1. Emerging therapies for COVID-19, guidelines and recommendations
On 11 March 2020, the World Health Organization declared coronavirus disease 2019 (COVID-19) a pandemic. In recent months, with the spread of COVID-19, there has been growing interest in preventive and therapeutic treatments, but no effective strategies for either purpose have been confirmed by the available randomized controlled trials. Although future clinical trials may renew hope [1], management of COVID-19 is still exclusively supportive. The mechanism of infection with the causative agent of COVID-10, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), requires binding of viral spike (S) proteins to the angiotensin-converting enzyme-2 (ACE2) receptor, a process facilitated by transmembrane serine protease 2 (TMPRSS2) and followed by downregulation of ACE2 [2]. The viral membrane then fuses with the host cell, and the viral ribonucleic acid (RNA) is taken up via endocytosis. Within the host cell, it undergoes translation, proteolysis, RNA synthesis, new assembly, and exocytosis to further infect other cells. Secondary hemophagocytic lymphohistiocytosis, with a characteristic ‘cytokine storm’ component, has been recently proposed as a severe hyperinflammatory complication of COVID-19, driven by viral immune response [3]. Moreover, many COVID-19 patients suffer from abnormal coagulation, which has been associated with poor prognosis [4].
Recent advances have focused on a multi-target therapeutic strategy, taking inspiration from previous outbreaks of coronavirus infections, namely SARS and the Middle East respiratory syndrome (MERS). Nevertheless, no specific drugs are available for the treatment of either infection nor for COVID-19. With the current paucity of data, therapeutic choices that usually would never been adopted before appraising their utility is now being employed in an empiric and presumptive manner, moving away from core tenets of evidence-based medicine. Effective therapies are urgently needed.
Renin-angiotensin-aldosterone system (RAAS) inhibitors [2] have been proposed because they bind to ACE2. Data from Wuhan reported that a huge number of COVID-19 patients present with hypertension and RAAS inhibitors as daily antihypertensive therapy [2]. However, preclinical studies demonstrated that RAAS inhibitors may upregulate ACE2 expression, raising concerns as to their efficacy in COVID-19 (Figure 1) [2]. Conversely, abrupt withdrawal of RASS inhibitors in high-risk patients may result in clinical instability and adverse outcome. Clinical trials to determine whether the use of RASS inhibitors may affect the outcome in COVID-19 are ongoing (Table 1) [2].
Figure 1.
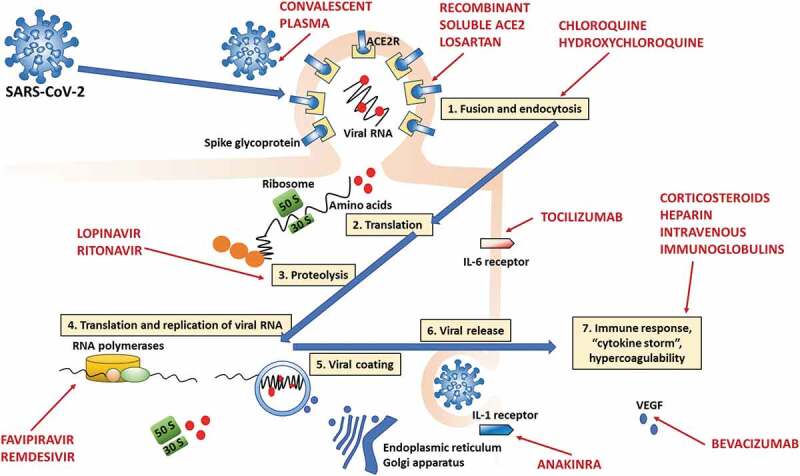
Main drugs tested to date in SARS-CoV-2 infection and their potential mechanisms and sites of action in the viral infection and replication cycle. 1. SARS-CoV-2 enters cells by binding the ACE2 receptor, with subsequent fusion and endocytosis (recombinant soluble ACE2 and losartan would act at this level). 2. Viral RNA is released, and transduction begins in the ribosome. 3. This is followed by proteolysis (may be inhibited by lopinavir/ritonavir), which in turn is followed by translation and replication (RNA polymerases; may be inhibited by favipiravir and remdesivir). 5. Then, the ribosome synthesizes nucleocapsid proteins, which will encase RNA, and the viral envelope is assembled with the aid of the endoplasmic reticulum and Golgi apparatus. The now-complete virions are released, ready to infect other cells. 7. In parallel, a local and systemic immune response is now activated, and hypercoagulability ensues (modulated by corticosteroids and heparin). The IL-6 receptor can be inhibited by tocilizumab, and the IL-1 receptor, by anakinra. Bevacizumab acts by inhibiting vascular endothelial growth factor (VEGF). Convalescent plasma reduces viral load.
Table 1.
Main proposed drugs for the management of COVID-19 patients.
| Proposed mechanism of action | Potential drawbacks | Supporting evidence | Ongoing trials (clinicaltrials.gov) | |
|---|---|---|---|---|
| RAAS inhibitors | ||||
| Losartan | ACE2 receptor binding | Unclear effects on ACE2 expression in vivo | Preclinical studies [2] |
NCT04312009 NCT04311177 |
| Recombinant soluble ACE2 | Restoring balance in the RAAS system and preventing organ injury | Limited data in vivo | In vitro [18] | NCT04287686 |
| Aminoquinolines | ||||
| Chloroquine | Attenuation of cytokine production Inhibition of virus in host cells |
Safety concerns arising from observational studies Several mild side-effects Hemolysis in G6PD-deficient patients Retinal damage QTc prolongation |
Preclinical studies [5,6] |
NCT04328493 NCT04333628 |
| Hydroxychloroquine | Same as chloroquine | Similar to chloroquine, safety profile probably better | Preclinical studies [5,6] |
NCT04333654 NCT04328272 (with and without adjunctive azithromycin) |
| Antivirals | ||||
| Lopinavir/ritonavir Darunavir/ritonavir Darunavir/cobicistat Other anti-HIV drugs |
Inhibition of viral 3C-like protease | Several drug interactions Common side effects, including hepatotoxicity One negative trial in late moderate-severe patients [7] |
Preclinical studies [7] |
NCT04315948 NCT04252274 |
| Remdesivir | RNA polymerase inhibitor | Hepatotoxicity Nephrotoxicity One negative trial in severe patients [9] |
Preclinical studies [9] |
NCT04292899 NCT04257656 |
| Favipiravir | RNA polymerase inhibitor | Hyperuricemia Neutropenia Hepatotoxicity |
Preclinical studies [9] |
NCT04336904 NCT04319900 |
| Convalescent plasma from recovered donors containing SARS-CoV-2 neutralizing antibodies | Reduce viral load and improve outcome | Potential for pathogen transmission Antibody-dependent enhancement of infection |
Pilot clinical study [11] | NCT04292340 |
| Immunomodulators | ||||
| Steroids (methylprednisolone and dexamethasone in particular) | Modulating the host inflammatory response and limiting organ damage [12] | Controversial effects on superinfections and viral clearance [12] Might increase superinfections |
Observational data [19] |
NCT04325061 NCT04323592 |
| Tocilizumab | Persistent immune modulation through inhibition of IL6 receptor (3). Reduction of cytokine storm |
Might increase superinfections Blocks C-reactive protein synthesis, limiting its usefulness in monitoring inflammation and bacterial superinfections |
Clinical rationale [3] |
NCT04320615 NCT04317092 |
| Anakinra | Short-term reversible suppression of inflammation through inhibition of IL1 receptor | Might increase superinfections | Clinical rationale [1] | NCT04324021 |
| Bevacizumab | Reduce pulmonary edema and protein levels in bronchoalveolar lavage fluid through inhibition of vascular endothelial growth factor | Nosebleed, rectal bleeding, increased blood pressure, headache | Preclinical study [1] | NCT04275414 |
| Intravenous immunoglobulins | Immunomodulation Specific antiviral activity if extracted from recovered patients (hyperimmune plasma) |
Unclear efficacy | Small case series [1] | NCT04321421 |
| Mesenchymal stromal cells | Immunomodulation, reduction in inflammation and fibrosis | Unclear efficacy | Preclinical and clinical studies in ARDS [16] | NCT03042143, NCT04269525, NCT04252118, NCT04339660, NCT04288102 NCT04293692 |
| Adjuvant therapy | ||||
| Unfractionated or low-molecular-weight heparin | Prophylaxis of thrombosis Protects endothelial cells from oxidative stress May modulate cytokine storm |
Increased bleeding risk | Observational data [20] | |
RAAS: renin–angiotensin–aldosterone system; ACE2: angiotensin-converting enzyme 2; G6PD: glucose-6-phosphate-dehydrogenase; IL6: interleukin 6; IL1: interleukin 1; HIV: human immunodeficiency virus.
Chloroquine and hydroxychloroquine have broad-spectrum antiviral activity in vitro by inhibiting virus entry into host cells through the inhibition of glycosylation, proteolysis, and endosomal acidification of host receptors. Both drugs also have immunomodulatory effects, attenuating cytokine production [5]. Very recently, the U.S. Food and Drug Administration (FDA) reviewed the available literature, as well as data from the Adverse Event Reporting System Database and the American Association of Poison Control Centers National Poison Data System, suggesting that these drugs should be used only very cautiously in patients at increased risk of heart rhythm problems, especially considering the limitation of their use to hospital settings [5]. On May 7, an observational study of 1376 consecutive patients who received hydroxychloroquine was published in the New England Journal of Medicine. The study concluded that hydroxychloroquine did not influence the time from enrollment to intubation or death [6], implying limited clinical utility and perhaps detrimental effects. Thus, we recommend that physicians not use hydroxychloroquine until new data from randomized-controlled trials become available (Table 1).
Antiretroviral agents, such as protease inhibitors and integrase strand transfer inhibitors, have also been suggested for the treatment of COVID-19. The use of lopinavir/ritonavir did not result in benefits in terms of viral clearance or mortality rate in a randomized-controlled study of 199 COVID-19 patients. Randomization was made 13 days after symptom onset, suggesting that delayed initiation of therapy was probably associated with the inefficacy of treatment [7]. Other antiretrovirals are still undergoing preclinical testing, with possible evidence of efficacy against SARS-CoV-2 [1]. Favipiravir, a purine nucleoside leading to inaccurate viral RNA synthesis, has recently been approved for clinical trials to treat COVID-19 [8]. Remdesivir, a nucleotide analogue, although was developed as a therapeutic agent for Ebola and Marburg virus infections with interesting results, preliminary data in COVID-19 did not show clinical benefit [9]; phase III clinical trials are ongoing (Table 1). In addition to various antiviral drugs already on the market, there are also several small-molecule compounds currently in research and development which have shown significant inhibitory effects on many key proteins from similar coronaviruses, such as SARS-CoV and MERS-CoV. These drug candidates mostly inhibit viral enzymes, including proteases and components of RNA-dependent RNA polymerase (RdRp). Since the 3C-like proteinase (3CLpro) has a high level of sequence homology between SARS-CoV and SARS-CoV-2, inhibitors tested against SARS-CoV 3CLpro may also be active against SARS-CoV-2 [10]. Because of their ability to interfere with viral replication, interferons and interferon fusion proteins have been utilized as therapeutic agents for the treatment of viral infections for more than 20 years. Finally, several RNA therapies have been developed, and, in addition to directly targeting the virus, antisense oligonucleotides could be used to target disease-related proteins involved in the inflammatory process. Convalescent plasma has also been shown to reduce viral load and improve outcome in COVID-19 patients [11].
The use of corticosteroids to modulate the inflammatory response in COVID-19 remains controversial. Based on an expert consensus [12–14], their use should be carefully weighed, according to clinical evidence: in patients already taking corticosteroids for chronic diseases, increased use should be evaluated with caution, with doses not exceed 0.5–1 mg/kg/day (methylprednisolone or equivalent) and for no longer than 7 days. The safety and efficacy of methylprednisolone in COVID-19 patients have been investigated in the clinical setting (Table 1). Interleukin (IL)-6 plays a crucial role in the inflammatory response, showing both anti-and pro-inflammatory effects on cells that express the IL-6 receptor (IL-6R), such as macrophages, neutrophils, and T-cells. Tocilizumab, a recombinant humanized monoclonal antibody, is designed to bind IL-6R. In 2017, it was approved for the treatment of cytokine release syndrome, which has prompted its possible use for COVID-19 in patients with higher levels of IL-6; efficacy remains unclear and requires confirmation [3]. Anakinra, an IL-1 receptor inhibitor, may likewise induce a short-term reversible suppression of inflammation. However, both of these drugs might increase the risk of superinfections, making their clinical use for COVID-19 questionable [3]. Bevacizumab, a human monoclonal antibody against vascular endothelial growth factor, has been studied in COVID-19 patients to reduce lung edema [15].
Immunoglobulin therapy is considered another potential treatment for COVID-19 patients. Two small case series (five and three, respectively) reported the use of immunoglobulins extracted from convalescent patients and the use of intravenous immunoglobulin for the treatment of SARS-CoV-2, respectively [1].
Mesenchymal stromal cells (MSCs) have demonstrated a good safety profile and possible efficacy in patients with acute respiratory distress syndrome (ARDS), but whether these therapies are effective for treating COVID-19 remain unknown. Few studies have been published [16] and several others are ongoing. COVID-19 patients with different degrees of severity were treated with MSCs or placebo, and no adverse events were observed. However, further studies on dose, dosing strategies, and timing of administration are needed.
COVID-19 patients may also develop severe coagulopathy. During SARS-CoV-2 infection, alveolar macrophages and T cells, among other immune cells, are recruited and activated in an uncontrolled immune response leading to a cytokine storm, characterized by elevated serum levels of interleukin-1β, tumor necrosis factor (TNF)-α, and IL-6, which foster thrombin activity [4]. Heparin is commonly administered to these patients. IL-1β and TNF-α target the glycocalyx via activation of metalloproteinases and heparanase, consequently reducing the content of proteoglycans (e.g., syndecan-1) and heparan sulfate, respectively. Glycocalyx shedding is linked to endothelial barrier rupture, which facilitates the increase of vascular permeability, leukocyte trafficking, and microthrombi. Heparin inhibits thrombin activity, protects endothelial cells from oxidative stress, and may modulate the cytokine storm [17].
Taken together, this evidence suggests that biologics have the potential to broaden the spectrum of treatment options for coronavirus-induced diseases. Prior knowledge and experience with the SARS and MERS outbreaks provide potential strategies for developing new target-specific therapeutic agents. However, COVID-19 has peculiar characteristics, and no strategies have demonstrated clear benefit to date; costs are also a concern.
Table 1 and Figure 1 summarize the current studies ongoing, and the mechanisms implicated in the treatment of COVID-19.
In conclusion, the main mechanism involved in the pathogenesis of COVID-19 appears to be activation of the inflammatory cascade with a massive pro-coagulable state, which can lead to rapid deterioration including potentially fatal secondary conditions (e.g., thromboembolic events, superinfections). In our opinion, ‘common’ drugs such as steroids and heparin should be used routinely to modulate inflammation and coagulability. The possibility of superinfection is a concern with corticosteroid therapy, while increased risk of bleeding may be seen with unfractionated or low-molecular-weight heparin. Strict monitoring of inflammatory (C-reactive protein, lymphocyte count, IL-6, ferritin, procalcitonin, etc.) and coagulation parameters (antithrombin III, prothrombin, D-dimer, fibrinogen, international normalized ratio, activated partial thromboplastin time, activated factor X) must be enforced and used to guide daily therapeutic management.
2. Expert opinion
The pandemic of novel coronavirus disease 2019 (COVID-19) has posed significant therapeutic challenges. High fatality rates have been observed in COVID-19 patients who require mechanical ventilation. Therefore, there has been growing interest in the development of new therapies capable of improving outcomes. At present, there is no evidence from randomized-controlled trials to support any therapeutic strategy as effective for COVID-19. Extensive studies have evaluated different potential strategies; however, to date, no treatment had improved outcomes or reduced fatality rates. Several drugs, including RAAS inhibitors (losartan, recombinant soluble ACE2), aminoquinolines (hydroxychloroquine/chloroquine), antivirals (remdesivir, favipiravir), immunomodulators (steroids, anti-interleukin agents, intravenous immunoglobulin, mesenchymal stromal cells), and antithrombotics (heparin) have been studied. These studies have had several limitations, which seem to be associated with: 1) a lack of understanding of the pathophysiological mechanisms of SARS-CoV-2-induced pneumonia; 2) the difficulty of translating in vitro findings into clinical practice; and 3) the lack of proper clinical study designs to elucidate optimal dosage, timing, and population. SARS-CoV-2 is still a poorly known virus, and a better understanding of its transmission mechanisms, clinical spectrum of disease, diagnosis, and fatality rates is urgently needed. Future investigations should focus on new drugs targeting the pathophysiological pathways implicated in COVID-19. Moreover, several therapies which have been investigated may lead to more adverse effects than benefits. Evidence suggests there are different phenotypes of COVID-19, potentially distinguishable through distinct biomarkers, which may respond differently to treatments. Therefore, personalized approaches to COVID-19 management should be recommended, including the use of ‘common’ drugs such as heparin and steroids as part of daily clinical management. While we await the development of an effective vaccine, clinical trials should help define the best strategy to treat COVID-19 [18,19,20].
Funding Statement
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (421067/2016-0), Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (E-26/210.910/2016).
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19). JAMA. 2020. DOI: 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]
- 2.Vaduganathan M, Vardeny O, Michel T, et al. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Eng J Med. 2020;382(17):1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang C, Wu Z, Li J-W, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;105954. DOI: 10.1016/j.ijantimicag.2020.105954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hällgren R, Samuelsson T, Laurent TC, et al. Accumulation of hyaluronan (Hyaluronic Acid) in the lung in adult respiratory distress syndrome. Am Rev Respir Dis. 1989;139:682–687. [DOI] [PubMed] [Google Scholar]
- 5.FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 . Outside of the hospital setting or a clinical trial due to risk of heart rhythm problems | FDA. FDA; 2020. [Google Scholar]
- 6.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. New Engl J Med. 2020;NEJMoa2012410. DOI: 10.1056/NEJMoa2012410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maxmen A. More than 80 clinical trials launch to test coronavirus treatments. Nature. 2020;578:347–348. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ortiz-Alcantara J, Bhardwaj K, Palaninathan S, et al. Small molecule inhibitors of the SARS-CoV Nsp15 endoribonuclease. Virus Adapt Treat. 2010;2:125. [Google Scholar]
- 11.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Pnas. 2020;117(17):9490–9496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao J, Hu Y, Du R, et al. Expert consensus on the use of corticosteroid in patients with 2019-nCoV pneumonia. Zhonghua Jie He He Hu Xi Za Zhi. 2020;43(0):E007. [DOI] [PubMed] [Google Scholar]
- 13.Alhazzani W, Hylander Møller M, Arabi YM, et al. Surviving sepsis campaign: guidelines on the management of critically Ill adults with coronavirus disease 2019 (COVID-19). Critical Care Medicine. 2020;1. DOI: 10.1097/CCM.0000000000004363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson KC, Chotirmall SH, Bai C, et al. COVID-19: interim guidance on management pending empirical evidence. 2020. [Google Scholar]
- 15.Barratt S, Medford AR, Millar AB. Vascular endothelial growth factor in acute lung injury and acute respiratory distress syndrome. Respiration. 2014;87:329–342. [DOI] [PubMed] [Google Scholar]
- 16.Khoury M, Cuenca J, Cruz FF, et al. Current status of cell-based therapies for respiratory virus infections: applicability to COVID-19. Eur Respir J. 2020;2000858. DOI: 10.1183/13993003.00858-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kropf J, Grobe E, Knoch M, et al. The prognostic value of extracellular matrix component concentrations in serum during treatment of adult respiratory distress syndrome with extracorporeal CO2 removal. Eur J Clin Chem Clin Biochem. 1991;29:805–812. [DOI] [PubMed] [Google Scholar]
- 18.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues using clinical-Grade Soluble Human ACE2. Cell. 2020; 181(4):905-913.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu C, Chen X, Cai Y, et al. Risk factors associated with coronavirus disease 2019 pneumonia in wuhan, china. Jama Intern Med. 2020; e200994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18(5):1094-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]


