Abstract
Ion mobility separations coupled to mass spectrometry (IM-MS) have received much attention for their ability to provide complementary structural information to solution-phase-based separations, as well as to aid in the identification of unknown compounds. While IM-MS is an increasingly powerful analytical technique, significant bottlenecks related to the resolution of measurements have kept it from becoming broadly applied for biological analyses. Presently, IM-MS-based measurements also remain limited in terms of their sensitivity as compared to state of the art MS-based approaches alone. Structures for Lossless Ion Manipulations (SLIM)-based IM separations provide a basis for overcoming these bottlenecks, addressing issues associated with resolution and sensitivity in the omics, and potentially opening the door to much broader application.
1. Introduction
The accurate identification and quantification of biomolecules is of importance for understanding their potential biological, disease, and immunological implications [1]. While much progress has been made in developing techniques for biomolecule analyses, there remain major bottlenecks. Hyphenated techniques, notably liquid chromatography separations coupled to mass spectrometry (LC-MS), remain the current norm for the separation, identification, and quantification of biologically-relevant analytes [2]. LC-based separations benefit from the wide availability of various stationary phases (e.g., C18, phenyl hexyl, porous graphitic carbon) and the toolbox of chromatographic modes (e.g., reversed-phase, HILIC, normal phase) [3], but the need for several separations and approximate timescales that often approach or exceed an hour for each, drastically limits throughput. Ion mobility spectrometry separations, usually coupled to mass spectrometry (i.e., IM-MS), provide a potentially attractive alternative since analytes are separated in a gas based on their structure/shape and mass-to-charge (m/z) on a typically sub-second timescale [4–6]. Unfortunately, commercially available IM-MS platforms are limited in their achievable IM resolution and sensitivity of measurements (e.g., detection limits and dynamic range). Resolution is constrained by the limited device path length for separation, and sensitivity is limited by effective ion utilization and transmission efficiencies.
Recently, structures for lossless ion manipulations (SLIM)-based ion mobility separations have enabled significant gains in IM resolution by enabling long path lengths and multi-pass capabilities. Also, the sensitivity of analytes was improved by improvements to the ion accumulation process to better characterize low abundance analytes of interest [7]. This review article will describe SLIM IM-MS-based platform developments, and outline their unprecedented achievable resolution in the context of challenging bioanalytical applications.
2. Discussion
2.1. Structures for Lossless Ion Manipulations
SLIM uses electrodes patterned on two closely spaced surfaces to apply radiofrequency electric fields that can confine ions between the surfaces [8, 9]. Additional potentials are applied to a separate set of electrodes to create traveling waves (TW) to drive ion motion, and cause the mobility (and thus size) dependent transport of ions [10, 11].
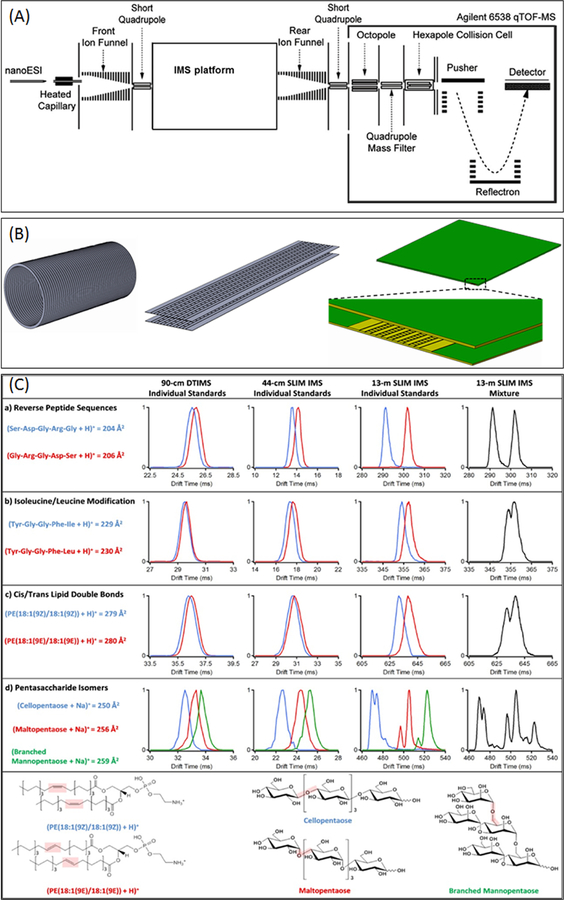
Currently available IM separation platforms include drift tube IM [12] (Agilent Technologies, Santa Clara, USA), traveling wave IM [13] (Waters Inc., Milford MA, USA), trapped ion mobility [14] (Bruker), and FAIMS [15] (Sciex, Concord, ON, Canada). A key parameter defining the IM separation power for different ion species, which often defines its utility for the analysis of complex biological samples, is the IM path length. A key development in SLIM was TW electrode arrangements allowing ions to execute 90° turns [16] done in a manner that ions are not lost and resolution is not degraded [17]. This enabled multiple turns to be incorporated into SLIM allowing extremely long serpentine path lengths in a compact format. Figure 1A shows an overall instrumental schematic with the insets showing drift tube, linear short (44 cm) SLIM, and long (serpentine) path SLIM. An initial 12”×18” module SLIM design incorporated 44 turns (green structure on Figure 1A, right inset, with a detailed view of the inlet region), to create a path length of ~13 m.
Figure 1.
A) Overall instrument schematic. B) Three configurations i.e. drift tube (left), short (straight) path SLIM (center), and serpentine path SLIM (right) board with zoomed in portion of the entrance region. C) Comparison of platform for a host of bio-relevant molecules. Reprinted with permission from [18].
The separation performance of a 1 m drift tube, short 44 cm TW SLIM module and the serpentine 13 m path length SLIM module is compared for several challenging biological compounds (reverse peptides, leucine/iso-leucine substitutions, positional lipid isomers, and pentasaccharide isomers) in Figure 1B [18]. The performance of the 44 cm TW SLIM module was comparable with the separation performance of a 1 m drift tube. The separation of two reverse peptides (GAGAS and SAGAG) was similar between the 1 m drift tube and 44 cm SLIM module, with the TW SLIM module performing marginally better with individual standards separated by a slightly larger mean arrival time. However, with a 13 m long TW SLIM device, baseline separation was achieved. Similarly, improved separations were achieved for leucine/iso-leucine and cis/trans lipid double bond isomers using the 13 m TW SLIM IM platform. The benefit of a 13 m long serpentine SLIM is particularly evident in the separation of various pentasaccharide isomers. While in the current commercial 1 m drift tube and 44 cm SLIM module, only 3 distinct conformers were identified, the use of a 13 m long path length enabled at least five distinct ion conformers to be identified.
2.2. Quest for higher resolution IM separations
In order to address currently intractable isomeric separations through IM-MS comes the need for even further gains in achievable IM resolution and resolving power. While TW SLIM IM separations over path lengths of ~13 meters [18] have shown utility for previously intractable separations, many applications will nonetheless require much longer path lengths due to the subtle differences in collision cross sections of the analytes of interest. Since resolution is expected to increase with the square root of separation path length, the ability to perform ultra-long path length (>100 m) separations is very much desired. The first attempt to increase IM resolution through multipass separations was by Clemmer and co-workers [19, 20], where a cyclic drift region was utilized to achieve resolving powers of ~1000. Unfortunately, a tradeoff between achievable resolution and corresponding sensitivity due to ion losses greatly limited sensitivity, while the limited path length increasingly restricted the range of ion mobilities that could be simultaneously transmitted.
Building upon the TW ion mobility separations in a serpentine SLIM design, an ion switch was introduced permitting ‘serpentine ultra-long path with extended routing’ (SUPER) IM separations [21]. This ion switch (Figure 2A), utilizes the option to route ions to the TOF-MS for detection or back to the start of the serpentine path for another pass, through the application of a DC blocking voltage to an electrode set. To evaluate ion transmission during SLIM SUPER IM separations, Agilent tuning mix ions were subjected to 81 passes (~1094 m), under conditions where the ions move at the same speed as the traveling waves (i.e., ‘surfing’ conditions where ions do not separate), where lossless ion transmission was observed [21]. To evaluate attainable resolution, Agilent tuning mix ions m/z 622 and m/z 922 were assessed in separation conditions (i.e., ions moving at a slower speed than the traveling waves where ion separations take place). After 40 passes (~540 m) a resolution of ~400 for these m/z 622 and m/z 922 ions [21] was achieved. The corresponding resolving power of individual peaks was ~1870.
Figure 2.
A) Ion switch diagram for SLIM SUPER IM separations. B) Lipid isomer separations and C) human milk oligosaccharide isomer separations. Adapted with permission from [21,22].
The SLIM SUPER IM-MS platform has been initially evaluated in the context of targeted lipidomics, proteomics, and glycomics-related applications, exploring ultra-long path length separations for labile/fragile compounds, as well as various biologically-relevant isomers. Figure 2 (B, C, D) illustrates the SLIM SUPER IM separations of two glycosphingolipid (GSL) isomers, which differ only in their monosaccharide constituent (galactose or glucose) attached to an identical lipid chain [22]. After a 1.25 m separation, the two GSL isomers were indistinguishable, but after 59.9 m of separation partial resolution of these species was observed. Other lipidomics-related examples with this SLIM SUPER IM-MS platform included the separation of various glycosylceramide and glycerophosphatidylcholine isomers [22]. Such work has laid the foundation to potentially explore isomer-specific diseases and other biological processes. Figure 2 (E, F) depicts the resolution of two human milk oligosaccharide isomers, with two distinct conformers present for one of the species, after a 121.5 m SLIM SUPER IM separation [21]. This conformational fingerprint was unseen with conventional approaches, thus potentially permitting the elucidation of unknown species in future glycan-related applications.
Because of the importance in understanding protein aging and how it relates to Alzheimer’s disease, much attention has been paid in resolving D, L, and iso-aspartic acid-containing beta amyloid (Aβ) peptides. With a conventional, 90 cm, drift tube (DT) IM-MS instrument, four Aβ peptide epimers were completely unresolved; however, after 67.5 m of SLIM SUPER IM separation, all four epimers were baseline separated [23]. Future work may potentially involve surveying other such peptide fragments associated with age-related diseases. While these ultra-long path separations afforded by this SLIM SUPER IM platform have shown tremendous benefit for the resolution of very structurally similar biomolecules, there remain certain drawbacks related to decreased sensitivity (i.e., peak intensities, S/N) over extended passes (due to peak broadening from analyte diffusion), which will be the subject of discussion in section 2.3.
2.3. Overcoming diffusion and injection contributions to IM separations
A distinction between drift tube IM and traveling wave IM as implemented in SLIM is in the nature of the ion peak distributions. While traditional drift tube IM produces continuous ion packet distributions (i.e., peaks), in SLIM the presence of potential troughs of the traveling waves results in some amount of discreteness to the ion distribution of an IM peak. This effective peak “quantization” [24] means, by combining multiple troughs ions can be ‘repopulated’ to produce a peak with increased signal intensity and narrower width. This increase in peak intensity (and S/N) is performed by controlling the traveling waves in two different SLIM regions. And if the compression ratio is small enough (typically less than 10 for peaks spanning hundreds to thousands of bins) the peak heights are increased but not the subtle shape of the peak, and hence resolution is essentially unaffected. We refer to this process as Compression Ratio Ion Mobility Programming (CRIMP) [24, 25]. CRIMP provides flexibility and is advantageous because it can help overcome the effects of peak diffusional broadening enhancing the signal intensity and overcoming diffusional broadening.
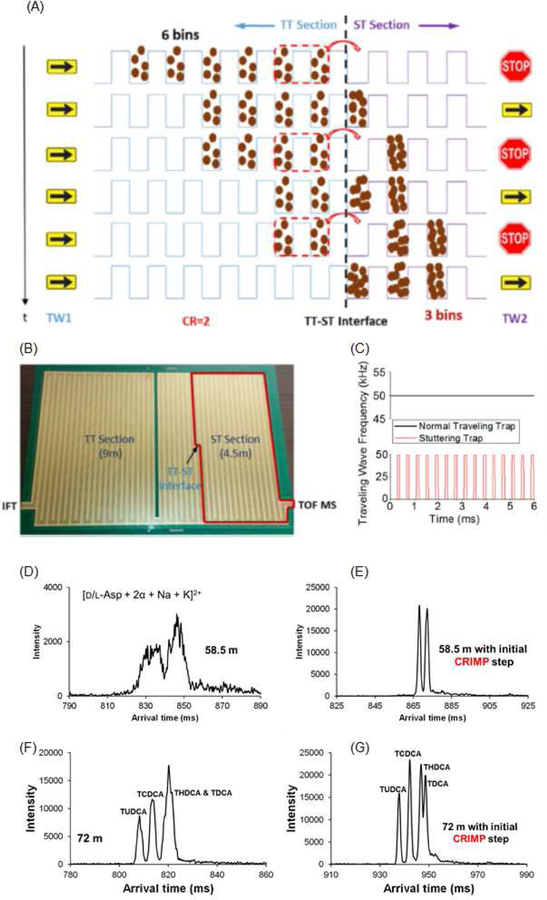
SLIM devices implementing CRIMP incorporate (at least) two different TW regions, the first operating with a conventional traveling wave (where the traveling wave ‘bins’ move constantly in the traveling trap, or TT, mode), and a second region where the voltage profile pauses periodically (i.e., in a stuttering manner, or ST mode). The key compression step occurs at the interface between the two regions where the ST in the second region presents a trapping region for the accumulation of ions spread over multiple TTs as the ions approach the TT/ST interface from the first region. Once this spatial compression is accomplished, the ST region is switched back into the normal TT mode (i.e., separation conditions) to retain the spatial compression in the temporal domain (Figure 3A) [24]. When the right side ST section is halted, multiple bins from the TT section will merge into the first ST section. The compression ratio (CR) is defined by the number of bins from the TT merging into each ST bin (i.e. the relative speed of the TW in the two sections). When the CR is 1, it is the trivial case of a normal TT or TW section(i.e. the same TW speed) throughout. At CR of 2, the ions are redistributed to exactly half the number of bins (rounded to an integer value). Figure 3A illustrates 6 bins being redistributed to 3 bins. Typically, a single peak will span multiple bins, ideally no fewer than ~5, and potentially hundreds of bins. Also, a peak often constitutes multiple species (including potential isomers and naturally occurring isotopomers/isotopologues) that may be only partially resolved. The implementation of this CRIMP scheme in SLIM (or the frequency with which the traveling wave profile advances from electrode to the next in the SLIM), along with the optimization of TW amplitude and frequency, is crucial to successful separations (Figure 3B).
Figure 3.
A) Depiction of CRIMP process. B) SLIM device with the TT and ST sections marked. C) The frequencies (or speed) of traveling wave profile in the TT and ST regions. Separation of enantiomers as their cyclodextrin complexes D) without compression and E) with compression. Bile acid isomers separations F) without compression and G) with compression.
There is a second significant way a CRIMP step significantly impacts S/N: CRIMP can offset the detrimental effects of increasingly large initial ion packet widths in much the same way as it overcomes diffusional broadening during a separation. This enables making use of large ion populations, otherwise prohibited or impractical in normal/commercial IMS instruments. Typical commercial IMS instruments can accumulate and release ions in pulses with temporal widths of ~/< 1 ms whereas the time-scale of separation is often on the order of 10 to 100 ms. SLIM enables much longer accumulation times thus significantly increases the ion utilization efficiency with continuous sources (e.g. ESI) during a separation event.
For ion accumulation, a potential ‘wall’ is applied along the ion path (e.g. at the entrance to the second section) so that ions accumulate at the interface of two SLIM regions, and allowing the creation of much larger initial trapped ion populations than has been previously feasible [25]. Once a separation is resumed with this large initial packet, CRIMP can then be used to reduce the width of a broad initial ion distribution after some initial separation (to lower total charge density to reduce space charge effects), and aid in providing increased signal intensities and narrower widths for IM peaks. Traditionally IM resolution for a given path length is limited by two factors – the injection (gate width) ion packet distribution and its subsequent diffusional broadening during separation. Both these limitations contribute to poorer S/N of measurements, as any diffusional broadening leads to lower signal intensities and the limited injection packet limits the number of ions that can be sent into the device hindering detection. This would specifically hamper the detectability of very low concentration species (or the overall dynamic range of measurements) as too few ions are distributed over a given IM peak to precisely define its centroid. Reduction of the peak widths without compromising separation power, would offset these two limitations and open avenues for practically achieving unlimited IM resolution.
Figure 3C and 3D show examples using large ion population accumulation and CRIMP for previously intractable IM measurements. ‘In SLIM’ ion accumulation extending over 2 s was used to provide large initial ion populations for separations. Enantiomers (L and D aspartic acid) formed a non-covalent complex with a chiral cyclodextrin adduct, and their respective complexes could be resolved based on slight differences in their gas-phase shape/structures [26]. Without the use of a CRIMP step, these chiral non-covalent gas-phase complexes could be partially separated after 58.5 m of SLIM SUPER separation. However, the observed resolution remains quite poor due to their broader peaks, and poor S/N, resulting from the initial starting ion packet width associated with the 2 s in-SLIM accumulation step. When a CRIMP step was applied (CR of 25) after the first pass (13.5 m of separation prior to CRIMP), the observed resolution was much higher and the peak intensities were approximately an order of magnitude greater than when no CRIMP step was applied [26]. Figure 3D shows the application of CRIMP for a long path length separation of several tauro-conjugated bile acid isomers as their cyclodextrin adducts [27]. It was observed that without the use of a CRIMP step, only three bile acid isomers could be resolved; all four isomers were evident with the use of an initial CRIMP step. By overcoming the broad initial width of the ion packet (from the multi-second accumulation step) via a CRIMP step, the final isomeric resolution was increased. These capabilities have been initially explored for these, and other applications, such as for resolving various isomeric glycans [28], human milk oligosaccharides [28], and metabolites present in carbon-fixing communities [29]. From these applications, it is evident that increased S/N of measurements, and eventual gains in resolution, can be accomplished by introducing a large ion population followed by a subsequent CRIMP step. It is important to note that this described increase in S/N is relative to conventional IM approaches where ions are introduced by an ion funnel trap. It has been shown that the charge capacity of the ion funnel trap is ~100–1000 times smaller [25] than with use of several seconds of in-SLIM ion accumulation. The subsequent CRIMP step after some initial separation and relaxation of space charge, can be used to spatially compress the ion packet widths to what would result from an ion funnel trap after a similar separation time. We envision that by combining these technological capabilities (large in-SLIM ion accumulation, CRIMP, and additional SLIM SUPER IM separations), broad utility for fast analyses of very small sample sizes will be enabled [30].
2.4. Broad mobility range SLIM IM-MS applications
While many of our initial explorations of SLIM SUPER IM separations have focused on targeted, isomeric, separations, we particularly see utility of SLIM for MS-based omics measurements. Ion mobility-based separations are also attractive when coupled with an initial orthogonal first dimension separation, such as liquid chromatography (LC) [31]. LC separations usually occur on the order of minutes to hours, while IM separations occur on the order of milliseconds to second timescales (e.g., for SLIM SUPER IM). Due to the much faster second dimension IM separations, sufficient chromatographic peak sampling (i.e., the number of IM separations sampled across an LC peak) can be achieved. Such ‘nested’ two-dimensional separations in conjunction with MS bring great benefits, typically including additional compound discrimination and identifications, broader sample coverage, and improved limits of detection [31].
The SLIM-based IM-MS ability to accumulate large ion populations prior to IM separations provides significant gains for ion introduction (up to 3 orders of magnitude more ions) as compared to commercially available platforms that utilize an ion funnel trap for ion introduction [25].These capabilities have been demonstrated for phosphopeptide measurements, by coupling a conventional reversed-phase LC gradient with a 2 second in-SLIM ion accumulation, followed by an 18 m SLIM IM separation, to provide sensitivities (i.e., limits of detection) comparable to LC-MRM measurements [32]. Figure 4A illustrates the linear dynamic range to be approximately 3–4 orders of magnitude for heavy-labeled phosphopeptides spiked in a complex background matrix. Figure 4B and 4C also depict the benefit of coupling this SLIM IM separation with a first dimension LC separation; the phosphopeptide analyte ion of interest co-eluted in LC with several background ions, but was successfully “mobility filtered” in the IM separation, improving its limits of detection as compared to LC-MRM measurements [32]. This work demonstrates the first steps taken to permit the broad applicability of SLIM-based measurements for omics-related applications. Future work in further developing the ion accumulation process will only further improve the limits of detection for both targeted and untargeted global omics applications, to provide complementary information to conventional MS-based approaches.
Figure 4.
(A) Linear dynamic range achieved by coupling reversed-phase LC separations with 18 m SLIM SUPER IM-MS separations and 2 s in-SLIM ion accumulation. Mobility filtering of phosphopeptides analytes of interest from background matrix ions at (B) 1nM concentration and (C) 50 pM concentration. (D) Much longer path length multilevel SLIM implementations are presently being developed to permit greatly increased peak capacity for broad and high peak capacity applications. Reprinted with permission from [32].
Finally, we note the current SLIM SUPER IM-MS platform is limited in its achievable peak capacity, or range of mobilities, that can be studied in broad LC-SLIM SUPER IM-MS applications due to the ~13.5 m single pass path length. To address this limitation, a new multilevel SLIM IM-MS platform is under development that utilizes an ‘ion elevator’ design to transport ions between different SLIM ‘levels’ (Figure 4D) [33]. Through these ion elevators, single pass SLIM separations exceeding 100 meters should become feasible, thus also greatly increasing the achievable overall separation peak capacity, as well as resolving power for broad mobility range (e.g., global proteomics) applications.
2.5. Continuing developments with SLIM IM-MS
While SLIM IM-MS-based applications have shown promise for better resolving isomeric species on a fast time scale, as well as providing an avenue for comparable limits of detection to MS-based omics applications, ongoing work is essential for its broad implementation in the analytical chemistry community. A SLIM platform has a known nominal path length for a single pass (typically 13 meters). The use of the previously described ion switch and SUPER separations enable longer paths. When used for complex (i.e., broad range of mobilities) samples, the faster ions will inevitably ‘lap’ the slower ions even after one or two passes around the SLIM making the technique of multi-pass extended separations limited to targeted mobility ranges. This sample-dependent switching to permit longer path lengths is a limitation of current SLIM devices. As discussed in section 2.4, developments are underway to implement an ‘ion elevator’ design to permit single pass path lengths of >100 meters, thereby eliminating any need for such ion switching and preventing ion lapping. Another limitation of current SLIM devices is the suboptimal duty cycle associated with having distinct accumulation and separation steps during an IM-MS experiment. Specifically, current SLIM devices do not permit ions to be accumulated while an IM separation is taking place. To circumvent this, new SLIM designs are being implemented that would permit concurrent accumulation and separation steps, so as to approach 100% ion utilization efficiency. This would enable greater peak sampling when coupled to online separation methods, such as LC. Work is also ongoing to further optimize the SLIM TW conditions (amplitude and frequency) during both in-SLIM ion accumulation and CRIMP, to optimize IM resolution achieved etc. Lastly, much work remains to be done regarding platform control (currently all homebuilt software) to provide a more straightforward user interface and enable more complex sequences of ion manipulations. Work is actively being done to address these aforementioned current limitations of SLIM devices to promote its broad utility and implementation. Finally, we note that other groups are adopting SLIM technology to help address their respective research directions [34–37].
3. Conclusions
This review has highlighted recent advances in traveling wave SLIM-based IM-MS-based platforms, which have opened new paths for a wide array of biological applications. By coupling the use of large ion populations with ultra-long serpentine path high resolution IM separations and IM peak compression, such SLIM-based developments are anticipated to greatly improve and extend analytical capabilities for a broad range of important applications.
Opens new paths for broad global analyses
Enables increased resolution and sensitivity for biological applications
Overcomes key analytical bottlenecks in current omics measurements
4. Acknowledgements
Portions of this research were supported by grants from National Institute of General Medical Sciences (P41 GM103493) and the Laboratory Directed Research and Development Program at Pacific Northwest National Laboratory. This work was performed in the W. R. Wiley Environmental Molecular Sciences Laboratory (EMSL), a DOE national scientific user facility at the Pacific Northwest National Laboratory (PNNL). PNNL is operated by Battelle for the DOE under contract DE-AC05-76RL0 1830.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- 1.Couvillion SP, Zhu Y, Nagy G, Adkins JN, Ansong C, Renslow RS, Piehowski PD, Ibrahim YM, Kelly RT and Metz TO, New mass spectrometry technologies contributing towards comprehensive and high throughput omics analyses of single cells Analyst 144 (2019) 794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pirok BWJ, Stoll DR and Schoenmakers PJ, Recent Developments in Two-Dimensional Liquid Chromatography: Fundamental Improvements for Practical Applications Anal Chem 91 (2019) 240–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meng M, Wang L, Voelker T, Reuschel S, Van Horne KC and Bennett P, A systematic approach for developing a robust LC-MS/MS method for bioanalysis Bioanalysis 5 (2012) 91–115. [DOI] [PubMed] [Google Scholar]
- 4.Giles K, Williams JP and Campuzano I, Enhancements in travelling wave ion mobility resolution Rapid Communications in Mass Spectrometry 25 (2011) 1559–1566. [DOI] [PubMed] [Google Scholar]
- 5.Pringle SD, Giles K, Wildgoose JL, Williams JP, Slade SE, Thalassinos K, Bateman RH, Bowers MT and Scrivens JH, An investigation of the mobility separation of some peptide and protein ions using a new hybrid quadrupole/travelling wave IMS/oa-ToF instrument International Journal of Mass Spectrometry 261 (2007) 1–12. [Google Scholar]
- 6.Giles K, Pringle SD, Worthington KR, Little D, Wildgoose JL and Bateman RH, Applications of a travelling wave-based radio-frequency-only stacked ring ion guide Rapid Communications in Mass Spectrometry 18 (2004) 2401–2414. [DOI] [PubMed] [Google Scholar]
- 7.Ibrahim YM, Hamid AM, Deng L, Garimella SV, Webb IK, Baker ES and Smith RD, New frontiers for mass spectrometry based upon structures for lossless ion manipulations Analyst 142 (2017) 1010–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garimella SV, Ibrahim YM, Webb IK, Tolmachev AV, Zhang X, Prost SA, Anderson GA and Smith RD, Simulation of electric potentials and ion motion in planar electrode structures for lossless ion manipulations (SLIM) J Am Soc Mass Spectrom 25 (2014) 1890–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tolmachev AV, Webb IK, Ibrahim YM, Garimella SV, Zhang X, Anderson GA and Smith RD, Characterization of ion dynamics in structures for lossless ion manipulations Anal Chem 86 (2014) 9162–9168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Webb IK, Garimella SV, Tolmachev AV, Chen TC, Zhang X, Norheim RV, Prost SA, LaMarche B, Anderson GA, Ibrahim YM and Smith RD, Experimental evaluation and optimization of structures for lossless ion manipulations for ion mobility spectrometry with time-of-flight mass spectrometry Anal Chem 86 (2014) 9169–9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabelica V and Marklund E, Fundamentals of ion mobility spectrometry Curr. Opin. Chem. Biol 42 (2018) 51–59. [DOI] [PubMed] [Google Scholar]
- 12.Stow SM, Causon TJ, Zheng X, Kurulugama RT, Mairinger T, May JC, Rennie EE, Baker ES, Smith RD, McLean JA, Hann S and Fjeldsted JC, An Interlaboratory Evaluation of Drift Tube Ion Mobility-Mass Spectrometry Collision Cross Section Measurements Anal Chem 89 (2017) 9048–9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shvartsburg AA and Smith RD, Fundamentals of traveling wave ion mobility spectrometry Anal Chem 80 (2008) 9689–9699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michelmann K, Silveira JA, Ridgeway ME and Park MA, Fundamentals of trapped ion mobility spectrometry J Am Soc Mass Spectrom 26 (2015) 14–24. [DOI] [PubMed] [Google Scholar]
- 15.Cumeras R, Figueras E, Davis CE, Baumbach JI and Gracia I, Review on ion mobility spectrometry. Part 1: current instrumentation Analyst 140 (2015) 1376–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garimella SV, Ibrahim YM, Webb IK, Ipsen AB, Chen TC, Tolmachev AV, Baker ES, Anderson GA and Smith RD, Ion manipulations in structures for lossless ion manipulations (SLIM): computational evaluation of a 90 degrees turn and a switch Analyst 140 (2015) 6845–6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamid AM, Garimella SVB, Ibrahim YM, Deng L, Zheng X, Webb IK, Anderson GA, Prost SA, Norheim RV, Tolmachev AV, Baker ES and Smith RD, Achieving High Resolution Ion Mobility Separations Using Traveling Waves in Compact Multiturn Structures for Lossless Ion Manipulations Anal Chem 88 (2016) 8949–8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng L, Ibrahim YM, Baker ES, Aly NA, Hamid AM, Xhang X, Zheng X, Garimella SVB, Webb IK, Prost SA, Sandoval JA, Norheim RV, Anderson GA, Tolmachev AV and Smith RD, Ion Mobility Separations of Isomers based upon Long Path Length Structures for Lossless Ion Manipulations Combined with Mass Spectrometry ChemistrySelect 1 (2016) 2396–2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glaskin RS, Ewing MA and Clemmer DE, Ion trapping for ion mobility spectrometry measurements in a cyclical drift tube Anal Chem 85 (2013) 7003–7008. [DOI] [PubMed] [Google Scholar]
- 20.Merenbloom SI, Glaskin RS, Henson ZB and Clemmer DE, High-resolution ion cyclotron mobility spectrometry Anal Chem 81 (2009) 1482–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deng L, Webb IK, Garimella SVB, Hamid AM, Zheng X, Norheim RV, Prost SA, Anderson GA, Sandoval JA, Baker ES, Ibrahim YM and Smith RD, Serpentine Ultralong Path with Extended Routing (SUPER) High Resolution Traveling Wave Ion Mobility-MS using Structures for Lossless Ion Manipulations Anal Chem 89 (2017) 4628–4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wojcik R, Webb IK, Deng L, Garimella SV, Prost SA, Ibrahim YM, Baker ES and Smith RD, Lipid and Glycolipid Isomer Analyses Using Ultra-High Resolution Ion Mobility Spectrometry Separations Int J Mol Sci 18 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng X, Deng L, Baker ES, Ibrahim YM, Petyuk VA and Smith RD, Distinguishing d- and l-aspartic and isoaspartic acids in amyloid β peptides with ultrahigh resolution ion mobility spectrometry Chem. Commun 53 (2017) 7913–7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garimella SV, Hamid AM, Deng L, Ibrahim YM, Webb IK, Baker ES, Prost SA, Norheim RV, Anderson GA and Smith RD, Squeezing of Ion Populations and Peaks in Traveling Wave Ion Mobility Separations and Structures for Lossless Ion Manipulations Using Compression Ratio Ion Mobility Programming Anal. Chem 88 (2016) 11877–11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng L, Garimella SVB, Hamid AM, Webb IK, Attah IK, Norheim RV, Prost SA, Zheng X, Sandoval JA, Baker ES, Ibrahim YM and Smith RD, Compression Ratio Ion Mobility Programming (CRIMP) Accumulation and Compression of Billions of Ions for Ion Mobility-Mass Spectrometry Using Traveling Waves in Structures for Lossless Ion Manipulations (SLIM) Anal. Chem 89 (2017) 6432–6439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagy G, Chouinard CD, Attah IK, Webb IK, Garimella SVB, Ibrahim YM, Baker ES and Smith RD, Distinguishing enantiomeric amino acids with chiral cyclodextrin adducts and structures for lossless ion manipulations ELECTROPHORESIS 39 (2018) 3148–3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chouinard CD, Nagy G, Webb IK, Garimella SVB, Baker ES, Ibrahim YM and Smith RD, Rapid Ion Mobility Separations of Bile Acid Isomers Using Cyclodextrin Adducts and Structures for Lossless Ion Manipulations Anal. Chem 90 (2018) 11086–11091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagy G, Attah IK, Garimella SVB, Tang K, Ibrahim YM, Baker ES and Smith RD, Unraveling the isomeric heterogeneity of glycans: ion mobility separations in structures for lossless ion manipulations Chem. Commun 54 (2018) 11701–11704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagy G, Veliìkoviç D, Chu RK, Carrell AA, Weston DJ, Ibrahim YM, Anderton CR and Smith RD, Towards resolving the spatial metabolome with unambiguous molecular annotations in complex biological systems by coupling mass spectrometry imaging with structures for lossless ion manipulations Chem. Commun 55 (2019) 306–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dou M, Chouinard CD, Zhu Y, Nagy G, Liyu AV, Ibrahim YM, Smith RD and Kelly RT, Nanowell-mediated multidimensional separations combining nanoLC with SLIM IM-MS for rapid, high-peak-capacity proteomic analyses Analytical and Bioanalytical Chemistry, DOI: 10.1007/s00216-018-1452-5 (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Venter P, Muller M, Vestner J, Stander MA, Tredoux AGJ, Pasch H and de Villiers A, Comprehensive Three-Dimensional LC x LC x Ion Mobility Spectrometry Separation Combined with High-Resolution MS for the Analysis of Complex Samples Anal Chem 90 (2018) 11643–11650. [DOI] [PubMed] [Google Scholar]
- 32.Chouinard CD, Nagy G, Webb IK, Shi T, Baker ES, Prost SA, Liu T, Ibrahim YM and Smith RD, Improved Sensitivity and Separations for Phosphopeptides using Online Liquid Chromotography Coupled with Structures for Lossless Ion Manipulations Ion Mobility-Mass Spectrometry Anal Chem 90 (2018) 10889–10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ibrahim YM, Hamid AM, Cox JT, Garimella SV and Smith RD, Ion Elevators and Escalators in Multilevel Structures for Lossless Ion Manipulations Anal Chem 89 (2017) 1972–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Warnke S, Ben Faleh A, Pellegrinelli RP, Yalovenko N and Rizzo TR, Combining ultra-high resolution ion-mobility spectrometry with cryogenic IR spectroscopy for the study of biomolecular ions Faraday Discussions, DOI: 10.1039/C8FD00180D (2018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Allen SJ, Eaton RM and Bush MF, Structural Dynamics of Native-Like Ions in the Gas Phase: Results from Tandem Ion Mobility of Cytochrome c Anal Chem 89 (2017) 7527–7534. [DOI] [PubMed] [Google Scholar]
- 36.Allen SJ, Eaton RM and Bush MF, Analysis of Native-Like Ions Using Structures for Lossless Ion Manipulations Anal Chem 88 (2016) 9118–9126. [DOI] [PubMed] [Google Scholar]
- 37.Ben Faleh A, Warnke S and Rizzo TR, Combining Ultrahigh-Resolution Ion-Mobility Spectrometry with Cryogenic Infrared Spectroscopy for the Analysis of Glycan Mixtures Anal Chem, DOI: 10.1021/acs.analchem.9b00659(2019) [DOI] [PubMed] [Google Scholar]