Abstract
15N spin-lattice relaxation dynamics in metronidazole-15N3 and metronidazole-15N2 isotopologues are studied for rational design of 15N-enriched biomolecules for Signal Amplification by Reversible Exchange in microtesla fields. 15N relaxation dynamics mapping reveals the deleterious effects of interactions with polarization transfer catalyst and quadrupolar 14N nucleus within the spin-relayed 15N-15N network.
Graphical Abstract
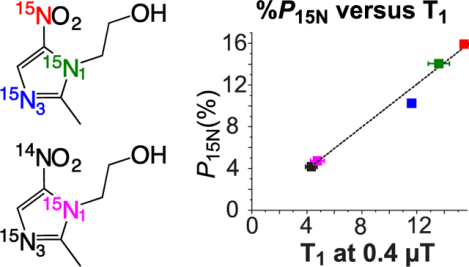
Presence of 14N nucleus in the scalar coupling network causes deleterious effects in SABRE hyperpolarization in microtesla fields resulting in 3-fold decrease of 15N T1 and polarization values for all 15N sites in 15N2-isotopologue versus 15N3-isotopologue.
The nuclear spin polarization P at thermal equilibrium is governed by a Boltzmann distribution of nuclear spins among Zeeman energy levels. P increases linearly with magnetic field strength. For a conventional high-field NMR spectrometer (e.g., 9.4 T) or clinical Magnetic Resonance Imaging (MRI) scanner (e.g., 3 T) at room temperature, P is typically on the order of 10−5 to 10−6, resulting in a relatively low sensitivity of NMR-based applications. For NMR applications where this sensitivity is too low to be useful, various hyperpolarization strategies may be employed to increase P by as much as 4–5 orders of magnitude,1, 2 with corresponding signal gains.
One such technique is Signal Amplification by Reversible Exchange (SABRE), pioneered by Duckett et al. in 2009,3 which utilizes simultaneous reversible chemical exchange of parahydrogen (p-H2) and to-be-hyperpolarized substrate molecules at a metal center. In SABRE, the transfer of nuclear spin polarization from parahydrogen-derived hydrides to a spin-polarizable substrate occurs spontaneously via the network of spin-spin couplings established in a transient polarization transfer catalyst (PTC) complex (Figure 1a).3–5 While several approaches have been developed for polarization transfer in SABRE,6–11 a variant of SABRE, SABRE-SHEATH (SABRE in SHield Enables Alignment Transfer to Heteronuclei),12, 13 facilitates the generation of highly polarized spin states of heteronuclei14–17 including nitrogen-15 with high polarization (P15N >30%18) persisting for tens of minutes.19 Nitrogen is found in a wide range of biomolecules including nucleic acids, amino acids, proteins, and drugs. Since SABRE-SHEATH is performed at very low magnetic fields (< 1 μT) and near room temperature, the production of such HP 15N spin-labeled biomolecules is comparatively simple, fast and inexpensive.10
Figure 1.
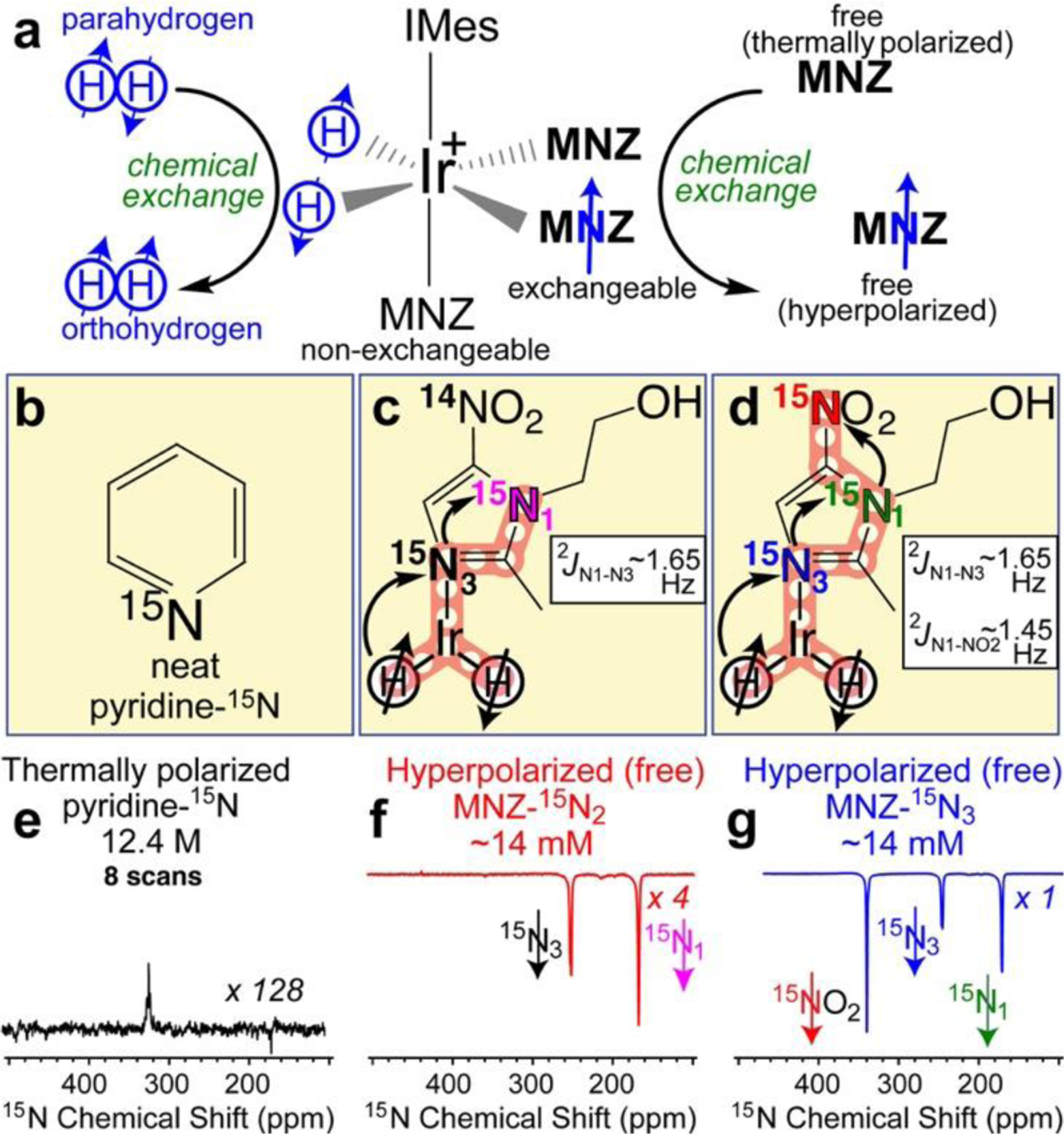
a) Molecular exchange between p-H2 and substrate, e.g., metronidazole (MNZ) employed here, in SABRE hyperpolarization. b) Structure of pyridine-15N employed as a signal reference. c-d) Corresponding structures and polarization transfer spin-relays (red overlay) between p-H2 and 15N nuclei in corresponding MNZ 15N-isotopologues. e) Signal reference 15N NMR spectrum of a thermally polarized neat pyridine-15N acquired with 8 scans and 10-minute recovery time. f-g) Corresponding 15N NMR spectra of HP metronidazole-15N2 and metronidazole-15N3.
Nitroimidazoles can be readily reduced in anaerobic environments. This property has been widely employed in a number of antibiotic drugs,20 Positron Emission Tomography (PET) tracers for hypoxia sensing,21 and also in a number of emerging cancer therapeutics: e.g., evofosfamide (a.k.a. TH-302)22 and the radiosensitizer nimorazole.23
Metronidazole is an FDA-approved antibiotic,24 belonging to the nitroimidazole class of compounds. We envision that 15N-hyperpolarized metronidazole can be potentially employed for hypoxia sensing in a manner similar to that of nitroimidazole-based Positron Emission Tomography (PET) tracers. One such tracer, 18F-fluoromisonidazole (FMISO),21 undergoes reduction in hypoxic environment (including most notably hypoxic tumors) and the metabolic products of this reduction process become trapped in hypoxic cells, providing contrast in FMISO PET images.25 The enormous potential for using HP MRI to sense metabolic transformations in vivo has been well demonstrated10, 26; correspondingly, HP MRI of metronidazole may obviate the limitations of FMISO PET imaging, including the use of ionizing radiation, the requirement for long clearance time from surrounding tissues, and the inability to spectrally distinguish parent compounds from downstream products.
We have demonstrated efficient SABRE-SHEATH hyperpolarization of the 15N3 site in natural abundance metronidazole with %P15N exceeding 30%.18 This nitrogen site directly interacts with the PTC, and therefore gains its polarization directly from p-H2-derived hydrides. Subsequently, commercially available metronidazole-15N2-13C2 (Sigma-Aldrich, #32744) was employed for SABRE-SHEATH, but unfortunately yielded significantly lower %P15N (roughly by an order of magnitude), although all 15N- and 13C-labeled sites have been successfully hyperpolarized.27, 28 Most recently we have synthesized metronidazole-15N3 and demonstrated 15N→15N spin-relayed SABRE-SHEATH hyperpolarization via two-bond 15N-15N spin-spin couplings, Figure 1d. This metronidazole-15N3 isotopologue exhibited a remarkable %P15N of ~16% on all three 15N sites including 15NO2, which has a polarization relaxation decay constant T1 approaching 10 minutes. However, in order to facilitate more effective production of HP biomolecules for bioimaging applications, it is clearly necessary to gain improved understanding of the underlying spin-relaxation phenomena to inform the rational design of these promising imaging agents.
Here, we report a quantitative study of spin relaxation dynamics of metronidazole-15N2 and metronidazole-15N3 isotopologues, Figure 1c and Figure 1d respectively, using previously described experimental setup (Figure S2).27, 29 Catalyst activation was performed for approximately 2 h for each sample studied to ensure reproducibility, see Figure S1 in the Supporting Information (SI). Other experimental parameters (temperature, p-H2 pressure, flow rate and in-shield magnetic field) were optimized for each isotopologue, Figure S1. Using an overpressure of 94 psig, p-H2 was bubbled through a solution of MNZ 15N-isotopologue substrate and Ir-IMes catalyst in 0.6 mL methanol-d4 for one minute to facilitate polarization transfer in microtesla fields. Following polarization build-up, the sample solution was rapidly transferred (2–4 s to minimize the relaxation losses) to a 1.4 T NMR Pro (Nanalysis, Canada) bench-top NMR spectrometer for 15N NMR detection (Figure 1h). Each relaxation/build-up curve was obtained by varying the time duration that the sample spent in a given magnetic field.
The key results related to 15N T1 relaxation at the optimal magnetic field during the SABRE-SHEATH polarization transfer process (ca. 0.4 μT, Figure S1) for metronidazole-15N3 and metronidazole-15N2 are shown in Figures 2e and 2f, respectively. As expected for microtesla magnetic fields, all 15N sites within a given molecule share approximately the same relaxation rate (e.g., 13.8–15.4 s for MNZ-15N3, Figure 2e). However, the 15NO2 group replacement by 14NO2 leads to dramatic, 3-fold shortening of the 15N T1 (4.3–4.8 s corresponding T1 values for MNZ-15N2, Figure 2f). This striking effect can be explained by the enhanced scalar relaxation of the second kind30, 31 induced by the quadrupolar 14NO2 site within the N-N spin-spin coupling network. These results are further supported by the overall similar 15N T1 trend at the Earth’s magnetic field (ca. 10 μT in the basement of our lab at Detroit, MI, Table S1). Moreover, we find that each 15N polarization build-up constant (Tb, Figure 2c and Figure 2d) at 0.4 μT is closely correlated with the corresponding T1 value. In practice, this means that the increased relaxation rate caused by the presence of the quadrupolar 14N spin in MNZ-15N2 (despite the peripheral position of the NO2 group) allows for achieving the steady-state %P15N faster on 15N3 and 15N1 sites—but at significantly lower levels. The correlation plot of %P15N versus T1 at 0.4 μT indeed exhibits a linear trend with R2>0.99 (Figure 2inset). When the magnetic field is sufficiently high (e.g., 1.4 T, Figure 2g and Figure 2h), the increased frequency dispersion of the nuclear spins puts them in a weakly coupled regime with 15N sites having significantly longer T1 decay constants—on the order of many minutes.
Figure 2.
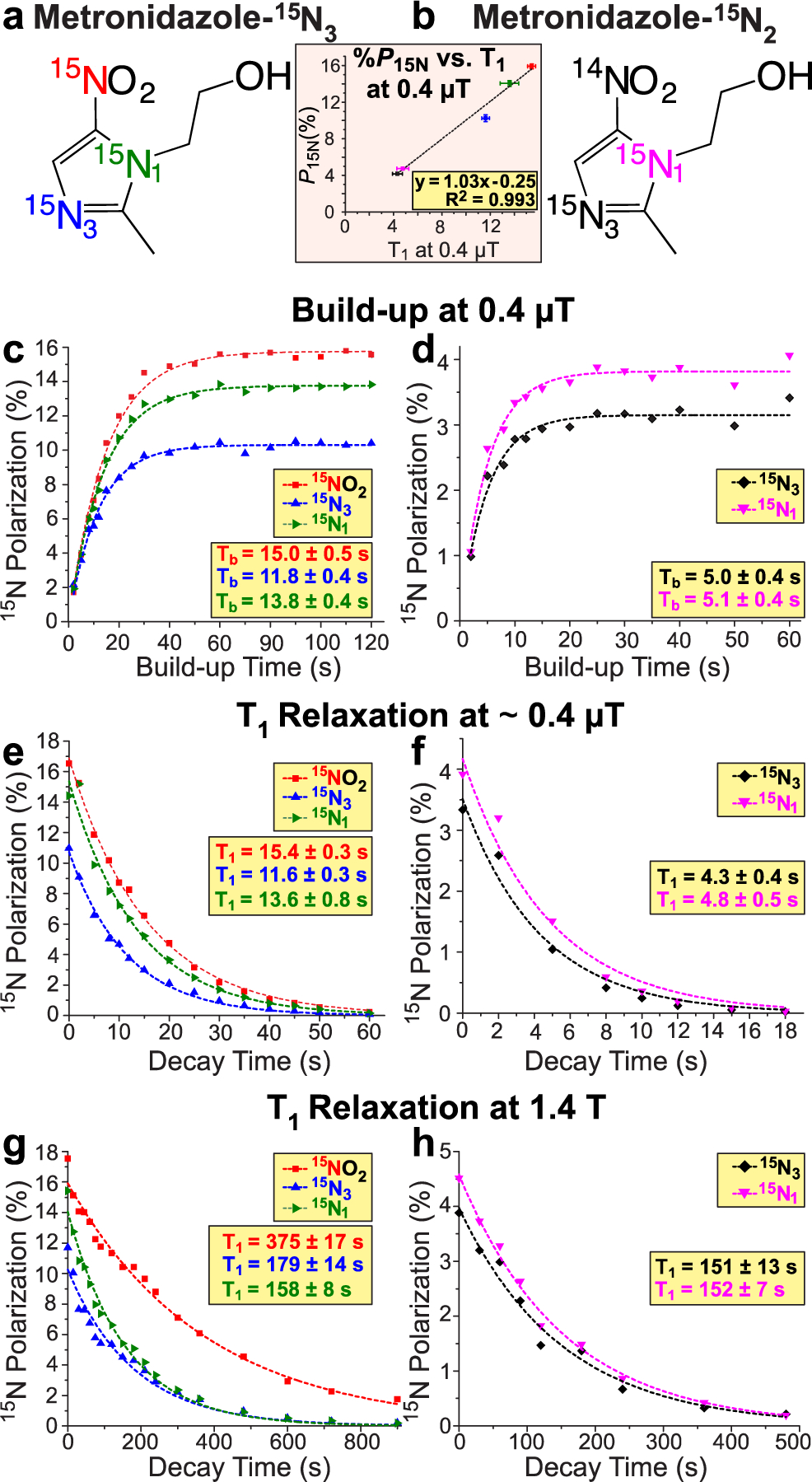
a-b) Structures of two metronidazole 15N-isotopologues. c-d) Corresponding 15N polarization build-up curves at 0.4 μT. e-f) Corresponding 15N T1 decay curves at 0.4 μT. g-h) Corresponding 15N T1 decay curves at 1.4 T. The presented data was recorded using a 2 mM IrIMes catalyst concentration and a corresponding 20 mM MNZ isotopologue concentration. All experiments are performed in CD3OD.
On another note, the realization that scalar-coupled 14N spins are highly deleterious in the context of SABRE-SHEATH suggests that if these quadrupolar effects would have been avoided, near-unity P15N would have been potentially achievable in the previous studies.18
It should be pointed out that substrate exchange of Ir-IMes catalyst may act as the potential source of additional undesirable 15N relaxation (e.g., due to compounding effects of quadrupolar Ir nucleus and the chemical exchange process).13 Consequently, a series of control experiments were performed, where the catalyst concentration was systematically varied from 0.5 mM to 1 mM to 2 mM at a fixed concentration of metronidazole isotopologue (Figures S3a–d). The [catalyst] increase from 0.5 mM to 2 mM results in a stepwise decrease in 15N T1 and Tb by approximately 2-fold at 0.4 μT (Figure S3b and Figure S3c). However, because the interplay of T1 relaxation and catalyst concentration is complex in the SABRE process,32, 33 these decreases in 15N T1 and Tb at 0.4 μT are offset by the overall increased catalyst-to-substrate ratio (i.e., better substrate access to p-H2 spin bath), resulting in somewhat greater %P15N in metronidazole-15N3 and similar %P15N in metronidazole-15N2 at higher [catalyst], Figure S3d. Of note, the Ir-IMes catalyst decreases 15N T1 even at high magnetic fields (1.4 T, weakly coupled regime) for 15NO2 (Figure S3a). Moreover, this observation clearly indicates a second reason (beyond agent purification) that SABRE catalyst removal18, 34–36 is warranted as soon polarization build-up is completed to minimize 15N polarization losses prior to biomedical utilization of HP metronidazole-15N3 as a contrast agent.
The high levels of 15N polarization obtained in 15N-labeled metronidazole isotopologues enable direct 15N MRI. Figure 3 demonstrates the 2D 15N projection images of 5 mm NMR tubes filled with HP solutions of 15N-labeled metronidazole isotopologues with the highest spatial resolution reported to date to the best of our knowledge.
Figure 3.
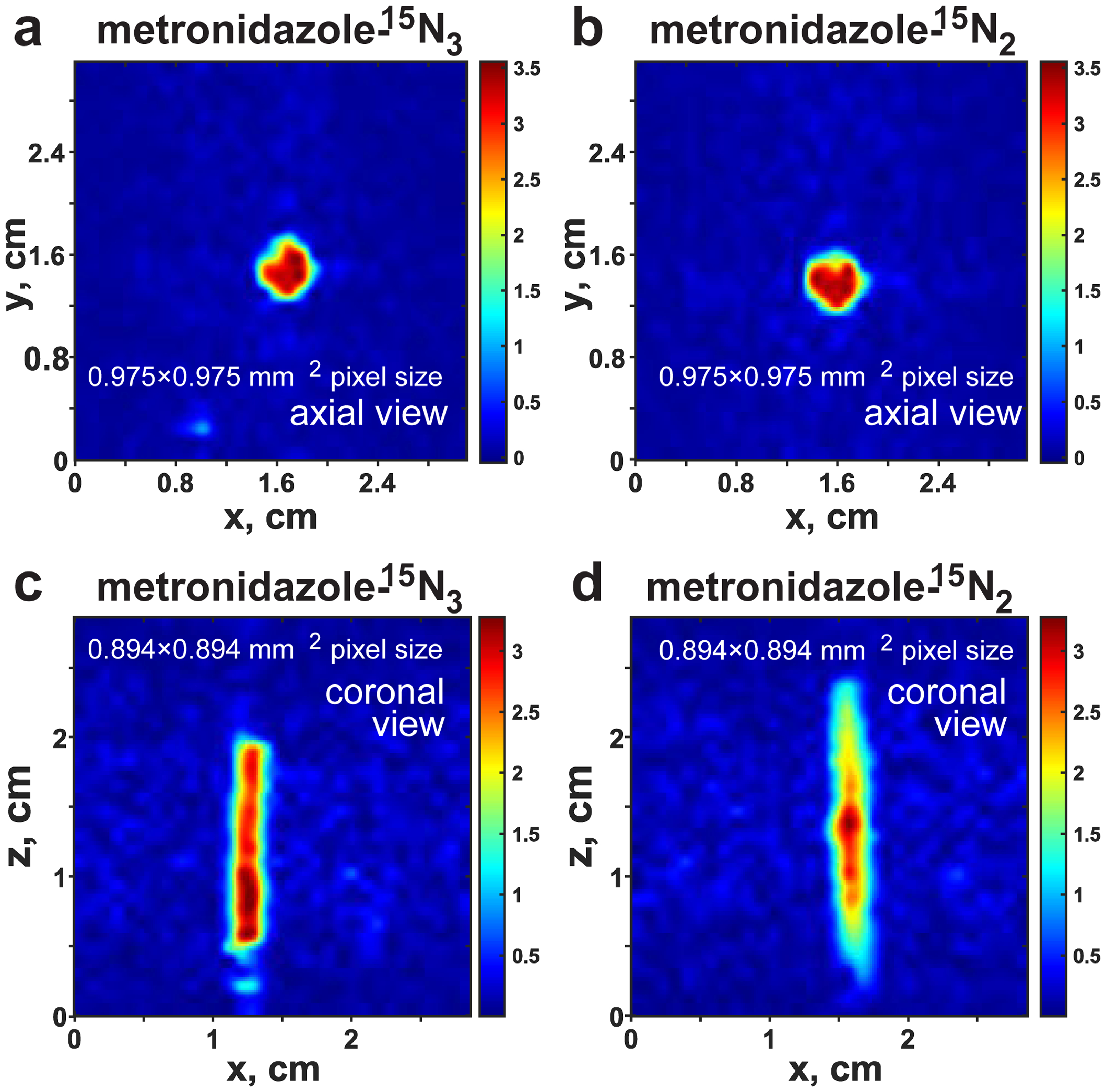
15N MRI of 5 mm NMR tubes filled with hyperpolarized solutions of 0.1 M metronidazole-15N3 (MNZ-15N3) and metronidazole-15N2 (MNZ-15N2) respectively using 5 mM of Ir(COD)(IMes)Cl in methanol-d4 obtained by TrueFISP pulse sequence. Imaging parameters employed: TR = 62.5 ms, TE = 3.6 ms, scan time = 2.0 seconds, flip angle = 15˚, matrix size = 32×32 (zero-filled to 512×512). a) axial projection 2D image of metronidazole-15N3 using 1 average (maximum SNR(SNRMAX) is ~500, b) axial projection 2D image of metronidazole-15N2 using 1 average, SNRMAX is ~410, c) coronal projection 2D image of metronidazole-15N3 using 8 averages, SNRMAX is ~450, d) coronal projection 2D image of metronidazole-15N2 using 8 averages, SNRMAX is ~340.
Conclusions
In summary, the spin-relayed SABRE-SHEATH hyperpolarization approach allows efficient polarization of scalar coupled 15N-15N spin networks. These networks may be created via two-bond 15N-15N J-couplings at most. The presence of 14N spins in such networks must be avoided to prevent deleterious polarization losses due to quadrupolar relaxation effects (manifested on 15N as scalar relaxation of the second kind16), in order to maximize the resulting %P15N. Although the catalyst decreases the 15N spin-relaxation time constant, T1, of metronidazole isotopologues in the microtesla regime in a concentration-dependent manner, the overall impact on the achievable 15N polarization level is relatively minor. On the other hand, the presence of a 14N nucleus in the scalar coupling network results in an approximately 3-fold decrease of microtesla 15N T1 values for all 15N sites in the 15N2-isotopologue versus the 15N3-isotopologue over a wide range of catalyst concentrations. This 15N T1 reduction results in a corresponding 3-fold decrease of 15N polarization levels. These findings have substantial translational relevance for the rational design of hyperpolarized MRI contrast agents comprising 15N- and 13C-labeled biomolecules—both in general, and in the specific case of SABRE-hyperpolarized metronidazole, an antibiotic that can be potentially employed for non-invasive hypoxia sensing antibiotics,20 emerging cancer therapeutics such as evofosfamide22 and nimorazole radiosensitizers,23 etc. Feasibility of high resolution MRI of HP metronidazole is shown, which bodes well for potential biomedical applications.
Supplementary Material
Acknowledgements
This work was supported by the NSF under grants CHE-1904780 and CHE-1905341, NIH R21CA220137, DOD CDMRP W81XWH-12-1-0159/BC112431, W81XWH-15-1-0271 and W81XWH-15-1-0272. O.G.S. thanks the RFBR (#19-33-60045) for the support of mechanistic studies with the use of parahydrogen. N.V.C. and K.V.K. thank the RFBR and Novosibirsk region government (#18-43-543023) for their support in the synthesis of 15N-labeled compounds. I.V.K. thanks the Russian Ministry of Science and Higher Education (project AAAA-A16-116121510087-5) and the RFBR (#19-29-10003, #17-54-33037) for financial support.
Footnotes
Electronic Supplementary Information (ESI) available: Numerical values of 15N polarization build-up and polarization decay constants, additional experimental details (file type, PDF). See DOI: 10.1039/x0xx00000x
Conflicts of interest
BMG, EYC declare stake ownership in XeUS Technologies, LTD.
Notes and References
- 1.Ardenkjaer-Larsen JH, J. Magn. Reson, 2016, 264, 3–12. [DOI] [PubMed] [Google Scholar]
- 2.Kovtunov KV, Pokochueva EV, Salnikov OG, Cousin S, Kurzbach D, Vuichoud B, Jannin S, Chekmenev EY, Goodson BM, Barskiy DA and Koptyug IV, Chem. Asian J, 2018, 13, 1857–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams RW, Aguilar JA, Atkinson KD, Cowley MJ, Elliott PIP, Duckett SB, Green GGR, Khazal IG, Lopez-Serrano J and Williamson DC, Science, 2009, 323, 1708–1711. [DOI] [PubMed] [Google Scholar]
- 4.Rayner PJ and Duckett SB, Angew. Chem. Int. Ed, 2018, 57, 6742–6753. [DOI] [PubMed] [Google Scholar]
- 5.Cowley MJ, Adams RW, Atkinson KD, Cockett MCR, Duckett SB, Green GGR, Lohman JAB, Kerssebaum R, Kilgour D and Mewis RE, J. Am. Chem. Soc, 2011, 133, 6134–6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Green RA, Adams RW, Duckett SB, Mewis RE, Williamson DC and Green GGR, Prog. Nucl. Mag. Res. Spectrosc, 2012, 67, 1–48. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov KL, Pravdivtsev AN, Yurkovskaya AV, Vieth H-M and Kaptein R, Prog. Nucl. Mag. Res. Spectrosc, 2014, 81, 1–36. [DOI] [PubMed] [Google Scholar]
- 8.Pravdivtsev AN, Yurkovskaya AV, Zimmermann H, Vieth H-M and Ivanov KL, RSC Adv, 2015, 5, 63615–63623. [Google Scholar]
- 9.Pravdivtsev AN, Yurkovskaya AV, Vieth H-M and Ivanov KL, J. Phys. Chem. B, 2015, 119, 13619–13629. [DOI] [PubMed] [Google Scholar]
- 10.Hövener J-B, Pravdivtsev AN, Kidd B, Bowers CR, Glöggler S, Kovtunov KV, Plaumann M, Katz-Brull R, Buckenmaier K, Jerschow A, Reineri F, Theis T, Shchepin RV, Wagner S, Bhattacharya P, Zacharias NM and Chekmenev EY, Angew. Chem. Int. Ed, 2018, 57, 11140–11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barskiy DA, Kovtunov KV, Koptyug IV, He P, Groome KA, Best QA, Shi F, Goodson BM, Shchepin RV, Truong ML, Coffey AM, Waddell KW and Chekmenev EY, ChemPhysChem, 2014, 15, 4100–4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Theis T, Truong ML, Coffey AM, Shchepin RV, Waddell KW, Shi F, Goodson BM, Warren WS and Chekmenev EY, J. Am. Chem. Soc, 2015, 137, 1404–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Truong ML, Theis T, Coffey AM, Shchepin RV, Waddell KW, Shi F, Goodson BM, Warren WS and Chekmenev EY, J. Phys. Chem. C, 2015, 119, 8786–8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duckett S, Roy S, Norcott P, Rayner PJ and Green GGR, Chem. Eur. J, 2017, 23, 10496–10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Olaru AM, Robertson TBR, Lewis JS, Antony A, Iali W, Mewis RE and Duckett SB, ChemistryOpen, 2018, 7, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barskiy DA, Shchepin RV, Tanner CPN, Colell JFP, Goodson BM, Theis T, Warren WS and Chekmenev EY, ChemPhysChem, 2017, 18, 1493–1498. [DOI] [PubMed] [Google Scholar]
- 17.Shchepin RV, Goodson BM, Theis T, Warren WS and Chekmenev EY, ChemPhysChem, 2017, 18, 1961–1965. [DOI] [PubMed] [Google Scholar]
- 18.Kidd BE, Gesiorski JL, Gemeinhardt ME, Shchepin RV, Kovtunov KV, Koptyug IV, Chekmenev EY and Goodson BM, J. Phys. Chem. C, 2018, 122, 16848–16852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shchepin RV, Birchall JR, Chukanov NV, Kovtunov KV, Koptyug IV, Theis T, Warren WS, Gelovani JG, Goodson BM, Shokouhi S, Rosen MS, Yen Y-F, Pham W and Chekmenev EY, Chem. Eur. J, 2019, 25, 8829–8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nepali K, Lee H-Y and Liou J-P, J. Med. Chem, 2018, 62, 2851–2893. [DOI] [PubMed] [Google Scholar]
- 21.Hendrickson K, Phillips M, Smith W, Peterson L, Krohn K and Rajendran J, Radiother. Oncol, 2011, 101, 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Connor LJ, Cazares-Körner C, Saha J, Evans CNG, Stratford MRL, Hammond EM and Conway SJ, Org. Chem. Front, 2015, 2, 1026–1029. [Google Scholar]
- 23.ClinicalTrials.gov, Clinical tiral #NCT01507467.
- 24.Roy RB, Laird SM and Heasman L, Br. J. Vener. Dis, 1975, 51, 281–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masaki Y, Shimizu Y, Yoshioka T, Tanaka Y, Nishijima K.-i., Zhao S, Higashino K, Sakamoto S, Numata Y, Yamaguchi Y, Tamaki N and Kuge Y, Sci. Rep, 2015, 5, 16802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurhanewicz J, Vigneron DB, Brindle K, Chekmenev EY, Comment A, Cunningham CH, DeBerardinis RJ, Green GG, Leach MO, Rajan SS, Rizi RR, Ross BD, Warren WS and Malloy CR, Neoplasia, 2011, 13, 81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shchepin RV, Jaigirdar L, Theis T, Warren WS, Goodson BM and Chekmenev EY, J. Phys. Chem. C, 2017, 121, 28425–28434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shchepin RV, Jaigirdar L and Chekmenev EY, J. Phys. Chem. C, 2018, 122, 4984–4996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theis T, Ariyasingha NM, Shchepin RV, Lindale JR, Warren WS and Chekmenev EY, J. Phys. Chem. Lett, 2018, 9, 6136–6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernatowicz P, Kubica D, Ociepa M, Wodyński A and Gryff-Keller A, J. Phys. Chem. A, 2014, 118, 4063–4070. [DOI] [PubMed] [Google Scholar]
- 31.Blanchard JW, Sjolander TF, King JP, Ledbetter MP, Levine EH, Bajaj VS, Budker D and Pines A, Phys. Rev. B, 2015, 92, 220202. [Google Scholar]
- 32.Barskiy DA, Pravdivtsev AN, Ivanov KL, Kovtunov KV and Koptyug IV, Phys. Chem. Chem. Phys, 2016, 18, 89–93. [DOI] [PubMed] [Google Scholar]
- 33.Barskiy DA, Knecht S, Yurkovskaya AV and Ivanov KL, Prog. Nucl. Mag. Res. Spectrosc, 2019, 114.-, 33–70. [DOI] [PubMed] [Google Scholar]
- 34.Barskiy DA, Ke LA, Li X, Stevenson V, Widarman N, Zhang H, Truxal A and Pines A, J. Phys. Chem. Lett, 2018, 9, 2721–2724. [DOI] [PubMed] [Google Scholar]
- 35.Mewis RE, Fekete M, Green GGR, Whitwood AC and Duckett SB, Chem. Comm, 2015, 51, 9857–9859. [DOI] [PubMed] [Google Scholar]
- 36.Manoharan A, Rayner P, Iali W, Burns M, Perry V and Duckett S, ChemMedChem, 2018, 13, 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


