Abstract
Background
The early death of patients is a global cancer issue. We aimed to identify the risk factors for early death in stage IV breast cancer. Predictive nomograms for early death evaluation were generated based on the risk factors.
Material/Methods
Based on the Surveillance, Epidemiology, and End Results (SEER) database, patients diagnosed with IV breast cancer were selected. The risk factors for early death (survival time ≤1 year) were identified using logistic regression model analysis. Predictive nomograms were constructed and internal validation was performed.
Results
A total of 5998 (32.6%) breast cancer patients were diagnosed as early death in the construction cohort. Age older than 50 years, unmarried status, black race, uninsured status, triple-negative type, grade (II and III), tumor size >5 cm, and metastasis to lung, liver, and brain were risk factors for total early death, while Luminal B subtype, N1 stage, and surgical interventions were associated with lower risk of early death. As for cancer-specific and non-cancer-specific early death, several factors were not consistent between the 2 groups. Nomograms for all-cause, cancer-specific, and non-cancer-specific early death were constructed. The calibration curve showed satisfactory agreement. The areas under the ROC curve (AUC) were 78.3% (95% CI: 77.7–78.9%), 75.8% (75.1–76.4%), and 72.3% (71.6–72.9%), respectively. In the validation cohort, a total of 689 (19.3%) patients were diagnosed as early death and the calibration curve showed satisfactory agreement. The AUCs of the all-cause, cancer-specific, and non-cancer-specific early death prediction were 74.0% (95% CI: 72.5–75.4%), 73.5% (72.0–74.9%), and 68.6% (67.0–70.1%), respectively.
Conclusions
Nomograms were generated to predict early death, with good calibration and discrimination. The predictive model can provide a reference for identifying cases with high risk of early death among stage IV breast cancer patients and play an auxiliary role in guiding individual treatment.
MeSH Keywords: Breast Neoplasms, Nomograms, SEER Program
Background
Breast cancer is a worldwide problem, and is the leading cause of cancer morbidity and mortality in females [1]. The long-term survival of breast cancer patients has been investigated, and the global 5- and 10-year pooled survival rates were reported to be 73% (95% CI: 71–75%) and 61% (95% CI: 54–67%), respectively [2].
Early death is a crucial issue in the management of cancer. A series of studies have been conducted to investigate early death in cancer, including lung cancer [3], bladder cancer [4], and colorectal cancer [5]. The related factors for early death revealed in these studies provided evidence for cancer treatment. For primary breast cancer, around 10% of the patients died during the first year after initial diagnosis. Age over 80 years and distant metastases at presentation were proved to be the risk factors leading to early death. Meanwhile, surgery was reported to be able to decrease the risk of early death (OR=0.29, 95% CI: 0.24–0.35) [6].
As to the United States, approximately 5% of women with newly diagnosed breast cancer were reported to present with stage IV at diagnosis [7]. The proportion of stage IV breast cancer was even higher in the Asian population [8]. Compared with patients in early stage, patients in advanced stage were reported to have worse survival. The reported unadjusted median overall survival was 3.52 years in patients after surgery and 2.36 years in those without surgery [9].
The identification of factors correlated with early death in stage IV breast cancer is important. Based on a large cohort, we sought to identify the patients who died within 12 months after stage IV breast cancer diagnosis and to investigate the risk factors. Furthermore, predictive nomograms based on the results from the logistic regression model were constructed to predict the early death in stage IV breast cancer and to guide personalized early death risk screening.
Material and Methods
Data source and cohort selection
The National Cancer Institute Surveillance, Epidemiology and End Results (SEER) database (https://seer.cancer.gov/) covers approximately 28% of the population in the USA. The data used in the present study were obtained from the latest version by April 2019, which was named as Incidence – SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (1975–2016 varying).
Breast cancer patients at stage IV diagnosed between 2010 and 2015 were included into the construction cohort. In order to validate performance of the prediction model, temporal validation was conducted as internal validation. In the validation cohort, breast cancer patients at stage IV diagnosed in 2016 were used to validate the performance. The exclusion criteria were as follows: patients diagnosed at autopsy or via death certificate, male patients, and those without detailed information about site-specific metastases. The flow-chart of subject selection is listed in Figure 1. As in a previous study, early death in breast cancer patients was defined as death within 1 year after initial diagnosis [6].
Figure 1.
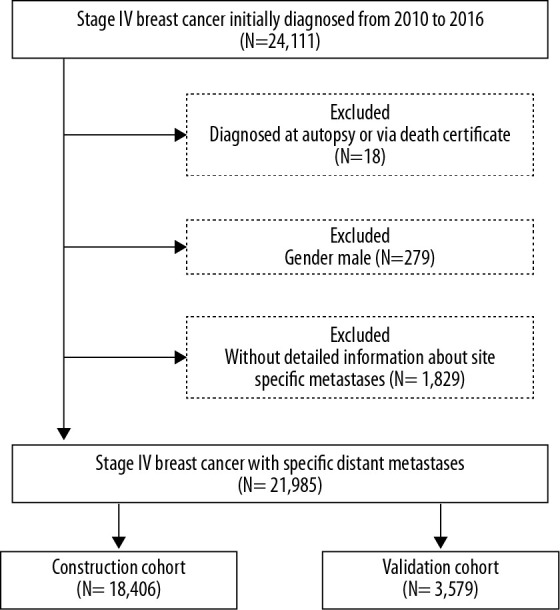
Selection procedure for patients with stage IV breast cancer.
Statistical analysis
The variables of the extracted data from the SEER database included age at diagnosis (<50, 50–59, 60–69, 70–79, and ≥80 years), marital status (married and unmarried), race (white, black and others), insurance recode (insured and uninsured), breast cancer subtypes (Luminal A, Luminal B, HER2 enriched, and triple-negative), tumor grade (I to IV: well, moderately, poorly, and undifferentiated, respectively), primary tumor site (C50.4-outer quadrant, UOQ; C50.5-lower outer quadrant, LOQ; C50.3-lower inner quadrant, LIQ; C50.2-upper inner quadrant, UIQ; C50.1-central portion, CEN; others including C50.0-Nipple, C50.6-Axillary tail of breast and C50.8-Overlapping lesion of breast), laterality (left-sided, right-sided, one side, NOS and paired sides), tumor size (0–2, 2–5 and >5 cm), N stage (N0, N1, N2 and N3), bone metastasis (no or yes), brain metastasis (no or yes), liver metastasis (no or yes), lung metastasis (no or yes), surgery treatment (no surgery; partial mastectomy, PM; total mastectomy, TM; radical mastectomy, RM; and others, including local tumor destruction, subcutaneous mastectomy, and bilateral mastectomy).
All of the variables in our study were categorical data, which are presented as numbers and percentages. A logistics regression model was used to identify the risk factors for early death. Variables with P<0.05 in the univariate analysis were further used in the multivariate analysis to determine the potential risk factors. The cases with missing data were excluded from the logistics analysis. Based on the results from the logistic regression model, the nomograms for all-cause, cancer-specific, and non-cancer-specific early death were formulated using the rms package in R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria; www.r-project.org). The calibration plots produced by bootstrapping with 1000 resamples were used to test the calibration of the nomograms. The discrimination of the nomograms was evaluated by the receiver operating characteristics (ROC). A greater area under the curve (AUC) close to 1.0 indicated better ability of discrimination.
Data extraction was performed using the SEER*Stat Software and statistical analyses were performed using SPSS 23.0 (IBM Corporation, Armonk, NY, USA). All statistical tests were two-sided, and P<0.05 was considered significant.
Ethics statement
The SEER dataset is a freely available database, and the data released by SEER do not require informed patient consent because cancer is a reportable disease in every state in the USA. The present study complied with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Results
Clinical characteristics and incidence of early death
After excluding patients diagnosed by autopsy or death certificate, male patients, and those without complete records, a total of 18 406 breast cancer patients at stage IV were enrolled in the construction cohort. Patients’ basic characteristics are summarized in Table 1. According to the pre-definition, 5998 (32.6%) patients were categorized as early death, among whom 4416 patients died due to cancer-specific cause. Most patients with early death were diagnosed after age 50 years (89.2%). The most common cancer subtype was Luminal A (38.3%), followed by triple-negative (19.4%), Luminal B, and HER2 enriched type. Except for the unknown grade, grade III (37.0%) and II (23.9%) were the most common types. Distant metastasis was found in 3842 (64.1%) patients in bone, 2471 (41.2%) patients in lung, 2178 (36.3%) patients in liver, and 820 (13.7%) patients in brain. A total of 5094 (84.9%) patients did not receive any surgical intervention.
Table 1.
Description of the early death for patients with IV breast cancer in the construction cohort.
| Subject characteristics | Patients No. (%) | |||
|---|---|---|---|---|
| No early death | Total early death | Cancer specific early death | Non-cancer specific early death | |
| Age | ||||
| <50 | 2790 (22.5) | 645 (10.8) | 521 (11.8) | 124 (7.8) |
| 50–59 | 3256 (26.2) | 1178 (19.6) | 923 (20.9) | 255 (16.1) |
| 60–69 | 3267 (26.3) | 1506 (25.1) | 1128 (25.5) | 378 (23.9) |
| 70–79 | 1952 (15.7) | 1318 (22.0) | 940 (21.3) | 378 (23.9) |
| ≥80 | 1143 (9.2) | 1351 (22.5) | 904 (20.5) | 447 (28.3) |
| Marital status | ||||
| Married | 5790 (46.7) | 1993 (33.2) | 1477 (33.4) | 516 (32.6) |
| Unmarried | 5922 (47.7) | 3674 (61.3) | 2697 (61.1) | 977 (61.8) |
| Unknown | 696 (5.6) | 331 (5.5) | 242 (5.5) | 89 (5.6) |
| Race | ||||
| White | 9466 (76.3) | 4409 (73.5) | 3252 (73.6) | 1157 (73.1) |
| Black | 1906 (15.4) | 1208 (20.1) | 881 (20) | 327 (20.7) |
| Others | 987 (8.0) | 369 (6.2) | 274 (6.2) | 95 (6.0) |
| Unknown | 49 (0.4) | 12 (0.2) | 9 (0.2) | 3 (0.2) |
| Insurance | ||||
| Insured | 11727 (94.5) | 5527 (92.1) | 4033 (91.3) | 1494 (94.4) |
| Uninsured | 437 (3.5) | 308 (5.1) | 265 (6.0) | 43 (2.7) |
| Unknown | 244 (2.0) | 163 (2.7) | 118 (2.7) | 45 (2.8) |
| Subtypes | ||||
| Luminal A | 7274 (58.6) | 2295 (38.3) | 1645 (37.3) | 650 (41.1) |
| Luminal B | 1961 (15.8) | 544 (9.1) | 427 (9.7) | 117 (7.4) |
| HER2 enriched | 891 (7.2) | 424 (7.1) | 323 (7.3) | 101 (6.4) |
| Triple negative | 908 (7.3) | 1164 (19.4) | 871 (19.7) | 293 (18.5) |
| Unknown | 1374 (11.1) | 1571 (26.2) | 1150 (26) | 421 (26.6) |
| Grade | ||||
| Grade I | 979 (7.9) | 212 (3.5) | 140 (3.2) | 72 (4.6) |
| Grade II | 4401 (35.5) | 1432 (23.9) | 1021 (23.1) | 411 (26) |
| Grade III | 4220 (34.0) | 2220 (37.0) | 1689 (38.2) | 531 (33.6) |
| Grade IV | 56 (0.5) | 45 (0.8) | 37 (0.8) | 8 (0.5) |
| Unknown | 2752 (22.2) | 2089 (34.8) | 1529 (34.6) | 560 (35.4) |
| Tumor site | ||||
| UOQ | 2930 (23.6) | 1141 (19) | 832 (18.8) | 309 (19.5) |
| LOQ | 684 (5.5) | 226 (3.8) | 162 (3.7) | 64 (4.0) |
| LIQ | 453 (3.7) | 154 (2.6) | 104 (2.4) | 50 (3.2) |
| UIQ | 794 (6.4) | 284 (4.7) | 204 (4.6) | 80 (5.1) |
| CEN | 761 (6.1) | 288 (4.8) | 212 (4.8) | 76 (4.8) |
| Others | 2577 (20.8) | 1059 (17.7) | 767 (17.4) | 292 (18.5) |
| Unknown | 4209 (33.9) | 2846 (47.4) | 2135 (48.3) | 711 (44.9) |
| Laterality | ||||
| Left-sided | 6030 (48.6) | 2848 (47.5) | 2110 (47.8) | 738 (46.6) |
| Right-sided | 5799 (46.7) | 2684 (44.7) | 1972 (44.7) | 712 (45.0) |
| One side, NOS | 51 (0.4) | 35 (0.6) | 29 (0.7) | 6 (0.4) |
| Paired sides | 528 (4.3) | 431 (7.2) | 305 (6.9) | 126 (8.0) |
| Tumor size | ||||
| 0–2 cm | 1937 (15.6) | 705 (11.8) | 438 (9.9) | 267 (16.9) |
| 2–5 cm | 4721 (38.0) | 1871 (31.2) | 1346 (30.5) | 525 (33.2) |
| >5 cm | 3263 (26.3) | 1580 (26.3) | 1274 (28.8) | 306 (19.3) |
| Unknown | 2487 (20.0) | 1842 (30.7) | 1358 (30.8) | 484 (30.6) |
| N stage | ||||
| N0 | 2860 (23.0) | 1490 (24.8) | 991 (22.4) | 499 (31.5) |
| N1 | 4321 (34.8) | 1634 (27.2) | 1266 (28.7) | 368 (23.3) |
| N2 | 1131 (9.1) | 370 (6.2) | 276 (6.3) | 94 (5.9) |
| N3 | 3092 (24.9) | 1613 (26.9) | 1237 (28) | 376 (23.8) |
| Unknown | 1004 (8.1) | 891 (14.9) | 646 (14.6) | 245 (15.5) |
| Bone Met | ||||
| No | 3562 (28.7) | 2156 (35.9) | 1525 (34.5) | 631 (39.9) |
| Yes | 8846 (71.3) | 3842 (64.1) | 2891 (65.5) | 951 (60.1) |
| Brain Met | ||||
| No | 11786 (95.0) | 5178 (86.3) | 3792 (85.9) | 1386 (87.6) |
| Yes | 622 (5.0) | 820 (13.7) | 624 (14.1) | 196 (12.4) |
| Liver Met | ||||
| No | 9722 (78.4) | 3820 (63.7) | 2712 (61.4) | 1108 (70) |
| Yes | 2686 (21.6) | 2178 (36.3) | 1704 (38.6) | 474 (30) |
| Lung Met | ||||
| No | 8861 (71.4) | 3527 (58.8) | 2521 (57.1) | 1006 (63.6) |
| Yes | 3547 (28.6) | 2471 (41.2) | 1895 (42.9) | 576 (36.4) |
| Surgery | ||||
| No surgery | 8177 (65.9) | 5094 (84.9) | 3785 (85.7) | 1309 (82.7) |
| PM | 1174 (9.5) | 298 (5.0) | 195 (4.4) | 103 (6.5) |
| TM | 984 (7.9) | 201 (3.4) | 132 (3) | 69 (4.4) |
| RM | 1851 (14.9) | 339 (5.7) | 251 (5.7) | 88 (5.6) |
| Others | 38 (0.3) | 9 (0.2) | 5 (0.1) | 4 (0.3) |
| Unknown | 184 (1.5) | 57 (1.0) | 48 (1.1) | 9 (0.6) |
UOQ – upper outer quadrant; LOQ – lower outer quadrant; LIQ – lower inner quadrant; UIQ – upper inner quadrant; CEN – central portion; NOS – not otherwise specified; PM – partial mastectomy; TM – total mastectomy; RM – radical mastectomy.
Risk factors for early death
In the univariate logistic regression, diagnosis age older than 50 years, unmarried status, black race, uninsured status, HER2-enriched and triple-negative types, grade (II–IV), tumor size >5 cm, paired sides, and distant metastases (lung, liver and brain) were associated with greater risk of total early death. Other race, Luminal B subtype, N1 and N2 stage, and surgical interventions significantly decreased the risk of early death. As for cause of death, uninsured status, Luminal B subtype, tumor size 2–5 cm and >5 cm, and N3 stage showed different trends in the cancer-specific and non-cancer-specific early death. Table 2 provides detailed information.
Table 2.
Univariate logistic regression for analyzing the risk factors for early death in patients with IV breast cancer.
| Subject characteristics | Total early death | Cancer specific early death | Non-cancer specific early death | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | |
| Age | ||||||
| <50 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| 50–59 | 1.57 (1.40–1.74) | <0.001 | 1.47 (1.31–1.66) | <0.001 | 1.63 (1.31–2.03) | <0.001 |
| 60–69 | 1.99 (1.80–2.22) | <0.001 | 1.73 (1.54–1.94) | <0.001 | 2.30 (1.87–2.83) | <0.001 |
| 70–79 | 2.92 (2.62–3.26) | <0.001 | 2.26 (2.00–2.54) | <0.001 | 3.49 (2.83–4.30) | <0.001 |
| ≥80 | 5.11 (4.55–5.74) | <0.001 | 3.18 (2.81–3.60) | <0.001 | 5.83 (4.74–7.17) | <0.001 |
| Marital status | ||||||
| Married | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Unmarried | 1.80 (1.69–1.92) | <0.001 | 1.67 (1.55–1.79) | <0.001 | 1.60 (1.43–1.78) | <0.001 |
| Race | ||||||
| White | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Black | 1.36 (1.26–1.48) | <0.001 | 1.29 (1.18–1.41) | <0.001 | 1.29 (1.13–1.47) | <0.001 |
| Others | 0.80 (0.71–0.91) | 0.001 | 0.83 (0.72–0.95) | 0.007 | 0.83 (0.67–1.03) | 0.089 |
| Insurance | ||||||
| Insured | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Uninsured | 1.50 (1.29–1.74) | <0.001 | 1.81 (1.55–2.11) | <0.001 | 0.65 (0.47–0.88) | 0.006 |
| Subtypes | ||||||
| Luminal A | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Luminal B | 0.88 (0.79–0.98) | 0.017 | 0.99 (0.88–1.11) | 0.864 | 0.67 (0.55–0.82) | <0.001 |
| HER2 enriched | 1.51 (1.33–1.71) | <0.001 | 1.57 (1.37–1.80) | <0.001 | 1.14 (0.92–1.42) | 0.234 |
| Triple negative | 4.06 (3.68–4.48) | <0.001 | 3.49 (3.15–3.87) | <0.001 | 2.26 (1.95–2.62) | <0.001 |
| Grade | ||||||
| Grade I | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Grade II | 1.50 (1.28–1.76) | <0.001 | 1.59 (1.32–1.92) | <0.001 | 1.18 (0.91–1.53) | 0.214 |
| Grade III | 2.43 (2.08–2.84) | <0.001 | 2.67 (2.22–3.21) | <0.001 | 1.40 (1.08–1.80) | 0.010 |
| Grade IV | 3.71 (2.44–5.65) | <0.001 | 4.34 (2.79–6.75) | <0.001 | 1.34 (0.63–2.86) | 0.454 |
| Tumor site | ||||||
| UOQ | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| LOQ | 0.85 (0.72–1.00) | 0.051 | 0.84 (0.70–1.02) | 0.072 | 0.92 (0.70–1.22) | 0.564 |
| LIQ | 0.87 (0.72–1.06) | 0.173 | 0.81 (0.64–1.01) | 0.058 | 1.09 (0.80–1.49) | 0.577 |
| UIQ | 0.92 (0.79–1.07) | 0.272 | 0.91 (0.77–1.08) | 0.271 | 0.98 (0.76–1.26) | 0.852 |
| CEN | 0.97 (0.84–1.13) | 0.712 | 0.99 (0.83–1.17) | 0.870 | 0.95 (0.73–1.23) | 0.705 |
| Others | 1.06 (0.96–1.17) | 0.287 | 1.04 (0.93–1.16) | 0.477 | 1.06 (0.90–1.26) | 0.472 |
| Laterality | ||||||
| Left-sided | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Right-sided | 0.98 (0.92–1.05) | 0.534 | 0.97 (0.91–1.04) | 0.419 | 1.01 (0.91–1.13) | 0.848 |
| One side, NOS | 1.45 (0.94–2.24) | 0.090 | 1.63 (1.04–2.56) | 0.033 | 0.83 (0.36–1.90) | 0.655 |
| Paired sides | 1.73 (1.51–1.98) | <0.001 | 1.50 (1.30–1.73) | <0.001 | 1.67 (1.36–2.04) | <0.001 |
| Tumor size | ||||||
| 0–2 cm | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| 2–5 cm | 1.09 (0.98–1.21) | 0.100 | 1.29 (1.15–1.45) | <0.001 | 0.77 (0.66–0.90) | 0.001 |
| >5 cm | 1.33 (1.20–1.48) | <0.001 | 1.80 (1.59–2.03) | <0.001 | 0.60 (0.51–0.71) | <0.001 |
| N stage | ||||||
| N0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| N1 | 0.73 (0.67–0.79) | <0.001 | 0.92 (0.83–1.01) | 0.065 | 0.51 (0.44–0.59) | <0.001 |
| N2 | 0.63 (0.55–0.72) | <0.001 | 0.76 (0.66–0.89) | <0.001 | 0.52 (0.41–0.65) | <0.001 |
| N3 | 1.00 (0.92–1.09) | 0.976 | 1.21 (1.10–1.33) | <0.001 | 0.67 (0.58–0.77) | <0.001 |
| Bone Met | ||||||
| No | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Yes | 0.72 (0.67–0.77) | <0.001 | 0.81 (0.76–0.87) | <0.001 | 0.65 (0.59–0.73) | <0.001 |
| Brain Met | ||||||
| No | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Yes | 3.00 (2.69–3.35) | <0.001 | 2.65 (2.37–2.96) | <0.001 | 1.77 (1.51–2.08) | <0.001 |
| Liver Met | ||||||
| No | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Yes | 2.06 (1.93–2.21) | <0.001 | 2.15 (2.00–2.32) | <0.001 | 1.21 (1.08–1.36) | 0.001 |
| Lung Met | ||||||
| No | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Yes | 1.75 (1.64–1.87) | <0.001 | 1.80 (1.68–1.93) | <0.001 | 1.20 (1.08–1.33) | 0.001 |
| Surgery | ||||||
| No surgery | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| PM | 0.41 (0.36–0.47) | <0.001 | 0.38 (0.33–0.45) | <0.001 | 0.69 (0.56–0.85) | <0.001 |
| TM | 0.33 (0.28–0.38) | <0.001 | 0.31 (0.26–0.38) | <0.001 | 0.57 (0.44–0.73) | <0.001 |
| RM | 0.29 (0.26–0.33) | <0.001 | 0.32 (0.28–0.37) | <0.001 | 0.38 (0.31–0.48) | <0.001 |
| Others | 0.38 (0.18–0.79) | 0.009 | 0.30 (0.12–0.76) | 0.011 | 0.85 (0.31–2.37) | 0.756 |
SEER – Surveillance, Epidemiology, and End Result; OR – odds ratio; CI – confidence interval; Ref – reference; NA – not applicable; UOQ – upper outer quadrant; LOQ – lower outer quadrant; LIQ – lower inner quadrant; UIQ – upper inner quadrant; CEN – central portion; NOS – not otherwise specified; PM – partial mastectomy; TM – total mastectomy; RM – radical mastectomy.
When adjusting these factors in the multivariate logistic regression, the independent risk factors for total early death included age older than 50 years, unmarried status, black race, uninsured status, triple-negative type, grade (II and III), tumor size >5 cm, and metastasis to lung, liver, and brain. Luminal B subtype, N1 stage, and surgical interventions were significantly associated with lower risk of early death. Unmarried status, uninsured status, Luminal B subtype, grade (II and III), tumor size 2–5 cm and >5 cm, N1 stage, and metastasis to bone and lung were not consistent between the cancer-specific and non-cancer-specific early death groups. Table 3 provides detailed information.
Table 3.
Multivariate logistic regression for analyzing the risk factors for early death in patients with IV breast cancer.
| Subject characteristics | Total early death | Cancer specific early death | Non-cancer specific early death | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | ||||||
| <50 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| 50–59 | 1.59 (1.35–1.89) | <0.001 | 1.51 (1.26–1.80) | <0.001 | 1.52 (1.09–2.13) | 0.014 |
| 60–69 | 2.22 (1.88–2.63) | <0.001 | 1.84 (1.54–2.20) | <0.001 | 2.5 (1.83–3.44) | <0.001 |
| 70–79 | 3.48 (2.90–4.17) | <0.001 | 2.41 (1.98–2.93) | <0.001 | 4.25 (3.08–5.86) | <0.001 |
| ≥80 | 7.04 (5.78–8.57) | <0.001 | 3.95 (3.21–4.87) | <0.001 | 7.37 (5.31–10.22) | <0.001 |
| Marital status | ||||||
| Married | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Unmarried | 1.40 (1.26–1.56) | <0.001 | 1.39 (1.23–1.56) | <0.001 | 1.19 (1–1.42) | 0.052 |
| Race | ||||||
| White | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Black | 1.36 (1.19–1.57) | <0.001 | 1.28 (1.10–1.48) | 0.001 | 1.34 (1.08–1.67) | 0.009 |
| Others | 0.91 (0.74–1.12) | 0.381 | 0.94 (0.75–1.17) | 0.570 | 0.88 (0.61–1.27) | 0.505 |
| Insurance | ||||||
| Insured | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Uninsured | 1.31 (1.00–1.72) | 0.048 | 1.52 (1.15–2.00) | 0.003 | 0.61 (0.33–1.11) | 0.103 |
| Subtypes | ||||||
| Luminal A | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Luminal B | 0.78 (0.67–0.91) | 0.002 | 0.81 (0.68–0.96) | 0.014 | 0.83 (0.63–1.08) | 0.164 |
| HER2 enriched | 1.17 (0.97–1.42) | 0.107 | 1.11 (0.90–1.36) | 0.318 | 1.23 (0.9–1.69) | 0.193 |
| Triple Negative | 3.76 (3.23–4.37) | <0.001 | 3.06 (2.62–3.57) | <0.001 | 2.09 (1.66–2.62) | <0.001 |
| Grade | ||||||
| Grade I | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Grade II | 1.56 (1.25–1.95) | <0.001 | 1.59 (1.22–2.06) | 0.001 | 1.27 (0.91–1.79) | 0.165 |
| Grade III | 2.22 (1.76–2.79) | <0.001 | 2.29 (1.76–2.99) | <0.001 | 1.42 (1–2.02) | 0.053 |
| Grade IV | 1.44 (0.70–2.93) | 0.321 | 1.95 (0.94–4.07) | 0.075 | 0.57 (0.13–2.49) | 0.454 |
| Laterality | ||||||
| Left-sided | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Right-sided | 0.99 (0.90–1.10) | 0.888 | 1.00 (0.89–1.12) | 0.998 | 0.98 (0.83–1.15) | 0.768 |
| One side, NOS | – | 1.000 | – | 1.000 | – | 1.000 |
| Paired sides | 2.66 (0.72–9.79) | 0.142 | 2.45 (0.67–8.94) | 0.176 | 1.28 (0.15–10.76) | 0.821 |
| Tumor size | ||||||
| 0–2 cm | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| 2–5 cm | 1.05 (0.91–1.22) | 0.519 | 1.21 (1.02–1.43) | 0.027 | 0.82 (0.66–1.02) | 0.070 |
| >5 cm | 1.32 (1.13–1.54) | 0.001 | 1.69 (1.42–2.01) | <0.001 | 0.67 (0.53–0.86) | 0.001 |
| N stage | ||||||
| N0 | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| N1 | 0.77 (0.67–0.89) | <0.001 | 0.97 (0.84–1.13) | 0.731 | 0.56 (0.45–0.69) | <0.001 |
| N2 | 0.82 (0.67–1.01) | 0.066 | 0.91 (0.72–1.14) | 0.419 | 0.77 (0.56–1.06) | 0.112 |
| N3 | 0.94 (0.81–1.09) | 0.395 | 1.03 (0.87–1.20) | 0.769 | 0.84 (0.67–1.05) | 0.117 |
| Bone Met | ||||||
| No | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Yes | 1.11 (0.99–1.24) | 0.088 | 1.15 (1.02–1.31) | 0.027 | 0.91 (0.76–1.1) | 0.325 |
| Brain Met | ||||||
| No | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Yes | 3.67 (3.04–4.40) | <0.001 | 2.94 (2.44–3.54) | <0.001 | 2.03 (1.55–2.65) | <0.001 |
| Liver Met | ||||||
| No | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Yes | 2.28 (2.03–2.56) | <0.001 | 2.2 (1.95–2.48) | <0.001 | 1.41 (1.17–1.7) | <0.001 |
| Lung Met | ||||||
| No | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| Yes | 1.30 (1.16–1.45) | <0.001 | 1.36 (1.21–1.53) | <0.001 | 0.96 (0.8–1.15) | 0.675 |
| Surgery | ||||||
| No surgery | Ref | 1.0 | Ref | 1.0 | Ref | 1.0 |
| PM | 0.46 (0.38–0.56) | <0.001 | 0.44 (0.35–0.54) | <0.001 | 0.76 (0.57–1) | 0.049 |
| TM | 0.42 (0.34–0.51) | <0.001 | 0.41 (0.32–0.52) | <0.001 | 0.71 (0.52–0.98) | 0.036 |
| RM | 0.39 (0.33–0.46) | <0.001 | 0.41 (0.34–0.49) | <0.001 | 0.59 (0.45–0.77) | <0.001 |
| Others | 0.75 (0.29–1.94) | 0.557 | 0.65 (0.22–1.93) | 0.435 | 1.23 (0.28–5.34) | 0.786 |
SEER – Surveillance, Epidemiology, and End Result; OR – odds ratio; CI – confidence interval; Ref – reference; NA – not applicable; UOQ – upper outer quadrant; LOQ – lower outer quadrant; LIQ – lower inner quadrant; UIQ – upper inner quadrant; CEN – central portion; NOS – not otherwise specified; PM – partial mastectomy; TM – total mastectomy; RM = radical mastectomy.
Performance of the nomograms for predicting early death
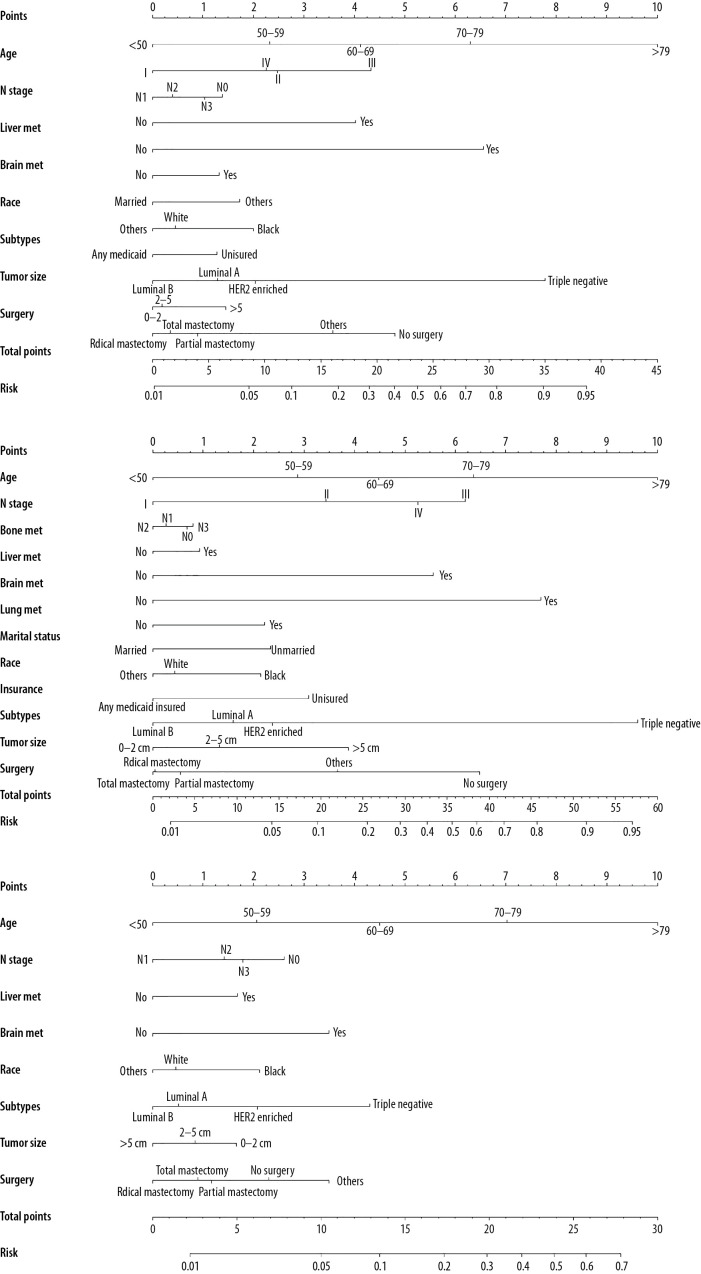
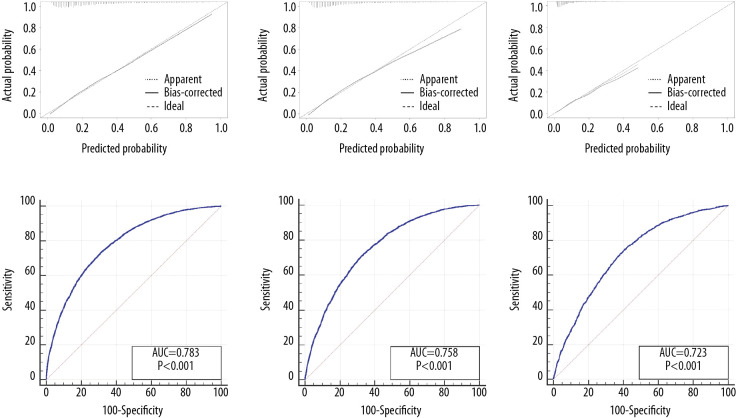
Nomograms predicting the risk of early death are shown in Figure 2 (Figure 2A–2C for all-cause, cancer-specific, and non-cancer-specific early death, respectively). Excellent ability of calibration was achieved in the 3 predictive models, with all calibration curve close to the 45-degree line (Figure 3A–3C for all-cause, cancer-specific, and non-cancer-specific early death, respectively). All 3 nomograms showed satisfactory strength of discrimination (Figure 3D–3F). The AUC for nomograms of the all-cause, cancer-specific, and non-cancer-specific early death prediction were 78.3% (95% CI: 77.7–78.9%), 75.8% (95% CI: 75.1–76.4%), and 72.3% (95% CI: 71.6–72.9%), respectively.
Figure 2.
Nomograms for predicting (A) all-cause early death; (B) cancer-specific early death and (C) non-cancer-specific early death in stage IV breast cancer patients.
Figure 3.
The calibration curve and ROC curve for assessing the calibration and discrimination of the nomograms in predicting all-cause early death (A, D), cancer-specific early death (B, E) and non-cancer-specific early death (C, F) in the construction cohort.
In the validation cohort, a total of 689 (19.3%) breast cancer patients were diagnosed as early death, among whom 508 patients died due to cancer-specific causes. Patients’ basic characteristics are shown in Supplementary Table 1. The calibration curve showed satisfactory agreement (Supplementary Figure 1A–1C). As shown in Supplementary Figure 1D–1F, the AUC for nomograms of the all-cause, cancer-specific, and non-cancer-specific early death prediction were 74.0% (95% CI: 72.5–75.4%), 73.5% (72.0–74.9%), and 68.6% (67.0–70.1%), respectively.
Discussion
The present study in a large cohort is the first to prove that the survival of one-third of the stage IV breast cancer patients was less than 12 months. Compared with the previously reported proportion of early death by the national cancer database (19.4%), the proportion in the present study is higher [10]. Cardiovascular disease and breast cancer were previously reported to be the primary causes of mortality in breast cancer survivors [11]. In the present study, the cancer-specific cause was proved to be the most important reason, resulting in 73.6% of all the early deaths in the patients with stage IV breast cancer. Efforts to reduce the risk of cancer-specific early death are crucial to improving the total survival.
Several risk factors were identified in the present study for patients with early death. Age was found to be a common risk factor for early death, and most of the early death cases were patients older than 50 years. The proportion of early death due to the 2 causes showed disparities in age at diagnosis, with a trend of more non-cancer-specific early death in older patients, which is consistent with a previous study [12]. Therefore, health care should be provided to meet the needs of patients in specific age groups.
Since the pioneering work by Perou et al. [13] and Sorlie et al. [14], breast cancer has been divided into different subtypes, and enormous studies have been conducted to investigate the relationship between survival and each subtype. In the present study, different subtypes were associated with various odds of early death. Compared with the Luminal A breast cancer, the triple-negative type was a risk factor resulting in more cancer-specific and non-cancer-specific early death. Luminal B was a protective factor for cancer-specific early death. The trend was also reported in the latest study [15]. Therefore, clear pathological diagnosis is important to predict early death and more studies should be performed among patients with triple-negative breast cancer.
Distant metastasis was the main characteristic of stage IV breast cancer. Bone was the most common metastatic site in our study, followed by lung, liver, and brain. Multivariate analysis showed that metastasis to liver and brain was a common risk factor for cancer-specific and non-cancer-specific early death, while metastases to bone and lung were associated with higher odds of cancer-specific early death. Due to the fact that bone and lung accounted for three-quarters of all metastases, more screenings should be scheduled to test these organs.
Socioeconomic variables were significantly associated with cancer management [16]. Because unmarried and uninsured patients are less likely to receive adequate mental and financial support, such patients have limited resources for their cancer treatment. Compared with white patients, black patients also had higher risk for early death. Previous studies have investigated the racial differences in breast cancer molecular features, and indicated black patients were more likely to have basal-like and HER2-enriched breast cancer subtypes [17]. Another study reported that black women were significantly more likely to develop triple-negative breast cancer [18]. In addition to the biological disadvantage, neoadjuvant chemotherapy was less likely to be completed in non-white patients, after controlling other factors [19]. The underlying reasons should be investigated in further studies.
Among the investigated variables in this study, surgery was the most important protective factor for lower odds of early death. Partial, total, and radical mastectomy can significantly decrease the risk of early death. Surgery resulting in improved survival of breast cancer with distant metastasis has been reported in several studies [20,21]. However, only less than 15% of all stage IV patients received surgery in this cohort from the SEER database. As with all retrospective reviews, there was a possible patient selection bias contributing to the better results in the patients after surgery [9]. Patients with better conditions and less risk were reported to be more likely to receive surgical interventions [7]. Therefore, the actual benefit from surgery for stage IV breast cancer should be evaluated.
In the current clinical processing, it was not convenient to predict the possibility of early death for individual patients. Several risk factors for early death were revealed in the present study. Based on the revealed factors, the nomograms were constructed, and this predictive system can be an effective method for use in clinical practice [5].
Our study had several limitations. First, we defined the early death as those who died within 1 year after diagnosis. With larger populations, other intervals such as 3 and 6 months should be further investigated. Second, the SEER database only recorded the synchronous metastasis in breast cancer, and data on patients with metachronous metastasis were not available. Third, breast cancer has distinct heterogeneity, and patients with different subtypes should receive different individual treatment modalities. However, detailed information on targeted treatment and specific regimens of adjuvant chemotherapy were not available in the SEER database.
Conclusions
Early death occurs in one-third of stage IV breast cancer patients, and the cancer-specific death was the most common cause. A series of risk factors for early death were identified. Based on these factors, nomograms for predicting early death were constructed. This predictive system can guide and schedule targeted treatment regimens for patients with stage IV breast cancer.
Supplementary Data
Supplementary Table 1.
Description of the early death for patients with IV breast cancer in the validation cohort.
| Subject characteristics | Patients No. (%) | |||
|---|---|---|---|---|
| No early death | Total early death | Cancer specific early death | Non-cancer specifi early death | |
| Age | ||||
| <50 | 600 (20.8) | 64 (9.3) | 51 (10.0) | 13 (7.2) |
| 50–59 | 655 (22.7) | 131 (19) | 101 (19.9) | 30 (16.6) |
| 60–69 | 771 (26.7) | 178 (25.8) | 136 (26.8) | 42 (23.2) |
| 70–79 | 527 (18.2) | 133 (19.3) | 97 (19.1) | 36 (19.9) |
| ≥80 | 337 (11.7) | 183 (26.6) | 123 (24.2) | 60 (33.1) |
| Marital status | ||||
| Married | 1341 (46.4) | 213 (30.9) | 164 (32.3) | 49 (27.1) |
| Unmarried | 1390 (48.1) | 441 (64) | 321 (63.2) | 120 (66.3) |
| Unknown | 159 (5.5) | 35 (5.1) | 23 (4.5) | 12 (6.6) |
| Race | ||||
| White | 2206 (76.3) | 498 (72.3) | 369 (72.6) | 129 (71.3) |
| Black | 402 (13.9) | 147 (21.3) | 109 (21.5) | 38 (21) |
| Others | 260 (9.0) | 41 (6.0) | 27 (5.3) | 14 (7.7) |
| Unknown | 22 (0.8) | 3 (0.4) | 3 (0.6) | 0 (0) |
| Insurance | ||||
| Insured | 2737 (94.7) | 640 (92.9) | 466 (91.7) | 174 (96.1) |
| Uninsured | 75 (2.6) | 31 (4.5) | 27 (5.3) | 4 (2.2) |
| Unknown | 78 (2.7) | 18 (2.6) | 15 (3.0) | 3 (1.7) |
| Subtypes | ||||
| Luminal A | 1612 (55.8) | 239 (34.7) | 174 (34.3) | 65 (35.9) |
| Luminal B | 476 (16.5) | 76 (11.0) | 58 (11.4) | 18 (9.9) |
| HER2 enriched | 222 (7.7) | 55 (8.0) | 42 (8.3) | 13 (7.2) |
| Triple negative | 295 (10.2) | 110 (16.0) | 76 (15.0) | 34 (18.8) |
| Unknown | 285 (9.9) | 209 (30.3) | 158 (31.1) | 51 (28.2) |
| Grade | ||||
| Grade I | 204 (7.1) | 27 (3.9) | 22 (4.3) | 5 (2.8) |
| Grade II | 1065 (36.9) | 139 (20.2) | 98 (19.3) | 41 (22.7) |
| Grade III | 1006 (34.8) | 233 (33.8) | 172 (33.9) | 61 (33.7) |
| Grade IV | 5 (0.2) | 3 (0.4) | 2 (0.4) | 1 (0.6) |
| Unknown | 610 (21.1) | 287 (41.7) | 214 (42.1) | 73 (40.3) |
| Tumor site | ||||
| UOQ | 764 (26.4) | 128 (18.6) | 93 (18.3) | 35 (19.3) |
| LOQ | 162 (5.6) | 19 (2.8) | 12 (2.4) | 7 (3.9) |
| LIQ | 98 (3.4) | 20 (2.9) | 12 (2.4) | 8 (4.4) |
| UIQ | 161 (5.6) | 32 (4.6) | 24 (4.7) | 8 (4.4) |
| CEN | 188 (6.5) | 32 (4.6) | 26 (5.1) | 6 (3.3) |
| Others | 577 (20.0) | 116 (16.8) | 86 (16.9) | 30 (16.6) |
| Unknown | 940 (32.5) | 342 (49.6) | 255 (50.2) | 87 (48.1) |
| Laterality | ||||
| Left-sided | 1371 (47.4) | 312 (45.3) | 233 (45.9) | 79 (43.6) |
| Right-sided | 1387 (48.0) | 310 (45.0) | 226 (44.5) | 84 (46.4) |
| One side, NOS | 10 (0.3) | 3 (0.4) | 2 (0.4) | 1 (0.6) |
| Paired sides | 122 (4.2) | 64 (9.3) | 47 (9.3) | 17 (9.4) |
| Tumor size | ||||
| 0–2 cm | 414 (14.3) | 86 (12.5) | 63 (12.4) | 23 (12.7) |
| 2–5 cm | 1076 (37.2) | 196 (28.4) | 138 (27.2) | 58 (32.0) |
| >5 cm | 794 (27.5) | 177 (25.7) | 138 (27.2) | 39 (21.5) |
| Unknown | 606 (21.0) | 230 (33.4) | 169 (33.3) | 61 (33.7) |
| N stage | ||||
| N0 | 664 (23) | 196 (28.4) | 142 (28.0) | 54 (29.8) |
| N1 | 1200 (41.5) | 243 (35.3) | 180 (35.4) | 63 (34.8) |
| N2 | 329 (11.4) | 53 (7.7) | 40 (7.9) | 13 (7.2) |
| N3 | 430 (14.9) | 74 (10.7) | 60 (11.8) | 14 (7.7) |
| Unknown | 267 (9.2) | 123 (17.9) | 86 (16.9) | 37 (20.4) |
| Bone Met | ||||
| No | 1014 (35.1) | 254 (36.9) | 166 (32.7) | 88 (48.6) |
| Yes | 1876 (64.9) | 435 (63.1) | 342 (67.3) | 93 (51.4) |
| Brain Met | ||||
| No | 2747 (95.1) | 612 (88.8) | 448 (88.2) | 164 (90.6) |
| Yes | 143 (4.9) | 77 (11.2) | 60 (11.8) | 17 (9.4) |
| Liver Met | ||||
| No | 2289 (79.2) | 411 (59.7) | 282 (55.5) | 129 (71.3) |
| Yes | 601 (20.8) | 278 (40.3) | 226 (44.5) | 52 (28.7) |
| Lung Met | ||||
| No | 2055 (71.1) | 423 (61.4) | 306 (60.2) | 117 (64.6) |
| Yes | 835 (28.9) | 266 (38.6) | 202 (39.8) | 64 (35.4) |
| Surgery | ||||
| No Surgery | 2127 (73.6) | 633 (91.9) | 467 (91.9) | 166 (91.7) |
| PM | 220 (7.6) | 18 (2.6) | 13 (2.6) | 5 (2.8) |
| TM | 240 (8.3) | 17 (2.5) | 13 (2.6) | 4 (2.2) |
| RM | 263 (9.1) | 16 (2.3) | 12 (2.4) | 4 (2.2) |
| Others | 14 (0.5) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 26 (0.9) | 5 (0.7) | 3 (0.6) | 2 (1.1) |
UOQ – upper outer quadrant; LOQ – lower outer quadrant; LIQ – lower inner quadrant; UIQ – upper inner quadrant; CEN – central portion; NOS – not otherwise specified; PM – partial mastectomy; TM – total mastectomy; RM – radical mastectomy.
The calibration curve and ROC curve for assessing the calibration and discrimination of the nomograms in predicting all-cause early death (A, D), cancer-specific early death (B, E) and non-cancer-specific early death (C, F) in the validation cohort.
Footnotes
Conflicts of interest
None.
Source of support: The present study was sponsored by the Natural Science Foundation of China (8191101553, 81702161, 81701081, 81801781, 81802508, 81903398), the Top Talent Training Program of the First Affiliated Hospital of PLA Army Medical University (SWH2018BJKJ-12), the Laboratory of Tumor Immunology and Pathology (Army Medical University), Ministry of Education (2017jszl01), and the Chongqing Natural Science Foundation Program (cstc2019jcyj-msxmX0466)
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. Cancer J Clin. 2019;69(1):7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.Maajani K, Jalali A, Alipour S, et al. The global and regional survival rate of women with breast cancer: A systematic review and meta-analysis. Clin Breast Cancer. 2019;19(3):165–77. doi: 10.1016/j.clbc.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 3.Christensen NL, Kejs A, Jakobsen E, et al. Early death in Danish stage I lung cancer patients: A population-based case study. Acta Oncol. 2018;57(11):1561–66. doi: 10.1080/0284186X.2018.1497298. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Z, Wang X, Wang J, et al. Preoperative predictors of early death risk in bladder cancer patients treated with robot-assisted radical cystectomy. Cancer Med. 2019;8(7):3447–52. doi: 10.1002/cam4.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Mao M, Xu G, et al. The incidence, associated factors, and predictive nomogram for early death in stage IV colorectal cancer. Int J Colorectal Dis. 2019;34(7):1189–201. doi: 10.1007/s00384-019-03306-1. [DOI] [PubMed] [Google Scholar]
- 6.Stapelkamp C, Holmberg L, Tataru D, et al. Predictors of early death in female patients with breast cancer in the UK: A cohort study. BMJ Open. 2011;1(2):e247. doi: 10.1136/bmjopen-2011-000247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim SM, Kim JY, Park HS, et al. Effect of primary tumor resection on overall survival in patients with stage IV breast cancer. Breast J. 2019;25(5):908–15. doi: 10.1111/tbj.13344. [DOI] [PubMed] [Google Scholar]
- 8.Miao H, Hartman M, Bhoo-Pathy N, et al. Predicting survival of de novo metastatic breast cancer in Asian women: systematic review and validation study. Plos One. 2014;9(4):e93755. doi: 10.1371/journal.pone.0093755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bafford AC, Burstein HJ, Barkley CR, et al. Breast surgery in stage IV breast cancer: Impact of staging and patient selection on overall survival. Breast Cancer Res Treat. 2009;115(1):7–12. doi: 10.1007/s10549-008-0101-7. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Zhao J, Zhu L, et al. Development and validation of a nomogram predicting the overall survival of stage IV breast cancer patients. Cancer Med. 2017;6(11):2586–94. doi: 10.1002/cam4.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patnaik JL, Byers T, DiGuiseppi C, et al. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: A retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Printz C. Older breast cancer patients have higher risk of death. Cancer. 2012;118(13):3225. doi: 10.1002/cncr.27702. [DOI] [PubMed] [Google Scholar]
- 13.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–52. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 14.Sorlie T, Tibshirani R, Parker J, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–23. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waks AG, Winer EP. Breast cancer treatment: A review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 16.Coughlin SS. Social determinants of breast cancer risk, stage, and survival. Breast Cancer Res Treat. 2019;177(3):537–48. doi: 10.1007/s10549-019-05340-7. [DOI] [PubMed] [Google Scholar]
- 17.Huo D, Hu H, Rhie SK, et al. Comparison of breast cancer molecular features and survival by African and European Ancestry in The Cancer Genome Atlas. JAMA Oncol. 2017;3(12):1654–62. doi: 10.1001/jamaoncol.2017.0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walsh SM, Zabor EC, Stempel M, et al. Does race predict survival for women with invasive breast cancer? Cancer. 2019;125(18):3139–46. doi: 10.1002/cncr.32296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knisely AT, Michaels AD, Mehaffey JH, et al. Race is associated with completion of neoadjuvant chemotherapy for breast cancer. Surgery. 2018;164(2):195–200. doi: 10.1016/j.surg.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lane WO, Thomas SM, Blitzblau RC, et al. Surgical resection of the primary tumor in women with de novo stage IV breast cancer: Contemporary practice patterns and survival analysis. Ann Surg. 2019;269(3):537–44. doi: 10.1097/SLA.0000000000002621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang K, Shi Y, Li ZY, et al. Metastatic pattern discriminates survival benefit of primary surgery for de novo stage IV breast cancer: A real-world observational study. Eur J Surg Oncol. 2019;45(8):1364–72. doi: 10.1016/j.ejso.2019.02.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
Description of the early death for patients with IV breast cancer in the validation cohort.
| Subject characteristics | Patients No. (%) | |||
|---|---|---|---|---|
| No early death | Total early death | Cancer specific early death | Non-cancer specifi early death | |
| Age | ||||
| <50 | 600 (20.8) | 64 (9.3) | 51 (10.0) | 13 (7.2) |
| 50–59 | 655 (22.7) | 131 (19) | 101 (19.9) | 30 (16.6) |
| 60–69 | 771 (26.7) | 178 (25.8) | 136 (26.8) | 42 (23.2) |
| 70–79 | 527 (18.2) | 133 (19.3) | 97 (19.1) | 36 (19.9) |
| ≥80 | 337 (11.7) | 183 (26.6) | 123 (24.2) | 60 (33.1) |
| Marital status | ||||
| Married | 1341 (46.4) | 213 (30.9) | 164 (32.3) | 49 (27.1) |
| Unmarried | 1390 (48.1) | 441 (64) | 321 (63.2) | 120 (66.3) |
| Unknown | 159 (5.5) | 35 (5.1) | 23 (4.5) | 12 (6.6) |
| Race | ||||
| White | 2206 (76.3) | 498 (72.3) | 369 (72.6) | 129 (71.3) |
| Black | 402 (13.9) | 147 (21.3) | 109 (21.5) | 38 (21) |
| Others | 260 (9.0) | 41 (6.0) | 27 (5.3) | 14 (7.7) |
| Unknown | 22 (0.8) | 3 (0.4) | 3 (0.6) | 0 (0) |
| Insurance | ||||
| Insured | 2737 (94.7) | 640 (92.9) | 466 (91.7) | 174 (96.1) |
| Uninsured | 75 (2.6) | 31 (4.5) | 27 (5.3) | 4 (2.2) |
| Unknown | 78 (2.7) | 18 (2.6) | 15 (3.0) | 3 (1.7) |
| Subtypes | ||||
| Luminal A | 1612 (55.8) | 239 (34.7) | 174 (34.3) | 65 (35.9) |
| Luminal B | 476 (16.5) | 76 (11.0) | 58 (11.4) | 18 (9.9) |
| HER2 enriched | 222 (7.7) | 55 (8.0) | 42 (8.3) | 13 (7.2) |
| Triple negative | 295 (10.2) | 110 (16.0) | 76 (15.0) | 34 (18.8) |
| Unknown | 285 (9.9) | 209 (30.3) | 158 (31.1) | 51 (28.2) |
| Grade | ||||
| Grade I | 204 (7.1) | 27 (3.9) | 22 (4.3) | 5 (2.8) |
| Grade II | 1065 (36.9) | 139 (20.2) | 98 (19.3) | 41 (22.7) |
| Grade III | 1006 (34.8) | 233 (33.8) | 172 (33.9) | 61 (33.7) |
| Grade IV | 5 (0.2) | 3 (0.4) | 2 (0.4) | 1 (0.6) |
| Unknown | 610 (21.1) | 287 (41.7) | 214 (42.1) | 73 (40.3) |
| Tumor site | ||||
| UOQ | 764 (26.4) | 128 (18.6) | 93 (18.3) | 35 (19.3) |
| LOQ | 162 (5.6) | 19 (2.8) | 12 (2.4) | 7 (3.9) |
| LIQ | 98 (3.4) | 20 (2.9) | 12 (2.4) | 8 (4.4) |
| UIQ | 161 (5.6) | 32 (4.6) | 24 (4.7) | 8 (4.4) |
| CEN | 188 (6.5) | 32 (4.6) | 26 (5.1) | 6 (3.3) |
| Others | 577 (20.0) | 116 (16.8) | 86 (16.9) | 30 (16.6) |
| Unknown | 940 (32.5) | 342 (49.6) | 255 (50.2) | 87 (48.1) |
| Laterality | ||||
| Left-sided | 1371 (47.4) | 312 (45.3) | 233 (45.9) | 79 (43.6) |
| Right-sided | 1387 (48.0) | 310 (45.0) | 226 (44.5) | 84 (46.4) |
| One side, NOS | 10 (0.3) | 3 (0.4) | 2 (0.4) | 1 (0.6) |
| Paired sides | 122 (4.2) | 64 (9.3) | 47 (9.3) | 17 (9.4) |
| Tumor size | ||||
| 0–2 cm | 414 (14.3) | 86 (12.5) | 63 (12.4) | 23 (12.7) |
| 2–5 cm | 1076 (37.2) | 196 (28.4) | 138 (27.2) | 58 (32.0) |
| >5 cm | 794 (27.5) | 177 (25.7) | 138 (27.2) | 39 (21.5) |
| Unknown | 606 (21.0) | 230 (33.4) | 169 (33.3) | 61 (33.7) |
| N stage | ||||
| N0 | 664 (23) | 196 (28.4) | 142 (28.0) | 54 (29.8) |
| N1 | 1200 (41.5) | 243 (35.3) | 180 (35.4) | 63 (34.8) |
| N2 | 329 (11.4) | 53 (7.7) | 40 (7.9) | 13 (7.2) |
| N3 | 430 (14.9) | 74 (10.7) | 60 (11.8) | 14 (7.7) |
| Unknown | 267 (9.2) | 123 (17.9) | 86 (16.9) | 37 (20.4) |
| Bone Met | ||||
| No | 1014 (35.1) | 254 (36.9) | 166 (32.7) | 88 (48.6) |
| Yes | 1876 (64.9) | 435 (63.1) | 342 (67.3) | 93 (51.4) |
| Brain Met | ||||
| No | 2747 (95.1) | 612 (88.8) | 448 (88.2) | 164 (90.6) |
| Yes | 143 (4.9) | 77 (11.2) | 60 (11.8) | 17 (9.4) |
| Liver Met | ||||
| No | 2289 (79.2) | 411 (59.7) | 282 (55.5) | 129 (71.3) |
| Yes | 601 (20.8) | 278 (40.3) | 226 (44.5) | 52 (28.7) |
| Lung Met | ||||
| No | 2055 (71.1) | 423 (61.4) | 306 (60.2) | 117 (64.6) |
| Yes | 835 (28.9) | 266 (38.6) | 202 (39.8) | 64 (35.4) |
| Surgery | ||||
| No Surgery | 2127 (73.6) | 633 (91.9) | 467 (91.9) | 166 (91.7) |
| PM | 220 (7.6) | 18 (2.6) | 13 (2.6) | 5 (2.8) |
| TM | 240 (8.3) | 17 (2.5) | 13 (2.6) | 4 (2.2) |
| RM | 263 (9.1) | 16 (2.3) | 12 (2.4) | 4 (2.2) |
| Others | 14 (0.5) | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 26 (0.9) | 5 (0.7) | 3 (0.6) | 2 (1.1) |
UOQ – upper outer quadrant; LOQ – lower outer quadrant; LIQ – lower inner quadrant; UIQ – upper inner quadrant; CEN – central portion; NOS – not otherwise specified; PM – partial mastectomy; TM – total mastectomy; RM – radical mastectomy.
The calibration curve and ROC curve for assessing the calibration and discrimination of the nomograms in predicting all-cause early death (A, D), cancer-specific early death (B, E) and non-cancer-specific early death (C, F) in the validation cohort.