Abstract
Although clinical treatment has significant progress, acute pulmonary embolism is still a common disease with high morbidity and mortality. Janus Kinase 3, a member of JAK family, has been demonstrated to promote smooth muscle cell proliferation through STAT3. In this work, we explored the effect of JANEX-1 (a specific Janus Kinase 3 inhibitor) on platelet-derived growth factor (PDGF)-induced proliferation-related molecules in pulmonary artery smooth muscle cells (PVSMCs) in vitro and assessed the therapeutic potential of Janus Kinase 3 for vascular remodeling in acute pulmonary embolism mice. The results revealed that Janus Kinase 3 was overexpressed and active in PDGF-induced PVSMCs and acute pulmonary embolism mice, compared to a low expression in normal conditions. JANEX-1, blocking Janus Kinase 3 expression or activity, reduced Janus Kinase 3/STAT3 signaling pathway, VEGF expression, FAK activation, and PDGF-induced proliferation of PVSMCs, while overexpression of VEGF or FAK induced PVSMCs proliferation and resisted the negative effects of JANEX-1. Moreover, JANEX-1 improved right ventricular systolic pressure, survival and lung damage in acute pulmonary embolism-mice, and inhibited the thrombus-induced intimal hyperplasia and the expression of α-SMA, VEGF, and FAK activation under neointimal smooth muscle cells of acute pulmonary embolism mice. In conclusion, the data suggest that JANEX-1 exerts protective effects by inhibiting PVSMCs proliferation and vascular remodeling post-acute pulmonary embolism, in part through Janus Kinase 3/STAT3 signaling pathway-mediated VEGF expression and FAK activation. The data are helpful to elucidate the pharmacological mechanism and potential therapeutic effect of JANEX-1 in APE.
Impact statement
Accumulating evidence suggests that vascular remodeling due to immoderate proliferation and migration of SMCs is a common process occurring in APE. In this work, we tried to find a breakthrough in the pathological mechanism to alleviate the prognosis of APE by improving SMCs proliferation and explored the effect of JANEX-1 on PDGF-induced proliferation-related molecules in PVSMCs and assessed the therapeutic potential of JAK3 for vascular remodeling in APE mice. We demonstrated that JANEX-1, blocking JAK3 expression or activity, reduced JAK3/STAT3 signaling pathway, VEGF expression and FAK activation, and PDGF-induced proliferation of PVSMCs. Moreover, JANEX-1 inhibited the thrombus-induced intimal hyperplasia and the expression of VEGF and FAK activation in neointimal SMCs of APE mice. The data are helpful to elucidate the pharmacological mechanism and potential therapeutic effect of JANEX-1 in APE.
Keywords: Acute pulmonary embolism, pulmonary artery smooth muscle cells, JAK3/STAT3 signaling, JANEX-1, VEGF, FAK
Introduction
As one of the important factors of acute cardiovascular disease, acute pulmonary embolism (APE) can cause pulmonary hypertension (PH) by initiating pulmonary embolism, which may lead to heart failure and death.1 Studies have demonstrated that immoderate proliferation and migration of smooth muscle cells (SMCs) induce PH by remodeling the vascular system.2–5 Although clinical treatments including anticoagulant and thrombolytic methods have shown significant progress,6,7 many relative contraindications to treatments were exclusion criteria in the clinical trials and APE is still a common disease with high morbidity and mortality.8,9 Therefore, there is an urgent need to further study the pathological mechanism and treatment strategies of pulmonary embolism.
Janus Kinase (JAK) is a member of the non-receptor tyrosine Kinase family, and transmits signals from the transmembrane receptor to the nucleus, regulating the transcription of target genes to mediate the differentiation, proliferation, and death of cells, through the JAK/STAT pathways.10–12 The JAK family includes JAK1, JAK2, JAK3, and Tyk2: JAK1 regulates innate immunity, T-cell differentiation, and inflammation when it pairs with JAK2 and/or TYK2; JAK1 and JAK3 are associated with lymphocyte proliferation and homeostasis; While JAK2 pairs with itself to modulate erythropoiesis, myelopoiesis, and thrombocytopoiesis.13 JAK3, not as widely expressed and related to extensive cytokine receptors as other members of the family, is mainly expressed in the lymphoid or myeloid cell lineages, and it can be stimulated by some specific cytokine receptors which include the γ chain, such as IL-2, IL-4, and IL-7.14 JAK3 plays an important role in the immune system as a target of the immunosuppressive agent.15 JAK3 deficiency affects T lymphocyte differentiation and proliferation, causing serious combined immunodeficiency.16,17 In vascular cells and other non-hematopoietic cells, JAK3 is also expressed and induced by LPS, TNF-α, and IL-1.17,18 Recently, it has been demonstrated that JAK3 promotes SMCs proliferation through STAT3 and JNK, and JAK3 downregulation decreases platelet-derived growth factor (PDGF)-induced neointimal formation and improves SMCs proliferation and apoptosis levels.19 Furthermore, JAK3 inhibitor JANEX-1 suppresses TNF-α-induced ICAM-1, VCAM-1, and fractalkine in human umbilical vein endothelial cells via inhibiting STAT3 and NF-κB activation, and retards the high level of LPS-induced cardiac ICAM-1 in arteriolar and capillary endothelial cells, and alleviates myocardial vascular leakage.20 Thus, JAK3 may play a pivotal role in pulmonary embolism-induced artery SMCs (VSMCs) proliferation and intimal hyperplasia.
JANEX-1 inhibits JAK3 but not JAK1, JAK2, or the activity of other protein tyrosine kinases.21 In this work, we tried to find a breakthrough in the pathological mechanism to alleviate the prognosis of APE by improving SMCs proliferation, and explored the effect of JANEX-1 on PDGF-induced proliferation-related molecules in pulmonary artery SMCs (PVSMCs) and assessed the therapeutic potential of JAK3 for vascular remodeling in APE mice.
Materials and methods
Mice APE model
Eight-week male C57BL/6 mice were purchased from animal centers of Xi'an Jiaotong University and fed in the standard mouse feeding facility with a 12 h/12-h light/dark cycle and free foraging and activity under 22–24°C. All animal experiments were performed according to the NIH Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of Xi'an Jiaotong University (No. XJTULAC2019-1145). The APE model was produced using the autologous blood clot method.22–24 Briefly, after anesthetized by 6% chloral hydrate (600 mg/kg), 100 μL blood was drawn from the lateral tail veins of the mice (n = 96). After coagulation, blood clots were put in a 70°C water bath for 10 min and then cut into a size of 1.0 mm × 2 mm. A total of 25 autologous blood clots followed by 0.4 mL normal saline were injected. At 24 h post-operation, mice in the JANEX-1 treatment group were treated with JANEX-1 (Selleck, Shanghai, China; 20, 50 and 100 mg/kg/d), for seven continuous days. Mice in the control and APE groups were given a subcutaneous injection of the same volume of normal saline. All the mice had free access to water and food after the operation. The mortality of the mice in each group was monitored during 15 days (total 60 mice, n = 10 per group). A total of 66 mice were killed by carbon dioxide asphyxiation on days 7 and 15.
Right ventricular systolic pressure
RVSP was measured at seven days after treatment. Right heart catheterization was performed on anaesthetized, spontaneously breathing mice (total 36 mice, n = 6 per group). Mice were anaesthetized with inhaled isoflurane (1%–3%). The right ventricular pressure data were analyzed using the LabChart 7 program (AD Instruments). The animal was euthanized (mice were put to death by carbon dioxide asphyxiation (flow rate displacing no more than 30% of the chamber volume/minute) for 2–3 min) while still under anesthesia, and lung tissues were obtained for analyzing lung index (lung index = lung weight (mg)/body weight (g) × 100%), hematoxylin-eosin (HE) staining, and other experiments.
PDGF-induced PVSMCs model
Mouse PVSMCs were purchased from ScienCell, (San Diego, CA, USA). PVSMCs were cultured in DMEM medium (Gibco, Grand Island, NY, USA) with 10% FBS (Gibco); 70%–80% PVSMCs were treated with 10, 20, or 40 ng/mL PDGF (Sigma-Aldrich, St. Louis, MO, USA) to induce cell proliferation,25,26 and then treated with 20, 50, and 100 μM JANEX-1 or not for 24 h.
Transfection
JAK3 shRNA Particles (sc-35721-SH; Santa Cruz Biotechnology, Santa Cruz, CA, USA), VEGF Activation Particles (sc-423665-ACT; Santa Cruz Biotechnology), and FAK Activation Particles (sc-420280-ACT; Santa Cruz Biotechnology) were used for transfection. We performed according to the manufacturer's instructions (Santa Cruz Biotechnology): 70%–80% PVSMCs were treated with the moderate lentiviral particles, incubated at 37°C for 6 h and then replaced the fresh medium. PVSMCs were harvested and prepared for further testing after 48 h of transfection. qRT-PCR analysis was used for measuring the transfection efficiency (in Figure S1).
CCK8 assay
Cell proliferation was detected using the CCK8 assay. PVSMCs were plated in 96-well plates in triplicate at approximately 3 × 104 cells per well and cultured in the growth medium. After treatment, the 10 μL CCK8 solution (Dojindo, Kumamoto, Japan) was added to the culture medium, and the cultures were incubated for 1 h at 37°C, 5% CO2. The absorbance was measured at 450 nm using a microplate reader (Invitrogen, Waltham, MA, USA).
qRT-PCR assay
Total RNA was extracted from the pulmonary arteries or treated PVSMCs using TRIzol reagent (Invitrogen, Waltham, MA, USA). HiFi-MMLV cDNA First-Strand Synthesis Kit (CW Bio, Beijing, China) was used for reverse transcription. qRT-PCR experiments were conducted through SYBR Green Real-Time PCR Master Mix (TaKaRa, Tokyo, Japan). The primers used in this study are as follows: JAK3, forward 5′-TGACAAGTGGGGCTTTGGAG -3′ and reverse 5′-TCTGTCCATTTGAGAGCGGG-3′; PCNA, forward 5′-GAGAGCTTGGCAATGGGAAC -3′ and reverse 5′- TCTCTATGGTTACCGCCTCCT-3′; GAPDH, F 5′-AACTTTGGCATTGTGGAAGG-3′, R 5′-GGATGCAGGGATGATGTTCT-3′. The relative expression value was calculated using the 2−ΔΔCT method.
Western blot assay
Total protein was lysed in RIPA lysis buffer (Beyotime, Shanghai, China). After SDS-PAGE electrophoresis and Western transfer, the PVDF membrane (Millipore, Billerica, MA, USA) was blocked with 5% BSA (Sigma‐Aldrich) and incubated with the appropriate primary antibodies: JAK3 (CST, 1:1500), p-JAK3 (CST, 1:1000), STAT3 (CST, 1:1000), p-STAT3 (CST, 1:1000), VEGF (Abcam, 1:2000), FAK (Abcam, 1:1000), p-FAK (Abcam, 1:1000), PCNA(Abcam, 1:1000), and anti-GAPDH (CST, 1:2000) at 4°C overnight, and incubated with secondary antibody (Abbkine, Soochow, China; 1:30,000) for 1 h at room temperature, followed by chemiluminescent substrate development (Bio-Rad, Hercules, CA) and detected by an imaging system (Bio-Rad). GAPDH was defined as the internal reference, protein relative expression was defined as target protein versus GAPDH, and phosphorylation level was defined as phosphorylation versus total protein.
Immunohistochemistry
Lung tissues were obtained and immediately perfused with 4% paraformaldehyde in PBS after removal and embedded in paraffin for sectioning. Following rehydration of the paraffin section and two washes in PBS, endogenous peroxidase activity was blocked using 3% H2O2 for 10 min, and antigen crosslinking was conducted in an autoclave for 3 min. After blocking with normal bovine serum, the slides were incubated with primary antibody against VEGF and p-FAK overnight at 4°C. Subsequently, slides were incubated with streptavidin peroxidase-conjugated secondary goat anti-rabbit IgG (Abbkine), and then stained with a DAB kit (Beyotime, Shanghai, China).
Immunofluorescence
The slides of lung tissues were incubated with primary antibody against α-SMA overnight at 4°C, then with Alexa Fluor 594-conjugated secondary antibody at 37°C for 2 h. Cell nuclei were stained with DAPI (Sigma-Aldrich) and images were obtained using a fluorescence microscope (Olympus, Osaka, Japan).
Statistical analysis
Data are shown as the mean ± SEM. All statistical analyses were conducted with GraphPad Prism 7.0. Statistical differences were determined using the Student’s t-test or one-way ANOVA followed by Tukey’s multiple comparison test. A value of P < 0.05 was considered significant.
Results
PDGF-induced JAK3 expression and phosphorylation in PVSMCs
To show the effect of JAK3 on PVSMCs, JAK3 expression and phosphorylation were detected after treatment with 0, 10, 20, or 40 ng/mL PDGF in PVSMCs. As shown in Figure 1(a) and (b), PDGF significantly increased JAK3 mRNA and protein expression, and p-JAK3 with a dose-dependence, and along with the expression of PCNA, which is a proliferation-related protein. JAK3 activity and PCNA showed prominently high levels when treated with 20 ng/mL PDGF in PVSMCs, so 20 ng/mL PDGF was used in the following test. The expression of JAK3 was time-dependent within 48 h, while the peak for p-JAK3 was at 24 h, correlating with PCNA expression (Figure 1(c)). This suggests that JAK3 activity may play an important role in PDGF-induced PVSMCs proliferation.
Figure 1.
PDGF-induced JAK3 expression and phosphorylation in PVSMCs. After treatment with 0, 10, 20, or 40 ng/mL PDGF for 24 h in PVSMCs, JAK3 mRNA level (a), and JAK3, p-JAK3, and PCNA (b) were detected by qRT-PCR and WB assays and were dose-dependent. (c) JAK3, p-JAK3, and PCNA expression were time-dependent after treatment with 20 ng/mL PDGF during 48 h. Samples derived from the same experiment and gels/blots were processed in parallel. Data are presented as the mean ± SEM (n = 4). *P < 0.05 vs. control group. Ctrl: control.
JANEX-1 mitigated PDGF-induced PVSMCs proliferation
JANEX-1 is a specific inhibitor of JAK3, so its effect on PVSMCs was probed. In normal conditions, JANEX-1 did not influence PVSMCs proliferation (Figure 2(a)), while it suppressed PDGF-induced PVSMCs proliferation in a dose-dependent manner (Figure 2(b)). As there was no significant difference in the inhibition of cell activity between 50 μM and 100 μM treatment of JANEX (Figure 2(b)), a 50 μM JANEX treatment was used in the subsequent experiments. As shown in Figure 2(c), 50 μM JANEX could reduce PCNA expression in PDGF-nontreated PVSMCs, and it distinctly alleviated 20 ng/mL PDGF-induced PCNA expression of PVSMCs.
Figure 2.
JANEX-1 alleviated PDGF-induced PVSMCs proliferation. PVSMCs were treated with 20 ng/mL PDGF or not, and then treated with 20, 50 μM and 100 μM JANEX-1 or not, subsequently, cell viability was monitored by CCK8 assay. (a) JANEX-1 did not inhibit PVSMCs proliferation under normal conditions. (b) JANEX-1 dose-dependently suppressed PDGF-induced PVSMCs proliferation. (c) 50 μM JANEX alleviated PDGF-induced PCNA expression in PVSMCs. Samples derived from the same experiment and gels/blots were processed in parallel. Data are presented as the mean ± SEM (n = 4). *P < 0.05 vs. control group. #P < 0.05 vs. PDGF group. JANEX-1 L, 20 μM; JANEX-1 M, 50 Μm; JANEX-1 H, 100 Μm.
JANEX-1 inhibited VEGF and p-FAK expression in PDGF-induced PVSMCs
To confirm regulators of JANEX-1 regulating PVSMCs proliferation, VEGF and p-FAK levels were monitored after PDGF treatment, and they were found to be related to cell proliferation. JANEX-1 suppressed p-JAK3, p-STAT3, VEGF, and p-FAK expression in PVSMCs, and JANEX-1 significantly inhibited the PDGF-induced JAK3/STAT3 signal, VEGF and p-FAK levels (Figure 3(a)). As shown in Figure 3(b), knockdown of JAK3 (sh-JAK3) also suppressed PDGF-induced PVSMCs proliferation through the JAK3/STAT3 signal, VEGF and p-FAK. To further determine that JANEX-1 suppressed PVSMCs proliferation through blocking VEGF expression and FAK activation, VEGF activation particles (Act-VEGF) and FAK activation particles (Act-FAK) were used to manipulate VEGF and FAK expression in PVSMCs. Figure 4(a) and (b) shows that VEGF and FAK cDNA induced PVSMCs proliferation and JANEX-1 inhibited this facilitation. Act-VEGF stimulated VEGF and PCNA expression, and JANEX-1 dramatically alleviated Act-VEGF-induced VEGF and PCNA upregulation (Figure 4(c)). Meanwhile, Act-FAK stimulated FAK activation and PCNA expression, and JANEX-1 significantly inhibited the Act-FAK-induced high levels of p-FAK and PCNA (Figure 4(d)). That is to say, overexpression of VEGF or FAK induced PVSMCs proliferation and resisted the negative effects of JANEX-1.
Figure 3.
JANEX-1 inhibited PDGF-induced JAK3/STAT3 signal, VEGF and p-FAK. (a) PVSMCs treated with PDGF or not, and then treated with JANEX-1 for 24 h. WB analysis of p-JAK3, p-STAT3, VEGF, and p-FAK levels in PVSMCs. (b) PVSMCs with JAK3 knockdown (sh-JAK3) were used to detect the effect of JAK3 downregulation on PDGF induction. WB analysis of p-JAK3, p-STAT3, VEGF, and p-FAK levels in PVSMCs. sh-JAK3 suppressed PDGF-induced PVSMCs proliferation through JAK3/STAT3 signal, VEGF, and p-FAK. Samples derived from the same experiment and gels/blots were processed in parallel. Data are presented as the mean ± SEM (n = 4). *P < 0.05 vs. control group. #P < 0.05 vs. PDGF group.
Figure 4.
VEGF expression and FAK activation were involved in the blocking of JANEX-1 on PDGF-induced PVSMCs proliferation. To further determine whether JANEX-1 suppressed PVSMCs proliferation through blocking VEGF expression and FAK activation, VEGF-activated and FAK-activated PVSMCs were used respectively. CCK8 assay was performed to demonstrate the proliferation inhibition of JANEX-1 in VEGF-activated (a) and FAK-activated (b) PVSMCs. WB analysis of, VEGF, p-FAK, and PCNA expression in VEGF-activated (c) and FAK-activated (d) PVSMCs. Samples derived from the same experiment and gels/blots were processed in parallel. Data are presented as the mean ± SEM (n = 4). (c) *P < 0.05 vs. Act-NC group. #P < 0.05 vs. Act-VEGF group. &P < 0.05 vs. Act-VEGF + JANEX-1 group. (d) *P < 0.05 vs. Act-NC group. #P < 0.05 vs. Act-FAK group. &P < 0.05 vs. Act-FAK + JANEX-1 group. Act: activation; NC: negative control.
JANEX-1 improved mortality and pulmonary vascular remodeling in APE-mice
Based on the results of in vitro cell experiments, we further investigated the effects of JANEX-1 in APE mice. After JANEX-1 treatment, some mice were killed on day 7 to assess lung index and pulmonary vascular remodeling, while other mice were kept alive to assess survival rate until day 15. We found that control recipient mice injected with autologous blood clot developed APE, as evidenced by increased RVSP seven days after injection, whereas JANEX-1 (50 and 100 mg/kg) treated mice had lower RVSP compared with the APE group and the 100 mg/kg JANEX-1 group presented a minimum RVSP in APE mice (Figure 5(a)). As shown in Figure 5(b) and (c), a high concentration of JANEX-1 (100 mg/kg) did not influence normal mice mortality rate and lung index. APE caused mass death in mice within seven days, and the survival rate was about 30% in the APE group on day 7, but all the remaining mice died on day 10. JANEX-1 reduced the risk of death, and the survival rate remained above 60% on day 7 and above 20% on day 15 in JANEX-1 groups (20, 50, and 100 mg/kg), and the high concentration JANEX-1 showed a better reduction in APE-induced mice mortality (Figure 5(b)). JANEX-1 showed the same trend of protective effect on the lung index (Figure 5(c)).
Figure 5.
JANEX-1 improved survival and lung damage in APE-mice. RVSP was measured at seven days after JANEX-1 (20, 50, and 100 mg/kg) treatment (n = 6) (a). After JANEX-1 treatment, some mice were kept alive with recording the death of the mice every day and analyzing the survival rate until day 15 (n = 10) (b). Meanwhile, other mice were put to death at day 7, and lung tissue was obtained for analyzing lung index (lung index = lung weight (mg)/body weight (g) × 100%) for assessing lung index (n = 6) (c). Data are presented as the mean ± SEM. *P < 0.05 vs. Ctrl (control) group. #P < 0.05 vs. APE group. (A color version of this figure is available in the online journal.)
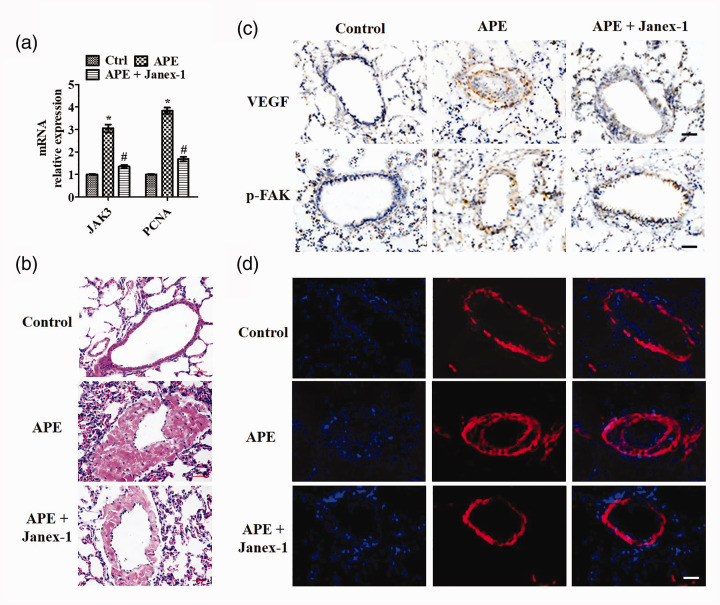
Since SMC proliferation is a pivotal process of vascular remodeling, we monitored the mRNA levels of JAK3 and PCNA. As shown in Figure 6(a), APE induced expression of JAK3 and PCNA mRNA in the pulmonary artery, and JAK3 was about 3.05-fold and PCNA was about 3.83-fold related to the control group. After JANEX-1 (100 mg/kg) treatment, JAK3 and PCNA levels were significantly decreased compared with the APE group, with these levels being 1.35-fold and 1.68-fold, respectively, compared to the control group. To demonstrate that JANEX-1 also inhibited pulmonary vascular remodeling in vivo, HE staining and α-SMA staining were used to detect the intimal formation after vascular injury. HE staining reflected that APE induced narrowed blood vessels and intimal formation (Figure 6(b) and Figure S2(a)), upregulated VEGF and p-FAK expression (Figure 6(c) and Figure S2(b)), and increased α-SMA expression demonstrated a remarkable increase of VSMCs number in the neointimal layer (Figure 6(d) and Figure S2(c)) . However, JANEX-1 improved the neointima area and abolished the APE induced high levels of VEGF p-FAK, and α-SMA in the neointima of pulmonary vasculature (Figure 6(b) to (d) and Figure S2). The data suggested that JANEX-1 improved pulmonary vascular remodeling via inhibiting PVSMCs proliferation in APE mice.
Figure 6.
JANEX-1 suppressed pulmonary vascular remodeling in APE-mice. After JANEX-1 (100 mg/kg) treatment, mice were put to death on day 7, and pulmonary artery and lung tissue were obtained for analysis. (a) qRT-PCR analysis of JAK3 and PCNA mRNA in the pulmonary artery. Pulmonary vascular remodeling was evaluated by measuring the intimal formation and expression of VEGF, p-FAK, and α-SMA in lung sections. (b) HE staining of lung sections. (c) IHC analysis of VEGF and p-FAK expression in pulmonary vascular. Scale bar: 20 μm. (d) IF results of α-SMA expression. Scale bar: 20 μm. Data are presented as the mean ± SEM (n = 6). *P < 0.05 vs. Ctrl (control) group. #P < 0.05 vs. APE group. (A color version of this figure is available in the online journal.)
Discussion
Accumulating evidence has suggested that vascular remodeling due to immoderate proliferation and migration of SMCs is a common process occurring in APE. In our study, we demonstrated that JAK3 was overexpressed and active in PDGF-induced PVSMCs and APE mice, compared to a low expression under normal conditions. JANEX-1, blocking JAK3 expression or activity, reduced the JAK3/STAT3 signaling pathway, VEGF expression and FAK activation, and PDGF-induced proliferation of PVSMCs. Moreover, JANEX-1 inhibited the thrombus-induced intimal hyperplasia and the expression of VEGF and FAK activation in neointimal SMCs of APE mice.
Initially, JAK3 was widely studied in the differentiation and proliferation of T lymphocytes. JAK3 deficiency inhibits innate lymphoid (ILC) cells differentiation in the bone marrow at the ILC precursor and the pre-NK cell progenitor. Tofacitinib (pan-JAK inhibitor) and PF-06651600 (specific JAK3 inhibitor) break the production of IFN-γ in human intraepithelial ILC1, but not the production of IL-22 in ILC3,17 causing severe combined immunodeficiency, such as rheumatoid arthritis.27 Curculigoside exhibits prominent anti-arthritic effects via the JAK/STAT/NF‑κB signaling pathway.28 Furthermore, JAK3 is also present in other non-hematopoietic cells, such as vascular cells.17–19 JAK3 regulates the stability of the mucosa by mediating IL-2-induced migration, proliferation, and apoptosis of intestinal epithelial cells.29 JAK3 is involved in the progression of human colon cancer, renal fibrosis, vascular calcification, myocardial ischemia reperfusion injury, and various physiological changes,30,31 the JAK3/STAT1 pathway shows abnormal activation in brain microvascular endothelial cells (BMVECs) after brain injury, and JAK3 inhibitor decreases the permeability of BMVECs during brain inflammatory events.32 Oncostatin M induces osteoblastic differentiation of human VSMCs through the JAK3/STAT3 pathway, and JAK3 downregulation can prevent the ALP activity and matrix mineralization in umbilical artery human VSMCs.33 Hypoxia induces a high level of JAK3 expression in SMCs.34 In cigarette smoke-induced chronic obstructive pulmonary disease mice, ergosterol suppresses pathological injury to lung tissue through the JAK3/STAT3/NF-κB pathway.35 It has been verified that JAK3 promotes SMC proliferation and vascular remodeling via STAT3 and JNK activation.18,19 STAT3 and JNK are considered to be important regulatory factors of differentiation, proliferation, and death of SMCs and cancer cells.36 Activated STAT3 increases SMC proliferation and survival, contributing to the injury-induced neointimal formation.37,38 JAK3 may also mediate Bax/Bcl-2 level and cleaved-caspase 3, coordinating SMC survival and death, by igniting STAT3 and JNK signaling.18,19 We showed that JANEX-1 blocks the JAK3/STAT3 pathway and cell proliferation in PDGF-induced PVSMCs, and attenuates injury-induced neointimal formation in APE mice, which is consistent with VEGF and FAK suppression.
Studies confirm that VEGF is involved in vessel adaptation to an external stimulus such as hypoxia, inflammation, and degenerative processes related to cardiovascular disease.39 VEGF is a central pro-angiogenic growth factor necessary to promote the proliferation and migration of vascular endothelial cells and SMCs, thus promoting normal and pathological angiogenesis.40 Lv et al.41 have demonstrated that STAT3 regulated VEGF activation by directly interacting with the binding site on the 5ʹ region of the VEGF gene. VEGF is involved in STAT3-mediated pro-angiogenic activity of SMCs, suggesting that it promotes SMC activation in angiogenesis during vascular remodeling, and this regulatory activity is related to the signaling cascade of STAT3/VEGF.41,42 FAK, a tyrosine kinase, mainly localized to cellular focal contacts, is associated with cell adhesion, migration, and invasion, and over-expressed and highly activated FAK is found in a variety of tumors.43 Via transmitting signals from the extracellular matrix by binding to integrins and transmitting signals from soluble bioactive factors through special receptors, the mechanism of FAK also has been demonstrated in human PVSMCs.44 Lin et al.44 have recently found that RELM-β promotes human PVSMCs proliferation and increases FAK and survivin levels, suggesting that FAK is an important upstream of survivin in the signaling of RELM-β-mediated PVSMCs proliferation. Banerjee et al.45 demonstrated that loss of FAK signaling during endoplasmic reticulum stress causes mitochondrial dysfunction by reducing the protective effects of mitochondrial STAT3, leading to endothelial cell death. Visavadiya et al.46 show that FAK inhibition reduced pS727-STAT3 within mitochondria and reduced mitochondrial function in a non-transcriptional manner, while S727-STAT3 activators rescue mitochondrial function and cells against FAK inhibition in bEnd5 cells. The increase in STAT3-induced pro-angiogenic activity of airway SMCs is significantly decreased by administration of VEGF inhibition41 and activated STAT3 signaling modulates the cytoskeleton of human AECs by regulating phosphorylation of FAK.47 Although several papers indicate that JAK/STAT signaling acts as an upstream pathway of VEGF expression and Src/FAK signal activation,41,42,46,47 whether JAK3 is involved in FAK signaling and JANEX-1 regulates VEGF and FAK in PVSMCs and APE mice remain to be further confirmed. We demonstrate that JANEX-1 extinguished exogenous upregulation of VEGF and FAK in PVSMCs, which induced PCNA expression and cell proliferation. Furthermore, JANEX-1 exerts protective effects by inhibiting PVSMCs proliferation and vascular remodeling post-APE, in part through the JAK3/STAT3 signaling pathway-mediated VEGF expression and FAK activation. However, whether JAK3 is an upstream regulator of VEGF and FAK still needs to be verified by more precise experiments in future studies.
Conclusions
A JAK3 inhibitor, JANEX-1, prevented the PDGF-induced VEGF expression and FAK activation in PVSMCs. The decreased levels of these molecules by JANEX-1 were regulated by inhibiting the JAK3/STAT3 signaling pathway. In APE mice, pretreatment with JANEX-1 attenuated not only upregulation of VEGF expression in the pulmonary artery by embolism but also neointimal formation. To summarize, JANEX-1 mitigates the levels of PDGF-induced proliferation-related molecules in PVSMCs and alleviates intimal hyperplasia. Thus, the data suggest that JANEX-1 exerts its protective effects by inhibiting PVSMCs proliferation and vascular remodeling post-APE, in part through JAK3/STAT3 signaling pathway-mediated VEGF expression and FAK activation. The data are helpful to elucidate the pharmacological mechanism and potential therapeutic effect of JANEX-1 in APE.
Supplemental Material
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220942474 for JANEX-1 improves acute pulmonary embolism through VEGF and FAK in pulmonary artery smooth muscle cells by Longfei Pan, Zhuo Peng, Ruipeng Zhang, Rui Zhang, Dean Liang, Heming Chen and Hongyan Tian in Experimental Biology and Medicine
Authors’ contributions
LFP, ZP, RPZ, RZ, and DAL conducted the experiments, LFP, HMC, and HYT designed the experiments and LFP wrote the manuscript. All the authors have read and approved the final version of the manuscript.
DECLARATION OF CONFLICTING INTERESTS
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
FUNDING
The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Heming Chen https://orcid.org/0000-0003-0609-0142
Supplemental material
Supplemental material for this article is available online.
References
- 1.Konstantinides SV. ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2014; 35:3145–6 [DOI] [PubMed] [Google Scholar]
- 2.Alruwaili N, Kandhi S, Sun D, Wolin MS. Metabolism and redox in pulmonary vascular physiology and pathophysiology. Antioxid Redox Signal 2019; 31:752–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Z, Trautz B, Kračun D, Vogel F, Weitnauer M, Hochkogler K, Petry A, Görlach A. Stabilization of p22phox by hypoxia promotes pulmonary hypertension. Antioxid Redox Signal 2019; 30:56–73 [DOI] [PubMed] [Google Scholar]
- 4.Zhang H, Liu Y, Yan L, Wang S, Zhang M, Ma C, Zheng X, Chen H, Zhu D. Long noncoding RNA Hoxaas3 contributes to hypoxia-induced pulmonary artery smooth muscle cell proliferation. Cardiovasc Res 2019; 115:647–57 [DOI] [PubMed] [Google Scholar]
- 5.Zemskov EA, Lu Q, Ornatowski W, Klinger CN, Desai AA, Maltepe E, Yuan JX, Wang T, Fineman JR, Black SM. Biomechanical forces and oxidative stress: implications for pulmonary vascular disease. Antioxid Redox Signal 2019; 31:819–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.1, Leentjens J, Peters M, Esselink AC, Smulders Y, Kramers C. Initial anticoagulation in patients with pulmonary embolism: thrombolysis, unfractionated heparin, LMWH, fondaparinux, or DOACs? Br J Clin Pharmacol 2017; 83:2356–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nishanth KR, Math RS, Shankar M, Ravindranath KS, Manjunath CN. Thrombolysis with reteplase in acute pulmonary embolism. Indian Heart J 2019; 71:464–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lixon A, Rahman EU, Mohan CA, Bhattarai B, Schmidt F. An unusual case of bilateral pulmonary embolism in a patient on dual venous thromboprophylaxis, secondary to heparin induced thrombocytopenia. J Community Hosp Intern Med Perspect 2018; 8:376–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Galen J, Pava L, Wright C, Elbadawi A, Hamer A, Chaturvedi A, Cameron SJ. Effect of platelet inhibitors on thrombus burden in patients with acute pulmonary embolism. Platelets 2020. doi: 10.1080/09537104.2020.1732329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotthardt D, Trifinopoulos J, Sexl V, Putz EM. JAK/STAT cytokine signaling at the crossroad of NK cell development and maturation. Front Immunol 2019; 10:2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Han CL, Ge M, Liu YP, Zhao XM, Wang KL, Chen N, Meng WJ, Hu W, Zhang JG, Li L, Meng FG. LncRNA H19 contributes to hippocampal glial cell activation via JAK/STAT signaling in a rat model of temporal lobe epilepsy. J Neuroinf lammation 2018; 15:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh A, Pechota A, Coleman D, Upchurch GR, Jr, Eliason JL. Cigarette smoke-induced MMP2 and MMP9 secretion from aortic vascular smooth cells is mediated via the JAK/STAT pathway. Hum Pathol 2015; 46:284–94 [DOI] [PubMed] [Google Scholar]
- 13.Biggioggero M, Becciolini A, Crotti C, Agape E, Favalli EG. Upadacitinib and filgotinib: the role of JAK1 selective inhibition in the treatment of rheumatoid arthritis. Drugs Context 2019; 8:212595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yamaoka K, Saharinen P, Pesu M, Holt VE, Silvennoinen O, 3rd, O'Shea JJ. The Janus kinases (JAKS). Genome Biol 2004; 5:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Changelian PS, Flanagan ME, Ball DJ, Kent CR, Magnuson KS, Martin WH, Rizzuti BJ, Sawyer PS, Perry BD, Brissette WH, McCurdy SP, Kudlacz EM, Conklyn MJ, Elliott EA, Koslov ER, Fisher MB, Strelevitz TJ, Yoon K, Whipple DA, Sun J, Munchhof MJ, Doty JL, Casavant JM, Blumenkopf TA, Hines M, Brown MF, Lillie BM, Subramanyam C, Shang-Poa C, Milici AJ, Beckius GE, Moyer JD, Su C, Woodworth TG, Gaweco AS, Beals CR, Littman BH, Fisher DA, Smith JF, Zagouras P, Magna HA, Saltarelli MJ, Johnson KS, Nelms LF, Des Etages SG, Hayes LS, Kawabata TT, Finco-Kent D, Baker DL, Larson M, Si MS, Paniagua R, Higgins J, Holm B, Reitz B, Zhou YJ, Morris RE, O'Shea JJ, Borie DC. Prevention of organ allograft rejection by a specific Janus kinase 3 inhibitor. Science 2003; 302:875–8 [DOI] [PubMed] [Google Scholar]
- 16.Stabile H, Scarno G, Fionda C, Gismondi A, Santoni A, Gadina M, Sciumè G. JAK/STAT signaling in regulation of innate lymphoid cells: the gods before the guardians. Immunol Rev 2018; 286:148–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robinette ML, Cella M, Telliez JB, Ulland TK, Barrow AD, Capuder K, Gilfillan S, Lin LL, Notarangelo LD, Colonna M. Jak3 deficiency blocks innate lymphoid cell development. Mucosal Immunol 2018; 11:50–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verbsky JW, Bach EA, Fang YF, Yang L, Randolph DA, Fields LE. Expression of Janus kinase 3 in human endothelial and other non-lymphoid and non-myeloid cells. J Biol Chem 1996; 271:13976–80 [DOI] [PubMed] [Google Scholar]
- 19.Wang YC, Cui XB, Chuang YH, Chen SY. Janus kinase 3, a novel regulator for smooth muscle proliferation and vascular remodeling. Arterioscler Thromb Vasc Biol 2017; 37:1352–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JE, Lee AS, Kim DH, Jung YJ, Lee S, Park BH, Lee SH, Park SK, Kim W, Kang KP. Janex-1, a JAK3 inhibitor, ameliorates tumor necrosis factor-alpha-induced expression of cell adhesion molecules and improves myocardial vascular permeability in endotoxemic mice. Int J Mol Med 2012; 29:864–70 [DOI] [PubMed] [Google Scholar]
- 21.Cetkovic-Cvrlje M, Dragt AL, Vassilev A, Liu XP, Uckun FM. Targeting JAK3 with JANEX-1 for prevention of autoimmune type 1 diabetes in NOD mice. Clin Immunol 2003; 106:213–25 [DOI] [PubMed] [Google Scholar]
- 22.Wan J, Lu LJ, Miao R, Liu J, Xu XX, Yang T, Hu QH, Wang J, Wang C. Alterations of bone marrow-derived endothelial progenitor cells following acute pulmonary embolism in mice. Exp Biol Med 2010; 235:989–98 [DOI] [PubMed] [Google Scholar]
- 23.Miao R, Wang Y, Wan J, Leng D, Gong J, Li J, Zhang Y, Pang W, Zhai Z, Yang Y. Microarray analysis and detection of MicroRNAs associated with chronic thromboembolic pulmonary hypertension. Biomed Res Int 2017; 2017:8529796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu ZY, Li H, Tang YJ. Effect of simvastatin on the SIRT2/NF-κB pathway in rats with acute pulmonary embolism. Pharm Biol 2018; 56:511–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothman A, Wolner B, Button D. Immediate-early gene expression in response to hypertrophic and proliferative stimuli in pulmonary arterial smooth muscle cells. J Biol Chem 1994; 269:6399–05 [PubMed] [Google Scholar]
- 26.Wu JH, Zhou YF, Hong CD, Chen AQ, Luo Y, Mao L, Xia YP, He QW, Jin HJ, Huang M, Li YN, Hu B. Semaphorin-3A protects against neointimal hyperplasia after vascular injury. EBioMedicine 2019; 39:95–08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malemud CJ. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther Adv Musculoskelet Dis 2018; 10:117–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tan S, Xu J, Lai A, Cui R, Bai R, Li S, Liang W, Zhang G, Jiang S, Liu S, Zheng M, Wang W. Curculigoside exerts significant anti‑arthritic effects in vivo and in vitro via regulation of the JAK/STAT/NF‑κB signaling pathway. Mol Med Rep 2019; 19:2057–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra J, Waters CM, Kumar N. Molecular mechanism of interleukin-2-induced mucosal homeostasis. Am J Physiol Cell Physiol 2012; 302:C735–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan J, Zhang Z, Yang J, Mitch WE, Wang Y. JAK3/STAT6 stimulates bone Marrow-Derived fibroblast activation in renal fibrosis. J Am Soc Nephrol 2015; 26:3060–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh YB, Ahn M, Lee SM, Mitch WE, Wang Y. Inhibition of janus activated kinase-3 protects against myocardial ischemia and reperfusion injury in mice. Exp Mol Med 2013; 45:e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang G, Qian P, Xu Z, Zhang J, Wang Y, Cheng S, Cai W, Qian G, Wang C, Decoster MA. Regulatory effects of the JAK3/STAT1 pathway on the release of secreted phospholipase A(2)-IIA in microvascular endothelial cells of the injured brain. J Neuroinf lammation 2012; 9:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kakutani Y, Shioi A, Shoji T, Okazaki H, Koyama H, Emoto M, Inaba M. Oncostatin M promotes osteoblastic differentiation of human vascular smooth muscle cells through JAK3-STAT3 pathway. J Cell Biochem 2015; 116:1325–33 [DOI] [PubMed] [Google Scholar]
- 34.Wang GS, Qian GS, Zhou DS, Zhou DS, Zhao JQ. JAK-STAT signaling pathway in pulmonary arterial smooth muscle cells is activated by hypoxia. Cell Biol Int 2005; 29:598–03 [DOI] [PubMed] [Google Scholar]
- 35.Huan W, Tianzhu Z, Yu L, Shumin W. Effects of ergosterol on COPD in mice via JAK3/STAT3/NF-kappaB pathway. Inf lammation 2017; 40:884–93 [DOI] [PubMed] [Google Scholar]
- 36.Daniel JM, Dutzmann J, Bielenberg W. Inhibition of STAT3 signaling prevents vascular smooth muscle cell proliferation and neointima formation. Inhibition of STAT3 signaling prevents vascular smooth muscle cell proliferation and neointima formation. Basic Res Cardiol 2012; 107:261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al Zaid Siddiquee K, Turkson J. STAT3 as a target for inducing apoptosis in solid and hematological tumors. Cell Res 2008; 18:254–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mingo-Sion AM, Marietta PM, Koller E, Wolf DM. Van den berg CL. Inhibition of JNK reduces G2/M transit independent of p53, leading to endoreduplication, decreased proliferation, and apoptosis in breast cancer cells. Oncogene 2004; 23:596–04 [DOI] [PubMed] [Google Scholar]
- 39.Dashkevich A, Hagl C, Beyersdorf F, Nykänen AI, Lemström KB. VEGF pathways in the lymphatics of healthy and diseased heart. Microcirculation 2016; 23:5–14 [DOI] [PubMed] [Google Scholar]
- 40.Kumar AH, Martin K, Doyle B, Huang CL, Pillai GK, Ali MT, Skelding KA, Wang S, Gleeson BM, Jahangeer S, Ritman EL, Russell SJ, Caplice NM. Intravascular cell delivery device for therapeutic VEGF-induced angiogenesis in chronic vascular occlusion. Biomaterials 2014; 35:9012–22 [DOI] [PubMed] [Google Scholar]
- 41.Lv J, Sun B, Mai Z, Jiang M, Du J. STAT3 potentiates the ability of airway smooth muscle cells to promote angiogenesis by regulating VEGF signalling. Exp Physiol 2017; 102:598–06 [DOI] [PubMed] [Google Scholar]
- 42.Demyanets S, Kaun C, Rychli K, Pfaffenberger S, Kastl SP, Hohensinner PJ, Rega G, Katsaros KM, Afonyushkin T, Bochkov VN, Paireder M, Huk I, Maurer G, Huber K, Wojta J. Oncostatin M-enhanced vascular endothelial growth factor expression in human vascular smooth muscle cells involves PI3K-, p38 MAPK-, Erk1/2- and STAT1/STAT3-dependent pathways and is attenuated by interferon-gamma. Basic Res Cardiol 2011; 106:217–31 [DOI] [PubMed] [Google Scholar]
- 43.Sulzmaier FJ, Jean C, Schlaepfer DD. FAK in cancer: mechanistic findings and clinical applications. Nat Rev Cancer 2014; 14:598–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin C, Li X, Luo Q, Yang H, Li L, Zhou Q, Li Y, Tang H, Wu L. RELM-beta promotes human pulmonary artery smooth muscle cell proliferation via FAK-stimulated surviving. Exp Cell Res 2017; 351:43–50 [DOI] [PubMed] [Google Scholar]
- 45.Banerjee K, Keasey MP, Razskazovskiy V, Visavadiya NP, Jia C, Hagg T. Reduced FAK-STAT3 signaling contributes to ER stress-induced mitochondrial dysfunction and death in endothelial cells. Cell Signal 2017; 36:154–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Visavadiya NP, Keasey MP, Razskazovskiy V, Banerjee K, Jia C, Lovins C, Wright GL, Hagg T. Integrin-FAK signaling rapidly and potently promotes mitochondrial function through STAT3. Cell Commun Signal 2016; 14:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei Z, Jiang W, Wang H, Li H, Tang B, Liu B, Jiang H, Sun X. The IL-6/STAT3 pathway regulates adhesion molecules and cytoskeleton of endothelial cells in thromboangiitis obliterans. Cell Signal 2018; 44:118–26 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-ebm-10.1177_1535370220942474 for JANEX-1 improves acute pulmonary embolism through VEGF and FAK in pulmonary artery smooth muscle cells by Longfei Pan, Zhuo Peng, Ruipeng Zhang, Rui Zhang, Dean Liang, Heming Chen and Hongyan Tian in Experimental Biology and Medicine