Abstract
Lyme disease (LD) is a tick-transmitted disease caused by Borrelia burgdorferi (Bb). Temporal studies of maternal antibody (Ab) profiles in Bb infected pregnant dogs and their pups have not been conducted. In this study, Ab profiles of a client-owned Bb C6 Ab positive Rottweiler and her nine pups were assessed. The dam presented with lameness 12 days prior to parturition and was C6 Ab positive with a Quant C6 Ab concentration of 237 U/mL. Treatment with amoxicillin was initiated and 11 days later nine pups were delivered.
Screening of the sera from the dam and pups against Bb cell lysates and a panel of antigens revealed similar immunoreactivity profiles. While antigen-specific IgG and IgM reactivity persisted in the dam for at least 7 months, a rapid decline in IgG specific for BBA36, BBK53, BB0238, BBA73 and outer surface protein (Osp) E in the pups occurred between days 29 and 52 post-parturition. In contrast, Ab specific for DbpA and the diagnostic antigens variable major protein (VMP)-like sequence (Vls) E lipoprotein (VlsE) VlsE (C6) and OspF, remained elevated in the pups. Sera from the dam displayed potent complement-dependent bactericidal activity against Bb. Sera from the pups was also bactericidal but primarily through a complement-independent mechanism. Lastly, single dose vaccination of the dam at day 51 post-parturition with a LD subunit vaccine consisting of OspA and an OspC chimeritope triggered a broad anti-OspC Ab response indicative of an anamnestic response. Although this study focused on a single case, these findings add to our knowledge of maternal Ab profiles and will aid the interpretation of serological assays in pups delivered by a Bb C6 Ab positive dog.
Keywords: Borreliella, Dogs, Lyme disease, Maternal antibody, OspC, VlsE
Introduction
Lyme disease (LD) is the most common tick-borne infection in North America and Europe (Nelson et al., 2015; Sykes and Makiello, 2016; Eisen et al., 2017; Heyman et al., 2018). Borrelia burgdorferi (Bb) was identified as the etiological agent in 1982 (Benach et al., 1983; Burgdorfer et al., 1982). Several additional species that cause LD have since been delineated. The genus Borrelia has been split into two genera with those species associated with LD assigned to the genus Borreliella (Adeolu and Gupta, 2014). We collectively refer to Borreliella species as the LD spirochetes. The LD spirochetes are maintained in nature in an enzootic cycle involving mammalian reservoirs and Ixodes ticks (Barbour and Hayes, 1986). The endemic range for LD is expanding due to an increasing Ixodes tick population (Eisen et al., 2016).
The Companion Animal Parasite Council (CAPC) reports serological testing data for LD and other infectious diseases of veterinary concern in North America1. In 2019, approximately 360,000 positive Bb antibody (Ab) tests were reported in dogs in the US alone1. CAPC states that this number represents approximately 30% of the total number of tests run each year in the US and hence the actual number of positive tests per year may exceed 1,000,000. In Canada, approximately 5,500 positive Bb Ab tests in dogs were reported in 2019. In dogs, early stage LD may present with variable and non-descript clinical signs (Littman et al., 2018). When delivered early, antibiotic therapy generally results in positive clinical outcomes. However, if untreated intermittent lameness, polyarthritis and nephritis can develop. While it has been suggested that <5% of Bb-infected dogs are symptomatic (Littman et al., 2018), several studies have demonstrated abnormal histopathology and other manifestations in experimentally-infected dogs that would not be evident upon routine examination (Appel et al., 1993; Straubinger et al., 1998; Summers et al., 2005). Preventive strategies in dogs consist of vaccination and the use of acaricides.
Intrauterine transmission of Bb in dogs has been investigated in two studies (Appel et al., 1993; Gustafson et al., 1993) each of which reached different conclusions. Appel et al (1993) infected pregnant dogs using infected Ixodes scapularis ticks and then tested the fetuses from one dam and the pups from another for Bb DNA and maternally derived Ab. The fetuses and pups were negative for Bb DNA. Fetal blood was Bb Ab negative whereas the pups possessed maternally-derived Ab reactive with Bb whole cell lysate antigens. The maternally-derived Ab was no longer detectable 4 weeks postpartum. In contrast, Gustafson et al (1993) reported positive cultures and PCR results in a limited number of pups. Additional studies are required to determine the potential for intrauterine transmission in dogs and to better characterize maternally derived Ab profiles.
In this report, a temporal analysis of Ab responses to whole cell lysates (WCL) from in vitro cultivated Bb B31 and to antigens that are upregulated during infection (in vivo antigens) was conducted using sera collected from a client-owned Bb C6 Ab positive and symptomatic Rottweiler dam and her nine pups. Passive transfer of bactericidal Ab, Ig isotype profiles and the specificity of outer surface protein (Osp) C Ab responses pre- and post-vaccination with a LD vaccine were also assessed. The results of this study advance our understanding of the temporal nature of maternally-derived Ab and immune responses to vaccination. This information will assist the interpretation of serological assays and assist decision-making in treatment of pups born to a Bb Ab positive dam.
Materials and methods
Clinical history
A 4-year-old female Rottweiler presented to a veterinary hospital in Alexandria, Ontario, Canada for progressive lameness. The dog’s condition was complicated by advanced pregnancy with 12 days until anticipated parturition. The onset of lameness was noted a few days prior to initial presentation and started with the right forelimb, progressing to reluctance to move. On examination, the dam was reluctant to bear weight on the right front limb with pain on flexion of the right carpus. The owner reported a similar episode 3–4 months prior that had resolved without intervention. Information regarding prior vaccination for LD or active use of acaricides was not available from the owner. Ab testing revealed the dam to be SNAP 4DX PLUS (IDEXX) positive for Bb C6 Ab and to be negative for heartworm (Dirofilaria immitis), anaplasmosis (Anaplasma phagocytophilum and A. platys) and ehrlichiosis (Ehrlichia canis and Ehrlichia ewingii). The Bb Ab results were confirmed using the Quant C6 Ab test (IDEXX) which revealed a high C6 Ab concentration of 237 U/mL. For reference, values >30 U/mL are considered clinically relevant (IDEXX). Hematological analyses using ProCyte Dx (IDEXX) revealed some abnormalities that are listed as follows: haematocrit, 32.3% (reference interval, 37.3–61.7%); haemoglobin, 11.6 g/dL (reference interval, 13.1–20.5 g/dL); MCV, 56.7 (reference interval, 61.6–73.5); RETIC-HGB, 19.7 pg (reference interval, 22.3–29.6 pg); neutrophils, 13.3×109/L (reference interval, 2.95–11.64×109/L) and monocytes, 1.26×109/L (reference interval, 0.16–1.12×109/L). Catalyst Dx (IDEXX) revealed a globulin concentration of 52 g/L (reference interval, 25–45 g/L) and alkaline phosphatase of 248 U/L (reference interval, 23–212 U/L). Urinary analyses revealed a BLD of 10 erythrocytes/µL. Based on the collective findings, a diagnosis of LD was made and a 28-day course of amoxicillin was prescribed and initiated 10 days prior to whelping. Nine puppies were delivered without complication. Lameness in the dam resolved after initiation of treatment and prior to re-evaluation on day 14 post-partum. The dam and pups were vaccinated on days 65 and 51 post-partum, respectively and the dam received a second dose on day 92. Additional sera and clinical information were not available after transfer of the pups to new owners.
Bacterial strains, bacterial cultivation, serum samples and research ethics
Bb B31 (5A4 clone) was cultivated in BSK-H media (Sigma) supplemented with 6% rabbit serum (37 °C; 5% CO2) and growth was monitored using dark-field microscopy. Blood samples were drawn by a licensed veterinarian and sera collected using standard procedures. Informed consent was obtained from the pet owner for blood collection and analysis. A timeline of sampling and treatment procedures is presented in Fig. 1. The earliest sera available for analyses were collected on day 29 post-partum from the dam and pups and labeled as MD1 and PD1, respectively. Sera from individual pups were sequentially numbered (PD1-1 through PD1-9). Previously characterized sera from Bb Ab positive and Ab negative dogs served as controls (Oliver Jr et al., 2016).
Fig. 1.
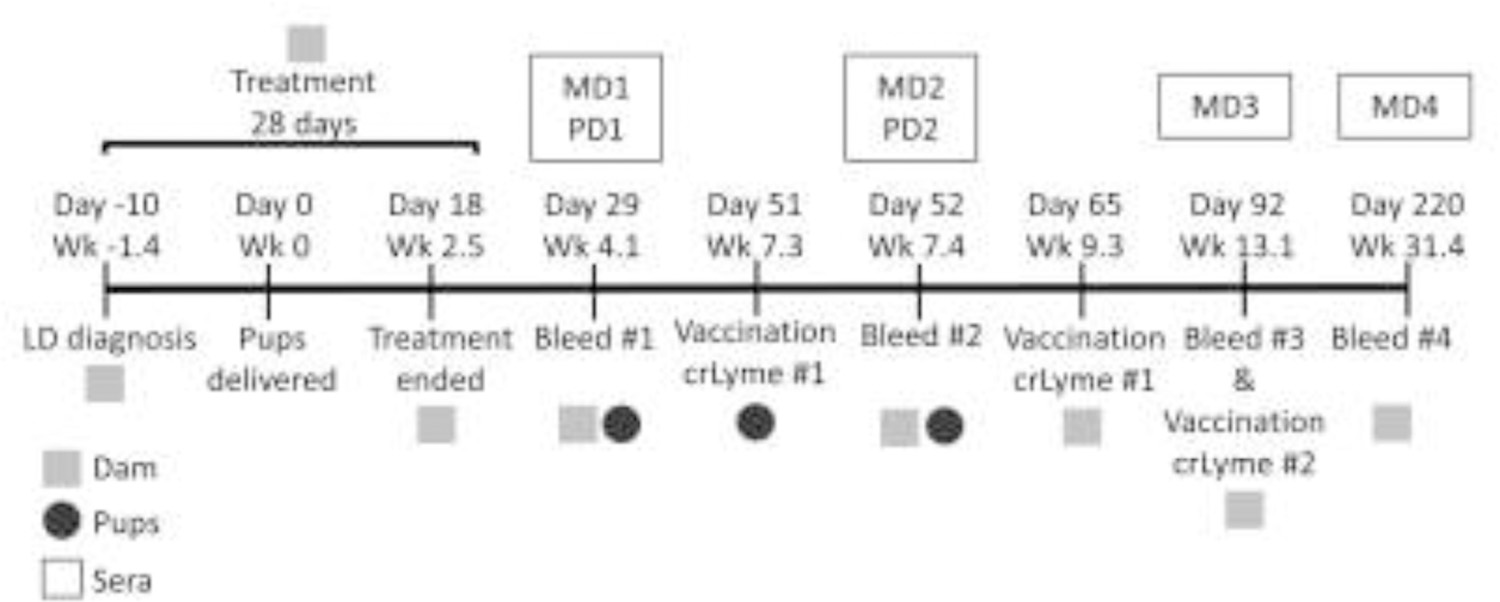
Timeline for sampling and treatment procedures. A pregnant Rottweiler presented with lameness (Day −10) and a diagnosis of Lyme disease (LD) was confirmed using SNAP4Dx and Lyme Quant C6 (IDEXX). Antibiotic treatment was prescribed. Blood for serology was collected at different time points from the dam (designated as MD1 through MD4) and her pups (PD1 and PD2). The dogs were vaccinated against Borrelia burgdorferi (Bb) as indicated. The timeline is presented as days relative to whelping.
Cloning, protein production and purification
Cloning of Bb proteins was completed as previously described (Oliver Jr et al., 2016). Briefly, genes were PCR amplified, the amplicons were digested with BamHI-HF and Eag1-HF (NEB) and ligated into the pET-45b(+) expression vector. All proteins were produced with N-terminal hexahistidine-tags and purified by FPLC (Izac et al., 2019). The recombinant proteins and their properties are presented in Table 1.
Table 1.
Information and general properties of Borreliella proteins employed in this study.
| Protein and ORF designationa | Genome map locationb | General information | MW (kDa) |
|---|---|---|---|
| DbpA (BBA24) | lp54 | In vivo antigen; binds to decorin (Brown et al., 1999) | 23.7 |
| BBA36 | lp54 | In vivo antigen (Baum et al., 2014) | 26.6 |
| BBK53 | lp36 | In vivo antigen (Iyer et al., 2015) | 24.0 |
| BB0238 | Chromosome | In vivo antigen; required for infection of mammals (Groshong et al., 2014) | 32.2 |
| BBA73 | lp54 | In vivo antigen (Hughes et al., 2008) | 37.2 |
| OspF (BBR42) | cp32-4 | In vivo antigen; facilitates binding to connective tissue (Antonara et al., 2007); diagnostic antigen | 27.9 |
| OspE (BBL39) | cp32-8 | In vivo antigen; contributes to complement evasion though Factor H binding (Hellwage et al., 2001) | 22.0 |
| VlsE (BBF41) | lp28-1 | In vivo antigen; contributes to immune evasion through antigenic variation (Zhang and Norris, 1998); diagnostic antigen | 38.6 |
| OspC Type A (BBB19) | cp26 | In vivo antigen; upregulated during the tick bloodmeal; essential virulence factor in mammals; immunodominant early antigen (Tilly et al., 2006); diagnostic and vaccine antigen | 25.0 |
| FhbB (TDE0108) | Chromosome | Immunodominant T. denticola antigen (Miller et al., 2016); used in this study as a negative control protein | 11.4 |
lp, linear and circular plasmid; cp, circular plasmid; variable major protein (VMP)-like sequence (Vls) E lipoprotein (VlsE); Osp, Outer surface protein
ORF designations are indicated by the ‘BB’ prefix. ORF designations are as originally assigned for the annotated genome of Bb B31 (Fraser et al., 1997).
The number following lp or cp indicates the size of the plasmid in kb.
SDS-PAGE and immunoblot analyses
B. burgdorferi B31 whole-cell lysate (WCL) was generated and fractionated by SDS-PAGE using Any-Kd Criterion Precast gels (Biorad) and standard methods (Izac et al., 2017). Proteins were visualized with Coomassie brilliant blue (CBB-R 250) or transferred to PVDF membranes (Pierce) for immunoblot analyses (Oliver Jr et al., 2016). Dot-blot procedures were as previously described (Oliver Jr et al., 2016). In brief, 125 ng of protein was spotted onto nitrocellulose and dried overnight. Equal protein loading was confirmed by staining (MemCode Reversible Protein Stain Kit; Thermo Scientific). Immunoblots and dot-blots were screened with diluted sera (1:1,000 and 1:200, respectively) and Ab binding detected using horseradish peroxidase (HRP)-conjugated anti-dog IgG Ab (1:40,000; Pierce) and Clarity Western ECL Substrate (Biorad). Images were captured using a ChemiDoc imaging system (Biorad). All blots from a given experiment were imaged simultaneously using identical parameters. The dot-blot images were cropped into a grid for visualization and comparison. All experiments were performed three times.
Indirect immunofluorescence assay (IFA) analyses
IFAs were performed as previously described (Earnhart et al., 2010). Briefly, Bb B31 strain cells were harvested, washed and dried onto Superfrost Plus slides (Fisher Scientific). After blocking, the samples were overlaid with serum (1:100; 3% BSA; PBST) and bound IgG detected with FITC-conjugated goat anti-dog IgG (1:500; Life Technologies). Dark-field and fluorescence microscopy using a FITC filter were used for visualization. The images were cropped and the contrast adjusted as needed. Due to limited serum volumes, the pup sera used in this and subsequent experiments were pooled and designated as PD1(1–9) and PD2(1–9).
ELISA analyses
ELISA plates (Costar 3590) were coated with 500 ng protein (variable major protein [VMP]-like sequence [Vls] E lipoprotein [VlsE], DbpA, BB0238, OspF (BBR42) in bicarbonate buffer (15 h; 4 °C), sera were added (1:100), and plates developed with HRP-conjugated goat anti-dog IgG or HRP-conjugated goat anti-dog IgM (Invitrogen; 1:15,000). ABTS was added and absorbance was measured at A405 nm. Statistical significance was determined by Tukey’s multiple comparison test using GraphPad Prism. The ELISA methods were as previously published (Earnhart and Marconi, 2007a).
Immunoglobulin (Ig) isotype and IgG subtype responses against Bb whole cell lysate and VlsE were then determined. Sera were tested at a dilution of 1:1,000. HRP-conjugated goat/sheep anti-dog IgG, IgG1, IgG2, IgM, IgA, and IgE secondary Abs (Novus Biologicals ) were used at a dilution of 1:10,000.
Measurement of serum bactericidal activity
Bactericidal activity was measured as previously described (Izac et al. 2017, 2019). Cells were incubated (37 °C; 18 h) in 40% BSK-H (Sigma), 20% complement-certified guinea pig serum (GPS; Complement Tech), and 40% heat inactivated (HI) sera from the dam and pups. Viable Bb cells (intact and motile) in 10 fields of view were counted using dark-field microscopy. The numbers were averaged and % killing determined by dividing the number of live cells in the experimental reaction by the number of live cells after incubation with serum from a seronegative dog. To determine if bactericidal activity is complement-dependent or independent, reactions were also run with HI-GPS as the exogenous complement source. Statistical significance was assessed via unpaired, two-tailed Student’s t-test using GraphPad Prism.
Results
Whole-cell lysate (WCL) immunoblot and dot blot analyses
To test for IgG to Bb antigens that are produced during in vitro cultivation, immunoblots of Bb B31 WCL were screened with sera collected over time (Fig. 2; Panel A). The earliest sera available for analyses were collected on day 29 post-partum from the dam and pups and labeled as MD1 and PD1, respectively. Sera from individual pups were sequentially numbered (PD1-1 through PD1-9). MD1 and the PD1 samples reacted with the same set of antigens present in the WCL. The immunoreactivity of the dam sera was steady out to day 220 (MD4; final sample). Notably, IgG levels in the dam did not decline after treatment with amoxicillin. In contrast, the pups’ IgG levels declined between day 29 (PD1) and 52 (PD2).
Fig. 2.
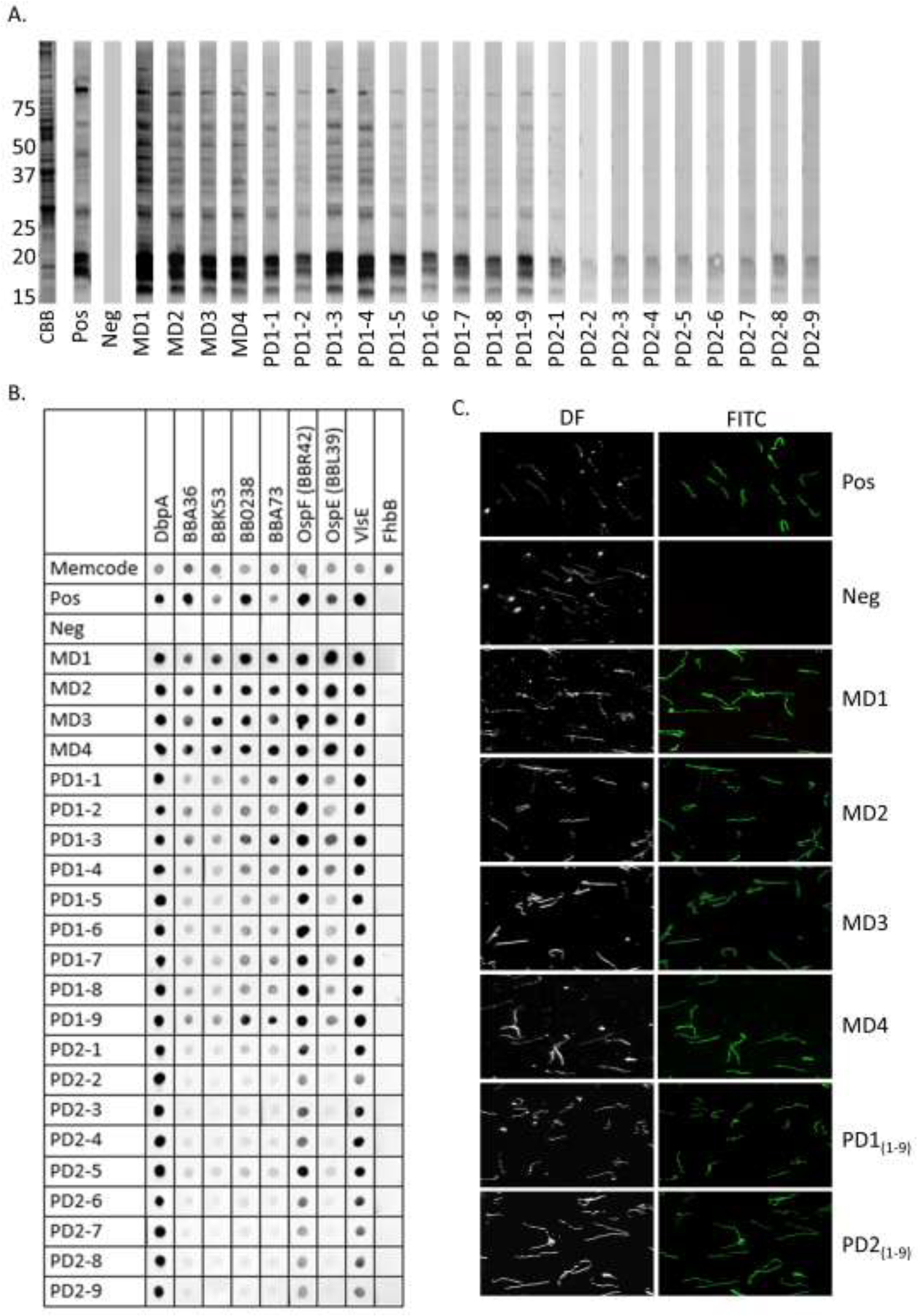
Immunoblot, dot blot and immunofluorescence assay (IFA) analyses. Panel A presents immunoblots of Borrelia burgdorferi (Bb) strain B31 WCL that were incubated with sera from each dog as indicated along the bottom of the panel A. Collection timepoints for the sera are detailed in Fig. 1. MW standards are indicated to the left. The image labeled CBB (Coomassie brilliant blue) is a representative stained gel strip. Panel B presents the results of dot blot analyses in which sera (indicated at left) were screened against Bb in vivo antigens indicated along the top of panel B. Equal loading of protein was demonstrated by staining of a representative dot blot with MemCode stain. The T. denticola FhbB protein served as a negative control. Panel C presents IFA analyses in which non-permeabilized Bb B31 cells were screened with sera from the dam (temporal bleeds) and pups (pooled sera from each timepoint) as indicated on the right. Images were captured using a FITC filter or by dark field (DF) microscopy. In panels A, B and C, sera from client-owned dogs confirmed to be Bb antibody positive (Pos) or negative (Neg) served as controls.
Analysis of Ab responses to proteins upregulated during infection
The sera were screened for Abs to mammalian stage in vivo antigens (DbpA, BBA36, BBK53, BB0238, BBA73, OspF paralog BBR42, and OspE paralog BBL39) using a dot blot format (Fig. 2; Panel B). Serum samples MD1 -MD4 bound strongly to all proteins except the negative control, FhbB. Ab in the PD1 sera also bound to each protein but binding was strongest to DbpA, OspF and VlsE. While the Ab levels in the MD1 through MD4 sera remained steady between days 29 and 220, a decline in Ab levels was observed in the pups. Specifically, Ab levels to BBA36, BBK53, BB0238, BBA73, and OspE (BBL39) declined between day 29 and 52 (PD1 and PD2) while Ab specific for DbpA, OspF (BBR42) and VlsE remained relatively unchanged.
Ab binding to Bb surface antigens
IFA analyses were performed to screen for Abs to Bb surface antigens (Fig. 2; Panel C). Surface labeling of Bb was observed with no clear differences in labeling noted between the sera from the dam and pups at any time point.
ELISA analyses
IgG against VlsE, BB0238, DbpA and OspF (BBR42) was detected in all serum samples with the exception of the second pooled bleed from the pups (PD2(1–9)) which was near baseline for B0238. The IgG reactivity in the dam for DbpA, BB0238 and OspF (BBR42) remained remain elevated throughout the study but declined for VlsE when MD1 and MD4 were compared (P<0.01). IgG responses against all antigens, with the exception of BB0238, declined in the pups when PD1(1–9) (day 29) and PD2(1–9) (day 52) were compared (P<0.01). In the dam, IgM responses against each antigen were detected in all sera collected and Ab reactivity to DbpA, BB0238 and OspF (BBR42) actually increased at one or more later timepoints (MD2 or MD3). Antigen specific IgM in the pup sera was detected but was low for VlsE and DbpA and near baseline for BB0238 and OspF. IgG2 was the dominant IgG isotype in all sera (Fig. 4) with IgA, IgE and IgG1 levels at or near baseline.
Fig. 4.
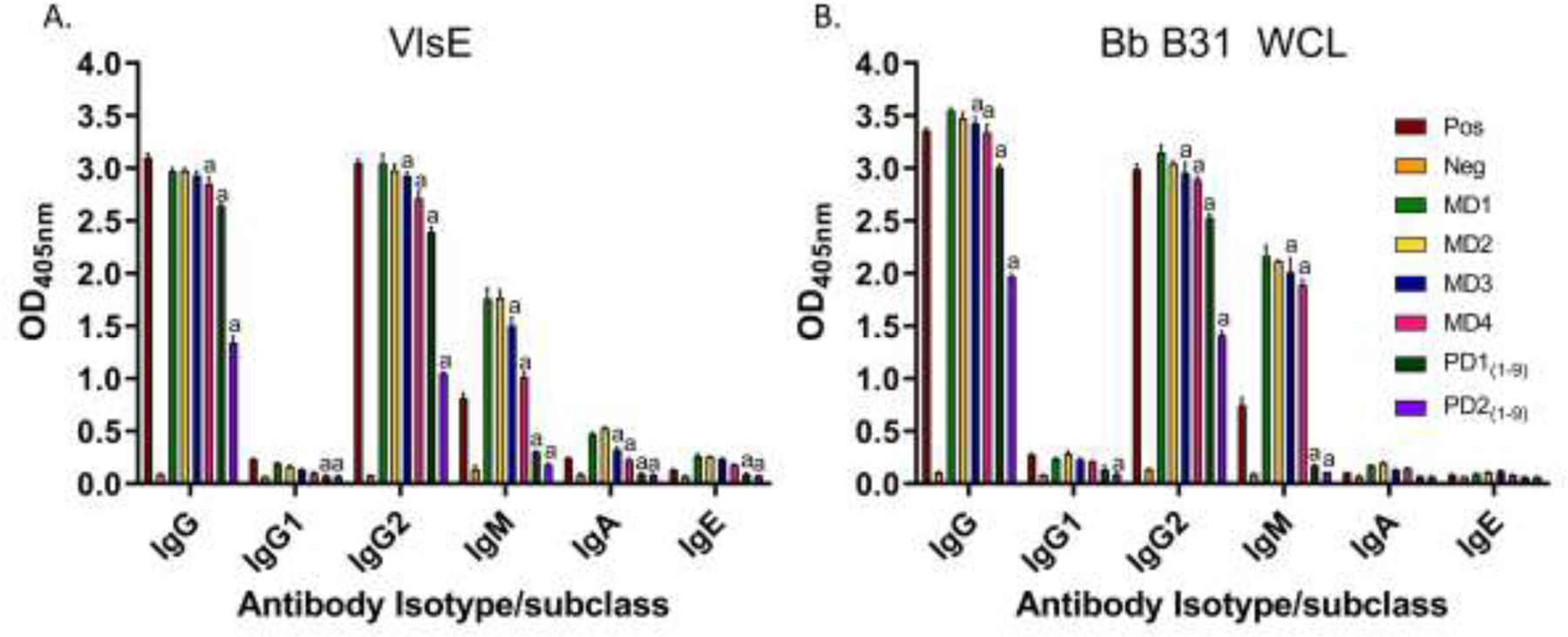
Antibody (Ab) isotype and IgG subclass responses to variable major protein (VMP)-like sequence (Vls) E lipoprotein (VlsE; Panel A) or Borrelia burgdorferi (Bb) B31 WCL (Panel B). All assays were performed in triplicate and the means ± standard deviation values are shown. a Denotes values that were significantly different from the first specimen collected for serology from the dam (MD1; P<0.01).
Anti-OspC Ab responses pre- and post-vaccination
To assess the potential diversity of the strains that infected the dam, 20 OspC type proteins were screened with pre-vaccination serum samples, MD1 and MD2. IgG directed against OspC was detected that preferentially bound to OspC types A, D, G, K and N (Fig. 5). The same immunoreactivity pattern was observed with the pups but Ab reactivity was weak. Immunoblot analyses of the serum (MD3) collected just prior to the second vaccine dose (day 92) revealed strong Ab reactivity with all OspC types tested. The same was true for the MD4 sample collected 108 days after the second vaccine dose. A post-vaccination Ab analysis of the pups was not performed due to ownership transfer.
Fig. 5.
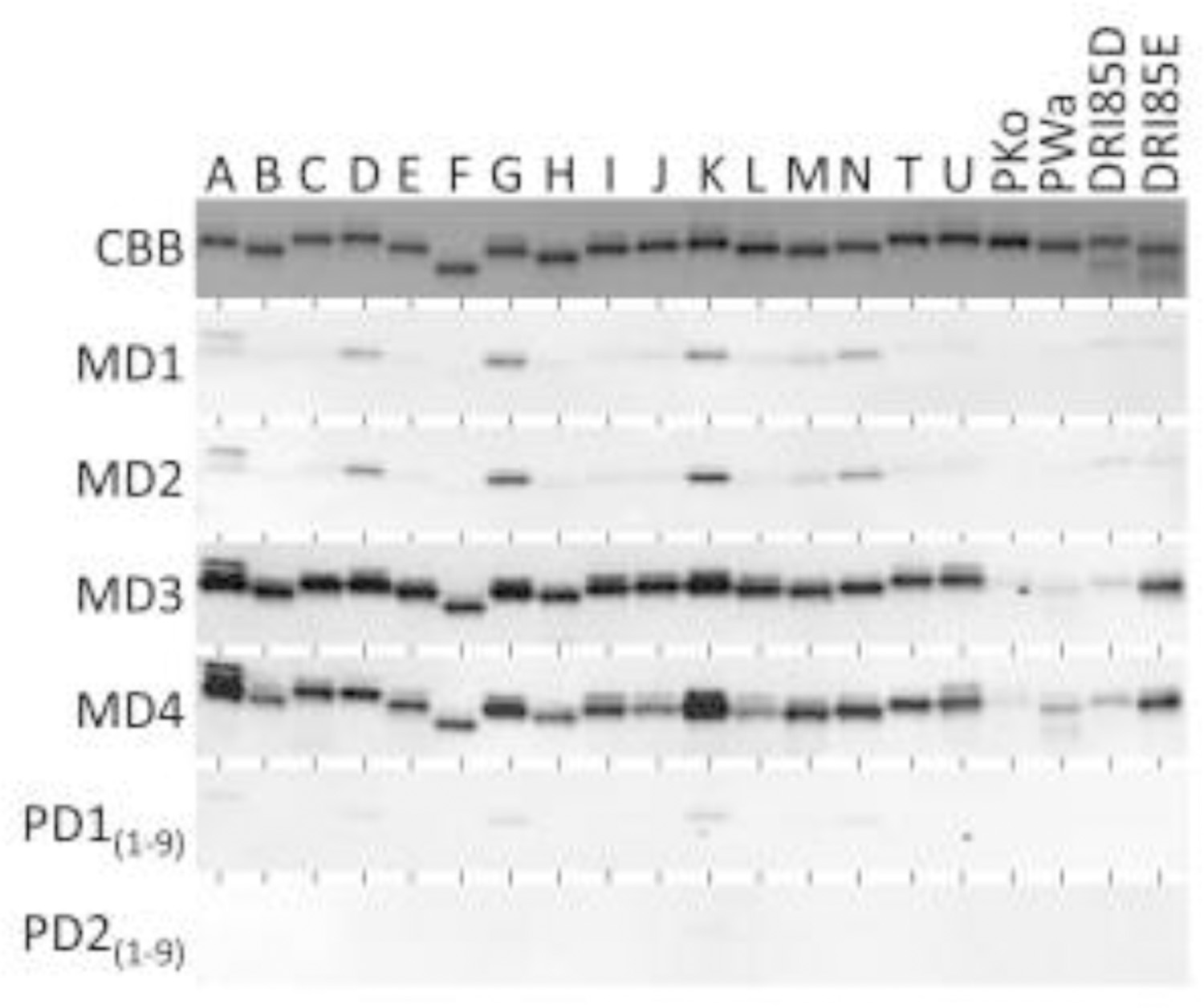
Analysis of antibody responses to diverse outer surface protein (Osp) C types pre- and post-vaccination. Twenty different recombinant OspC proteins (OspC types; indicated at top) were produced, immunoblotted and screened with the serum samples indicated to the left. The top panel is an image of an SDS-PAGE gel stained with Coomassie brilliant blue (CBB-).
Bactericidal activity of antibodies from dam and pups
Bactericidal assays were performed using HI-sera from the dam (pre- and post-vaccination) and pups. In the presence of active complement (GPS), MD1 through MD4 displayed 100% killing of Bb B31 (Fig. 6). In contrast, when HI-GPS was used, bactericidal activity declined from 70% at MD1 to 40% at MD4. The pup Abs also displayed potent bactericidal activity. With the PD1(1–9) serum, percent killing was 85% and 55% with GPS and HI-GPS, respectively. The level of killing observed with PD2(1–9) was 50% with either GPS or HI-GPS. Both complement-dependent and complement-independent killing were observed. All pairwise comparisons were determined to be significant (P<0.01).
Fig. 6.
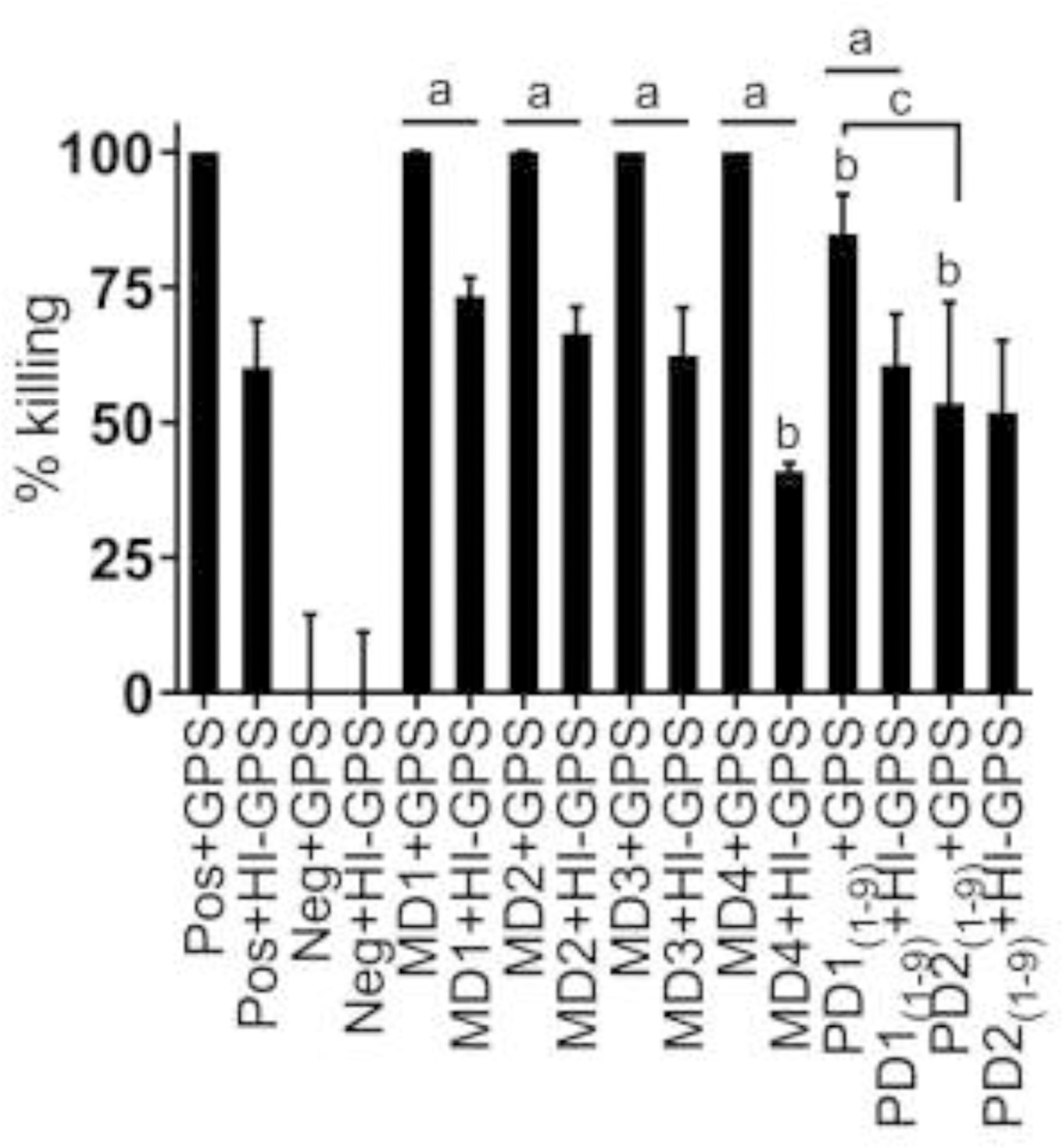
Analysis of bactericidal activity of sera from the dam and pups. Borrelia burgdorferi (Bb) B31 was incubated with sera from the dam and pups as indicated along the x axis. Complement certified guinea pig serum (GPS) or heat inactivated (HI) GPS was added as indicated. The positive (POS) and negative (NEG) controls are Borrelia burgdorferi (Bb) antibody positive or negative dog sera, respectively. The data are expressed as the percentage of cells killed (mean ± standard deviation). a Denotes significant differences between GPS and HI-GPS for each sample (P<0.01). Blood for serology was collected at different time points from the dam (designated as MD1 through MD4) and her pups (PD1 and PD2). b Denotes significant difference compared to MD1 sample (P<0.01). c Denotes significant difference when PD1(1–9) and PD2(1–9) are compared (P<0.01)
Discussion
While Ab responses to Bb infections have been studied in mature mammals including dogs (Baum et al., 2014; Oliver Jr et al., 2016), little is known about maternally derived Ab profiles in neonates born to Bb infected dams. An understanding of the origins and evolution of Ab profiles will provide information that will facilitate interpretation of diagnostic assays in pups and inform vaccination and treatment strategies. To investigate this area, Ab profiles of a symptomatic Bb C6 Ab+ Rottweiler dam and her nine pups were compared. Sera was collected from the dam on days 29, 52, 92 and 220 post-whelping and on days 29 and 52 for the pups (Fig. 1). The immunoreactivity of each serum sample against Bb B31 WCL, a panel of eight recombinant in vivo antigens (DbpA, BBA36, BBK53, BB0238, BBA73, BBR42 (OspF paralog), BBL39 (OspE paralog and VlsE), 20 different OspC type proteins and intact Bb B31 cells was assessed by immunoblot, dot-blot, ELISA and IFA.
Focusing first on the dam, IgG against the WCL (immunoblots) and in vivo antigens (dot blots and ELISA) remained high from day 29 (MD1) through day 220 (MD4) (Fig. 2). In contrast to the dam, antigen specific IgG reactivity to WCL in the pups declined between day 29 (PD1) and day 52 (PD2). ELISA analyses revealed that IgM against VlsE, OspF, BB0238 and DbpA was elevated in the dam throughout (Fig. 3; Panel B). The persistent IgM response in this dam is intriguing and raises the possibility that IgM may not be a reliable marker for early stage infection with Bb in dogs.
Fig. 3.
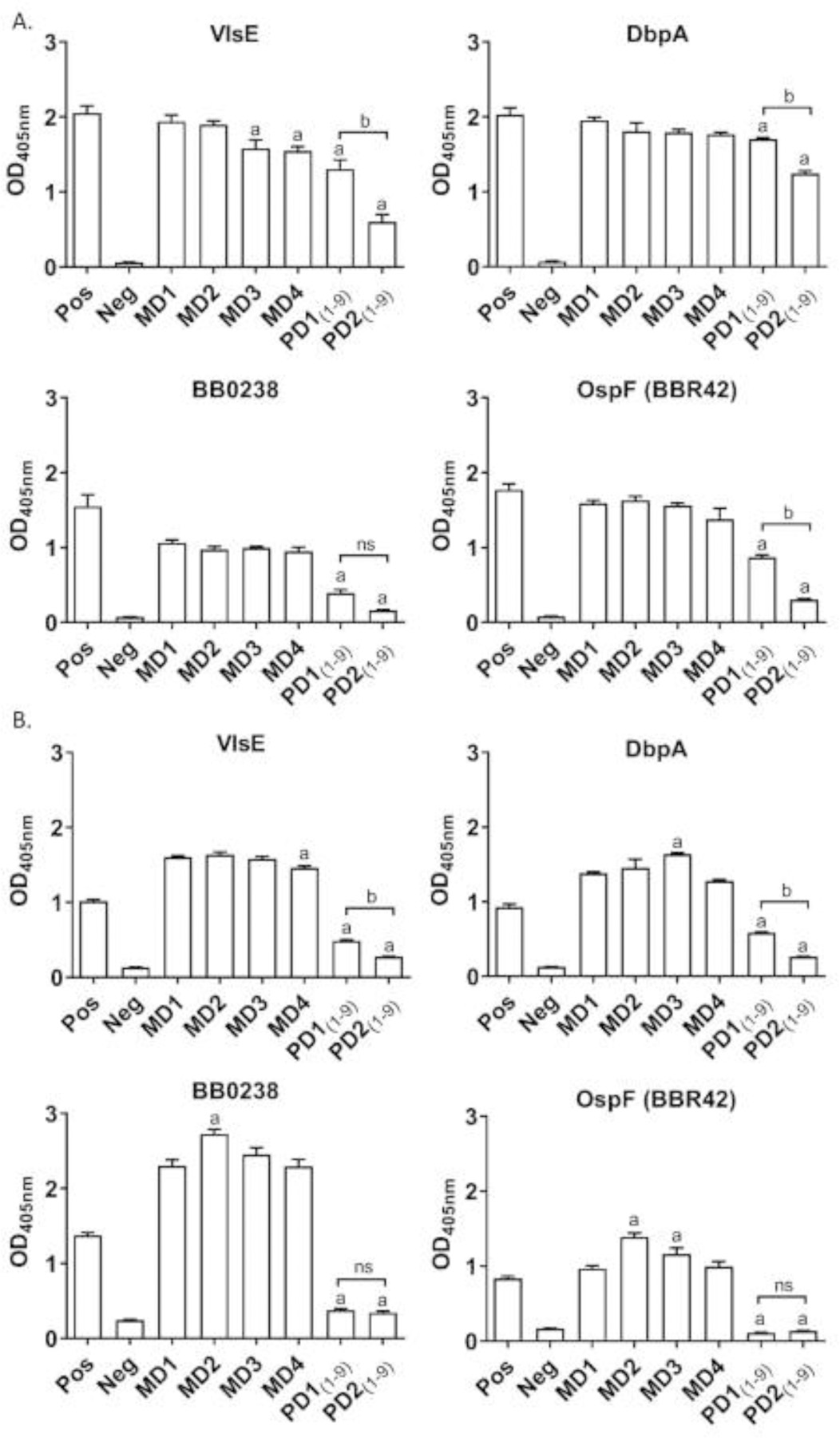
Single dilution ELISAs measuring antigen specific IgG (Panel A) and IgM (Panel B) reactivity of individual or pooled serum samples. The immobilised protein antigens are indicated above each graph and serum samples tested are indicated along the X axis. Assays were performed in triplicate and the means ± standard deviation values are shown. Blood for serology was collected at different time points from the dam (designated as MD1 through MD4) and her pups (PD1 and PD2). a Denotes values that were significantly different when compared with MD1 and b denotes a significant difference between PD1(1–9) and PD2(1–9) (P<0.01). NS indicates ‘not significant’
The majority of IgG and secretory IgM in a newborn pup is from colostrum (Chastant and Mila, 2019). IgG accounts for approximately 75% of total colostral Ig and originates from maternal blood. Colostral secretory IgM originates from plasma cells localized in mammary tissue. Direct Ab transfer during gestation is minimal due to the endotheliochorial structure of the canine placenta which limits transfer of macromolecules. The rapid decline in IgG and IgM in the pups observed here, coupled with the fact that a functional immune system does not mature in a pup until 8–12 weeks post-whelping, indicates that the Ab detected in the pups is maternally derived Ab and likely to have been obtained from colostrum.
The detection antigens used in this study included OspC (Fuchs et al., 1992), an essential plasmid-encoded (Marconi et al., 1993) virulence determinant that is upregulated by the tick bloodmeal (Schwan and Piesman, 2000) and is required for establishment of infection in mammals (Tilly et al., 2006; Earnhart et al., 2010). Phylogenetically distinct variants of OspC, referred to as OspC ‘types’, have been identified (Seinost et al., 1999a; Seinost et al., 1999b; Wang et al., 1999; Earnhart and Marconi, 2007b). OspC types are differentiated by letter or other designations (i.e., type A, type B, etc.). Ab responses to OspC in dogs and other mammals are robust (Wagner et al., 2012; Wagner et al., 2013) and largely OspC type specific (Earnhart et al., 2005; Buckles et al., 2006; Oliver Jr et al., 2016; Izac et al., 2019; Izac et al., 2020). OspC Ab analyses can be used to infer ospC genotype of the infecting strain(s) and to assess the diversity of LD spirochetes that infect an individual animal (Oliver Jr et al., 2016; Izac et al., 2019). The dam harbored Ab to five OspC types (A, D, G, K, and N) suggesting infection with a heterogenous population of strains. Coinfection with multiple strains has been reported in humans, dogs and other mammals (Seinost et al., 1999b; Rhodes et al., 2013; Golovchenko et al., 2014; Oliver Jr et al., 2016; Izac et al., 2019) and as many as 21 OspC types have been demonstrated in a single tick (Di et al., 2018). Some of the OspC types identified in the dam have been associated with invasive (disseminated) infections which could in part explain why lameness developed (Seinost et al., 1999a; Lagal et al., 2006).
The OspC Ab analyses shed insight into immune responses to vaccination in a Bb Ab positive dog. As detailed above, the dam was Ab positive for five OspC types before the first dose of subunit vaccine was delivered. According to the vaccine manufacturer (Zoetis), this vaccine contains OspA and a custom designed chimeric epitope-based protein referred to in the literature as a chimeritope (Izac et al., 2020). The chimeritope harbors two epitopes from seven different OspC types and thus contains at least 14 distinct epitopes. Four weeks after the first vaccine dose, a broad IgG response to all OspC types tested was observed. This observation, coupled with the robust nature of the anti-OspC IgG response after a single vaccine dose is suggestive of an anamnestic response. These data, while limited to the individual dog studied here, may suggest potential benefits associated with vaccination of an immunologically primed or Bb Ab positive dog with an OspC-containing subunit vaccine.
The high and persistent Ab reactivity in the dam and pups for VlsE and OspF suggests that caution should be exercised in reaching conclusions on the infection status of pups based on the results of serological assays that employ these antigens. Although the pup sera weren’t screened with commercially available LD diagnostic assays, based on the level of Ab to VlsE and OspF demonstrated here, it is likely that the pups would have been Ab positive with most tests. Hence, serological tests that screen pups born to a Bb C6 Ab positive dam for Ab to VlsE, OspF or DbpA may not be informative in dogs <8 weeks of age. If Ab testing in young dogs is considered, quantitative ELISAs that do not use VlsE (or C6), OspF or DbpA as diagnostic antigens may be preferable. An approach that measures the Ab response to antigens such as BBA36, BBK53, BB0238, BBA73 and OspE, may provide a better indication of infection status. If high levels to these antigens are observed on initial analysis and do not decrease over time, treatment of the pups might be warranted.
Neonatal puppies have low systemic immunity to infectious agents. Studies of maternally derived Ab protective immunity have focused on distemper and parvovirus (Pereira et al., 2019). The protective potential of maternally-derived Ab against Bb has not been investigated. In this study, bactericidal assays were employed as an in vitro indicator of protection against LD (Izac et al., 2017; Izac et al., 2019). Sera from the dam (MD1 through MD4) displayed high bactericidal activity against Bb (100%) in the presence of GPS. Interestingly, high level bactericidal activity (70% for MD1) when HI-GPS was added was observed, indicating that Ab triggered by infection killed through both complement-dependent and independent mechanisms. The PD1 sera from the pups displayed bactericidal activity of 85% with active complement and 55% inactive complement. However, sera collected 3 weeks later (PD2) killed equally in the presence or absence of active complement indicating a transition to an antibody mediated-complement independent mechanism over time. Complement-independent killing by maternally derived Ab is important in the context of neonates who lack a fully developed complement system.
A difficult decision for veterinarians is whether to treat or not treat a dog that is Bb C6 Ab positive but asymptomatic. For the case presented in this study, the decision to treat was made based on the observed intermittent lameness (a common manifestation of LD), positive SNAP4Dx PLUS, and the Lyme Quant C6 (IDEXX) antibody concentration of 237 U/mL (where >30 U/mL is considered to be clinically relevant). These findings support the conclusion that the intermittent lameness was of infectious etiology. Consistent with recommended treatment protocols for pregnant dogs (Littman et al., 2018), amoxicillin was prescribed for the dam. However, Ab levels remained elevated and steady 230 days after initiation of treatment.
There is uncertainty regarding the clinical significance of Bb C6 Ab positive test results post-treatment, as it is generally assumed that dogs remain positive long after treatment. However, controlled laboratory studies suggest otherwise as Quant C6 Ab levels in antibiotic treated laboratory dogs drop below baseline within approximately 5 weeks of the end of therapy (Wagner et al., 2015). As detailed here, Ab reactivity in this dog remained high 7 months after treatment (the last time point tested). There are several possible explanations for persistent Ab, some of which are summarized here. Firstly, cases refractory to treatment have been reported in dogs (Appel et al., 1993; Straubinger et al., 1997), humans (Shen et al., 2010; Strle et al., 2017) and non-human primates (Embers et al., 2012). Secondly, compliance with antibiotic treatment can be a factor. Thirdly, the possibility of long-lived plasma cells cannot be excluded. Lastly, it is important to note that successful treatment does not prevent future infection. Hence, reexposure prior to vaccination could have occurred and stimulated Ab production. Presently there is no definitive answer for the best course of action for dogs that remain Bb C6 Ab positive for an extended time.
This study is among the first to investigate the temporal nature and antigen specificity of maternally derived Ab in dogs. Since this study focused on a single case, our findings require confirmation using larger numbers of patients. Nonetheless, this study can serve as a starting point for future analyses of maternally derived Ab in Bb infected dogs.
Conclusions
Immunological analyses demonstrated similar Ab reactivity profiles in sera from a dam and her nine pups against a panel of Bb antigens and WCL. This, coupled with a rapid decline in antigen specific Ab levels in the pups, suggest the Abs were of maternal origin and likely to have been obtained from colostrum. These maternally-derived Ab in the pup sera retained bactericidal activity and killed in part through a complement-independent mechanism. OspC Ab analyses suggest that the dam had been infected with multiple Bb strains that produce different OspC types. The pronounced expansion of the Ab response to OspC after a single dose of a vaccine containing an OspC based chimeritope antigen (Izac et al., 2020), provides suggestive evidence for an anamnestic response upon vaccination in this dog. Lastly, the persistence of Ab to widely used diagnostic antigens including VlsE and OspF long after maternally-derived Ab to other antigens declined suggests that serological tests for Bb should be interpreted with caution in pups delivered by an Bb infected or Ab positive dam.
Highlights.
This is the first profiling of Borrelia burgdorferi specific maternal antibody in pups
Sera from dam and pups had similar immunoreactivity patterns and were bactericidal
Antibodies against diagnostic antigens (VlsE, OspF) persisted longer than other proteins
Pup antibodies were derived from colostrum and not an ongoing infection
Vaccination triggered an immune memory response to diverse OspC types
Acknowledgements
This study was supported, in part, by grants, donations or funds from the Steven and Alexandra Cohen Foundation2, Virginia Commonwealth University Medical Center, Bay Area Lyme Foundation, the Christian Family and the National Institutes of Health (1R01AI141801). We thank the clinicians and staff at Glengarry Animal Hospital (Alexandria, ON K0C) for their dedication and contributions to this study. We thank Dr. Edward B. Breitschwerdt (Comparative Medicine Institute, College of Veterinary Medicine, North Carolina State University, USA) for providing control serum samples and members of the Marconi laboratory for valuable discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
RT Marconi is an inventor of and has a financial interest in the canine Bb vaccine, Vanguard crLyme (Zoetis). RTM is a paid speaker and consultant for Zoetis. K Stasiak is a paid employee of Zoetis. None of the authors has any other financial or personal relationships that could inappropriately influence or bias the content of the paper.
See: Companion Animal Parasite Council Parasite Prevalence Maps. https://capcvet.org/maps/#2019/all/lyme-disease/dog/united-states/ (Accessed 2 July 2020).
See: Steven and Alexandra Cohen Foundation. https://www.steveandalex.org/ (Accessed 2 July 2020)
References
- Adeolu M, Gupta RS, 2014. A phylogenomic and molecular marker based proposal for the division of the genus Borrelia into two genera: the emended genus Borrelia containing only the members of the relapsing fever Borrelia, and the genus Borreliella gen. nov. containing the members of the Lyme disease Borrelia (Borrelia burgdorferi sensu lato complex). Antonie Van Leeuwenhoek 105, 1049–1072. [DOI] [PubMed] [Google Scholar]
- Antonara S, Chafel RM, Lafrance M, Coburn J, 2007. Borrelia burgdorferi adhesins identified using in vivo phage display. Molecular Microbiology 66, 262–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel MJ, Allan S, Jacobson RH, Lauderdale TL, Chang YF, Shin SJ, Thomford JW, Todhunter RJ, Summers BA, 1993. Experimental Lyme disease in dogs produces arthritis and persistent infection. Journal of Infectious Diseases 167, 651–664. [DOI] [PubMed] [Google Scholar]
- Barbour AG, Hayes SF, 1986. Biology of Borrelia species. Microbiological Reviews 50, 381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum E, Grosenbaugh DA, Barbour AG, 2014. Diversity of antibody responses to Borrelia burgdorferi in experimentally infected beagle dogs. Clinical and Vaccine Immunology 21, 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum E, Hue F, Barbour AG, 2012. Experimental infections of the reservoir species Peromyscus leucopus with diverse strains of Borrelia burgdorferi, a Lyme disease agent. MBio 3, e00434–00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, Cameron DJ, Ziegler JL, Barbour AG, Burgdorfer W, Edelman R, Kaslow RA, 1983. Spirochetes isolated from the blood of two patients with Lyme disease. New England Journal of Medicine 308, 740–742. [DOI] [PubMed] [Google Scholar]
- Brown EL, Guo BP, O’Neal P, Hook M, 1999. Adherence of Borrelia burgdorferi. Identification of critical lysine residues in DbpA required for decorin binding. Journal of Biological Chemistry 274, 26272–26278. [DOI] [PubMed] [Google Scholar]
- Buckles EL, Earnhart CG, Marconi RT, 2006. Analysis of antibody response in humans to the type A OspC loop 5 domain and assessment of the potential utility of the loop 5 epitope in Lyme disease vaccine development. Clinical and Vaccine Immunology 13, 1162–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP, 1982. Lyme disease-a tick-borne spirochetosis? Science 216, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Chastant S, Mila H, 2019. Passive immune transfer in puppies. Animal Reproductive Science. Reprod. Sci 207, 162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di L, Wan Z, Akther S, Ying C, Larracuente A, Li L, Di C, Nunez R, Cucura DM, Goddard NL, Krampis K, Qiu WG, 2018. Genotyping and quantifying Lyme pathogen strains by deep sequencing of the outer surface protein C (ospC) locus. Journal of Clinical Microbiology [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Buckles EL, Dumler JS, Marconi RT, 2005. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infection and Immunity 73, 7869–7877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Leblanc DV, Alix KE, Desrosiers DC, Radolf JD, Marconi RT, 2010. Identification of residues within ligand-binding domain 1 (LBD1) of the Borrelia burgdorferi OspC protein required for function in the mammalian environment. Molecular Microbiology 76, 393–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Marconi RT, 2007a. Construction and analysis of variants of a polyvalent Lyme disease vaccine: approaches for improving the immune response to chimeric vaccinogens. Vaccine 25, 3419–3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnhart CG, Marconi RT, 2007b. OspC phylogenetic analyses support the feasibility of a broadly protective polyvalent chimeric Lyme disease vaccine. Clinical and Vaccine Immunology 14, 628–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Eisen L, Beard CB, 2016. County-Scale Distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the Continental United States. Journal of Medical Entomology 53, 349–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen RJ, Kugeler KJ, Eisen L, Beard CB, Paddock CD, 2017. Tick-Borne Zoonoses in the United States: Persistent and Emerging Threats to Human Health. ILAR J 58, 319–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Embers ME, Barthold SW, Borda JT, Bowers L, Doyle L, Hodzic E, Jacobs MB, Hasenkampf NR, Martin DS, Narasimhan S, Phillippi-Falkenstein KM, Purcell JE, Ratterree MS, Philipp MT, 2012. Persistence of Borrelia burgdorferi in rhesus macaques following antibiotic treatment of disseminated infection. PLoS One 7, e29914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, Lathigra R, White O, Ketchum KA, Dodson R, Hickey EK, Gwinn M, Dougherty B, Tomb JF, Fleischmann RD, Richardson D, Peterson J, Kerlavage AR, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams MD, Gocayne J, Weidman J, Utterback T, Watthey L, McDonald L, Artiach P, Bowman C, Garland S, Fuji C, Cotton MD, Horst K, Roberts K, Hatch B, Smith HO, Venter JC, 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390, 580–586. [DOI] [PubMed] [Google Scholar]
- Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, Soutschek E, 1992. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Molecular Microbiology 6, 503–509. [DOI] [PubMed] [Google Scholar]
- Golovchenko M, Sima R, Hajdusek O, Grubhoffer L, Oliver JH Jr., Rudenko N, 2014. Invasive potential of Borrelia burgdorferi sensu stricto ospC type L strains increases the possible disease risk to humans in the regions of their distribution. Parasites and Vectors 7, 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groshong AM, Fortune DE, Moore BP, Spencer HJ, Skinner RA, Bellamy WT, Blevins JS, 2014. BB0238, a presumed tetratricopeptide repeat-containing protein, is required during Borrelia burgdorferi mammalian infection. Infection and Immunity, 82, 4292–4306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson JM, Burgess EC, Wachal MD, Steinberg H, 1993. Intrauterine transmission of Borrelia burgdorferi in dogs. American Journal of Veterinary Research 54, 882–890. [PubMed] [Google Scholar]
- Hellwage J, Meri T, Heikkila T, Alitalo A, Panelius J, Lahdenne P, Seppala IJT, Meri S, 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. Journal of Biological Chemistry 276, 8427–8435. [DOI] [PubMed] [Google Scholar]
- Heyman P, Cochez C, Hofhuis A, van der Giessen J, Sprong H, Porter SR, Losson B, Saegerman C, Donoso-Mantke O, Niedrig M, Papa A, 2018. A clear and present danger: tick-borne diseases in Europe. Expert Review of Anti-Infective Therapy 8, 33–50. [DOI] [PubMed] [Google Scholar]
- Hughes JL, Nolder CL, Nowalk AJ, Clifton DR, Howison RR, Schmit VL, Gilmore RD Jr., Carroll JA, 2008. Borrelia burgdorferi surface-localized proteins expressed during persistent murine infection are conserved among diverse Borrelia spp. Infection and Immunity 76, 2498–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer R, Caimano MJ, Luthra A, Axline D Jr., Corona A, Iacobas DA, Radolf JD, Schwartz I, 2015. Stage-specific global alterations in the transcriptomes of Lyme disease spirochetes during tick feeding and following mammalian host adaptation. Molecular Microbiology 95, 509–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izac JR, Camire AC, Earnhart CG, Embers ME, Funk RA, Breitschwerdt EB, Marconi RT, 2019. Analysis of the antigenic determinants of the OspC protein of the Lyme disease spirochetes: Evidence that the C10 motif is not immunodominant or required to elicit bactericidal antibody responses. Vaccine 37, 2401–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izac JR, O’Bier NS, Oliver LD Jr., Camire AC, Earnhart CG, LeBlanc Rhodes DV, Young BF, Parnham SR, Davies C, Marconi RT, 2020. Development and optimization of OspC chimeritope vaccinogens for Lyme disease. Vaccine 38, 1915–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izac JR, Oliver LD Jr., Earnhart CG, Marconi RT, 2017. Identification of a defined linear epitope in the OspA protein of the Lyme disease spirochetes that elicits bactericidal antibody responses: Implications for vaccine development. Vaccine 35, 3178–3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagal V, Portnoi D, Faure G, Postic D, Baranton G, 2006. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes and Infection 8, 645–652. [DOI] [PubMed] [Google Scholar]
- Littman MP, Gerber B, Goldstein RE, Labato MA, Lappin MR, Moore GE, 2018. ACVIM consensus update on Lyme borreliosis in dogs and cats. Journal of Veterinary Internal Medicine 32, 887–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marconi RT, Samuels DS, Garon CF, 1993. Transcriptional analyses and mapping of the ospC gene in Lyme disease spirochetes. Journal of Bacteriology 175, 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DP, Oliver LD Jr., Tegels BK, Reed LA, O’Bier NS, Kurniyati K, Faust LA, Lawson CK, Allard AM, Caimano MJ, Marconi RT, 2016. The Treponema denticola FhbB Protein Is a Dominant Early Antigen That Elicits FhbB Variant-Specific Antibodies That Block Factor H Binding and Cleavage by Dentilisin. Infection and Immunity 84, 2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, Mead PS, 2015. Incidence of Clinician-Diagnosed Lyme Disease, United States, 2005–2010. Emerging Infectious Diseases 21, 1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver LD Jr, Earnhart CG, Virgina-Rhodes D, Theisen M, Marconi R, 2016. Antibody profiling of canine IgG responses to the OspC protein of the Lyme disease spirochetes supports a multivalent approach in vaccine and diagnostic assay development. The Veterinary Journal, 27–33. [DOI] [PubMed] [Google Scholar]
- Pereira M, Valerio-Bolas A, Saraiva-Marques C, Alexandre-Pires G, Pereira da Fonseca I, Santos-Gomes G, 2019. Development of Dog Immune System: From in Uterus to Elderly. Veterinary Science 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DV, Earnhart CG, Mather TN, Meeus PF, Marconi RT 2013. Identification of Borrelia burgdorferi ospC genotypes in canine tissue following tick infestation: Implications for Lyme disease vaccine and diagnostic assay design. The Veterinary Journal. 198, 412–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwan TG, Piesman J, 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. Journal of Clinical Microbiology 38, 382–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinost G, Dykhuizen DE, Dattwyler RJ, Golde WT, Dunn JJ, Wang IN, Wormser GP, Schriefer ME, Luft BJ, 1999a. Four clones of Borrelia burgdorferi sensu stricto cause invasive infection in humans. Infection and Immunity 67, 3518–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seinost G, Golde WT, Berger BW, Dunn JJ, Qiu D, Dunkin DS, Dykhuizen DE, Luft BJ, Dattwyler RJ, 1999b. Infection with multiple strains of Borrelia burgdorferi sensu stricto in patients with Lyme disease. Archives of Dermatology. 135, 1329–1333. [DOI] [PubMed] [Google Scholar]
- Shen S, Shin JJ, Strle K, McHugh G, Li X, Glickstein LJ, Drouin EE, Steere AC, 2010. Treg cell numbers and function in patients with antibiotic-refractory or antibiotic-responsive Lyme arthritis. Arthritis and Rheumatology 62, 2127–2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straubinger RK, Straubinger AF, Summers BA, Jacobson RH, Erb HN, 1998. Clinical manifestations, pathogenesis, and effect of antibiotic treatment on Lyme borreliosis in dogs. Wiener Klinische Wochenschrift 110, 874–881. [PubMed] [Google Scholar]
- Straubinger RK, Summers BA, Chang YF, Appel MJ, 1997. Persistence of Borrelia burgdorferi in experimentally infected dogs after antibiotic treatment. Journal of Clinical Microbiology 35, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strle K, Sulka KB, Pianta A, Crowley JT, Arvikar SL, Anselmo A, Sadreyev R, Steere AC, 2017. T-Helper 17 Cell Cytokine Responses in Lyme Disease Correlate With Borrelia burgdorferi Antibodies During Early Infection and With Autoantibodies Late in the Illness in Patients With Antibiotic-Refractory Lyme Arthritis. Clinical Infectious Diseases 64, 930–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers BA, Straubinger AF, Jacobson RH, Chang YF, Appel MJ, Straubinger RK, 2005. Histopathological studies of experimental lyme disease in the dog. Journal of Comparative Pathology 133, 1–13. [DOI] [PubMed] [Google Scholar]
- Sykes RA, Makiello P, 2017. An estimate of Lyme borreliosis incidence in Western Europe. Journal of Public Health (Oxf) 39, 74–81 [DOI] [PubMed] [Google Scholar]
- Tilly K, Krum JG, Bestor A, Jewett MW, Grimm D, Bueschel D, Byram R, Dorward D, Vanraden MJ, Stewart P, Rosa P, 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infection and Immunity 74, 3554–3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B, Freer H, Rollins A, Garcia-Tapia D, Erb HN, Earnhart C, Marconi R, Meeus P, 2012. Antibodies to Borrelia burgdorferi OspA, OspC, OspF, and C6 antigens as markers for early and late infection in dogs. Clinical and Vaccine Immunology 19, 527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B, Goodman LB, Rollins A, Freer HS, 2013. Antibodies to OspC, OspF and C6 antigens as indicators for infection with Borrelia burgdorferi in horses. Equine Veterinary Journal 45, 533–537. [DOI] [PubMed] [Google Scholar]
- Wagner B, Johnson J, Garcia-Tapia D, Honsberger N, King V, Strietzel C, Hardham JM, Heinz TJ, Marconi RT, Meeus PF, 2015. Comparison of effectiveness of cefovecin, doxycycline, and amoxicillin for the treatment of experimentally induced early Lyme borreliosis in dogs. BMC Veterinary Research 11, 163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang IN, Dykhuizen DE, Qiu W, Dunn JJ, Bosler EM, Luft BJ, 1999. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics 151, 15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JR, Norris SJ, 1998. Genetic variation of the Borrelia burgdorferi gene vlsE involves cassette-specific, segmental gene conversion. Infection and Immunity 66, 3698–3704. [DOI] [PMC free article] [PubMed] [Google Scholar]


