Abstract
Substantial advances have been made recently in the pathobiology of pituitary tumors. Similar to many other endocrine tumors, over the last few years we have recognized the role of germline and somatic mutations in a number of syndromic or nonsyndromic conditions with pituitary tumor predisposition. These include the identification of novel germline variants in patients with familial or simplex pituitary tumors and establishment of novel somatic variants identified through next generation sequencing. Advanced techniques have allowed the exploration of epigenetic mechanisms mediated through DNA methylation, histone modifications and noncoding RNAs, such as microRNA, long noncoding RNAs and circular RNAs. These mechanisms can influence tumor formation, growth, and invasion. While genetic and epigenetic mechanisms often disrupt similar pathways, such as cell cycle regulation, in pituitary tumors there is little overlap between genes altered by germline, somatic, and epigenetic mechanisms. The interplay between these complex mechanisms driving tumorigenesis are best studied in the emerging multiomics studies. Here, we summarize insights from the recent developments in the regulation of pituitary tumorigenesis.
Keywords: pituitary neoplasm, pituitary tumorigenesis, pituitary adenoma, PitNET, pituitary tumor
Graphical Abstract
Graphical Abstract.
Essential Points.
An increasing number of genes with germline mutations are known now to be associated with pituitary tumors, some causing syndromic disease while others isolated pituitary adenomas.
Gain-of-function somatic mutations are common in somatotropinomas in the GNAS gene and in corticotropinomas in USP8.
Other, less common somatic variants recently identified through next generation sequencing need to be confirmed in independent cohorts and elucidated through functional studies in the future.
Epigenetic modifications (DNA methylation, histone modification, and noncoding RNAs) can greatly influence tumorigenesis and tumor characteristics such as subtype differentiation and local invasion.
An integrated multiomics approach to characterize genetic and epigenetic pathways allows better understanding the molecular mechanisms that underlie pituitary tumorigenesis within and across the various subtypes and may lead to the identification of better prognostic factors.
Pituitary tumors (PTs) are common intracranial neoplasms with an overall prevalence estimated at 17% in a systematic review using post-mortem (14%) and radiologic studies (22%) (1). While the majority of these would represent incidentalomas and usually of little clinical significance, the prevalence of clinically-presenting adenomas is higher in epidemiological studies conducted over the last 10–15 years compared to older data, probably due to better diagnostic modalities, with 68–110 PTs clinically-presenting cases identified per 100 000 inhabitants (2–8). Using incidence data from population-based state cancer registries in the United States, the age-adjusted annual incidence rate of PTs increases from 2.52 in 2004 to 3.13 in 2009 (per 100 000 subjects) (9).
Tumors of the anterior pituitary usually do not metastasize, and hence have been referred to as “adenomas.” However, as a significant minority can show clinically aggressive behavior and similar characteristics to true metastasizing lesions (10), the term “pituitary neuroendocrine tumor” or “PitNET” has been coined recently, receiving mixed acceptance (11–14). Here we use the term pituitary tumor representing tumors arising from the potentially hormone-producing cells of the anterior pituitary.
Pituitary tumors are clinically categorized by their hormone-secreting characteristics, with over-secretion of growth hormone (GH), prolactin, adrenocorticotropic hormone (ACTH), thyroid stimulating hormone (TSH), luteinizing hormone (LH) and follicle-stimulating hormone (FSH) or clinically nonfunctioning tumors. Histological characterization has been based on immunohistochemical staining of pituitary hormones, with more recently transcription factors (PIT1 for GH, prolactin and TSH lineages, SF1 for gonadotroph lineages and TPIT for ACTH lineage) being added to the classification (15). Therefore, the final diagnosis relies on the combination of the clinical picture (excess hormone secreting or not) and histological assessment (hormone and transcription factor immunostaining (16)). Molecular characterization based on methylation patterns, gene expression and DNA mutations may add further granularity to the assessment of these tumors in the future (17).
Over the last decade, we have witnessed major advances in the biology of pituitary tumors, with the identification of several germline and somatic mutations and epigenetic mechanisms, such as DNA methylation, histone modifications, and noncoding RNAs. In this review, genetic and epigenetic mechanisms contributing to pituitary tumorigenesis will be succinctly summarized with an emphasis on novel insights over the last ten years.
Genetic Mechanisms Of Tumorigenesis
Germline mutations driving tumorigenesis
Pituitary tumors associated with germline mutations may present as part of a syndromic disease or in isolation (Fig. 1, 2). The nonsyndromic group consists of patients in whom no other organ than the pituitary is involved, and is known as familial isolated pituitary adenoma (FIPA) (18). We summarize here the key genetic aspects, while refer to other reviews on the detailed clinical characteristics of these diseases (19, 20).
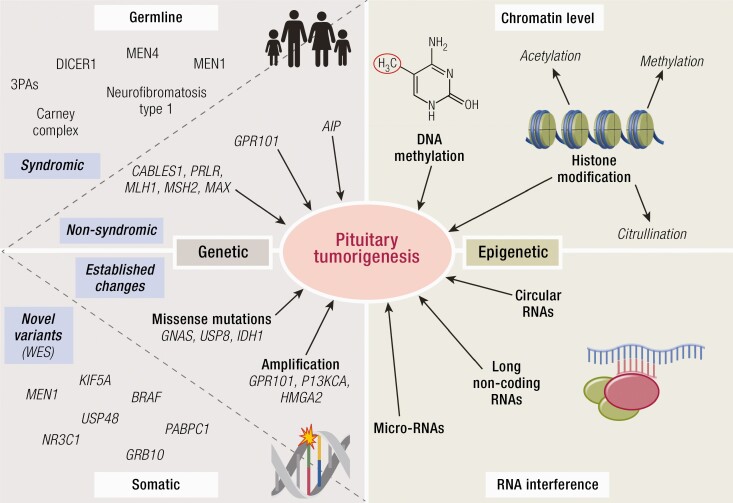
Figure 1.
Genetic and epigenetic mechanisms of pituitary tumorigenesis. Genetic mechanisms may be secondary to germline or somatic mutations, while epigenetic mechanisms can be mediated at the chromatin level (such as in the case of DNA methylation or histone modifications) or via noncoding RNAs.
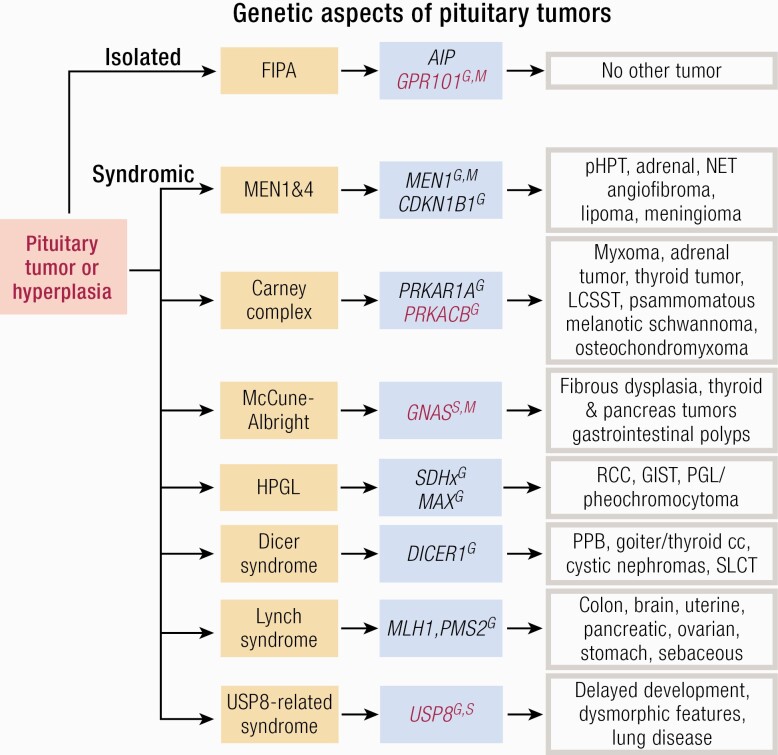
Figure 2.
Germline or mosaic mutations causing pituitary tumors. Pituitary tumors presenting in isolation (familial isolated pituitary adenoma, FIPA) or part of a tumor syndrome. Hyperplasia has been described in Carney complex, McCune-Albright syndrome, 20% of XLAG cases and rarely in AIP mutation positive cases. Genes marked with red letter types are oncogenes, while the black ones are tumor suppressor genes. Abbreviations: G, germline; GIST, gastrointestinal stromal tumor; HPGL, hereditary paraganglioma; LCCSCT, large-cell calcifying Sertoli cell tumors; M, mosaic; NET, neuroendocrine tumor; pHPT, primary hyperparathyroidism; PPB, pleuropulmonary blastoma; RCC, renal cell carcinoma; S, somatic; SLCT, Sertoli–Leydig cell tumor.
Familial isolated pituitary adenoma (FIPA).
The prevalence of FIPA among all PT patients was found to be 1.9% to 3.8% in pituitary referral centers (2, 21). The first identified gene underlying FIPA is AIP (22), which accounts for 10% to 20% of FIPA kindreds (23, 24). Duplication of GPR101 in X-linked acrogigantism (XLAG), although mostly identified as de novo mutation, has also been described in families (three kindreds described so far in the literature (25–28)). However, patients with a suggestive family history with no known genetic cause form the majority of patients with FIPA.
AIP mutation-positive pituitary tumors.
The AIP gene maps to chromosome 11q13.2, incidentally close to the locus of the MEN1 gene, although there are no sequence similarities between the two genes. It encodes a ubiquitously expressed co-chaperone protein with multiple partners, but currently its role in pituitary tumorigenesis is incompletely understood. It behaves as a tumor suppressor with a unique primarily somatotroph/lactotroph specificity, although global lack of AIP is lethal in mouse, Drosophila and C. elegans studies (29–31). The cAMP/protein kinase A/phosphodiesterase pathway plays a key role in somatotroph physiology and acromegaly-related genetic syndromes (Fig. 3). Not surprisingly, therefore, a link has been found between this pathway and AIP at several levels: at the inhibitory Gαi-2 protein (32, 33), at cAMP (34), at phosphodiesterase 4A (35-37), at protein kinase A (38, 39), downstream of somatostatin receptors, and Zac1 (40, 41) levels. AIP has also found interact and inhibit the endoplasmatic reticulum calcium channel ryanodine receptor in C. elegans (31), another pathway closely linked with hormone release, with somatic variants identified in calcium-related pathways in somatotropinomas (42, 43). Given the particular role of RET in somatotroph cells (44), the link with RET (45) could be a link to the specific role of AIP in somatotrophs. Increased GH release has been found in AIP-disrupted cells, probably associated with the increased STAT3 phosphorylation (41, 46), while an altered microenvironment may also explain the aggressive phenotype of some of these tumors (47). However, the role of other AIP partners—nuclear receptors (AHR, ER, GR, PPRα, TRβ1), mitochondrial proteins, survivin (reviewed in (48)) or BCL6 (49) in the pituitary-specific effects is unclear.
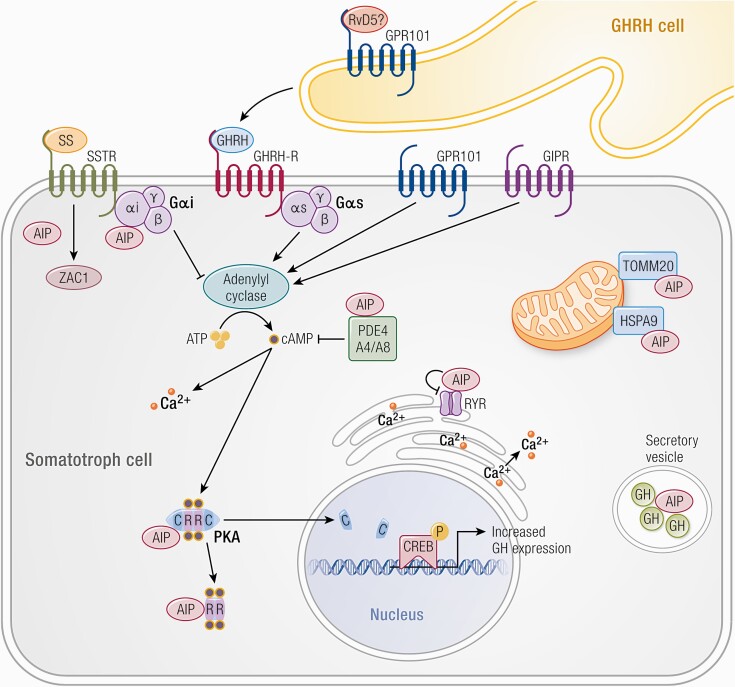
Figure 3.
Tumorigenic mechanism in somatotroph cells. cAMP-associated pathways are key for somatotroph tumorigenesis. GHRH released by the hypothalamus interacts with its receptor (GHRH-R) on the somatotroph cell membrane to increase activation of adenylyl cyclase through Gαs. Consequent increased cAMP production leads to the dissociation of the regulatory subunits (R) of protein kinase A (PKA) from the catalytic subunits (C), which then translocate to the nucleus and phosphorylate CREB (cAMP response element) and other targets, eventually leading to increased GH expression and cell proliferation. Mosaic (McCune-Albright syndrome) or somatic activating mutations in GNAS (coding for Gαs) lead to upregulation of the cAMP pathway. In Carney complex, increased PKA activity, either due to the inhibitory action of the regulatory subunit PRKAR1A, or increased catalytic subunit activity (PRKACB) leads to tumorigenesis. Loss of AIP has been shown to increase cAMP signaling through (1) decreased expression of the G inhibitory protein Gαi-2, which mediates the inhibitory effects of somatostatin (SS) on adenylyl cyclase. AIP deficiency is associated with reduced Gαi-2 expression in human and mouse GH-PTs (2, 32) an interaction with phosphodiesterases type 4 (PDE4) (36); expression of type 4 phosphodiesterase is lower in AIP-mutated GH-PTs compared to sporadic GH-PTs (37) and AIP mutations disrupt the interaction of AIP with PDE4A5 in GH3 cell (3, 35) interaction of AIP with members of the PKA complex (38, 39). AIP deficiency results in reduced ZAC1 levels (40, 41) and is associated with mitochondrial proteins TOMM20 and HSPA9 (39, 69), the endoplasmatic reticulum calcium channel RYR (31) and with secretory vesicles (35), but the exact mechanisms as to how these interactions might lead to tumorigenesis are unclear. GPR101 is Gsα-coupled constitutively active receptor leading to increased cAMP signaling. The mechanism of GPR101-related tumorigenesis may occur via a dual mechanism: hypothalamic dysregulation as elevated GHRH levels can be measured in some patients, while there may be a direct pituitary action due to increased GPR101 expression on pituitary cells. Recently an endogenous ligand has been identified, the lipid mediator Resolvin D5 (RvD5), the role of this mediator in the regulation of the GH axis and its levels in patients with XLAG is currently unknown. Ectopic expression of GIPR may also lead to an activated cAMP pathway (70–72).
Pituitary tumors are significantly more frequent in global heterozygous Aip+/- mice compared to wild-type mice (50). The majority of the tumors developed were GH-PTs, which were negative for AIP immunostaining. This predisposition to developing GH-PTs in the heterozygous state with loss of expression of AIP in the tumor is similar to the human clinical phenotype. Interestingly, full penetrance is achieved in global heterozygous Aip+/- mice by 15 months in contrast to the 23% penetrance observed in a representative, large, thoroughly screened human family (51). The discrepancy between the mouse and human phenotype is likely to be due to genetic variability in humans. Supporting this hypothesis is that fact that, although using the same mouse line, phenotypic variability has been noted by another laboratory in the penetrance of pituitary tumors in global heterozygous Aip+/- model with no pituitary tumors detected at 12 months of age (52), in contrast to Raitila et al where pituitary tumor incidence was greater than 80% by 12 months. Homozygous somatotroph-specific knockout showed over 80% penetrance of GH-PTs by 10 months (53), with animals showing features similar to acromegaly (increased body size and elevated serum GH and insulin-like growth factor 1). High penetrance has also been observed in pituitary-specific Aip-knockout both in heterozygote and homozygote cases by 12–15 months (47, 54).
Patients with germline AIP mutations have a clinical phenotype that is distinct from sporadic tumors: they show earlier disease onset and are diagnosed at a younger age, are larger, and are predominantly sparsely-granulated GH-secreting tumors, locally invasive and develop apoplexy (23, 24, 35, 55–57). These tumors are less likely to respond to first-generation somatostatin analogues (SSA) (35, 55, 58), while some cases have shown responses to the second-generation SSA pasireotide (59).
Families with AIP mutations show an incomplete penetrance of around 15% to 30% (22, 24, 51, 55). This probably explains why 50% to 70% of the identified AIP mutation positive kindreds do not have a known family history (simplex cases) (24, 55), while de novo mutations (60) are exceedingly rare. Genetic screening can identify carrier family members, and clinical screening leads to a surprisingly high percentage of earlier recognition of clinically relevant disease (24).
The prevalence of AIP mutations in patients with sporadic pituitary tumors varies significantly depending on age of disease onset, family history and tumor type (61). It is highest among patients with gigantism, 29% to 41% (62, 63), 12% in sporadic patients with age at diagnosis less than 30 years (64), and 3.6% of unselected population of a pituitary referral center (65). However, other studies found no pathogenic or likely pathogenic mutations, for example in a cohort of 127 adult PT patients less than 40 years at diagnosis (66), or in a group of 50 of SSA-resistant adult patients with acromegaly (58). Current recommendations suggest screening for AIP mutations in patients with no syndromic features and any of the following criteria: (1) childhood onset PT, (2) familial PT, or (3) a macroadenoma at age 30 years or younger (18, 67, 68).
X-linked acrogigantism (XLAG).
XLAG is an extremely rare condition showing early-onset gigantism secondary to germline or somatic microduplication of the Xq26.3 chromosomal region containing the GPR101 gene (25).
GPR101 is an orphan G-protein coupled receptor, with constitutive activity of the human protein. GPR101 is predicted to couple to the Gα stimulatory protein (73), which activates adenylyl cyclase and increases cAMP production. Indeed, culture of pituitary tissue of an XLAG patient showed increased GH and prolactin release (74), and heterologous in vitro overexpression of human GPR101 leads to increased cAMP signaling in HEK293 cells (73) and GH3 cells (25). GPR101 is expressed in the normal human hypothalamus and in the embryonic and pubertal pituitary, but less in prepubertal and adult pituitary, suggesting a role in development and at the peak of growth (75). GPR101 is overexpressed in XLAG pituitary tumors, while expression in sporadic GH-PTs is low (25). The hypothalamic expression could explain the increased GHRH levels measured in some of the patients, and points to the role of hypothalamic dysregulation in this disease (74).
Recently, a lipid mediator n-3 docosapentaenoic-derived resolvin D5 (7S,17S-dihydroxy-8E,10Z,13Z,15E,19Z-docosapentaenoic acid, RvD5n-3 DPA) has been shown to activate GPR101, representing a potential endogenous ligand to this previously orphan receptor (76). This bioactive lipid mediator enzymatically derived from essential fatty acid n-3 docosapentaenoic acid is a member of the specialized proresolving mediators. It plays a role in the regulation of leukocytes and macrophages, intestinal barrier protection, and in joint inflammation. It is currently unknown whether this ligand has a role in the physiological regulation of the GH axis and how it behaves in patients with XLAG. A previously suggested putative ligand for GPR101 is GnRH- (1–5), a short fragment of GnRH. GnRH- (1–5) has been suggested to activate GPR101 to increase epidermal growth factor release and increase MMP-9 enzymatic activity in endometrial cancer cell lines, facilitating cellular migration and leading to an increase in cellular invasion (77). Similar proproliferation and proinvasive effects may underlie pituitary tumorigenesis in XLAG.
The majority of the reported XLAG cases are sporadic (26 patients) due to de novo mutations, while three kindreds have been reported in the literature to date (25–28, 63, 74, 78–80). The majority of the patients are females (24/33, 73%), with all female cases showing de novo germline duplication, making transmission to future generations possible (81). In males, XLAG is secondary to somatic mosaicism in simplex cases described so far (78, 82), or to germline duplications inherited from an affected mother (full penetrance was seen in all three kindreds) (25–28). Two of 3 familial female patients described so far are mothers of affected sons and have de novo mutations; all 4 familial male patients are affected sons who have inherited the duplication from their mothers (25–28). It is unclear why de novo germline mutations have not been described in males so far.
Patients with XLAG have a distinct clinical phenotype with the onset of symptoms and diagnosis in early childhood (25, 63). Clinical presentation in most cases is due to accelerated growth velocity in infancy or early childhood (<5 years of age; most commonly during the first 2 years of life) (63), with acromegaly-type features such as acral enlargement and coarse facial features, signs which are often not seen in patients with other types of childhood-onset acromegaly (28). The majority (~80%) of the patients present with a GH and prolactin-secreting macroadenoma (25, 63). Some of the patients have a normal-sized pituitary gland or diffusely enlarged histologically-proven pituitary hyperplasia (63, 78, 83), despite very high levels of GH and IGF-1, with or without prolactin elevation. A prenatally diagnosed familial XLAG case showed a pituitary tumor on MRI already at 3 weeks of age, associated with high prolactin and growth hormone (26, 27). Given the full penetrance observed so far in familial XLAG, preimplantation diagnosis or prenatal screening is worth considering in affected mothers, and theoretically in female fetuses of affected males (although male-to-female transmission has not yet been demonstrated). Histological features show mixed GH-PRL tumors with a mixed sparsely and densely granulated pattern (27, 28, 63, 79). No other PT type has been associated with GPR101 duplications (84). Missense variants of GPR101 do not seem to be associated with PTs (63, 85, 86).
Novel germline variants.
Comprehensive reviews of clinical and genetic aspects of germline syndromes are available elsewhere: here we briefly summarize here the more recent developments (Fig. 1).
The vast majority of FIPA does not have an established genetic basis: approximately 85% of the FIPA cohort were negative for AIP mutations in a study (23). Consequently, there has been significant interest in identifying other germline variants, which may predispose to familial tumors (Table 1), but none of the published data convincingly supports the established presence of a further gene causing FIPA.
Table 1.
Suggested germline variants, which may underlie FIPA or GH excess. Gene locations are according to the using human genome hg19/GRCh37 assembly.
| Gene (Symbol) | Gene (Name) | Location | Association with Hormone-Secreting Subtype | Function of Gene Product and Mechanism of Tumorigenesis, if Known | In Vitro Evidence | In Vivo Evidence | Loss of Heterozygosity | Familial Presentation | References |
|---|---|---|---|---|---|---|---|---|---|
| CABLES1 | Cdk5 and Abl enzyme substrate 1 | 18: 20,714,528-20,840,431 | ACTH-PTs | Cell cycle progression: inhibits corticotroph cell proliferation. | Increased proliferation seen in corticotroph cells following knockdown using Cables1 small interfering RNA (88). All identified variants located close to the predicted cyclin-dependent kinase-3 (CDK3)-binding region of Cables1 and showed impaired ability to block cell proliferation in response to dexamethasone in corticotroph cells (87). | None available. | Not found for all variants. | Simplex for all identified variant. | (87) |
| PRLR | Prolactin receptor | 5: 35,048,861-35,230,794 | PRL-PTs | Prolactin receptor | Increased prolactin-induced AKT signaling and proliferation seen in p.Asn516Ile only (gain-of-function) (89). p.Ile100Val, p.Ile170Leu, p.Glu108Lys, and p.Glu554Gln have no effect on PRLR expression, localization, and signaling after prolactin stimulation in vitro, suggestive of minimal functional relevance (89, 90). | Female mice with a germline loss-of-function mutation in PRLR show large PRL-PTs (91) with a penetrance of 100% from 12 months of age (92). | Not investigated. | Gorvin et al: Familial in p.Ile100Val, simplex in p.Glu400Gln, p.Asp492Ile, unavailable for other variants (89). Bernard et al: simplex cases in all variants identified (p.Ile100Val, p.Ile170Leu, p.Glu108Lys and p.Glu554Gln) (90). |
(89, 90) |
| RXRG | Retinoid X receptor gamma | 1: 165,370,159-165,414,433 | PRL-PTs | Forms dimers with ligands, increasing their DNA binding and transcriptional function. The identified variant p.R317H localizes to the ligand-binding domain of the protein and may disrupt interactions. | None available. | None available. | Not investigated.. | Familial | (93) |
| TH | Tyrosine hydroxylase | 11: 2,185,159-2,193,107 | PRL-PTs | Converts L-tyrosine into L-3,4-dihydroxyphenylalanine (L-DOPA), the essential and rate-limiting step to formation of dopamine. Reduced dopaminergic activity leads to reduced inhibitory effects on lactotroph cells, increasing prolactin secretion. | primary cultures of human lactotroph tumor cells were transfected with an adenovirus vector containing a cDNA encoding a human tyrosine hydroxylase: transfection induced increased production of dopamine, resulting in the predicted biologic effect of decreased prolactin secretion (94). | adenovirus-mediated delivery of tyrosine hydroxylase reduces pituitary growth and circulating prolactin levels in a model of estrogen-induced pituitary tumors in rats (95). | Not investigated. | Familial. | (93) |
| CDH23 | Cadherin related 23 | 10: 73,156,691-73,575,702 | None specific | Calcium-dependent cell-cell adhesion glycoprotein | None available | None available. | Not investigated. | Familial . | (96) |
| IGSF1 | Immunoglobulin superfamily member 1 | X: 130,407,480-130,533,677 | Somatomammotroph hyperplasia | Membrane glycoprotein with modified residue possibly altering interaction with an extracellular ligand. | Transfection of GH3 cells with the p.N604T IGSF1 variant did not significantly affect GH production compared to wild-type. The mutant protein showed the same pattern of maturation and stability as wild-type when expressed in heterologous cells and was detected in the plasma membrane (97). |
Male Igsf1Δexon1 null mice show increased serum IGF1 at 10 weeks. Assessment of the knockout model (Igsf1Δ312) demonstrated enhanced pituitary Gh mRNA expression (98). | Not investigated. | Familial. | (99) |
Four heterozygous germline missense variants were identified in CABLES1 in four sporadic patients from a cohort of 182 patients with ACTH-PTs with functional evidence of loss of function for some of them (Table 1). No familial cases have been reported to date (87).
While loss of the Prlr leads to large pituitary tumors in mice, homozygous loss-of-function PRLR mutation in a human patient with hyperprolactinemia and agalactia had no pituitary tumor (100). On the contrary, a gain-of-function variant was identified in 9 out of 46 patients with PRL-PTs, representing a possible novel mechanism for prolactinoma tumorigenesis. In addition, 3 other rare and 2 low-frequency variants found in this cohort may represent benign changes (89). Further data are needed to confirm these findings. Furthermore, no loss or gain-of-function mutations could be identified in a cohort of young 88 patients with PRL-PTs (90) or in a cohort of 16 PRL-PT (17).
There are some further reports of germline variants in patients with pituitary tumors but without functional elucidation to define pathogenicity or mechanisms. Investigation into a family with isolated PRL-PTs (3 affected siblings) with whole exome sequencing showed novel, germline, potentially pathogenic variants in RXRG and TH (93), the latter of which may be relevant as it encodes tyrosine hydroxylase which mediates the rate-limiting step in the formation of dopamine which, in turn, negatively regulates prolactin secretion in the pituitary. Further cases or functional studies will strengthen this report.
Using whole exome sequencing, a study of 12 FIPA families identified four families with germline variants in CDH23, which were predicted to be pathogenic using in silico analysis. Tumors of these patients showed a reduced frequency of cavernous sinus invasion, compared to the rest of the familial patients. The identified variants were predicted to be loss-of-function changes and occurred in conserved motifs, suggestive for impaired protein function, although CDH23 is a large gene and therefore is, in general, more likely to harbor sequence variants. Homozygous mutations in CDH23 result in Usher syndrome, characterized by congenital sensorineural hearing loss, vestibular dysfunction and early-onset retinitis pigmentosa (101). Pituitary tumors have not been described in association with any of these problems (102). This study also describes 2 (out of 125) sporadic pituitary tumor patients with homozygous CDH23 variants (96), but it is not specified whether these individuals showed clinical manifestations of Usher syndrome.
Interestingly, a recently-described syndrome, X-linked IGSF1 deficiency characterized by central hypothyroidism, macro-orchidism (103) and prolactin deficiency (104), can be associated with acromegaloid facial features, increased head circumference and increased total GH secretion and IGF-1 levels (98). Given that patients show hyperplasia rather than adenomas, this may be secondary to a failure of regulatory and feedback mechanisms. A germline variant in IGSF1 was identified in three family members with gigantism (due to somatomammotroph hyperplasia, rather than adenoma) (97). No effect of the variant on protein expression, maturation, stability, or membrane trafficking was observed. The authors speculate that, given the prediction that the modified residue changes the surface charge in the 6th immunoglobulin loop, this may alter IGSF1’s interaction with an extracellular partner, although this variant is reasonably common in the general population (minor allele frequency is 0.009, Table 2).
Table 2.
Germline nonynonymous missense variants identified with predictions of pathogenicity using SIFT, PolyPhen, and Condel. Variants that could not be identified unambiguously have been excluded (PRLR, p.Arg477Trp (89), p.Glu108Lys (90), CDH23, p.Arg3138Trp, p.Arg2115His, p.Arg3138Trp, and p.Asp3296Asn (96)) and have not been included in the following table. The variant associated with TH is a truncating mutation and does not have any predictions of pathogenicity using the missense tools.
| Gene (Symbol) | Location | HGVSc | HGVSp | gnomAD Allele Frequency | SIFT Interpretation | PolyPhen Interpretation | Condel Interpretation | References |
|---|---|---|---|---|---|---|---|---|
| CABLES1 | 18:20716258 | ENST00000256925.7:c.532G>A | ENSP00000256925.7:p.Glu178Lys | 0.0101 | Deleterious low confidence | Benign | N/A | (87) |
| 18:20716444 | ENST00000256925.7:c.718C>T | ENSP00000256925.7:p.Leu240Phe | 0.000773 | Deleterious low confidence | Probably damaging | Deleterious | ||
| 18:20774429 | ENST00000256925.7:c.935G>A | ENSP00000256925.7:p.Gly312Asp | 0.0000601 | Deleterious | Probably damaging | Deleterious | ||
| 18:20817151 | ENST00000256925.7:c.1388A>G | ENSP00000256925.7:p.Asp463Gly | not present | Deleterious | Probably damaging | Deleterious | ||
| PRLR | 5:35084704 | ENST00000382002.5:c.241G>A | ENSP00000371432.5:p.Gly81Ser | 0.0001328 | Tolerated | Benign | Neutral | (89) |
| 5:35065862 | ENST00000382002.5:c.1198G>C | ENSP00000371432.5:p.Glu400Gln | 0.000898 | Tolerated | Possibly damaging | Deleterious | ||
| 5:35065513 | ENST00000382002.5:c.1547A>T | ENSP00000371432.5:p.Asn516Ile | 0.0008925 | Deleterious | Possibly damaging | Deleterious | ||
| 5:35084647 | ENST00000382002.5:c.298A>G | ENSP00000371432.5:p.Ile100Val | 0.04221 | Tolerated | Benign | neutral | (89, 90) | |
| 5:35072712 | ENST00000382002.5:c.508A>C | ENSP00000371432.5:p.Ile170Leu | 0.01884 | Tolerated | Benign | neutral | ||
| 5:35065328 | ENST00000382002.5:c.1732G>C | ENSP00000371432.5:p.Glu578Gln | 0.001195 | Tolerated | Possibly damaging | Deleterious | (90) | |
| RXRG | 1:165378891 | ENST00000359842.5:c.950G>A | ENSP00000352900.5:p.Arg317His | 0.00002495 | Deleterious | Probably damaging | Deleterious | (93) |
| TH | 11:2186469 | ENST00000381178.1:c.1420A>T | ENSP00000370571.1:p.Lys474Ter | not present | N/A | N/A | N/A | (93) |
| CDH23 | 10:73494028 | ENST00000224721.6:c.4151G>T | ENSP00000224721.6:p.Arg1384Leu | not present | Deleterious | Probably damaging | Deleterious | (96) |
| IGSF1 | X:130412680 | ENST00000370903.3:c.1811A>C | ENSP00000359940.3:p.Asn604Thr | 0.009383 | Tolerated | Probably damaging | Deleterious | (97) |
Abbreviations: HGVSc, Human Genome Variation Society (HGVS) coding sequence name; HGVSp, HGVS protein sequence name; SIFT, sorting intolerant from tolerant prediction tool.
Syndromes associated with pituitary tumors.
In addition to the previously well-described syndromes with multiple tumor types where pituitary tumors represent one of the possible manifestations, several novel syndromes have been described over the last few years (Fig. 2). The MEN1 syndrome is due to germline loss-of-function mutations of the MEN1 gene. Ten percent of the cases could be de novo mutations, sometimes identified as mosaicism in the proband (105–107).
An MEN1-like clinical picture can be seen in MEN4 syndrome due to mutation in cyclin dependent kinase inhibitors, primarily p27 (CDKN1B) and rarely in p21 (CDKN1A), p15 (CDKN2B), and p18 (CDKN2C) (108, 109).
In Carney complex, in addition to loss-of-function mutations in the regulatory protein kinase A subunit PRKAR1A, gain-of-function has been described in the catalytic protein kinase A subunit PRKACB (110). The disease-causing gene associated to the 2p16 locus in Carney complex cases is unknown.
While pituitary tumors and pheochromocytoma are rarely seen in MEN1 syndrome, the constellation of paraganglioma, pheochromocytoma, and pituitary tumor (“3P” association) is now increasingly recognized in patients with SDHx mutations (111, 112) with a characteristic histological phenotype (111) and pituitary adenomas developing in a Sdhb-knockout mouse model (113). MAX mutations have been identified in 5 patients with pituitary tumor and pheochromocytoma (114–116). While loss-of-heterozygosity has not been shown yet, further data are needed to confirm a causal relationship between MAX mutations and pituitary tumors.
Corticotroph tumors have been recently identified in three tumor syndromes. DICER1 syndrome (loss-of-of-function DICER1 mutations) has shown infantile-onset large pituitary blastoma in a few patients (117). Germline mutations in the mismatch repair pathway (MLH1, PMS2, MSH2, MSH6) lead to Lynch syndrome, an autosomal dominant inherited cancer syndrome associated with colorectal, endometrial, ovarian, and other carcinomas. Germline mutations in MLH1 (118) and MSH2 (119) have been identified in patients with aggressively growing ACTH-secreting tumors. While somatic variants were found in a single nonfunctioning tumor in 4 mismatch-related genes (120), microsatellite instability was not found in 107 sporadic pituitary tumor samples (121). While somatic USP8 mutations are commonly seen in corticotroph adenomas, a germline mutation has now also been described in a child with dysmorphic features, developmental delay and a corticotroph tumor (122), with a second similar case now under workup (Constantine Stratakis, NIH, personal communication).
Rarely, optic pathway gliomas cause high GH levels in neurofibromatosis type 1 (NF1), while true pituitary adenomas are extremely rare. Pituitary tumors have been reported in patients with tuberous sclerosis (123–126). It is currently unclear if these are indeed related to the TSC1 or TSC2 mutations or are coincidental findings.
Somatic mutations driving tumorigenesis
The most common recurrent somatic mutations occur in GNAS in somatotroph tumors, and in USP8 in corticotroph tumors. Other somatic changes suggested to be associated with pituitary tumors include: PIK3CA amplification (127, 128), IDH1 mutations (129, 130), TP53 in pituitary carcinomas (131) and ACTH-PTs (132), and HMGA2 amplification in PRL-PTs (133–135). HRAS mutations have been seen in pituitary carcinoma (136), while the report of complex 1 mitochondrial mutations in oncocytomas (137) await confirmation. A somatic frameshift mutation in the glucocorticoid receptor gene (NR3C1) resulting in premature termination of the coding sequence has been described in a patient with Nelson’s syndrome, which may contribute to tumor development by reducing glucocorticoid feedback on tumor cells (138). No coding region NR3C1 mutation was found in 18 ACTH-PTs using Sanger sequencing (139), or in 18 USP8 mutation-negative PTs using exome sequencing (132). A single patient with a de novo missense germline NR3C1 mutation associated with an ACTH-PA has also been described (138), while a child with corticotroph adenoma and partial glucocorticoid resistance had no detectable NR3C1 mutation (140). A recent study reported a novel somatic recurrent (“hotspot”) mutation in splicing factor 3 subunit B1 (p.R625H in SF3B1) in about 20% of prolactinomas in altogether 227 prolactinomas (141). This variant causes aberrant splicing of the estrogen related receptor gamma (ESRRG), causing stronger activation of PIT-1 leading to prolactin secretion and lactotroph proliferation. This variant was not reported in another study sequencing prolactinoma 16 tissues (17). Variants discovered recently through next generation sequencing-based approaches are also discussed below.
GNAS.
GNAS encodes the stimulatory α subunit of G-proteins and shows the most frequent somatic mutations in GH-PTs, more recently confirmed through whole genome (WGS) (42) and whole exome sequencing (WES) (17, 120, 142, 143). Mutations affect codon 201 or 227, disrupting the GTPase activity of the protein (144) and leading to prolonged adenylyl cyclase activity and increased cAMP levels, driving tumorigenesis (Fig. 3). GNAS-mutated tumors are smaller (145–149), less likely to be locally invasive (149), and more likely to respond to SSAs (149, 150), although a Brazilian cohort showed no differences in tumor extension or response to SSAs between mutated and nonmutated tumors (151). Dopamine receptor 2 expression is increased in GNAS-mutated tumors, potentially allowing for GNAS mutation status in predicting the response to dopamine agonists in GH-PTs (17). Recently, DNA methylation-activated inhibitory Gα (Gαi) -signaling was found in GNAS-mutation-positive GH-PTs (152). Patients with somatic mosaicism for codon 201 GNAS develop McCune-Albright syndrome, characterized by somato- or somatomammotroph hyperplasia or tumor, polyostotic fibrous dysplasia, cafe-au-lait spots, and precocious puberty (153).
USP8 and USP48.
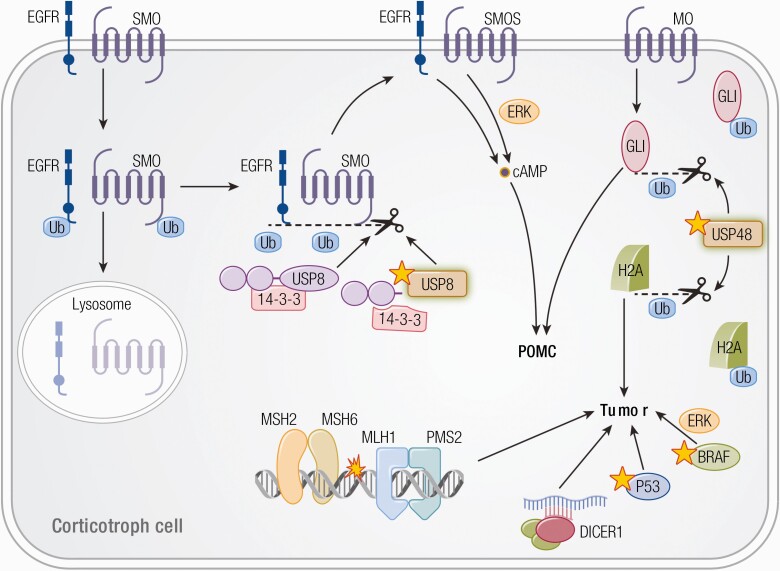
Gain-of-function mutations in the deubiquitinase enzymes USP8 and USP48 are associated with ACTH-PTs (120, 132, 142, 154–161). USP8 mutations disrupt the interaction between USP8 and the 14-3-3 protein, thereby allowing USP8 cleavage and increased enzymatic activity (154–156); this protects EGFR from lysosomal degradation, which leads to increased expression of EGFR (154–156) (Fig. 4) and pro-opiomelanocortin (POMC) (154, 159). Inhibition of USP8 leads to increased degradation of EGFR with suppresses corticotroph cell growth and ACTH secretion in vitro (162). Lapatinib, an EGFR inhibitor, decreases proliferation in vitro and reduces tumor weight in vivo (163). A recent meta-analysis showed an overall prevalence of 32% of USP8 mutations in ACTH-PTs with a higher prevalence in females (164). USP8-mutated tumors are associated with an earlier onset (156, 165), smaller size (154), and increased ACTH production (154, 165). In patients who showed biochemical remission after surgery, the incidence of recurrence in a 10-year follow-up was higher in patients with USP8 mutant tumors (165). In pediatric patients with Cushing’s disease, all recurrences after initial remission (in five patients) occurred in tumors with USP8 mutations (166). When remission status was investigated, the remission rates were higher in patients with USP8-mutated-alleles, although no recurrence was detected for at least 6 months after surgery (164). These data suggest that patients with USP8-mutated-tumors may be more likely to go into initial remission post-surgery but may also more likely to show recurrence later in the clinical course. USP8-mutation-negative tumors are more likely to show sphenoid invasion with an increased epithelial-mesenchymal-transition signature (17). Recently, SSTR5 expression has been shown to be higher in USP8-mutated tumors (17, 159), potentially allowing the mutation status to be used as a predictor of response to pasireotide (a second generation SSA with greater affinity for SSTR5 (167)). In vitro studies found increased expression of pCREB and protein kinase A Cα on immunoblotting in AtT20 cells transfected with mutant USP8 (160).
Figure 4.
Tumorigenic mechanism in corticotroph cells (154). USP8 removes ubiquitin tags through its deubiquitinase action from its targets, such as EGFR and smoothened (SMO), preventing them from being degraded in the lysosome and allowing recycling back to the cell surface. Increased EGFR and SMO activity leads to increased cAMP signaling and POMC levels. Mutated USP8 cannot bind 14-3-3 protein and undergoes cleavage, which increases its enzymatic activity, leading to increased deubiquitination of EGFR and SMO with higher expression on the cell membrane. Similarly, GLI1 and histone 2a (H2A) are suggested to be target of USP48 leading to increased activity with USP48 mutations. Loss-of-function of DICER1, TP53, MLH1 and MSH2 and gain-of-function of BRAF has also been suggested to be associated with corticotroph tumorigenesis.
Activating USP48 mutations were found in 10% to 20% of ACTH-PTs (30, 132). USP48 variants are associated with smaller tumors and better response to corticotropin releasing hormone (CRH) stimulation (132). Interestingly, both USP8 and USP48 have targets in the hedgehog signaling pathway: Smoothened for USP8 (168) and GLI1 for USP48 (132), suggesting that upregulation of this pathway may play a role in corticotroph mutagenesis (Fig. 4).
Novel Somatic Variants.
Analysis of whole exome and whole genome sequencing data from pituitary tumors has revealed a low number of somatic mutations per tumor across all subtypes (120, 142, 143). This is consistent with their generally low proliferation rate. Only a handful of genes show recurrent mutations (120, 141, 169). Such recurrently mutated genes are reported in more detail in Table 3. Identifying somatic variants in circulating free DNA has been attempted recently (170), and may develop into a useful method to follow patients with pituitary tumors.
Table 3.
Novel recurrently mutated somatic variants identified through whole exome sequencing studies.
| Gene Associated with Variant, Symbol | Gene Name | Hormone Subtype | Mechanism of Tumorigenesis | References |
|---|---|---|---|---|
| NR3C1 | Nuclear receptor subfamily 3 group C member 1 | ACTH-PT | Glucocorticoid receptor. If mutated, this receptor may become insensitive to feedback from cortisol leading to ACTH over-production (171). | (142, 154, 161, 172) |
| MEN1 | Menin 1 | Plurihormonal (GH/PRL) | Inactivating mutations underlie multiple endocrine neoplasia type 1, an autosomal dominant syndrome with pituitary tumors as part of the phenotype. | (118, 142) |
| KIF5A | Kinesin heavy chain isoform 5A | PRL, GT | Modulates cell proliferation. Somatic mutations also found in prostate cancer (173). | (142) |
| GRB10 | Growth factor receptor bound protein 10 | GH-PT | Suppresses signals from activated receptors tyrosine kinases, including insulin-like growth factor type 1 receptors. Inactivating mutations may allow increased signaling facilitating somatotroph tumorigenesis. | (142) |
| BRAF | BRAF proto-oncogene, serine/threonine kinase | ACTH-PT | Elevated kinase activity with activation of MAPK pathway and transactivation of POMC, which is the precursor of ACTH. Well-established oncogenic roles in melanoma and multiple carcinomas. |
(160, 161) |
| USP48 | Ubiquitin specific peptidase 48 | ACTH-PT | USP48 has been suggested to increase transcriptional activation of POMC through the NF-κB pathway, increase response to CRH and possibly involve the hedgehog pathway. | (160, 161) |
| PABPC1 | poly (A) binding protein cytoplasmic 1 | ACTH-PT | Binds the poly (A) tail of mRNA and is involved in regulatory processes such as pre-mRNA splicing and regulation of nonsense-mediated decay. | (120) |
| TP53 | Cellular tumor antigen p53 | ACTH-PT | Well-established tumor suppressor with role in cell cycle arrest, DNA repair and apoptosis induction. | (132) |
| SF3B1 | Splicing factor 3b subunit 1 | PRL | – | (141) |
Novel sequencing approaches have provided an opportunity to develop interesting insights into pituitary tumorigenesis:
Novel candidate genes may point towards dysregulated pathways in tumorigenesis. The first genome-wide association study of sporadic pituitary tumors identified new candidates (174): CDK8 (cell cycle regulation) and NEBL and PCDH15 (cell-cell adhesion). CDK8 is an oncogene in colorectal (175) and gastric carcinoma (176) with differentially expressed genes from animal PRL-PT models showing enrichment for CDK8 targets (92). Targeted mutation profiling of canonical cancer-associated genes has identified recurrently mutated genes, with three main pathways implicated in tumorigenesis in one study: cell cycle regulation and growth, chromatin modification and transcriptional regulation, and DNA damage response (143).
Subtype-specific mechanisms can be unveiled through WES and WGS studies focusing on tumor subgroups (Table 4): WES and WGS studies on GH-PTs have identified dysregulation of multiple signaling pathways as potential tumorigenic mechanisms, although they need to be characterized further using functional studies. Mutations in different genes can affect the same pathways and mediate tumorigenesis. For example, several genes involved in cAMP signaling were found to have somatic variants in 14 of 36 somatotroph tumors, including G-protein-coupled receptors CCR10 and OR51B4, which were suggested to increase cAMP signaling similar to classical GNAS mutations (43).
Table 4.
Somatic variants identified from WES studies using specific tumor subtypes.
| Tumor Subtype | Sequencing Technique | Insight into Tumorigenesis | References |
|---|---|---|---|
| GH-PT | WGS (42), WES (43) | No recurrent mutations; somatic variants mediating calcium signaling (42, 43), ATP signaling (42) and cAMP signaling (43) identified. | (42, 43) |
| ACTH-PT | WES | Enhanced promoter activity and increased transcription of POMC through different mechanisms can lead to tumorigenesis. | (161) |
| TSH-PT | WES | Six candidate variants identified, of which 2 have previously characterized tumorigenic roles: (a) Increased expression of SMOX is associated with gastric cancer, and (b) SYTL3 encodes proteins which interact with RAB27 and deregulation of this pathway is associated with bladder cancer. | (177) |
| NFPT | WES | Somatic variants in putative driver genes including platelet-derived growth factor D (PDGFD), N-myc down-regulated gene family member 4 (NDRG4), and Zipper sterile-motif kinase (ZAK) identified. However, these mutations were not replicated in the validation set. | (178) |
Pathways mediating aggressive behaviors such as local invasion (irrespective of subgroups) can be identified for further functional characterization: WES comparing invasive and noninvasive NFPTs and PRL-secreting tumors identified fifteen variants, which were mainly associated with processes involved in the regulation of invasion such as angiogenesis and cytoskeleton organization (179). ACTH-PTs with disrupted genomes and chromosomal instability are more likely to show cavernous sinus invasion (180).
Whole exome/genome sequencing studies can be used to identify copy number alterations and degree of chromosomal instability, which can provide further insights into subtype-specific tumorigenesis. Two distinct subgroups of pituitary tumors with low and high fractions of genomic disruption have been identified. The percentage of genome disruption used to define these 2 subgroups varies across studies (WES (17, 120, 142, 169, 180) and array-CGH (181)). Tumors with high genome disruption are enriched for hormone-secreting tumors, suggesting differing mechanisms of tumorigenesis between functioning and nonfunctioning tumors (17, 169). Chromosomal instability is not related to aggressiveness (17). GH-PTs show greater genomic disruption than ACTH-PTs as well as inactive tumors with no clinical evidence of hormone secretion (120). Subgroups of GNAS-mutation negative GH-secreting tumors show high levels of genomic instability (152) with 20 q amplification (GNAS locus) (17, 143, 181), which may be an alternative mechanism of increased signaling through GNAS driving somatotroph tumorigenesis. GH-PTs with recurrent aneuploidy showed high expression of pituitary tumor transforming gene 1 (PTTG1), which is a regulator of sister chromatid segregation, this may subsequently drive chromosomal instability (152, 182, 183).
Next generation sequencing approaches can also be used in sequencing extrachromosomal DNA and identifying their role in tumorigenesis: tumors with the highest number of mitochondrial variants show the highest Ki-67 indices, irrespective of tumor subtype (184), indicating a role for the mitochondrial genome in modulating tumor biology.
Epigenetic Mechanisms of Tumorigenesis
While germline and somatic genetic alterations have provided interesting insights into pituitary tumorigenesis, still the majority of pituitary tumors are sporadic with no known somatic driver mutations. Epigenetic changes are heritable phenotypic changes, which do not alter the DNA sequence. These can regulate transcription and/or translation. Epigenetic mechanisms may occur at the chromatin level, such as DNA methylation and histone modification, or at the RNA level, mainly mediated by noncoding RNAs, such as microRNAs, long noncoding RNAs (lncRNA), circular RNA (circRNA), and others. Such mechanisms affect gene expression and, consequently, tumorigenesis, and have assumed great significance, especially given the paucity of somatic mutations.
DNA methylation
Recently, it has been shown that PIT-1 lineage tumors (GH-, PRL- and TSH-PTs) show global hypomethylation and cluster as a group with hypomethylation, chromosome alterations, and transposable element overexpression (17), suggesting that DNA hypomethylation may induce chromosomal instability through upregulation of transposable elements. GNAS-mutation positive GH-PTs also showed hypomethylation but with limited chromosomal alterations (17); GNAS-mutation positive tumors are associated with low levels of copy number alterations, as mentioned above.
Promoter methylation can prevent access to transcriptional machinery, leading to decreased expression. A systematic review of genes implicated in epigenetic dysfunction in pituitary tumors identified 16 tumor suppressor genes that underwent silencing secondary to methylation, of which 11 mediate apoptosis and cell cycle progression (185). These genes included CDKN2A and RB1 (186, 187):
Methylation of CpG islands in CDKN2A is seen in up to 90% of sporadic pituitary tumors (185, 186, 188–190) with loss of expression of p16 on immunohistochemistry (188, 189).
Methylation of CpG islands in the RB1 promoter in sporadic tumors (186, 190) is significantly associated with loss of expression on immunohistochemistry (191).
Transgenic mice with a disrupted Rb1 allele develop pituitary tumors (192).
The systematic review also identified aberrant methylation in more than 50% of tumors in GADD45γ, CASP-8, PTAG, and FGFR2 (185). GADD45γ, CASP8, and PTAG are involved in apoptosis modulation, while FGFR2 is a growth factor receptor. Growth factors and growth factors receptors can also be over-expressed in pituitary tumors (193, 194).
The epigenome may be modified by the de novo methyltransferases DNMT3A and DNMT3B. DNMT3A and DNMT3B is overexpressed in pituitary tumors (195, 196) and frequently detected in invasive tumors (195). Reducing the expression of DNMT3B was associated with increased levels of hypo-phosphorylated Rb, p21, and p27 proteins and reduced proliferation (196), suggesting that increased expression DNMT3B may mediate proliferation through promoter hypermethylation and silencing of tumor suppressor genes.
Gene imprinting is a process in which transcription of 1 allele is repressed through methylation, depending on the parent of origin. Relaxation of imprinting through loss of methylation can lead to increased transcription, such as in GNAS-mediated tumorigenesis (197). Imprinted genes such as MEG3 (discussed later) have only 1 transcriptionally active copy, rendering them especially susceptible to inactivation through mutation or increased promoter methylation.
DNA methylation is associated with clinical characteristics, such as:
Tumor subtype: Microarray profiles differentiate between functioning tumors (including hormone subtypes) and NFPTs (120, 198–201) with GH-PTs divided into sparsely and 2 densely granulated groups (199).
Local invasion: While a study has shown distinct microarray profiles between invasive and noninvasive, nonfunctioning tumors (202), others have shown no significant differences, both when only NFPTs were used (203) or with a diverse group of functioning and nonfunctioning tumors (200). Interestingly, differentially methylated genes were enriched for cell adhesion pathways (202). Decreased LAMA2 (204) and WIF1 (205) expression (RT-qPCR and immunoblotting) with increased promoter methylation is seen in invasive NFPTs (204). LAMA2 overexpression in a xenograft model significantly suppressed tumor growth (204). Greater promoter methylation of ESR1 and RASSF1 is seen in noninvasive compared to invasive tumors (201).
Tumor size: CDKN2A methylation and reduced expression of p16 are significantly related to larger tumor size (188, 189). Macroadenomas show a greater frequency of promoter methylation of MSH6 and CADM1 than microadenomas (201).
Disease progression: TERT encodes a component of telomerase, which lengthens the telomere, mediates cell immortalization, and is a well-recognized oncogene. Aberrant TERT promoter methylation is associated with TERT upregulation in malignancies (206–208) and shorter progression-free survival and tumor recurrence (209) in pituitary tumors.
Implications for therapy: MGMT promoter methylation and consequent low expression are noted in a subset of pituitary tumors (210, 211). Temozolomide is currently used in the management of pituitary carcinomas and aggressive pituitary tumors (defined clinically as tumors that recur despite various treatment modalities such as surgery, radiotherapy, and pharmacological therapy (212–214)). Immunohistochemistry to assess MGMT expression has been recommended prior to commencing temozolomide, as high expression is associated with a lack of response, although the methodologies are not standardized and most clinicians would not use the result to discard temozolomide therapy (213, 214). Loss of MSH2 and MSH6 has also been linked to developing rapid resistance to temozolomide (215). Patients with somatotropinomas and GSTP1 promoter methylation are more resistant to SSAs (216).
Histone modification
Acetylation, methylation, and citrullination of histone tails are associated with active and inactive regions of the genome respectively. These epigenetic marks can be modified by chromatin regulators such as histone acetyltransferases, histone deacetylases, histone methyltransferases, and citrullination enzymes.
Several studies suggest that changes in histone acetylation play a crucial role in pituitary tumorigenesis:
Tumor-specific ikaros isoform Ik6 promotes pituitary cell survival through enhanced antiapoptotic activity by upregulation of Bcl-XL through promoter histone acetylation (217).
Expression of bone morphogenetic protein 4, which is a growth factor known to drive pituitary tumorigenesis (193), is controled through histone acetylation and methylation (218).
Tumor subtype-specific changes: Sirtuins are conserved histone deacetylases, which show differences in expression profiles in pituitary tumors based on size and hormone-secreting subtype (219). Histone deacetylase-2 deficiency is seen in glucocorticoid-resistant ACTH-PTs (220), with histone deacetylase-11 mediating decreased p53 expression in corticotroph AtT-20 cells (221). Inhibition of histone deacetylase activity reduces survival and ACTH secretion in corticotroph cells (222).
Aggressive behavior: Histone acetyltransferases p300 upregulates human pituitary tumor transforming gene (PTTG1) (223). PTTG1 is a growth factor with a well-established role in carcinogenesis and invasion, partly regulated through the c-myc pathway (224). A meta-analysis has confirmed increased PTTG1 expression in invasive pituitary tumors (225) (this has also been replicated in NFPTs (226)). Pituitary tumors show a global increase in H3K9 acetylation compared to normal pituitary, with increased acetylation seen in tumors with increased Ki-67 index (227).
Histone methylation and citrullination (conversion of arginine to citrulline catalyzed by peptidylarginine deiminase enzymes) may also mediate tumorigenesis. Enhanced H3K27 methylation and reduced H3K4/H3K9 methylation are found in tumors with increased RIZ1 expression (which is thought to be a histone methyltransferase), which also correlates with longer progression-free survival (228). Noninvasive tumors also show significantly increased RIZ1 expression compared to invasive tumors (228). Citrullination of histone H3 in GH3 cells represses the expression of specific tumor suppressor microRNAs, which leads to increased expression of known drivers such as N-MYC, and IGF-1 and increased proliferation (229).
MicroRNAs
These are short noncoding RNAs that mediate post-transcriptional regulation of gene expression through RNA interference and mRNA destabilization. The role of microRNAs in pituitary tumorigenesis has been extensively reviewed elsewhere (230–232). The recent advances (mainly from the last 5 years) with novel insights into tumorigenesis are summarized in Table 5.
Table 5.
Varying functions of microRNAs in pituitary tumorigenesis with illustrative examples from publications from the last 5 years.
| Major Function | Mechanisms of Action and/or Relevant Examples | Supporting Evidence | ||
|---|---|---|---|---|
| MicroRNAs can demonstrate a tumor suppressor action by targeting oncogenic gene products for degradation | MicroRNAs regulate the cell cycle, facilitating increased proliferation when deregulated (230). | miR-23b and miR-130b, targeting HMGA2 and cyclin A2 respectively, are downregulated in GH-PTs, GT-PTs and NFPTs (233). HMGA2 is a high mobility group protein, which shows increased expression in pituitary tumors (234, 235). HMGA2 overexpression enhances E2F1 activity and drives cell cycle (236, 237) microRNAs targeting HMGA2 and E2F1 are downregulated in pituitary tumors (235, 238). | ||
| miR-410 targeting the cyclin B1 gene is downregulated in GT-PTs (239). | ||||
| miR-186 targets SKP2, which inhibits expression of p27, a negative regulator of G1 cell cycle progression, increasing proliferation. In human pituitary tumors, miR-186 and p27 expression is downregulated, while SKP2 expression is upregulated (240). In vitro, SKP2 overexpression decreases p27 expression and increases cell growth (240). | ||||
| Multiple microRNAs, when down regulated, lead to increased expression of PTTG1 and its partners. | p53 activates transcription of miR-329, miR-300, miR-381, and miR-655 in pituitary tumor cells, which target PTTG1 (241). | |||
| miR-423-5p (targeting PTTG1) shows decreased expression in GH-PTs with increased PTTG1 expression compared to normal pituitary (242). | ||||
| Overexpression of miR-524-5p downregulates expression of PTTG1 binding factor, which interacts with PTTG1 to mediate downstream effects (243) and significantly attenuates proliferation, migration, and invasion in folliculostellate cells (244); downregulation of this microRNA may mediate increased proliferation in the pituitary through PTTG1. | ||||
| Other tumor-suppressive microRNAs which show reduced expression in human pituitary tumors or relevant cell lines. | miR-205-5p targeting CBX1 in pituitary cell lines (245). | |||
| miR‑1 targeting G6PD in human pituitary tumors (246). | ||||
| miR-34a targeting SOX7 in GH4C1 cells (247). | ||||
| miR-378 targeting RNF31 in human pituitary tumors (248). | ||||
| Increased expression of certain microRNAs can drive tumorigenesis by targeting gene products with tumor suppressor roles for degradation. | High levels of miR-107 (249) and miR-34 (250) target AIP mRNA in pituitary tumors. | miR-107 expression is significantly upregulated in GH-secreting and nonfunctioning pituitary tumors and inhibits in vitro AIP expression (249) | ||
| miR-34 is highly expressed in tumors with low AIP protein levels compared to tumors with high levels (250). miR-34 overexpression in HEK293 and GH3 cells inhibits endogenous AIP expression (250). | ||||
| MicroRNAs may regulate subtype-specific mechanisms of tumorigenesis. | Distinct profiles identified in tumor subtypes with differential microRNA expression specific to subtype. | Next generation sequencing and other techniques in GH-PTs, GT-PTs and NFPT subtypes (251, 252). | ||
| TSP-1, which has a tumor suppressor role, shows decreased expression in ACTH-PTs with increased miR-449c expression inhibiting its expression (253). | ||||
| Four groups, miR1 to miR4, are strongly associated with tumor type with PIT1-lineage tumors being distinctly different from GT-PTs and ACTH-PTs (17). | ||||
| MicroRNAs play a prominent role in driving tumor invasion. | Decreased expression of mi-RNAs can have an anti-apoptotic effect, mediating invasion: | Downregulation of miR-132 and miR-15a/16 with upregulation of SOX5 is seen in invasive tumors (254). MiR-15a and miR-16-1 are also downregulated in pituitary tumors that develop after 12 months of age in mice with heterozygous Men1 knockout (255). MiR-16 expression, which induces apoptosis (via Bax) and decreases proliferation, is reduced in pituitary tumors (256). | ||
| Invasive pituitary tumors show lower miR-21 expression with increased expression of its target, PITX2, which has an antiapoptotic role (257). | ||||
| MiR-145-5p expression (targeting TPT1) correlates negatively with NFPT invasiveness. MiR-145-5p brings about apoptosis through Bcl-xL downregulation and Bax upregulation (258). | ||||
| MiR-543 expression is increased in invasive tumors (259) and activates the Wnt/ β-catenin pathway by downregulating Smad7. Overexpression of miR-543 in HP75 cells increases cell proliferation, migration and invasion and decreases apoptosis (259). | ||||
| microRNAs driving invasion specific to tumor subtype have also been identified: | MiR-183, which targets KIAA0101 (a cell cycle activator), is downregulated in aggressive PRL-PTs and demonstrates an inverse correlation with Ki-67 indices (260). | |||
| MicroRNA 106b~25 cluster shows increased expression in invasive ACTH-PTs and Crooke cell adenomas (261). MiR-106b is upregulated in pituitary tumors and can increase migration and invasion of pituitary tumor cells through the phosphatidylinositol 3-kinase (PI3K)/AKT pathway (262, 263). | ||||
| Differential microRNA profiles have been identified in invasive NFPTs (264). | ||||
| MiR-26b (targeting PTEN) is upregulated and miR-128 (targeting BMI1) is down-regulated in GH-PTs compared to control and is shown to mediate growth and invasiveness of pituitary tumor cells (265). MiR-338-3p expression is increased in invasive GH-PTs and is mediated through upregulation of PTTG1 (266). | ||||
| The same microRNAs may even play different roles in different tumor subtypes: miR-410-3p significantly upregulates proliferation, invasiveness, cyclin B1 levels and activation of MAPK, PTEN/AKT, and STAT3 signaling pathways in gonadotroph and corticotroph cells but not in somatotroph cells (267). | ||||
| Other microRNAs discovered recently through comparison of invasive and noninvasive pituitary tumors (target gene in parentheses): | Reduced expression of microRNA in invasive tumors: | |||
| microRNA | Targeted gene | Reference | ||
| miR-145 | FSCN1 | (268) | ||
| miR-124 | PTTG1IP | (268) | ||
| miR-183 | EZR | (268) | ||
| miR-148-3p and miR-152 | ALCAM | (269) | ||
| miR-200b | PKCα | (270) | ||
| miRNA-145 | AKT3 | (271) | ||
| Increased expression of microRNA in invasive tumors: | ||||
| miR-26a | PLAG1 | (272) | ||
| miR-20a and miR-17-5p | PTEN and TIMP2 | (273) | ||
Other noncoding RNAs
These include long noncoding RNA (lncRNA) (200 nt to ~100 kilobases long with no open reading frames (274)) and circular RNA (generated from exons of protein-coding genes and lacking a 5’ cap or 3’ poly (A) tail (275)). LncRNA and circRNAs have various functions, such as regulation of gene transcription and depletion of microRNA (“microRNA sponge”). Their up- or downregulation can result in tumorigenesis (275). Many lncRNAs are imprinted, allowing for dysregulation of imprinting resulting in abnormal function (274) (see above).
H19 expression is downregulated in pituitary tumors compared to normal pituitary (276) and H19 suppresses proliferation in vitro and in vivo (277) by inhibiting mTORC1 (276). CCAT2 is significantly upregulated in pituitary tumors with elevated expression correlating with poor prognosis (278). CCAT2 enhances proliferation in HP75 cells by suppressing PTTG1 degradation (278). IFNG-AS1 expression is greater in pituitary tumors with increased proliferation noted in HP75 cells on over-expression, probably mediated through ESRP2 (279). AFAP1-AS1 expression is also increased in pituitary tumors (280) and promotes proliferation by acting as a competing endogenous RNA of miR-103a-3p leading to activation of the PI3K/AKT pathway (281).
LncRNAs may drive subtype differentiation:
Genome-wide analysis of lncRNAs identified 839 differentially-expressed lncRNAs in GT-PTs compared to normal pituitary (282). Similar analysis in NFPTs identified 113 lncRNAs (283).
RPSAP52 expression is highly upregulated in GT-PTs and PRL-PTs, where it increases HMGA2 levels, compared with normal pituitary tissues (284). This relationship is not seen in GH-PTs.
LncRNA clarin 1 antisense RNA 1 (CLRN1-AS1) is downregulated in PRL-PTs therefore relieving inhibition from the Wnt/β-catenin pathway (285).
Overexpression of a subgroup of long noncoding RNAs, termed “highly up-regulated in liver cancer” (HULC), promoted GH3 cell viability, migration, invasion, and PRL and GH secretion with knockdown inducing apoptosis (286).
LncRNAs may also drive tumor invasion:
MEG3: This is an imprinted lncRNA, which is downregulated in NFPTs (287, 288). Loss of expression is not seen in other tumor subtypes (289, 290). Promoter hypermethylation mediates loss of expression (291). Ectopic expression inhibits growth in human cancer cell lines (287). MEG3 causes cell cycle arrest at the G1 phase with p53-dependent (292) and p53-independent (293) mechanisms. MEG3 expression is significantly reduced in invasive compared to noninvasive NFPTs (294).
HOTAIR: Expression is significantly higher in NFPTs compared to normal pituitary and in invasive compared to noninvasive NFPTs (294). HOTAIR interacts with the Polycomb Repressive Complex 2 (PRC2) (295, 296) and promotes invasion in pancreatic (297) and nonsmall cell lung cancer (298).
Lnc-SNHG1: Overexpression is seen in invasive pituitary tumors. Lnc-SNHG1 interacts with and decreases the activity of miR-302/372/373/520 in vitro, activating the TGFBR2/SMAD3 and RAB11A/Wnt/β-Catenin (299).
C5orf66-AS1: Its expression is decreased in invasive null cell adenomas compared to noninvasive tumors (300).
XIST: XIST and bFGF exhibited high expression while miR-424-5p showed low expression in invasive compared to noninvasive pituitary tumor tissue. XIST was found to up-regulate bFGF expression by competitively binding to miR-424-5p (301): bFGF (basic fibroblast growth factor) acts as a growth factor and can also promote angiogenesis.
Interestingly, multiple studies have shown a role for circRNAs in tumor progression in NFPTs. As discussed previously, miR-145-5p induces apoptosis in NFPTs; circOMA1 can promote tumor progression by sponging this microRNA (258). Differential circRNA expression profiles of invasive and noninvasive NFPTs have been shown (302, 303), with gene enrichment for cell adhesion and PI3K/AKT pathways (302). Ten circRNAs are upregulated in recurrent compared to primary tumors (302). In fact, a signature of two circRNAs (hsa_circ_0000066 and hsa_circ_0069707) is suggested to predict tumor recurrence in NFPTs (304).
Global gene expression profiles
Novel sequencing and array-based approaches have allowed for:
Identification of distinct profiles in subtypes. Using RNA sequencing, 6 distinct transcriptomic profiles have been identified which match the World Health Organization (WHO) 2017 tumor classification (17), with the following discrepancies: (1) null cell subtype matches GT-PTs; (2) silent and secreting ACTH-PTs show distinct profiles; (3) mixed GH-PRL tumors cluster with GH-PTs, rather than PRL-PTs; and (4) sparsely granulated GH-PTs cluster with thyrotroph and plurihormonal PIT1-positive tumors rather than densely granulated GH-PTs. Distinct tumor type-specific profiles were shown in earlier studies as well: GT-PTs (282, 305-307), ACTH-PTs (120, 308-310), GH-PTs (120, 311), PRL-PTs (312, 313) and NFPTs (120, 314, 315). Insights from such studies include a confirmation of the importance of deregulation of the cell cycle in pituitary tumorigenesis across subtypes (17, 306, 307, 311, 314, 316), deregulation of the mTOR signaling pathway in GT-PTs (282), and alterations in the Notch pathways in PRL-PTs (312) and NFPTs (315). A subtype of NFPTs may actively suppress the immune system, raising the possibility of immunotherapy in treatment (317). Integration of microarray datasets has also been used to identify immune-related genes and further explore this possibility (318). Recently, evaluation of splicing machinery components in GH-PTs, ACTH-PTs, and PRL-PTs showed severe dysregulation in all subtypes compared to normal pituitary (319).
In a subgroup of tumors from patients with acromegaly, ectopic GIPR overexpression was associated with a paradoxical increase in GH after an oral glucose tolerance test (70, 71). These GIPR-expressing somatotropinomas are negative for GNAS mutations (70, 72). An overall hypermethylator phenotype was identified in GIPR-expressing samples (compared with GNAS-mutated tumors), which also showed hypermethylation in the GIPR gene body, potentially driving ectopic expression (70). This represents a novel tumorigenic mechanism, similar to the well-described ectopic receptor induced adrenal tumors.
A study on invasive pituitary tumors identified that the TNFα network—including genes coding for proteins (TNFα, CCL3, CXCL12, and CCL2), microRNAs (miR-181c-5p and miR-454-3p) and lncRNAs (NR_033258 and lncRNA_SNHG24)—is upregulated in bone-invasive pituitary adenomas compared nonbone-invasive counterparts, suggesting that targeting the TNFα pathway may be beneficial for these invasive tumors (320).
Proteomics
Novel techniques such as nanoscale liquid chromatography coupled to tandem mass spectrometry (nano LC-MS/MS) have been used recently (321) in determining proteomic profiles. Integration of such techniques with transcriptomics has allowed for identification of invasion-related biomarkers (322) and pathways (323) in NFPTs. This technique has even allowed for the identification of novel pattern of phosphorylation in GH-PTs (321), with enrichment of differentially phosphorylated proteins in glycolysis and AMPK signaling. High-performance liquid chromatography coupled to mass spectrometry has been used to identify differentially-expressed molecules in fibroblasts isolated from bone-destructive NFPTs (324) (with significant upregulation of osteopontin, which can stimulate cell migration and invasion (325)). The first study to investigate protein ubiquitination profiling in pituitary tumors compared to normal pituitary showed enrichment for the PI3K/AKT signaling pathway in NFPTs (326).
High-resolution Fourier transform mass spectrometry is another promising novel technique which has identified 105 novel proteins in the normal anterior pituitary compared with previous high-throughput proteomic-based studies (327). Using this approach may also identify new candidate proteins and/or pathways driving pituitary tumorigenesis. Previously used techniques in proteome profiling of pituitary tumors include two-dimensional gel electrophoresis-based comparative proteomics (312, 315, 328, 329) and protein immunoblot array analysis (330).
Other Factors Influencing Pituitary Tumorigenesis
In this review we have concentrated on the novel genetic changes in pituitary adenomas. We refer to data on other important aspects of pituitary tumorigenesis, such as the role of senescence (331, 332), cytokines (47, 333) or tumor-associated fibroblasts (334, 335) in establishing the clinical phenotype of tumors is an emerging focus of research interest (336).
Conclusions
Novel mechanisms of pituitary tumorigenesis have been identified in recent years, both pertaining to germline mutations underlying familial tumors and somatic mutations and epigenetic changes driving sporadic tumors. Epigenetic modifications have become increasingly important in understanding tumorigenesis, as most pituitary tumors are sporadic with no known genetic driver mutations (with the exception of a significant proportion of GH-PTs and ACTH-PTs with GNAS and USP8 mutations, respectively). While novel somatic variants have been identified through whole genome and exome sequencing, their role in driving sporadic tumors remains to be established through further functional studies. Epigenetic changes at the chromatin (pretranscription) and RNA levels (post-transcription) are especially crucial in determining clinical characteristics such as subtype differentiation and local invasion (occasionally through epigenetic mechanisms specific to subtype). Indeed, a novel recent multiomic classification system using somatic mutations, chromosomal alterations and the miRNome, methylome, and transcriptome has shown the PIT1 lineage to be the main separator driving distinct group classification (17). Such integrated approaches to these genetic and epigenetic mechanisms will permit identification of molecular mechanisms of tumorigenesis common across different subtypes, as well as specific to tumor subtype, allowing for the development of novel therapeutic strategies.
Acknowledgments
We are grateful to Prof Ashley Grossman for the careful review of this manuscript.
VSN is an NIHR academic clinical fellow and received support from Cancer Research UK and the Pathological Society. MK’s work on pituitary adenomas is supported by the Medical Research Council and the Rosetrees Trust.
Financial Support: V.S.N. received support from Cancer Research UK and the Pathological Society. M.K.’s work on pituitary adenomas was supported by the Medical Research Council and the Rosetrees Trust.
Glossary
Abbreviations
- ACTH-PT
adrenocorticotropic hormone-secreting pituitary tumor
- circRNA
circular RNA
- FIPA
familial isolated pituitary adenomas
- GH-PT
growth hormone-secreting pituitary tumor
- GT-PT
gonadotropin (FSH/LH)- secreting pituitary tumor
- Gαi
inhibitory Gα protein subunit
- HGVS
Human Genome Variation Society
- lncRNA
long noncoding RNAs
- PitNET
pituitary neuroendocrine tumor
- PRL-PT
prolactin-secreting pituitary tumor
- PT
pituitary tumor
- SSA
somatostatin analogue
- TSH-PT
thyroid stimulating hormone-secreting pituitary tumor
- WES
whole exome sequencing
- WGS
whole genome sequencing
- XLAG
X-linked acrogigantism
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Ezzat S, Asa SL, Couldwell WT, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101(3):613–619. [DOI] [PubMed] [Google Scholar]
- 2. Daly AF, Rixhon M, Adam C, Dempegioti A, Tichomirowa MA, Beckers A. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006;91(12):4769–4775. [DOI] [PubMed] [Google Scholar]
- 3. Fernandez A, Karavitaki N, Wass JA. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010;72(3):377–382. [DOI] [PubMed] [Google Scholar]
- 4. Fontana E, Gaillard R. [Epidemiology of pituitary adenoma: results of the first Swiss study]. Rev Med Suisse. 2009;5(223):2172–2174. [PubMed] [Google Scholar]
- 5. Raappana A, Koivukangas J, Ebeling T, Pirila T. Incidence of pituitary adenomas in Northern Finland in 1992–2007. J Clin Endocrinol Metab. 2010;95(9):4268–4275. [DOI] [PubMed] [Google Scholar]
- 6. Al-Dahmani K, Mohammad S, Imran F, et al. Sellar masses: an epidemiological study. Can J Neurol Sci. 2016;43(2):291–297. [DOI] [PubMed] [Google Scholar]
- 7. Agustsson TT, Baldvinsdottir T, Jonasson JG, et al. The epidemiology of pituitary adenomas in Iceland, 1955–2012: a nationwide population-based study. Eur J Endocrinol. 2015;173(5):655–664. [DOI] [PubMed] [Google Scholar]
- 8. Gruppetta M, Formosa R, Falzon S, et al. Expression of cell cycle regulators and biomarkers of proliferation and regrowth in human pituitary adenomas. Pituitary. 2017;20(3):358–371. [DOI] [PubMed] [Google Scholar]
- 9. Gittleman H, Ostrom QT, Farah PD, et al. Descriptive epidemiology of pituitary tumors in the United States, 2004–2009. J Neurosurg. 2014;121(3):527–535. [DOI] [PubMed] [Google Scholar]
- 10. Trouillas J, Burman P, McCormack A, et al. Aggressive pituitary tumours and carcinomas: two sides of the same coin? Eur J Endocrinol. 2018;178(6):C7–C9. [DOI] [PubMed] [Google Scholar]
- 11. Asa SL, Casar-Borota O, Chanson P, et al. ; attendees of 14th Meeting of the International Pituitary Pathology Club, Annecy, France, November 2016. From pituitary adenoma to pituitary neuroendocrine tumor (PitNET): an International Pituitary Pathology Club proposal. Endocr Relat Cancer. 2017;24(4):C5–C8. [DOI] [PubMed] [Google Scholar]
- 12. Ho KKY, Fleseriu M, Wass J, et al. A tale of pituitary adenomas: to NET or not to NET: Pituitary Society position statement. Pituitary. 2019;22(6):569–573. [DOI] [PubMed] [Google Scholar]
- 13. Ho KKY, Fleseriu M, Wass J, et al. The tale in evolution: clarity, consistency and consultation, not contradiction and confusion. Pituitary. 2020;23(4):476–477. [DOI] [PubMed] [Google Scholar]
- 14. Asa SL, Asioli S, Bozkurt S, et al. Pituitary neuroendocrine tumors (PitNETs): nomenclature evolution, not clinical revolution. Pituitary. 2020;23(3):322–325. [DOI] [PubMed] [Google Scholar]
- 15. Osamura RY, Grossman AB, Korbonits M, et al. Pituitary adenoma. In: Lloyd RV, Osamura RY, Klöppel G, Rosai J, eds. World Health Organization Classification of Tumours of Endocrine Organs. Vol. 10. 4th ed. Geneva: International Agency for Cancer Research; 2017:15–19. [Google Scholar]
- 16. Drummond J, Roncaroli F, Grossman AB, Korbonits M. Clinical and pathological aspects of silent pituitary adenomas. J Clin Endocrinol Metab. 2019;104(7):2473–2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neou M, Villa C, Armignacco R, et al. Pangenomic classification of pituitary neuroendocrine tumors. Cancer Cell. 2020;37(1):123–134 e125. [DOI] [PubMed] [Google Scholar]
- 18. Beckers A, Aaltonen LA, Daly AF, Karhu A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr Rev. 2013;34(2):239–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loughrey PB, Korbonits M. Genetics of pituitary tumours. In: Igaz P, Patocs A, eds. Genetics of Endocrine Diseases and Syndromes. Experientia Supplementum. Vol. 111. Cham: Springer;2019:171–211. [DOI] [PubMed] [Google Scholar]
- 20. Vandeva S, Daly AF, Petrossians P, Zacharieva S, Beckers A. Somatic and germline mutations in the pathogenesis of pituitary adenomas. Eur J Endocrinol. 2019;181(6):R235–R254. [DOI] [PubMed] [Google Scholar]
- 21. Marques NV, Kasuki L, Coelho MC, Lima CHA, Wildemberg LE, Gadelha MR. Frequency of familial pituitary adenoma syndromes among patients with functioning pituitary adenomas in a reference outpatient clinic. J Endocrinol Invest. 2017;40(12):1381–1387. [DOI] [PubMed] [Google Scholar]
- 22. Vierimaa O, Georgitsi M, Lehtonen R, et al. Pituitary adenoma predisposition caused by germline mutations in the AIP gene. Science. 2006;312(5777):1228–1230. [DOI] [PubMed] [Google Scholar]
- 23. Daly AF, Vanbellinghen JF, Khoo SK, et al. Aryl hydrocarbon receptor-interacting protein gene mutations in familial isolated pituitary adenomas: analysis in 73 families. J Clin Endocrinol Metab. 2007;92(5):1891–1896. [DOI] [PubMed] [Google Scholar]
- 24. Hernández-Ramírez LC, Gabrovska P, Dénes J, et al. ; International FIPA Consortium . Landscape of familial isolated and young-onset pituitary adenomas: prospective diagnosis in AIP mutation carriers. J Clin Endocrinol Metab. 2015;100(9):E1242–E1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trivellin G, Daly AF, Faucz FR, et al. Gigantism and acromegaly due to Xq26 microduplications and GPR101 mutation. N Engl J Med. 2014;371(25):2363–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gordon RJ, Bell J, Chung WK, David R, Oberfield SE, Wardlaw SL. Childhood acromegaly due to X-linked acrogigantism: long term follow-up. Pituitary. 2016;19(6):560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wise-Oringer BK, Zanazzi GJ, Gordon RJ, et al. Familial X-linked acrogigantism: postnatal outcomes and tumor pathology in a prenatally diagnosed infant and his mother. J Clin Endocrinol Metab. 2019;104(10):4667–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beckers A, Lodish MB, Trivellin G, et al. X-linked acrogigantism syndrome: clinical profile and therapeutic responses. Endocr Relat Cancer. 2015;22(3):353–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin BC, Sullivan R, Lee Y, Moran S, Glover E, Bradfield CA. Deletion of the aryl hydrocarbon receptor-associated protein 9 leads to cardiac malformation and embryonic lethality. J Biol Chem. 2007;282(49):35924–35932. [DOI] [PubMed] [Google Scholar]
- 30. Aflorei ED, Klapholz B, Chen C, et al. In vivo bioassay to test the pathogenicity of missense human AIP variants. J Med Genet. 2018;55(8):522–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen B, Liu P, Hujber EJ, Li Y, Jorgensen EM, Wang ZW. AIP limits neurotransmitter release by inhibiting calcium bursts from the ryanodine receptor. Nat Commun. 2017;8(1):1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tuominen I, Heliövaara E, Raitila A, et al. AIP inactivation leads to pituitary tumorigenesis through defective Gαi-cAMP signaling. Oncogene. 2015;34(9):1174–1184. [DOI] [PubMed] [Google Scholar]
- 33. Ritvonen E, Pitkänen E, Karppinen A, et al. Impact of AIP and inhibitory G protein alpha 2 proteins on clinical features of sporadic GH-secreting pituitary adenomas. Eur J Endocrinol. 2017;176(2):243–252. [DOI] [PubMed] [Google Scholar]
- 34. Formosa R, Xuereb-Anastasi A, Vassallo J. Aip regulates cAMP signalling and GH secretion in GH3 cells. Endocr Relat Cancer. 2013;20(4):495–505. [DOI] [PubMed] [Google Scholar]
- 35. Leontiou CA, Gueorguiev M, van der Spuy J, et al. The role of the aryl hydrocarbon receptor-interacting protein gene in familial and sporadic pituitary adenomas. J Clin Endocrinol Metab. 2008;93(6):2390–2401. [DOI] [PubMed] [Google Scholar]
- 36. Bolger GB, Bizzi MF, Pinheiro SV, et al. cAMP-specific PDE4 phosphodiesterases and AIP in the pathogenesis of pituitary tumors. Endocr Relat Cancer. 2016;23(5):419–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bizzi MF, Pinheiro SVB, Bolger GB, et al. Reduced protein expression of the phosphodiesterases PDE4A4 and PDE4A8 in AIP mutation positive somatotroph adenomas. Mol Cell Endocrinol. 2018;476:103–109. [DOI] [PubMed] [Google Scholar]
- 38. Schernthaner-Reiter MH, Trivellin G, Stratakis CA. Interaction of AIP with protein kinase A (cAMP-dependent protein kinase). Hum Mol Genet. 2018;27(15):2604–2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hernández-Ramírez LC, Morgan RML, Barry S, D’Acquisto F, Prodromou C, Korbonits M. Multi-chaperone function modulation and association with cytoskeletal proteins are key features of the function of AIP in the pituitary gland. Oncotarget. 2018;9(10):9177–9198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chahal HS, Trivellin G, Leontiou CA, et al. Somatostatin analogs modulate AIP in somatotroph adenomas: the role of the ZAC1 pathway. J Clin Endocrinol Metab. 2012;97(8):E1411–E1420. [DOI] [PubMed] [Google Scholar]
- 41. Cai F, Hong Y, Xu J, et al. A novel mutation of Aryl hydrocarbon receptor interacting protein gene associated with familial isolated pituitary adenoma mediates tumor invasion and growth hormone hypersecretion. World Neurosurg. 2019;123:e45–e59. [DOI] [PubMed] [Google Scholar]
- 42. Välimäki N, Demir H, Pitkänen E, et al. Whole-genome sequencing of Growth Hormone (GH)-secreting pituitary adenomas. J Clin Endocrinol Metab. 2015;100(10):3918–3927. [DOI] [PubMed] [Google Scholar]
- 43. Ronchi CL, Peverelli E, Herterich S, et al. Landscape of somatic mutations in sporadic GH-secreting pituitary adenomas. Eur J Endocrinol. 2016;174(3):363–372. [DOI] [PubMed] [Google Scholar]
- 44. Cañibano C, Rodriguez NL, Saez C, et al. The dependence receptor Ret induces apoptosis in somatotrophs through a Pit-1/p53 pathway, preventing tumor growth. Embo J. 2007;26(8):2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vargiolu M, Fusco D, Kurelac I, et al. The tyrosine kinase receptor RET interacts in vivo with aryl hydrocarbon receptor-interacting protein to alter survivin availability. J Clin Endocrinol Metab. 2009;94(7):2571–2578. [DOI] [PubMed] [Google Scholar]
- 46. Fukuda T, Tanaka T, Hamaguchi Y, Kawanami T, Nomiyama T, Yanase T. Augmented growth hormone secretion and Stat3 phosphorylation in an Aryl hydrocarbon receptor interacting protein (AIP)-disrupted somatotroph cell line. PLoS One. 2016;11(10):e0164131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barry S, Carlsen E, Marques P, et al. Tumor microenvironment defines the invasive phenotype of AIP-mutation-positive pituitary tumors. Oncogene. 2019;38(27):5381–5395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Trivellin G, Korbonits M. AIP and its interacting partners. J Endocrinol. 2011;210(2):137–155. [DOI] [PubMed] [Google Scholar]
- 49. Sun D, Stopka-Farooqui U, Barry S, et al. Aryl hydrocarbon receptor interacting protein maintains germinal center B cells through suppression of BCL6 degradation. Cell Rep. 2019;27(5):1461–1471 e1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Raitila A, Lehtonen HJ, Arola J, et al. Mice with inactivation of aryl hydrocarbon receptor-interacting protein (Aip) display complete penetrance of pituitary adenomas with aberrant ARNT expression. Am J Pathol. 2010;177(4):1969–1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Williams F, Hunter S, Bradley L, et al. Clinical experience in the screening and management of a large kindred with familial isolated pituitary adenoma due to an aryl hydrocarbon receptor interacting protein (AIP) mutation. J Clin Endocrinol Metab. 2014;99(4):1122–1131. [DOI] [PubMed] [Google Scholar]
- 52. Lecoq AL, Zizzari P, Hage M, et al. Mild pituitary phenotype in 3- and 12-month-old Aip-deficient male mice. J Endocrinol. 2016;231(1):59–69. [DOI] [PubMed] [Google Scholar]
- 53. Gillam MP, Ku CR, Lee YJ, et al. Somatotroph-specific Aip-deficient mice display pretumorigenic alterations in cell-cycle signaling. J Endocr Soc. 2017;1(2):78–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Solomou A, Herincs M, Roncaroli F, Vignola ML, Gaston-Massuet C, Korbonits M. Investigating the role of AIP in mouse pituitary adenoma formation. Paper presented at: Endocrine Abstracts2017;Harrogate BES; 2017. [Google Scholar]
- 55. Daly AF, Tichomirowa MA, Petrossians P, et al. Clinical characteristics and therapeutic responses in patients with germ-line AIP mutations and pituitary adenomas: an international collaborative study. J Clin Endocrinol Metab. 2010;95(11):E373–E383. [DOI] [PubMed] [Google Scholar]
- 56. Xekouki P, Mastroyiannis SA, Avgeropoulos D, et al. Familial pituitary apoplexy as the only presentation of a novel AIP mutation. Endocr Relat Cancer. 2013;20(5):L11–L14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Villa C, Lagonigro MS, Magri F, et al. Hyperplasia-adenoma sequence in pituitary tumorigenesis related to aryl hydrocarbon receptor interacting protein gene mutation. Endocr Relat Cancer. 2011;18(3):347–356. [DOI] [PubMed] [Google Scholar]
- 58. Oriola J, Lucas T, Halperin I, et al. Germline mutations of AIP gene in somatotropinomas resistant to somatostatin analogues. Eur J Endocrinol. 2013;168(1):9–13. [DOI] [PubMed] [Google Scholar]
- 59. Daly AF, Rostomyan L, Betea D, et al. AIP-mutated acromegaly resistant to first-generation somatostatin analogs: long-term control with pasireotide LAR in two patients. Endocr Connect. 2019;8(4):367–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ramírez-Rentería C, Hernández-Ramírez LC, Portocarrero-Ortiz L, et al. AIP mutations in young patients with acromegaly and the Tampico Giant: the Mexican experience. Endocrine. 2016;53(2):402–411. [DOI] [PubMed] [Google Scholar]
- 61. Caimari F, Hernández-Ramírez LC, Dang MN, et al. ; International FIPA consortium . Risk category system to identify pituitary adenoma patients with AIP mutations. J Med Genet. 2018;55(4):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rostomyan L, Daly AF, Petrossians P, et al. Clinical and genetic characterization of pituitary gigantism: an international collaborative study in 208 patients. Endocr Relat Cancer. 2015;22(5):745–757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Iacovazzo D, Caswell R, Bunce B, et al. Germline or somatic GPR101 duplication leads to X-linked acrogigantism: a clinico-pathological and genetic study. Acta Neuropathol Commun. 2016;4(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tichomirowa MA, Barlier A, Daly AF, et al. High prevalence of AIP gene mutations following focused screening in young patients with sporadic pituitary macroadenomas. Eur J Endocrinol. 2011;165(4):509–515. [DOI] [PubMed] [Google Scholar]
- 65. Cazabat L, Bouligand J, Salenave S, et al. Germline AIP mutations in apparently sporadic pituitary adenomas: prevalence in a prospective single-center cohort of 443 patients. J Clin Endocrinol Metab. 2012;97(4):E663–E670. [DOI] [PubMed] [Google Scholar]
- 66. Preda V, Korbonits M, Cudlip S, Karavitaki N, Grossman AB. Low rate of germline AIP mutations in patients with apparently sporadic pituitary adenomas before the age of 40: a single-centre adult cohort. Eur J Endocrinol. 2014;171(5):659–666. [DOI] [PubMed] [Google Scholar]
- 67. Korbonits M, Storr H, Kumar AV. Familial pituitary adenomas - who should be tested for AIP mutations? Clin Endocrinol (Oxf). 2012;77(3):351–356. [DOI] [PubMed] [Google Scholar]
- 68. Lecoq AL, Kamenický P, Guiochon-Mantel A, Chanson P. Genetic mutations in sporadic pituitary adenomas–what to screen for? Nat Rev Endocrinol. 2015;11(1):43–54. [DOI] [PubMed] [Google Scholar]
- 69. Yano M, Terada K, Mori M. AIP is a mitochondrial import mediator that binds to both import receptor Tom20 and preproteins. J Cell Biol. 2003;163(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hage M, Chaligné R, Viengchareun S, et al. Hypermethylator phenotype and ectopic GIP receptor in GNAS mutation-negative somatotropinomas. J Clin Endocrinol Metab. 2019;104(5):1777–1787. [DOI] [PubMed] [Google Scholar]
- 71. Occhi G, Losa M, Albiger N, et al. The glucose-dependent insulinotropic polypeptide receptor is overexpressed amongst GNAS1 mutation-negative somatotropinomas and drives growth hormone (GH)-promoter activity in GH3 cells. J Neuroendocrinol. 2011;23(7):641–649. [DOI] [PubMed] [Google Scholar]
- 72. Regazzo D, Losa M, Albiger NM, et al. The GIP/GIPR axis is functionally linked to GH-secretion increase in a significant proportion of gsp- somatotropinomas. Eur J Endocrinol. 2017;176(5):543–553. [DOI] [PubMed] [Google Scholar]
- 73. Bates B, Zhang L, Nawoschik S, et al. Characterization of Gpr101 expression and G-protein coupling selectivity. Brain Res. 2006;1087(1):1–14. [DOI] [PubMed] [Google Scholar]
- 74. Daly AF, Lysy PA, Desfilles C, et al. GHRH excess and blockade in X-LAG syndrome. Endocr Relat Cancer. 2016;23(3):161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Trivellin G, Bjelobaba I, Daly AF, et al. Characterization of GPR101 transcript structure and expression patterns. J Mol Endocrinol. 2016;57(2):97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Flak MB, Koenis DS, Sobrino A, et al. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J Clin Invest. 2020;130(1):359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Cho-Clark M, Larco DO, Semsarzadeh NN, Vasta F, Mani SK, Wu TJ. GnRH-(1-5) transactivates EGFR in Ishikawa human endometrial cells via an orphan G protein-coupled receptor. Mol Endocrinol. 2014;28(1):80–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Rodd C, Millette M, Iacovazzo D, et al. Somatic GPR101 duplication causing X-Linked Acrogigantism (XLAG)-diagnosis and management. J Clin Endocrinol Metab. 2016;101(5):1927–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Naves LA, Daly AF, Dias LA, et al. Aggressive tumor growth and clinical evolution in a patient with X-linked acro-gigantism syndrome. Endocrine. 2016;51(2):236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Beckers A, Fernandes D, Fina F, et al. Paleogenetic study of ancient DNA suggestive of X-linked acrogigantism. Endocr Relat Cancer. 2017;24(2):L17–L20. [DOI] [PubMed] [Google Scholar]
- 81. Iacovazzo D, Korbonits M. X-linked acrogigantism. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, Amemiya A, eds. GeneReviews((R)). Seattle, WA: University of Washington, Seattle; 1993. [PubMed] [Google Scholar]
- 82. Daly AF, Yuan B, Fina F, et al. Somatic mosaicism underlies X-linked acrogigantism syndrome in sporadic male subjects. Endocr Relat Cancer. 2016;23(4):221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Moran A, Asa SL, Kovacs K, et al. Gigantism due to pituitary mammosomatotroph hyperplasia. N Engl J Med. 1990;323(5):322–327. [DOI] [PubMed] [Google Scholar]
- 84. Trivellin G, Correa RR, Batsis M, et al. Screening for GPR101 defects in pediatric pituitary corticotropinomas. Endocr Relat Cancer. 2016;23(5):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ferraù F, Romeo PD, Puglisi S, et al. Analysis of GPR101 and AIP genes mutations in acromegaly: a multicentric study. Endocrine. 2016;54(3):762–767. [DOI] [PubMed] [Google Scholar]
- 86. Lecoq AL, Bouligand J, Hage M, et al. Very low frequency of germline GPR101 genetic variation and no biallelic defects with AIP in a large cohort of patients with sporadic pituitary adenomas. Eur J Endocrinol. 2016;174(4):523–530. [DOI] [PubMed] [Google Scholar]
- 87. Hernández-Ramírez LC, Gam R, Valdés N, et al. Loss-of-function mutations in the CABLES1 gene are a novel cause of Cushing’s disease. Endocr Relat Cancer. 2017;24(8):379–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Roussel-Gervais A, Couture C, Langlais D, et al. The Cables1 gene in glucocorticoid regulation of pituitary corticotrope growth and cushing disease. J Clin Endocrinol Metab. 2016;101(2):513–522. [DOI] [PubMed] [Google Scholar]
- 89. Gorvin CM, Newey PJ, Rogers A, et al. Association of prolactin receptor (PRLR) variants with prolactinomas. Hum Mol Genet. 2019;28(6):1023–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Bernard V, Bouilly J, Beau I, et al. Germline prolactin receptor mutation is not a major cause of sporadic prolactinoma in humans. Neuroendocrinology. 2016;103(6):738–745. [DOI] [PubMed] [Google Scholar]
- 91. Schuff KG, Hentges ST, Kelly MA, et al. Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and -independent mechanisms. J Clin Invest. 2002;110(7):973–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bernard V, Villa C, Auguste A, et al. Natural and molecular history of prolactinoma: insights from a Prlr-/- mouse model. Oncotarget. 2018;9(5):6144–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Melo FM, Couto PP, Bale AE, et al. Whole-exome identifies RXRG and TH germline variants in familial isolated prolactinoma. Cancer Genet. 2016;209(6):251–257. [DOI] [PubMed] [Google Scholar]
- 94. Freese A, During MJ, Davidson BL, et al. Transfection of human lactotroph adenoma cells with an adenovirus vector expressing tyrosine hydroxylase decreases prolactin release. J Clin Endocrinol Metab. 1996;81(6):2401–2404. [DOI] [PubMed] [Google Scholar]
- 95. Williams JC, Stone D, Smith-Arica JR, Morris ID, Lowenstein PR, Castro MG. Regulated, adenovirus-mediated delivery of tyrosine hydroxylase suppresses growth of estrogen-induced pituitary prolactinomas. Mol Ther. 2001;4(6):593–602. [DOI] [PubMed] [Google Scholar]
- 96. Zhang Q, Peng C, Song J, et al. Germline mutations in CDH23, encoding cadherin-related 23, are associated with both familial and sporadic pituitary adenomas. Am J Hum Genet. 2017;100(5):817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Faucz FR, Horvath AD, Azevedo MF, et al. Is IGSF1 involved in human pituitary tumor formation? Endocr Relat Cancer. 2015;22(1):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Joustra SD, Roelfsema F, Endert E, et al. 2017 Male IGSF1 deficient humans and mice exhibit somatotroph neurosecretory hyperfunction. Poster presented at: Society for Endocrinology BES; Harrogate, UK; 06 - 08 Nov 2017; 2017-10-20, 2017. [Google Scholar]
- 99. Joustra SD, Roelfsema F, van Trotsenburg ASP, et al. IGSF1 deficiency results in human and murine somatotrope neurosecretory hyperfunction. J Clin Endocrinol Metab. 2020;105(3):e70–e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kobayashi T, Usui H, Tanaka H, Shozu M. Variant Prolactin Receptor in Agalactia and Hyperprolactinemia. N Engl J Med. 2018;379(23):2230–2236. [DOI] [PubMed] [Google Scholar]
- 101. Bolz H, von Brederlow B, Ramírez A, et al. Mutation of CDH23, encoding a new member of the cadherin gene family, causes Usher syndrome type 1D. Nat Genet. 2001;27(1):108–112. [DOI] [PubMed] [Google Scholar]
- 102. Mathur P, Yang J. Usher syndrome: Hearing loss, retinal degeneration and associated abnormalities. Biochim Biophys Acta. 2015;1852(3):406–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Sun Y, Bak B, Schoenmakers N, et al. Loss-of-function mutations in IGSF1 cause an X-linked syndrome of central hypothyroidism and testicular enlargement. Nat Genet. 2012;44(12):1375–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Joustra SD, Heinen CA, Schoenmakers N, et al. ; IGSF1 Clinical Care Group . IGSF1 deficiency: lessons from an extensive case series and recommendations for clinical management. J Clin Endocrinol Metab. 2016;101(4):1627–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Beijers HJBH, Stikkelbroeck NML, Mensenkamp AR, et al. Germline and somatic mosaicism in a family with multiple endocrine neoplasia type 1 (MEN1) syndrome. Eur J Endocrinol. 2019;180(2):K15–K19. [DOI] [PubMed] [Google Scholar]
- 106. Coppin L, Ferrière A, Crépin M, et al. Diagnosis of mosaic mutations in the MEN1 gene by next generation sequencing. Eur J Endocrinol. 2019;180(2):L1–L3. [DOI] [PubMed] [Google Scholar]
- 107. Mauchlen R, Carty D, Talla M, Drummond R. Multiple endocrine neoplasia type 1 (MEN1) mosaicism caused by a c.124G>A variant in the MEN1 gene. Endocrine Abstracts. 2019;65:CC4. [Google Scholar]
- 108. Pellegata NS. MENX. Ann Endocrinol (Paris). 2012;73(2):65–70. [DOI] [PubMed] [Google Scholar]
- 109. Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab. 2009;94(5):1826–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Forlino A, Vetro A, Garavelli L, et al. PRKACB and Carney complex. N Engl J Med. 2014;370(11):1065–1067. [DOI] [PubMed] [Google Scholar]
- 111. Dénes J, Swords F, Rattenberry E, et al. Heterogeneous genetic background of the association of pheochromocytoma/paraganglioma and pituitary adenoma: results from a large patient cohort. J Clin Endocrinol Metab. 2015;100(3):E531–E541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Xekouki P, Pacak K, Almeida M, et al. Succinate dehydrogenase (SDH) D subunit (SDHD) inactivation in a growth-hormone-producing pituitary tumor: a new association for SDH? J Clin Endocrinol Metab. 2012;97(3):E357–E366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Xekouki P, Szarek E, Bullova P, et al. Pituitary adenoma with paraganglioma/pheochromocytoma (3PAs) and succinate dehydrogenase defects in humans and mice. J Clin Endocrinol Metab. 2015;100(5):E710–E719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Roszko KL, Blouch E, Blake M, et al. Case report of a prolactinoma in a patient with a novel MAX mutation and bilateral pheochromocytomas. J Endocr Soc. 2017;1(11):1401–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Daly AF, Castermans E, Oudijk L, et al. Pheochromocytomas and pituitary adenomas in three patients with MAX exon deletions. Endocr Relat Cancer. 2018;25(5):L37–L42. [DOI] [PubMed] [Google Scholar]
- 116. Kobza AO, Dizon S, Arnaout A. Case report of bilateral pheochromocytomas due to a novel max mutation in a patient known to have a pituitary prolactinoma. AACE Clin Case Rep. 2018;4(6):e453–e456. [Google Scholar]
- 117. de Kock L, Sabbaghian N, Plourde F, et al. Pituitary blastoma: a pathognomonic feature of germ-line DICER1 mutations. Acta Neuropathol. 2014;128(1):111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Uraki S, Ariyasu H, Doi A, et al. Atypical pituitary adenoma with MEN1 somatic mutation associated with abnormalities of DNA mismatch repair genes; MLH1 germline mutation and MSH6 somatic mutation. Endocr J. 2017;64(9):895–906. [DOI] [PubMed] [Google Scholar]
- 119. Bengtsson D, Joost P, Aravidis C, et al. Corticotroph pituitary carcinoma in a patient with lynch syndrome (LS) and pituitary tumors in a nationwide LS cohort. J Clin Endocrinol Metab. 2017;102(11):3928–3932. [DOI] [PubMed] [Google Scholar]
- 120. Salomon MP, Wang X, Marzese DM, et al. The epigenomic landscape of pituitary adenomas reveals specific alterations and differentiates among acromegaly, Cushing’s disease and endocrine-inactive subtypes. Clin Cancer Res. 2018;24(17):4126–4136. [DOI] [PubMed] [Google Scholar]
- 121. Bujko M, Kober P, Zbijewska J, Kunicki J, Bonicki W. Microsatellite instability analysis in pituitary adenomas. Neuro Endocrinol Lett. 2015;36(5):511–514. [PubMed] [Google Scholar]
- 122. Cohen M, Persky R, Stegemann R, et al. Germline USP8 mutation associated with pediatric cushing disease and other clinical features: a new syndrome. J Clin Endocrinol Metab. 2019;104(10):4676–4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hoffman WH, Perrin JC, Halac E, Gala RR, England BG. Acromegalic gigantism and tuberous sclerosis. J Pediatr. 1978;93(3):478–480. [DOI] [PubMed] [Google Scholar]
- 124. Tigas S, Carroll PV, Jones R, et al. Simultaneous Cushing’s disease and tuberous sclerosis; a potential role for TSC in pituitary ontogeny. Clin Endocrinol (Oxf). 2005;63(6):694–695. [DOI] [PubMed] [Google Scholar]
- 125. Nandagopal R, Vortmeyer A, Oldfield EH, Keil MF, Stratakis CA. Cushing’s syndrome due to a pituitary corticotropinoma in a child with tuberous sclerosis: an association or a coincidence? Clin Endocrinol (Oxf). 2007;67(4):639–641. [DOI] [PubMed] [Google Scholar]
- 126. Regazzo D, Gardiman MP, Theodoropoulou M, Scaroni C, Occhi G, Ceccato F. Silent gonadotroph pituitary neuroendocrine tumor in a patient with tuberous sclerosis complex: evaluation of a possible molecular link. Endocrinol Diabetes Metab Case Rep. 2018;2018(1):18–0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Lin Y, Jiang X, Shen Y, et al. Frequent mutations and amplifications of the PIK3CA gene in pituitary tumors. Endocr Relat Cancer. 2009;16(1):301–310. [DOI] [PubMed] [Google Scholar]
- 128. Murat CB, Braga PB, Fortes MA, Bronstein MD, Corrêa-Giannella ML, Giorgi RR. Mutation and genomic amplification of the PIK3CA proto-oncogene in pituitary adenomas. Braz J Med Biol Res. 2012;45(9):851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hao S, Hong CS, Feng J, et al. Somatic IDH1 mutation in a pituitary adenoma of a patient with Maffucci syndrome. J Neurosurg. 2016;124(6):1562–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Nejo T, Tanaka S, Ikemura M, et al. Maffucci syndrome complicated by three different central nervous system tumors sharing an IDH1 R132C mutation: case report. J Neurosurg. 2018;131(6):1829–1834. [DOI] [PubMed] [Google Scholar]
- 131. Tanizaki Y, Jin L, Scheithauer BW, Kovacs K, Roncaroli F, Lloyd RV. P53 gene mutations in pituitary carcinomas. Endocr Pathol. 2007;18(4):217–222. [DOI] [PubMed] [Google Scholar]
- 132. Sbiera S, Perez-Rivas LG, Taranets L, et al. Driver mutations in USP8 wild-type Cushing’s disease. Neuro Oncol. 2019;21(10):1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Finelli P, Pierantoni GM, Giardino D, et al. The High Mobility Group A2 gene is amplified and overexpressed in human prolactinomas. Cancer Res. 2002;62(8):2398–2405. [PubMed] [Google Scholar]
- 134. Pierantoni GM, Finelli P, Valtorta E, et al. High-mobility group A2 gene expression is frequently induced in non-functioning pituitary adenomas (NFPAs), even in the absence of chromosome 12 polysomy. Endocr Relat Cancer. 2005;12(4):867–874. [DOI] [PubMed] [Google Scholar]
- 135. Fedele M, Palmieri D, Fusco A. HMGA2: A pituitary tumour subtype-specific oncogene? Mol Cell Endocrinol. 2010;326(1-2):19–24. [DOI] [PubMed] [Google Scholar]
- 136. Pei L, Melmed S, Scheithauer B, Kovacs K, Prager D. H-ras mutations in human pituitary carcinoma metastases. J Clin Endocrinol Metab. 1994;78(4):842–846. [DOI] [PubMed] [Google Scholar]
- 137. Kurelac I, MacKay A, Lambros MB, et al. Somatic complex I disruptive mitochondrial DNA mutations are modifiers of tumorigenesis that correlate with low genomic instability in pituitary adenomas. Hum Mol Genet. 2013;22(2):226-238. [DOI] [PubMed] [Google Scholar]
- 138. Karl M, Von Wichert G, Kempter E, et al. Nelson’s syndrome associated with a somatic frame shift mutation in the glucocorticoid receptor gene. J Clin Endocrinol Metab. 1996;81(1):124–129. [DOI] [PubMed] [Google Scholar]
- 139. Antonini SR, Latronico AC, Elias LL, et al. Glucocorticoid receptor gene polymorphisms in ACTH-secreting pituitary tumours. Clin Endocrinol (Oxf). 2002;57(5):657–662. [DOI] [PubMed] [Google Scholar]
- 140. Briassoulis G, Horvath A, Christoforou P, et al. Lack of mutations in the gene coding for the hGR (NR3C1) in a pediatric patient with ACTH-secreting pituitary adenoma, absence of stigmata of Cushing’s syndrome and unusual histologic features. J Pediatr Endocrinol Metab. 2012;25(1-2):213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Li C, Xie W, Rosenblum JS, et al. Somatic SF3B1 hotspot mutation in prolactinomas. Nat Commun. 2020;11(1):2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Song ZJ, Reitman ZJ, Ma ZY, et al. The genome-wide mutational landscape of pituitary adenomas. Cell Res. 2016;26(11):1255–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bi WL, Greenwald NF, Ramkissoon SH, et al. Clinical identification of oncogenic drivers and copy-number alterations in pituitary tumors. Endocrinology. 2017;158(7):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Landis CA, Masters SB, Spada A, Pace AM, Bourne HR, Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989;340(6236):692–696. [DOI] [PubMed] [Google Scholar]
- 145. Landis CA, Harsh G, Lyons J, Davis RL, McCormick F, Bourne HR. Clinical characteristics of acromegalic patients whose pituitary tumors contain mutant Gs protein. J Clin Endocrinol Metab. 1990;71(6):1416–1420. [DOI] [PubMed] [Google Scholar]
- 146. Shi Y, Tang D, Deng J, Su C. Detection of gsp oncogene in growth hormone-secreting pituitary adenomas and the study of clinical characteristics of acromegalic patients with gsp-positive pituitary tumors. Chin Med J (Engl). 1998;111(10):891–894. [PubMed] [Google Scholar]
- 147. Freda PU, Chung WK, Matsuoka N, et al. Analysis of GNAS mutations in 60 growth hormone secreting pituitary tumors: correlation with clinical and pathological characteristics and surgical outcome based on highly sensitive GH and IGF-I criteria for remission. Pituitary. 2007;10(3):275–282. [DOI] [PubMed] [Google Scholar]
- 148. Larkin S, Reddy R, Karavitaki N, Cudlip S, Wass J, Ansorge O. Granulation pattern, but not GSP or GHR mutation, is associated with clinical characteristics in somatostatin-naive patients with somatotroph adenomas. Eur J Endocrinol. 2013;168(4):491–499. [DOI] [PubMed] [Google Scholar]
- 149. Buchfelder M, Fahlbusch R, Merz T, Symowski H, Adams EF. Clinical correlates in acromegalic patients with pituitary tumors expressing GSP oncogenes. Pituitary. 1999;1(3-4):181–185. [DOI] [PubMed] [Google Scholar]
- 150. Efstathiadou ZA, Bargiota A, Chrisoulidou A, et al. Impact of gsp mutations in somatotroph pituitary adenomas on growth hormone response to somatostatin analogs: a meta-analysis. Pituitary. 2015;18(6):861–867. [DOI] [PubMed] [Google Scholar]
- 151. Foltran RK, Amorim PVGH, Duarte FH, et al. Study of major genetic factors involved in pituitary tumorigenesis and their impact on clinical and biological characteristics of sporadic somatotropinomas and non-functioning pituitary adenomas. Braz J Med Biol Res. 2018;51(9):e7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Välimäki N, Schalin-Jäntti C, Karppinen A, et al. Genetic and epigenetic characterization of growth hormone-secreting pituitary tumors. Mol Cancer Res. 2019;17(12):2432–2443. [DOI] [PubMed] [Google Scholar]
- 153. Albright F, Butler AM, Hampton AO, Smith P. Syndrome characterized by osteitis fibrosa disseminata, areas of pigmentation and endocrine dysfunction, with precocious puberty in females. N Engl J Med. 1937;216:727–746. [Google Scholar]
- 154. Ma ZY, Song ZJ, Chen JH, et al. Recurrent gain-of-function USP8 mutations in Cushing’s disease. Cell Res. 2015;25(3):306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Reincke M, Sbiera S, Hayakawa A, et al. Mutations in the deubiquitinase gene USP8 cause Cushing’s disease. Nat Genet. 2015;47(1):31–38. [DOI] [PubMed] [Google Scholar]
- 156. Perez-Rivas LG, Theodoropoulou M, Ferraù F, et al. The gene of the ubiquitin-specific protease 8 is frequently mutated in adenomas causing Cushing’s disease. J Clin Endocrinol Metab. 2015;100(7):E997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Losa M, Mortini P, Pagnano A, Detomas M, Cassarino MF, Pecori Giraldi F. Clinical characteristics and surgical outcome in USP8-mutated human adrenocorticotropic hormone-secreting pituitary adenomas. Endocrine. 2019;63(2): 240–246. [DOI] [PubMed] [Google Scholar]
- 158. Ballmann C, Thiel A, Korah HE, et al. USP8 mutations in pituitary Cushing adenomas-targeted analysis by next-generation sequencing. J Endocr Soc. 2018;2(3):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hayashi K, Inoshita N, Kawaguchi K, et al. The USP8 mutational status may predict drug susceptibility in corticotroph adenomas of Cushing’s disease. Eur J Endocrinol. 2016;174(2):213–226. [DOI] [PubMed] [Google Scholar]
- 160. Weigand I, Knobloch L, Flitsch J, et al. Impact of USP8 gene mutations on protein deregulation in Cushing disease. J Clin Endocrinol Metab. 2019;104(7):2535–2546. [DOI] [PubMed] [Google Scholar]
- 161. Chen J, Jian X, Deng S, et al. Identification of recurrent USP48 and BRAF mutations in Cushing’s disease. Nat Commun. 2018;9(1):3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Jian FF, Li YF, Chen YF, et al. Inhibition of ubiquitin-specific peptidase 8 suppresses adrenocorticotropic hormone production and tumorous corticotroph cell growth in AtT20 cells. Chin Med J (Engl). 2016;129(17):2102–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Asari Y, Kageyama K, Sugiyama A, Kogawa H, Niioka K, Daimon M. Lapatinib decreases the ACTH production and proliferation of corticotroph tumor cells. Endocr J. 2019;66(6):515–522. [DOI] [PubMed] [Google Scholar]
- 164. Wanichi IQ, de Paula Mariani BM, Frassetto FP, et al. Cushing’s disease due to somatic USP8 mutations: a systematic review and meta-analysis. Pituitary. 2019;22(4):435–442. [DOI] [PubMed] [Google Scholar]
- 165. Albani A, Perez-Rivas LG, Dimopoulou C, et al. The USP8 mutational status may predict long-term remission in patients with Cushing’s disease. Clin Endocrinol (Oxf). 2018;89(4):454–458. [DOI] [PubMed] [Google Scholar]
- 166. Faucz FR, Tirosh A, Tatsi C, et al. Somatic USP8 gene mutations are a common cause of pediatric Cushing disease. J Clin Endocrinol Metab. 2017;102(8):2836–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146(5):707–716. [DOI] [PubMed] [Google Scholar]
- 168. Xia R, Jia H, Fan J, Liu Y, Jia J. USP8 promotes smoothened signaling by preventing its ubiquitination and changing its subcellular localization. Plos Biol. 2012;10(1):e1001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Bi WL, Horowitz P, Greenwald NF, et al. Landscape of Genomic Alterations in Pituitary Adenomas. Clin Cancer Res. 2017;23(7):1841–1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Megnis K, Peculis R, Rovite V, et al. Evaluation of the possibility to detect circulating tumor DNA from pituitary adenoma. Front Endocrinol (Lausanne). 2019;10:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Drouin J, Bilodeau S, Vallette S. Of old and new diseases: genetics of pituitary ACTH excess (Cushing) and deficiency. Clin Genet. 2007;72(3):175–182. [DOI] [PubMed] [Google Scholar]
- 172. Karl M, Lamberts SW, Koper JW, et al. Cushing’s disease preceded by generalized glucocorticoid resistance: clinical consequences of a novel, dominant-negative glucocorticoid receptor mutation. Proc Assoc Am Physicians. 1996;108(4):296–307. [PubMed] [Google Scholar]
- 173. Lindberg J, Klevebring D, Liu W, et al. Exome sequencing of prostate cancer supports the hypothesis of independent tumour origins. Eur Urol. 2013;63(2):347–353. [DOI] [PubMed] [Google Scholar]
- 174. Ye Z, Li Z, Wang Y, et al. Common variants at 10p12.31, 10q21.1 and 13q12.13 are associated with sporadic pituitary adenoma. Nat Genet. 2015;47(7):793–797. [DOI] [PubMed] [Google Scholar]
- 175. Firestein R, Bass AJ, Kim SY, et al. CDK8 is a colorectal cancer oncogene that regulates beta-catenin activity. Nature. 2008;455(7212):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Kim MY, Han SI, Lim SC. Roles of cyclin-dependent kinase 8 and β-catenin in the oncogenesis and progression of gastric adenocarcinoma. Int J Oncol. 2011;38(5):1375–1383. [DOI] [PubMed] [Google Scholar]
- 177. Sapkota S, Horiguchi K, Tosaka M, Yamada S, Yamada M. Whole-exome sequencing study of thyrotropin-secreting pituitary adenomas. J Clin Endocrinol Metab. 2017;102(2):566–575. [DOI] [PubMed] [Google Scholar]
- 178. Newey PJ, Nesbit MA, Rimmer AJ, et al. Whole-exome sequencing studies of nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2013;98(4):E796–E800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Lan X, Gao H, Wang F, et al. Whole-exome sequencing identifies variants in invasive pituitary adenomas. Oncol Lett. 2016;12(4):2319–2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Tatsi C, Pankratz N, Lane J, et al. Large genomic aberrations in corticotropinomas are associated with greater aggressiveness. J Clin Endocrinol Metab. 2019;104(5):1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Hage M, Viengchareun S, Brunet E, et al. Genomic alterations and complex subclonal architecture in sporadic GH-secreting pituitary adenomas. J Clin Endocrinol Metab. 2018;103(5):1929–1939. [DOI] [PubMed] [Google Scholar]
- 182. Pei L, Melmed S. Isolation and characterization of a pituitary tumor-transforming gene (PTTG). Mol Endocrinol. 1997;11(4):433–441. [DOI] [PubMed] [Google Scholar]
- 183. Zhang X, Horwitz GA, Heaney AP, et al. Pituitary tumor transforming gene (PTTG) expression in pituitary adenomas. J Clin Endocrinol Metab. 1999;84(2):761–767. [DOI] [PubMed] [Google Scholar]
- 184. Németh K, Darvasi O, Likó I, et al. Next-generation sequencing identifies novel mitochondrial variants in pituitary adenomas. J Endocrinol Invest. 2019;42(8):931–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Pease M, Ling C, Mack WJ, Wang K, Zada G. The role of epigenetic modification in tumorigenesis and progression of pituitary adenomas: a systematic review of the literature. PLoS One. 2013;8(12):e82619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ogino A, Yoshino A, Katayama Y, et al. The p15(INK4b)/p16(INK4a)/RB1 pathway is frequently deregulated in human pituitary adenomas. J Neuropathol Exp Neurol. 2005;64(5):398–403. [DOI] [PubMed] [Google Scholar]
- 187. Simpson DJ, Frost SJ, Bicknell JE, et al. Aberrant expression of G(1)/S regulators is a frequent event in sporadic pituitary adenomas. Carcinogenesis. 2001;22(8):1149–1154. [DOI] [PubMed] [Google Scholar]
- 188. Seemann N, Kuhn D, Wrocklage C, et al. CDKN2A/p16 inactivation is related to pituitary adenoma type and size. J Pathol. 2001;193(4):491–497. [DOI] [PubMed] [Google Scholar]
- 189. Abd El-Moneim HM, Abd El-Rehim D. Immunohistochemical and molecular study of p16INK4A expression in pituitary adenoma. J Egypt Natl Canc Inst. 2009;21(4):351–360. [PubMed] [Google Scholar]
- 190. Yoshino A, Katayama Y, Ogino A, et al. Promoter hypermethylation profile of cell cycle regulator genes in pituitary adenomas. J Neurooncol. 2007;83(2):153–162. [DOI] [PubMed] [Google Scholar]
- 191. Simpson DJ, Hibberts NA, McNicol AM, Clayton RN, Farrell WE. Loss of pRb expression in pituitary adenomas is associated with methylation of the RB1 CpG island. Cancer Res. 2000;60(5):1211–1216. [PubMed] [Google Scholar]
- 192. Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359(6393):295–300. [DOI] [PubMed] [Google Scholar]
- 193. Shorts-Cary L, Xu M, Ertel J, et al. Bone morphogenetic protein and retinoic acid-inducible neural specific protein-3 is expressed in gonadotrope cell pituitary adenomas and induces proliferation, migration, and invasion. Endocrinology. 2007;148(3):967–975. [DOI] [PubMed] [Google Scholar]
- 194. Theodoropoulou M, Arzberger T, Gruebler Y, et al. Expression of epidermal growth factor receptor in neoplastic pituitary cells: evidence for a role in corticotropinoma cells. J Endocrinol. 2004;183(2):385–394. [DOI] [PubMed] [Google Scholar]
- 195. Ma HS, Wang EL, Xu WF, et al. Overexpression of DNA (Cytosine-5)-Methyltransferase 1 (DNMT1) And DNA (Cytosine-5)-Methyltransferase 3A (DNMT3A) is associated with aggressive behavior and hypermethylation of tumor suppressor genes in human pituitary adenomas. Med Sci Monit. 2018;24:4841–4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Zhu X, Mao X, Hurren R, Schimmer AD, Ezzat S, Asa SL. Deoxyribonucleic acid methyltransferase 3B promotes epigenetic silencing through histone 3 chromatin modifications in pituitary cells. J Clin Endocrinol Metab. 2008;93(9):3610–3617. [DOI] [PubMed] [Google Scholar]
- 197. Hayward BE, Barlier A, Korbonits M, et al. Imprinting of the G(s)alpha gene GNAS1 in the pathogenesis of acromegaly. J Clin Invest. 2001;107(6):R31–R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Duong CV, Emes RD, Wessely F, Yacqub-Usman K, Clayton RN, Farrell WE. Quantitative, genome-wide analysis of the DNA methylome in sporadic pituitary adenomas. Endocr Relat Cancer. 2012;19(6):805–816. [DOI] [PubMed] [Google Scholar]
- 199. Capper D, Jones DTW, Sill M, et al. DNA methylation-based classification of central nervous system tumours. Nature. 2018;555(7697):469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Ling C, Pease M, Shi L, et al. A pilot genome-scale profiling of DNA methylation in sporadic pituitary macroadenomas: association with tumor invasion and histopathological subtype. Plos One. 2014;9(4):e96178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. García-Martínez A, Sottile J, Sánchez-Tejada L, et al. DNA methylation of tumor suppressor genes in pituitary neuroendocrine tumors. J Clin Endocrinol Metab. 2019;104(4):1272–1282. [DOI] [PubMed] [Google Scholar]
- 202. Gu Y, Zhou X, Hu F, et al. Differential DNA methylome profiling of nonfunctioning pituitary adenomas suggesting tumour invasion is correlated with cell adhesion. J Neurooncol. 2016;129(1):23–31. [DOI] [PubMed] [Google Scholar]
- 203. Kober P, Boresowicz J, Rusetska N, et al. DNA methylation profiling in nonfunctioning pituitary adenomas. Mol Cell Endocrinol. 2018;473:194–204. [DOI] [PubMed] [Google Scholar]
- 204. Wang RQ, Lan YL, Lou JC, et al. Expression and methylation status of LAMA2 are associated with the invasiveness of nonfunctioning PitNET. Ther Adv Endocrinol Metab. 2019;10:2042018818821296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Song W, Qian L, Jing G, et al. Aberrant expression of the sFRP and WIF1 genes in invasive non-functioning pituitary adenomas. Mol Cell Endocrinol. 2018;474:168–175. [DOI] [PubMed] [Google Scholar]
- 206. Castelo-Branco P, Choufani S, Mack S, et al. Methylation of the TERT promoter and risk stratification of childhood brain tumours: an integrative genomic and molecular study. Lancet Oncol. 2013;14(6):534–542. [DOI] [PubMed] [Google Scholar]
- 207. Lindsey JC, Schwalbe EC, Potluri S, Bailey S, Williamson D, Clifford SC. TERT promoter mutation and aberrant hypermethylation are associated with elevated expression in medulloblastoma and characterise the majority of non-infant SHH subgroup tumours. Acta Neuropathol. 2014;127(2):307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Fürtjes G, Köchling M, Peetz-Dienhart S, et al. hTERT promoter methylation in meningiomas and central nervous hemangiopericytomas. J Neurooncol. 2016;130(1):79–87. [DOI] [PubMed] [Google Scholar]
- 209. Miyake Y, Adachi JI, Suzuki T, et al. TERT promoter methylation is significantly associated with TERT upregulation and disease progression in pituitary adenomas. J Neurooncol. 2019;141(1):131–138. [DOI] [PubMed] [Google Scholar]
- 210. Salehi F, Scheithauer BW, Kros JM, et al. MGMT promoter methylation and immunoexpression in aggressive pituitary adenomas and carcinomas. J Neurooncol. 2011;104(3):647–657. [DOI] [PubMed] [Google Scholar]
- 211. Arya S, Majaid MA, Shwetha SD, et al. Implications of MGMT methylation status in pituitary adenoma. Pathol Res Pract. 2014;210(7):407–411. [DOI] [PubMed] [Google Scholar]
- 212. Syro LV, Rotondo F, Camargo M, Ortiz LD, Serna CA, Kovacs K. Temozolomide and pituitary tumors: current understanding, unresolved issues, and future directions. Front Endocrinol (Lausanne). 2018;9:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Raverot G, Burman P, McCormack A, et al. ; European Society of Endocrinology . European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol. 2018;178(1):G1–G24. [DOI] [PubMed] [Google Scholar]
- 214. McCormack A, Dekkers OM, Petersenn S, et al. Treatment of aggressive pituitary tumours and carcinomas: results of a European Society of Endocrinology (ESE) survey 2016. Eur J Endocrinol. 2018;178(3):265–276. [DOI] [PubMed] [Google Scholar]
- 215. Bengtsson D, Schrøder HD, Andersen M, et al. Long-term outcome and MGMT as a predictive marker in 24 patients with atypical pituitary adenomas and pituitary carcinomas given treatment with temozolomide. J Clin Endocrinol Metab. 2015;100(4):1689–1698. [DOI] [PubMed] [Google Scholar]
- 216. Ferraù F, Romeo PD, Puglisi S, et al. GSTP1 gene methylation and AHR rs2066853 variant predict resistance to first generation somatostatin analogs in patients with acromegaly. J Endocrinol Invest. 2019;42(7):825–831. [DOI] [PubMed] [Google Scholar]
- 217. Ezzat S, Zhu X, Loeper S, Fischer S, Asa SL. Tumor-derived Ikaros 6 acetylates the Bcl-XL promoter to up-regulate a survival signal in pituitary cells. Mol Endocrinol. 2006;20(11):2976–2986. [DOI] [PubMed] [Google Scholar]
- 218. Yacqub-Usman K, Duong CV, Clayton RN, Farrell WE. Epigenomic silencing of the BMP-4 gene in pituitary adenomas: a potential target for epidrug-induced re-expression. Endocrinology. 2012;153(8):3603–3612. [DOI] [PubMed] [Google Scholar]
- 219. Grande IPP, Amorim PVGH, Freire ACTB, et al. Differential gene expression of sirtuins between somatotropinomas and nonfunctioning pituitary adenomas. Pituitary. 2018;21(4):355–361. [DOI] [PubMed] [Google Scholar]
- 220. Bilodeau S, Vallette-Kasic S, Gauthier Y, et al. Role of Brg1 and HDAC2 in GR trans-repression of the pituitary POMC gene and misexpression in Cushing disease. Genes Dev. 2006;20(20):2871–2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Wang W, Fu L, Li S, Xu Z, Li X. Histone deacetylase 11 suppresses p53 expression in pituitary tumor cells. Cell Biol Int. 2017;41(12):1290–1295. [DOI] [PubMed] [Google Scholar]
- 222. Lu J, Chatain GP, Bugarini A, et al. Histone deacetylase inhibitor SAHA is a promising treatment of Cushing disease. J Clin Endocrinol Metab. 2017;102(8):2825–2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Li T, Huang H, Huang B, Huang B, Lu J. Histone acetyltransferase p300 regulates the expression of human pituitary tumor transforming gene (hPTTG). J Genet Genomics. 2009;36(6):335–342. [DOI] [PubMed] [Google Scholar]
- 224. Wang X, Duan W, Li X, et al. PTTG regulates the metabolic switch of ovarian cancer cells via the c-myc pathway. Oncotarget. 2015;6(38):40959–40969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Xiao JQ, Liu XH, Hou B, et al. Correlations of pituitary tumor transforming gene expression with human pituitary adenomas: a meta-analysis. Plos One. 2014;9(3):e90396. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 226. Trott G, Ongaratti BR, de Oliveira Silva CB, et al. PTTG overexpression in non-functioning pituitary adenomas: correlation with invasiveness, female gender and younger age. Ann Diagn Pathol. 2019;41:83–89. [DOI] [PubMed] [Google Scholar]
- 227. Ebrahimi A, Schittenhelm J, Honegger J, Schluesener HJ. Histone acetylation patterns of typical and atypical pituitary adenomas indicate epigenetic shift of these tumours. J Neuroendocrinol. 2011;23(6):525–530. [DOI] [PubMed] [Google Scholar]
- 228. Xue Y, Chen R, Du W, Yang F, Wei X. RIZ1 and histone methylation status in pituitary adenomas. Tumour Biol. 2017;39(7):1010428317711794. [DOI] [PubMed] [Google Scholar]
- 229. DeVore SB, Young CH, Li G, et al. Histone citrullination represses MicroRNA expression, resulting in increased oncogene mRNAs in somatolactotrope cells. Mol Cell Biol. 2018;38(19):e00084_00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Zhang T, Yang Z, Gao H. Advancements in the study of miRNA regulation during the cell cycle in human pituitary adenomas. J Neurooncol. 2017;134(2):253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Wierinckx A, Roche M, Legras-Lachuer C, Trouillas J, Raverot G, Lachuer J. MicroRNAs in pituitary tumors. Mol Cell Endocrinol. 2017;456:51-61. [DOI] [PubMed] [Google Scholar]
- 232. Gadelha MR, Kasuki L, Dénes J, Trivellin G, Korbonits M. MicroRNAs: Suggested role in pituitary adenoma pathogenesis. J Endocrinol Invest. 2013;36(10):889–895. [DOI] [PubMed] [Google Scholar]
- 233. Leone V, Langella C, D’Angelo D, et al. Mir-23b and miR-130b expression is downregulated in pituitary adenomas. Mol Cell Endocrinol. 2014;390(1-2):1–7. [DOI] [PubMed] [Google Scholar]
- 234. D’Angelo D, Esposito F, Fusco A. Epigenetic mechanisms leading to overexpression of HMGA proteins in human pituitary adenomas. Front Med (Lausanne). 2015;2:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Niu Y, Zhou H, Liu Y, et al. miR-16 regulates proliferation and apoptosis of pituitary adenoma cells by inhibiting HMGA2. Oncol Lett. 2019;17(2):2491–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Fedele M, Pierantoni GM, Visone R, Fusco A. E2F1 activation is responsible for pituitary adenomas induced by HMGA2 gene overexpression. Cell Div. 2006;1:17. Doi: 10.1186/1747-1028-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Fedele M, Visone R, De Martino I, et al. HMGA2 induces pituitary tumorigenesis by enhancing E2F1 activity. Cancer Cell. 2006;9(6):459–471. [DOI] [PubMed] [Google Scholar]
- 238. D’Angelo D, Palmieri D, Mussnich P, et al. Altered microRNA expression profile in human pituitary GH adenomas: down-regulation of miRNA targeting HMGA1, HMGA2, and E2F1. J Clin Endocrinol Metab. 2012;97(7):E1128–E1138. [DOI] [PubMed] [Google Scholar]
- 239. Müssnich P, Raverot G, Jaffrain-Rea ML, et al. Downregulation of miR-410 targeting the cyclin B1 gene plays a role in pituitary gonadotroph tumors. Cell Cycle. 2015;14(16):2590–2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. He Z, Chen L, Wang Q, et al. MicroRNA-186 targets SKP2 to induce p27Kip1-mediated pituitary tumor cell cycle deregulation and modulate cell proliferation. Korean J Physiol Pharmacol. 2019;23(3):171–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Liang HQ, Wang RJ, Diao CF, Li JW, Su JL, Zhang S. The PTTG1-targeting miRNAs miR-329, miR-300, miR-381, and miR-655 inhibit pituitary tumor cell tumorigenesis and are involved in a p53/PTTG1 regulation feedback loop. Oncotarget. 2015;6(30):29413–29427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Zhao S, Li J, Feng J, et al. Identification of serum miRNA-423-5p expression signature in somatotroph adenomas. Int J Endocrinol. 2019;2019:8516858. Doi: 10.1155/2019/8516858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Chien W, Pei L. A novel binding factor facilitates nuclear translocation and transcriptional activation function of the pituitary tumor-transforming gene product. J Biol Chem. 2000;275(25):19422–19427. [DOI] [PubMed] [Google Scholar]
- 244. Zhen W, Qiu D, Zhiyong C, et al. MicroRNA-524-5p functions as a tumor suppressor in a human pituitary tumor-derived cell line. Horm Metab Res. 2017;49(7):550–557. [DOI] [PubMed] [Google Scholar]
- 245. Hu A, Zhang Y, Zhao X, Li J, Ying Y. CBX1 is a direct target of miR-205-5p and contributes to the progression of pituitary tumor. Pharmazie. 2019;74(3):154–156. [DOI] [PubMed] [Google Scholar]
- 246. He C, Yang J, Ding J, et al. Downregulation of glucose‑6‑phosphate dehydrogenase by microRNA‑1 inhibits the growth of pituitary tumor cells. Oncol Rep. 2018;40(6):3533–3542. [DOI] [PubMed] [Google Scholar]
- 247. Yang Z, Zhang T, Wang Q, Gao H. Overexpression of microRNA-34a attenuates proliferation and induces apoptosis in pituitary adenoma cells via SOX7. Mol Ther Oncolytics. 2018;10:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Qiu P, Xu TJ, Lu XD, Yang W, Zhang YB, Xu GM. MicroRNA-378 regulates cell proliferation and migration by repressing RNF31 in pituitary adenoma. Oncol Lett. 2018;15(1):789–794. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 249. Trivellin G, Butz H, Delhove J, et al. MicroRNA miR-107 is overexpressed in pituitary adenomas and inhibits the expression of aryl hydrocarbon receptor-interacting protein in vitro. Am J Physiol Endocrinol Metab. 2012;303(6):E708–E719. [DOI] [PubMed] [Google Scholar]
- 250. Dénes J, Kasuki L, Trivellin G, et al. Regulation of aryl hydrocarbon receptor interacting protein (AIP) protein expression by MiR-34a in sporadic somatotropinomas. Plos One. 2015;10(2):e0117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Nemeth K, Darvasi O, Liko I, et al. Comprehensive analysis of circulating microRNAs in plasma of patients with pituitary adenomas. J Clin Endocrinol Metab. 2019;104(9):4151–4168. [DOI] [PubMed] [Google Scholar]
- 252. He Z, Chen L, Hu X, et al. Next-generation sequencing of microRNAs reveals a unique expression pattern in different types of pituitary adenomas. Endocr J. 2019;66(8):709–722. [DOI] [PubMed] [Google Scholar]
- 253. Ren J, Gu C, Yang Y, et al. TSP-1 is downregulated and inversely correlates with miR-449c expression in Cushing’s disease. J Cell Mol Med. 2019;23(6):4097–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Renjie W, Haiqian L. MiR-132, miR-15a and miR-16 synergistically inhibit pituitary tumor cell proliferation, invasion and migration by targeting Sox5. Cancer Lett. 2015;356(2 Pt B):568–578. [DOI] [PubMed] [Google Scholar]
- 255. Lines KE, Newey PJ, Yates CJ, et al. MiR-15a/miR-16-1 expression inversely correlates with cyclin D1 levels in Men1 pituitary NETs. J Endocrinol. 2018;240(1):41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Lu B, Liu GL, Yu F, Li WJ, Xiang XX, Xiao HZ. MicroRNA‑16/VEGFR2/p38/NF‑κB signaling pathway regulates cell growth of human pituitary neoplasms. Oncol Rep. 2018;39(3):1235–1244. [DOI] [PubMed] [Google Scholar]
- 257. Cui M, Zhang M, Liu HF, Wang JP. Effects of microRNA-21 targeting PITX2 on proliferation and apoptosis of pituitary tumor cells. Eur Rev Med Pharmacol Sci. 2017;21(13):2995–3004. [PubMed] [Google Scholar]
- 258. Du Q, Hu B, Feng Y, et al. circOMA1-mediated miR-145-5p suppresses tumor growth of nonfunctioning pituitary adenomas by targeting TPT1. J Clin Endocrinol Metab. 2019;104(6):2419–2434. [DOI] [PubMed] [Google Scholar]
- 259. Shen DW, Li YL, Hou YJ, Xu ZD, Li YZ, Chang JY. MicroRNA-543 promotes cell invasion and impedes apoptosis in pituitary adenoma via activating the Wnt/β-catenin pathway by negative regulation of Smad7. Biosci Biotechnol Biochem. 2019;83(6):1035–1044. [DOI] [PubMed] [Google Scholar]
- 260. Roche M, Wierinckx A, Croze S, et al. Deregulation of miR-183 and KIAA0101 in aggressive and malignant pituitary tumors. Front Med (Lausanne). 2015;2:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Garbicz F, Mehlich D, Rak B, et al. Increased expression of the microRNA 106b~25 cluster and its host gene MCM7 in corticotroph pituitary adenomas is associated with tumor invasion and Crooke’s cell morphology. Pituitary. 2017;20(4):450–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Zheng Z, Zhang Y, Zhang Z, Yang Y, Song T. Effect of miR-106b on invasiveness of pituitary adenoma via PTEN-PI3K/AKT. Med Sci Monit. 2017;23:1277–1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Zhou K, Zhang T, Fan Y, et al. MicroRNA-106b promotes pituitary tumor cell proliferation and invasion through PI3K/AKT signaling pathway by targeting PTEN. Tumour Biol. 2016;37(10):13469–13477. [DOI] [PubMed] [Google Scholar]
- 264. Wu S, Gu Y, Huang Y, et al. Novel biomarkers for non-functioning invasive pituitary adenomas were identified by using analysis of microRNAs expression profile. Biochem Genet. 2017;55(3):253–267. [DOI] [PubMed] [Google Scholar]
- 265. Palumbo T, Faucz FR, Azevedo M, Xekouki P, Iliopoulos D, Stratakis CA. Functional screen analysis reveals miR-26b and miR-128 as central regulators of pituitary somatomammotrophic tumor growth through activation of the PTEN-AKT pathway. Oncogene. 2013;32(13):1651–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Lee YJ, Cho JM, Moon JH, et al. Increased miR-338-3p expression correlates with invasiveness of GH-producing pituitary adenomas. Endocrine. 2017;58(1):184–189. [DOI] [PubMed] [Google Scholar]
- 267. Grzywa TM, Klicka K, Rak B, et al. Lineage-dependent role of miR-410-3p as oncomiR in gonadotroph and corticotroph pituitary adenomas or tumor suppressor miR in somatotroph adenomas via MAPK, PTEN/AKT, and STAT3 signaling pathways. Endocrine. 2019;65(3):646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Yang W, Xu T, Qiu P, Xu G. Caveolin-1 promotes pituitary adenoma cells migration and invasion by regulating the interaction between EGR1 and KLF5. Exp Cell Res. 2018;367(1):7–14. [DOI] [PubMed] [Google Scholar]
- 269. He W, Huang L, Li M, Yang Y, Chen Z, Shen X. MiR-148b, MiR-152/ALCAM axis regulates the proliferation and invasion of pituitary adenomas cells. Cell Physiol Biochem. 2017;44(2):792–803. [DOI] [PubMed] [Google Scholar]
- 270. Wang Y, Yin X, Zhao L, et al. MicroRNA-200b inhibits pituitary tumor cell proliferation and invasion by targeting PKCα. Exp Ther Med. 2017;14(2):1706–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Zhou K, Fan YD, Wu PF, et al. MicroRNA-145 inhibits the activation of the mTOR signaling pathway to suppress the proliferation and invasion of invasive pituitary adenoma cells by targeting AKT3 in vivo and in vitro. Onco Targets Ther. 2017;10:1625–1635. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 272. Yu C, Li J, Sun F, Cui J, Fang H, Sui G. Expression and clinical significance of miR-26a and pleomorphic adenoma gene 1 (PLAG1) in invasive pituitary adenoma. Med Sci Monit. 2016;22:5101–5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Wei Z, Zhou C, Liu M, et al. MicroRNA involvement in a metastatic non-functioning pituitary carcinoma. Pituitary. 2015;18(5):710–721. [DOI] [PubMed] [Google Scholar]
- 274. Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer. 2011;10:38. Doi: 10.1186/1476-4598-10-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Patop IL, Kadener S. circRNAs in Cancer. Curr Opin Genet Dev. 2018;48:121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Wu ZR, Yan L, Liu YT, et al. Inhibition of mTORC1 by lncRNA H19 via disrupting 4E-BP1/Raptor interaction in pituitary tumours. Nat Commun. 2018;9(1):4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Zhang Y, Liu YT, Tang H, et al. Exosome-transmitted lncRNA H19 inhibits the growth of pituitary adenoma. J Clin Endocrinol Metab. 2019;104(12):6345–6356. [DOI] [PubMed] [Google Scholar]
- 278. Fu D, Zhang Y, Cui H. Long noncoding RNA CCAT2 is activated by E2F1 and exerts oncogenic properties by interacting with PTTG1 in pituitary adenomas. Am J Cancer Res. 2018;8(2):245–255. [PMC free article] [PubMed] [Google Scholar]
- 279. Lu G, Duan J, Zhou D. Long-noncoding RNA IFNG-AS1 exerts oncogenic properties by interacting with epithelial splicing regulatory protein 2 (ESRP2) in pituitary adenomas. Pathol Res Pract. 2018;214(12):2054–2061. [DOI] [PubMed] [Google Scholar]
- 280. Tang H, Hou B, Ye Z, Ling C, Guo Y. Knockdown of long non-coding RNA AFAP1-AS1 inhibits growth and promotes apoptosis in pituitary adenomas. Int J Clin Exp Pathol. 2018;11(3): 1238–1246. [PMC free article] [PubMed] [Google Scholar]
- 281. Tang H, Zhu D, Zhang G, Luo X, Xie W. AFAP1-AS1 promotes proliferation of pituitary adenoma cells through miR-103a-3p to activate PI3K/AKT signaling pathway. World Neurosurg. 2019;130:e888–e898. [DOI] [PubMed] [Google Scholar]
- 282. Li J, Li C, Wang J, et al. Genome-wide analysis of differentially expressed lncRNAs and mRNAs in primary gonadotrophin adenomas by RNA-seq. Oncotarget. 2017;8(3):4585–4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Xing W, Qi Z, Huang C, et al. Genome-wide identification of lncRNAs and mRNAs differentially expressed in non-functioning pituitary adenoma and construction of an lncRNA-mRNA co-expression network. Biol Open. 2019;8(1):bio037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. D’Angelo D, Mussnich P, Sepe R, et al. RPSAP52 lncRNA is overexpressed in pituitary tumors and promotes cell proliferation by acting as miRNA sponge for HMGA proteins. J Mol Med (Berl). 2019;97(7):1019–1032. [DOI] [PubMed] [Google Scholar]
- 285. Wang C, Tan C, Wen Y, et al. FOXP1-induced lncRNA CLRN1-AS1 acts as a tumor suppressor in pituitary prolactinoma by repressing the autophagy via inactivating Wnt/β-catenin signaling pathway. Cell Death Dis. 2019;10(7):499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Rui QH, Ma JB, Liao YF, Dai JH, Cai ZY. Effect of lncRNA HULC knockdown on rat secreting pituitary adenoma GH3 cells. Braz J Med Biol Res. 2019;52(4):e7728. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 287. Zhang X, Zhou Y, Mehta KR, et al. A pituitary-derived MEG3 isoform functions as a growth suppressor in tumor cells. J Clin Endocrinol Metab. 2003;88(11):5119–5126. [DOI] [PubMed] [Google Scholar]
- 288. Gejman R, Batista DL, Zhong Y, et al. Selective loss of MEG3 expression and intergenic differentially methylated region hypermethylation in the MEG3/DLK1 locus in human clinically nonfunctioning pituitary adenomas. J Clin Endocrinol Metab. 2008;93(10):4119–4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Cheunsuchon P, Zhou Y, Zhang X, et al. Silencing of the imprinted DLK1-MEG3 locus in human clinically nonfunctioning pituitary adenomas. Am J Pathol. 2011;179(4):2120–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Mezzomo LC, Gonzales PH, Pesce FG, et al. Expression of cell growth negative regulators MEG3 and GADD45γ is lost in most sporadic human pituitary adenomas. Pituitary. 2012;15(3):420–427. [DOI] [PubMed] [Google Scholar]
- 291. Zhao J, Dahle D, Zhou Y, Zhang X, Klibanski A. Hypermethylation of the promoter region is associated with the loss of MEG3 gene expression in human pituitary tumors. J Clin Endocrinol Metab. 2005;90(4):2179–2186. [DOI] [PubMed] [Google Scholar]
- 292. Chunharojrith P, Nakayama Y, Jiang X, et al. Tumor suppression by MEG3 lncRNA in a human pituitary tumor derived cell line. Mol Cell Endocrinol. 2015;416:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Zhou Y, Zhong Y, Wang Y, et al. Activation of p53 by MEG3 non-coding RNA. J Biol Chem. 2007;282(34):24731–24742. [DOI] [PubMed] [Google Scholar]
- 294. Li Z, Li C, Liu C, Yu S, Zhang Y. Expression of the long non-coding RNAs MEG3, HOTAIR, and MALAT-1 in non-functioning pituitary adenomas and their relationship to tumor behavior. Pituitary. 2015;18(1):42–47. [DOI] [PubMed] [Google Scholar]
- 295. Rinn JL, Kertesz M, Wang JK, et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297. Kim K, Jutooru I, Chadalapaka G, et al. HOTAIR is a negative prognostic factor and exhibits pro-oncogenic activity in pancreatic cancer. Oncogene. 2013;32(13):1616–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Nakagawa T, Endo H, Yokoyama M, et al. Large noncoding RNA HOTAIR enhances aggressive biological behavior and is associated with short disease-free survival in human non-small cell lung cancer. Biochem Biophys Res Commun. 2013;436(2):319–324. [DOI] [PubMed] [Google Scholar]
- 299. Wang H, Wang G, Gao Y, et al. Lnc-SNHG1 activates the TGFBR2/SMAD3 and RAB11A/Wnt/β-catenin pathway by sponging MiR-302/372/373/520 in invasive pituitary tumors. Cell Physiol Biochem. 2018;48(3):1291–1303. [DOI] [PubMed] [Google Scholar]
- 300. Yu G, Li C, Xie W, et al. Long non-coding RNA C5orf66-AS1 is downregulated in pituitary null cell adenomas and is associated with their invasiveness. Oncol Rep. 2017;38(2):1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. Zhou Q, Zhang W, Wang Z, Liu S. Long non-coding RNA PTTG3P functions as an oncogene by sponging miR-383 and up-regulating CCND1 and PARP2 in hepatocellular carcinoma. BMC Cancer. 2019;19(1):731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Wang J, Wang D, Wan D, et al. Circular RNA in invasive and recurrent clinical nonfunctioning pituitary adenomas: expression profiles and bioinformatic analysis. World Neurosurg. 2018;117:e371–e386. [DOI] [PubMed] [Google Scholar]
- 303. Hu Y, Zhang N, Zhang S, et al. Differential circular RNA expression profiles of invasive and non-invasive non-functioning pituitary adenomas: a microarray analysis. Medicine (Baltimore). 2019;98(26):e16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Guo J, Wang Z, Miao Y, et al. A two‑circRNA signature predicts tumour recurrence in clinical non‑functioning pituitary adenoma. Oncol Rep. 2019;41(1):113–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Cai T, Xiao J, Wang ZF, Liu Q, Wu H, Qiu YZ. Identification of differentially coexpressed genes in gonadotrope tumors and normal pituitary using bioinformatics methods. Pathol Oncol Res. 2014;20(2):375–380. [DOI] [PubMed] [Google Scholar]
- 306. Hou Z, Yang J, Wang G, Wang C, Zhang H. Bioinformatic analysis of gene expression profiles of pituitary gonadotroph adenomas. Oncol Lett. 2018;15(2):1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Lee M, Marinoni I, Irmler M, et al. Transcriptome analysis of MENX-associated rat pituitary adenomas identifies novel molecular mechanisms involved in the pathogenesis of human pituitary gonadotroph adenomas. Acta Neuropathol. 2013;126(1):137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. Cassarino MF, Ambrogio AG, Cassarino A, et al. Gene expression profiling in human corticotroph tumours reveals distinct, neuroendocrine profiles. J Neuroendocrinol. 2018;30(9):e12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Jiang ZQ, Gui SB, Zhang YZ. Differential gene expression by fiber-optic beadarray and pathway in adrenocorticotrophin-secreting pituitary adenomas. Chin Med J (Engl). 2010;123(23):3455–3461. [PubMed] [Google Scholar]
- 310. Wang R, Yang Y, Sheng M, et al. Phenotype-genotype association analysis of ACTH-secreting pituitary adenoma and its molecular link to patient osteoporosis. Int J Mol Sci. 2016;17(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. de Lima DS, Martins CS, Paixao BM, et al. SAGE analysis highlights the putative role of underexpression of ribosomal proteins in GH-secreting pituitary adenomas. Eur J Endocrinol. 2012;167(6):759–768. [DOI] [PubMed] [Google Scholar]
- 312. Evans CO, Moreno CS, Zhan X, et al. Molecular pathogenesis of human prolactinomas identified by gene expression profiling, RT-qPCR, and proteomic analyses. Pituitary. 2008;11(3):231–245. [DOI] [PubMed] [Google Scholar]
- 313. Jiang Z, Gui S, Zhang Y. Analysis of differential gene expression by fiber-optic BeadArray and pathway in prolactinomas. Endocrine. 2010;38(3):360–368. [DOI] [PubMed] [Google Scholar]
- 314. Jiang Z, Gui S, Zhang Y. Analysis of differential gene expression by bead-based fiber-optic array in nonfunctioning pituitary adenomas. Horm Metab Res. 2011;43(5):325–330. [DOI] [PubMed] [Google Scholar]
- 315. Moreno CS, Evans CO, Zhan X, Okor M, Desiderio DM, Oyesiku NM. Novel molecular signaling and classification of human clinically nonfunctional pituitary adenomas identified by gene expression profiling and proteomic analyses. Cancer Res. 2005;65(22):10214–10222. [DOI] [PubMed] [Google Scholar]
- 316. Morris DG, Musat M, Czirják S, et al. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur J Endocrinol. 2005;153(1):143–151. [DOI] [PubMed] [Google Scholar]
- 317. Richardson TE, Shen ZJ, Kanchwala M, et al. Aggressive behavior in silent subtype III pituitary adenomas may depend on suppression of local immune response: a whole transcriptome analysis. J Neuropathol Exp Neurol. 2017;76(10):874–882. [DOI] [PubMed] [Google Scholar]
- 318. Yang Q, Wang Y, Zhang S, et al. Biomarker discovery for immunotherapy of pituitary adenomas: enhanced robustness and prediction ability by modern computational tools. Int J Mol Sci. 2019;20(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Vazquez-Borrego MC, Fuentes-Fayos AC, Venegas-Moreno E, et al. Splicing machinery is dysregulated in pituitary neuroendocrine tumors and is associated with aggressiveness features. Cancers (Basel). 2019;11(10):1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Zhu H, Guo J, Shen Y, et al. Functions and mechanisms of tumor necrosis factor-α and noncoding RNAs in bone-invasive pituitary adenomas. Clin Cancer Res. 2018;24(22): 5757–5766. [DOI] [PubMed] [Google Scholar]
- 321. Zhao S, Feng J, Li C, et al. Phosphoproteome profiling revealed abnormally phosphorylated AMPK and ATF2 involved in glucose metabolism and tumorigenesis of GH-PAs. J Endocrinol Invest. 2019;42(2):137–148. [DOI] [PubMed] [Google Scholar]
- 322. Yu SY, Hong LC, Feng J, Wu YT, Zhang YZ. Integrative proteomics and transcriptomics identify novel invasive-related biomarkers of non-functioning pituitary adenomas. Tumour Biol. 2016;37(7):8923–8930. [DOI] [PubMed] [Google Scholar]
- 323. Feng J, Yu SY, Li CZ, Li ZY, Zhang YZ. Integrative proteomics and transcriptomics revealed that activation of the IL-6R/JAK2/STAT3/MMP9 signaling pathway is correlated with invasion of pituitary null cell adenomas. Mol Cell Endocrinol. 2016;436:195–203. [DOI] [PubMed] [Google Scholar]
- 324. Zhang LY, Ge XL, Li Z, et al. Fibroblasts play a potential role in bone destruction via osteopontin related caldesmon expression and polymerization in human non-functioning pituitary adenomas. Sci Rep. 2017;7(1):17523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Rodrigues LR, Teixeira JA, Schmitt FL, Paulsson M, Lindmark-Mänsson H. The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev. 2007;16(6):1087–1097. [DOI] [PubMed] [Google Scholar]
- 326. Qian S, Zhan X, Lu M, et al. Quantitative analysis of ubiquitinated proteins in human pituitary and pituitary adenoma tissues. Front Endocrinol (Lausanne). 2019;10:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Yelamanchi SD, Tyagi A, Mohanty V, et al. Proteomic analysis of the human anterior pituitary gland. Omics. 2018;22(12):759–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Zhan X, Wang X, Long Y, Desiderio DM. Heterogeneity analysis of the proteomes in clinically nonfunctional pituitary adenomas. BMC Med Genomics. 2014;7:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Liu Z, Liu Y, Fang W, Chen W, Li C, Xiao Z. Establishment of differential expression profiles from invasive and non-invasive pituitary adenomas. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2009;34(7):569–575. [PubMed] [Google Scholar]
- 330. Ribeiro-Oliveira A Jr, Franchi G, Kola B, et al. Protein western array analysis in human pituitary tumours: insights and limitations. Endocr Relat Cancer. 2008;15(4):1099–1114. [DOI] [PubMed] [Google Scholar]
- 331. Chesnokova V, Zonis S, Ben-Shlomo A, Wawrowsky K, Melmed S. Molecular mechanisms of pituitary adenoma senescence. Front Horm Res. 2010;38:7–14. [DOI] [PubMed] [Google Scholar]
- 332. Manojlovic-Gacic E, Skender-Gazibara M, Popovic V, et al. Oncogene-induced senescence in pituitary adenomas–an immunohistochemical study. Endocr Pathol. 2016;27(1):1–11. [DOI] [PubMed] [Google Scholar]
- 333. Lu JQ, Adam B, Jack AS, Lam A, Broad RW, Chik CL. Immune cell infiltrates in pituitary adenomas: more macrophages in larger adenomas and more T cells in growth hormone adenomas. Endocr Pathol. 2015;26(3):263–272. [DOI] [PubMed] [Google Scholar]
- 334. Marques P, Barry S, Carlsen E, et al. Chemokines modulate the tumour microenvironment in pituitary neuroendocrine tumours. Acta Neuropathol Commun. 2019;7(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335. Marques P, Barry S, Carlsen E, et al. Pituitary tumour fibroblast-derived cytokines influence tumour aggressiveness. Endocr Relat Cancer. 2019;26(12):853–865. [DOI] [PubMed] [Google Scholar]
- 336. Ilie MD, Vasiljevic A, Raverot G, Bertolino P. The microenvironment of pituitary tumors-biological and therapeutic implications. Cancers (Basel). 2019;11(10):1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.