic member. Being a urea derivative, its underlying mechanism involves its selective uptake by pancreatic β cells due to its structural similarity to glucose. It induces necrosis and destruction of β cells due to its oxidation of essential thiols and generation of free radicals, and the net results are inhibition of insulin secretion. This is mostly attributed to the inhibition of beta cell glucose sensor glucokinase along with disturbances in intra calcium hemostasis.39,40
Materials and methods
Male albino rats (Wistar strain), weighing 150 – 170 g, were used. The experimental design and animal handling were approved by the Ethical committee of animal research at Zagazig University (ZU-IACUC/3/F/79/2018).
Rats were fed on normal chow diet, which consisted of 20% wheat, 10% wheat bran, 24% soybean cake, 10% corn,20% rice, 10% fish flour, 1% salt, 3% bone meal, and 1% multivitamins as supplied from a local manufacturing company.41 The rats were allowed one week for acclimatization and then administered Alloxan monohydrate (Acros-Organics Chemical Co., USA) at a dose of 125 mg/kg body weight subcutaneously freshly prepared in saline (0.9% NaCl). After 1 h of alloxan administration, a 5% to 10% glucose (alpha chemika) was added to drinking water for 24 h to protect the rats from the hypoglycemic shock.42–45 Blood glucose level was measured for each rat after 48 h. Rats that achieved a blood sugar level ≥200 mg/dl were selected as diabetic.46–48 Diabetic rats were fed later on cholesterol diet (CCT) that contains 4% cholesterol (Alpha Chemical Co., India), 1% cholic acid (Sigma Chemical company) mixed with chow diet along with drinking water contain 0.5% thiouracil (Riedel-De Haen Ag Seelize-Hannover, Germany) for a period of 14 days41,42 and continued later for eight weeks along with the treatment intake. The rats were then categorized into four groups (six rats/each). The first one received no treatment (only CCT diet) and referred to as a control group or diabetic hyperlipidemic control (DHC) group), group 2 received 10-DHGD (extracted from ginger fresh rhizomes (Zingiber officinale) 10 mg/kg orally,31,49 group 3 received vitamin D3 (the Medical Union of Pharmaceuticals (MUP, Egypt)) 500 IU/kg weekly administered by oral gavage,50–52 group 4 received a combination therapy of 10-dehydrogingerdione and vitamin D3. Another group of normal rats (group 5) that received only chow diet was included and referred to normal group. These drugs are water insoluble, so they were freshly prepared as suspensions before the oral intake using Tween 80 and continued daily for eight weeks along with CCT.
For the preparation of 10-DHGD, fresh rhizomes of ginger (Zingiber officinale) was used and processed as described before.53,54 These rhizomes were cut into fragments and then macerated in methanol for extraction at room temperature for three days. The methanol extract was then filtered and concentrated under reduced pressure at 45°C forming a dark brown residue. The collected residue was suspended later in water and mixed with n-hexane. The n-hexane layer was separated and concentrated; the residue collected was applied to silica gel for chromatography steps (Merk, silica gel 60, 230–400 mesh, 250 g) with a gradient of n-hexane–ethyl acetate (10:0, 9:1, 8:2, 7:3, 6:4, and 5:5 v/v, 1000 ml each) to obtain a yellowish solid mass. Fraction 5 was separated and applied to GLC-MS analysis of the volatile oil. This was carried out using Clarus 600 Gas Chromatography, and Mass Spectrometer Model: Clarus 600 T Mass Spectrometer, USA.
Sampling
At the experimental end, blood samples were collected from fasted rats. A portion of blood samples was collected in EDTA blood tubes for the determination of HBA1C immediately, and the other portion was processed for serum preparation and the immediate determination of glucose, lipid profile, and kept later at −20°C for the determination of CRP and myeloperoxidase.
After the rats were sacrificed, part of the myocardium was removed and stored at –80°C for HFABP and copeptin gene expression analysis. Another part of myocardium along with aorta samples were kept in 10% formalin in saline, kept at 4°C and processed later for histopathological examination.55
Laboratory analysis
Blood glucose was determined using diagnostic kits provided by Beckman, USA. Total, HDL cholesterol, and TG were determined using kits provided by Spinreact Co., Spain. Friedewald formula was used for LDL-C calculation. HBA1c was determined by rat hemoglobin A1C assay kits supplied by Crystal Chem Europe. CRP and MPO levels were determined using ELISA kits supplied by BD Biosciences Co. and BioVisions incorporated company, Germany, respectively. HFABP and copeptin were determined by quantitative RT PCR. For RNA extraction, we used kits supplied from Qiagen (Qiagen, USA). For cDNA synthesis, we used cDNA reverse transcription kits (Fermentas, USA). qPCR amplification and analysis were performed using an Applied Biosystem with software version 3.1 (StepOne™, USA). The primer sequence is as shown in Table 1.
Table 1.
RT-qPCR primer sequence.
| Name | Sequence (5′-3′) |
|---|---|
| HFABP gene | Forward primer: 5′-TGGCCAAACCAGACTGCAT-3′ Reverse primer: 5′-CTCTCCCAAGGTGCAAGAAAA-3′ |
| Copeptin | Forward primer: 5′-GCCTCAGGACCAGACAGAAG-3′ Reverse primer: 5′-AATCACTGCCAGCACAGC-3′ |
| Beta actin | Forward primer: 5′-CAACTACATGGTTTACATGTTC-3′ Reverse primer: 5′-GCCAGTGGACTCCACGAC-3′ |
Statistical analysis
Statistical analyses were done using GraphPad (CA, USA) Prism 6. All values were expressed as M ± SD using One-way analysis of variances (ANOVA) followed by Tukey–Kramer test for comparison between groups. Correlation coefficient was done using Pearson’s correlation coefficient (r) test taking P ≤ 0.05 as statistically significant.
Results
Histological examination of myocardium and aorta tissues
Effect of DHC and concurrent all different treatment on myocardium tissues: Figure 1 and Table 4
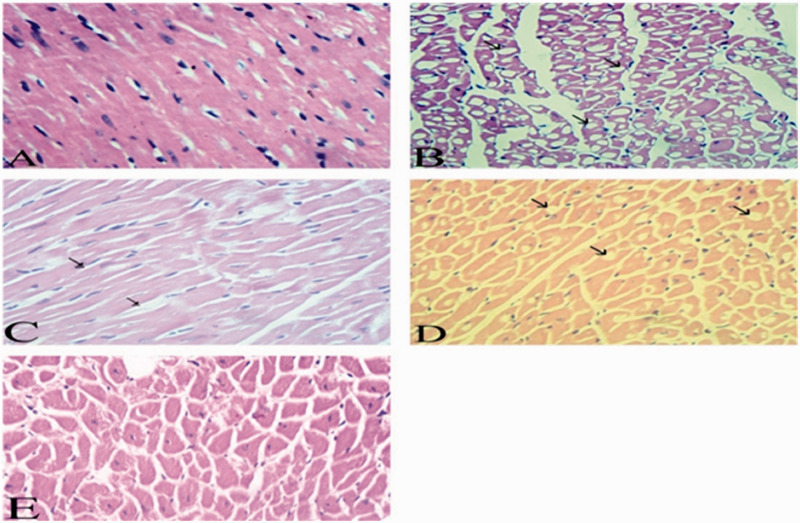
Figure 1.
Effect of DHC and all the different treatments on microscopic examination of myocardium (H &E). Normal group (a). Diabetic hyperlipidemic group (b). 10-DHGD group (c). Vit. D3 group (d). The combination therapy (e). (A color version of this figure is available in the online journal.)
Table 4.
Effect of DHC and concurrent different treatments on HFABP, copeptin, CRP, and MPO.
| Parameters | NG | DHC | 10-DHGD | VIT.D | 10-DHGD+VIT.D |
|---|---|---|---|---|---|
| HFABP mRNA | 1.01 ± 0.014 | 7.01 ± 0.142* | 2.70 ± 0.377# | 4.32 ± 0.215#a | 1.84 ± 0.176#ab |
| Copeptin mRNA | 1.04 ± 0.023 | 8.92 ± 0.147* | 3.14 ± 0.137# | 5.00 ± 0.142#a | 2.02 ± 0.171#ab |
| CRP (mg/l) | 0.37 ± 0.08 | 3.73 ± 0.67* | 0.59 ± 0.05# | 0.89 ± 0.10# | 0.40 ± 0.03# |
| MPO (ng/ml) | 2.89 ± 0.43 | 19.04 ± 2.75* | 6.60 ± 0.40# | 8.88 ± 0.44#a | 4.1 ± 0.49#ab |
NG: normal groups; DHC: diabetic hyperlipidemic rats received no drugs; 10-DHGD: 10-dehydrogingerdione, VIT.D: vitamin D3, 10-DHGD+VIT.D: combination therapy; HFABP: heart fatty acid binding protein; copeptin; CRP: C-reactive protein, MPO: myeloperoxidase.
*p < 0.0001: NG vs. DHC; #p < 0.0001: DHC vs. treated groups.
ap < 0.0001; 10-DHGD vs. vit.D3 and combined therapy.
bp < 0.0001; vit.D3 vs. combined therapy.
Myocardium tissues of normal group demonstrated normal vasculature and muscle fibers (Figure 1(a)). The control group showed intense vacuolation (micro steatosis) and necrosis of cardiac myocytes, which appeared as empty clear vacuoles of variable sizes and evident edema. Clusters of fat cells appeared between the myocardial fibers along with blood vessels congestion, intimal destruction and appearance of plaques (Figure 1(b)). 10-DHGD group appeared ameliorative, and few myocardial fibers were swallowed, showed vacuolation, little lipocytes between the myocardial muscle fibers and the blood vessels appeared normal (Figure 1(c)). Vitamin D3 group demonstrated mild myocardial lesions, and the myocytes have minute fatty vacuoles and fat cells within the myocardial fibers. The blood vessels were dilated, hyperemic without any intimal lesions (Figure 1(d)). Myocardial muscle fibers of the combination therapy group showed normal morphological features, but a few myocytes demonstrated some vacuolar (fatty) degeneration without any fat deposits between the affected myocardial muscles. Blood vessels showed normal morphological picture (Figure 1(e)).
Effect of DHC and all the different treatment on aortic tissues: Figure 2
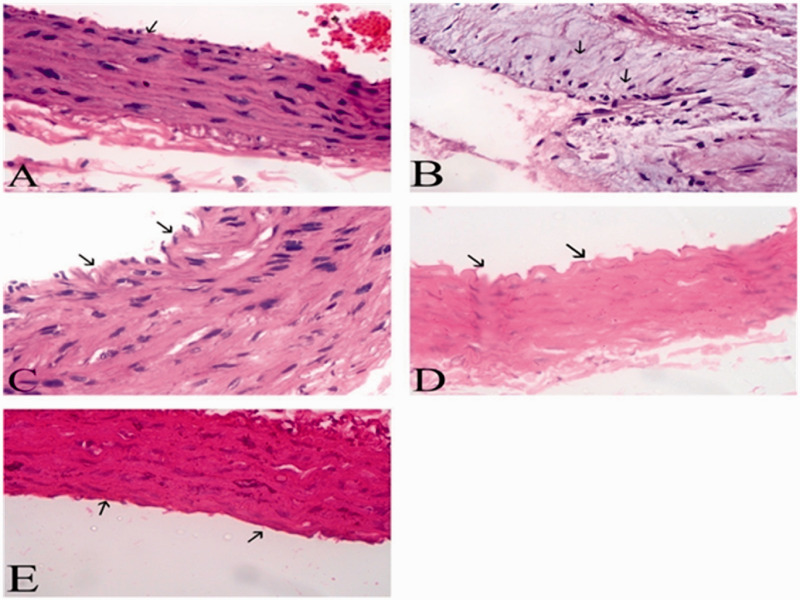
Figure 2.
Effect of DHC and all the different treatments on microscopic examination of aorta sections (H & E) normal rats (a). Diabetic hyperlipidemic (b). 10-DHGD group (c). Vit.D3 group (d). Combination therapy (e). (A color version of this figure is available in the online journal.)
Examination of aortic section of normal group demonstrated normal morphological pattern (Figure 2(a)). Control group showed subintimal infiltration of lipid (foamy cells), thickened intima, and evident plaques (Figure 2(b)). 10-DHGD treated rats showed minute subintimal thickening with irregular intima, and the majority of the remaining tunics are apparently normal (Figure 2(c)). Vitamin D3-treated rats showed irregular intima, few foam cells, and little edema in the media region (Figure 2(d)). Combination therapy group showed small irregularities of the tunica intima, and most of the vascular wall was restored to its normal morphological form (Figure 2(e)).
Changes of glucose, HBA1c, lipid profile, and other myocardial injury biomarkers
Effect of treatment with vitamin D3, 10-DHGD, and their combination on blood glucose, hemoglobin A1c, and lipid profile in DHC rats: Table 2
Table 2.
Lesion score of myocardial changes among experimental groups of albino rats.
| Groups | Microsteatosis and necrosis of muscle fibers | Intermuscular lipocytes |
Blood vessels |
|
|---|---|---|---|---|
| Hyalinization of the wall | Intimal destruction (plaques) | |||
| NG | − | − | − | − |
| DHC | ++++ | ++ | + | + |
| 10-DHGD | ++ | − | − | − |
| VIT. D3 | ++ | + | + | − |
| 10.DHGD+VIT.D3 | + | − | − | − |
Note: ++++ Very intense, +++ intense, ++ moderate, + mild, - absence.
NG: normal group; DHC: diabetic hyperlipidemic group; 10-DHGD: 10-dehydrogingerdione group; VIT.D3: vitamin D3 group; 10-DHGD+VIT.D3: combination therapy group.
Diabetic hyperlipidemia (DHC) rats showed significant increase in blood glucose (BG), hemoglobin A1c (HBA1C), and serum lipogram pattern compared to the normal group (NG). Values obtained by individual treatment with 10-DHGD and vitamin D were significantly lower in comparison to the DHC group. Combined treatments with 10-DHGD and vitamin D3 resulted in greater decreases in BG, HBA1C, and lipid profile than the individual treatment groups.
Effect of treatment with vitamin D3, 10-DHGD, and their combination on CRP and myeloperoxidase in DHC rats: Table 3
Table 3.
Effect of DHC and concurrent different treatments on blood glucose, hemoglobin A1c, and lipid profile.
| Parameters | NG | DHC | 10-DHGD | VIT.D | 10-DHGD+VIT.D |
|---|---|---|---|---|---|
| BG (mg/dl) | 90 ± 5.69 | 389.5 ± 48.93* | 101.66 ± 6.56# | 130 ± 8.94# | 93.166 ± 9.49# |
| HBA1c % | 3.5 ± 0.45 | 9.8 ± 0.89* | 3.96 ± 0.49# | 4.98 ± 0.38# | 3.46 ± 0.30#a |
| TC (mg/dl) | 87 ± 7.62 | 264 ± 15.88* | 112.41 ± 4.05# | 139 ± 8.18# | 105 ± 4.18#a |
| TG (mg/dl) | 59 ± 4.39 | 131 ± 7.16* | 70.73 ± 3.68# | 90 ± 8.87# | 62.03 ± 6.17#a |
| HDL-C (mg/dl) | 44 ± 3.81 | 26 ± 4.84* | 51.21 ± 4.20# | 39 ± 4.28# | 59.12 ± 5.81# |
| LDL-C (mg/dl) | 31.2 ± 11.03 | 211.8 ± 20.17* | 47.05 ± 5.91# | 82 ± 9.15# | 34.13 ± 9.45#a |
NG: normal groups; DHC: diabetic hyperlipidemic rats received no drug treatment; 10-DHGD: 10-dehydrogingerdione, VIT.D: vitamin D3, 10-DHGD+VIT.D: combination therapy; TC: total cholesterol; TG: triglycerides; HDL-C: high density lipoprotein cholesterol, LDL-C: low density lipoprotein cholesterol; HBA1C: hemoglobin A1c; BG: blood glucose.
*p < 0.0001: NG vs. DHC; #p < 0.0001: DHC vs. treated groups.
ap < 0.0001; combined therapy vs. vit.D3.
CRP and myeloperoxidase (MPO) demonstrated significant increase in control group compared with normal rats (NG). Treated groups with vitamin D3 and 10-DHGD either alone or in combination showed significant decrease in CRP and MPO levels.
mRNA gene expression of HFABP and copeptin
Effect of administration of vitamin D3, 10-DHGD, and their combination on myocardium mRNA gene expression of HFABP and copeptin in control group: Table 3
HFABP and Copeptin gene expressions of control group demonstrated significant increase compared to normal rats (NG). However, the administration of all treatment regimens induced a significant decrease in myocardial HFABP and Copeptin gene expressions as compared to DHC group. The combination of 10-DHGD with VIT-D3 reduced their myocardial gene expressions significantly compared to their corresponding monotherapies.
Discussion
As previously indicated, diabetic hyperlipidemic rats showed disturbances in serum glucose, lipid profile, blood HBA1c, markers specific for cardiovascular diseases, and MI.56,57 Many studies indicated that diabetic hyperlipidemia represents a major risk factor for MI.58 It is clear that diabetic rats receiving a hypercholesterolemic diet showed marked levels of hyperglycemia, hyperlipidemia, inflammatory state, atherosclerotic lesions, and myocardial injury. These changes are associated with biochemical markers and histological features typically seen in MI.30
The present study elucidated that vitamin D3 and its combination therapy with 10-dehydrogingerdione exerted a more marked effect than monotherapy. As previously reported, 10-DHGD demonstrates a hypolipidemic activity, potent anti-inflammatory, and hypoglycemic activity and offers anti-oxidant and cardiovascular protection.31,32,54
Pancreatic β cells dysfunction in diabetic individuals may be attributed to hypoxia and increased oxidative stress within these cells. Accordingly, Alloxan administration to the experimental rats induced oxidative stress state and excessive generation of free radicals. 10-DHGD is generally known to have antioxidant and anti-inflammatory properties. Therefore it may significantly reduce the oxidative stress in the β cells, ameliorate the potentials of free radical generations along with upregulation of insulin secretion, and indeed hypoglycemic effect. Recent published work dealing with polyphenol extract of Caesapinia bonduc demonstrated hypoglycemic effect in alloxan diabetic rats. The authors attributed the hypoglycemia to the suppression of JNK signaling pathway.59 The net effect is the regeneration of β cells and improvement of their free radicals’ scavenging potential and upregulation of antioxidant gene expression.59
10-DHGD acts as a cholesteryl ester transfer protein (CEPT) inhibitor conferring protection against lipid-mediated atherosclerosis.29,54 Moreover, it has been shown to improve the atherogenic lipid profile by modifying the potential of PCSK-9.31 The observed decrease in CRP and MPO levels in rats treated with 10-dehydrogingerdione may be attributed to its inhibition of vascular inflammation induced by hypercholesterolemia state.60 Its anti-inflammatory effect may be mediated through the suppression of NF-κB-regulated expression of inflammatory genes.54,61 Its hypolipidemic effect may be attributed to its stimulatory increase of LDL-C catabolism and inhibition of PCSK-9.31,62
10-DHGD also has a protective effect against aortic calcification, which in turn decreases cardiovascular diseases.63 In this study, the myocardial injury biomarkers HFABP and copeptin were significantly decreased by 10-DHGD as shown in Table 3.
Vitamin D3 demonstrated hypoglycemic effect as shown in Table 2. The mechanism behind may be through certain modifications of pancreatic β cells, since many vitamin D receptors are located in these cells.64,65 Vitamin D can also suppress the inflammatory markers, which in turn can improve insulin sensitivity66,67 as mediated through its muscle cell receptors.66,68 Vitamin D directly activates transcription of the human insulin receptor gene.64,69 Another mechanism is mediated through an increase in GLUT4 gene expression and improvement in the function of glucagon-like peptide 1 (GLP-1).70,71 Accordingly, the significant decrease in blood glucose and HBA1c levels can be attributed to vitamin D3 regulatory effect on insulin synthesis and secretion.72
Vitamin D treatment significantly decreased total, LDL-C cholesterol, triglycerides along with HDL-C increase as shown in Table 2. This may be due to enhancement of PPAR-α expression which affects hepatic lipid metabolism.73 Certain studies demonstrated positive correlation between vitamin D intake, apo A (HDL constituent), and negative one with total, LDL-C. This is mostly attributed to certain regulations of adipogenesis.74 TG decrease in the present study may be due to decreased hepatic TG accumulation and lipogenesis attenuation through Ca + 2/calmodulin-dependent kinase.75
Vitamin D also significantly decreased CRP (Table 3). This is mostly due to inhibition of inflammation and production of inflammatory markers76,77 taken in consideration that Vitamin D receptors are also located on leukocytes, T-helper cells additionally the monocytes.
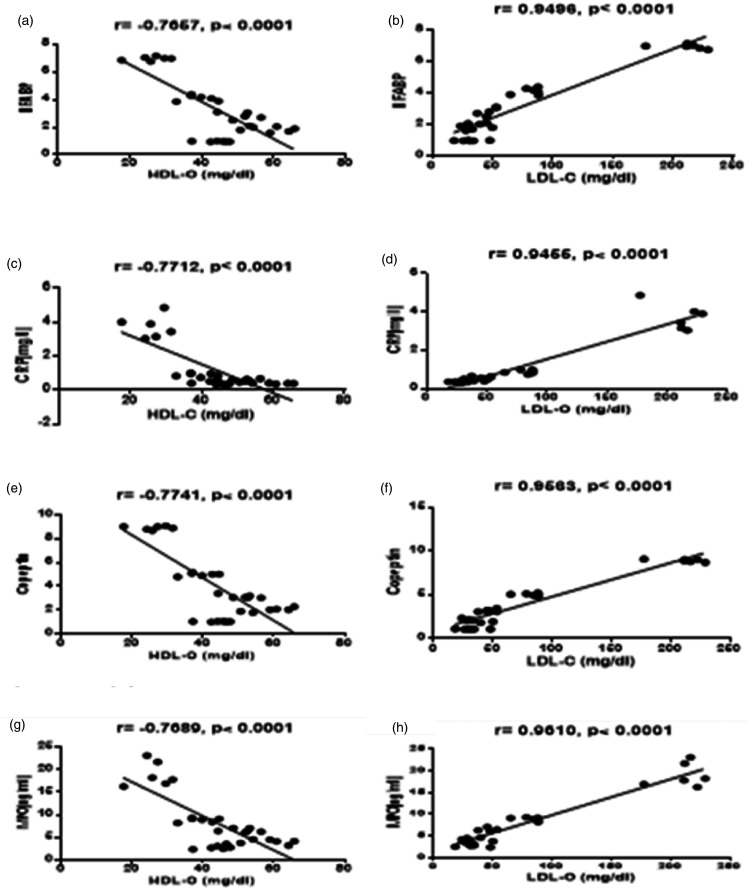
Reported study in agreement with the previous reports demonstrated that vitamin D significantly inhibited IL-6 synthesis in monocytes where IL-6 represents a stimulant for liver CRP production and secretion.76,78,79 In the present study, vitamin D3 supplementation was negatively associated with serum MPO levels as shown in Figure 3. A higher serum MPO level is associated with the systemic inflammation related to CV disease80 and vitamin D3 treatment significantly reduced MPO.81 This may refer to its important inhibitory influence on this inflammatory pathway.82
Figure 3.
Correlation between HDL-C and LDL-C with HFABP, CRP, copeptin, and MPO. (a) Correlation between serum HDL-C level and myocardium HFABP mRNA. (b) Correlation between serum LDL-C level and myocardium HFABP mRNA. (c) Correlation between serum HDL-C level and serum CRP level. (d) Correlation between serum LDL-C level and serum CRP level. (e) Correlation between serum HDL-C level and myocardium copeptin mRNA (f) Correlation between serum LDL-C level and myocardium copeptin mRNA. (g) Correlation between serum HDL-C level and serum MPO level. (h) Correlation between serum LDL-C level and serum MPO level.
Collected data therefore may refer to the protective role of vitamin D against the development of cardiovascular disease.79,83 Therefore, vitamin D demonstrated in the present study (Table 4) a significant influence on the early biomarkers of myocardial injury such as HFABP and Copeptin where the latter represents a diagnostic marker for myocardial injury and MI.84,85 HFABP as a sensitive marker is useful to detect myocardial injury and its early diagnosis.11,13 10-DHGD and vitamin D3 in combination demonstrated more significant hypolipidemic, hypoglycemic, anti-inflammatory potential and additionally exerted marked improvement on the biomarkers of MI (copeptin and HFABP) compared to monotherapy.
Conclusions
10-DHGD and vitamin D3 each individually demonstrated hypoglycemic and hypolipemic inhibitory effects on the studied myocardial injury markers (Copeptin, MPO, CRP, and H FABP). In addition, a combined intake showed evident synergy between the two as reflected by the biochemical and the histopathological findings.
Acknowledgments
We acknowledge the great help provided by Dr. Samieh Eldahmy for isolation and purification of 10DHGD from Ginger Rhizome and we thank Dr. Abd Elmonem Aly for his great efforts in the Histopathology section and Dr. Mohamed Shawky Zaghloul for his help during the processing of the article.
Authors’ contributions
MM conceived the idea, designed, supervised the study and wrote the article, NI conducted most of the experiments, SI conducted the statistical analysis.
DECLARATION OF CONFLICTING INTERESTS
The authors declare no potential conflicts of interest with respect to the research, authorship and/or publication of this article
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Mohamed M Elseweidy https://orcid.org/0000-0001-8167-7563
References
- 1.Ji X, Shi S, Liu B, Shan M, Tang D, Zhang W, Zhang Y, Zhang L, Zhang H, Lu C, Wang Y. Bioactive compounds from herbal medicines to manage dyslipidemia. Biomed Pharmacother 2019; 118:109338. [DOI] [PubMed] [Google Scholar]
- 2.Gupta M, Tummala R, Ghosh RK, Blumenthal C, Philip K, Bandyopadhyay D, Ventura H, Deedwania P. An update on pharmacotherapies in diabetic dyslipidemia. Prog Cardiovasc Dis 2019;62(4), 334–341 [DOI] [PubMed] [Google Scholar]
- 3.Mishra RM. Determinants of cardiovascular disease and sequential decision-making for treatment among women: a Heckman's approach. SSM Popul Health 2019; 7:100365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flora GD, Nayak MK. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr Pharm Des 2019;25(38),4063–4084 [DOI] [PubMed] [Google Scholar]
- 5.Shattat G. A review article on hyperlipidemia: types, treatments and new drug targets. Biomed Pharmacol J 2014; 7:399–409 [Google Scholar]
- 6.Nickolas TL, Radhakrishnan J, Appel GB. Hyperlipidemia and thrombotic complications in patients with membranous nephropathy. Semin Nephrol 2003; 23:406–11 [DOI] [PubMed] [Google Scholar]
- 7.Chan D, Ng LL. Biomarkers in acute myocardial infarction. BMC Med 2010; 8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilotto L. Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA project populations. Ital Heart J Suppl 2000; 1:836–7 [PubMed] [Google Scholar]
- 9.Wang J, Tan G-J, Han L-N, Bai Y-Y, He M, Liu H-B. Novel biomarkers for cardiovascular risk prediction. J Geriatr Cardiol 2017; 14:135–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O'Donoghue ML, Morrow DA, Cannon CP, Jarolim P, Desai NR, Sherwood MW, Murphy SA, Gerszten RE, Sabatine MS. Multimarker risk stratification in patients with acute myocardial infarction. J Am Heart Assoc 2016; 5:e002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Das UN. Heart-type fatty acid-binding protein (H-FABP) and coronary heart disease. Indian Heart J 2016; 68:16–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleine AH, Glatz JF, Van Nieuwenhoven FA, Van der Vusse GJ. Release of heart fatty acid-binding protein into plasma after acute myocardial infarction in man. Mol Cell Biochem 1992; 116:155–62 [DOI] [PubMed] [Google Scholar]
- 13.Vupputuri A, Sekhar S, Krishnan S, Venugopal K, Natarajan KU. Heart-type fatty acid-binding protein (H-FABP) as an early diagnostic biomarker in patients with acute chest pain. Indian Heart J 2015; 67:538–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li CJ, Li JQ, Liang XF, Li XX, Cui JG, Yang ZJ, Guo Q, Cao KJ, Huang J. Point-of-care test of heart-type fatty acid-binding protein for the diagnosis of early acute myocardial infarction. Acta Pharmacol Sin 2010; 31:307–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boeckel J-N, Oppermann J, Anadol R, Fichtlscherer S, Zeiher AM, Keller T. Analyzing the release of copeptin from the heart in acute myocardial infarction using a. Sci Rep 2016; 6:20812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsutamoto T, Wada A, Maeda K, Hisanaga T, Maeda Y, Fukai D, Ohnishi M, Sugimoto Y, Kinoshita M. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation 1997; 96:509–16 [DOI] [PubMed] [Google Scholar]
- 17.Morgenthaler NG, Struck J, Alonso C, Bergmann A. Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 2006; 52:112–9 [DOI] [PubMed] [Google Scholar]
- 18.Lattuca B, Sy V, Nguyen LS, Bernard M, Zeitouni M, Overtchouk P, Yan Y, Hammoudi N, Ceccaldi A, Collet J-P, Kerneis M, Diallo A, Montalescot G, Silvain J. Copeptin as a prognostic biomarker in acute myocardial infarction. Int J Cardiol 2019; 274:337–41 [DOI] [PubMed] [Google Scholar]
- 19.Choi HJ, Kim MC, Sim DS, Hong YJ, Kim JH, Jeong MH, Kim SH, Shin MG, Ahn Y. Serum copeptin levels predict clinical outcomes after successful percutaneous coronary intervention in patients with acute myocardial infarction. Ann Lab Med 2018; 38:538–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasan R, Lindarto D, Siregar GA, Mukhtar Z. The effect of Bay leaf extract Syzygium polyanthum (wight) Walp. on C-reactive protein (CRP) and myeloperoxidase (MPO) level in the heart of rat model of myocardial infarction. Med Glas (Zenica) 2020; 17(1):41–45 [DOI] [PubMed] [Google Scholar]
- 21.Libby P. Inflammation in atherosclerosis. Arterioscler Thromb Vasc Biol 2012; 32:2045–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudolph V, Goldmann BU, Bos C, Rudolph TK, Klinke A, Friedrichs K, Lau D, Wegscheider K, Haddad M, Meinertz T, Baldus S. Diagnostic value of MPO plasma levels in patients admitted for suspected myocardial infarction. Int J Cardiol 2011; 153:267–71 [DOI] [PubMed] [Google Scholar]
- 23.Sacks FM. Do statins play a role in the early management of the acute coronary syndrome? Eur Heart J Suppl 2004; 6:A32–A36 [Google Scholar]
- 24.Yao H, Lv J. Statin attenuated myocardial inflammation induced by PM2.5 in rats. Acta Cardiologica Sinica 2017; 33:637–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cozlea DL, Farcas DM, Nagy A, Keresztesi AA, Tifrea R, Cozlea L, Carasca E. The impact of C reactive protein on global cardiovascular risk on patients with coronary artery disease. Curr Health Sci J 2013; 39:225–31 [PMC free article] [PubMed] [Google Scholar]
- 26.Gronholdt ML, Sillesen H, Wiebe BM, Laursen H, Nordestgaard BG. Increased acute phase reactants are associated with levels of lipoproteins and increased carotid plaque volume. Eur J Vasc Endovasc Surg 2001; 21:227–34 [DOI] [PubMed] [Google Scholar]
- 27.Miyao Y, Yasue H, Ogawa H, Misumi I, Masuda T, Sakamoto T, Morita E. Elevated plasma interleukin-6 levels in patients with acute myocardial infarction. Am Heart J 1993; 126:1299–304 [DOI] [PubMed] [Google Scholar]
- 28.Elseweidy MM, Elswefy SE, Younis NN, Tarek S. The modulation of PCSK-9 and GAGs by 10-dehydrogingerdione and pentoxifylline in hyperlipidemic rabbits. Nat Prod Res 2018;1–6 [DOI] [PubMed] [Google Scholar]
- 29.El-Seweidy MM, Asker M-S, Eldahmy SI, Atteia HH, Abdallah MA. Haemostatic risk factors in dyslipidemic rabbits: role of 10-dehydrogingerdione as a new hypolipemic agent. J Thromb Thrombolysis 2015; 39:196–202 [DOI] [PubMed] [Google Scholar]
- 30.Elseweidy MM, Mohamed HE, Elrashidy RA, Atteia HH, Elnagar GM, Ali A-M. Potential therapeutic roles of 10-dehydrogingerdione and/or pentoxifylline against calcium deposition in aortic tissues of high dietary cholesterol-fed rabbits. Mol Cell Biochem 2019; 453:131–42 [DOI] [PubMed] [Google Scholar]
- 31.Elseweidy MM, Elswefy SE, Younis NN, Tarek S. Contribution of aorta glycosaminoglycans and PCSK9 to hyperlipidemia in experimental rabbits: the role of 10-dehdrogingerdione as effective modulator. Mol Biol Rep 2019; 46:3921–28 [DOI] [PubMed] [Google Scholar]
- 32.Elseweidy MM, Zaghloul MS, Younis NN. 10-DHGD ameliorates cisplatin-induced nephrotoxicity in rats. Biomed Pharmacother 2016; 83:241–46 [DOI] [PubMed] [Google Scholar]
- 33.Saponaro F, Marcocci C, Zucchi R. Vitamin D status and cardiovascular outcome. J Endocrinol Invest 2019; [DOI] [PubMed] [Google Scholar]
- 34.Kunadian V, Ford GA, Bawamia B, Qiu W, Manson JE. Vitamin D deficiency and coronary artery disease: a review of the evidence. Am Heart J 2014; 167:283–91 [DOI] [PubMed] [Google Scholar]
- 35.Liu Y, Peng W, Li Y, Wang B, Yu J, Xu Z. Vitamin D deficiency harms patients with coronary heart disease by enhancing inflammation. Med Sci Monit 2018; 24:9376–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reid IR, Bolland MJ. Role of vitamin D deficiency in cardiovascular disease. Heart 2012; 98:609–14 [DOI] [PubMed] [Google Scholar]
- 37.Zittermann A. Vitamin D and cardiovascular disease. Anticancer Res 2014; 34:4641–8 [PubMed] [Google Scholar]
- 38.Yousefi Rad E, Djalali M, Koohdani F, Saboor-Yaraghi AA, Eshraghian MR, Javanbakht MH, Saboori S, Zarei M, Hosseinzadeh-Attar MJ. The effects of vitamin D supplementation on glucose control and insulin resistance in patients with diabetes type 2: a randomized clinical trial study. Iran J Public Health 2014; 43:1651–6 [PMC free article] [PubMed] [Google Scholar]
- 39.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia 2008; 51:216–26 [DOI] [PubMed] [Google Scholar]
- 40.Rohilla A, Ali S. Alloxan induced diabetes: mechanisms and effects. Int J Res Pharm Biomed Sci 2012; 3(2):819–23 [Google Scholar]
- 41.Yang W, Shi H, Zhang J, Shen Z, Zhou G, Hu M. Effects of the duration of hyperlipidemia on cerebral lipids, vessels and neurons in rats. Lipids Health Dis 2017; 16:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elseweidy MM, Zein N, Aldhamy SE, Elsawy MM, Saeid SA. Policosanol as a new inhibitor candidate for vascular calcification in diabetic hyperlipidemic rats. Exp Biol Med (Maywood) 2016; 241:1943–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fallah Huseini H, Abdolghaffari AH, Ahwazi M, Jasemi E, Yaghoobi M, Ziaee M. Topical application of Teucrium polium can improve wound healing in diabetic rats. Int J Lower Extremity Wounds 2019;19(2):132--138 [DOI] [PubMed] [Google Scholar]
- 44.Rodríguez-Magaña MP, Cordero-Pérez P, Rivas-Morales C, Oranday-Cárdenas MA, Moreno-Peña DP, García-Hernández DG, Leos-Rivas C. Hypoglycemic activity of Tilia americana, Borago Officinalis, Chenopodium nuttalliae, and piper sanctum on wistar rats. J Diabetes Res 2019; 2019:7836820–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah NA, Khan MR. Antidiabetic effect of Sida cordata in alloxan induced diabetic rats. Biomed Res Int 2014; 2014:671294–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben Salem M, Ben Abdallah Kolsi R, Dhouibi R, Ksouda K, Charfi S, Yaich M, Hammami S, Sahnoun Z, Zeghal KM, Jamoussi K, Affes H. Protective effects of Cynara scolymus leaves extract on metabolic disorders and oxidative stress in alloxan-diabetic rats. BMC Complement Altern Med 2017; 17:328–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guadarrama-López AL, Valdés-Ramos R, Martínez-Carrillo BE. Type 2 diabetes, PUFAs, and vitamin D: their relation to inflammation. J Immunol Res 2014; 2014:860703–03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qinna NA, Badwan AA. Impact of streptozotocin on altering normal glucose homeostasis during insulin testing in diabetic rats compared to normoglycemic rats. Drug Des Devel Ther 2015; 9:2515–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deepa PR, Varalakshmi P. Atheroprotective effect of exogenous heparin-derivative treatment on the aortic disturbances and lipoprotein oxidation in hypercholesterolemic diet fed rats. Clin Chim Acta 2005; 355:119–30 [DOI] [PubMed] [Google Scholar]
- 50.Farhangi MA, Mesgari-Abbasi M, Nameni G, Hajiluian G, Shahabi P. The effects of vitamin D administration on brain inflammatory markers in high fat diet induced obese rats. BMC Neurosci 2017; 18:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farhangi MA, Nameni G, Hajiluian G, Mesgari-Abbasi M. Cardiac tissue oxidative stress and inflammation after vitamin D administrations in high fat-diet induced obese rats. BMC Cardiovasc Disord 2017; 17:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salum E, Kampus P, Zilmer M, Eha J, Butlin M, Avolio AP, Põdramägi T, Arend A, Aunapuu M, Kals J. Effect of vitamin D on aortic remodeling in streptozotocin-induced diabetes. Cardiovasc Diabetol 2012; 11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi S-Y, Park G-S, Lee SY, Kim JY, Kim YK. The conformation and CETP inhibitory activity of [10]-dehydrogingerdione isolated from Zingiber officinale. Arch Pharm Res 2011; 34:727–31 [DOI] [PubMed] [Google Scholar]
- 54.Elseweidy MM, Abdallah FR, Younis NN, Aldohmy S, Kassem HM. 10-Dehydrogingerdione raises HDL-cholesterol through a CETP inhibition and wards off oxidation and inflammation in dyslipidemic rabbits. Atherosclerosis 2013; 231:334–40 [DOI] [PubMed] [Google Scholar]
- 55.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc 2008; 2008:pdb prot4986 [DOI] [PubMed] [Google Scholar]
- 56.Chait A, Goldberg I. Treatment of dyslipidemia in diabetes: recent advances and remaining questions. Curr Diab Rep 2017; 17:112. [DOI] [PubMed] [Google Scholar]
- 57.Ojiako OA, Chikezie PC, Ogbuji AC. Blood glucose level and lipid profile of alloxan-induced hyperglycemic rats treated with single and combinatorial herbal formulations. J Tradit Complement Med 2016; 6:184–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin MJ, Chang YJ, Chen CY, Huang CC, Chuang TY, Wu HP. Influence of hypercholesterolemia and diabetes on long-term outcome in patients with stable coronary artery disease receiving percutaneous coronary intervention. Medicine 2019; 98:e16927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iftikhar A, Aslam B, Iftikhar M, Majeed W, Batool M, Zahoor B, Amna N, Gohar H, Latif I. Effect of Caesalpinia bonduc polyphenol extract on alloxan-induced diabetic rats in attenuating hyperglycemia by upregulating insulin secretion and inhibiting JNK signaling pathway. Oxid Med Cell Longev 2020; 2020:9020219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koh EM, Kim HJ, Kim S, Choi WH, Choi YH, Ryu SY, Kim YS, Koh WS, Park SY. Modulation of macrophage functions by compounds isolated from Zingiber officinale. Planta Med 2009; 75:148–51 [DOI] [PubMed] [Google Scholar]
- 61.Lee HY, Park SH, Lee M, Kim HJ, Ryu SY, Kim ND, Hwang BY, Hong JT, Han SB, Kim Y. 1-Dehydro-[10]-gingerdione from ginger inhibits IKKbeta activity for NF-kappaB activation and suppresses NF-kappaB-regulated expression of inflammatory genes. Br J Pharmacol 2012; 167:128–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bekkouch O, Harnafi M, Touiss I, Khatib S, Harnafi H, Alem C, Amrani S. In vitro antioxidant and in vivo lipid-lowering properties of Zingiber officinale crude aqueous extract and methanolic fraction: a follow-up study. Evid Based Complement Alternat Med 2019; 2019:9734390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Elseweidy MM, Mohamed HE, Elrashidy RA, Atteia HH, Elnagar GM, Ali AE. Potential therapeutic roles of 10-dehydrogingerdione and/or pentoxifylline against calcium deposition in aortic tissues of high dietary cholesterol-fed rabbits. Mol Cell Biochem 2018; 453(1-2):131–142 [DOI] [PubMed] [Google Scholar]
- 64.Elseweidy MM, Amin RS, Atteia HH, Ali MA. Vitamin D3 intake as regulator of insulin degrading enzyme and insulin receptor phosphorylation in diabetic rats. Biomed Pharmacother 2017; 85:155–59 [DOI] [PubMed] [Google Scholar]
- 65.Johnson JA, Grande JP, Roche PC, Kumar R. Immunohistochemical localization of the 1,25(OH)2D3 receptor and calbindin D28k in human and rat pancreas. Am J Physiol 1994; 267:E356–60 [DOI] [PubMed] [Google Scholar]
- 66.Milajerdi A, Ostadmohammadi V, Amirjani S, Kolahdooz F, Asemi Z. The effects of vitamin D treatment on glycemic control, serum lipid profiles, and C-reactive protein in patients with chronic kidney disease: a systematic review and meta-analysis of randomized controlled trials. Int Urol Nephrol 2019; 51:1567–80 [DOI] [PubMed] [Google Scholar]
- 67.Al-Shoumer KA, Al-Essa TM. Is there a relationship between vitamin D with insulin resistance and diabetes mellitus? WJD 2015; 6:1057–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kumar S, Davies M, Zakaria Y, Mawer EB, Gordon C, Olukoga AO, Boulton AJ. Improvement in glucose tolerance and beta-cell function in a patient with vitamin D deficiency during treatment with vitamin D. Postgrad Med J 1994; 70:440–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Maestro B, Molero S, Bajo S, Davila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3). Cell Biochem Funct 2002; 20:227–32 [DOI] [PubMed] [Google Scholar]
- 70.Derakhshanian H, Djalali M, Mohammad Hassan MH, Alvandi E, Eshraghian MR, Mirshafiey A, Nadimi H, Jahanabadi S, Zarei M, Djazayery A. Vitamin D suppresses cellular pathways of diabetes complication in liver. Iranian J Basic Med Sci 2019; 22:690–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dunlop TW, Vaisanen S, Frank C, Molnar F, Sinkkonen L, Carlberg C. The human peroxisome proliferator-activated receptor Delta gene is a primary target of 1alpha,25-dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol 2005; 349:248–60 [DOI] [PubMed] [Google Scholar]
- 72.Derakhshanian H, Djazayery A, Javanbakht MH, Eshraghian MR, Mirshafiey A, Jahanabadi S, Ghadbeigi S, Zarei M, Alvandi E, Djalali M. Vitamin D downregulates key genes of diabetes complications in cardiomyocyte. J Cell Physiol 2019; 234:21352–58 [DOI] [PubMed] [Google Scholar]
- 73.Ning C, Liu L, Lv G, Yang Y, Zhang Y, Yu R, Wang Y, Zhu J. Lipid metabolism and inflammation modulated by vitamin D in liver of diabetic rats. Lipids Health Dis 2015; 14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Imga NN, Karci AC, Oztas D, Berker D, Guler S. Effects of vitamin D supplementation on insulin resistance and dyslipidemia in overweight and obese premenopausal women. AOMS 2019; 15:598–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdel-Rehim WM, El-Tahan RA, El-Tarawy MA, Shehata RR, Kamel MA. The possible antidiabetic effects of vitamin D receptors agonist in rat model of type 2 diabetes. Mol Cell Biochem 2019; 450:105–12 [DOI] [PubMed] [Google Scholar]
- 76.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr 2004; 80:1717S–20S [DOI] [PubMed] [Google Scholar]
- 77.Liefaard MC, Ligthart S, Vitezova A, Hofman A, Uitterlinden AG, Kiefte-de Jong JC, Franco OH, Zillikens MC, Dehghan A. Vitamin D and C-reactive protein: a Mendelian randomization study. PLoS One 2015; 10:e0131740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhang Y, Leung DY, Richers BN, Liu Y, Remigio LK, Riches DW, Goleva E. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol 2012; 188:2127–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou W, Ye S-D, Chen C, Wang W. Involvement of RBP4 in diabetic atherosclerosis and the role of vitamin D intervention. J Diabetes Res 2018; 2018:7329861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sabry HH, Sabry JH, Daifalla AEH, Akl EM, Hamed AM, Torky AA. Serum markers for asymptomatic atherosclerosis in Egyptian psoriatic patients: study controlled by Doppler estimation of carotid intima-media thickness. Vasc Health Risk Manag 2018; 14:145–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei-Hong T, Min-Chang G, Zhen X, Jie S. Pharmacological and pharmacokinetic studies with vitamin D-loaded nanoemulsions in asthma model. Inflammation 2014; 37:723–28 [DOI] [PubMed] [Google Scholar]
- 82.da Silva JLG, Passos DF, Bernardes VM, Cabral FL, Schimites PG, Manzoni AG, de Oliveira EG, de Bona da Silva C, Beck RCR, Jantsch MH, Maciel RM, Leal D. Co-Nanoencapsulation of vitamin D3 and curcumin regulates inflammation and purine metabolism in a model of arthritis. Inflammation 2019; 42:1595–610 [DOI] [PubMed] [Google Scholar]
- 83.Mellor-Pita S, Tutor-Ureta P, Rosado S, Alkadi K, Granado F, Jimenez-Ortiz C, Castejon R. Calcium and vitamin D supplement intake may increase arterial stiffness in systemic lupus erythematosus patients. Clin Rheumatol 2019; 38:1177–86 [DOI] [PubMed] [Google Scholar]
- 84.Niizuma S, Iwanaga Y, Yahata T, Miyazaki S. Renocardiovascular biomarkers: from the perspective of managing chronic kidney disease and cardiovascular disease. Front Cardiovasc Med 2017; 4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reinstadler SJ, Klug G, Feistritzer H-J, Metzler B, Mair J. Copeptin testing in acute myocardial infarction: ready for routine use? Dis Markers 2015; 2015:614145. [DOI] [PMC free article] [PubMed] [Google Scholar]