Abstract
Introduction
Type 1 diabetes is a lifelong disease with high management burden. The majority of people with type 1 diabetes fail to achieve glycaemic targets. Algorithm-driven automated insulin delivery (closed-loop) systems aim to address these challenges. This review provides an overview of commercial and emerging closed-loop systems.
Areas covered
We review safety and efficacy of commercial and emerging hybrid closed-loop systems. A literature search was conducted and clinical trials using day-and-night closed-loop systems during free-living conditions were used to report on safety data. We comment on efficacy where robust randomised controlled trial data for a particular system are available. We highlight similarities and differences between commercial systems.
Expert opinion
Study data shows that hybrid closed-loop systems are safe and effective, consistently improving glycaemic control when compared to standard therapy. While a fully closed-loop system with minimal burden remains the end-goal, these hybrid closed-loop systems have transformative potential in diabetes care.
Keywords: Artificial pancreas, automated insulin delivery, closed-loop, continuous glucose monitoring, insulin pump, type 1 diabetes
1. Introduction
1.1. Type 1 diabetes and the need for automated insulin delivery
In type 1 diabetes, insulin-producing pancreatic beta cells are destroyed by an immune-mediated process. Beta and alpha cells produce insulin, amylin and glucagon, all of which are hormones required to maintain the body’s glucose homeostasis. Prior to insulin therapy, type 1 diabetes was universally fatal. Nowadays, insulin is either replaced with multiple daily injections (MDI) using both short- and long-acting insulins, or by continuous infusion of rapid-acting insulin via an insulin pump. Even with insulin replacement therapy and frequent self-monitoring of blood glucose levels, people with diabetes are at risk of multiple long-term complications if glucose control is suboptimal (1), as well as the immediate risks posed by dangerously low (hypoglycaemia) or high (hyperglycaemia) glucose levels if insulin is acutely over- or under-dosed.
A person with type 1 diabetes is required to make frequent and complex dosing decisions to maintain glucose levels in the desired range whilst avoiding hypoglycaemia. A multitude of factors influence glucose levels and insulin sensitivity, causing considerable day-to-day variability of glucose levels as well as insulin requirements (2, 3). This leads to a high burden of diabetes management. Consequently, only one third of people with type 1 diabetes achieve recommended levels of glycaemic control with current treatment options (4). Automated insulin delivery has the potential to address these issues, decrease disease burden, and improve glycaemic outcomes.
1.2. Evolution of closed-loop systems
The concept of closed-loop glucose control has been present since the 1960s (5). The approach was not feasible until much more recently. The availability of smaller and reliable insulin pumps, the emergence of accurate and reliable continuous glucose monitoring systems, and the access to secure and safe wireless communication technologies made the development of wearable closed-loop systems possible.
The first rudimental glucose-responsive systems were able to automatically suspend insulin delivery at low glucose levels, addressing the issue of hypoglycaemia (low glucose suspend feature). This was quickly followed by systems able to predict hypoglycaemia and suspend insulin delivery accordingly (PLGS: predictive low glucose suspend feature). Both systems were shown to reduce the frequency and severity of hypoglycaemia but did not address hyperglycaemia (6–8). Two such PLGS systems, the MiniMed 640G insulin pump with SmartGuard technology (Medtronic, Northridge, CA, USA) and the t:slim X2 insulin pump with Basal-IQ (Tandem Diabetes Care, San Diego, CA, USA) are commercially available and rapidly being integrated into standard of care in many countries.
Subsequently, several research groups began developing more complex algorithms with the goal of an automated glucose-responsive insulin delivery closed-loop system. Initially, an insulin-dosing component was added to the existing PLGS feature to create a predictive hyperglycaemia and hypoglycaemia minimisation (PPHM) system. These systems delivered small automated correction boluses when glucose was predicted to exceed a pre-defined level, but did not modulate insulin delivery continuously. Systems were shown to improve time in target range overnight without increasing time in hypoglycaemia in both children and adults (9, 10). Following this, more advanced algorithms were developed that automatically and continuously modulate insulin delivery based on sensor glucose readings to maintain glucose in a target range. Initial studies focused on assessing safety and feasibility in the inpatient settings (5). This was followed by supervised camp studies as well as overnight outpatient studies (11–19). These trials showed that closed-loop delivery overnight was able to significantly increase time in the target range and reduce hypoglycaemia in children and adults (11–16, 18, 20). Day-and-night closed-loop glucose control presented additional challenges in the form of mealtimes and exercise. Postprandial glucose control is challenging due to delays in absorption of subcutaneously administered insulin and the unpredictable pattern of glucose absorption from meals. Fully closed-loop systems, not requiring user input at meal times, are therefore currently challenged by postprandial hyperglycaemia and the risk of subsequent hypoglycaemia. Hybrid closed-loop systems that require user-initiated meal-time boluses represent a more practical approach. These systems have demonstrated efficacy and safety in the home setting in children, adolescents, adults and pregnant women (21, 22). The first commercially available hybrid closed-loop system was the 670G system (Medtronic, Northridge, CA, USA), released in 2016. More recently the Tandem Control-IQ system (Tandem Diabetes Care, San Diego, CA, USA ) and the CamAPS FX closed-loop app (CamDiab Ltd., Cambridge, UK) received regulatory approval and are now commercially available, while several other systems await approval and commercialisation.
1.3. Scope of this review
We review the safety and efficacy of current and emerging insulin-only closed-loop systems. A search of the existing literature was conducted via PubMed and Google Scholar. Keywords included were ‘type 1 diabetes’, ‘closed-loop’, ‘artificial pancreas’, ‘insulin’ and ‘clinical trial’. Further studies were identified from cited articles. Our search was restricted to reports published in English. Only clinical trials using day-and-night closed-loop systems during free-living conditions were used to report on safety and efficacy data. We comment on the efficacy where robust randomised controlled trial data for a particular system are available.
2.0. The building blocks of closed-loop systems
Three technological building blocks are required to facilitate automated glucose-responsive insulin delivery: an insulin pump able to continuously deliver insulin; a continuous glucose monitoring system to make glucose measurements; and a computer algorithm that directs insulin pump’s delivery based on real-time glucose measurements. Most current closed-loop systems adopt the hybrid closed-loop approach requiring input from the user at mealtimes.
2.1. Insulin pump therapy
Insulin pumps are programmable, battery-operated devices that deliver short- or rapid acting insulin into the subcutaneous tissue via Teflon or steel catheters at pre-programmed rates with user-initiated meal-time boluses (23) Compared to MDI, pump therapy allows for greater flexibility in terms of prandial bolusing and accommodating changes in insulin sensitivity due to exercise, early morning insulin resistance, illness or periods of fasting (23). A variety of pumps are commercially available. Tethered pumps have a separate cannula and tubing while patch pumps have an embedded infusion set. More than half of people with type 1 diabetes in the USA use pump therapy (4). Pump therapy has been shown to affect modest reductions in HbA1c of 0.3 to 0.6% as well as lowering rates of severe hypoglycaemia and diabetic ketoacidosis (24, 25). However, these devices still require frequent user input and adjustments to settings.
2.2. Continuous glucose monitoring
With the emergence of continuous glucose monitoring (CGM) devices in the early 2000s (26), the possibility of creating a feedback loop came within reach. CGM systems consist of a disposable sensor placed subcutaneously that measures glucose concentration in the interstitial fluid approximately every 5 minutes and a transmitter that sends values to a receiver or mobile device, providing near real-time glucose measurements. Some systems display glucose concentration only on demand, referred to as intermittently viewed or intermittently scanned CGM (26, 27), while some but not all require regular finger-stick calibrations. Sensor accuracy is measured through the mean absolute relative difference, which is the average of the absolute error between CGM and matched reference values (27). Current best performing CGMs have a mean absolute relative difference of just below 10%, an accuracy broadly comparable to most blood glucose meters, making them safe for insulin dosing decisions (19, 27). The first interoperable CGM system, the Dexcom G6, was licensed by the FDA in 2018 and is factory-calibrated obviating the need for finger-stick calibrations (28).
2.3. Control algorithms
First glucose-responsive automated insulin systems were trialled long before insulin pumps and CGM were widely available (26). However, only with the advent of modern pumps and CGM systems has closed-loop become a feasible and achievable therapy option. Three main types of closed-loop control algorithms have been developed.
2.3.1. Proportional, integral, derivative controllers
The proportional, integral, derivative (PID) controller is an algorithm treating to target by directing insulin doses based on the difference from target glucose at the current point of time (proportional), the rate of change in measured glucose level over time (derivative) and the area under the curve between measured and target glucose levels (integral) (19, 29). In a systematic review by Weisman et al, PID algorithms had less improved time in target compared to model predictive control (MPC) and fuzzy logic algorithms (22). This is consistent with the results of a direct comparison between PID and MPC algorithms, where time in target range was greater for MPC with a lower mean glucose (30).
2.3.2. Model predictive control controllers
The model predictive control (MPC) algorithm uses a mathematical model that links insulin delivery to glucose excursions. The model can be dynamic and multi-compartmental, predicting glucose levels whilst simultaneously adjusting insulin delivery to treat-to-target. These controllers are able to accommodate insulin absorption delays as well as events that influence glucose levels such as carbohydrates and active insulin (19). The MPC approach allows for modelling of diurnal variations and exercise (19) and is the most commonly used type of closed-loop algorithm.
2.3.3. Fuzzy logic controllers
The Fuzzy logic approach adjusts insulin delivery by applying approximate rules aiming to imitate the line of reasoning of diabetes practitioners or experts (31). Like the other two algorithms it frequently uses safety modules to limit insulin delivery such as suspending insulin delivery when glucose is low or falling quickly.
3.0. Clinical efficacy and safety of commercial and emerging insulin closed-loop systems
In the very near future people with type 1 diabetes will have the choice between multiple hybrid closed-loop systems. This choice will be based on a combination of safety, efficacy, usability, feature set, and personal preference. We review closed-loop systems that are commercially available, approved or pending approval, and systems that are in the clinical trial phase.
3.1. Safety and efficacy measures
We comment on safety of closed-loop systems by assessing diabetes-related adverse events. These include diabetic ketoacidosis (DKA), severe hyperglycaemia with ketosis, and severe hypoglycaemia events. Both DKA and severe hypoglycaemia have the potential to be life-threatening events requiring hospitalisation, and can occur in any person with type 1 diabetes irrespective of type of insulin therapy.
Therapeutic efficacy in type 1 diabetes is traditionally assessed by measuring average glycaemia through glycated haemoglobin (HbA1c), which reflects glucose concentrations over the previous three months (32). However, HbA1c is only an indirect marker of glycaemia and measurements can vary in the same individual as well as being affected by ethnicity and conditions affecting red blood cell turnover (32). By using CGM technology it is now possible to reliably determine mean glucose levels, which more accurately reflect the duration and degree of hyperglycaemia than HbA1c. It is now suggested to use CGM metrics alongside HbA1c when assessing glycaemic control (32, 33). Based on consensus statements on the reporting of CGM data, the most common outcome measures of glycaemic control are (27, 34): mean sensor glucose; time in the normoglycaemic range defined as between 3.9mmol/L and 10mmol/L with an aim of >70% time in range; time below range defined as <3.9mmol/L with an aim of <4% time spent in hypoglycaemia; time above range defined as >10mmol/L; and the coefficient of variation, reflecting glycaemic variability. We focus on these metrics alongside HbA1c to assess efficacy of closed-loop systems in the present review.
3.2. Medtronic 670G system
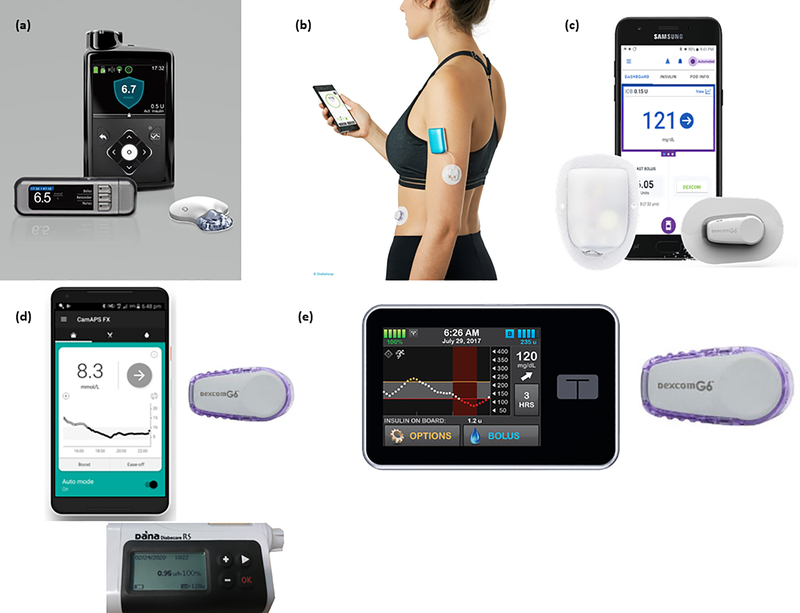
The Medtronic 670G Hybrid Closed-loop System (Medtronic, Northridge, CA, USA) was approved by the FDA in 2016 and is commercially available in the USA and Europe. It comprises the 670G insulin pump with an embedded PID algorithm, the Guardian Sensor 3 with Guardian Link 3 transmitter and the Contour Next Link blood glucose meter (Fig. 1). Similar to all other commercial closed-loop systems, it is a hybrid closed-loop system requiring manual meal-time boluses. It delivers variable basal rates directed by the control algorithm, and is a treat-to-target system. The glucose target is set at 6.7mmol/L, which cannot be lowered, but can be temporarily raised to 8.3mmol/L. The algorithm learns based on total daily dose and adjusts maximum basal rates and suggested corrections based on this information. Corrections are not automated. The system requires accurate basal rate, insulin-to-carbohydrate and insulin sensitivity settings in order to modulate insulin delivery appropriately. The glucose sensor requires a minimum of two calibrations per day (35). Remote glucose monitoring is not available (Table 1)
Figure 1.
Hybrid closed loop systems. (a) Medtronic 670G Hybrid Closed System with Guardian Link 3 transmitter and the COUNTOUR NEXT LINK blood glucose meter (reproduced with permission of Medtronic, Inc.) (b) Diabeloop system with DLBG1 Algorithm with Kaleido insullin patch-pump (reproduced with permission of Diabelop SA) (c) Omnipod Horizon Hybrid Closed Loop System (Insulet Crop., Acton, MA, USA) paired with a Dexcom G6 sensor (Dexcom, San Diego, CA, USA)(Copyrighted image used with permission. © 2019 Insulet Corporation. All rights reserved.) (d) CamAPS FX algorithm with Dexcom G6 sensor and Dana Diabecare RS Sooil insulin pump (Sooil Development, Seoul, Korea) (reproduced with permission of CamDiab Ltd.) (e) Tandem tslim X2 insulin pump (Tandem Diabetes Care, San Diego, CA, USA) paired with a Dexcom G6 Sensor (Dexcom, San Diego, CA, USA).
Table 1.
Comparison of commercially available hybrid closed-loop systems.
| CamAPS FX | MiniMed 670G System | Tandem Control-IQ | |
|---|---|---|---|
| Regulatory approvals | |||
| Age licensed for | 1 year + | 7 years + | 14 years + |
| Countries licensed in | Europe | USA & Europe | USA & Europe |
| Location of algorithm | App-based | Pump-integrated | Pump-integrated |
| Compatible with factory calibrated CGM | Yes – Dexcom G6 | No – regular finger-prick calibrations required | Yes – Dexcom G6 |
| Insulins licensed for | Rapid- and ultra-rapid acting | Rapid-acting | Rapid-acting |
| System features | |||
| Parameters required for system setup | TDD, weight | TDD, weight, ICR, ISF, basal rates | TDD, weight, ICR, ISF, basal rates |
| Adaptive learning | Overall, diurnal, meals | Overall | None |
| Target type | Treat-to-target: default 5.8mmol/L | Treat-to-target: 6.7mmol/L | Treat-to-range: 6.2–8.9mmol/L |
| Adjustable glucose target | Personalised target 4.4 – 11mmol/L; Personalised time-blocks | Temporary target option of 8.3mmol/L | Overnight target range 6.1–6.7mmol/L (Sleep Activity) Exercise target range 7.8–8.9mmol/L |
| Active insulin time | Automatically adjusted based on adaptive learning | Personalised active insulin time of 2–8 hours | Factory-set at 5 hours; not adjustable |
| Automated correction bolus | No | No | Yes, when glucose is predicted to be >10mmol/L and not in Sleep Activity |
| Automode suspension | No | Yes | No |
| Automated data upload to cloud | Diasend | No | No |
| User features | |||
| Phone-based bolusing | Yes | No | No |
| Activity mode | Yes | Yes | Yes |
| Boost mode | Yes | No | No |
| Remote glucose monitoring | Yes – SMS alerts | No | Yes – live Followers |
[CGM – continuous glucose monitoring; TDD – total daily dose; ICR – insulin-to-carbohydrate ratio; ISF – insulin sensitivity factor; Boost mode in CamAPS FX is user-initiated and intensifies algorithm-driven insulin delivery for use during periods of high glucose e.g. minor illness, pre-menstrual, growth hormone pulses etc.]
The pivotal trial for the 670G system was a single-arm non-randomised trial including 124 pump-users, 30 adolescents and 94 adults, who used the 670G system for three months at home (36). All outcome data were compared to baseline values only. Adolescents used Auto Mode a median 76% (IQR 68% to 88%) of the time and adults a median 88% (IQR 78% to 97%) of the time with high sensor usage in both groups. There were no episodes of severe hypoglycaemia or DKA over 12,389 patient days, confirming the safety of the system in the home setting (36, 37) (Table 2). In a retrospective cohort study including 127 adults who transitioned from sensor augmented pump therapy to closed-loop therapy, Akturk et al similarly found no events of severe hypoglycaemia or DKA during closed-loop use (38). Unusually, severe hypoglycaemia was defined as requiring a glucagon injection, which is different to the more common definition of requiring third party assistance.
Table 2.
Hybrid closed loop systems that are approved, pending approval or in the clinical trial phase.
| Closed loop system | Safety | Efficacy | Approval status | Key references |
|---|---|---|---|---|
| Medtronic 670G Hybrid Closed Loop System | No severe adverse events related to algorithm | No controlled trial data available | Approved in the USA and Europe; Commercially available |
Garg 2017 (30) Forlenza 2019 (33) |
| Tandem Control-IQ | 1 DKA event unrelated to algorithm, no severe hypoglycaemia |
TIR increased 11 percentage points1 (95% CI, 9 to 14; p<0.001) TIR 11 increased percentage points2 (p<0.0001) HbA1c reduction of 0.33%1 (95% CI, 0.53 to 0.13; p=0.001) |
Approved in the USA and Europe; Commercially available |
1Brown 2019 (43) 2Kovatchev 2020 (41) |
| CamAPS FX (CamDiab) | 1 DKA event unrelated to algorithm, 1 severe hypoglycaemia unrelated to algorithm |
TIR increased 11 percentage points
3 (95% CI, 8 to 14; p<0.001) TIR increased 11 percentage points4 (95% CI, 8 to 13; p<0.0001) HbA1c reduction of 0.36%4 (95% CI, 0.53 to 0.19; p<0.0001) |
Approved in Europe; Commercially available in the UK |
3Thabit 2015 (48) 4Tauschmann 2018 (49) |
| DLBG1 Hybrid Closed Loop System (Diabeloop) | 9 severe hypoglycaemia events unrelated to algorithm, no DKA events |
TIR increased 9 percentage points (95% CI, 6 to 12; p<0.0001) HbA1c reduction not significant |
Approved in Europe; Not yet commercially available |
Benhamou 2019 (85) |
| Omnipod Horizon Closed Loop System (Insulet) | No severe adverse events reported | No controlled trial data available | Awaiting approval | Sherr 2019 (61) |
| iLet (insulin-only; Beta Bionics) | No severe adverse events reported | No controlled trial data available | Awaiting approval | Ekhlaspour 2019 (13) |
| Tidepool Loop | No data available | No data available | Awaiting approval | No published data |
[TIR – time in range]
The 670G system is licensed from age 7 years based on a nonrandomised single-arm multi-centre study in 106 children aged 7–13 years using the 670G system for three months in the home setting (39). Over 13,738 patient days there were no episodes of severe hypoglycaemia, no serious adverse events and no adverse device events. There were twenty-seven severe hyperglycaemia events during the run-in phase and seventy-six during the study phase (39). However, given that hyperglycaemia is an expected adverse event in people with type 1 diabetes this data is difficult to interpret without a control arm. This trial also evaluated safety of the system in children aged 2–7 years; this data is yet to be published.
Although the 670G system is not currently licensed for children under the age of 7 years, it is being prescribed off-label by diabetes healthcare providers. Salehi et al report a retrospective cohort study comprising 16 children under the age of 7 years using the 670G system for a minimum of three months. They reported no DKA events, hypoglycaemic seizures, glucagon use or diabetes-related hospitalisation, but four subjects experienced severe hypoglycaemia (40).
To date there are no published randomised controlled trials assessing the efficacy of the 670G system. Two six month prospective open-label randomised controlled trials comparing the system to sensor-augmented pump therapy or standard therapy (either pump or MDI) are under way (41, 42).
An enhanced system incorporating a combination of fuzzy logic and PID algorithms is in development. It administers automated correction boluses, has an optional lower target glucose level, and other features aimed at increasing utilisation of Auto Mode. Results from a feasibility study in 12 adults showed no severe hypoglycaemia or DKA events during a three week home study period (43).
3.3. Tandem Control-IQ
After receiving FDA approval in December 2019, the Tandem Control-IQ algorithm (Tandem Diabetes Care, San Diego, CA, USA) became commercially available in the USA in 2020 from age 14 years upwards. This hybrid closed-loop system uses an MPC algorithm originally implemented on the DiAS platform, which was developed at the University of Virginia. Unlike the Medtronic 670G system, the Control-IQ algorithm has been approved for use with any alternate controller-enabled insulin pump (44). The system requires manual meal boluses, while closed-loop variable basal rates and correction boluses are automated. The system requires accurate basal rate, insulin-to-carbohydrate and insulin sensitivity settings to modulate insulin delivery appropriately. There is no adaptive learning. The algorithm is treat-to-range defined by a target range from 6.2 to 8.9mmol/L and can be set to intensify overnight with target range between 6.2 and 6.7mmol/L. Remote glucose monitoring is available via the Dexcom Follow function (Table 1).
The system was first tested for safety in the unmonitored home setting in an uncontrolled study of 30 adults who used the algorithm for a two week period (45), which was extended to six months for 14 participants (46). There were no severe hypoglycaemia or DKA events. Two episodes of moderate hyperglycaemia with ketosis were related to insulin pump occlusions.
Two randomised trials compared the Control-IQ algorithm as a mobile version in combination with the Roche Accu-Chek Spirit Combo insulin pump (Roche; Indianapolis, IN, USA) and a Dexcom G4 Platinum CGM system (Dexcom; San Diego, CA, USA) to sensor-augmented pump therapy (47, 48). The crossover trial included periods of 24 hour closed-loop therapy and evening and overnight only closed-loop therapy. There were no cases of DKA, three cases of hyperglycaemia with ketosis managed in the home setting, and five cases of severe hypoglycaemia (47). Only one of these events occurred while the closed-loop system was active and was not attributable to device malfunction. The trial showed an improvement in time in range of 11 percentage points (p<0.0001) (47). In the three month parallel group study there were no DKA events and one severe hypoglycaemia event not related to device malfunction (48). Fourteen versus four days of hyperglycaemia with ketosis were recorded in the closed-loop versus sensor-augmented pump therapy arm respectively. Both studies showed a reduction in time below range and time above range in the closed-loop periods (47, 48).
In the pivotal trial by Brown et al the Control-IQ algorithm was integrated into a Tandem t:slim X2 insulin pump (Tandem Diabetes Care, San Diego, CA, USA) paired with a Dexcom G6 (Dexcom, San Diego, CA, USA) continuous glucose monitor (49) (Fig. 1). This multi-centre parallel-group randomised trial compared the Control-IQ system to sensor-augmented pump therapy over a six month period in the home setting in 168 participants, previously on MDI or pump therapy, aged 14–71 years. The study had a 100% follow-up rate and closed-loop usage was high at median 90%, enabling robust assessment of efficacy and safety. While the initial feasibility study conducted for two days in a supervised setting demonstrated no adverse events (50), seventeen adverse events occurred in the pivotal study over 20,571 days of closed-loop usage. No severe hypoglycaemia was reported, but one DKA event occurred in the closed-loop group as a result of a pump infusion set failure. Thirteen further adverse events of hyperglycaemia or ketosis occurred in 12 closed-loop users compared to two in the control group. A certain number of hyperglycaemia events may be anticipated as it is well known that commencing pump therapy or switching pump model increases the risk of hypo- and hyperglycaemia events as user errors and infusion set issues are more common.
The closed-loop group demonstrated a time in range of 71%±12%, an increase of 11 percentage points (95% CI 9 to 14; p<0.001) compared to the control group, equating to 2.6 more hours per day spent in the target range (49). This effect remained consistent over the six month study period. All main secondary outcomes, including time above range, mean glucose level, HbA1c, and time below range favoured closed-loop. There was a significant reduction in HbA1c of 0.33% (95% CI, 0.13 to 0.53; p=0.001) favouring closed-loop groups. Closed-loop continued to be favoured in terms of time in range and time below range when adjusted for a broad range of baseline characteristics. These results show Control-IQ to be safe and efficacious, with similar efficacy to other closed-loop systems (Table 2). A potential advantage may be the advertised inter-operability of the algorithm across different pump and CGM devices.
3.4. CamAPS FX
While both the Control-IQ and Medtronic 670G systems currently function as pump-integrated systems, the CamAPS FX algorithm, developed at Cambridge University (Cambridge, UK), runs on an Android smartphone and communicates with insulin pump and CGM sensor wirelessly via Bluetooth. In earlier studies, the MPC based algorithm was used with a modified Medtronic 640G insulin pump and an Enlite 3 glucose sensor (Medtronic, Northridge, CA, USA). The follow-up algorithm implemented by the CamAPS FX Android app (CamDiab, Cambridge, UK) received CE marking from age 1 year in 2020. It will be initially used with the Dexcom G6 CGM and the Dana Diabecare RS Sooil insulin pump (Sooil Development, Seoul, Korea), with the intention to connect to other insulin pumps and CGM devices in future (Fig. 1) (51). It is the only commercially available closed-loop system licensed for very young children.
The CamAPS FX app is a hybrid closed-loop system and requires prandial bolusing. Algorithm-driven insulin delivery is continuously adjusted to achieve a default glucose target of 5.8mmol/L, user-adjustable to between 4.4 and 11 mmol/L. Other user-adjustable settings include the ability to make the algorithm more or less aggressive for a set time period. Unlike other closed-loop systems, CamAPS FX requires only the user’s weight and total daily insulin dose for set-up, insulin sensitivity and active insulin time are automatically calculated and adjusted. Adaptive learning is incorporated with regards to total daily insulin requirements, diurnal variations and meal patterns. Multiple alert functions including remote alarms are available (Table 1)
A four week randomised crossover study investigated the safety and efficacy of the algorithm in 29 adults with good baseline glycaemic control at HbA1c of <58mmol/mol (52). Time in range improved by 10 percentage points (95% CI 8 to 13; p<0.0001) during the closed-loop period, even though participants already had tight control on standard therapy. This was achieved while significantly reducing hypoglycaemia and glucose variability. No severe hypoglycaemia or DKA events were reported indicating that the system is safe and effective in a well-controlled population at higher risk of hypoglycaemia.
The algorithm was studied in adolescents with suboptimal glycaemic control and very young children. Tauschmann et al conducted a three week randomised crossover trial comparing closed-loop with sensor augmented pump therapy in 12 adolescents with a mean HbA1c of 69 ± 8 mmol/mol (53). Adolescents omit prandial insulin boluses more frequently and are therefore at higher risk of significant hypo- and hyperglycaemia. Significantly, there were no severe hypoglycaemia or hyperglycaemia events reported during closed-loop use. Time in range was significantly increased with no increase in hypoglycaemia events in the closed-loop period, suggesting the system is safe and effective in adolescents with suboptimal glycaemic control. A multi-centre, three week randomised crossover study in 24 children aged between 1 and 7 years compared the closed-loop algorithm using standard strength and diluted insulin aspart (U100 and U20 respectively) (54). No severe hypoglycaemia or DKA events were reported. Time in range was consistent with previous studies at 72 ± 8% for closed-loop with diluted insulin and 70 ± 7% for closed-loop with standard insulin (p = 0.16).
Thabit et al conducted a twelve week open-label randomised crossover study comparing the closed-loop system to sensor augmented pump therapy in 33 adults (55), while Tauschmann et al reported a similarly designed study enrolling 86 participants aged 6 years and older with suboptimal glycaemic control (56). In the study by Thabit et al, there was one severe hypoglycaemia event that occurred when closed-loop was non-operational and the participant was receiving pre-set basal rates via their pump. No DKA events were reported. Time in range improved by 11 percentage points (95% CI 8 to 14; p<0.001) in the closed-loop group, with a significant reduction in HbA1c of 0.3 (95% CI 0.1 to 0.5; p<0.002) (55) (Table 1). In the study by Tauschmann et al, there were no severe hypoglycaemia events and one DKA event in the closed-loop group due to infusion set failure (56). Time in range improved by 11 percentage points (95% CI 8 to 13; p<0.0001) and HbA1c was reduced significantly by 0.4% (95% CI 0.2 to 0.5%; p<0.0001) in the closed-loop group (Table 2). There was a significant reduction in both hypoglycaemia as well as glucose variability in the closed-loop group showing the efficacy of the system across a wider demographic than shown in other closed-loop systems.
Several longer-term studies of the CamAPS FX hybrid closed-loop system in children, adolescents and adults are under way, including newly diagnosed children and young people with type 1 diabetes (57–61).
3.5. Diabeloop System
The Diabeloop system, developed by a research group in France, relies on an MPC algorithm based on the Hovorka model (62). The DLBG1 algorithm received CE marking in Europe in 2018. Diabeloop plan to commercialise the system for people aged from 22 years in the first instance (63). The system applies the hybrid closed-loop approach and the algorithm resides on a locked down Android handset. The user applies manual meal bolusing and exercise input. In contrast to other systems, up to five settings are user-adjustable. These include the personal glucose target (pre-set at 6.1mmol/L), the hypoglycaemia threshold (pre-set at 3.9mmol/L), the reactivity in the hyperglycaemic range, the reactivity in the normoglycaemic range and the prandial insulin dose (62). During the studies, the control algorithm was installed on an android smartphone and communicated wirelessly with the Dexcom G5 CGM system and a Cellnovo insulin patch-pump (Cellnovo Group, Paris, France). Cellnovo has since ceased its operation and the Diabeloop system is now used with the Kaleido insulin patch-pump (ViCentra B.V., Utrecht, Netherlands) and the newer Dexcom G6 CGM system (Fig. 1).
In the initial pilot study, 8 adults used the closed-loop system for three weeks at home with remote monitoring throughout (62). There were no reports of severe hypoglycaemia or DKA events. There were twenty-seven reports of hypoglycaemia with sensor glucose <2.8mmol/L, ten of which required intervention by the study nurse, and three reports of hyperglycaemia >20mmol/L, one of which required intervention by the study nurse. The group then proceeded with their pivotal trial, which was an open-label randomised controlled crossover study comparing the Diabeloop system with sensor-augmented pump therapy in 68 adults over a twelve week period (64). Three severe hypoglycaemia events were reported during the first twelve week treatment period and a fault in a safety sensor of the Cellnovo pump was identified. Subsequently the study protocol was amended to include the Kaleido insulin patch-pump for the second twelve week treatment period, resulting in a longer washout period of thirty weeks. Sensitivity analysis revealed no carryover effect (p=0.96) and results were analysed using a modified intention-to-treat analysis. Seventeen serious adverse events occurred during both treatment periods but none were associated with inappropriate recommendations by the control algorithm. There were no DKA events, but nine severe hyperglycaemic events were reported in the closed-loop group. These were universally associated with hardware issues. Eight severe hypoglycaemic events were reported, of which five were in the closed-loop group. Three of these were due to the faulty Cellnovo pumps and two were due to human error, which may explain the higher number of severe hypoglycaemia events compared to other hybrid closed-loop system studies
Time in range was higher in the closed-loop group with a mean difference of 9 percentage points (95% CI 6 to 12; p<0.0001) (Table 2). Although mean HbA1c reduced in the closed-loop group this was not statistically significant. Time below range was significantly lower in the closed-loop group with a mean difference of 2.4 percentage points (95% CI 1.7 to 3.0; p<0.0001). It is important to note that participants were remotely monitored throughout the trial with automated text messages being sent to healthcare professionals when certain alarm thresholds were reached. While Benhamou et al show that intervention from remote monitors decreased steadily during each twelve week treatment period, this limits the generalisability of both efficacy and safety findings in terms of usage of the algorithm in an unmonitored setting.
Two clinical trials using the Diabeloop system are under way, a shorter crossover study in younger children aged 6–12 years and a nine-month randomised trial in adults, both assessing safety and efficacy of the system (65, 66).
3.6. Omnipod Horizon System
The Omnipod Horizon Hybrid Closed-loop System is not yet approved, but initial safety and feasibility trials have been reported. The developmental system applies an MPC algorithm running on a handheld device, the Personal Diabetes Manager (PDM), which connects with the patch pump Omnipod Insulin Management System (Insulet Corporation, Acton, MA, USA), and a CGM sensor (Fig. 1). The system requires prandial bolusing. Insulin-to-carbohydrate ratios as well as insulin sensitivity factors are user-adjustable. Basal insulin-dosing decision are made every 5 minutes based on CGM values.
In the study setting, a modified version of the Omnipod Insulin Management System (Insulet Crop., Acton, MA) tubeless insulin pump (Pod), a modified Personal Diabetes Manager, the Dexcom G4 505 Share Artificial Pancreas (AP) System and the Omnipod personalised MPC algorithm on a Windows 10 tablet configured with the portable AP system were used. A 36 hour inpatient safety and feasibility study showed the algorithm was safe in adult, adolescent, and paediatric participants (67). A further study assessed the algorithm over 96 hours in a supervised hotel setting. The single-arm study included 36 participants, aged 6 to 65 years and was preceded by a 7 day standard outpatient therapy phase (68). No serious adverse events and no DKA or severe hypoglycaemia events were reported. There were twenty-one hyperglycaemia events, none with ketosis (Table 2).
A single-arm 3-month pivotal trial for the Omnipod Horizon system is due to be completed later this year, which will provide safety data in the unmonitored home setting (69, 70)
3.7. iLet (insulin only)
Beta Bionics, a public benefit corporation, has developed a closed-loop system the iLet (Beta Bionics Inc., Boston, MA, USA). This is not yet FDA approved and is being trialled as an insulin-only and a bihormonal closed-loop system utilising an MPC algorithm. Unlike other algorithms it does not require inputting of insulin-to-carbohydrate ratios and instead relies solely on a meal-announcement function with three meal size options. It also does not require a pre-set basal rate, but instead imputes its own basal rate based on recent data. In the bihormonal version glucagon dosing is directed by a separate PID algorithm.
A recent single-arm feasibility study by Ekhlaspour et al assessed the insulin-only version of the algorithm in 13 adults (17). The study included 3 seven day periods in the home setting with remote monitoring: seven days of standard therapy, seven days of insulin-only closed-loop therapy with a fixed glucose target of 7.2mmol/L and seven days of insulin-only closed-loop therapy with a dynamic glucose target between 6.1 to 7.2mmol/L. Throughout closed-loop use there were 157 alerts for connectivity issues or hypoglycaemia and the study team chose to alert participants for 71% of them. No severe hypo- or hyperglycaemic events occurred (Table 2). A 3 month pivotal randomised trial is due to commence this year and will assess safety and efficacy of the system (71)
3.8. Tidepool – Loop
Tidepool is a non-profit organisation initially created to provide free software for people with type 1 diabetes and health care professional to visualise data from a variety of different devices. More recently Tidepool has started a project to develop an FDA-regulated version of Loop (Tidepool Loop), a DIY artificial pancreas system open-source software for iPhone. This system is intended to work inter-operably with a variety of inter-operable insulin pumps and CGM devices. The Loop algorithm is an MPC algorithm that adjusts insulin delivery every 5 minutes applying the hybrid closed-loop approach, but the open-source version does not currently have a ‘learning’ ability (72). Following a partnership with Insulet, the Omnipod System is the first commercial system announced to be compatible with Tidepool Loop (Palo Alto, CA, USA) (73). More recently Medtronic is also partnering with Tidepool Loop (74).
While Tidepool have partnered with the Jaeb Centre for Health Research to conduct a large observational study of the Loop DIY open-source software using data from DIY Loop users across the globe (73), there is not yet any clinical trial data available on the Loop algorithm (Table 2).
3.9. DIYers
The DIY movement began in 2013 when a community of people with type 1 diabetes and their families worked together online to promote the development of open source diabetes management systems using the hashtag ‘#WeAreNotWaiting’ (75). These DIY hybrid closed-loop systems use open-source software, namely OpenAPS, AndroidAPS and Loop. Unlike commercial systems they are not regulated and no clinical trial data exists. They may require a hardware radio ‘bridge’ (i.e. RileyLink) to communicate between the pump and the algorithm controller. Lack of oversight and clear lines of accountability as well as the absence of clinical trial data are clear drawbacks to DIY systems. However, continuous user-driven optimisation of the system, low-cost availability and high interoperability will continue to make these algorithms an attractive option for some people with type 1 diabetes (75).
4.0. Current challenges and future directions
4.1. Fully closed-loop systems
Hybrid closed-loop systems improve glycaemic outcomes compared to gold-standard insulin therapy. However, daytime glucose control remains challenging due to mealtimes and exercise, necessitating increased user interaction and ongoing diabetes management burden. A fully closed-loop system without the need for user interaction is the ultimate goal but remains difficult to implement in practice. The two main challenges in fully closed-loop systems are early postprandial hyperglycaemic excursions and late postprandial hypoglycaemia. These are secondary to the delay in insulin action of currently available rapid-acting insulin analogues. Studies applying fully closed-loop systems have been limited to small study populations in inpatient or supervised settings. Weinzimer et al compared PID-type fully and hybrid closed-loop systems and found that time in range during the day was higher and postprandial peak glucose levels lower during hybrid closed-loop use with mealtime bolusing (76). A similar study using an MPC-type algorithm found that mean sensor glucose was significantly lower for announced versus unannounced meals (77). A fully closed-loop study by Dovc et al also reported a lower time in range compared to other hybrid closed-loop studies (78). In a fully closed-loop study in inpatients with unannounced meals there were more late post-prandial hypoglycaemia events than would be expected during standard therapy or hybrid closed-loop (79). However, a study by Dassau et al showed a mean time in range of 70% in a one day inpatient study with unannounced meals (80); a figure similar to the time in range in hybrid closed-loop studies, although the study duration was short. These studies highlight the challenges around fully closed-loop systems, which are unlikely to be resolved with the pharmacodynamic properties of currently available subcutaneous insulins analogues.
4.2. Dual hormone systems
One way to address the challenges around delayed insulin action as well as the hypoglycaemia risk associated with tighter glycaemic targets is to use adjunctive hormone therapy. In healthy individuals, pancreatic alpha cells secret glucagon in response to falling glucose levels to maintain normoglycaemia. This response is lost in people with type 1 diabetes, so even when insulin delivery is suspended, hypoglycaemia may occur. Dual hormone closed-loop systems aim to emulate this physiological process more closely by directing the delivery of both insulin and glucagon with the latter being delivered when hypoglycaemia occurs or is predicted. There are several barriers to dual hormone systems including the need for a second or dual chamber infusion pump and a lack of stable formulations of glucagon (81). Studies applying these systems have all been of limited size and duration. Although more recent results show an increased time in range for dual hormone systems compared to insulin-only therapy (82–84), long-term safety and efficacy data are not yet available. Glucagon is associated with increased gastrointestinal side-effects and long-term effects of regular subcutaneous glucagon administration on hepatic and cardiovascular systems are yet to be assessed (19, 81). Another approach that has been trialled in dual hormone systems is pramlintide. It is a synthetic analogue of amylin, a pancreatic protein that slows gastric emptying and is largely absent in people with type 1 diabetes. Pramlintide has been shown to reduce postprandial spikes in blood glucose more effectively than insulin alone (19). A recent inpatient study by Haidar et al showed an increased TIR when using a rapid insulin-and-pramlintide artificial pancreas compared to a rapid insulin-only artificial pancreas (84% compared to 74% TIR, p=0.0014), which was mainly due to a daytime increase in TIR on the dual hormone system (85). Similar to glucagon, pramlintide requires a separate infusion pump, although co-formulations with insulin are in development. Other limitations are its dosage cap and potential side-effects such as nausea and increased risk of hypoglycaemia with pre-meal administration (86).
4.3. Other adjunctive therapies
Glucagon-like peptide-1 (GLP1) is another potential adjunctive option in closed-loop systems. Much like pramlintide it increases satiety, delays gastric emptying and suppresses glucagon release (86). It is already being used in type 2 diabetes as it increases residual insulin secretion in this group. There are several potential oral adjunctive medications such as dipeptidyl peptidase-4 inhibitors and SGLT2 inhibitors that may be combined with closed-loop systems to improve glucose control (86), although these drugs increase the risk of DKA and must therefore be approached with caution in people with type 1 diabetes.
4.4. Usability & inter-operability
While the goal of improving glycaemic control is vital in focusing closed-loop technology development and research, its eventual real-life use and success is dependent on the usability of such systems. Systems should decrease management burden, otherwise participants may cease utilising what they perceive to be more complex therapy even with improved glycaemic control (87). Ensuring users understand the limitations of current closed-loop systems prior to commencing therapy is vital and provision of adequate training and support services remains imperative. Increased inter-operability of closed-loop components is likely to improve system performance and user experience. FDA approval of the first inter-operable pump and inter-operable CGM sensor in the last 2 years is a major step towards potentially allowing people with type 1 diabetes to choose combinations of devices that suit their own personal needs and preferences (44, 88).
4.5. Healthcare provider education & training
To support rollout and access to closed-loop systems, it is important to consider healthcare provider (HCP) burden and ease-of-training. Appropriate and readily available HCP training is vital to ensure people with T1D have access to these new technologies in future. Kimbell et al assessed HCP views with regards to training, support and resourcing for closed-loop technology (89). It was emphasised that HCPs should be proficient with current pump and CGM technologies, and that accredited closed-loop training as well as demonstration systems could help improve understanding and confidence in these systems. After the initial training period, closed-loop users required less HCP input than those on pump therapy or multiple daily injections, therefore potentially reducing burden on diabetes clinics. The study recommended the development of clinical guidance to support HCPs in delivering closed-loop consultations.
4.6. Reducing burden and improving quality of life
Alongside clinical efficacy, hybrid closed-loop systems have been shown to reduce diabetes management burden and improve quality of life. Users report improved sleep quality, less time spent managing diabetes as well as decreased anxiety with regards to hypo- and hyperglycaemia in several studies (90–93). However, concerns about technical glitches, CGM inaccuracy and the burden of wearing multiple devices remain prevalent (90–93) and are issues that will need to be addressed in future generations of closed-loop systems.
5.0. Conclusion
The development of closed-loop systems has progressed rapidly through improvements in CGM technology, improved wireless connectivity and FDA approval of inter-operable devices. Multiple robust controlled trials have shown that closed-loop systems are safe and effective, significantly improving glycaemic control and therefore having the potential to reduce long-term complications. By reducing diabetes management burden and improving quality of life, these systems have transformative potential, but further research is needed to improve usability, wearability and inter-operability. Large-scale trials with different hybrid closed-loop algorithms are under way to assess longer-term efficacy and usage in real-world settings. Widespread adoption will depend on adequate training infrastructures for health care professionals, sufficient economic data demonstrating cost-effectiveness, and robust reimbursement structures to ensure accessibility. While fully closed-loop systems are still out of reach, people with type 1 diabetes will soon have a range of hybrid closed-loop systems to choose from that offer improved glycaemic control and quality of life compared to standard treatment options.
6.0. Expert Opinion
Closed-loop technology has the potential to be transformative for diabetes care. The impending commercialisation of several new closed-loop systems will enable people with type 1 diabetes to choose systems tailored to personal needs and preferences. Study data not only shows that closed-loop systems are safe, with no difference in severe hypoglycaemia or DKA events. Where randomised controlled data is available, closed-loop use consistently increases time in target glucose range, reduces HbA1c and reduces glucose variability. Unlike previous new treatment options, closed-loop systems achieve this tighter glucose control while simultaneously reducing management burden and improving quality of life. These data are consistent across different closed-loop systems and algorithms. Closed-loop relies on real-time sensor glucose data, so CGM availability and funding are vital to enable wider uptake. Equal opportunity access to diabetes technology presents a significant challenge and robust evidence using health-economic models is needed to help secure wider funding availability. An increasing amount of closed-loop data showing improved outcomes should prompt insurance bodies to adapt policies to include accessible funding for CGM and closed-loop systems. There is potential for significant reduction in the risk of long-term diabetes complications, but longer term usage and efficacy data is required to inform health-economic analyses. Several longer-term closed-loop studies are ongoing aiming to demonstrate cost-effectiveness of these systems.
Another potential barrier to closed-loop uptake is that many healthcare providers are unfamiliar with closed-loop systems. In a foreseeable future, new pumps coming onto the market will have automated insulin delivery capability. Access to this technology is dependent on a clinic’s ability to provide structured education and support. Training healthcare providers in closed-loop system application and data interpretation needs to be a priority to enable timely access to this novel technology for people with type 1 diabetes.
While current hybrid closed-loop systems offer far more refined and individualised insulin therapy than any previous treatment options, they do not fully mimic a physiological response and user input remains integral. Delayed insulin action time and the lack of stable hormonal adjuncts are limiting the development of a true artificial pancreas. Further research within the pharmaceutical industry is needed to improve the pharmacodynamics of subcutaneous insulins and to develop hormonal adjuncts that can be mixed with insulin and remain stable for several days. Modification of currently available algorithms incorporating insulins and adjuncts with improved pharmacodynamics and pharmacokinetics will be the next step in automated systems coping with unannounced carbohydrate intake, activity, illness, stress and menstrual cycles. Improved insulin formulations would allow pumps to become smaller and improved integration with consumer electrical devices would enable people to receive updates on any smart device, improving usability.
Making hybrid closed-loop systems available widely will be a remarkable step in the advancement of diabetes care, but if these remaining challenges are addressed, the truly artificial pancreas could be a reality in the not-to-distant future.
Article Highlights.
Algorithm-directed automated insulin delivery is possible via hybrid closed-loop systems
People with type 1 diabetes will soon have a choice of multiple hybrid closed-loop systems, tailored to individual needs and preferences
Study data show that hybrid closed-loop systems are safe to use
Hybrid closed-loop systems increase the time spent in the target glucose range while reducing hypoglycaemia and lowering HbA1c compared to gold-standard insulin therapy
Funding restrictions and lack of health-economic data, as well as insufficient healthcare provider education and knowledge, are the main barriers to making hybrid closed-loop systems widely available
Acknowledgments
Funding
This paper was supported by the National Institute of Health Research Cambridge Biomedical Research Centre, Efficacy and Mechanism Evaluation National Institute for Health Research, The Leona M. & Harry B. Helmsley Charitable Trust, JDRF, National Institute of Diabetes and Digestive and Kidney Diseases and Diabetes UK.
Footnotes
Declaration of Interest
R Hovorka reports having received speaker honoraria from Eli Lilly and Novo Nordisk, serving on advisory panel for Eli Lilly and Novo Nordisk; receiving license fees from BBraun and Medtronic; having served as a consultant to BBraun, patents and patent applications related to closed-loop insulin delivery, shareholder and director at CamDiab Ltd. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Reviewer Disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Nathan DM, Genuth S, Lachin J, Cleary P, Crofford O, Davis M, et al. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. Epub 1993/09/30. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Dovc K, Boughton C, Tauschmann M, Thabit H, Bally L, Allen JM, et al. Young children have higher variability of insulin requirements: observations during hybrid closed-loop insulin delivery. Diabetes Care. 2019;42(7):1344–7. Epub 2019/06/22. doi: 10.2337/dc18-2625.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruan Y, Thabit H, Leelarathna L, Hartnell S, Willinska ME, Dellweg S, et al. Variability of Insulin Requirements Over 12 Weeks of Closed-Loop Insulin Delivery in Adults With Type 1 Diabetes. Diabetes Care. 2016;39(5):830–2. Epub 2016/03/12. doi: 10.2337/dc15-2623. [DOI] [PubMed] [Google Scholar]
- 4.Miller KM, Foster NC, Beck RW, Bergenstal RM, DuBose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38(6):971–8. Epub 2015/05/23. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 5.Boughton CK, Hovorka R. Is an artificial pancreas (closed-loop system) for Type 1 diabetes effective? Diabet Med. 2019;36(3):279–86. Epub 2018/09/06. doi: 10.1111/dme.13816. [DOI] [PubMed] [Google Scholar]
- 6.Tauschmann M, Hovorka R. Technology in the management of type 1 diabetes mellitus - current status and future prospects. Nat Rev Endocrinol. 2018;14(8):464–75. Epub 2018/06/28. doi: 10.1038/s41574-018-0044-y. [DOI] [PubMed] [Google Scholar]
- 7.Forlenza GP, Li Z, Buckingham BA, Pinsker JE, Cengiz E, Wadwa RP, et al. Predictive Low-Glucose Suspend Reduces Hypoglycemia in Adults, Adolescents, and Children With Type 1 Diabetes in an At-Home Randomized Crossover Study: Results of the PROLOG Trial. Diabetes Care. 2018;41(10):2155–61. doi: 10.2337/dc18-0771. [DOI] [PubMed] [Google Scholar]
- 8.Bergenstal RM, Klonoff DC, Garg SK, Bode BW, Meredith M, Slover RH, et al. Threshold-based insulin-pump interruption for reduction of hypoglycemia. N Engl J Med. 2013;369(3):224–32. Epub 2013/06/25. doi: 10.1056/NEJMoa1303576. [DOI] [PubMed] [Google Scholar]
- 9.Forlenza GP, Raghinaru D, Cameron F, Wayne Bequette B, Peter Chase H, Paul Wadwa R, et al. Predictive hyperglycemia and hypoglycemia minimization: In-home double-blind randomized controlled evaluation in children and young adolescents. Pediatr Diabetes. 2018;19(3):420–8. doi: 10.1111/pedi.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaic T, Driscoll M, Raghinaru D, Buckingham BA, Wilson DM, Clinton P, et al. Predictive Hyperglycemia and Hypoglycemia Minimization: In-Home Evaluation of Safety, Feasibility, and Efficacy in Overnight Glucose Control in Type 1 Diabetes. Diabetes Care. 2017;40(3):359–66. Epub 2017/01/20. doi: 10.2337/dc16-1794.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hovorka R, Kumareswaran K, Harris J, Allen JM, Elleri D, Xing D, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ. 2011;342:d1855 Epub 2011/04/16. doi: 10.1136/bmj.d1855.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hovorka R, Elleri D, Thabit H, Allen JM, Leelarathna L, El-Khairi R, et al. Overnight closed-loop insulin delivery in young people with type 1 diabetes: a free-living, randomized clinical trial. Diabetes Care. 2014;37(5):1204–11. Epub 2014/04/24. doi: 10.2337/dc13-2644.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke WL, Anderson S, Breton M, Patek S, Kashmer L, Kovatchev B. Closed-loop artificial pancreas using subcutaneous glucose sensing and insulin delivery and a model predictive control algorithm: the Virginia experience. J Diabetes Sci Technol. 2009;3(5):1031–8. Epub 2010/02/11. doi: 10.1177/193229680900300506.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nimri R, Muller I, Atlas E, Miller S, Fogel A, Bratina N, et al. MD-Logic overnight control for 6 weeks of home use in patients with type 1 diabetes: randomized crossover trial. Diabetes Care. 2014;37(11):3025–32. [DOI] [PubMed] [Google Scholar]
- 15.Phillip M, Battelino T, Atlas E, Kordonouri O, Bratina N, Miller S, et al. Nocturnal glucose control with an artificial pancreas at a diabetes camp. N Engl J Med. 2013;368(9):824–33. Epub 2013/03/01. doi: 10.1056/NEJMoa1206881. [DOI] [PubMed] [Google Scholar]
- 16.Kropff J, Del Favero S, Place J, Toffanin C, Visentin R, Monaro M, et al. 2 month evening and night closed-loop glucose control in patients with type 1 diabetes under free-living conditions: a randomised crossover trial. The Lancet Diabetes & Endocrinology. 2015;3(12):939–47. doi: 10.1016/s2213-8587(15)00335-6. [DOI] [PubMed] [Google Scholar]
- 17.Ekhlaspour L, Nally LM, El-Khatib FH, Ly TT, Clinton P, Frank E, et al. Feasibility Studies of an Insulin-Only Bionic Pancreas in a Home-Use Setting. J Diabetes Sci Technol. 2019;13(6):1001–7. Epub 2019/09/01. doi: 10.1177/1932296819872225.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Breton MD, Chernavvsky DR, Forlenza GP, DeBoer MD, Robic J, Wadwa RP, et al. Closed-loop control during intense prolonged outdoor exercise in adolescents with type 1 diabetes: the artificial pancreas ski study. Diabetes Care. 2017;40(12):1644–50. Epub 2017/09/01. doi: 10.2337/dc17-0883.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lal RA, Ekhlaspour L, Hood K, Buckingham B. Realizing a Closed-Loop (Artificial Pancreas) System for the Treatment of Type 1 Diabetes. Endocr Rev. 2019;40(6):1521–46. Epub 2019/07/06. doi: 10.1210/er.2018-00174.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ekhlaspour L, Forlenza GP, Chernavvsky D, Maahs DM, Wadwa RP, Deboer MD, et al. Closed loop control in adolescents and children during winter sports: Use of the Tandem Control-IQ AP system. Pediatr Diabetes. 2019;20(6):759–68. Epub 2019/05/18. doi: 10.1111/pedi.12867.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bekiari E, Kitsios K, Thabit H, Tauschmann M, Athanasiadou E, Karagiannis T, et al. Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ. 2018;361:k1310 Epub 2018/04/20. doi: 10.1136/bmj.k1310.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weisman A, Bai J-W, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. The Lancet Diabetes & Endocrinology. 2017;5(7):501–12. doi: 10.1016/s2213-8587(17)30167-5. [DOI] [PubMed] [Google Scholar]
- 23.Pickup JC. Is insulin pump therapy effective in Type 1 diabetes? Diabet Med. 2019;36(3):269–78. Epub 2018/08/12. doi: 10.1111/dme.13793. [DOI] [PubMed] [Google Scholar]
- 24.Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010(1):CD005103 Epub 2010/01/22. doi: 10.1002/14651858.CD005103.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johnson SR, Cooper MN, Jones TW, Davis EA. Long-term outcome of insulin pump therapy in children with type 1 diabetes assessed in a large population-based case-control study. Diabetologia. 2013;56(11):2392–400. Epub 2013/08/22. doi: 10.1007/s00125-013-3007-9. [DOI] [PubMed] [Google Scholar]
- 26.Dovc K, Battelino T. Evolution of diabetes technology. Endocrinology and Metabolism Clinics of North America. 2019. doi: 10.1016/j.ecl.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Danne T, Nimri R, Battelino T, Bergenstal RM, Close KL, DeVries JH, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–40. Epub 2017/11/23. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shah VN, Laffel LM, Wadwa RP, Garg SK. Performance of a factory-calibrated real-time continuous glucose monitoring system utilizing an automated sensor applicator. Diabetes Technol Ther. 2018;20(6):428–33. Epub 2018/06/21. doi: 10.1089/dia.2018.0143.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thabit H, Hovorka R. Coming of age: the artificial pancreas for type 1 diabetes. Diabetologia. 2016;59(9):1795–805. Epub 2016/07/02. doi: 10.1007/s00125-016-4022-4.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinsker JE, Lee JB, Dassau E, Seborg DE, Bradley PK, Gondhalekar R, et al. Randomized Crossover Comparison of Personalized MPC and PID Control Algorithms for the Artificial Pancreas. Diabetes Care. 2016;39(7):1135–42. Epub 2016/06/12. doi: 10.2337/dc15-2344.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atlas E, Nimri R, Miller S, Grunberg EA, Phillip M. MD-Logic Artificial Pancreas System: A pilot study in adults with type 1 diabetes. Diabetes Care. 2010;33(5):1072–6. doi: 10.2337/dc09-1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beck RW, Connor CG, Mullen DM, Wesley DM, Bergenstal RM. The Fallacy of Average: How Using HbA1c Alone to Assess Glycemic Control Can Be Misleading. Diabetes Care. 2017;40(8):994–9. doi: 10.2337/dc17-0636.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beck RW, Bergenstal RM, Riddlesworth TD, Kollman C, Li ZM, Brown AS, et al. Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care. 2019;42(3):400–5. doi: 10.2337/dc18-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Battelino T, Danne T, Bergenstal RM, Amiel SA, Beck R, Biester T, et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations From the International Consensus on Time in Range. Diabetes Care. 2019;42(8):1593–603. Epub 2019/06/10. doi: 10.2337/dci19-0028.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medtronic. CALIBRATING YOUR SENSOR - Device: MiniMed™ 670G insulin pump (MMT-1780K) 2020 [13th of February, 2020]. Available from: http://www.medtronicdiabetes.com/customer-support/minimed-670g-system-support/cgm-calibrating.
- 36.Garg SK, Weinzimer SA, Tamborlane WV, Buckingham BA, Bode BW, Bailey TS, et al. Glucose outcomes with the in-home use of a hybrid closed-loop insulin delivery system in adolescents and adults with type 1 diabetes. Diabetes Technology & Therapeutics. 2017;19(3):155–63. doi: 10.1089/dia.2016.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergenstal RM, Garg S, Weinzimer SA, Buckingham BA, Bode BW, Tamborlane WV, et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316(13):1407–8. Epub 2016/09/16. doi: 10.1001/jama.2016.11708. [DOI] [PubMed] [Google Scholar]
- 38.Akturk HK, Giordano D, Champakanath A, Brackett S, Garg S, Snell-Bergeon J. Long-term real-life glycemic outcomes with hybrid closed-loop system when compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes Obes Metab. 2019. Epub 2019/12/04. doi: 10.1111/dom.13933. [DOI] [PubMed] [Google Scholar]
- 39.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, Shulman DI, Bailey TS, Bode BW, et al. Safety evaluation of the MiniMed 670G System in children 7–13 years of age with type 1 diabetes. Diabetes Technol Ther. 2019;21(1):11–9. doi: 10.1089/dia.2018.0264.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salehi P, Roberts AJ, Kim GJ. Efficacy and safety of real-life usage of MiniMed 670G Automode in children with type 1 diabetes less than 7 years old. Diabetes Technol Ther. 2019;21(8):448–51. Epub 2019/06/06. doi: 10.1089/dia.2019.0123. [DOI] [PubMed] [Google Scholar]
- 41.de Bock M, McAuley SA, Abraham MB, Smith G, Nicholas J, Ambler GR, et al. Effect of 6 months hybrid closed-loop insulin delivery in young people with type 1 diabetes: a randomised controlled trial protocol. BMJ Open. 2018;8(8):e020275 Epub 2018/08/15. doi: 10.1136/bmjopen-2017-020275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medtronic Diabetes. Multi-center Trial in Adult and Pediatric Patients With Type 1 Diabetes Using Hybrid Closed Loop System and Control at Home: NIH US National Library of Medicine - ClinicalTrials.gov; 2019. [23rd of January, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT02748018?term=Medtronic&recrs=abdf&cond=Type+1+Diabetes&draw=2&rank=7.
- 43.Lee MH, Vogrin S, Paldus B, Jones HM, Obeyesekere V, Sims C, et al. Glucose control in adults with type 1 diabetes using a Medtronic prototype enhanced-hybrid closed-loop system: A feasibility study. Diabetes Technol Ther. 2019;21(9):499–506. Epub 2019/07/03. doi: 10.1089/dia.2019.0120. [DOI] [PubMed] [Google Scholar]
- 44.FDA authorizes first interoperable, automated insulin dosing controller designed to allow more choices for patients looking to customize their individual diabetes management device system 2019. [5th of January 2020]. Available from: https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-interoperable-automated-insulin-dosing-controller-designed-allow-more-choices.
- 45.Anderson SM, Raghinaru D, Pinsker JE, Boscari F, Renard E, Buckingham BA, et al. Multinational home use of closed-loop control is safe and effective. Diabetes Care. 2016;39(7):1143–50. Epub 2016/05/22. doi: 10.2337/dc15-2468.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kovatchev B, Cheng P, Anderson SM, Pinsker JE, Boscari F, Buckingham BA, et al. Feasibility of long-term closed-loop control: A multicenter 6-month trial of 24/7 automated insulin delivery. Diabetes Technol Ther. 2017;19(1):18–24. Epub 2016/12/17. doi: 10.1089/dia.2016.0333. [DOI] [PubMed] [Google Scholar]
- 47.Kovatchev BP, Kollar L, Anderson SM, Barnett C, Breton MD, Carr K, et al. Evening and overnight closed-loop control versus 24/7 continuous closed-loop control for type 1 diabetes: a randomised crossover trial. Lancet Digit Health. 2020;2(2):E64–E73. doi: 10.1016/S2589-7500(19)30218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kovatchev B, Anderson SM, Raghinaru D, Kudva YC, Laffel LM, Levy C, et al. Randomized Controlled Trial of Mobile Closed-Loop Control. Diabetes Care. 2020. Epub 2020/01/16. doi: 10.2337/dc19-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown SA, Kovatchev BP, Raghinaru D, Lum JW, Buckingham BA, Kudva YC, et al. Six-Month Randomized, Multicenter Trial of Closed-Loop Control in Type 1 Diabetes. N Engl J Med. 2019;381(18):1707–17. Epub 2019/10/17. doi: 10.1056/NEJMoa1907863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown S, Raghinaru D, Emory E, Kovatchev B. First Look at Control-IQ: A New-Generation Automated Insulin Delivery System. Diabetes Care. 2018;41(12):2634–6. Epub 2018/10/12. doi: 10.2337/dc18-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.JDRF. World’s first artificial pancreas app licensed for people with type 1 diabetes in the UK 2020 [6th of June, 2020]. Available from: https://jdrf.org.uk/news/worlds-first-artificial-pancreas-app-licensed-for-people-with-type-1-diabetes-in-the-uk/.
- 52.Bally L, Thabit H, Kojzar H, Mader JK, Qerimi-Hyseni J, Hartnell S, et al. Day-and-night glycaemic control with closed-loop insulin delivery versus conventional insulin pump therapy in free-living adults with well controlled type 1 diabetes: an open-label, randomised, crossover study. The Lancet Diabetes & Endocrinology. 2017;5(4):261–70. doi: 10.1016/s2213-8587(17)30001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tauschmann M, Allen JM, Wilinska ME, Thabit H, Acerini CL, Dunger DB, et al. Home use of day-and-night hybrid closed-loop insulin delivery in suboptimally controlled adolescents with type 1 diabetes: A 3-week, free-living, randomized crossover trial. Diabetes Care. 2016;39(11):2019–25. Epub 2016/09/11. doi: 10.2337/dc16-1094.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tauschmann M, Allen JM, Nagl K, Fritsch M, Yong J, Metcalfe E, et al. Home use of day-and-night hybrid closed-loop insulin delivery in very young children: A multi-center, 3-week, randomized trial. Diabetes Care. 2019;42(4):594–600. doi: 10.2337/dc18-1881. [DOI] [PubMed] [Google Scholar]
- 55.Thabit H, Tauschmann M, Allen JM, Leelarathna L, Hartnell S, Wilinska ME, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373(22):2129–40. Epub 2015/09/18. doi: 10.1056/NEJMoa1509351.**; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tauschmann M, Thabit H, Bally L, Allen JM, Hartnell S, Wilinska ME, et al. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet. 2018;392(10155):1321–9. Epub 2018/10/08. doi: 10.1016/S0140-6736(18)31947-0.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hovorka R Closed Loop From Onset in Type 1 Diabetes (CLOuD) - NCT02871089: ClinicalTrials.gov; 2019. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT02871089?term=cloud&cond=Diabetes&draw=2&rank=1.
- 58.JCfHR. Day and Night Closed-loop in Young People With Type 1 Diabetes (DAN05) - NCT02925299: ClinicalTrials.gov; 2020. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT02925299?term=dan05&cond=Diabetes&draw=2&rank=1.
- 59.Hovorka R The Artificial Pancreas in Very Young Children With T1D (KidsAP02) - NCT03784027: ClinicalTrials.gov; 2019. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT03784027?term=kidsap02&cond=Diabetes&draw=2&rank=1. [Google Scholar]
- 60.Hovorka R 24/7 Closed-loop in Older Subjects With Type 1 Diabetes (DAN06) - NCT04025762: ClinicalTrials.gov; 2019. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT04025762?term=camaps+fx&cond=Diabetes&draw=2&rank=3.
- 61.Hovorka R Closing the Loop in Adults With Type 1 Diabetes Under Free Living Conditions (AP@Home04_P3) - NCT04055480: ClinicalTrials.gov; 2020 [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT04055480?term=Hovorka+closed+loop&cond=Diabetes&draw=2&rank=5.
- 62.Benhamou PY, Huneker E, Franc S, Doron M, Charpentier G, Diabeloop C. Customization of home closed-loop insulin delivery in adult patients with type 1 diabetes, assisted with structured remote monitoring: the pilot WP7 Diabeloop study. Acta Diabetol. 2018;55(6):549–56. Epub 2018/03/10. doi: 10.1007/s00592-018-1123-1. [DOI] [PubMed] [Google Scholar]
- 63.Diabeloop SA. Who can use the DBLG1 system? 2019. [15th of January, 2020]. Available from: https://support.diabeloop.com/hc/en-us/articles/360024497034-Who-can-use-the-DBLG1-System-.
- 64.Benhamou P, Franc S. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. The Lancet Digital Health 2019. [DOI] [PubMed] [Google Scholar]
- 65.Centre d’Etudes et de Recherche pour l’Intensification du Traitement du Diabète. Safety Assessment of DBLUS System in Adolescent and Adult Patients With Type 1 Diabetes and Assessment of Its Clinical Efficacy (DIABELOOP SP8) (DIABELOOP SP8) - NCT04190277: ClinicalTrials.gov; 2019. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT04190277?term=diabeloop&cond=Diabetes&draw=2&rank=1.
- 66.Centre d’Etudes et de Recherche pour l’Intensification du Traitement du Diabète. Diabeloop for Kids (DBL4K) - NCT03671915: ClinicalTrials.gov; 2019. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT03671915?term=diabeloop&cond=Diabetes&draw=2&rank=5
- 67.Buckingham BA, Forlenza GP, Pinsker JE, Christiansen MP, Wadwa RP, Schneider J, et al. Safety and Feasibility of the OmniPod Hybrid Closed-Loop System in Adult, Adolescent, and Pediatric Patients with Type 1 Diabetes Using a Personalized Model Predictive Control Algorithm. Diabetes Technol Ther. 2018;20(4):257–62. doi: 10.1089/dia.2017.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sherr JL, Buckingham BA, Forlenza GP, Galderisi A, Ekhlaspour L, Wadwa RP, et al. Safety and performance of the omnipod hybrid closed-loop system in adults, adolescents, and children with type 1 diabetes over 5 days under free-living conditions. Diabetes Technol Ther. 2019;22(3):174–84. Epub 2019/10/10. doi: 10.1089/dia.2019.0286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Insulet C. Prepivotal Omnipod Horizon™ Automated Glucose Control System - NCT04176731: ClinicalTrials.gov; 2020. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT04176731?term=Insulet&cond=Diabetes&draw=2&rank=7.
- 70.Insulet C. Pivotal Omnipod Horizon™ Automated Glucose Control System - NCT04196140: ClinicalTrials.gov; 2020. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT04196140?term=Insulet&cond=Diabetes&draw=2&rank=6.
- 71.Jaeb Centre for Health Research. The Insulin-Only Bionic Pancreas Pivotal Trial - NCT04200313: ClinicalTrials.gov; 2020. [13th of February, 2020]. Available from: https://clinicaltrials.gov/ct2/show/NCT04200313?term=ilet&cond=Diabetes&draw=2&rank=3.
- 72.Loop. LoopDocs [06/01/2020]. Available from: https://loopkit.github.io/loopdocs/operation/algorithm/overview/.
- 73.TidePool.org TidePool delivering loop [04/01/2020]. Available from: https://www.tidepool.org/blog/tidepool-delivering-loop.
- 74.Snider C Tidepool Loop, one year in: A development update 2020 [13th of February, 2020]. Available from: https://www.tidepool.org/blog/tidepool-loop-development-update?mkt_tok=eyJpIjoiWlRCaE16RmxPR0V6WXpWaCIsInQiOiJSN2lyalpyMXRWRDA3NGVTWVYwUnMzM0UyUzJLYytheGVjSHVxOUJnM1wvbEVTOERVcWNodmx6UmVFRU1ERXlOUEdnS21uNTl1ZXdITGl3U2swd0ZMR2Q4UFVGU3FcL2JkNGVIT1N0Wkpudm4wRVlqRXhiNXVYTlU5ZUhXWm5GTlZvIn0%3D
- 75.Jennings P, Hussain S. Do-it-yourself artificial pancreas systems: A review of the emerging evidence and insights for healthcare professionals. J Diabetes Sci Technol. 2019:1932296819894296 Epub 2019/12/19. doi: 10.1177/1932296819894296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weinzimer SA, Steil GM, Swan KL, Dziura J, Kurtz N, Tamborlane WV. Fully Automated Closed-Loop Insulin Delivery Versus Semiautomated Hybrid Control in Pediatric Patients With Type 1 Diabetes Using an Artificial Pancreas. Diabetes Care. 2008;31(5):934–9. doi: 10.2337/dc07-1967. [DOI] [PubMed] [Google Scholar]
- 77.Forlenza GP, Cameron FM, Ly TT, Lam D, Howsmon DP, Baysal N, et al. Fully Closed-Loop Multiple Model Probabilistic Predictive Controller Artificial Pancreas Performance in Adolescents and Adults in a Supervised Hotel Setting. Diabetes Technol Ther. 2018;20(5):335–43. doi: 10.1089/dia.2017.0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dovc K, Piona C, Yesiltepe Mutlu G, Bratina N, Jenko Bizjan B, Lepej D, et al. Faster compared with standard insulin aspart during day-and-night fully closed-loop insulin therapy in type 1 diabetes: a double-blind randomized crossover trial. Diabetes Care. 2019. Epub 2019/10/03. doi: 10.2337/dc19-0895. [DOI] [PubMed] [Google Scholar]
- 79.Cameron FM, Ly TT, Buckingham BA, Maahs DM, Forlenza GP, Levy CJ, et al. Closed-loop control without meal announcement in type 1 diabetes. Diabetes Technol Ther. 2017;19(9):527–32. Epub 2017/08/03. doi: 10.1089/dia.2017.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dassau E, Zisser H, Harvey RA, Percival MW, Grosman B, Bevier W, et al. Clinical evaluation of a personalized artificial pancreas. Diabetes Care. 2013;36(4):801–9. Epub 2012/11/30. doi: 10.2337/dc12-0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Beato-Vibora PI, Arroyo-Diez FJ. New uses and formulations of glucagon for hypoglycaemia. Drugs Context. 2019;8:212599 Epub 2019/08/14. doi: 10.7573/dic.212599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Khatib FH, Balliro C, Hillard MA, Magyar KL, Ekhlaspour L, Sinha M, et al. Home use of a bihormonal bionic pancreas versus insulin pump therapy in adults with type 1 diabetes: a multicentre randomised crossover trial. The Lancet. 2017;389(10067):369–80. doi: 10.1016/s0140-6736(16)32567-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Haidar A, Rabasa-Lhoret R, Legault L, Lovblom LE, Rakheja R, Messier V, et al. Single- and Dual-Hormone Artificial Pancreas for Overnight Glucose Control in Type 1 Diabetes. J Clin Endocrinol Metab. 2016;101(1):214–23. Epub 2015/11/03. doi: 10.1210/jc.2015-3003. [DOI] [PubMed] [Google Scholar]
- 84.Blauw H, van Bon AC, Koops R, DeVries JH, consortium obotP. Performance and safety of an integrated bihormonal artificial pancreas for fully automated glucose control at home. Diabetes Obes Metab. 2016;18(7):671–7. doi: 10.1111/dom.12663.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haidar A, Tsoukas MA, Bernier-Twardy S, Yale JF, Rutkowski J, Bossy A, et al. A Novel Dual-Hormone Insulin-and-Pramlintide Artificial Pancreas for Type 1 Diabetes: A Randomized Controlled Crossover Trial. Diabetes Care. 2020. Epub 2020/01/25. doi: 10.2337/dc19-1922. [DOI] [PubMed] [Google Scholar]
- 86.Gingras V, Taleb N, Roy-Fleming A, Legault L, Rabasa-Lhoret R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes Metab. 2018;20(2):245–56. Epub 2017/07/05. doi: 10.1111/dom.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lal RA, Basina M, Maahs DM, Hood K, Buckingham B, Wilson DM. One Year Clinical Experience of the First Commercial Hybrid Closed-Loop System. Diabetes Care. 2019;42(12):2190–6. Epub 2019/09/25. doi: 10.2337/dc19-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.FDA authorizes first fully interoperable continuous glucose monitoring system, streamlines review pathway for similar devices 2018 [4th of January, 2020]. Available from: https://www.fda.gov/news-events/press-announcements/fda-authorizes-first-fully-interoperable-continuous-glucose-monitoring-system-streamlines-review.
- 89.Kimbell B, Rankin D, Ashcroft NL, Varghese L, Allen JM, Boughton CK, et al. What Training, Support, and Resourcing Do Health Professionals Need to Support People Using a Closed-Loop System? A Qualitative Interview Study with Health Professionals Involved in the Closed Loop from Onset in Type 1 Diabetes (CLOuD) Trial. Diabetes Technol Ther. 2020;22(6). Epub 2020/02/13. doi: 10.1089/dia.2019.0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Musolino G, Dovc K, Boughton CK, Tauschmann M, Allen JM, Nagl K, et al. Reduced burden of diabetes and improved quality of life: Experiences from unrestricted day-and-night hybrid closed-loop use in very young children with type 1 diabetes. Pediatr Diabetes. 2019;20(6):794–9. Epub 2019/05/30. doi: 10.1111/pedi.12872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weissberg-Benchell J, Hessler D, Fisher L, Russell SJ, Polonsky WH. Impact of an automated bihormonal delivery system on psychosocial outcomes in adults with type 1 diabetes. Diabetes Technol Ther. 2017;19(12):723–9. Epub 2017/11/07. doi: 10.1089/dia.2017.0174. [DOI] [PubMed] [Google Scholar]
- 92.Farrington C, Stewart Z, Hovorka R, Murphy H. Women’s Experiences of Day-and-Night Closed-Loop Insulin Delivery During Type 1 Diabetes Pregnancy. J Diabetes Sci Technol. 2018;12(6):1125–31. Epub 2018/10/06. doi: 10.1177/1932296818800065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawton J, Blackburn M, Rankin D, Allen JM, Campbell FM, Leelarathna L, et al. Participants’ experiences of, and views about, daytime use of a day-and-night hybrid closed-loop system in real life settings: longitudinal qualitative study. Diabetes Technology & Therapeutics. 2019;21(3):119–27. doi: 10.1089/dia.2018.0306. [DOI] [PMC free article] [PubMed] [Google Scholar]