Abstract
The biomarkers commonly utilized in diagnostic evaluations of kidney disease suffer from low sensitivity, especially in the early stages of renal damage. On the other hand, obtaining a renal biopsy to augment clinical decision making can lead to potentially serious complications. In order to overcome the shortcomings of currently available diagnostic tools, recent studies suggest that exosomes, cell-secreted extracellular vesicles containing a large array of active molecules to facilitate cell-to-cell communication, may represent a rich source of novel disease biomarkers. Because of their endocytic origin, exosomes carry markers typical for their parent cells, which could permit the localization of biochemical cellular alterations in specific kidney compartments. Different types of exosomes can be isolated from noninvasively obtained biofluids; however, in the context of kidney disease, evidence has emerged on the role of urinary exosomes in the diagnostic and predictive modeling of renal pathology. The current review summarizes the potential application of exosomes in the detection of acute and chronic inflammatory, metabolic, degenerative, and genetic renal diseases.
1. Introduction
Kidney disease, which encompasses various acute, chronic, or end-stage conditions, incurs a considerable health burden due to high prevalence and costly management [1]. While most cases of renal dysfunction are attributed to diabetes and hypertension, other inflammatory, immune-mediated, and genetic conditions have been implicated in kidney damage. A timely and accurate diagnosis is crucial for improved outcomes. Renal biopsy is an invaluable diagnostic tool for the establishment of the exact diagnosis and can aid in determining a prognosis and likelihood of response to treatment. As a result of the invasive technique used in obtaining the tissue samples, complications are numerous and can range from acute bleeding to the loss of the biopsied kidney. Renal biopsies are contraindicated in cases of increased bleeding risk, solitary kidneys, or renal anatomical abnormalities making diagnostic efforts in those cases very challenging [2].
The conventional biomarkers or renal disease in clinical practice are suboptimal: serum creatinine is limited by poor sensitivity in mild-to-moderate kidney failure and eGFR by its dependence on creatinine measurement in the early stage of renal dysfunction [3]; in addition, recent data question microalbuminuria as a reliable predictor of progression to end-stage renal disease [3].
Exosomes are bilipid membrane-bound vesicles measuring 40-120 nm in diameter; they are distinct from other extracellular vesicles (EVs) such as microvesicles and apoptotic bodies because their biogenesis is linked to the endosomal pathway. They are generated by an inward blebbing of the endosomal membrane that produces multivesicular bodies (MVBs), which are then fused with the plasma membrane and released via exocytosis (EL [4]). The exosomal cargo includes a variety of proteins, nucleic acids, lipids, and metabolites depending on their cell of origin and microenvironmental factors. All exosomes are highly enriched in proteins such as annexins, tetraspanins, and flotillin [5], which can be targeted in the process of exosome isolation and purification. The contents packaged into exosomes constitute intercellular mediators that can regulate certain physiologic processes including innate immunity, coagulation, spermatogenesis, central nervous system functions [6] , and bone remodeling [7]. In cancer biology, exosomes favor tumor progression by conditioning tumor microenvironment as well as remote premetastatic sites termed “premetastatic niches” [8] and can serve as liquid biopsies for various types of cancer [9, 10]. Recently, there has been an increasing interest in identifying exosomal biomarkers for nonneoplastic diseases [11–14].
There are multiple methods of exosome isolation. Centrifugation-based techniques, differential centrifugation, and density gradient ultracentrifugation are considered the gold standard. Differential centrifugation involves multiple steps of increasing centrifugation speed to first remove cells, apoptotic debris, and subsequently larger vesicles, so as to ultimately precipitate exosomes. Coprecipitation of EVs with apoptotic bodies and protein aggregates may occur. A way to avoid this is using a sucrose density gradient with centrifugation steps, separating the vesicles according to flotation density [15]. Filtration-based techniques, which separate vesicles depending on size and molecular weight, can be used independently, or as a replacement of the first two spins in differential centrifugation, so as to increase purity [15]. Tangential flow filtration is a technique that combines membrane filtration and flow, whereby the exosome-containing fluid flows tangentially across the membrane surface [16]. Size-exclusion chromatography is another size-based technique; it consists in EVs passing through diluted porous particles instead of a membrane, which results in different elution times for vesicles depending on whether they are small enough to enter the pores [15]. The best technique by far is the combination of tangential flow filtration and size-exclusion chromatography. Immunoaffinity-based separation takes advantage of exosomal membrane proteins, usually members of the tetraspanin family, such as CD9, CD63, and CD81 [15], and tissue-specific surface proteins when isolation of tissue-specific exosomes is desired, such as FABP4 for adipocyte-derived EVs using Western Blots [17]. Antibody-coated magnetic beads are commonly applied [18]. An elution buffer is required to release the exosomes from immunocomplexes [15]. Polymer-based precipitation methods consist in mixing the exosome-containing fluids with a polymer solution, usually polyethylene glycol, followed by recovering of the precipitated exosomes with low-speed centrifugation [15]. More recently, miniaturized microfluidic apparatuses using immunoaffinity-based or size-dependent separation techniques, or even contact-free particle sorting mechanisms (e.g., elastic lift force, acoustic, and dielectrophoresis), have been developed [15]. Nowadays, progress in the analytical procedures on exosome isolation bioassays proved helpful for better quantification of disease-specific exosomes in clinical samples [18].
The actual disease biomarkers are the miRNAs or proteins carried by the exosomes (exosomal cargo). Those miRNAs can be analyzed by RNA sequencing. There are currently bedside RNA sequencing techniques that give results within a few hours [19, 20].
Human models of renal disease have demonstrated that kidney damage is primarily driven by immune dysregulation and alterations in hemostasis, vascular integrity, and matrix modulation that are regulated by exosomes [21]. Circulating exosomes, capable of traversing basement membranes, are excreted in the urine and reuptaken by the collecting duct cells in a vasopressin-dependent manner. Renal tubular- and glomerular-derived exosomes are thought to participate in renal clearance and tissue regeneration [21]. Exosomes entrapped in the polymeric Tamm-Horsfall protein are hypothesized to mediate effects along tubular lumina, for instance, inducing the expression of proximal tubular proteins aquaporin-1 and glutaminase in downstream segments of the nephron [22].
The potential of exosomes to serve as therapeutic agents or drug delivery vehicles in chronic kidney disease [23] to alleviate systemic consequences [24, 25] makes them ideal treatment candidates. Multiple studies using preclinical, clinical, and ex vivo models have examined possible therapeutic applications of exosomes in diabetic nephropathy [26], hypertension-related cardiorenal syndrome [27], acute kidney injury [28, 29], IgA nephropathy [30], cadmium nephropathy [31], obstructive kidney disease [32], and ischemia/reperfusion injury [33].
MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression mainly through RNA silencing [34]. Exosomes transport miRNA clusters which mediate autocrine and paracrine effects in target sites [34]. In chronic kidney disease, miRNAs are implicated in fibrosis, podocyte damage and apoptosis, mesangial cell hypertrophy or proliferation, and oxidative stress and inflammation [35]. Abundant miRNAs have been associated with kidney disease. Despite discrepancies in the literature, certain miRNAs seem to be consistently dysregulated, such as miR-21-5p, miR-29a-3p, miR-126-3p, miR-192-5p, miR-214-3p, and miR-342-3p in diabetic kidney disease [36]. Interestingly, amplification-free detection methods of exosomal miRNAs have been developed [37].
Exosomal cargo which determines cell-targeting can give us a wealth of information about the original cytosolic environment and relevant biochemical changes and also serves as a potential source of biomarkers. In this review, we aim to synthesize published data from human studies to date. The candidate biomarkers are presented in Tables 1–4 by potential clinical utility and are discussed in the text below by clinical condition.
Table 1.
Urinary exosomal biomarkers potentially useful in the recognition of early damage patients (pts)/control (ctr).
| Condition | Potential exosomal biomarker | Study subjects | Reference |
|---|---|---|---|
| CKD | Ceruloplasmin ↑ | 51 pts-15 ctrs; rats | [38] |
| miR-181a-5p ↑, among 30 differentially expressed ncRNAs | 15 pts-10 ctrs | [39] | |
| Exosomal miR-451 ↑ | 38 pts-23 ctrs | [40] | |
|
| |||
| Lupus nephritis | miR-150 and miR-21 ↑; miR-29c ↓ | 45 pts-20 ctrs | [77] |
| miR-146a ↑ | 38 pts-12 ctrs | [78] | |
|
| |||
| Diabetic kidney disease | Among 22 proteins: MASP2 and CALB1 ↑; S100A8 and S100A9 ↓ | 60 pts-15 ctrs | [53] |
| miR-21-5p ↑; miR-30b-5p ↓ | 66 pts | [51] | |
| miR-15b, miR-34a, and miR-636 | 54 pts-12 ctrs | [52] | |
| Myeloblastin, elafin, cystatin B and neutrophil gelatinase-associated Lipocalin ↑ | 37 pts-12 ctrs | [54] | |
| Regucalcin ↓ | 4 pts-3 ctrs; rats | [55] | |
|
| |||
| Nephronophthisis | 156 differentially expressed proteins | 12 pts-12 ctrs | [89] |
|
| |||
| Acute kidney injury in critical disease | Activating transcriptional factor 3 ↑ | 8 pts-8 ctrs; mice | [61] |
| Fetuin-A ↑ | 6 pts; rats | [62] | |
Table 2.
Urinary exosomal biomarkers potentially useful in disease monitoring and/or management patients (pts)/control (ctr).
| Condition | Potential exosomal biomarker | Study subjects | Reference | |
|---|---|---|---|---|
| Lupus nephritis | Prediction of clinical response | miR-31, miR-107, and miR-135b-5p ↑ | 57 pts | [81] |
| Disease flare | let-7a and miR-21 ↓ | 34 pts | [79] | |
| Cellular crescent formation in type IV lupus nephritis | miR-3135b, miR-654-5p, and miR-146a-5p ↑ | 14 pts-3 ctrs | [80] | |
|
| ||||
| IgA nephropathy | Tubulointerstitial inflammation and C3 deposition | CCL2 mRNA ↑ | 55 pts-24 ctrs | [76] |
|
| ||||
| Nephropathy in type 1 diabetes | Various degrees of albuminuria | Various differentially expressed miRNAs | 48 pts | [56] |
|
| ||||
| Nephropathy in type 2 diabetes | Decline in renal function | Uromodulin mRNA ↑ | 242 pts and ctrs | [57] |
| Progression of albuminuria | C-megalin | 33 pts-11 ctrs | [58] | |
| Macroalbuminuria | miR-362-3p, miR-877-3p, and miR-150-5p ↑; urinary miR-15a-5p ↓ | 5 pts-5 ctrs | [106] | |
|
| ||||
| AL amyloidosis | Active amyloid formation | Light chain oligomers | 4 pts-1 ctr | [71] |
| 13 pts-1 ctr | [72] | |||
|
| ||||
| Autosomal dominant polycystic kidney disease | eGFR decline | AQP-2 ↓; APO-A1 ↑ | 46 pts-11 ctrs | [85] |
|
| ||||
| Cystinuria | eGFR value | 165 differentially expressed proteins | 8 pts-10 ctrs | [90] |
Table 3.
Exosomal biomarkers associated with specific etiological factors of renal disease patients (pts)/control (ctr).
| Condition | Potential exosomal biomarker | Study subjects | Reference | |
|---|---|---|---|---|
| Medullary sponge kidney | vs. idiopathic calcium nephrolithiasis | Blood FCN1 and C4BPB proteins ↑; blood MASP2 protein ↓ | 15 pts-15 ctrs | [87] |
| vs. autosomal dominant polycystic kidney disease | Mainly urinary CD133 ↓, among 34 discriminative urinary EV proteins | 15 pts-15 ctrs | [88] | |
|
| ||||
| Autosomal dominant polycystic kidney disease | Urinary periplakin, envoplakin, villin-1, and complement C3 and C9 ↑, among 30 proteins | 34 pts-32 ctrs | [84] | |
| Urinary PC1/TMEM2 or PC2/TMEM2 ↓ | 13 pts-18 ctrs | [83] | ||
|
| ||||
| Diabetic nephropathy | vs. minimal change nephrotic syndrome | Urinary WT1 mRNA ↑ | 20 pts-5 ctrs | [49] |
|
| ||||
| Cadmium-induced nephrotoxicity | Blood MT1DP lncRNA ↑ | 100 persons | [107] | |
|
| ||||
| Idiopathic membranous nephropathy | Blood and urinary MUC3A circRNA and various snoRNAs ↑ | 10 pts-10 ctrs | [70] | |
|
| ||||
| Pediatric idiopathic nephrotic syndrome | Urinary miR-194-5p, miR-146b-5p, miR-378a-3p, miR-23b-3p, and miR-30a-5p ↑ | 129 pts-126 ctrs | [66] | |
|
| ||||
| Pediatric primary focal segmental glomerulosclerosis | vs. minimal change disease | Urinary miR-193a | 13 pts | [67] |
|
| ||||
| IgA nephropathy | vs. thin basement membrane nephropathy | Urinary miR-215-5p and miR-378i ↑; urinary miR-29c and miR-205-5p ↓ | 18 pts-18 ctrs | [73] |
| Urinary aminopeptidase N, vasorin precursor, α-1-antitrypsin, and ceruloplasmin ↑ | 12 pts-7 ctrs | [74] | ||
|
| ||||
| Acute rejection | vs. BK nephropathy or chronic allograft injury | Urinary CLCA1, PROS1, KIAA0753, and ApoM ↑ | 30 pts-20 ctrs | [64] |
|
| ||||
| Focal segmental glomerulosclerosis | vs. steroid-sensitive nephrotic syndrome | Urinary WT-1 ↑ | 25 pts-5 ctrs | [68] |
|
| ||||
| Bartter syndrome type 1 | Urinary NKCC2 protein ↓ | 2 pts | [91] | |
Table 4.
Urinary exosomal biomarkers associated with injury localized to a specific cellular or subcellular component of the nephron patients (pts)/control (ctr).
| Condition | Potential exosomal biomarker | Study subjects | Reference | |
|---|---|---|---|---|
| Podocyte injury | In diabetic nephropathy | Elf3 protein ↑ | 50 pts-5 ctrs | [48] |
| WT1, podocin, Actn4, CD2AP, and nephrin mRNA ↑ | 20 pts-5 ctrs | [49] | ||
| In minimal change nephrotic syndrome | Podocin, Actn4, CD2AP, and nephrin mRNA ↑ | |||
| In metabolic syndrome-related kidney disease | Podocyte-derived exosomes (nephrin+/podocalyxin+) ↑ | 16 pts-15 ctrs | [43] | |
| In CKD | miR-21 ↑ | 41 pts-5 ctrs | [44] | |
| In cellular crescent formation | SFP1 ↑ | 37 pts | [69] | |
| In renovascular hypertension | Podocyte-derived exosomes (nephrin+/podocalyxin+) ↑ | 31 pts-45 ctrs | [60] | |
| In lupus nephritis | miR-29c ↓ | 24 pts-8 ctrs; mice | [75] | |
| In IgA nephropathy | ||||
| In focal segmental glomerulosclerosis | WT-1 ↑ | 25 pts-5 ctrs | [68] | |
|
| ||||
| Proximal tubular injury | In decompensated cirrhosis | Maltase glucoamylase ↑ | 24 pts | [63] |
|
| ||||
| Renal fibrosis | In CKD | Nonproximal tubule-derived miR-200b ↑ | 38 pts | [45] |
| miR-29c ↓ | 32 pts-7 and ctrs | [46] | ||
| CD2AP mRNA ↓ | 32 pts-7 ctrs | [47] | ||
| In lupus nephritis | miR-29c ↓ | 47pts-20 ctrs | [82] | |
|
| ||||
| Peritubular capillary loss | In hypertension | Endothelial-derived EVs (PL-VAP+/CD31+/CD144+) ↑ | 38 pts-14 ctrs | [59] |
|
| ||||
| Mitochondrial dysfunction | In diabetic nephropathy | 12 mitochondria-specific metabolites ↓ | 149 pts-23 ctrs | [50] |
2. Materials and Methods
We searched the online MEDLINE® database of the U.S. National Library of Medicine with the complex term (exosomes OR “extracellular vesicles”) AND (“kidney disease” OR “renal disease” OR “renal transplantation” OR “renal transplant” OR “renal failure” OR “kidney injury” OR nephritis OR “nephrotic syndrome” OR “nephritic syndrome”) which produced 393 results (last assessed on April 27th, 2020). Articles referring to “extracellular vesicles” were included as long as the described experimental method included exosomal isolation. Ultimately, the literature cited herein includes 95 peer-reviewed, original articles of studies in humans published in English. We used simple narrative analysis to summarize the data from the studies selected for review.
3. Results
3.1. Chronic Kidney Disease
Chronic kidney disease (CKD), which is characterized by the gradual irreversible deterioration of kidney function, is a multifactorial condition caused mainly by metabolic and inflammatory changes and is typically diagnosed and staged based on the estimated glomerular filtration rate (eGFR). In CKD, urinary exosomal cargo is characterized by higher levels of ceruloplasmin [38] and the overexpression of miR-181a-5p [39] and miR-451 [40] compared to healthy controls. Mir-181a-5p has been found to downregulate lipid metabolism regulator PPARα [41] and is in silico predicted to downregulate MAT2A, TIMP3, and LGSF11 [42]; miR-451 downregulates YWHAZ and CAB39, which could be implicated in renal fibrosis and mesangial hypertrophy [40]. These biomarkers are identifiable early in CKD, and more specifically, ceruloplasmin can be detected at the premicroalbuminuric stage [38]. It is suggested that the extent of impairment of specific parts of the nephron is dependent on the underlying causative factors and disease stage. Studies have shown that podocyte injury may be related to a higher urinary concentration of exosomes expressing podocytal markers nephrin and podocalyxin [43], or containing higher amounts of miR-21 [44]. Renal fibrosis is the hallmark of permanent damage in CKD and has been associated with higher levels of miR-200b [45] and lower levels of miR-29c [46] as well as CD2AP mRNA [47].
3.2. Diabetic Nephropathy
Hyperglycemia secondary to diabetic nephropathy gradually damages all compartments of the kidney, beginning with glomerular capillary dysfunction with hyperfiltration and microalbuminuria and ultimately leading to interstitial fibrosis, tubular atrophy, and interstitial inflammation in advanced disease. Evidence of exosome-mediated podocytal injury is evident by either increased Elf3 protein [48] or WT1, podocin, Actn4, CD2AP, and nephrin mRNA [49]. Lower concentrations of mitochondria-specific metabolites such as 3-hydroxyisovalerate, citric acid, and 2-ethyl hydracrylic acid suggest mitochondrial dysfunction [50] that might be responsible for energy production dysregulation. Recognition of incipient damage is important due to the lack of early clinical manifestations. Other potential biomarkers for diabetic nephropathy include miR-21-5p [51], miR-15b, miR-34a, miR-636 [52], MASP2, CALB1 [53], myeloblastin, elafin, cystatin B, and neutrophil gelatinase-associated lipocalin [54], all of which increase in the presence of the condition. Decreased levels of miR-30b-5p [51], S100A8, S100A9 [53] and regucalcin [55] have also been described in diabetic nephropathy. The urinary exosomal miRNA profile [56], uromodulin mRNA levels [57], and C-megalin content [58] seem to be correlated with the degree of albuminuria.
3.3. Hypertensive Nephropathy
Hypertensive nephropathy is the result of either long-standing essential hypertension causing vascular-glomerular damage and remodeling or a primary renovascular lesion leading to renal hypoperfusion and secondary hypertension. The level of urinary exosomal plasmalemma vesicle-associated protein (PLVAP), a protein expressed in the peritubular capillaries, is associated with clinical measurements such as blood pressure and eGFR, and also the histological count of peritubular capillaries and degree of fibrosis in renal patients with essential or renovascular hypertension. This association makes PLVAP a potentially specific biomarker of microcirculation injury [59]. Urinary exosomes positive for nephrin and podocalyxin, proteins normally expressed in podocytes, have been isolated in the urine of patients with renovascular hypertension, indicating podocytal damage [60].
3.4. Acute Kidney Injury
Preliminary data from small clinical studies in critical care medicine have identified two urinary exosomal proteins as candidate biomarkers of acute kidney injury (AKI): (i) activating transcriptional factor 3 (ATF3), which is activated in models of ischemic reperfusion injury [61], and (ii) fetuin-A which is expressed in the cytoplasm of renal tubular cells, especially those detached from the basal lamina [62]. In the setting of decompensated cirrhosis, urine exosome protein characterization in AKI patients revealed increased secretion of maltase glucoamylase, a renal brush border disaccharidase [63]. In renal transplant patients, Sigdel et al. observed that acute rejection and BK-virus-associated nephropathy, two main causes of acute loss of renal function in this population, present with different urinary exosomal protein expression profiles; higher abundance of CLCA1, PROS1, KIAA0753, and ApoM was linked to acute rejection [64].
3.5. Nephrotic Syndrome
Nephrotic syndrome represents a constellation of symptoms including peripheral edema, heavy proteinuria, hypoalbuminemia, and often hyperlipidemia and is thought to result from increased glomerular permeability to albumin and other plasma proteins [65]. The various causes of nephrotic syndrome can be grouped together according to the microscopic pattern of injury into the following: minimal change disease (MCD), focal segmental glomerulosclerosis (FGSG), membranous glomerulonephritis, mesangiocapillary glomerulonephritis, and other, such as amyloidosis [65].
In the pediatric population, a urinary exosomal miRNA profile of upregulated miR-194-5p, miR-146b-5p, miR-378a-3p, miR-23b-3p, and miR-30a-5p has been identified in various histological patterns of injury in idiopathic nephrotic syndrome [66]. At the same time, miR-193a levels may be useful in distinguishing between pediatric primary FGSG and MCD [67]. In addition, the detection of WT-1, a marker of podocytal injury, may aid in diagnosing FGSG when also steroid-sensitive nephrotic syndrome is considered [68]. WT-1 mRNA is generally not detected in the urine of MCD patients, but it is isolated in cases of diabetic nephropathy. Other presumed markers of podocytal injury (podocin, Actn4, CD2AP and Nephrin mRNA) seem insufficient to help differentiate between those two conditions [49].
Crescent formation, the hallmark of RPGN, has been associated with the presence of fibroblast-specific protein 1 (FSP1), a cytosolic protein expressed by increased number of renal cells in kidneys exhibiting ongoing injury [69]. In idiopathic membranous nephropathy, upregulation of blood and urinary MUC3A circular RNA (circRNA) and various small nucleolar RNAs (snoRNAs) such as SNORA51, SNORA31, SNORA70, SNORA75, and SNORD112 has been reported [70]. Lastly, Ramirez-Alvarado et al. demonstrated the presence of amyloidogenic light chains in urinary exosomes of patients with amyloidosis but not in patients with multiple myeloma without amyloidosis [71, 72].
3.6. Nephritic Syndrome
Nephritic syndrome, defined by the presence of hematuria in association with hypertension, oliguria, fluid retention, and a decline in renal function, is an inflammatory process with a histological picture of glomerular basement membrane ruptures and usually diagnosed by renal biopsy. Common causes of nephritic syndrome include anti-GMB disease, IgA nephropathy, and lupus nephritis.
In IgA nephropathy, increased expression of urinary exosomal miR-215-5p and miR-378i and decreased expression of miR-29c and miR-205-5p have been described compared to healthy individuals [73]. Higher levels of aminopeptidase N, vasorin precursor, α-1-antitrypsin, and ceruloplasmin have been used to distinguish between IgA and thin basement membrane nephropathy which is another common cause of glomerular hematuria [74]. A decrease in urinary exosomal miR-29c may indicate podocytal injury [75], whereas an increase in CCL2 mRNA may represent tubulointerstitial inflammation and C3 deposition [76].
Lupus nephritis is characterized by downregulation of urinary exosomal miR-29c [77] and upregulation of miR-146a [78], miR-150, and miR-21 [77]. A decrease in miR-21 along with let-7a miRNA precursor may indicate disease flare [79]; an increase in urinary exosomal miR-3135b, miR-654-5p, and miR-146a-5p has been described in cellular crescent formation of lupus nephritis [80]. Conversely, higher urinary exosomal levels of miR-31, miR-107, and miR-135b-5p are associated with a better response to treatment [81]. Lower levels of miR-29c have been correlated with both renal fibrosis, even without a decline in renal function [82], and podocyte injury [75].
3.7. Genetic Disorders
Cystic kidney diseases (CKD) are heterogeneous in origin, distribution, and pathogenesis; many are related to genetic defects. Autosomal dominant polycystic kidney disease (ADPKD), the most common inherited CKD, mainly results from PKD1 mutations. Hogan et al. described lower PKD1 and higher transmembrane protein 2 (TMEM2) urinary exosomal protein secretion in ADPKD, suggesting that the PKD1/TMEM2 ratio may have some diagnostic utility [83]. Thirty differentially expressed urinary exosomal proteins between ADPKD patients and healthy controls have been identified: urinary periplakin, envoplakin, villin-1, and complement C3 and C9 were more abundant in ADPKD [84]. Additionally, lower AQP-2 and higher APO-A1 levels were correlated with eGFR decline [85]. There is evidence that urinary exosomes in ADPKD individuals may have a different surface glycosylation profile than that of healthy individuals [86].
Exosome isolation and characterization have assisted in the diagnostic challenge to differentiate between medullary sponge kidney (MSK), a cause of medullary nephrocalcinosis, and idiopathic calcium nephrolithiasis. Bruschi et al. found that higher FCN1 and C4PBP, as well as lower MASP2 serum exosome protein levels, were positively associated with MSK [87]. Furthermore, a lower urinary CD133 level seems to favor the diagnosis of MSK over ADPKD [88]. Nephronophthisis, another renal medullary cystic disorder, also presents with a distinct urinary exosomal protein profile [89].
A pilot study with patients with cystinuria highlighted that 165 urinary exosomal proteins, analyzed by mass spectrometry, could be utilized to identify patients and also determine the severity of disease [90]. In two patients with Bartter syndrome type 1, Gonzales et al. noted the absence from urinary exosomes of NKCC2, the protein encoded by the SLC12A1 gene which mutated in this disease [91].
4. Discussion
Treatment optimization of renal disease depends on the availability of diagnostic and prognostic biomarkers. The use of renal biopsy, which remains the gold standard in the diagnosis of kidney disease, is also affected by potentially serious postoperative complications and the possibility of improper or nonrepresentative sampling.
4.1. Advantages of Exosomal Biomarkers in Renal Disease
It is evident that exosomes may be the solution to finding accurate renal disease biomarkers without the need for invasive procedures. Proteomic profiling of urinary exosomes by mass spectroscopy and subsequent computational analysis, reveals an abundance of proteins implicated in pathophysiologic processes such as sodium ion transport, immune activation, and epithelial cell differentiation [92]. Additionally, a significant portion of exosomal protein cargo plays a well-established role in glomerular physiology. Some examples of relevant proteins isolated in exosomes include podocalyxin, lysosomal-associated membrane protein 2, Src substrate cortactin, Rab 23, ENPP6, ezrin, complement C4B, agrin, FAT4, CD59, talin 1, syntenin 1, neprilysin, Na+/H+ exchange regulatory cofactor 2, and angiotensin-converting enzyme [93]. Production of pathologic proteins regulated by defective genes in exosomes from certain genetic renal diseases may be either decreased (PKD1 in ADPKD) [83] or totally absent (SLC12A1 in Bartter syndrome type 1) [91].
Another advantage of exosomes as potential biomarkers is the expression of markers that are specific for their cell of origin that allows the tracking of alterations in specific cellular compartments within a tissue (Figure 1). Hogan et al. identified in urine exosomal cargo molecules specific for their place of biogenesis which includes mesangial and subendothelial cells, proximal tubule cells, glomerular basement membrane (GBM), podocytes and slit diaphragm, podocyte-GBM interface, glomerular endothelial cells, and capillary loops [93]. It is of great interest that, for example, an increase in podocyte- or endothelial-derived exosomes which were determined by the presence of podocytal proteins podocin [49], nephrin, and podocalyxin [43, 60] may indicate podocytal damage. Similarly, higher levels of exosomal endothelial proteins PL-VAP, CD31, and CD144 [59] suggest endothelial damage. Interestingly, urinary exosomal miR-200b was associated with renal fibrosis only when measured in CD13+ (i.e., nonproximal renal tubule-derived) exosomes in CKD [45]; this indicates that a biomarker may be of clinical significance when it is associated with exosomes derived from a specific cell population.
Figure 1.
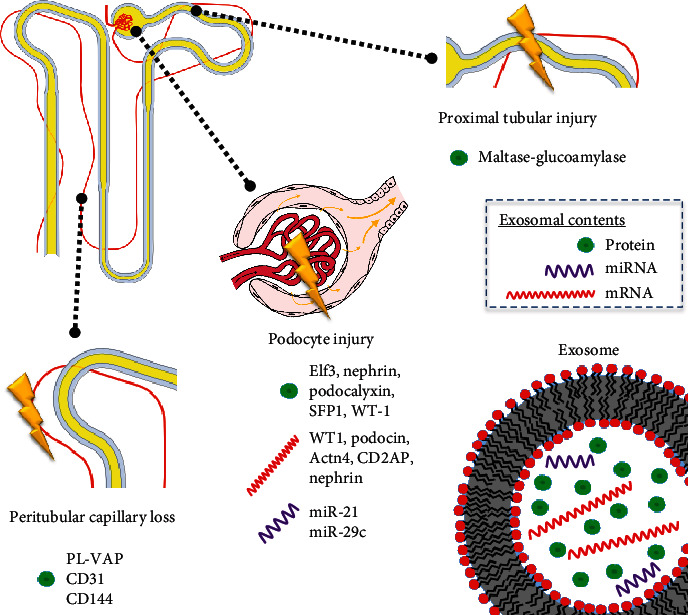
Exosomal biomarkers reflecting alterations in compartments of the nephron.
4.2. Challenges in the Use of Exosomal Biomarkers in Renal Disease
Despite all the theoretical advantages of exosomal biomarkers, there are many challenges, both technical and translational that need to be addressed before the routine application in the clinical practice.
4.2.1. Technical Challenges
The main challenge in exosome isolation is to differentiate exosomes from other EVs. Unfortunately, to date, there is no single isolation technique that guarantees purity, speed, cost-effectiveness, and ability to process large sample volumes at the same time. Ultracentrifugation-based techniques are inexpensive with a low contamination risk and are suitable for large volume preparation; however, they require expensive nonportable equipment and are labor-consuming, making them unsuitable for small volume sampling. Additionally, high centrifugation speeds may mechanically damage EVs [15]. Ultrafiltration is a low-cost, fast and portable procedure, but the end result suffers from moderate purity when used alone. The shear forces that develop during this process can lead to potential loss of exosomes due to entrapment in the filtration membrane [15]. Tangential flow filtration is a promising filtration technique that avoids membrane clogging and mechanical EV damage, while allowing for processing of large volumes in a time-efficient manner [16]. Size-exclusion chromatography is a quick, reproducible method, suitable for both large and small sample volumes resulting in highly pure EVs, but it is limited by the relatively high cost and the necessity for an additional exosome enrichment method [15]. Polymer precipitation is an easy-to-use technique, also versatile for both large and small sample volumes; nonetheless, it requires extended processing times, and exosomal concentrates may be contaminated with protein aggregates, other extracellular vesicles and polymeric contaminants [15]. Immunoaffinity capture is an easy-to-use, high-purity method, able to separate exosomes based on their origin, which may be appealing in the case of urinary exosomal biomarkers; however, the required antibodies may be costly, and the technique is dependable on the specificity of the exosomal marker which is used for exosome identification and cannot be used in larger sample volumes. This method also requires an extra step for exosome elution, which may damage the exosomal structure [15]. Microfluidics-based techniques are highly efficient, cost-effective, portable, and easily automated but suffer from limited sample capacity [15]. Any new exosome isolation technology should be validated before it becomes available for clinical use, a process which is oftentimes lengthy. Although there are reproducibility concerns due to the variability of isolation methods reported in the literature, the development of an exosome-specific nomenclature with descriptive definitions has been an important step towards improved standardization of results among studies [94].
4.2.2. Biological and Clinical Challenges
The exosomal cargo is speculated to be reflective of complex intracellular changes. However, it is unclear and dependent on the condition whether exosomal biomarkers can be more sensitive for the detection of a pathologic process than nonexosomal biomarkers. In general, exosomal and nonexosomal EV cargoes can overlap considerably but are not identical. For example, analysis of the proteomic composition of urinary EVs has revealed that some proteins are detected exclusively either in microvesicles or exosomes [87]. In lupus nephritis and more specifically in active disease, larger quantities of miRNA biomarkers were identified in urinary exosomes than in the cell-free fraction of urine preceding exosome isolation [78]. Some biomarkers seem to better correlate with the clinical condition or variable in question when measured in the exosomal content; for instance, exosomal ceruloplasmin and/or gelatinase are superior in reflecting changes in renal tissue compared to their direct urine measurements [38, 95]. However, other biomarkers such as the urinary NGAL and IL18 proteins in patients after renal transplantation correlated to day seven post op creatinine reduction ratio, whereas the corresponding urinary exosomal transcripts did not [96].
Another consideration is which biofluid is optimal for exosomal biomarkers in renal disease diagnostics. The vast majority of biomarkers examined in this review were identified in urinary exosomes and very few in serum. Other biofluids such as peritoneal dialysis aspirate contain exosomes which could carry biomarkers associated with membrane failure [97]. It has been observed that urinary and blood exosomal contents are very different [98]. There are currently very few studies that compare the usability of urinary vs. nonurinary exosomes in renal disease. However, Sun et al. noted that urinary endothelial-derived exosomes identified renal microcirculation injury better than systemically circulating endothelial-derived exosomes in hypertensive patients [59].
The diurnal variations of urine consistency should also be accounted for in the evaluation of urinary biomarkers. Urine creatinine is commonly used to normalize the values of soluble urinary biomarkers, but its relevance to exosomal biomarkers remains unknown [99]. As far as timing of biosampling is concerned, a circadian pattern in urinary exosomal excretion has been observed in rats with peak concentrations occurring between 19:00 and 23:00 hours, although the circadian variation seems to be normalized with TSG101 protein levels [100]. All types of circulating EVs are reduced following dialysis [101]. Fernández-LLama et al. suggest that Tamm-Horsfall protein levels can be useful in the normalization of urinary exosome concentration in spot urine samples [102].
Lastly, comorbidities should also be taken into account. Changes in circulating and urinary exosomal contents have been reported in patients treated with antihypertensive agents [103] or cyclosporine [104], respectively. It is worth mentioning though that even serious proteinuria secondary to glomerular damage does not seem to affect the concentration of urinary exosomes [105].
5. Conclusion
Exosomes represent a valuable source of candidate diagnostic and/or prognostic biomarkers for a variety of renal conditions. Their potential to reflect changes in specific cellular compartments of the nephron is of particular interest. Exosomes, particularly urinary ones, may provide a dynamic image of the processes taking place in the affected renal tissue. Exosomal biomarkers unlike renal biopsies are not limited by the possibility of obtaining unrepresentative sampling. Exosomal purification and analysis require minimally to noninvasive techniques depending on the biofluid of interest, and exosomal isolation technology is constantly improving. This allows for serial analyses in follow-up clinical visits for comparison. Moreover, exosomal miRNAs have a potential diagnostic and therapeutic potential mainly to their active role in disease pathophysiology. In many cases, exosomal biomarkers may complement renal biopsy in risk stratification and prognostic evaluation. Clinical correlations of currently available data on exosomes in kidney disease are difficult to make currently because of a considerably low to moderate sample size in most research efforts found in the literature and the lack of a standardized methodology of exosome isolation that prevents the direct comparison of study results. Further work is warranted in order to identify accurate and reliable exosomal biomarkers that could complement or replace currently available diagnostic tools. Although great progress has been achieved in exosome research so far, further work is warranted in order to identify accurate and reliable exosomal biomarkers that could complement or replace currently available diagnostic tools.
Acknowledgments
The following image files, all available on http://commons.wikimedia.org/, were used for the creation of Figure 1: “Glomerulus_and_tubule.svg” by ColnKurtz and “Structure of glomerulus.png” by Pharmattila, licensed under the Creative Commons Attribution-Share Alike 4.0 International license; “Kidney.gif” by Mikael Häggström and “Lipid vesicles.svg” by MDougM, released into the public domain.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Luyckx V. A., Tonelli M., Stanifer J. W. The global burden of kidney disease and the sustainable development goals. Bulletin of the World Health Organization. 2018;96(6):414–422D. doi: 10.2471/BLT.17.206441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bandari J., Fuller T. W., Turner R. M. D., II, Agostino L. A. Renal biopsy for medical renal disease: indications and contraindications. The Canadian Journal of Urology. 2016;23(1):8121–8126. [PubMed] [Google Scholar]
- 3.Campion C. G., Sanchez-Ferras O., Batchu S. N. Potential role of serum and urinary biomarkers in diagnosis and prognosis of diabetic nephropathy. Canadian Journal of Kidney Health and Disease. 2017;4(May):p. 2054358117705371. doi: 10.1177/2054358117705371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andaloussi E. L., Samir I. M., Breakefield X. O., Wood M. J. A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nature Reviews. Drug Discovery. 2013;12(5):347–357. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 5.Kourembanas S. Exosomes: vehicles of intercellular signaling, biomarkers, and vectors of cell therapy. Annual Review of Physiology. 2015;77(1):13–27. doi: 10.1146/annurev-physiol-021014-071641. [DOI] [PubMed] [Google Scholar]
- 6.Qin J., Qing X. Functions and application of exosomes. Acta Poloniae Pharmaceutica. 2014;71(4):537–543. [PubMed] [Google Scholar]
- 7.Masaoutis C., Theocharis S. The role of exosomes in bone remodeling: implications for bone physiology and disease. Disease Markers. 2019;2019:12. doi: 10.1155/2019/9417914.9417914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajagopal C., Harikumar K. B. The origin and functions of exosomes in cancer. Frontiers in Oncology. 2018;8(March):p. 66. doi: 10.3389/fonc.2018.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masaoutis C., Korkolopoulou P., Theocharis S. Exosomes in sarcomas: tiny messengers with broad implications in diagnosis, surveillance, prognosis and treatment. Cancer Letters. 2019;449(May):172–177. doi: 10.1016/j.canlet.2019.02.025. [DOI] [PubMed] [Google Scholar]
- 10.Masaoutis C., Mihailidou C., Tsourouflis G., Theocharis S. Exosomes in lung cancer diagnosis and treatment. From the translating research into future clinical practice. Biochimie. 2018;151(August):27–36. doi: 10.1016/j.biochi.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Wu W.-P., Pan Y.-H., Cai M.-Y., et al. Plasma-derived exosomal circular RNA hsa circ0005540 as a novel diagnostic biomarker for coronary artery disease. Disease Markers. 2020;2020:7. doi: 10.1155/2020/3178642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Z., Li H., Yuan M., Dong L., Sun C., Wang G. Serum exosomal microRNAs as potential circulating biomarkers for endometriosis. Disease Markers. 2020;2020:10. doi: 10.1155/2020/2456340.2456340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alipoor S. D., Tabarsi P., Varahram M., et al. Serum exosomal miRNAs are associated with active pulmonary tuberculosis. Disease Markers. 2019;2019:9. doi: 10.1155/2019/1907426.1907426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perez-Hernandez J., Cortes R. Extracellular vesicles as biomarkers of systemic lupus erythematosus. Disease Markers. 2015;2015:7. doi: 10.1155/2015/613536.613536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang D., Zhang W., Zhang H., et al. Progress, opportunity, and perspective on exosome isolation - efforts for efficient exosome-based theranostics. Theranostics. 2020;10(8):3684–3707. doi: 10.7150/thno.41580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busatto S., Vilanilam G., Ticer T., et al. Tangential flow filtration for highly efficient concentration of extracellular vesicles from large volumes of fluid. Cell. 2018;7(12):p. 273. doi: 10.3390/cells7120273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Connolly K. D., Wadey R. M., Mathew D., Errin J., Aled Rees D., James P. E. Evidence for adipocyte-derived extracellular vesicles in the human circulation. Endocrinology. 2018;159(9):3259–3267. doi: 10.1210/en.2018-00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boriachek K., Islam M. N., Gopalan V., Lam A. K., Nguyen N.-T., Shiddiky M. J. A. Quantum dot-based sensitive detection of disease specific exosome in serum. The Analyst. 2017;142(12):2211–2219. doi: 10.1039/C7AN00672A. [DOI] [PubMed] [Google Scholar]
- 19.Beigh M. M. Next-generation sequencing: the translational medicine approach from ‘bench to bedside to population. Medicines. 2016;3(2):p. 14. doi: 10.3390/medicines3020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pauly R., Schwartz C. E. The Future of Clinical Diagnosis: Moving Functional Genomics Approaches to the Bedside. Advances in Molecular Pathology. 2019;2(1):13–19. doi: 10.1016/j.yamp.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 21.Karpman D., Ståhl A.-L., Arvidsson I. Extracellular vesicles in renal disease. Nature Reviews Nephrology. 2017;13(9):545–562. doi: 10.1038/nrneph.2017.98. [DOI] [PubMed] [Google Scholar]
- 22.van Balkom B. W. M., Pisitkun T., Verhaar M. C., Knepper M. A. Exosomes and the kidney: prospects for diagnosis and therapy of renal diseases. Kidney International. 2011;80(11):1138–1145. doi: 10.1038/ki.2011.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nassar W., El-Ansary M., Sabry D., et al. Umbilical cord mesenchymal stem cells derived extracellular vesicles can safely ameliorate the progression of chronic kidney diseases. Biomaterials Research. 2016;20(1):p. 21. doi: 10.1186/s40824-016-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang B., Wang J., He W., et al. Exogenous miR-29a attenuates muscle atrophy and kidney fibrosis in unilateral ureteral obstruction mice. Human Gene Therapy. 2020;31(5-6):367–375. doi: 10.1089/hum.2019.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang B., Zhang A., Wang H., et al. miR-26aLimits muscle wasting and cardiac fibrosis through exosome-mediated microRNA transfer in chronic kidney disease. Theranostics. 2019;9(7):1864–1877. doi: 10.7150/thno.29579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grange C., Tritta S., Tapparo M., et al. Stem cell-derived extracellular vesicles inhibit and revert fibrosis progression in a mouse model of diabetic nephropathy. Scientific Reports. 2019;9(1):p. 4468. doi: 10.1038/s41598-019-41100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindoso R. S., Lopes J. A., Binato R., et al. Adipose mesenchymal cells-derived EVs alleviate DOCA-salt-induced hypertension by promoting cardio-renal protection. Molecular Therapy Methods & Clinical Development. 2020;16(March):63–77. doi: 10.1016/j.omtm.2019.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Collino F., Lopes J. A., Tapparo M., et al. Extracellular vesicles derived from induced pluripotent stem cells promote renoprotection in acute kidney injury model. Cell. 2020;9(2):p. 453. doi: 10.3390/cells9020453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu C., Wang J., Hu J., et al. Extracellular vesicles for acute kidney injury in preclinical rodent models: a meta-analysis. Stem Cell Research & Therapy. 2020;11(1):p. 11. doi: 10.1186/s13287-019-1530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bai L., Li J., Li H., et al. Renoprotective effects of artemisinin and hydroxychloroquine combination therapy on IgA nephropathy via suppressing NF-κB signaling and NLRP3 inflammasome activation by exosomes in rats. Biochemical Pharmacology. 2019;169(November):p. 113619. doi: 10.1016/j.bcp.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 31.Matsukura T., Inaba C., Weygant E. A., et al. Extracellular vesicles from human bone marrow mesenchymal stem cells repair organ damage caused by cadmium poisoning in a Medaka model. Physiological Reports. 2019;7(14, article e14172) doi: 10.14814/phy2.14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang A., Wang H., Wang B., Yuan Y., Klein J. D., Wang X. H. Exogenous miR-26a suppresses muscle wasting and renal fibrosis in obstructive kidney disease. FASEB Journal. 2019;33(12):13590–13601. doi: 10.1096/fj.201900884R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu G., Pei L., Lin F., et al. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. Journal of Cellular Physiology. 2019;234(12):23736–23749. doi: 10.1002/jcp.28941. [DOI] [PubMed] [Google Scholar]
- 34.Kabekkodu S. P., Shukla V., Varghese V. K., D' Souza J., Chakrabarty S., Satyamoorthy K. Clustered miRNAs and their role in biological functions and diseases. Biological Reviews of the Cambridge Philosophical Society. 2018;93(4):1955–1986. doi: 10.1111/brv.12428. [DOI] [PubMed] [Google Scholar]
- 35.Zhao H., Ma S.-X., Shang Y.-Q., Zhang H.-Q., Wei S. MicroRNAs in chronic kidney disease. Clinica Chimica Acta. 2019;491(April):59–65. doi: 10.1016/j.cca.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Assmann T. S., Recamonde-Mendoza M., de Souza B. M., Bauer A. C., Crispim D. MicroRNAs and diabetic kidney disease: systematic review and bioinformatic analysis. Molecular and Cellular Endocrinology. 2018;477(December):90–102. doi: 10.1016/j.mce.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 37.Boriachek K., Umer M., Islam M. N., et al. An amplification-free electrochemical detection of exosomal miRNA-21 in serum samples. The Analyst. 2018;143(7):1662–1669. doi: 10.1039/c7an01843f. [DOI] [PubMed] [Google Scholar]
- 38.Gudehithlu K. P., Hart P., Joshi A., et al. Urine exosomal ceruloplasmin: a potential early biomarker of underlying kidney disease. Clinical and Experimental Nephrology. 2019;23(8):1013–1021. doi: 10.1007/s10157-019-01734-5. [DOI] [PubMed] [Google Scholar]
- 39.Khurana R., Ranches G., Schafferer S., et al. Identification of urinary exosomal noncoding RNAs as novel biomarkers in chronic kidney disease. RNA. 2017;23(2):142–152. doi: 10.1261/rna.058834.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumari M., Mohan A., Ecelbarger C. M., Gupta A., Prasad N., Tiwari S. miR-451 loaded exosomes are released by the renal cells in response to injury and associated with reduced kidney function in human. Frontiers in Physiology. 2020;11(April):p. 234. doi: 10.3389/fphys.2020.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J., Song J., Li Y., Shao J., Xie Z., Sun K. Down-regulation of LncRNA CRNDE aggravates kidney injury via increasing MiR-181a-5p in sepsis. International Immunopharmacology. 2020;79(February):p. 105933. doi: 10.1016/j.intimp.2019.105933. [DOI] [PubMed] [Google Scholar]
- 42.Zhu K., Zheng T., Chen X., Wang H. Bioinformatic analyses of renal ischaemia-reperfusion injury models: identification of key genes involved in the development of kidney disease. Kidney & Blood Pressure Research. 2018;43(6):1898–1907. doi: 10.1159/000496001. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L.-H., Zhu X.-Y., Eirin A., et al. Early podocyte injury and elevated levels of urinary podocyte-derived extracellular vesicles in swine with metabolic syndrome: role of podocyte mitochondria. American Journal of Physiology Renal Physiology. 2019;317(1):F12–F22. doi: 10.1152/ajprenal.00399.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lange T., Artelt N., Kindt F., et al. MiR-21 is up-regulated in urinary exosomes of chronic kidney disease patients and after glomerular injury. Journal of Cellular and Molecular Medicine. 2019;23(7):4839–4843. doi: 10.1111/jcmm.14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yu Y., Bai F., Qin N., et al. Non-proximal renal tubule-derived urinary exosomal miR-200b as a biomarker of renal fibrosis. Nephron. 2018;139(3):269–282. doi: 10.1159/000487104. [DOI] [PubMed] [Google Scholar]
- 46.Lv L.-L., Cao Y.-H., Ni H.-F., et al. MicroRNA-29c in urinary exosome/microvesicle as a biomarker of renal fibrosis. American Journal of Physiology Renal Physiology. 2013;305(8):F1220–F1227. doi: 10.1152/ajprenal.00148.2013. [DOI] [PubMed] [Google Scholar]
- 47.Lv L.-L., Cao Y.-H., Pan M.-M., et al. CD2AP mRNA in urinary exosome as biomarker of kidney disease. Clinica Chimica Acta. 2014;428(January):26–31. doi: 10.1016/j.cca.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Sakurai A., Ono H., Ochi A., et al. Involvement of Elf3 on Smad3 activation-dependent injuries in podocytes and excretion of urinary exosome in diabetic nephropathy. PLoS One. 2019;14(5, article e0216788) doi: 10.1371/journal.pone.0216788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Abe H., Sakurai A., Ono H., et al. Urinary exosomal mRNA of WT1 as diagnostic and prognostic biomarker for diabetic nephropathy. The Journal of Medical Investigation. 2018;65(3.4):208–215. doi: 10.2152/jmi.65.208. [DOI] [PubMed] [Google Scholar]
- 50.Sharma K., Karl B., Mathew A. V., et al. Metabolomics reveals signature of mitochondrial dysfunction in diabetic kidney disease. Journal of the American Society of Nephrology. 2013;24(11):1901–1912. doi: 10.1681/ASN.2013020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zang J., Maxwell A. P., Simpson D. A., McKay G. J. Differential expression of urinary exosomal microRNAs miR-21-5p and miR-30b-5p in individuals with diabetic kidney disease. Scientific Reports. 2019;9(1):p. 10900. doi: 10.1038/s41598-019-47504-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Eissa S., Matboli M., Aboushahba R., Bekhet M. M., Soliman Y. Urinary exosomal microRNA panel unravels novel biomarkers for diagnosis of type 2 diabetic kidney disease. Journal of Diabetes and its Complications. 2016;30(8):1585–1592. doi: 10.1016/j.jdiacomp.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 53.Gu D., Chen Y., Masucci M., Xiong C., Zou H., Holthofer H. Potential urine biomarkers for the diagnosis of prediabetes and early diabetic nephropathy based on ISN CKHDP program. Clinical Nephrology. 2020;93(1):129–133. doi: 10.5414/CNP92S123. [DOI] [PubMed] [Google Scholar]
- 54.Musante L., Tataruch D., Dongfeng G., et al. Proteases and protease inhibitors of urinary extracellular vesicles in diabetic nephropathy. Journal of Diabetes Research. 2015;2015:14. doi: 10.1155/2015/289734.289734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zubiri I., Posada-Ayala M., Benito-Martin A., et al. Kidney tissue proteomics reveals regucalcin downregulation in response to diabetic nephropathy with reflection in urinary exosomes. Translational Research. 2015;166(5):474–84.e4. doi: 10.1016/j.trsl.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 56.Ghai V., Wu X., Bheda-Malge A., et al. Genome-wide profiling of urinary extracellular vesicle microRNAs associated with diabetic nephropathy in type 1 diabetes. Kidney International Reports. 2018;3(3):555–572. doi: 10.1016/j.ekir.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamamoto C. M., Murakami T., Oakes M. L., et al. Uromodulin mRNA from urinary extracellular vesicles correlate to kidney function decline in type 2 diabetes mellitus. American Journal of Nephrology. 2018;47(5):283–291. doi: 10.1159/000489129. [DOI] [PubMed] [Google Scholar]
- 58.De S., Kuwahara S., Hosojima M., et al. Exocytosis-mediated urinary full-length megalin excretion is linked with the pathogenesis of diabetic nephropathy. Diabetes. 2017;66(5):1391–1404. doi: 10.2337/db16-1031. [DOI] [PubMed] [Google Scholar]
- 59.Sun I. O., Santelli A., Abumoawad A., et al. Loss of renal peritubular capillaries in hypertensive patients is detectable by urinary endothelial microparticle levels. Hypertension. 2018;72(5):1180–1188. doi: 10.1161/HYPERTENSIONAHA.118.11766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwon S. H., Woollard J. R., Saad A., et al. Elevated urinary podocyte-derived extracellular microvesicles in renovascular hypertensive patients. Nephrology, Dialysis, Transplantation. 2017;32(5):800–807. doi: 10.1093/ndt/gfw077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Panich T., Chancharoenthana W., Somparn P., Issara-Amphorn J., Hirankarn N., Leelahavanichkul A. Urinary exosomal activating transcriptional factor 3 as the early diagnostic biomarker for sepsis-induced acute kidney injury. BMC Nephrology. 2017;18(1):p. 10. doi: 10.1186/s12882-016-0415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou H., Pisitkun T., Aponte A., et al. Exosomal fetuin-A identified by proteomics: a novel urinary biomarker for detecting acute kidney injury. Kidney International. 2006;70(10):1847–1857. doi: 10.1038/sj.ki.5001874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awdishu L., Tsunoda S., Pearlman M., et al. Identification of maltase glucoamylase as a biomarker of acute kidney injury in patients with cirrhosis. Critical Care Research and Practice. 2019;2019:8. doi: 10.1155/2019/5912804.5912804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sigdel T. K., Ng Y. W., Lee S., et al. Perturbations in the urinary exosome in transplant rejection. Frontiers of Medicine. 2014;1:p. 57. doi: 10.3389/fmed.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mahalingasivam V., Booth J., Sheaff M., Yaqoob M. Nephrotic syndrome in adults. Acute Medicine. 2018;17(1):36–43. [PubMed] [Google Scholar]
- 66.Chen T., Cheng W., Yu H., et al. Increased urinary exosomal microRNAs in children with idiopathic nephrotic syndrome. eBioMedicine. 2019;39(January):552–561. doi: 10.1016/j.ebiom.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang Z., Zhang Y., Zhou J., Yu Z. Urinary exosomal miR-193a can be a potential biomarker for the diagnosis of primary focal segmental glomerulosclerosis in children. BioMed Research International. 2017;2017:6. doi: 10.1155/2017/7298160.7298160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou H., Kajiyama H., Tsuji T., et al. Urinary exosomal Wilms’ tumor-1 as a potential biomarker for podocyte injury. American Journal of Physiology Renal Physiology. 2013;305(4):F553–F559. doi: 10.1152/ajprenal.00056.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morikawa Y., Takahashi N., Kamiyama K., et al. Elevated levels of urinary extracellular vesicle fibroblast-specific protein 1 in patients with active crescentic glomerulonephritis. Nephron. 2019;141(3):177–187. doi: 10.1159/000495217. [DOI] [PubMed] [Google Scholar]
- 70.Ma H., Xu Y., Zhang R., Guo B., Zhang S., Zhang X. Differential expression study of circular RNAs in exosomes from serum and urine in patients with idiopathic membranous nephropathy. Archives of Medical Science. 2019;15(3):738–753. doi: 10.5114/aoms.2019.84690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ramirez-Alvarado M., Barnidge D. R., Murray D. L., et al. Assessment of renal response with urinary exosomes in patients with AL amyloidosis: a proof of concept. American Journal of Hematology. 2017;92(6):536–541. doi: 10.1002/ajh.24717. [DOI] [PubMed] [Google Scholar]
- 72.Ramirez-Alvarado M., Ward C. J., Huang B. Q., et al. Differences in immunoglobulin light chain species found in urinary exosomes in light chain amyloidosis (Al) PLoS One. 2012;7(6, article e38061) doi: 10.1371/journal.pone.0038061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Min Q.-H., Chen X.-M., Zou Y.-Q., et al. Differential expression of urinary exosomal microRNAs in IgA nephropathy. Journal of Clinical Laboratory Analysis. 2018;32(2):p. e22226. doi: 10.1002/jcla.22226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moon P.-G., Lee J.-E., You S., et al. Proteomic analysis of urinary exosomes from patients of early IgA nephropathy and thin basement membrane nephropathy. Proteomics. 2011;11(12):2459–2475. doi: 10.1002/pmic.201000443. [DOI] [PubMed] [Google Scholar]
- 75.Ichii O., Otsuka-Kanazawa S., Horino T., et al. Decreased miR-26a expression correlates with the progression of podocyte injury in autoimmune glomerulonephritis. PLoS One. 2014;9(10, article e110383) doi: 10.1371/journal.pone.0110383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Feng Y., Lv L.-L., Wu W.-J., et al. Urinary Exosomes and Exosomal CCL2 mRNA as Biomarkers of Active Histologic Injury in IgA Nephropathy. The American Journal of Pathology. 2018;188(11):2542–2552. doi: 10.1016/j.ajpath.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 77.Solé C., Moliné T., Vidal M., Ordi-Ros J., Cortés-Hernández J. An exosomal urinary miRNA signature for early diagnosis of renal fibrosis in lupus nephritis. Cell. 2019;8(8):p. 773. doi: 10.3390/cells8080773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perez-Hernandez J., Forner M. J., Pinto C., Chaves F. J., Cortes R., Redon J. Increased urinary exosomal microRNAs in patients with systemic lupus erythematosus. PLoS One. 2015;10(9, article e0138618) doi: 10.1371/journal.pone.0138618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tangtanatakul P., Klinchanhom S., Sodsai P., et al. Down-regulation of Let-7a and miR-21 in urine exosomes from lupus nephritis patients during disease flare. Asian Pacific Journal of Allergy and Immunology / Launched by the Allergy and Immunology Society of Thailand. 2019;37(4):189–197. doi: 10.12932/AP-130318-0280. [DOI] [PubMed] [Google Scholar]
- 80.Li Y., Xu X., Tang X., et al. MicroRNA expression profile of urinary exosomes in type IV lupus nephritis complicated by cellular crescent. Journal of Biological Research. 2018;25(December):p. 16. doi: 10.1186/s40709-018-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia-Vives E., Solé C., Moliné T., et al. The urinary exosomal miRNA expression profile is predictive of clinical response in lupus nephritis. International Journal of Molecular Sciences. 2020;21(4):p. 1372. doi: 10.3390/ijms21041372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Solé C., Cortés-Hernández J., Felip M. L., Vidal M., Ordi-Ros J. MiR-29c in urinary exosomes as predictor of early renal fibrosis in lupus nephritis. Nephrology, Dialysis, Transplantation. 2015;30(9):1488–1496. doi: 10.1093/ndt/gfv128. [DOI] [PubMed] [Google Scholar]
- 83.Hogan M. C., Bakeberg J. L., Gainullin V. G., et al. Identification of biomarkers forPKD1Using urinary exosomes. Journal of the American Society of Nephrology. 2015;26(7):1661–1670. doi: 10.1681/ASN.2014040354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salih M., Demmers J. A., Bezstarosti K., et al. Proteomics of urinary vesicles links Plakins and complement to polycystic kidney disease. Journal of the American Society of Nephrology. 2016;27(10):3079–3092. doi: 10.1681/ASN.2015090994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pocsfalvi G., Raj D. A. A., Fiume I., Vilasi A., Trepiccione F., Capasso G. Urinary extracellular vesicles as reservoirs of altered proteins during the pathogenesis of polycystic kidney disease. Proteomics. Clinical Applications. 2015;9(5-6):552–567. doi: 10.1002/prca.201400199. [DOI] [PubMed] [Google Scholar]
- 86.Gerlach J. Q., Krüger A., Gallogly S., et al. Surface glycosylation profiles of urine extracellular vesicles. PLoS One. 2013;8(9, article e74801) doi: 10.1371/journal.pone.0074801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bruschi M., Granata S., Candiano G., et al. Proteomic analysis of urinary extracellular vesicles reveals a role for the complement system in medullary sponge kidney disease. International Journal of Molecular Sciences. 2019;20(21) doi: 10.3390/ijms20215517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bruschi M., Granata S., Santucci L., et al. Proteomic analysis of urinary microvesicles and exosomes in medullary sponge kidney disease and autosomal dominant polycystic kidney disease. Clinical Journal of the American Society of Nephrology. 2019;14(6):834–843. doi: 10.2215/CJN.12191018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stokman M. F., Bijnsdorp I. V., Schelfhorst T., et al. Changes in the urinary extracellular vesicle proteome are associated with nephronophthisis-related ciliopathies. Journal of Proteomics. 2019;192(February):27–36. doi: 10.1016/j.jprot.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 90.Bourderioux M., Nguyen-Khoa T., Chhuon C., et al. A new workflow for proteomic analysis of urinary exosomes and assessment in cystinuria patients. Journal of Proteome Research. 2014;14(1):567–577. doi: 10.1021/pr501003q. [DOI] [PubMed] [Google Scholar]
- 91.Gonzales P. A., Pisitkun T., Hoffert J. D., et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. Journal of the American Society of Nephrology: JASN. 2009;20(2):363–379. doi: 10.1681/ASN.2008040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pisitkun T., Gandolfo M. T., Das S., Knepper M. A., Bagnasco S. M. Application of systems biology principles to protein biomarker discovery: urinary exosomal proteome in renal transplantation. Proteomics. Clinical Applications. 2012;6(5-6):268–278. doi: 10.1002/prca.201100108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hogan M. C., Johnson K. L., Zenka R. M., et al. Subfractionation, characterization, and in-depth proteomic analysis of glomerular membrane vesicles in human urine. Kidney International. 2014;85(5):1225–1237. doi: 10.1038/ki.2013.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Théry C., Witwer K. W., Aikawa E., et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. Journal of Extracellular Vesicles. 2018;7(1):p. 1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gudehithlu K. P., Garcia-Gomez I., Vernik J., et al. In diabetic kidney disease urinary exosomes better represent kidney specific protein alterations than whole urine. American Journal of Nephrology. 2016;42(6):418–424. doi: 10.1159/000443539. [DOI] [PubMed] [Google Scholar]
- 96.Peake P. W., Pianta T. J., Succar L., et al. A comparison of the ability of levels of urinary biomarker proteins and exosomal mRNA to predict outcomes after renal transplantation. PLoS One. 2014;9(6, article e98644) doi: 10.1371/journal.pone.0098644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Carreras-Planella L., Soler-Majoral J., Rubio-Esteve C., et al. Characterization and proteomic profile of extracellular vesicles from peritoneal dialysis efflux. PLoS One. 2017;12(5, article e0176987) doi: 10.1371/journal.pone.0176987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xie J. X., Fan X., Drummond C. A., et al. MicroRNA profiling in kidney disease: plasma versus plasma-derived exosomes. Gene. 2017;627:1–8. doi: 10.1016/j.gene.2017.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Street J. M., Koritzinsky E. H., Glispie D. M., Star R. A., Yuen P. S. T. Urine exosomes: an emerging trove of biomarkers. Advances in Clinical Chemistry. 2017;78:103–122. doi: 10.1016/bs.acc.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 100.Koritzinsky E. H., Street J. M., Chari R. R., et al. Circadian variation in the release of small extracellular vesicles can be normalized by vesicle number or TSG101. American Journal of Physiology Renal Physiology. 2019;317(5):F1098–F1110. doi: 10.1152/ajprenal.00568.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ruzicka M., Xiao F., Abujrad H., et al. Effect of hemodialysis on extracellular vesicles and circulating submicron particles. BMC Nephrology. 2019;20(1):p. 294. doi: 10.1186/s12882-019-1459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernández-Llama P., Khositseth S., Gonzales P. A., Star R. A., Pisitkun T., Knepper M. A. Tamm-Horsfall protein and urinary exosome isolation. Kidney International. 2010;77(8):736–742. doi: 10.1038/ki.2009.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Erdbrügger U., Thu H. L. Extracellular vesicles as a novel diagnostic and research tool for patients with HTN and kidney disease. American Journal of Physiology Renal Physiology. 2019;317(3):F641–F647. doi: 10.1152/ajprenal.00071.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Esteva-Font C., Guillén-Gómez E., Diaz J. M., et al. Renal sodium transporters are increased in urinary exosomes of cyclosporine-treated kidney transplant patients. American Journal of Nephrology. 2014;39(6):528–535. doi: 10.1159/000362905. [DOI] [PubMed] [Google Scholar]
- 105.McNicholas K., Li J. Y., Michael M. Z., Gleadle J. M. Albuminuria is not associated with elevated urinary vesicle concentration but can confound nanoparticle tracking analysis. Nephrology. 2017;22(11):854–863. doi: 10.1111/nep.12867. [DOI] [PubMed] [Google Scholar]
- 106.Xie Y., Jia Y., Xie C., Hu F., Xue M., Xue Y. Urinary exosomal microRNA profiling in incipient type 2 diabetic kidney disease. Journal of Diabetes Research. 2017;2017:10. doi: 10.1155/2017/6978984.6978984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gao M., Dong Z., Sun J., et al. Liver-derived exosome-laden lncRNA MT1DP aggravates cadmium-induced nephrotoxicity. Environmental Pollution. 2019;258(December):p. 113717. doi: 10.1016/j.envpol.2019.113717. [DOI] [PubMed] [Google Scholar]


