Abstract
Atherosclerosis is a major cause of mortality worldwide and is driven by multiple risk factors, including diabetes. Diabetes is associated with either an insulin deficiency in its juvenile form or with insulin resistance and obesity in Type 2 diabetes mellitus, and the latter is clustered with other comorbidities to define the metabolic syndrome. Diabetes and metabolic syndrome are complex pathologies and are associated with cardiovascular risk via vascular inflammation and other mechanisms. Several transcription factors are activated upon diabetes-driven endothelial dysfunction and drive the progression of atherosclerosis. In particular, the hypoxia-inducible factor (HIF) transcription factor family is a master regulator of endothelial biology and is raising interest in the field of atherosclerosis. In this review, we will present an overview of studies contributing to the understanding of diabetes-driven atherosclerosis, integrating the role of HIF in this disease with the knowledge of its functions in metabolic syndrome and diabetic scenario.
Keywords: atherosclerosis, hypoxia-inducible factors, type 2 diabetes
Introduction
Diabetes is a risk factor for cardiovascular disease (CVD), which causes around 17 millions deaths each year [1]. Diabetes is associated with myocardial infarction, heart failure and micro and macrovascular complications [2–4].
Diabetes is part of a constellation of metabolic abnormalities clinically recognised as metabolic syndrome. Metabolic syndrome is characterised by the co-existence of raised blood pressure, insulin resistance, obesity and dyslipidaemia, particularly hypertriglyceridaemia and reduced ratio of high density lipoproteins (HDL) to low density lipoproteins (LDL). Dyslipidaemia has a central role in atherosclerosis [5,6].
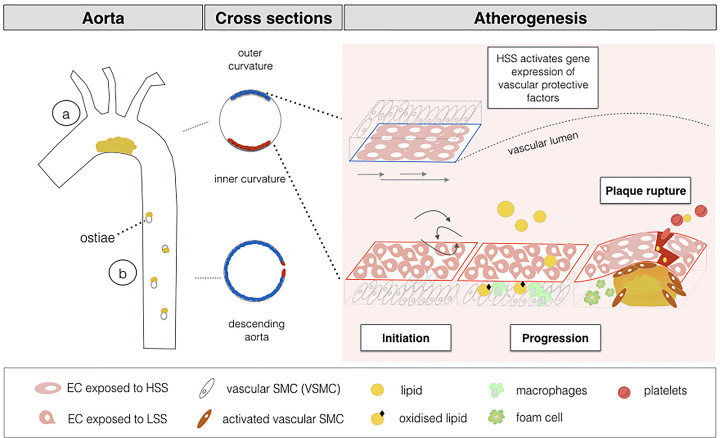
Atherosclerosis is a chronic inflammatory disease characterised by the thickening of the arterial wall with the formation of a lipid-enriched plaque, as illustrated and described in Figure 1 [6]. Atherosclerosis includes initiation, progression and plaque rupture. Blood flow frictional forces (shear stress) prime endothelial cells (ECs) for atherogenesis. Atheroprone areas are found at regions exposed to low shear stress (LSS), while high shear stress (HSS) protects ECs from atherosclerosis. Atheroprone ECs allow molecules to adhere on their surface and penetrate underneath the vascular layer, initiating an inflammatory process. Lipoproteins can pass through the ECs layer and become entrapped underneath the endothelium where they are oxidised. As the inflammatory process progresses, infiltrated macrophages engulf oxidised LDL and eventually turn into large foam cells. When foam cells die the inflammatory process is exacerbated resulting in a hypoxic, necrotic and highly pro- inflammatory core. Meanwhile, vascular smooth muscle cells (VSMCs) contribute to inflammation and produce collagen to form a fibrous cap. Importantly, epidemiological studies show a direct association between hyperglycaemia and atherosclerosis [7–10]; clinical studies corroborate this link, atherosclerosis being accelerated and worsened in diabetic patients [11].
Figure 1. Schematic representation of site-specific atherogenesis.
Inner curvature of the aortic arch (a) is exposed to low shear stress that primes ECs for atherosclerosis (red labelled cells), while outer curvature and descending aorta (b) are exposed to high shear stress and ECs are protected (blue labelled cells). The three step process of atherogenesis is also represented.
Multiple cell types contribute to the atheromatous plaque (or atheroma), and the relative abundance of these constituents differs between individuals, which in turn determines the stability of the atheroma. In humans, atheromatous plaques can break, releasing their contents into the bloodstream and activating platelet coagulation with a high risk of thrombosis. Despite the risk of a plaque partially or fully occluding the vessel lumen, thrombosis remains a major complication in plaque progression [12]. All the main cellular components of the atherogenesis, namely ECs, VSMCs and macrophages, are affected by diabetes.
Despite a large body of evidence that describe the contribution of diabetes to the development and progression of atherosclerosis, the molecular events leading to diabetes-induced atherosclerosis progression been not been revealed yet. For instance, multiple transcription factors contribute to atherosclerosis progression and some of these have key roles in controlling metabolic syndrome. This review will address the experimental evidence that underpins the role of diabetes in atherosclerosis, with a specific focus on the role of the hypoxia-inducible factor transcription factors (HIFs) in the development of diabetic atherosclerosis.
The role of diabetes in atherosclerosis
Much of our understanding of the contribution of metabolic syndrome to the development and progression of atherosclerosis is generated by animal studies. The fundamental cross-talk between atherosclerosis and hyperglycaemia has been established in murine models employing mutation of key molecules in the regulation of the lipid metabolism, apolipoprotein E (ApoE) and low density lipoprotein receptor (LDLR), often combined with feeding of high-fat high-cholesterol diets [13–15]. To molecularly dissect the effect of hyperglycaemia and hyperlipidaemia in murine diabetic atherosclerosis, studies have used an experimental approach of pancreatic β-cell destruction in ApoE and LDLR mice thereby leading to accelerated atherosclerosis [16–19]. While it is well understood that diabetes contributes to the initial stage of endothelial dysfunction, understanding the contribution of diabetes in the progression and rupture stages of the atheroma is more complex. A major drawback is that plaque rupture is difficult to achieve in murine models [20]. Nevertheless, when the plaque is formed and diabetes is exogenously triggered, hyperglycaemia causes inter-plaque haemorrhage and destabilization at the brachiocephalic artery [21]. Therefore, suggesting that diabetes contributes to plaque destabilization. In conclusion, hyperglycaemia and hyperlipidaemia exhibit considerable cross-talk. Hyperglycaemia contributes to accelerated atherosclerosis, while hyperlipidaemia is absolutely required for developing atheroma.
Molecular mechanisms of diabetes/atherosclerosis coupling
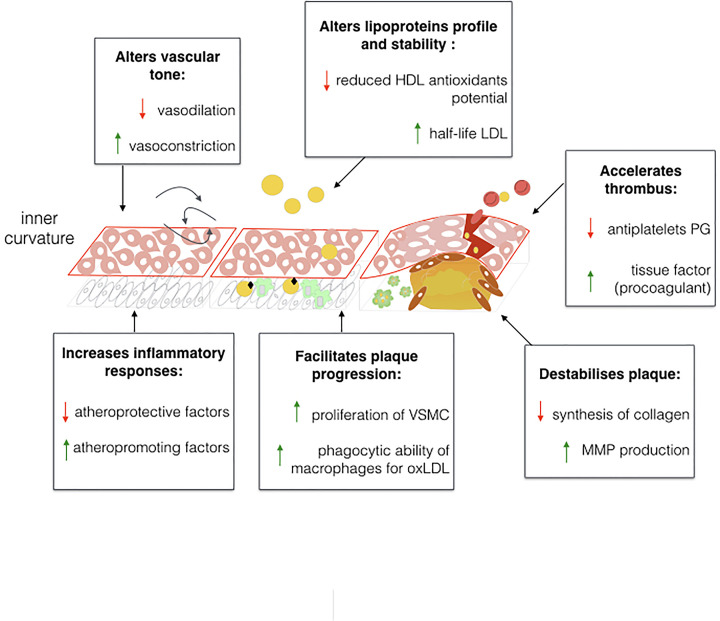
Vascular homeostasis is finely tuned by both hormonal, mechanical and inflammatory factors [22]. Flowing blood with high wall shear stress confers protection to the endothelium [23]. It primes the production of vasoactive factors such as nitric oxide (NO) via, amongst other mediators, endothelial nitric oxide synthase (eNOS), which inhibits pro-inflammatory and vasoconstrictor molecules, such as the endothelin 1 (ET-1) [24–28]. On the contrary, flowing blood with low wall shear stress enhances inflammatory and proliferative responses, contributing to disease progression [29,30]. Low wall shear stress is observed at sites of ramification and bends of the aortic vessel, intriguingly these are the sites where atheromatous plaque develops preferentially [31–33]. Hyperglycaemia interacts with the endothelium exacerbating vascular disease, as shown in Figure 2. For instance, in vitro high glucose concentration reduces eNOS availability and reduces vascular responses to shear stress through structural rearrangement of the glycocalyx [34]. Hyperglycaemia increases pro-inflammatory activity of nuclear factor-κB (NF-κB), together with increased plasminogen activator inhibitor-1 (PAI-1) expression through diacylglycerol (DAG)-protein kinase C (PKC) mechanisms [35,36], which participates in thrombotic processes. Hyperglycaemia also contributes to endothelial dysfunction and inflammation by increasing advanced glycated end-product (AGE) formation, radical oxygen species (ROS) and increasing fatty acid oxidation and free fatty acids [37,38]. In ECs, when AGEs bind to their receptor (RAGE), they activate pro-inflammatory signalling mediated by NF-κB and increase adhesion molecules such as vascular cell adhesion protein 1 (VCAM-1).
Figure 2. Effects of diabetes on atherogenesis.
Schematic summarization of some of the effects of diabetes on the vasculature. Hyperglycaemia, insulin and ROS, together with hyperlipidemia contribute to reinforce pro-inflammatory and pro-proliferative stimuli, reduce atheroprotective molecules and antioxidant defenses. High density lipoprotein, HDL; low density lipoprotein, LDL; or oxidized LDL, oxLDL; prostaglandin, PG; vascular smooth muscle cells, VSMC; matrix metalloproteinases, MMP.
Hyperglycaemia also contributes to atherosclerosis by altering VSMCs. The AGE/RAGE interaction promotes proliferation of VSMCs. Interestingly, soluble RAGE injection in diabetic mice suppressed atherosclerosis in a glycaemia and lipid-dependent manner [16,39]. Therefore, it is suggested that hyperglycaemia through activation of the AGE pathway can contribute to both initiation and progression steps of atheroma formation. At the same time, insulin has a controversial role in atherosclerosis, since it confers protection to ECs by inducing eNOS production [40]. On the other hand, insulin influences disease by inducing VSMCs proliferation [41], while macrophages lacking insulin receptors have an increased capacity for endocytosis of oxLDL, through augmented expression of the oxLDL receptor CD36 [42].
Meanwhile, diabetes associated with obesity leads to an unbalanced physiology of the leptin system. Leptin is a hormone that regulates energy balance and food intake, as well as glucose homeostasis [43,44], therefore making leptin a central regular of metabolic disease. Leptin deficient (ob/ob) or leptin receptor-deficient (db/db) murine models display obesity and transient diabetes [45]. Despite multiple studies suggesting that alteration of leptin signalling enhances atherosclerosis progression, the precise mechanism by which this occurs has not been clarified yet [39,46–51].
Furthermore, diabetes-induced activation of protein kinase-β (PKC-β) increases production of AGE from the polyol pathway and leads to accelerated atherosclerosis, reduced insulin-stimulated eNOS production and increased expression of the vasoconstrictive molecule, ET-1 [52,53]. On the other hand, inhibition of the PKC-β and PKC-θ isoforms in mice prevents atherosclerosis and improves cardiac functionality [54,55]. Therefore, insulin in vascular homeostasis is a beneficial signal in macrophages and endothelium, whereas insulin has detrimental effects on VSMCs. Thus, diabetes might also be involved in the later stages of the atherogenesis.
Taken together, glycaemia, insulin and leptin are key mechanistic components of the diabetes–atherosclerosis axis. The resultant excessive ROS activity causes abnormal ECs and VSMCs physiology. Therefore, diabetes both accelerates atheroma formation and contributes to plaque instability and thrombus formation.
A role for HIF transcription factors in diabetic atherosclerosis
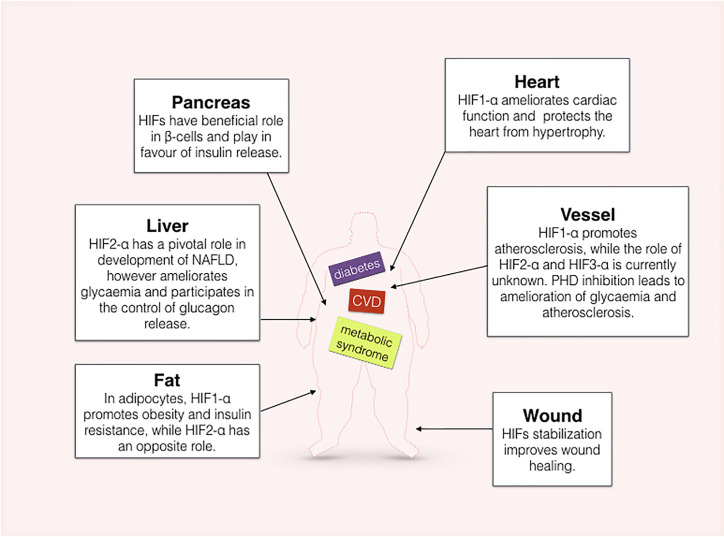
Advanced atherosclerotic plaques presents a core with a hypoxic state, and it is associated with neovascularization and inflammatory processes that potentially contribute to plaque instability [56–58]. The molecular basis of plaque neovascularization involves the hypoxia-vascular endothelial growth factor (VEGF) axis [59,60]. Hypoxia-inducible factor (HIF) is a heterodimeric transcription factor composed of an inducible alpha subunit (HIF-α) and a constitutive beta subunit (HIF-β), also known as aryl hydrocarbon receptor nuclear translocator (ARNT) [61]. Three alpha subunits have been recognised and are differentially distributed within tissues [62]. HIFs are regulated by an oxygen-dependent prolyl hydroxylase enzymes (PHDs), thus in presence of oxygen, HIF-α subunits are rapidly hydroxylated and prepared for proteasome degradation by the interaction of ubiquitin subunits with the Von Hippel-Lindau tumor suppressor protein (pVHL) [63–65]. Whilst another oxygen-sensitive inhibitor, the factor inhibiting HIF (FIH) blocks the recruitment of 300/CBP transcriptional co-factors [66,67]. HIF is the major effector of the response to hypoxia; upon this condition, the activity of PHDs are reduced and HIF-α is stabilised and, together with HIF-β, form the transcription factor that actively binds the hypoxia responsive elements (HREs), thus activating transcriptional reprogramming of the cell [68]. There are several lines of evidence showing that diabetes and HIF pathways interact at different levels. As an example, human single-nucleotide polymorphism at the exon 12 (P582S) of HIF1-α was recurrent in Japanese patients with Type 2 diabetes mellitus [69]. A schematic summary of the action of HIFs in different organs involved in diabetes and metabolic syndrome is proposed in Figure 3.
Figure 3. Role of HIF in diabetic organs.
Graphical representation of the role of HIFs at various organs.
HIF signalling in β cells
Glycaemia is balanced by the action of two main hormones: insulin and glucagon. Within the pancreas, β cells are designated to insulin production and release with a mechanism requiring an appropriate adenosine di- and tri-phosphate (ADP/ATP) ratio. During diabetes, HIF signalling is reduced [70] along with the reduction in glycolytic enzymes; therefore, the resultant reduction in metabolic rate reduces ATP production and prevents insulin release from β cells. Thus, loss of HIF contributes to β-cells dysfunction [71]. Both in vitro and in vivo evidence suggest that HIF signalling plays a central role in β-cell function and glucose homeostasis. HIF1-α knockout in β cells impaired insulin secretion [72]. Furthermore, lack of the HIF-α isoforms counterpart, ARNT, both in mice and in vitro studies, showed a reduction in glycolytic enzymes [72,73]. Mice with β-cell inactivation of the Vhlh gene encoding for pVHL led to a marked increase in expression of genes that promote glucose uptake (e.g. glucose transporter- 1 (GLUT-1)) mediated by HIF1-α activity, and also possess impaired glucose tolerance as well as reduction in insulin levels [74]. Whilst FIH deletion in mice led to amelioration of body weight, increased insulin sensitivity, and decreased triglycerides and cholesterol levels [75]. Thus, HIF stabilisation seems to have a beneficial role in β-cell function.
HIF signalling in diabetic adiposity and liver
Fat, metabolic disease and hypoxia signalling are intrinsically connected, as reviewed in [76]. Obesity and insulin resistance are observed in mice overexpressing HIF1-α [77,78]. However, adipose tissue specific HIF1-α or PHD inhibition, respectively, led to aggravation or amelioration of insulin sensitivity and obesity [79,80]. Furthermore, Lee et al. studied HIF1-α and HIF2-α in adipocytes and showed that lack of only HIF1-α or both isoforms led to reduced insulin resistance; on the contrary, solely lack of HIF2-α had the opposite effect [81]. Thus, it was thought that HIF1-α expression in adipose tissue acts in favour of obesity by leading to excess of fatty acid and triglycerides [76]. Furthermore, HIF2-α may have a more central role in regulation of obesity. It was demonstrated that HIFs transcriptionally activate the pro-opiomelanocortin (POMC) and that, unlike HIF1-α mRNA, HIF2-α mRNA was highly expressed in hypothalamic regions; while its protein level was enhanced after glucose or glucose metabolites infusion through the third ventricle. Loss of function of HIF2-α in hypothalamic POMC neurons favoured obesity [82].
Nevertheless, long-term hyperglycaemia and hyperlipidaemia present in diabetic patients leads to metabolic dysfunction of the liver. Diabetic patients often presented non-alcoholic fatty liver disease (NAFLD) and this pathology is associated with increased expression of HIF2-α isoform [83,84]. A model of HIF2-α stable expression by VHL and HIF1-α knockout showed that HIF2-α controls lipid metabolism in hepatocytes with reduction of fatty acid oxidation and severe hepatic fibrosis [83]. At the same time, in a model of hepatic PHD3 deletion, HIF2-α increased the expression of insulin receptor substrate 2 (IRS2), ameliorated hyperglycaemia and regulated hepatic glucose metabolism [85]. Furthermore, glucagon is the hormone responsible for counterbalancing the blood glucose levels and acts to avoid hypoglycaemia. Interestingly, transient post-prandial hypoxia in the liver activates HIF2- α and represses glucagon action by blocking the second messenger cyclic adenosine monophosphate (cAMP) [86]. Increased hepatic glucagon levels were thought to be responsible for hepatic insulin resistance in Type 2 diabetes mellitus [87]. Interestingly, patients with Chuvash polycythaemia, by VHL mutation have a modest increase in expression of HIF isoforms, and present hypoglycaemia [88]. Therefore, HIF2-α could contribute to the control glycaemia via hepatic signalling.
Despite the lack of understanding about the role of the third isoform at the moment, HIF3-α is emerging as an important regulator of body adiposity. Multiple epigenetic studies have shown a correlation between different methylation sites of the HIF3-α promoter with both adult obesity and perinatal body weight [89–91].
In conclusion, these data suggest that the three HIF-α isoforms might have different roles in obesity and metabolic syndrome. Particularly, both HIF1-α and HIF2-α might contribute to the control of glycaemic levels by their activation in pancreatic cells and hepatic cells, respectively.
HIF signalling in diabetic hearts
HIF1-α is a crucial regulator of heart development [92–94]. In the adult heart, the following evidence revealed that HIF1-α protects myocardium. It was demonstrated that intermittent hypoxia treatment protects the myocardium from ischemia–reperfusion injury, with reduction in infarct size; mice heterozygous for Hif1-α had reduced protection when compared with their wild-type controls [95]. Cardiac overexpression of Hif1-α ameliorated and restored the level of several glycolytic and angiogenic proteins [96]. Hearts of Hif1-α heterozygous mice with exogenously-induced diabetes had decreased levels of apoptosis and altered gene expression profiles of angiogenic genes [97]. Interestingly, the level of PHD3, a key molecule of HIF protein regulation, was increased in the heart of diabetic rats and these levels were associated with increased cardiomyocyte diameter, increased apoptosis and collagen deposition, in a HIF1A independent manner [98]. Furthermore, in vitro, cardiomyoblasts respond to high glucose stimulation and hypoxia exposure by increasing apoptosis in a HIF1-α and Forkhead Box O3 (FOXO3)-mediated mechanism [99].
Diabetes type 2 increases fatty acid metabolism against the utilization of glucose through glycolysis; this leads to a reduction of succinate content which decreases HIF1-α levels in diabetic hearts [100]. Interestingly, during diabetes, ARNT expression is also reduced and cardiac deletion of Arnt in mice causes an increase in lipid accumulation and consequent cardiomyopathy [101]. Moreover, mice carrying an endothelial cell specific Hif1-α deletion driven by the Tie2 gene and subjected to transaortic constriction to induce pressure overload manifested cardiac hypertrophy and fibrosis associated with reduction of myocardial capillary density and an increase in myocardial apoptosis [102].
Overall, these data suggest that HIF1-α prevents diabetic cardiopathy; however, the roles of HIF2-α and HIF3-α in diabetic hearts are poorly studied.
HIF signalling in diabetic vasculature and atherosclerosis
Diabetic patients have both macro and microvascular complications. The role of HIF in vascularisation in this context is evidenced by experiments where stabilisation of HIF by deferoxamine (DFO) or genetically results in improved wound-healing and angiogenesis [103,104]. Similarly, diabetic db/db mice have impaired wound healing associated with decrease in HIF signalling, and stabilisation of HIF1-α by DFO or dimethyloxalylglycine (DMOG) restores it [105]. These data suggest that HIF- driven angiogenesis may be suppressed in diabetes.
Notably, HIF proteins are major regulators of cellular glucose homeostasis, along with other essential cellular pathways (e.g. angiogenesis), HIF1-α and HIF2-α control glycolytic genes such as GLUT1 [106,107]. From a metabolic prospective, ECs have very low mitochondrial content and fatty acids or glutamine serve as alternative energetic substrates to glucose [108–111]. Therefore, ECs rely almost completely on glycolysis, either during their basal metabolism and even more during vessel sprouting [112,113]. Interestingly, the activity of 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 3 (PFKB3) promotes growth and migration of ECs and it is a master regulator of angiogenic ECs both in heath and disease [112,114].
Similarly, atheroprone areas of the vasculature are associated with a higher glycolytic rate [115,116]. Interestingly, atheroma progression is associated with endothelial-to-mesenchymal transition (EndMT) and fatty acid oxidation can protect ECs from undergoing EndMT [117–122].
Mechanosensory molecules activated by shear stress may also control metabolic enzymes. For instance, HIF1-α enhanced glycolytic enzymes in response to low wall shear stress forces, while activation of the Hippo pathway via YAP (Yes-associated protein) and TAZ (transcriptional coactivator with PDZ- binding motif) to form YAP/TAZ was demonstrated to enhance both glycolysis and glutaminolysis [115,123]. On the other hand, high wall shear stress through activation of krüppel-like factor 2 (KLF2) was suggested to inhibit glycolytic enzymes and glucose uptake [124]. Similarly, administration of the PHD inhibitor FG4497 in LDLR null mice and hypomorphism for PHD2 in a atheroprone model, conferred protection from atherosclerosis [125].
In summary, HIF1-α is a protagonist in atherosclerosis. However, its activation may be beneficial in diabetic microvascular consequences. In contrast, the role of HIF2-α and HIF3-α in atherosclerosis is much less understood. Collectively, these studies suggest that activation of the HIF family in endothelium might control metabolic rate and potentially atherosclerosis progression.
Conclusions
In conclusion, the HIF family has diverse roles in arterial homeostasis and disease which have not been fully elucidated. HIF isoforms might have opposite or synergistic roles in vascular disease depending on context. Hence, whether HIF isoforms could be a target for therapeutic intervention of atherosclerosis is not yet clear. In this context, it will be essential to analyse the effect of single deletion of HIF isoforms on vascular homeostasis and atherosclerosis in the presence of hyperglycaemia.
Acknowledgements
We would like to thank Dr Qian Yi Lee for the critical reading of the manuscript.
Abbreviations
- ARNT
aryl hydrocarbon receptor nuclear translocator
- CVD
cardiovascular disease
- EC
endothelial cell
- HDL
high density lipoproteins
- HIF
hypoxia-inducible factor
- HRE
hypoxia responsive element
- HSS
high shear stress
- LDL
low density lipoproteins
- LSS
low shear stress
- NF-κB
nuclear factor-κB
- PAI-1
plasminogen activator inhibitor-1
- PKC-β
protein kinase-β
- ROS
radical oxygen species
- VSMC
vascular smooth muscle cell
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
References
- 1.WHO https://www.who.int/cardiovascular_diseases/publications/atlas_cvd/en/ [Google Scholar]
- 2.Bell D.S.H. (2003) Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care 26, 2433–2441 10.2337/diacare.26.8.2433 [DOI] [PubMed] [Google Scholar]
- 3.Kannel W.B. and McGee D.L. (1979) Diabetes and cardiovascular disease. The Framingham study. JAMA 241, 2035–2038 10.1001/jama.1979.03290450033020 [DOI] [PubMed] [Google Scholar]
- 4.Stratton I.M., Adler A.I., Neil H.A., Matthews D.R., Manley S.E., Cull C.A. et al. (2000) Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321, 405–412 10.1136/bmj.321.7258.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P., Buring J.E., Badimon L., Hansson G.K., Deanfield J., Bittencourt M.S. et al. (2019) Atherosclerosis. Nat. Rev. Dis. Prim. 5, 1–18 [DOI] [PubMed] [Google Scholar]
- 6.Tuñón J., Bäck M., Badimón L., Bochaton-Piallat M.L., Cariou B., Daemen M.J. et al. (2018) Interplay between hypercholesterolaemia and inflammation in atherosclerosis: Translating experimental targets into clinical practice. Eur. J. Prev. Cardiol. 25, 948–955 10.1177/2047487318773384 [DOI] [PubMed] [Google Scholar]
- 7.Khaw K.-T., Wareham N., Bingham S., Luben R., Welch A. and Day N. (2004) Association of hemoglobin A1c with cardiovascular disease and mortality in adults: the European prospective investigation into cancer in Norfolk. Ann. Intern. Med. 141, 413–420 10.7326/0003-4819-141-6-200409210-00006 [DOI] [PubMed] [Google Scholar]
- 8.Gerstein H.C., Pogue J., Mann J.F.E., Lonn E., Dagenais G.R., McQueen M. et al. (2005) The relationship between dysglycaemia and cardiovascular and renal risk in diabetic and non-diabetic participants in the HOPE study: a prospective epidemiological analysis. Diabetologia 48, 1749–1755 10.1007/s00125-005-1858-4 [DOI] [PubMed] [Google Scholar]
- 9.Turner R.C., Millns H., Neil H.A., Stratton I.M., Manley S.E., Matthews D.R. et al. (1998) Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316, 823–828 10.1136/bmj.316.7134.823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selvin E., Coresh J., Shahar E., Zhang L., Steffes M. and Sharrett A.R. (2005) Glycaemia (haemoglobin A1c) and incident ischaemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Lancet Neurol. 4, 821–826 10.1016/S1474-4422(05)70227-1 [DOI] [PubMed] [Google Scholar]
- 11.Nicholls S.J., Tuzcu E.M., Kalidindi S., Wolski K., Moon K.-W., Sipahi I. et al. (2008) Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J. Am. Coll. Cardiol. 52, 255–262 10.1016/j.jacc.2008.03.051 [DOI] [PubMed] [Google Scholar]
- 12.Kolodgie F.D., Burke A.P., Farb A., Gold H.K., Yuan J. and Narula J.F.A. (2001) The thin-cap fibroatheroma: a type of vulnerable plaque: the major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 16, 285–292 10.1097/00001573-200109000-00006 [DOI] [PubMed] [Google Scholar]
- 13.Plump A.S., Smith J.D., Hayek T., Aalto-Setala K., Walsh A., Verstuyft J.G. et al. (1992) Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 71, 343–353 10.1016/0092-8674(92)90362-G [DOI] [PubMed] [Google Scholar]
- 14.Ishibashi S., Brown M.S., Goldstein J.L., Gerard R.D., Hammer R.E. and Herz J. (1993) Hypercholesterolemia in low density lipoprotein receptor knockout mice and its reversal by adenovirus-mediated gene delivery. J. Clin. Invest. 92, 883–893 10.1172/JCI116663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roche-Molina M., Sanz-Rosa D., Cruz F.M., Garcia-Prieto J., Lopez S., Abia R. et al. (2015) Induction of sustained hypercholesterolemia by single adeno-associated virus-mediated gene transfer of mutant hPCSK9. Arterioscler. Thromb. Vasc. Biol. 35, 50–59 10.1161/ATVBAHA.114.303617 [DOI] [PubMed] [Google Scholar]
- 16.Park L., Raman K.G., Lee K.J., Lu Y., Ferran L.J.J., Chow W.S. et al. (1998) Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat. Med. 4, 1025–1031 10.1038/2012 [DOI] [PubMed] [Google Scholar]
- 17.Candido R., Allen T.J., Lassila M., Cao Z., Thallas V., Cooper M.E. et al. (2004) Irbesartan but Not Amlodipine Suppresses. Circulation 109, 1536–1542 [DOI] [PubMed] [Google Scholar]
- 18.Keren P., George J., Shaish A., Levkovitz H., Janakovic Z., Afek A. et al. (2000) Effect of Hyperglycemia and Hyperlipidemia on Atherosclerosis in LDL Receptor - Deficient Mice. Diabetes 49, 1064–1069 [DOI] [PubMed] [Google Scholar]
- 19.Kunjathoor V.V., Wilson D.L. and Leboeuf R.C. (1996) Increased atherosclerosis in streptozotocin- induced diabetic mice. J Clin Invest 97, 1767–1773Find the latest version [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz S.M., Galis Z.S., Rosenfeld M.E. and Falk E. (2007) Plaque rupture in humans and mice. Arterioscler. Thromb. Vasc. Biol. 27, 705–713 10.1161/01.ATV.0000261709.34878.20 [DOI] [PubMed] [Google Scholar]
- 21.Johansson F., Kramer F., Barnhart S., Kanter J.E., Vaisar T., Merrill R.D. et al. (2008) Type 1 diabetes promotes disruption of advanced atherosclerotic lesions in LDL receptor-deficient mice. Proc Natl Acad Sci U S A 105, 2082–2087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alexander Y., Osto E., Schmidt-Trucksäss A., Shechter M., Trifunovic D., Duncker D.J. et al. (2020) Endothelial function in cardiovascular precision medicine : a position paper on behalf of the European society of cardiology. Cardiovasc. Res. cvaa085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryan M.T., Duckles H., Feng S., Hsiao S.T., Kim H.R., Serbanovic-Canic J. et al. (2014) Mechanoresponsive networks controlling vascular inflammation. Arterioscler. Thromb. Vasc. Biol. 34, 2199–2205 10.1161/ATVBAHA.114.303424 [DOI] [PubMed] [Google Scholar]
- 24.Chien S. (2008) Role of shear stress direction in endothelial mechanotransduction. Mol. Cell Biomech. 5, 1–8 [PubMed] [Google Scholar]
- 25.Parmar K.M., Nambudiri V., Dai G., Larman H.B., Gimbrone M.A. and García-Cardeña G. (2005) Statins exert endothelial atheroprotective effects via the KLF2 transcription factor. J. Biol. Chem. 280, 26714–26719 10.1074/jbc.C500144200 [DOI] [PubMed] [Google Scholar]
- 26.Wu C.-C., Wang N., Li Y.-S., Chien S., Hu Y., Young A. et al. (2006) Shear stress regulation of Krüppel- like factor 2 expression is flow pattern-specific. Biochem. Biophys. Res. Commun. 341, 1244–1251 [DOI] [PubMed] [Google Scholar]
- 27.Zakkar M., Van Der Heiden K., Luong L.A., Chaudhury H., Cuhlmann S., Hamdulay S.S. et al. (2009) Activation of Nrf2 in endothelial cells protects arteries from exhibiting a proinflammatory state. Arterioscler. Thromb. Vasc. Biol. 29, 1851–1857 10.1161/ATVBAHA.109.193375 [DOI] [PubMed] [Google Scholar]
- 28.Xanthis I., Souilhol C., Serbanovic-Canic J., Roddie H., Kalli A.C., Fragiadaki M. et al. (2019) β1 Integrin Is a Sensor of Blood Flow Direction. J. Cell Sci. 132, 10.1242/jcs.229542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gijsen F., Katagiri Y., Barlis P., Bourantas C., Collet C., Coskun U. et al. (2019) Expert recommendations on the assessment of wall shear stress in human coronary arteries: Existing methodologies, technical considerations, and clinical applications. Eur. Heart J. 40, 3421–3433 10.1093/eurheartj/ehz551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Souilhol C., Serbanovic-Canic J., Fragiadaki M., Chico T.J., Ridger V., Roddie H. et al. (2020) Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat. Rev. Cardiol. 17, 52–63, [Internet] 10.1038/s41569-019-0239-5 [DOI] [PubMed] [Google Scholar]
- 31.Kwak B.R., Bäck M., Bochaton-Piallat M.-L., Caligiuri G., Daemen M.J.A.P., Davies P.F. et al. (2014) Biomechanical factors in atherosclerosis: mechanisms and clinical implications†. Eur. Heart J. 35, 3013–3020, [Internet] http://www.ncbi.nlm.nih.gov/pubmed/25230814 10.1093/eurheartj/ehu353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies P.F., Civelek M., Fang Y. and Fleming I. (2013) The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc. Res. 99, 315–327 10.1093/cvr/cvt101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walpola P.L., Gotlieb A.I., Cybulsky M.I. and Langille B.L. (1995) Expression of ICAM-1 and VCAM-1 and monocyte adherence in arteries exposed to altered shear stress. Arterioscler. Thromb. Vasc. Biol. 15, 2–10 10.1161/01.ATV.15.1.2 [DOI] [PubMed] [Google Scholar]
- 34.Lopez-Quintero V.S., Cancel L.M., Pierides A., Antonetti D., Spray D.C. and Tarbell J.M. (2013) High glucose attenuates shear-induced changes in endothelial hydraulic conductivity by degrading the glycocalyx. PLoS ONE 8, e78954 10.1371/journal.pone.0078954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shah M.S. and Brownlee M. (2016) Molecular and Cellular Mechanisms of Cardiovascular Disorders in Diabetes. Circ. Res. 118, 1808–1829 10.1161/CIRCRESAHA.116.306923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du X.L., Edelstein D., Rossetti L., Fantus I.G., Goldberg H., Ziyadeh F. et al. (2000) Hyperglycemia- induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc. Natl. Acad. Sci. U.S.A. 97, 12222–12226 10.1073/pnas.97.22.12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beckman J.A., Creager M.A. and Libby P. (2002) Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA 287, 2570–2581 10.1001/jama.287.19.2570 [DOI] [PubMed] [Google Scholar]
- 38.Enesa K., Ito K., Luong L.A., Thorbjornsen I., Phua C., To Y. et al. (2008) Hydrogen peroxide prolongs nuclear localization of NF-κB in activated cells by suppressing negative regulatory mechanisms. J. Biol. Chem. 283, 18582–18590 10.1074/jbc.M801312200 [DOI] [PubMed] [Google Scholar]
- 39.Wendt T., Harja E., Bucciarelli L., Qu W., Lu Y., Rong L.L. et al. (2006) RAGE modulates vascular inflammation and atherosclerosis in a murine model of type 2 diabetes. Atherosclerosis 185, 70–77 10.1016/j.atherosclerosis.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 40.Kuboki K., Jiang Z.Y., Takahara N., Ha S.W., Igarashi M., Yamauchi T. et al. (2000) Regulation of endothelial constitutive nitric oxide synthase gene expression in endothelial cells and in vivo : a specific vascular action of insulin. Circulation 101, 676–681 10.1161/01.CIR.101.6.676 [DOI] [PubMed] [Google Scholar]
- 41.Banskota N.K., Taub R., Zellner K. and King G.L. (1989) Insulin, insulin-like growth factor I and platelet- derived growth factor interact additively in the induction of the protooncogene c-myc and cellular proliferation in cultured bovine aortic smooth muscle cells. Mol. Endocrinol. 3, 1183–1190 10.1210/mend-3-8-1183 [DOI] [PubMed] [Google Scholar]
- 42.Liang C.-P., Han S., Okamoto H., Carnemolla R., Tabas I., Accili D. et al. (2004) Increased CD36 protein as a response to defective insulin signaling in macrophages. J. Clin. Invest. 113, 764–773 10.1172/JCI19528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campfield L.A., Smith F.J., Guisez Y., Devos R. and Burn P. (1995) Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269, 546–549 10.1126/science.7624778 [DOI] [PubMed] [Google Scholar]
- 44.Dubuc P.U. (1976) The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism 25, 1567–1574 10.1016/0026-0495(76)90109-8 [DOI] [PubMed] [Google Scholar]
- 45.Coleman D.L. (1973) Effects of Parabiosis of Obese with Diabetes and Normal Mice. Diabetologia 298, 294–298 [DOI] [PubMed] [Google Scholar]
- 46.Nishina P.M., Lowe S., Wang J. and Paigen B. (1994) Characterization of plasma lipids in genetically obese mice: the mutants obese, diabetes, fat, tubby, and lethal yellow. Metabolism 43, 549–553 10.1016/0026-0495(94)90194-5 [DOI] [PubMed] [Google Scholar]
- 47.Hoffmann A., Ebert T., Klöting N., Dokas J., Jeromin F., Jessnitzer B. et al. (2016) Biochimica et Biophysica Acta Leptin dose-dependently decreases atherosclerosis by attenuation of hypercholesterolemia and induction of adiponectin. BBA - Mol. Basis Dis. 1862, 113–120, [Internet] 10.1016/j.bbadis.2015.10.022 [DOI] [PubMed] [Google Scholar]
- 48.Chiba T., Shinozaki S., Nakazawa T., Kawakami A., Ai M., Kaneko E. et al. (2008) Leptin deficiency suppresses progression of atherosclerosis in apoE-deficient mice. Atherosclerosis 196, 68–75 [DOI] [PubMed] [Google Scholar]
- 49.Gruen M.L., Saraswathi V., Nuotio-antar A.M., Plummer M.R., Coenen K.R. and Hasty A.H. (2006) Plasma insulin levels predict atherosclerotic lesion burden in obese hyperlipidemic mice, Atherosclerosis 186, 54–64 [DOI] [PubMed] [Google Scholar]
- 50.Wu K.K., Wu T.J., Chin J., Mitnaul L.J., Hernandez M., Cai T.Q. et al. (2005) Increased hypercholesterolemia and atherosclerosis in mice lacking both ApoE and leptin receptor. Atherosclerosis 181, 251–259 10.1016/j.atherosclerosis.2005.01.029 [DOI] [PubMed] [Google Scholar]
- 51.Bodary P.F., Gu S., Shen Y., Hasty A.H., Buckler J.M. and Eitzman D.T. (2005) Recombinant leptin promotes atherosclerosis and thrombosis in apolipoprotein E-deficient mice. Arterioscler. Thromb. Vasc. Biol. 25, e119–e122 10.1161/01.ATV.0000173306.47722.ec [DOI] [PubMed] [Google Scholar]
- 52.Kong L., Shen X., Lin L., Leitges M., Rosario R., Zou Y.S. et al. (2013) PKCbeta promotes vascular inflammation and acceleration of atherosclerosis in diabetic ApoE null mice. Arterioscler. Thromb. Vasc. Biol. 33, 1779–1787 10.1161/ATVBAHA.112.301113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Q., Park K., Li C., Rask-Madsen C., Mima A., Qi W. et al. (2013) Induction of vascular insulin resistance and endothelin-1 expression and acceleration of atherosclerosis by the overexpression of protein kinase C-beta isoform in the endothelium. Circ. Res. 113, 418–427 10.1161/CIRCRESAHA.113.301074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durpès M.C., Morin C., Paquin-Veillet J., Beland R., Paré M., Guimond M.O. et al. (2015) PKC-β activation inhibits IL-18-binding protein causing endothelial dysfunction and diabetic atherosclerosis. Cardiovasc. Res. 106, 303–313 10.1093/cvr/cvv107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li Z., Abdullah C.S. and Jin Z.-Q. (2014) Inhibition of PKC-theta preserves cardiac function and reduces fibrosis in streptozotocin-induced diabetic cardiomyopathy. Br. J. Pharmacol. 171, 2913–2924 10.1111/bph.12621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jeziorska M. and Woolley D.E. (1999) Local neovascularization and cellular composition within vulnerable regions of atherosclerotic plaques of human carotid arteries. J. Pathol. 188, 189–196 [DOI] [PubMed] [Google Scholar]
- 57.Caporali A., Bäck M., Daemen M.J., Hoefer I.E., Jones E.A., Lutgens E. et al. (2018) Future directions for therapeutic strategies in post-ischaemic vascularization: A position paper from European Society of Cardiology Working Group on Atherosclerosis and Vascular Biology. Cardiovasc. Res. 114, 1411–1421 10.1093/cvr/cvy184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luong L.A., Fragiadaki M., Smith J., Boyle J., Lutz J., Dean J.L.E. et al. (2013) Cezanne regulates inflammatory responses to hypoxia in endothelial cells by targeting TRAF6 for Deubiquitination. Circ. Res. 112, 1583–1591 10.1161/CIRCRESAHA.111.300119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Santilli S.M., Kronson J. and Payne W.D. (1998) The effect of hypercholesterolemia on the rabbit transarterial wall oxygen gradient. Ann. Vasc. Surg. 12, 418–423 10.1007/s100169900178 [DOI] [PubMed] [Google Scholar]
- 60.Wilson S.H., Herrmann J., Lerman L.O., Holmes D.R.J., Napoli C., Ritman E.L. et al. (2002) Simvastatin preserves the structure of coronary adventitial vasa vasorum in experimental hypercholesterolemia independent of lipid lowering. Circulation 105, 415–418 10.1161/hc0402.104119 [DOI] [PubMed] [Google Scholar]
- 61.Wang G.L. and Semenza G.L. (1993) Characterization of hypoxia-inducible factor 1 and regulation of DNA binding activity by hypoxia. J. Biol. Chem. 268, 21513–21518 [PubMed] [Google Scholar]
- 62.Gu Y.Z., Moran S.M., Hogenesch J.B., Wartman L. and Bradfield C.A. (1998) Molecular characterization and chromosomal localization of a third alpha-class hypoxia inducible factor subunit, HIF3alpha. Gene Expr. 7, 205–213 [PMC free article] [PubMed] [Google Scholar]
- 63.Maxwell P.H., Wiesener M.S., Chang G.W., Clifford S.C., Vaux E.C., Cockman M.E. et al. (1999) The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399, 271–275 10.1038/20459 [DOI] [PubMed] [Google Scholar]
- 64.Jaakkola P., Mole D.R., Tian Y.M., Wilson M.I., Gielbert J., Gaskell S.J. et al. (2001) Targeting of HIF- alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292, 468–472 10.1126/science.1059796 [DOI] [PubMed] [Google Scholar]
- 65.Epstein A.C., Gleadle J.M., McNeill L.A., Hewitson K.S., O'Rourke J., Mole D.R. et al. (2001) C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107, 43–54 10.1016/S0092-8674(01)00507-4 [DOI] [PubMed] [Google Scholar]
- 66.Mahon P.C., Hirota K. and Semenza G.L. (2001) FIH-1: a novel protein that interacts with HIF-1alpha and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 15, 2675–2686 10.1101/gad.924501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lando D., Peet D.J., Gorman J.J., Whelan D.A., Whitelaw M.L. and Bruick R.K. (2002) FIH-1 is an asparaginyl hydroxylase enzyme that regulates the transcriptional activity of hypoxia-inducible factor. Genes Dev. 16, 1466–1471 10.1101/gad.991402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang G.L., Jiang B.-H., Rue E.A. and Semenza G.L. (1995) Hypoxia-inducible factor 1 is a basic-helix-loop- helix-PAS heterodimer regulated by cellular 02 tension. Genetics 92, 5510–5514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yamada N., Horikawa Y., Oda N., Iizuka K., Shihara N., Kishi S. et al. (2005) Genetic variation in the hypoxia-inducible factor-1alpha gene is associated with type 2 diabetes in Japanese. J. Clin. Endocrinol. Metab. 90, 5841–5847 10.1210/jc.2005-0991 [DOI] [PubMed] [Google Scholar]
- 70.Gunton J.E., Kulkarni R.N., Yim S.H., Okada T., Hawthorne W.J., Tseng Y.H. et al. (2005) Loss of ARNT/HIF1β mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell 122, 337–349 10.1016/j.cell.2005.05.027 [DOI] [PubMed] [Google Scholar]
- 71.Girgis C.M., Cheng K., Scott C.H. and Gunton J.E. (2012) Novel links between HIFs, type 2 diabetes, and metabolic syndrome. Trends Endocrinol. Metab. 23, 372–380 10.1016/j.tem.2012.05.003 [DOI] [PubMed] [Google Scholar]
- 72.Cheng K., Ho K., Stokes R., Scott C., Lau S.M., Hawthorne W.J. et al. (2010) Hypoxia-inducible factor- 1α regulates β cell function in mouse and human islets, J Clin Invest 120, 2171–2183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pillai R., Huypens P., Huang M., Schaefer S., Sheinin T., Wettig S.D. et al. (2011) Aryl hydrocarbon receptor nuclear translocator/hypoxiainducible factor-1 β plays a critical role in maintaining glucose-stimulated anaplerosis and insulin release from pancreatic β-cells. J. Biol. Chem. 286, 1014–1024 10.1074/jbc.M110.149062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zehetner J., Danzer C., Collins S., Eckhardt K., Gerber P.A., Ballschmieter P. et al. (2008) pVHL is a regulator of glucose metabolism and insulin secretion in pancreatic β cells. Genes Dev. 22, 3135–3146 10.1101/gad.496908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang N., Fu Z., Linke S., Chicher J., Gorman J.J., Visk D. et al. (2010) The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab. 11, 364–78 10.1016/j.cmet.2010.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gonzalez F.J., Xie C. and Jiang C. (2018) The role of hypoxia-inducible factors in metabolic diseases. Nat. Rev. Endocrinol. 15, 21–32 10.1038/s41574-018-0096-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jun J.C., Devera R., Unnikrishnan D., Shin M.K., Bevans-Fonti S., Yao Q.R.A. et al. (2017) Adipose HIF-1α causes obesity by suppressing brown adipose tissue thermogenesis. 95, 287–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Halberg N., Khan T., Trujillo M.E., Wernstedt-Asterholm I., Attie A.D., Sherwani S. et al. (2009) Hypoxia-Inducible Factor 1 Induces Fibrosis and Insulin Resistance in White Adipose Tissue. Mol. Cell. Biol. 29, 4467–4483 10.1128/MCB.00192-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang X., Lam K.S.L., Ye H., Chung S.K., Zhou M., Wang Y. et al. (2010) Adipose tissue-specific inhibition of hypoxia-inducible factor 1α induces obesity and glucose intolerance by impeding energy expenditure in mice. J. Biol. Chem. 285, 32869–32877 10.1074/jbc.M110.135509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsuura H., Ichiki T., Inoue E., Nomura M., Miyazaki R., Hashimoto T. et al. (2013) Prolyl hydroxylase domain protein 2 plays a critical role in diet-induced obesity and glucose intolerance. Circulation 127, 2078–2087 10.1161/CIRCULATIONAHA.113.001742 [DOI] [PubMed] [Google Scholar]
- 81.Lee Y.S., Kim J., Osborne O., Oh D.Y., Sasik R., Schenk S. et al. (2015) Increased Adipocyte O2 Consumption Triggers HIF-1α Causing Inflammation and Insulin Resistance in Obesity. Cell 157, 1339–1352 10.1016/j.cell.2014.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang H., Zhang G., Gonzalez F.J., Park S.M. and Cai D. (2011) Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol. 9, 1–16 10.1371/journal.pbio.1001112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rankin E.B., Rha J., Selak M.A., Unger T.L., Keith B., Liu Q. et al. (2009) Hypoxia-Inducible Factor 2 Regulates Hepatic Lipid Metabolism. Mol. Cell. Biol. 29, 4527–4538 10.1128/MCB.00200-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morello E., Sutti S., Foglia B., Novo E., Cannito S., Bocca C. et al. (2018) Hypoxia-inducible factor 2α drives nonalcoholic fatty liver progression by triggering hepatocyte release of histidine-rich glycoprotein. Hepatology 67, 2196–2214 10.1002/hep.29754 [DOI] [PubMed] [Google Scholar]
- 85.Taniguchi C.M., Finger E.C., Krieg A.J., Wu C., Diep A.N., LaGory E.L. et al. (2013) Cross-talk between hypoxia and insulin signaling through Phd3 regulates hepatic glucose and lipid metabolism and ameliorates diabetes. Nat. Med. 19, 1325–1330 10.1038/nm.3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ramakrishnan S.K., Zhang H., Takahashi S., Centofanti B., Periyasamy S., Weisz K. et al. (2016) HIF2α Is an essential molecular brake for postprandial hepatic glucagon response independent of insulin signaling. Cell Metab. 23, 505–516 10.1016/j.cmet.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Baron A.D., Schaeffer L., Shragg P. and Kolterman O.G. (1987) Role of hyperglucagonemia in maintenance of increased rates of hepatic glucose output in type II diabetics. Diabetes 36, 274–283 10.2337/diab.36.3.274 [DOI] [PubMed] [Google Scholar]
- 88.McClain D.A., Abuelgasim K.A., Nouraie M., Salomon-Andonie J., Niu X., Miasnikova G. et al. (2013) Decreased serum glucose and glycosylated hemoglobin levels in patients with Chuvash polycythemia: a role for HIF in glucose metabolism. J. Mol. Med. 91, 59–67 10.1007/s00109-012-0961-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pan H., Lin X., Wu Y., Chen L., Teh A.L., Soh S.E. et al. (2015) HIF3A association with adiposity: the story begins before birth. Epigenomics 7, 937–950 10.2217/epi.15.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pfeiffer S., Krüger J., Maierhofer A., Böttcher Y., Klöting N., El Hajj N. et al. (2016) Hypoxia-inducible factor 3A gene expression and methylation in adipose tissue is related to adipose tissue dysfunction. Sci. Rep. 6, 27969, [Internet] http://www.nature.com/articles/srep27969 10.1038/srep27969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Main A.M., Gillberg L., Jacobsen A.L., Nilsson E., Gjesing A.P., Hansen T. et al. (2016) DNA methylation and gene expression of HIF3A : cross-tissue validation and associations with BMI and insulin resistance. Clin. Epigenetics 1–7, [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iyer N.V., Kotch L.E., Agani F., Leung S.W., Laughner E., Wenger R.H. et al. (1998) Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1 alpha. Genes Dev. 12, 149–162 10.1101/gad.12.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kotch L.E., Iyer N.V., Laughner E. and Semenza G.L. (1999) Defective Vascularization of HIF-1α-Null Embryos Is Not Associated with VEGF Deficiency but with Mesenchymal Cell Death. Dev Biol. 209, 254–267 10.1006/dbio.1999.9253 [DOI] [PubMed] [Google Scholar]
- 94.Compernolle V., Brusselmans K., Franco D., Moorman A., Dewerchin M., Collen D. et al. (2003) Cardia bifida, defective heart development and abnormal neural crest migration in embryos lacking hypoxia-inducible factor-1alpha. Cardiovasc. Res. 60, 569–579 10.1016/j.cardiores.2003.07.003 [DOI] [PubMed] [Google Scholar]
- 95.Cai Z., Manalo D.J., Wei G., Rodriguez E.R., Fox-Talbot K., Lu H. et al. (2003) Hearts from rodents exposed to intermittent hypoxia or erythropoietin are protected against ischemia-reperfusion injury. Circulation 108, 79–85 10.1161/01.CIR.0000078635.89229.8A [DOI] [PubMed] [Google Scholar]
- 96.Xue W., Cai L., Tan Y., Thistlethwaite P., Kang Y.J., Li X. et al. (2010) Cardiac-specific overexpression of HIF-1α prevents deterioration of glycolytic pathway and cardiac remodeling in streptozotocin-induced diabetic mice. Am. J. Pathol. 177, 97–105 10.2353/ajpath.2010.091091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bohuslavova R., Kolar F., Sedmera D., Skvorova L., Papousek F., Neckar J. et al. (2014) Partial deficiency of HIF-1α stimulates pathological cardiac changes in streptozotocin-induced diabetic mice. BMC Endocr. Disord. 14, 1–14, [Internet] 10.1186/1472-6823-14-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xia Y., Gong L., Liu H., Luo B., Li B., Li R. et al. (2015) Inhibition of prolyl hydroxylase 3 ameliorates cardiac dysfunction in diabetic cardiomyopathy. Mol. Cell. Endocrinol. 403, 21–29 10.1016/j.mce.2015.01.014 [DOI] [PubMed] [Google Scholar]
- 99.Chen Y.F., Pandey S., Day C.H., Chen Y.F., Jiang A.Z., Ho T.J. et al. (2018) Synergistic effect of HIF-1α and FoxO3a trigger cardiomyocyte apoptosis under hyperglycemic ischemia condition. J. Cell. Physiol. 233, 3660–3671 10.1002/jcp.26235 [DOI] [PubMed] [Google Scholar]
- 100.Dodd M.S., Sousa Fialho M.D.L., Montes Aparicio C.N., Kerr M., Timm K.N., Griffin J.L. et al. (2018) Fatty Acids Prevent Hypoxia-Inducible Factor-1α Signaling Through Decreased Succinate in Diabetes. JACC Basic to Transl. Sci. 3, 485–498 10.1016/j.jacbts.2018.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wu R., Chang H.C., Khechaduri A., Chawla K., Tran M., Chai X. et al. (2014) Cardiac-specific ablation of ARNT leads to lipotoxicity and cardiomyopathy. J. Clin. Invest. 124, 4795–4806 10.1172/JCI76737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wei H., Bedja D., Koitabashi N., Xing D., Chen J., Fox-Talbot K. et al. (2012) Endothelial expression of hypoxia-inducible factor 1 protects the murine heart and aorta from pressure overload by suppression of TGF-beta signaling. Proc. Natl. Acad. Sci. U.S.A. 109, E841–E850 10.1073/pnas.1202081109 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Grogan R.H., Wong V.W., Chang E.I., Shi Y., Galvez M.G., Januszyk M. et al. (2011) HIF-1α dysfunction in diabetes. Cell Cycle 9, 75–79 [DOI] [PubMed] [Google Scholar]
- 104.Mace K.A., Yu D.H., Paydar K.Z., Boudreau N. and Young D.M. (2007) Sustained expression of Hif-1α in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 15, 636–645 10.1111/j.1524-475X.2007.00278.x [DOI] [PubMed] [Google Scholar]
- 105.Botusan I.R., Sunkari V.G., Savu O., Catrina A.I., Grunler J., Lindberg S. et al. (2008) Stabilization of HIF-1 is critical to improve wound healing in diabetic mice. Proc. Natl Acad. Sci. 105, 19426–19431 10.1073/pnas.0805230105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Semenza G.L., Roth P.H., Fang H.M. and Wang G.L. (1994) Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J. Biol. Chem. 269, 23757–23763 [PubMed] [Google Scholar]
- 107.Tian H., Mcknight S.L. and Russell D.W. (1997) Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 11, 72–82 [DOI] [PubMed] [Google Scholar]
- 108.Dagher Z., Ruderman N., Tornheim K. and Ido Y. (2001) Acute regulation of fatty acid oxidation and AMP-activated protein kinase in human umbilical vein endothelial cells. Circ. Res. 88, 1276–1282 10.1161/hh1201.092998 [DOI] [PubMed] [Google Scholar]
- 109.Groschner L.N., Waldeck-Weiermair M., Malli R. and Graier W.F. (2012) Endothelial mitochondria-less respiration, more integration. Pflugers Arch. 464, 63–76 10.1007/s00424-012-1085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kim B., Li J., Jang C. and Arany Z. (2017) Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 36, 2321–2333 10.15252/embj.201796436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tang X., Luo Y.X., Chen H.Z. and Liu D.P. (2014) Mitochondria, endothelial cell function, and vascular diseases. Front. Physiol. 5, 1–17 10.3389/fphys.2014.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.De Bock K., Georgiadou M., Schoors S., Kuchnio A., Wong B.W., Cantelmo A.R. et al. (2013) Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 154, 651–663 10.1016/j.cell.2013.06.037 [DOI] [PubMed] [Google Scholar]
- 113.Mann G.E., Yudilevich D.L. and Sobrevia L. (2003) Regulation of Amino Acid and Glucose Transporters in Endothelial and Smooth Muscle Cells. Physiol. Rev. 83, 183–252 10.1152/physrev.00022.2002 [DOI] [PubMed] [Google Scholar]
- 114.Falkenberg K.D., Rohlenova K., Luo Y. and Carmeliet P. (2019) The metabolic engine of endothelial cells. Nat. Metab. 1, 937–946 10.1038/s42255-019-0117-9 [DOI] [PubMed] [Google Scholar]
- 115.Feng S., Bowden N., Fragiadaki M., Souilhol C., Hsiao S., Mahmoud M. et al. (2017) Mechanical Activation of Hypoxia-Inducible Factor 1alpha Drives Endothelial Dysfunction at Atheroprone Sites. Arterioscler. Thromb. Vasc. Biol. 37, 2087–2101 10.1161/ATVBAHA.117.309249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu D., Huang R.-T., Hamanaka R.B., Krause M., Oh M.-J., Kuo C.-H. et al. (2017) HIF-1alpha is required for disturbed flow-induced metabolic reprogramming in human and porcine vascular endothelium. Elife 6, e25217, 10.7554/eLife.25217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Xiong J., Kawagishi H., Yan Y., Liu J., Wells Q., Edmunds L.R. et al. (2018) A Metabolic Basis for Endothelial-to-Mesenchymal Transition, Mol Cell 69, 689–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mahmoud M.M., Kim H.R., Xing R., Hsiao S., Mammoto A., Chen J. et al. (2016) TWIST1 Integrates Endothelial Responses to Flow in Vascular Dysfunction and Atherosclerosis. Circ. Res. 119, 450–462 10.1161/CIRCRESAHA.116.308870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen P.Y., Qin L., Baeyens N., Li G., Afolabi T., Budatha M. et al. (2015) Endothelial-to-mesenchymal transition drives atherosclerosis progression. J. Clin. Invest. 125, 4514–4528 10.1172/JCI82719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Evrard S.M., Lecce L., Michelis K.C., Nomura-Kitabayashi A., Pandey G., Purushothaman K.R. et al. (2016) Endothelial to mesenchymal transition is common in atherosclerotic lesions and is associated with plaque instability. Nat. Commun. 7, 11853, 10.1038/ncomms11853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Souilhol C., Harmsen M.C., Evans P.C. and Krenning G. (2018) Endothelial-mesenchymal transition in atherosclerosis. Cardiovasc. Res. 114, 565–577 10.1093/cvr/cvx253 [DOI] [PubMed] [Google Scholar]
- 122.Mahmoud M.M., Serbanovic-Canic J., Feng S., Souilhol C., Xing R., Hsiao S. et al. (2017) Shear stress induces endothelial-To-mesenchymal transition via the transcription factor Snail. Sci. Rep. 7, 1–12 10.1038/s41598-017-03532-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bertero T., Fessel J., Chan S.Y., Bertero T., Oldham W.M., Cottrill K.A. et al. (2016) Vascular stiffness mechanoactivates YAP / TAZ- dependent glutaminolysis to drive pulmonary hypertension Find the latest version : Vascular stiffness mechanoactivates YAP / TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Invest. 126, 3313–3335 10.1172/JCI86387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Doddaballapur A., Michalik K.M., Manavski Y., Lucas T., Houtkooper R.H., You X. et al. (2015) Laminar Shear Stress Inhibits Endothelial Cell Metabolism via KLF2-Mediated Repression of PFKFB3. Arterioscler Thromb Vasc Biol 35, 137–145 [DOI] [PubMed] [Google Scholar]
- 125.Rahtu-Korpela L., Määttä J., Dimova E.Y., Hörkkö S., Gylling H., Walkinshaw G. et al. (2016) Hypoxia- Inducible Factor Prolyl 4-Hydroxylase-2 Inhibition Protects Against Development of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 36, 608–617 10.1161/ATVBAHA.115.307136 [DOI] [PubMed] [Google Scholar]