Abstract
Background
Liver transplant (LT) patients have an increased risk of postoperative respiratory failure requiring tracheostomy. This study sought to characterize objective clinical predictors of tracheostomy.
Material/Methods
The records for 2017 LT patients at a single institution were reviewed. Patients requiring tracheostomy were first compared with all other patients. A case-control subgroup analysis was conducted in which 98 tracheostomy patients were matched with 98 non-tracheostomy LT patients. For the case-control study, muscle mass was assessed using preoperative computed tomography scans.
Results
Among 2017 LT patients, 98 required tracheostomy (5%), with a 19% complication rate. Tracheostomy patients were older and had a higher model for end-stage liver disease score, a lower body mass index (BMI), and a greater smoking history. Tracheostomy patients had a longer hospital stay (45 vs. 10 days, P<0.001) and worse 1-year survival (65% vs. 91%, P<0.001). Ten-year Cox regression patient survival for tracheostomy patients was significantly worse (32% vs. 68%, P<0.001). In the case-control analysis, respiratory failure patients were older (P<0.01) and had a lower BMI (P=0.05). They also had a muscle mass deficit of −39% compared with matched LT controls (P<0.001). No significant differences were seen with pre-LT total protein or albumin or with forced expiratory volume in 1 s divided by forced vital capacity (FEV1/FVC) values.
Conclusions
Predictors for respiratory failure requiring post-LT tracheostomy include higher model for end-stage liver disease score, older age, lower BMI, greater smoking history, and worse sarcopenia. Patients requiring tracheostomy have dramatically longer hospital stays and worse survival.
MeSH Keywords: Frail Elderly; Liver Transplantation; Malnutrition; Postoperative Complications; Respiration, Artificial; Tracheostomy
Background
Prolonged mechanical ventilation after liver transplant (LT) has been shown to be associated with increased mortality [1]. Previous studies have identified the incidence of posttransplant respiratory complications to be approximately 59–87% [2,3]. Many clinical factors have been found to have an impact on post-LT respiratory failure requiring tracheostomy including older age, subjective nutritional deficiency, intraoperative bleeding, pre-LT mechanical ventilation, diagnosis of acute liver failure, and re-transplantation [2,4]. Further analysis is required to identify additional clinical factors useful in identifying these high-risk patients prior to transplant.
Screening of transplant candidates prior to LT is critical to optimizing the utility of scarce organs. Of note, a scoring system to calculate the risk of respiratory failure after liver transplantation was recently proposed. This scoring system defines respiratory failure as patients requiring >7.5 days on a respirator, and it takes into account many of the previously identified clinical factors to calculate risk [5]. However, preoperative pulmonary function testing (PFT) and objective measures of wasting and frailty were not included in the scoring system.
The purpose of this study was to further identify objective clinical predictors for posttransplant respiratory failure requiring tracheostomy. As LT patients are often deconditioned, chronically ill, and suffer from coagulopathy, this study also sought to better understand the impact of objective measures of pre-LT nutritional deficiency in patients with post-LT respiratory failure. Additionally, a thorough review of all tracheostomy procedures in these high-risk patients is reported to assess the incidence and types of tracheostomy-related complications.
Material and Methods
The electronic medical records of all LT recipients at a single center over a 16-year period were reviewed. Patients with post-LT respiratory failure were selected based on their requirement for posttransplant tracheostomy. Further chart review was then performed for these patients to determine demographics, pre-LT PFT results, smoking history, nutritional status, and clinical outcomes (hospital stay and patient survival). A case-control subgroup analysis was then conducted in which 98 tracheostomy patients were matched with 98 non-tracheostomy LT patients based on year of transplant, model for end-stage liver disease score (MELD), and sex. Data collection was complete except for 2 patients in the respiratory failure group who did not have computed tomography (CT) or magnetic resonance imaging (MRI) scans available for review. Additionally, pretransplant PFT results were unavailable for 8 patients in the control group and 15 patients in the respiratory failure group.
Preoperative nutritional status was assessed using preoperative CT scans (or MRI if CT was not available) and biochemical measures of serum albumin and total protein. CT measurements were taken at the level of the L2/L3 intervertebral disc space, which has been determined to be an accurate approximation of total body composition [6]. Total psoas muscle area was obtained by outlining both the right and left psoas muscles and summing these measurements (Figure 1). In order to account for variations in patient size, all measurements were scaled for height, as has been done previously [7]. The sarcopenic index was obtained by dividing the total psoas area (cm2) by the height in meters squared. Measurements were taken using Synapse picture archiving and communication software (PACS). The scaled measurements of the transplant patients were compared with the scaled measurements of MELD, transplant year, and sex-matched controls. The difference was calculated by subtracting the control measure from the transplant patient measure and a percent difference was calculated.
Figure 1.
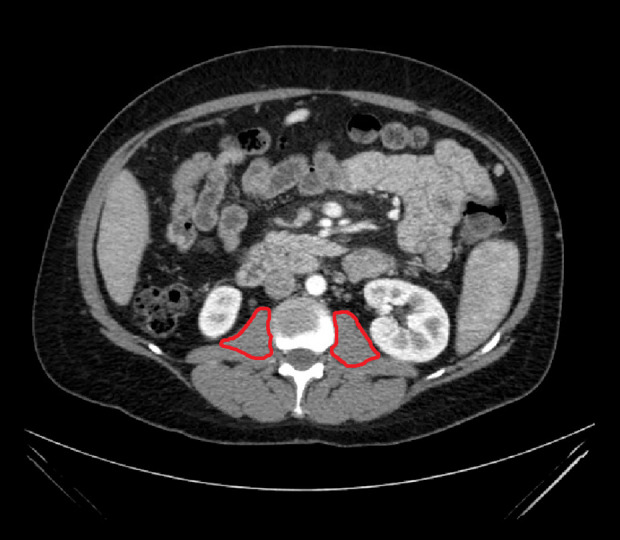
Cross section of patient CT scan at L2/L3 with bilateral psoas muscle areas highlighted.
The original operative reports for all tracheostomy procedures were reviewed in detail. Extracted data included ventilator days until tracheostomy, operating surgeon, technique, complications, days to decanulation, and risk of death with tracheostomy. Any complications were described and required interventions were noted.
For this patient cohort, the posttransplant immunosuppression protocol has been described previously and includes induction therapy with rabbit antithymocyte globulin and steroids, followed by Prograf (tacrolimus) monotherapy [8]. Donor livers were recovered using standard procurement techniques including aortic and portal vein flushing and cold storage as has been described previously [9]. Ninety-five percent of all transplants during the study period were performed using the piggyback hepatectomy technique, which has been described previously and was technically equivalent for all participating surgeons [10]. Overall median anesthesia time was 4 h 40 min. A temporary portal-cava shunt was not created in any of the cases, and there was no use of veno-venous bypass. An antifibrinolytic agent (aprotinin, or aminocaproic acid or tranexamic acid) was administered routinely during the transplant procedure.
Standard statistical testing was utilized for continuous and categorical variables, as indicated. The Cox proportional hazards model was constructed using a direct entry method. Co-variates were included in the final model for P-value <0.10. Statistical testing was performed using the Statistical Package for the Social Sciences software (IBM SPSS Statistics Version 25 [2018], IBM Corporation, Armonk, NY, USA). Retrospective analysis of data for LT patients at our center was reviewed and approved by the institutional review board of the Indiana University School of Medicine.
Results
Among 2017 liver transplants, 98 patients required tracheostomy (5%). Tracheostomy patients had a higher MELD, older age, and a lower body mass index (BMI). Tracheostomy patients had a greater exposure to smoking. They did not differ in race or sex. Compared with the entire cohort, patients requiring tracheostomy after LT had significantly longer hospital stay (45 vs. 10 days, P<0.001) and worse 1-year survival (65% vs. 91%, P<0.001) (Table 1).
Table 1.
Demographic data for liver transplant patients who did or did not require tracheostomy for respiratory failure in the first 6-months post transplant.
| Overall | No tracheostomy | Tracheostomy | p-Value |
|---|---|---|---|
| 1919 (95.2%) | 98 (4.8%) | ||
| Recipient characteristics | |||
| MELD (median (SD)) | 18 (7) | 22 (9) | <0.001 |
| Gender male | 67% | 66% | 0.88 |
| Race | |||
| White | 89% | 90% | 0.38 |
| Black | 6% | 3% | |
| Other | 5% | 7% | |
| Age in years (median (SD)) | 55 (10) | 58 (11) | 0.02 |
| Body mass index (median (SD)) | 28.2 (5) | 26.5 (5) | <0.01 |
| Retransplant | 4% | 2% | 0.71 |
| Tobacco use | |||
| Never smoker | 53% | 48% | 0.41 |
| Former smoker | 47% | 52% | |
| Current smoker at transplant | 19% | 17% | 0.65 |
| Pack-years smoking* | |||
| Zero | 53% | 48% | 0.01 |
| 1 to 20 | 13% | 26% | |
| 20 to 40 | 25% | 21% | |
| >40 | 9% | 5% | |
| Outcomes | |||
| Hospital stay (days, median (SD)) | 10 (31) | 45 (64) | <0.001 |
| 1-year survival | 91% | 65% | <0.001 |
Pack-years is the number of years of smoking multiplied by the average number of packs of cigarettes per day.
In the case-control analysis, tracheostomy patients were older (P<0.01) and had a lower BMI (P=0.05). These patients also had a muscle mass deficit of −39% compared with matched controls (P<0.001). No significant difference was found between groups in a comparison of biochemical markers of nutrition (serum total protein and albumin levels). Forced vital capacity (FVC) was found to be lower in the respiratory failure group (81% vs. 88%, P=0.03), as was the forced expiratory volume in 1 s (FEV1) (76% vs. 83%, P=0.02). No significant difference between the 2 groups was noted for FEV1/FVC. Similar to the findings from Table 1, the case-control analysis showed an extended length of hospital stay (45 vs. 10 days, P<0.001) and decreased 1-year patient survival (65% vs. 94%, P<0.001) for the respiratory failure group (Table 2).
Table 2.
Subgroup analysis of 98 liver transplant patients with respiratory failure who required tracheostomy in the first 6-months post liver transplant.
| Number | |
|---|---|
| Overall | 98 (100%) |
| Clinical outcomes | |
| Days to tracheostomy post transplant (median (SD)) | 19 (35) |
| Surgeon* | |
| Transplant surgeon | 92 (94%) |
| Other | 6 (6%) |
| Days from tracheostomy to decanulation (median (SD)) | 46 (430) |
| Died with tracheostomy | 18 (18%) |
| Pulmonary function tests** | |
| Forced vital capacity (FVC, % of predicted) | 81% (20) |
| Forced expiratory volume in 1 second | |
| (FEV1,% of predicted) | 76% (18) |
| FEV1/FVC (% of predicted) | 75% (8) |
| Complications | |
| Any complication related to tracheostomy | 19 (19%) |
| Operative revision/post-operative hemorrhage | 4 |
| Tracheocutaneous fistula/non-closure | 4 |
| Bleeding requiring operative intervention | 5 |
| Subcutaneous emphysema | 2 |
| Pneumothorax/pneumomediastinum | 3 |
| Intraoperative unstable atrial fibrillation (cardioverted) | 1 |
2 tracheostomies were performed percutaneously;
15 patients did not have PFT reports available.
Subgroup analysis of the 98 tracheostomy patients was performed. The median number of days between transplant and tracheostomy was 19 days, with a median time of 46 days from tracheostomy to decanulation (for survivors). Nearly all the patients failed multiple attempts at extubation prior to undergoing the tracheostomy procedure (Table 3). A transplant surgeon performed the tracheostomy procedure in 94% of cases. All but 2 tracheostomies were placed using an open technique in the operating room. Two tracheostomies were placed percutaneously early in this series, but both required early revision due to the poor quality of the tissue. Therefore, the percutaneous approach was abandoned at this center in post-LT patients. Pulmonary function tests among tracheostomy patients included FVC of 81% of predicted, forced expiratory volume in 1 s (FEV1) of 76% predicted, and FEV1/FVC of 75% predicted. Of the 98 tracheostomy patients, 18 (18%) died with their tracheostomy in place. A list of the most common complications in these 98 patients is provided in Table 2. Four patients required early reoperation for dislodged tracheostomy or hemorrhage. Five patients had late hemorrhage requiring operative exploration to control bleeding. Four patients required operative closure of the tracheostomy site after developing a persistent tracheocutaneous fistula.
Table 3.
Case control analysis of muscle mass in patients with tracheostomy and matched controls without tracheostomy*.
| No tracheostomy (controls) | Tracheostomy (cases) | p-Value | |
|---|---|---|---|
| Number | 98 | 98 | |
| Demographics | |||
| Age (median (SD)) | 53 (12) | 58 (11) | <0.01 |
| Model for end-stage liver disease score (SD) | 22 (9) | 22 (9) | 0.78 |
| Body mass index (median (SD)) | 27.7 (6) | 26.5 (5) | 0.05 |
| Pack-years smoking (mean, median SD) | 13.0 (18) | 12, 3 (20) | 0.82 |
| Skeletal muscle mass** | |||
| Psoas index group median | 4.6 | 3.2 | <0.001 |
| Psoas index matched difference | −39% (120) | ||
| Biochemical markers of nutrition | |||
| Serum albumin level (median SD) | 3.0 (0.7) | 2.9 (0.8) | 0.97 |
| Serum protein level (median SD) | 6.5 (0.9) | 6.4 (1.2) | 0.97 |
| Pulmonary function tests*** | |||
| Forced vital capacity (FVC, % of predicted (SD)) | 88% (19) | 81% (20) | 0.03 |
| Forced expiratory volume in 1 second | |||
| (FEV1,% of predicted (SD)) | 83% (18) | 76% (18) | 0.02 |
| FEV1/FVC (% of predicted (SD)) | 76% (7) | 75% (8) | 0.45 |
| Clinical outcomes | |||
| Length of hospital stay (median (SD)) | 10 (41) | 45 (64) | <0.001 |
| 1-year patient survival | 94% | 65% | <0.001 |
Cases and controls matched for age, gender, MELD score and year of transplant;
2 patients in the tracheostomy group did not have CT or MRI available for review;
15 patients in the tracheostomy group and 8 patients in the control group did not have PFTs available.
In a comparison of the tracheostomy group with the entire cohort, 10-year Cox patient survival was worse for the tracheostomy patients (32% vs. 68%, P<0.001; Figure 2).
Figure 2.
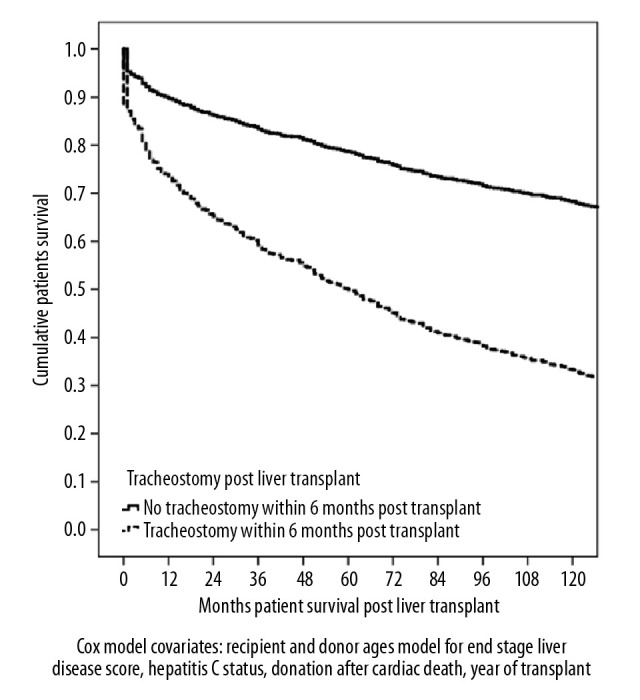
Cox proportional hazards patient survival post transplant for 98 patients with respiratory failure who required tracheostomy in the first 6-months post liver transplant (n=2017 transplants).
Discussion
Understanding clinical predictors of post-LT tracheostomy is essential because of its association with increased costs, lengthened hospital stay, and worse outcomes [1]. In support of previous studies, we report decreased 1- and 10-year survival and markedly lengthened hospital stay for post-LT tracheostomy patients [1,3]. To date, there have been many identified risk factors for respiratory failure after LT including older age, subjective nutritional deficiency, intraoperative bleeding, pre-LT mechanical ventilation, diagnosis of acute liver failure, and retransplantation [2,4]. However, to our knowledge, this study represents the first time sarcopenic index has been identified as a potential objective predictor of post-LT respiratory failure. The significant difference in sarcopenic index demonstrated between cohorts in the case-control portion of our analysis suggests that this measurement may prove useful, without adding cost, to the pre-LT screening process.
Recently, a study was done to validate the use of a scoring system to predict the risk of respiratory failure after LT. The investigators were aiming to predict the risk of post-LT 3-month mortality and prolonged ventilation. Their findings demonstrated that the respiratory risk score (RRS) could indeed be useful to discriminate between patients with and without post-LT respiratory failure. While the RRS includes many previously identified variables associated with post-LT respiratory failure, it does not appear to utilize any objective findings of nutritional status [5]. Further, many of the included variables may not be available unless the patient is already in the intensive care unit. Calculation of the sarcopenic index requires little time and utilizes imaging that the patient will have already undergone as part of their pretransplant workup.
Although hypoalbuminemia has previously been associated with postoperative respiratory failure in patients receiving noncardiac surgery, our case-control analysis demonstrates that serum albumin and total protein may not be reliable clinical predictors of post-LT respiratory failure [11]. This finding could be explained by the chronically ill state of the majority of LT patients. Regardless, this finding further illustrates the importance of identifying unique and objective signs of poor nutritional status, and the potential for sarcopenic index to fill that need. Both cachectic patients and patients that experience sarcopenic obesity may be at risk for worse outcomes. An objective measure of nutrition, such as sarcopenic index, can be an important clinical indicator to aid physicians in identifying patients at risk for perioperative and postoperative complications such as respiratory failure. There are also clinical measures of frailty such as the 6-min walk test, the 5- and 10-m walk tests, and grip strength testing [12,13]. With further study, these noninvasive measures of overall physiologic reserve may become important predictors for successful recovery from LT.
Pulmonary function testing is a standard portion of pre-LT evaluation. Unfortunately, the utility of pre-LT PFT has not been well documented in the literature. One recent study showed that patterns of restrictive lung disease were indeed associated with prolonged ventilation time after LT. The actual clinical significance is uncertain, as time on the ventilator was increased by approximately 1 day [14]. To our knowledge, these are the first data to present screening PFT for LT recipients who subsequently developed respiratory failure requiring tracheostomy. However, it must be noted that several PFT reports were missing for patients in our cohort including 8 patients in the control group and 15 patients in the respiratory failure group. We found that patients who experience respiratory failure after LT had a lower group average FVC and FEV1, which suggests that worse pre-LT pulmonary function may be associated with post-LT respiratory failure. However, similar to the aforementioned study, we found FEV1/FVC was not significantly different for patients with post-LT respiratory failure [14]. This finding could be explained by the screening of patients with extremely poor pulmonary function prior to LT. Further work and a larger cohort are needed to better understand the predictive value of pre-LT PFT for posttransplant respiratory failure.
The tracheostomy procedure is generally low risk, and it is often performed at the bedside. In this series, a 19% complication rate was observed, with 4 transplant surgeons performing 94% of the procedures. Although none of these complications were associated with death, many patients had to be returned to the operating room for bleeding or tracheostomy revision. The 6 other tracheostomies were placed by otolaryngology in patients with a known complex history of previous tracheostomy or neck surgery. In this series, 2 percutaneous dilational tracheostomies (PDTs) were placed in post-LT patients. Both had significant complications requiring operative revision. In both cases, the patients had significant disruption (tearing) of the tracheal tissue, which appeared to be related to overall poor tissue quality. PDT requires a relatively rigid and healthy trachea that can withstand the force of passing dilators of increasing size through the tracheal wall. Although a small body of literature supports the overall safety of PDT placement in patients with liver disease, our experience is that standard tracheostomy appears to be safer for the post-LT patient [15,16].
As with any retrospective study, this analysis has intrinsic limitations including potential documentation error and selection bias. Although data from large registries have the advantage of increased statistical power, the findings from this single-center study offer a more granular picture. This level of detail is needed to appreciate and identify clinical predictors such as sarcopenic index, which must be computed individually for each subject. For the case-control analysis, matching criteria include year of transplant, sex, and MELD. Cases and controls were not matched by age and BMI, which both differed significantly between respiratory failure LT cases and LT controls and could be potential confounders. Unfortunately, in the matching process for this analysis, matching beyond 3 variables became very difficult. However, the employed matching criteria did provide the opportunity to identify the clinically relevant data that older patients with cachexia (i.e., elderly patients with lower BMI and sarcopenia) are at increased risk for post-LT tracheostomy.
Conclusions
The need to identify pretransplant clinical predictors of post-LT respiratory failure is clearly highlighted by the poor clinical outcomes, including very poor survival, observed in patients who fail to wean from the ventilator. These findings suggest that although serum protein and albumin levels are not as useful in predicting post-LT clinical outcomes, sarcopenic index and other markers of frailty may serve as important objective measurements of nutritional status in patients on the LT waiting list. Accurately identifying patients at high risk for poor outcome will facilitate patient selection and improve organ utility.
Abbreviations
- BMI
body mass index
- CT
computed tomography
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- LT
liver transplant
- MRI
magnetic resonance imaging
- PACS
picture archiving and communication software
- PDT
percutaneous dilational tracheostomy
- PFT
pulmonary function testing
- SPSS
Statistical Package for the Social Sciences
- RRS
respiratory risk score
Footnotes
Source of support: Departmental sources
Conflicts of interest
Richard S. Mangus – F. Kohler Chemie (Germany) provides travel support and honoraria.
References
- 1.Yuan H, Tuttle-Newhall JE, Chawa V, et al. Prognostic impact of mechanical ventilation after liver transplantation: A national database study. Am J Surg. 2014;208(4):582–90. doi: 10.1016/j.amjsurg.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirat A, Ozgur S, Torgay A, et al. Risk factors for postoperative respiratory complications in adult liver transplant recipients. Transplant Proc. 2004;36(1):218–20. doi: 10.1016/j.transproceed.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 3.Golfieri R, Giampalma E, Morselli Labate AM, et al. Pulmonary complications of liver transplantation: Radiological appearance and statistical evaluation of risk factors in 300 cases. Eur Radiol. 2000;10(7):1169–83. doi: 10.1007/s003309900268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glanemann M, Langrehr JM, Muller AR, et al. Incidence and risk factors of prolonged mechanical ventilation and causes of reintubation after liver transplantation. Transplant Proc. 1998;30(5):1874–75. doi: 10.1016/s0041-1345(98)00466-7. [DOI] [PubMed] [Google Scholar]
- 5.Kleine M, Vondran FW, Johanning K, et al. Respiratory risk score for the prediction of 3-month mortality and prolonged ventilation after liver transplantation. Liver Transpl. 2013;19(8):862–71. doi: 10.1002/lt.23673. [DOI] [PubMed] [Google Scholar]
- 6.Shen W, Punyanitya M, Wang Z, et al. Total body skeletal muscle and adipose tissue volumes: Estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97(6):2333–38. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 7.Mangus RS, Bush WJ, Miller C, Kubal CA. Severe sarcopenia and increased fat stores in pediatric patients with liver, kidney, or intestine failure. J Pediatr Gastroenterol Nutr. 2017;65(5):579–83. doi: 10.1097/MPG.0000000000001651. [DOI] [PubMed] [Google Scholar]
- 8.Mangus RS, Fridell JA, Vianna RM, et al. Immunosuppression induction with rabbit anti-thymocyte globulin with or without rituximab in 1000 liver transplant patients with long-term follow-up. Liver Transpl. 2012;18(7):786–95. doi: 10.1002/lt.23381. [DOI] [PubMed] [Google Scholar]
- 9.Mangus RS, Tector AJ, Agarwal A, et al. Comparison of histidine-tryptophan-ketoglutarate solution (HTK) and University of Wisconsin solution (UW) in adult liver transplantation. Liver Transpl. 2006;12(2):226–30. doi: 10.1002/lt.20552. [DOI] [PubMed] [Google Scholar]
- 10.Mangus RS, Fridell JA, Vianna RM, et al. Use of the piggyback hepatectomy technique in liver transplant recipients with hepatocellular carcinoma. Transplantation. 2008;85(10):1496–99. doi: 10.1097/TP.0b013e31816feec0. [DOI] [PubMed] [Google Scholar]
- 11.Arozullah AM, Daley J, Henderson WG, Khuri SF. Multifactorial risk index for predicting postoperative respiratory failure in men after major noncardiac surgery. The National Veterans Administration Surgical Quality Improvement Program. Ann Surg. 2000;232(2):242–53. doi: 10.1097/00000658-200008000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bohannon RW. Hand-grip dynamometry predicts future outcomes in aging adults. J Geriatr Phys Ther. 2008;31(1):3–10. doi: 10.1519/00139143-200831010-00002. [DOI] [PubMed] [Google Scholar]
- 13.Carey EJ, Steidley DE, Aqel BA, et al. Six-minute walk distance predicts mortality in liver transplant candidates. Liver Transpl. 2010;16(12):1373–78. doi: 10.1002/lt.22167. [DOI] [PubMed] [Google Scholar]
- 14.Kia L, Cuttica MJ, Yang A, et al. The utility of pulmonary function testing in predicting outcomes following liver transplantation. Liver Transpl. 2016;22(6):805–11. doi: 10.1002/lt.24426. [DOI] [PubMed] [Google Scholar]
- 15.Auzinger G, O’Callaghan GP, Bernal W, et al. Percutaneous tracheostomy in patients with severe liver disease and a high incidence of refractory coagulopathy: A prospective trial. Crit Care. 2007;11(5):R110. doi: 10.1186/cc6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waller EA, Aduen JF, Kramer DJ, et al. Safety of percutaneous dilatational tracheostomy with direct bronchoscopic guidance for solid organ allograft recipients. Mayo Clin Proc. 2007;82(12):1502–8. doi: 10.1016/S0025-6196(11)61094-X. [DOI] [PubMed] [Google Scholar]


