Abstract
Current research indicates that changes in gut microbiota can impact the host, but it is not always clear how dietary and environmental factors alter gut microbiota. One potential factor is antimicrobial activity of compounds ingested by the host. The goal of this study was to determine the antimicrobial activity of common plant secondary metabolites against pure cultures of paired, structurally and phylogenetically distinct gastrointestinal bacteria of human or bovine origin: Prevotella bryantii B14, Bacteroides fragilis 25285, Acetoanaerobium (Clostridium) sticklandii SR and Clostridioides difficile 9689. When growth media were amended with individual phytochemicals (the alkaloids: berberine, capsaicin, nicotine, piperine and quinine and the phenolic: curcumin), growth of each species was inhibited to varying degrees at the three greatest concentrations tested (0.10–10.00 mg mL−1). The viable cell numbers of all the cultures were reduced, ≥4-logs, by berberine at concentrations ≥1.00 mg mL−1. Quinine performed similarly to berberine for B14, 25285, and SR at the same concentrations. The other phytochemicals were inhibitory, but not as much as quinine or berberine. Nicotine had activity against all four species (≥2-log reduction in viable cell number at 10.00 mg mL−1), but had stronger activity against the Gram-positive bacteria, SR and 9689, (≥4-log reductions at 10.00 mg mL−1). In conclusion, the phytochemicals had varying spectra of antimicrobial activity. These results are consistent with the hypothesis that ingested phytochemicals have the ability to differentially impact gut microbiota through antimicrobial activity.
Keywords: Antimicrobial activity, Alkaloids, Nicotine, Inhibitory concentration, Clostridioides difficile, Bacteroides fragilis
1. Introduction
It is now recognized that the gut microbiota influences many aspects of human and animal health [1]. Microbe-host interactions can alter digestive processes and host metabolic conditions, such as obesity and inflammatory diseases [2–6]. Furthermore, recent results indicate behavior and psychiatric conditions can be influenced by the gut microbiota. For example, the stress-related behaviors of mice can be influenced by colonization status and microbiological interventions [7].
There are many factors that could affect enteric microbiota. It is well known from ruminant literature that host genetics can determine the phylogenetic composition of the microflora [8]. Dietary factors including carbohydrates and protein can select for the microorganisms that use these substrates [9,10]. These dietary factors can include exogenous cultures (probiotics) or microbial substrates (prebiotics), as well [11,12]. Recent results indicate that environmental toxicants can alter gut microflora [13,14]. Additionally, drugs of abuse, such as nicotine, are now known to influence the phylogenetic composition of the microflora [15–18].
Many of these inputs impacting gut microbiota are of plant origin. Plant primary metabolites like fiber, sugars or protein are growth substrates, but plant secondary metabolites are often biologically active [19]. Specific phytochemicals, such as phenolics, terpenoids and alkaloids, affect microbial ecology through antimicrobial activities. The objective of the current study was to determine the antimicrobial activity of select secondary metabolites against structurally and phylogenetically distinct bacteria. Two species from Phylum Bacteroidetes, Bacteroides fragilis and Prevotella bryantii, and from Phylum Firmicutes, Clostridioides difficile and Acetoanaerobium (Clostridium) sticklandii, were selected. B. fragilis and C. difficile are of human origin, and P. bryantii and A. sticklandii are of bovine origin. Five common alkaloids were selected: berberine, capsaicin, nicotine, piperine and quinine, as well as the phenolic compound, curcumin. The hypothesis was that the phytochemicals would differ in their spectra of activity against the test species.
2. Materials and methods
2.1. Materials and chemicals
Quinine, berberine, capsaicin, and (–) nicotine hydrogen tartrate salt (nicotine) were purchased from Sigma Aldrich (St. Louis, MO, USA). Piperine was purchased from Alfa Aesar (Haverhill, MA, USA). Curcumin was purchased from Cayman Chemicals (Ann Arbor, MI, USA). The Avicel (microcrystalline cellulose/MCC) used was purchased from FMC Corporation (Philadelphia, PA, USA). SiO2 was purchased from Waters Corporation (Milford, MA, USA).
2.2. Cultures
P. bryantii (B14) was obtained from our stock culture collection maintained at the Forage-Animal Production Research Unit, ARS, USDA on the University of Kentucky campus (chain of custody: Marvin P. Bryant, James B. Russell, Michael D. Flythe). A. sticklandii (SR) was also obtained from our stock culture collection (chain of custody: James B. Russell, Michael D. Flythe). B. fragilis (ATCC 25285) was obtained from the American Type Culture Collection (Manassas, VA, USA). C. difficile (ATCC 9689) was also obtained from the American Type Culture Collection (Manassas, VA, USA).
2.3. Media and anaerobic technique
The growth medium was prepared, and cultures were transferred using the Hungate methods for anaerobic technique [20,21]. The growth medium for B14 was based on [22] and modified as indicated. It contained (per 1L): 500 mg yeast extract, 1g Trypticase, 600 mg cysteine HCl, 240 mg KH2PO4, 240 mg K2HPO4, 480 mg NaCl, 480 mg (NH4)2SO4, 100 mg MgSO4 ●7H2O, and 64 mg CaCl2 ●2H2O. The medium was adjusted to a pH 6.5 via addition of NaOH, then autoclaved (121 °C, 15 min) to remove O2, and finally cooled under CO2. Na2CO3 (4.0 g) was added as a buffer. The growth medium for 25285 was based on [23] and contained (per 1L): 2 g Trypticase, 600 mg cysteine HCl, 1 mL hemin (0.1% in 1M NaOH) solution, 240 mg KH2PO4, 240 mg K2HPO4, 480 mg NaCl, 480 mg (NH4)2SO4, 100 mg MgSO4 ●7H2O, and 64 mg CaCl2 ●2H2O, 1 mg pyridoxamine 2HCl, 2 mg riboflavin, 2 mg thiamine HCl, 2 mg nicotinamide, 2 mg CaD pantothenate, 1 mg lipoic acid, 0.1 mg p-aminobenzoic acid, 0.05 mg folic acid, 0.05 mg biotin, 0.05 mg cobalamin, 1 mg pyridoxal HCl, 1 mg pyridoxine, 2.5 mg Na4EDTA, 1 mg FeSO4● 7H2O, 0.05 mg ZnSO4 ●7H2O, 1 mg MnCl2 ●4H2O, 0.1 mg H3BO3, 0.1 mg CoCl2 ●6H2O, 5 μg CuCl2 ●2H2O, 0.01 mg NiCl2 ●6H2O, and 0.015 mg NaMoO4 ●2H2O. The medium was adjusted to a pH 6.5 via addition of NaOH, then autoclaved to remove O2, and finally cooled under CO2. Na2CO3 (4.0 g) was added as a buffer. The growth medium for SR was based on [24] and contained (per 1L): 15 g Trypticase, 600 mg cysteine HCl, 240 mg KH2PO4, 240 mg K2HPO4, 480 mg NaCl, 516 mg Na2SO4, 100 mg MgSO4 ●7H2O, and 64 mg CaCl2 ●2H2O, and the vitamins listed for strain 25285 above. The broth was adjusted to a pH 6.5 via addition of NaOH, then autoclaved to remove O2, and cooled under CO2. Again, 4.0 g Na2CO3 was added as a buffer. The growth medium for 9689 was prepared (per 1L) per manufacturer (BD Difco, Livonia, MI) instructions: 38 g reinforced clostridium media powder into deionized H2O, boiled and cooled under N2. For all media types described above, the media were anaerobically transferred to Hungate tubes, capped with rubber stoppers and hard plastic caps, and autoclaved (121 °C, 15 min) for sterilization following preparation. All four cultures (B14, 25285, SR, and 9689) were routinely transferred using anaerobic technique and incubated at either 39 °C (SR and B14) or 37 °C (25285 and 9689).
2.4. Alkaloid preparation
Alkaloid tubes were prepared via a 10-fold solid dilution series. As with a 10% liquid dilution series, an alkaloid (10 mg) was mixed into 90 mg MCC or SiO2 and vortexed for homogeneity. Then 10 mg was removed from the first tube to continue the dilution series. The tubes were then sanitized with 70% EtOH. The EtOH was evaporated, and the tubes were sealed under CO2 (B14, SR, 25285) or N2 (9689).
2.5. Inhibitory concentration experiments
Growth inhibition experiments were conducted in growth media (9.9 mL) in Balch tubes. When the alkaloid dilutions were completed, as described above, the appropriate growth medium was added to each tube using anaerobic and aseptic technique. Immediately prior to inoculation, the alkaloid tubes with media were sanitized via pasteurization at 65 °C for 20 min and allowed to cool to room temperature (~23 °C). Each tube was inoculated (1% v/v) from a stationary phase (24 h) culture (B14, SR, 9689, or 25285) and incubated while shaking at 39 °C for 24 h, or until a growth control reached stationary phase. The growth control for each culture included 10 mg MCC/SiO2 and no alkaloids. Additional controls included uninoculated media with alkaloids or MCC/SiO2 (10 mg) to test for contaminants. Viable cell number was determined via a 10-fold dilution series in unamended growth media (10 mL), incubated for 24 h at 37 °C (25285 and 9689) or 39 °C (SR and B14), or until the control reached stationary phase. Experiments were repeated at least three times with no variation in the data presented below.
3. Results
Prevotella bryantii B14 grew to 107 cells mL−1 in the stationary phase when no phytochemicals were included in the growth medium (Fig. 1). B14 was not inhibited by any of the tested phytochemicals at 0.01 mg mL−1, but was completely inhibited by berberine at greater concentrations. In fact, all cell viability was lost at concentrations >0.10 mg mL−1 (≥26.90 mM). At 1.00 mg mL−1, quinine and capsaicin were also inhibitory (0.31 mM and 0.33 mM, respectively). As with berberine, there were no viable cells when 1.00 mg mL−1 quinine was included. The viable cell number of B14 cultures treated with capsaicin was two logs lower (105 cells mL−1) than controls. At 10.00 mg mL−1 (2.16 mM and 3.27 mM, respectively), nicotine and capsaicin were both inhibitory (105 cells mL−1, in each case). Neither piperine nor curcumin were inhibitory to B14 at the concentrations tested.
Fig. 1.
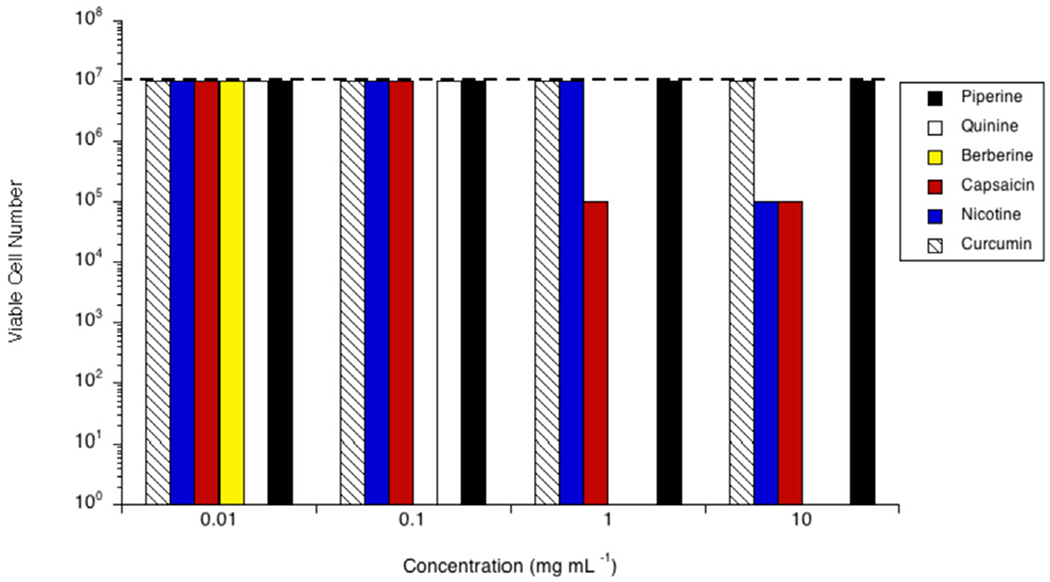
Effect of pipeline (black bars), quinine (white bars), berberine (yellow bars), capsaicin (red bars), nicotine (blue bars) or curcumin (hatched bars) on growth of Prevotella bryantii B14 in vitro. Cultures were grown (24 h; 39 °C; 160 rpm) in the presence of the amount of the phytochemical indicated. Viable cell number was determined via a 10-fold dilution series in unamended growth media, incubated for 24 h at 39 °C, or until the control reached stationary phase. The viable cell number of a control with no phytochemical is indicated by the dashed line. Experiments were repeated three or more times with no variation.
The growth of Bacteroides fragilis 25285 was impacted by the presence of MCC, but all cultures reliably grew to 106 cells mL−1 in the stationary phase (Fig. 2). None of the phytochemicals inhibited 25285 at 0.01 mg mL−1. Piperine was inhibitory at concentrations ≥0.10 mg mL−1 (105 cells mL−1). Berberine and capsaicin were inhibitory to 25285 at 0.10 mg mL−1, or 26.90 μM and 32.70 μM for each case, (104 cells mL−1). 25285 was completely inhibited by berberine at concentrations ≥1.00 mg mL−1 (≥0.27 mM). Quinine was inhibitory at concentrations ≥1.00 mg mL−1, or ≥0.31 mM, (102 cells mL−1). Capsaicin demonstrated inhibitory activity at 1.00 mg mL−1/0.33 mM (103 cells mL−1). 25285 was completely inhibited by capsaicin at 10.00 mg mL−1 (3.27 mM). Nicotine was inhibitory at 10.00 mg mL−1, or 2.16 mM, as well (103 cells mL−1). Curcumin showed no inhibitory activity against 25285 for any of the concentrations tested.
Fig. 2.
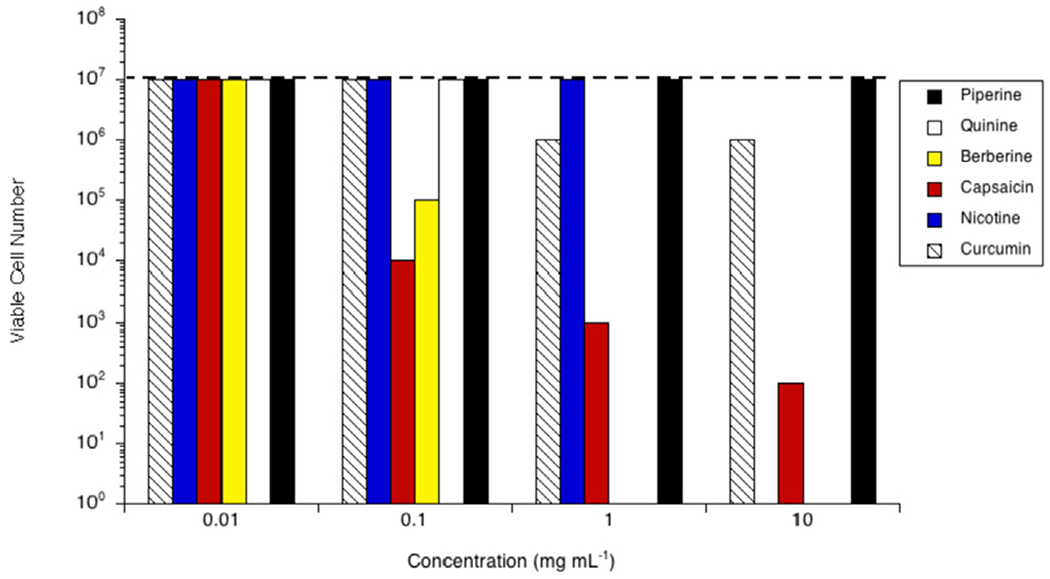
Effect of piperine (black bars), quinine (white bars), berberine (yellow bars), capsaicin (red bars), nicotine (blue bars) or curcumin (hatched bars) on growth of Bacteroides fragilis 25285 in vitro. Cultures were grown (24 h; 37 °C; 160 rpm) in the presence of the amount of the phytochemical indicated. Viable cell number was determined via a 10-fold dilution series in unamended growth media, incubated for 24 h at 37 °C, or until the control reached stationary phase. The viable cell number of a control with no phytochemical is indicated by the dashed line. Experiments were repeated three or more times with no variation.
Acetoanaerobium sticklandii SR grew to 107 cells mL−1 in the stationary phase (Fig. 3). No phytochemicals inhibited SR at 0.01 mg mL−1. Berberine and capsaicin were inhibitory at 0.10 mg mL−1 (26.90 μM and 32.70 μM, respectively), and those cultures were 2- and 3-logs lower than control, respectively. SR was completely inhibited by both quinine and berberine at concentrations >1.00 mg mL−1 (≥0.31 mM and ≥0.27 mM, in each case). Capsaicin was inhibitory at 1.00 mg mL−1, or 0.33 mM, (103 cells mL−1), and curcumin was inhibitory at concentrations ≥ 1.00 mg mL−1, or ≥ 0.27 mM, (106 cells mL−1). There were no viable cells observed when 10.00 mg mL−1 (2.16 mM) nicotine was included. SR was inhibited by capsaicin at a concentration of 10.00 mg mL−1 (3.27 mM) as well (102 cells mL−1). Piperine demonstrated no inhibitory activity toward SR at any of the concentrations tested.
Fig. 3.
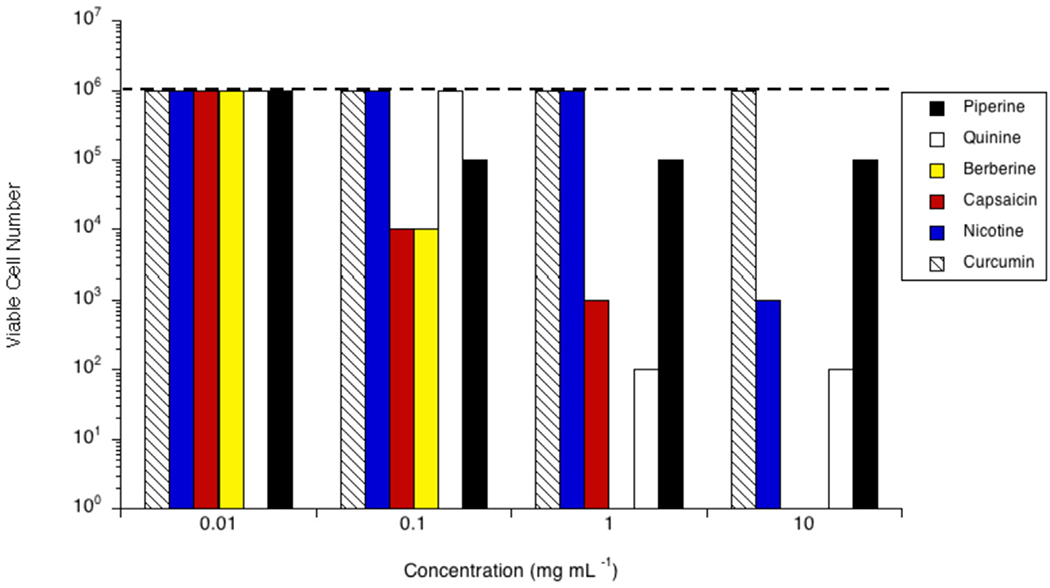
Effect of piperine (black bars), quinine (white bars), berberine (yellow bars), capsaicin (red bars), nicotine (blue bars) or curcumin (hatched bars) on growth of Acetoanaerobium sticklandii SR in vitro. Cultures were grown (24 h; 39 °C; 160 rpm) in the presence of the amount of the phytochemical indicated. Viable cell number was determined via a 10-fold dilution series in unamended growth media, incubated for 24 h at 39 °C, or until the control reached stationary phase. The viable cell number of a control with no phytochemical is indicated by the dashed line. Experiments were repeated three or more times with no variation.
Clostridioides difficile 9689 grew to 107 cells mL−1 in the stationary phase (Fig. 4). None of the phytochemicals inhibited 9689 at 0.01 mg mL−1. Curcumin was inhibitory at concentrations ≥0.10 mg mL−1, or ≥27.10 μM, (106 cells mL−1, in each case). Berberine was inhibitory at 1.00 and 10.00 mg mL−1 (0.27 mM and 2.69 mM, respectively), reducing the viable cell number to 102 cells mL−1 and no viable cells, respectively. At 10.00 mg mL−1 (3.27 mM and 2.16 mM, successively) both capsaicin and nicotine were inhibitory, 106 cells mL−1 and no viable cells, respectively. Neither piperine nor quinine were inhibitory at the tested concentrations.
Fig. 4.
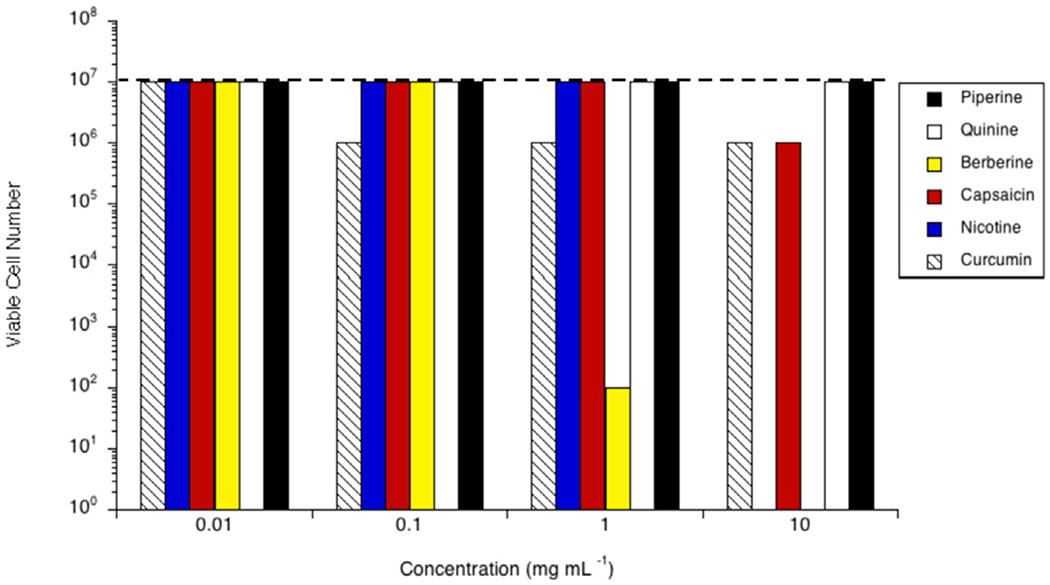
Effect of piperine (black bars), quinine (white bars), berberine (yellow bars), capsaicin (red bars), nicotine (blue bars) or curcumin (hatched bars) on growth of Clostridioides difficile 9689 in vitro. Cultures were grown (24 h; 37 °C; 160 rpm) in the presence of the amount of the phytochemical indicated. Viable cell number was determined via a 10-fold dilution series in unamended growth media, incubated for 24 h at 37 °C, or until the control reached stationary phase. The viable cell number of a control with no phytochemical is indicated by the dashed line. Experiments were repeated three or more times with no variation.
4. Discussion
These experiments were initiated with the hypothesis that the phytochemicals would differ in their spectra of activity against the test species, and they did. Piperine and curcumin were generally less inhibitory than the other four phytochemicals. Quinine and berberine were generally the most inhibitory, in that they caused 4-log or greater reductions in viable cell numbers. These results are consistent with previous observations that the latter two alkaloids can inhibit other bacterial species as well as eukaryotic microorganisms [25–29]. Berberine inhibited the growth and decreased final viable cell number of all four bacterial species. Nicotine and capsaicin also inhibited all four species, but generally required greater concentrations than berberine.
Antimicrobial compounds can be broadly characterized by their spectra of activity, which is a result of the mechanism of action and depends on the chemical structure of the compound. The relationships of activity to structural characteristics were considered, in that there was no identifiable trend between antimicrobial activity and molecular weight or aqueous solubility. Another hypothesis was that particular reactive groups (i.e., nitrogen, hydroxyl or carboxylic acid) contribute to the overall activity of the compound, but these data did not allow us to make such a conclusion. Similarly, we investigated differences in abundance and type of polarizable groups (primarily oxygens, nitrogens, and hydrogen bonding potential), which contributes to the overall polar surface area (PSA), measured in angstroms squared (Å2), of the compound(s) [30]. Computational studies suggest that PSA could be an indicator of a compound’s antimicrobial capability as it would influence cell membrane permeability, in that a compound with a lower relative PSA (≤90 Å2) would be more membrane permeable and vice versa [31]. However, there was no apparent relationship between polar/non-polar characteristics and activity of the phytochemicals tested in this study. For example, piperine had the lowest PSA of the compounds tested, 39 Å2, and demonstrated minimal antimicrobial activity against the test species. Whereas, nicotine had the highest PSA, 248 Å2, and demonstrated median antimicrobial activity.
Phylum Bacteroidetes, which was classically defined as the anaerobic Gram-negative bacteria, can be characterized by the presence of a thin peptidoglycan cell wall between an inner and outer membrane [32]. The presence of the second membrane decreases sensitivity among representatives of this phylum to a variety of antimicrobials, both naturally derived and synthetic [33–35]. Prevotella bryantii (B14) was originally described as a member of genus Bacteroides [36] and was later reclassified [37]. Phylogenetically and structurally, B14 is similar to Bacteroides fragilis (25285) [38]. Curcumin had no observable activity against either representative at the concentrations tested. Piperine was observed to have activity against only the human-derived species 25285 and not the ruminant species B14. Quinine and berberine had a 4-log or greater reduction in viable cell number for both representative species at the higher concentrations tested. Nicotine and capsaicin were also observed to have activity against both species, but generally to a lesser extent than quinine or berberine.
Phylum Firmicutes, classically defined as the low G + C Gram-positive bacteria, are sometimes considered to be more susceptible to a wider variety of antimicrobials than members of the Gram-negative Bacteroidetes [39,40]. The study employed A. sticklandii (SR) and C. difficile (9689) due to their close phylogenetic and metabolic relationships. Both of these bacteria were originally categorized in Clostridium Cluster XI [41]. More recently they have been reclassified into different genera [42,43]. Of the two representatives, SR was more sensitive to the phytochemicals tested than 9689. However, 9689 is notoriously antibiotic insensitive [44–49]. Thus, the observations regarding the activity of the phytochemicals against 9689 are not surprising. In general, we saw that piperine had no activity against either species at any concentration. Curcumin had limited activity, ≤ 1-log reduction in viable cell number, against both SR and 9689. Quinine and berberine had strong activity, ≥ 4-log reduction in viable cell number, against SR. Similarly, berberine was observed to have strong activity against 9689. However, quinine had no activity against 9689. This latter result was consistent with reports by others, who have observed either no, or only limited, activity of quinine against C. difficile in vivo [45,49]. Lastly, capsaicin and nicotine had observed activity against both species though to a much lesser degree against 9689.
It is widely accepted that antimicrobial chemotherapy can suppress normal intestinal flora, decrease competitive exclusion and lead to increased viable numbers of opportunistic pathogens, like C. difficile [50–55]. The current study demonstrated that six tested phytochemicals had antimicrobial activity against GI bacteria. It is plausible that nicotine and the other phytochemicals could impact normal intestinal flora in a manner similar to clinical antibiotics. The latter hypothesis would be consistent with observations that nicotine consumption is correlated with higher rates of C. difficile infections [56].
Berberine is structurally similar to the tetracyclines [57,58]. Historically, tetracyclines have been given to both humans and animals for clinical and non-clinical purposes due to their broad spectrum of activity. The mechanisms of action for tetracyclines and berberine are similar in that they alter protein production of the cell, but the ways in which they accomplish this differ. Tetracyclines interfere with transcription, whereas berberine interferes with translation [29,59]. In the current, limited study, berberine also had a broad spectrum of activity. It inhibited both the Gram-negative and Gram-positive bacteria, albeit at a higher concentration for the antibiotic-insensitive C. difficile.
The antimicrobial mechanism of action of capsaicin appears to be similar to that of the isoflavone, biochanin A. Biochanin A interferes with the activity of the multidrug efflux pump, TetA [60]. Capsaicin interferes with the activity of another multidrug efflux pump called NorA [61]. Drug efflux pump inhibitors are a current topic of interest in the scientific community, but less is known about them than the classical categories of antibiotics. The mechanism of action against the bacteria used in this study is not clear. However, capsaicin demonstrated limited activity at low concentrations against both representatives of the Bacteroidetes and Firmicutes.
There are limited data reporting on the intestinal concentrations of commonly ingested compounds. Additionally, the relevant concentrations in the intestine of the phytochemicals tested here are unclear. However, there are a limited number of fates for ingested compounds. For compounds that are absorbed, they may be excreted with or without having undergone some metabolism within the organism. Alternatively, the compounds might not be absorbed at all, but instead transit the digestive tract and ultimately be expelled without any adulteration to their original forms. For example, studies done in a rodent model indicated that capsaicin was only partially absorbed by the organism prior to excretion [62]. Additionally, the large intestinal microbiota will be exposed to absorbed compounds that undergo biliary clearance without metabolism. An example of this is curcumin, which is largely unabsorbed or metabolized, but is cleared via the bile duct [63].
Typical plasma concentrations of all the phytochemicals used in this study are known. Berberine, capsaicin, and curcumin all have average plasma concentrations reported in the nM range (6 nM, 8 nM, and 48 nM, respectively) [64–67]. Generally, capsaicin and curcumin are thought to have poor bioavailability in humans. Although, capsaicin and curcumin are believed to accumulate in the intestine prior to absorption through the epithelia with capsaicin potentially reaching peak concentrations between 500 μM and 1000 μM in the intestine [65,67]. Quinine reaches plasma concentrations of ~13 μM after ingestion of tonic water, and ~130 μM after anti-malaria medication administration [68], both of which are comparable to the lower two concentrations tested here. Piperine has been shown to bind, at a plasma concentration of ~10 μM–1 μM of human serum albumin, which is present in the blood at 0.6 mM [69]. In this way, a large concentration of piperine could theoretically be present in human plasma depending on how much was initially consumed. Lastly, nicotine has been shown to be present in plasma at ~1 μM [70]. However, nicotine is rapidly metabolized by the liver and is excreted to a much lesser extent due to reabsorption back into the intestine, meaning the concentration of nicotine and its metabolites in the intestine could be much greater than what is detectable in plasma. Due to our limited knowledge on the concentrations of these phytochemicals in the intestines, it is difficult to say, with certainty, whether the concentrations tested in this study (10 mg mL−1 − 0.01 mg mL−1) are directly applicable across all of the compounds. Due to the mechanisms of absorption reported by other studies, it is feasible that intestinal concentrations of all of the phytochemicals tested here exceed those reported in plasma [63–70].
More is known about the impact of phytochemicals on rumen microbiota than human gut microbiota. Historically, antibiotics have been given to ruminants to ameliorate rumen acidosis, improve feed efficiency and promote growth [71]. Phytochemicals have been explored as alternatives to feed antibiotics due to the rise of antibiotic-resistant bacteria [72]. Other phytochemicals such as isoflavones and the prenylated phloroglucinol compounds from the hops plant have spectra of activity that are useful in modulating rumen fermentation [73–75]. The rumen is the first major digestive compartment of the animal, but the major site of microbial activity in humans is the large intestine. We must consider that the host has the opportunity to absorb and transform phytochemicals, so pharmacokinetics and pharmacodynamics could alter the forms and amounts of the chemical that reach the microbiota. The results shared here merely demonstrate that it is a plausible hypothesis that ingested phytochemicals can impact gut microbiota, and subsequent host physiology, through antimicrobial mechanisms of action.
Funding/Acknowledgements
JEL was supported by a National Institutes of Health, National Institute on Drug Abuse T32 Training Grant (DA016176). MDF was supported by the USDA, Agricultural Research Service National Program NP-215, Grass, Forage and Rangeland Agroecosystems.
Footnotes
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Bercik P, Collins SM, Verdu EF, Microbes and the gut-brain axis, Neuro Gastroenterol. Motil 24 (2012) 405–413. [DOI] [PubMed] [Google Scholar]
- [2].Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. , Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43, Nature 461 (2009) 1282–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. , A core gut microbiome in obese and lean twins, Nature 457 (2009) 480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Takata K, Kinoshita M, Okuno T, Moriya M, Kohda T, Honorat JA, et al. , The lactic acid bacterium Pediococcus acidilactici suppresses autoimmune encephalomyelitis by inducing IL-10-producing regulatory T cells, PLoS One 6 (2011), e27644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sun H, Wang N, Cang Z, Zhu C, Zhao L, Nie X, et al. , Modulation of microbiota-gut-brain Axis by berberine resulting in improved metabolic status in high-fat diet-fed rats, Obes Facts 9 (2016) 365–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colpitts SL, Kasper LH, Influence of the gut microbiome on autoimmunity in the central nervous system, J. Immunol 198 (2017) 596–604. [DOI] [PubMed] [Google Scholar]
- [7].Cryan JF, O’Mahony SM, The microbiome-gut-brain axis: from bowel to behavior, Neuro Gastroenterol. Motil 23 (2011) 187–192. [DOI] [PubMed] [Google Scholar]
- [8].Weimer PJ, Stevenson DM, Mantovani HC, Man SL, Host specificity of the ruminal bacterial community in the dairy cow following near-total exchange of ruminal contents, J. Dairy Sci 93 (2010) 5902–5912. [DOI] [PubMed] [Google Scholar]
- [9].Nagaraja TG, Titgemeyer EC, Ruminal acidosis in beef cattle: the current microbiological and nutritional outlook, J. Dairy Sci 90 (Suppl 1) (2007) E17–E38. [DOI] [PubMed] [Google Scholar]
- [10].Singh V, Yeoh BS, Chassaing B, Xiao X, Saha P, Aguilera Olvera R, et al. , Dysregulated microbial fermentation of soluble fiber induces cholestatic liver cancer, Cell 175 (2018) 679–694 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, et al. , Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Luo J, Ranadheera CS, King S, Evans CA, Baines SK, Potential influence of dairy propionibacteria on the growth and acid metabolism of Streptococcus bovis and Megasphaera elsdenii, Benef. Microbes 8 (2017) 111–119. [DOI] [PubMed] [Google Scholar]
- [13].Petriello MC, Hoffman JB, Vsevolozhskaya O, Morris AJ, Hennig B, Dioxin-like PCB 126 increases intestinal inflammation and disrupts gut microbiota and metabolic homeostasis, Environ. Pollut 242 (2018) 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hoffman JB, Flythe MD, Hennig B, Environmental pollutant-mediated disruption of gut microbial metabolism of the prebiotic inulin, Anaerobe 55 (2019) 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Allais L, Kerckhof FM, Verschuere S, Bracke KR, De Smet R, Laukens D, et al. , Chronic cigarette smoke exposure induces microbial and inflammatory shifts and mucin changes in the murine gut, Environ. Microbiol 18 (2016) 1352–1363. [DOI] [PubMed] [Google Scholar]
- [16].Lee SH, Yun Y, Kim SJ, Lee EJ, Chang Y, Ryu S, et al. , Association between cigarette smoking status and composition of gut microbiota: population-based cross-sectional study, J. Clin. Med 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xiao HW, Ge C, Feng GX, Li Y, Luo D, Dong JL, et al. , Gut microbiota modulates alcohol withdrawal-induced anxiety in mice, Toxicol. Lett 287 (2018) 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wang R, Li S, Jin L, Zhang W, Liu N, Wang H, et al. , Four-week administration of nicotinemoderately impacts blood metabolic profile and gut microbiota in a diet-dependent manner, Biomed. Pharmacother 115 (2019) 108945. [DOI] [PubMed] [Google Scholar]
- [19].Hostettmann K, Wolfender J-L, The search for biologically active secondary metabolites, Pestic. Sci 51 (1997) 471–482. [Google Scholar]
- [20].Russell JB, A. New York State College of, S. Life, M. Department of. Rumen Microbiology and its Role in Ruminant Nutrition, Dept. of Microbiology, Cornell University, Ithaca, NY, 2002. [Google Scholar]
- [21].Hungate RE, Studies on Cellulose Fermentation: III. The culture and isolation for cellulose-decomposing bacteria from the rumen of cattle, J. Bacteriol 53 (1947) 631–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cotta MA, Russell JB, Effect of peptides and amino acids on efficiency of rumen bacterial protein synthesis in continuous culture, J. Dairy Sci 65 (1982) 226–234. [Google Scholar]
- [23].Varel VH, Bryant MP, Nutritional features of Bacteroides fragilis subsp. fragilis, Appl. Microbiol 28 (1974) 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chen G, Russell JB, More monensin-sensitive, ammonia-producing bacteria from the rumen, Appl. Environ. Microbiol 55 (1989) 1052–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hwang YB, Roberts SK, Chadwick LR, Wu CD, Kinghorn AD, Antimicrobial constituents from goldenseal (the rhizomes of Hydrastis canadensis) against selected oral pathogens, Planta Med. 69 (2003) 623–627. [DOI] [PubMed] [Google Scholar]
- [26].Chotivanich K, Sattabongkot J, Udomsangpetch R, Looareesuwan S, Day NP, Coleman RE, et al. , Transmission-blocking activities of quinine, primaquine, and artesunate, Antimicrob. Agents Chemother 50 (2006) 1927–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chahal JK, Sarin R, Malwal M, Efficacy of clerodendrum inerme L. (Garden quinine) against some human pathogenic strains, Int. J. Pharma Bio Sci 1 (2010). [Google Scholar]
- [28].Tegos G, Stermitz FR, Lomovskaya O, Lewis K, Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials, Antimicrob. Agents Chemother 46 (2002) 3133–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Amin AH, Subbaiah TV, Abbasi KM, Berberine sulfate: antimicrobial activity, bioassay, and mode of action, Can. J. Microbiol 15 (1968) 1067–1076. [DOI] [PubMed] [Google Scholar]
- [30].Pajouhesh H, Lenz GR, Medicinal chemical properties of successful central nervous system drugs, NeuroRx 2 (2005) 541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Umamatheswari S, Balaji B, Ramanathan M, Kabilan S, Synthesis, antimicrobial evaluation and QSAR studies of novel piperidin-4-yl-5-spiro-thiadia-zoline derivatives, Bioorg. Med. Chem. Lett 20 (2010) 6909–6914. [DOI] [PubMed] [Google Scholar]
- [32].Costerton JW, Ingram JM, Cheng KJ, Structure and function of the cell envelope of Gram-negative bacteria, Bacteriol. Rev 38 (1974) 87–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paterson DL, Resistance in gram-negative bacteria: enterobacteriaceae, Am. J. Infect. Contr 34 (2006) S20–S28. [DOI] [PubMed] [Google Scholar]
- [34].Slama TG, Gram-negative antibiotic resistance: there is a price to pay, Crit. Care 12 (2008) S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li X-Z, Plesiat P, Nikaido H, The challenge of efflux-mediated antibiotic resistance in Gram-negative bacteria, Clin. Microbiol. Rev 28 (2015) 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bryant MP, Small N, Bouma C, Chu H, Bacteroides ruminicola n. sp. and Succinimonas amylolytica; the new genus and species; species of succinic acid-producing anaerobic bacteria of the bovine rumen, J. Bacteriol 76 (1958) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Avgustin G, Wallace RJ, Flint HJ, Phenotypic diversity among ruminal isolates of Prevotella ruminicola: proposal of Prevotella brevis sp. nov., Prevotella bryantii sp. nov., and Prevotella albensis sp. nov. and redefinition of Prevotella ruminicola, Int. J. Syst. Evol. Microbiol 47 (1997) 284–288. [DOI] [PubMed] [Google Scholar]
- [38].Avgustin G, Wright F, Flint HJ, Genetic diversity and phylogenetic relationships among strains of Prevotella (Bacteroides) ruminicola from the rumen, Int. J. Syst. Bacteriol 44 (1994) 246–255. [DOI] [PubMed] [Google Scholar]
- [39].Benz MS, Scott IU, Flynn HW, Unonius N, Miller D, Endophthalmitis isolates and antibiotic sensitivities: a 6-year review of culture-proven cases, Am. J. Ophthalmol 137 (2004) 38–42. [DOI] [PubMed] [Google Scholar]
- [40].Moloney TP, Park J, Microbiological isolates and antibiotic sensitivities in culture-proven endophthalmitis: a 15-year review, Br. J. Ophthalmol 98 (2014) 1492–1497. [DOI] [PubMed] [Google Scholar]
- [41].Collins MD, Lawson PA, Willems A, Cordoba JJ, Fernandez-Garayzabal J, Garcia P, et al. , The phylogeny of the genus Clostridium: proposal of five new genera and eleven new species combinations, Int. J. Syst. Bacteriol 44 (1994) 812–826. [DOI] [PubMed] [Google Scholar]
- [42].Lawson PA, Citron DM, Tyrrell KL, Finegold SM, Reclassification of Clostridium difficile as Clostridioides difficile (Hall and O’Toole 1935) prevot 1938, Anaerobe 40 (2016) 95–99. [DOI] [PubMed] [Google Scholar]
- [43].Galperin MY, Brover V, Tolstoy I, Yutin N, Phylogenomic analysis of the family Peptostreptococcaceae (Clostridium cluster XI) and proposal for reclassification of Clostridium litorale (Fendrich et al. 1991) and Eubacterium acidaminophilum (Zindel et al. 1989) as Peptoclostridium litorale gen. nov. comb. nov. and Peptoclostridium acidaminophilum comb. nov, Int. J. Syst. Evol. Microbiol 66 (2016) 5506–5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Smith CJ, Markowitz SM, Macrina FL, Transferable tetracycline resistance in Clostridium difficile, Antimicrob. Agents Chemother 19 (1981) 997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Olson MM, Shanholtzer CJ, Lee JT, Gerding DN, Ten years of prospective Clostridium difficile-associated disease surveillance and treatment at the Minneapolis vA Medical Center, 1982–1991, Infect. Control Hosp. Epidemiol 15 (1994) 371–381. [DOI] [PubMed] [Google Scholar]
- [46].Sebaihia M, Wren BW, Mullany P, Fairweather NF, Minton N, Stabler R, et al. , The multidrug-resistant human pathogen Clostridium difficile has a highly mobile, mosaic genome, Nat. Genet 38 (2006) 779–786. [DOI] [PubMed] [Google Scholar]
- [47].Kelly CP, LaMont JT, Clostridium difficile — more difficult than ever, N. Engl. J. Med 359 (2008) 1932–1940. [DOI] [PubMed] [Google Scholar]
- [48].Huang H, Weintraub A, Fang H, Nord CE, Antimicrobial resistance in Clostridium difficile, Int. J. Antimicrob. Agents 34 (2009) 516–522. [DOI] [PubMed] [Google Scholar]
- [49].Obonyo CO, Juma EA, Clindamycin plus quinine for treating uncomplicated falciparum malaria: a systematic review and meta-analysis, Malar. J 11 (2012) 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Nord CE, Heimdahl A, Kager L, Malmborg AS, The impact of different antimicrobial agents on the normal gastrointestinal microflora of humans, Clin. Infect. Dis 6 (1984) S270–S275. [DOI] [PubMed] [Google Scholar]
- [51].Nord CE, Kager L, Heimdahl A, Impact of antimicrobial agents on the gastrointestinal micro flora and the risk of infections, Am. J. Med 76 (1984) 99–106. [DOI] [PubMed] [Google Scholar]
- [52].Sakata H, Fujita K, Yoshioka H, The effect of antimicrobial agents on fecal flora of children, Antimicrob. Agents Chemother 29 (1986) 225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nord CE, The effect of antimicrobial agents on the ecology of the human intestinal microflora, Vet. Microbiol 35 (1993) 193–197. [DOI] [PubMed] [Google Scholar]
- [54].Sullivan A, Edlund C, Nord CE, Effect of antimicrobial agents on the ecological balance of human microflora, Lancet Infect. Dis 1 (2001) 101–114. [DOI] [PubMed] [Google Scholar]
- [55].Rashid M-U, Weintraub A, Nord CE, Effect of new antimicrobial agents on the ecological balance of human microflora, Anaerobe 18 (2012) 249–253. [DOI] [PubMed] [Google Scholar]
- [56].Rogers MA, Greene MT, Saint S, Chenoweth CE, Malani PN, Trivedi I, et al. , Higher rates of Clostridium difficile infection among smokers, PLoS One 7 (2012), e42091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Iwasa K, Kamigauchi M, Ueki M, Taniguchi M, Antibacterial activity and structure-activity relationships of berberine analogs, Eur. J. Med. Chem 31 (1996) 469–478. [Google Scholar]
- [58].Fuoco D, Classification framework and chemical biology of tetracycline-structure-based drugs, Antibiotics (Basel) 1 (2012) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bryskier A, Antimicrobial Agents: Antibacterials and Antifungals, ASM press, 2005. [Google Scholar]
- [60].Lechner D, Gibbons S, Bucar F, Plant phenolic compounds as ethidium bromide efflux inhibitors in Mycobacterium smegmatis, J. Antimicrob. Chemother 62 (2008) 345–348. [DOI] [PubMed] [Google Scholar]
- [61].Kalia NP, Mahajan P, Mehra R, Nargotra A, Sharma JP, Koul S, et al. , Capsaicin, a novel inhibitor of the NorA efflux pump, reduces the intracellular invasion of Staphylococcus aureus, J. Antimicrob. Chemother 67 (2012) 2401–2408. [DOI] [PubMed] [Google Scholar]
- [62].Kawada T, Suzuki T, Takahashi M, Iwai K, Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats, Toxicol. Appl. Pharmacol 72 (1984) 449–456. [DOI] [PubMed] [Google Scholar]
- [63].Wahlstrom B, Blennow G, A study on the fate of curcumin in the rat, Acta Pharmacol. Toxicol 43 (1978) 86–92. [DOI] [PubMed] [Google Scholar]
- [64].Spinozzi S, Colliva C, Camborata C, Roberti M, Ianni C, Neri F, et al. , Berberine and its metabolites: relationship between physicochemical properties and plasma levels after administration to human subjects, J. Nat. Prod 77 (2014) 766–772. [DOI] [PubMed] [Google Scholar]
- [65].Bley K, Boorman G, Mohammad B, McKenzie D, Babbar S, A comprehensive review of the carcinogenic and anticarcinogenic potential of capsaicin, Toxicol. Pathol 40 (2012) 847–873. [DOI] [PubMed] [Google Scholar]
- [66].Kawada T, Suzuki T, Takahashi M, Iwai K, Gastrointestinal absorption and metabolism of capsaicin and dihydrocapsaicin in rats, Toxicol. Appl. Pharmacol 72 (1984) 449–456. [DOI] [PubMed] [Google Scholar]
- [67].Dei Cas M, Ghidoni R, Dietary Curcumin: correlation between bioavailability and health potential, Nutrients 11 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gisselmann G, Alisch D, Welbers-Joop B, Hatt H, Effects of quinine, quinidine and chloroquine on human muscle nicotinic acetylcholine receptors, Front. Pharmacol 9 (2018) 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Suresh DV, Mahesha HG, Rao AG, Srinivasan K, Binding of bioactive phytochemical piperine with human serum albumin: a spectrofluorometric study, Biopolymers 86 (2007) 265–275. [DOI] [PubMed] [Google Scholar]
- [70].Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, et al. , Guidelines on nicotine dose selection for in vivo research, Psychopharmacology (Berlin) 190 (2007) 269–319. [DOI] [PubMed] [Google Scholar]
- [71].Russell JB, Strobel H, Effect of ionophores on ruminal fermentation, Appl. Environ. Microbiol 55 (1989) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Wallace RJ, Antimicrobial properties of plant secondary metabolites, Proc. Nutr. Soc 63 (2004) 621–629. [DOI] [PubMed] [Google Scholar]
- [73].Flythe M, Kagan I, Antimicrobial effect of red clover (Trifolium pratense) phenolic extract on the ruminal hyper ammonia-producing bacterium, Clostridium sticklandii, Curr. Microbiol 61 (2010) 125–131. [DOI] [PubMed] [Google Scholar]
- [74].Harlow BE, Flythe MD, Kagan IA, Aiken GE, Biochanin A (an isoflavone produced by red clover) promotes weight gain of steers grazed in mixed Grass pastures and fed dried-distillers’ grains, Crop Sci. 57 (2017). [Google Scholar]
- [75].Flythe MD, Kagan IA, Wang Y, Narvaez N, Hops (Humulus lupulus L.) bitter acids: modulation of rumen fermentation and potential as an alternative growth promoter, Front Vet Sci 4 (2017) 131. [DOI] [PMC free article] [PubMed] [Google Scholar]


