ABSTRACT
Introduction
Over the last few months, coronavirus disease 2019 (COVID-19) pandemic caused by the novel coronavirus SARS-CoV-2 has posed a serious threat to public health on a global scale. Given the current lack of an effective vaccine, several drugs have been repurposed for treatment and prophylaxis of COVID-19 in an attempt to find an effective cure.
Areas covered
The antimalarial drug hydroxychloroquine (HCQ) initially garnered widespread attention following the publication of preliminary results showing that this drug exerts an anti-SARS-CoV-2 activity in vitro.
Expert opinion
To date, clinical evidence suggests lack of benefit from HCQ use for the treatment of hospitalized patients with COVID-19. In such patients, HCQ also appears to be associated with an increased risk of QT interval prolongation and potentially lethal ventricular arrhythmias. Therefore, FDA has recently revoked the Emergency Use Authorization (EUA) for emergency use of HCQ and chloroquine to treat COVID-19. Conversely, whether HCQ use may represent an effective prophylactic strategy against COVID-19 is a separate question that still remains to be answered. In addition, relevant aspects regarding the potential risks and benefits of HCQ need to be clarified, in pursuit of a rational use of this drug in the COVID-19 pandemic era.
KEYWORDS: COVID-19, drug repositioning, hydroxychloroquine, post-exposure prophylaxis, pre-exposure prophylaxis, prophylaxis, remdesivir, SARS-CoV-2, treatment, vitamin D
1. Introduction
Since its first identification in Wuhan (China) in December 2019 [1], coronavirus disease 2019 (COVID-19) caused by the novel coronavirus SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) has been posing a serious threat to public health. COVID-19 outbreak spread quickly across countries and was declared a global pandemic by the WHO on 11 March 2020, thus placing unprecedented strain on healthcare systems worldwide. Therefore, a robust research effort to develop an effective vaccine is currently underway and countries around the world keep on promoting face mask use, eye protection, hygiene measures and physical distancing as nonpharmacologic interventions to counteract the spread of COVID-19 outbreak. Although the risk of acquiring SARS-CoV-2 may decrease in the near future, a residual risk of unpredictable extent will likely persist [2]. For the foreseeable future, there is an urgent need to find safe and effective pre- and post-exposure prophylactic strategies (chemoprophylaxis) for high-risk individuals (such as healthcare workers and contacts of laboratory-confirmed cases), as well as safe pharmacological interventions able to effectively cure the established disease. In addition, the current pandemic scenario demands to prioritize the prophylaxis of selected individuals who are at high risk for greater disease severity and death from COVID-19, such as aged frail subjects, as well as individuals with obesity, cardiovascular disease and type 2 diabetes [3–5].
2. Drug repositioning and hydroxychloroquine use during the COVID-19 pandemic
Over the last few months, drug repositioning has allowed to investigate the off-label use of several drugs for prophylaxis and treatment of COVID-19 [6–8]. Amongst several potential drug candidates, the antimalarial drug hydroxychloroquine (HCQ) initially garnered widespread attention [9] following the publication of preliminary results showing that HCQ exerts an anti-SARS-CoV-2 activity in vitro [10,11]. Since the 1940s, chloroquine and its hydroxy-analogue HCQ have been used as disease-modifying antirheumatic drugs (DMARDs) for the treatment of various inflammatory rheumatic diseases, including rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) [12]. Overall, HCQ has shown a good safety and tolerability profile in most patients, even during pregnancy and breastfeeding [12].
After oral administration, HCQ is absorbed in the upper intestinal tract and displays a long half-life (~40–60 days) due to the large volume of distribution in the blood (47,257 L) [12–14]. Liver metabolism and renal clearance rate of HCQ amount to approximately 18 and 22%, respectively, and most of the administered dose is excreted unchanged in urine (Figure 1) [12,15]. HCQ is more soluble than chloroquine due to the hydroxyl group [16]. Moreover, HCQ can distribute to aqueous cellular and intercellular compartments, resulting in a long mean residence time (~1,300 h) [12,14]. HCQ is extensively sequestered in the tissues and the slow drug accumulation can account for its typical delay in achieving steady-state concentrations and elimination. For instance, the initial HCQ dose administered in patients with SLE ranges from 400 to 800 mg/day, although the therapeutic effect can appear after several weeks or months [17]. In addition, interindividual pharmacokinetic variability (probably arising from inherent differences in hepatic metabolism) [18] may be associated with variable tissue and circulating HCQ concentrations between individuals after the administration of standard doses. Altogether, these factors appear to contribute to the interindividual variability in the therapeutic onset of action of HCQ and in the magnitude of therapeutic response to HCQ [17].
Figure 1.
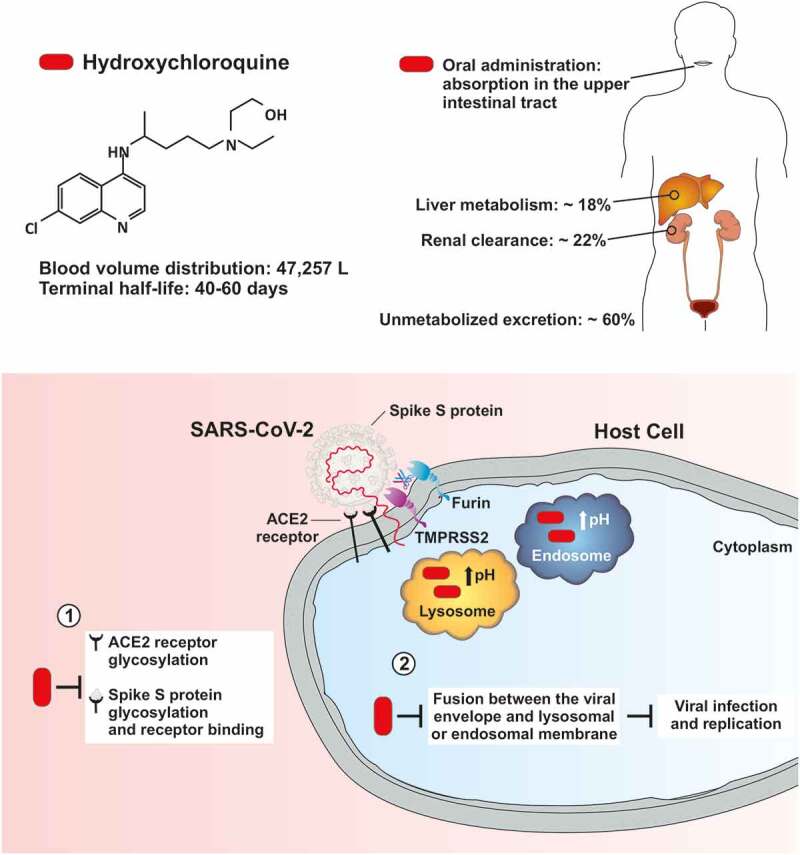
Pharmacokinetics of hydroxychloroquine and potential mechanisms underlying its postulated antiviral/prophylactic properties against SARS-CoV-2. Upper part) Pharmacokinetic properties of hydroxychloroquine (HCQ). HCQ displays a large volume of distribution and a long terminal half-life. Liver metabolism and renal clearance rate of HCQ amount to approximately 18% and 22%, respectively, and most of the administered dose is excreted unchanged in urine. Lower part) HCQ can interfere with the terminal glycosylation of ACE2 and viral spike S protein. The impaired glycosylation process of ACE2 and viral spike S protein may alter the affinity of SARS-CoV-2 for its receptor, potentially inhibiting key steps required for cell entry by SARS-CoV-2, such as receptor binding and membrane fusion. HCQ accumulates within cytoplasmic acidic organelles, such as lysosomes and endosomes, where it increases the pH, inhibits the activity of acidic pH-dependent lysosomal/endosomal proteases and ultimately prevents the fusion process between the viral envelope and lysosomal or endosomal membrane. Abbreviations: ACE2, angiotensin-converting enzyme 2; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TMPRSS2, transmembrane protease serine 2.
HCQ is a less toxic derivative of chloroquine [11] and may even display greater in vitro activity against SARS-CoV-2 compared to chloroquine [10]. In vitro studies conducted on different cell lines have reported different half maximal effective concentration (EC50) values of chloroquine and HCQ against SARS-CoV-2 [10,19,20]. The EC50 values of HCQ for SARS-CoV-2 have ranged from 0.72 to 17.31 μM across the studies [10,11]. Furthermore, EC50 values for chloroquine and HCQ have been shown to decrease with longer incubation times, suggesting that a longer incubation time allows for greater drug accumulation and higher intracellular concentrations, which subsequently result in a better antiviral effect [10,20]. Importantly, the in vitro EC50 of HCQ for SARS-CoV-2 is more than 20-fold higher compared to that for malaria [21]. Al-Kofahi et al. [21] simulated potential HCQ dosing regimens for pre-exposure prophylaxis and post-exposure prophylaxis of SARS-CoV-2 infection using HCQ pharmacokinetic data obtained from HCQ plasma concentrations in 91 subjects (22 healthy volunteers and 69 patients with malaria). The main aim of the study was to identify optimal HCQ dosing regimens for COVID-19 prophylactic studies by optimizing exposures above the EC50 generated in vitro. For the FDA recommended treatment dose of malaria (a loading dose of 800 mg, followed by a dose of 400 mg/day for 3 days), simulations predicted 89% of subjects would have troughs above the target on day 1, even though this number dropped to 7% by day 14 post-exposure after the initiation of prophylaxis. Then, authors simulated modified HCQ dosing scenarios for pre-exposure prophylaxis and found that a loading dose of 800 mg, followed by a dose of 400 mg given two or three times weekly can maintain weekly troughs above EC50 in 49–75% of the subjects (after reaching the steady-state) [21]. In a post-exposure prophylaxis setting, simulations showed that a loading dose of 800 mg followed in 6 hours by a dose of 600 mg/day for at least 5 days can maintain daily troughs above the EC50 in ≥50% of the subjects for at least 2 weeks [21]. Overall, these data suggest that higher HCQ doses may be required for prophylaxis of SARS-CoV-2 infection compared to those recommended for chemoprophylaxis of malaria, although they would need to be further validated in pharmacokinetic and clinical trials.
3. Possible mechanisms of action of chloroquine and HCQ against SARS-CoV-2 infection
SARS-CoV-2 is a single-stranded RNA-enveloped virus targeting host cells through the viral structural spike (S) protein, which binds to the angiotensin-converting enzyme 2 (ACE2) receptor on the host cell. Subsequently, host proteases, such as transmembrane protease serine 2 (TMPRSS2) and furin, favor the viral entry into the host cell by promoting the cleavage of viral spike S protein [22–26]. Several mechanisms of action have been postulated to underlie the antiviral properties of HCQ, accounting for its potential efficacy in preventing COVID-19 infection and progression. These mechanisms include HCQ ability to: (i) interfere with the terminal glycosylation of ACE2 receptor for viral entry, (ii) alter spike S protein glycosylation and binding to ACE2 receptor, (iii) inhibit proteolytic processing, endosomal acidification, lysosomal activity and autophagy in host cells, and (iv) exert immunomodulatory effects through reduction of cytokine production (Figure 1) [9,11,22,27]. Of note, chloroquine and HCQ may inhibit two key steps required for cell entry by SARS-CoV-2, namely: receptor binding and membrane fusion. Structure–function studies suggest that viral spike (S) protein of SARS-CoV-2 is highly glycosylated [28]. It has been hypothesized that concentration of glycosylated SARS-CoV-2 viral particles and glycosylated ACE2 in the lung epithelium may influence the susceptibility to COVID-19 infection and its subsequent severity [29]. Hence, impaired glycosylation of ACE2 and SARS-CoV-2 spike (S) protein mediated by HCQ may alter the binding efficiency between SARS-CoV-2 and ACE2 on the surface of host cells.
The non-protonated form of HCQ (and chloroquine) enters the host cell and becomes protonated, with the degree of protonation being inversely related to the pH [30]. Both chloroquine and HCQ are weak bases and accumulate within cytoplasmic acidic organelles (e.g., lysosomes, endosomes, Golgi vesicles), where these drugs bind to free protons, increase the pH, inhibit the activity of acidic pH-dependent lysosomal/endosomal proteases and ultimately prevent the cleavage of coronavirus surface spike proteins and the subsequent fusion between the viral envelope and lysosomal or endosomal membrane (Figure 1) [9,30–33]. Therefore, the use of chloroquine analogues (including HCQ) has been proposed as a valid strategy to inhibit infection by and replication of coronaviruses and other endosomal low pH-dependent viruses [34].
With regard to the effects of HCQ on immune system, the drug-mediated increase in intracellular pH and inhibition of lysosomal activity in antigen-presenting cells results in reduced toll-like receptor (TLR) signaling, antigen processing and antigen presentation mediated by major histocompatibility complex (MHC) class II molecules [9,35,36]. These molecular events underlie the anti-inflammatory properties of chloroquine and HCQ, which consist in the ability of these drugs to reduce the production of pro-inflammatory cytokines (e.g., IL-6, TNF-α) by immune cells [30,37]. Moreover, HCQ may influence the activity of the High mobility group box 1 protein (HMGB1) [38], which is a ubiquitous and highly conserved endogenous damage-associated molecular pattern (DAMP) molecule involved in the pathogenesis of several inflammatory conditions [39,40]. In light of these mechanisms of action, HCQ may theoretically exert an indirect antiviral activity by reducing the low-grade systemic inflammation, which is a common feature of several chronic diseases (e.g., obesity, type 2 diabetes, and metabolic syndrome) that are associated with an increased prevalence of SARS-CoV-2 infection and adversely affect the outcomes of patients with COVID-19 [41,42]. Intriguingly, relatively low rates of hospital PCR-confirmed COVID-19 cases have been reported in patients with SLE and inflammatory arthritis when compared to patients with systemic autoimmune or immune-mediated rheumatic diseases, despite an expected greater use of immunosuppressants and corticosteroids [43]. The authors argued that this may be partly explained by the frequent use of antimalarial drugs – such as HCQ – in this population, which might have played a protective role [43]. Further analyses and additional studies are needed, and clinical trials are currently ongoing to answer this question (ClinicalTrials.gov Identifier: NCT04355702).
Despite all these intriguing hypotheses, in vivo evidence for the efficacy of this drug as either prophylaxis or treatment of COVID-19 is lacking. Rosenke et al. [44] showed lack of prophylactic and therapeutic benefit of HCQ in two established COVID-like animal models, such as SARS-CoV-2 hamster and rhesus macaque disease models. Of note, neither the standard human malaria dose (6.5 mg/Kg) nor a high dose (50 mg/Kg) of HCQ led to beneficial effects on SARS-CoV-2 kinetics (replication and shedding) and clinical disease in the Syrian hamster disease model. Similarly, HCQ administration (at a dose of 6.5 mg/Kg) for both prophylaxis and treatment of COVID-19 did not significantly improve clinical outcome nor reduce SARS-CoV-2 replication and shedding in the upper and lower respiratory tract of the rhesus macaque disease model. Remarkably, in all prophylactically and therapeutically treated macaques HCQ was detected in lung tissue at time of necropsy, and plasma HCQ levels fell within or near human therapeutically relevant ranges for other diseases such as malaria and SLE (15 to 100 ng/mL plasma) [44].
4. Hydroxychloroquine for treatment of COVID-19
Due to the abovementioned preliminary findings, the negligible cost and the known safety and tolerability profile [12], the off-label use of HCQ as an investigational drug for treatment of COVID-19 has rapidly increased over the last few months [9,22]. As the interest in HCQ use for prophylaxis and treatment of COVID-19 also grew among governments and institutional leaders on a global scale, an open debate subsequently arose within the scientific community in pursuit of a middle ground between the two principles of ‘right to try’ and ‘primum non nocere’ [16,45–49].
The majority of clinical studies investigating the use of HCQ for treatment of COVID-19 have been conducted in hospitalized patients. A small case-control study conducted in hospitalized patients with confirmed COVID-19 infection initially suggested a potential efficacy of HCQ in promoting viral clearance, although major limitations included the small sample size and lack of information about clinical and safety outcomes [50]. Thereafter, two large observational studies conducted in hospitalized patients with moderate to severe COVID-19 showed lack of benefit from HCQ administration (alone or in combination with azithromycin) in reducing the need for intubation and in-hospital mortality [51,52].
Importantly, two retrospective studies conducted in hospitalized patients or admitted to intensive care units found that HCQ was significantly associated with an increased risk of QT interval prolongation, particularly when the drug was administered in combination with azithromycin [53–55]. Based on these findings, on April 24 2020 FDA cautioned against the use of HCQ or chloroquine for COVID-19 outside of hospital- or clinical trial settings because of the risk of potentially lethal cardiac arrhythmias [56]. On June 5 2020, the chief investigators of the RECOVERY trial (a large randomized trial involving 12,000 hospitalized patients with COVID-19 to assess the efficacy of different investigational drugs, including HCQ, in reducing all-cause mortality within 28 days; ClinicalTrials.gov Identifier: NCT04381936) announced closure of the HCQ arm due to lack of benefit [57]. Thereafter, on June 15 2020, FDA definitively revoked the Emergency Use Authorization (EUA) for emergency use of HCQ and chloroquine to treat COVID-19, based on a review of new information and a reevaluation of information available at the time the EUA was issued [58]. On July 4 2020, WHO accepted the recommendation from the Solidarity Trial’s International Steering Committee to discontinue the HCQ treatment arm for hospitalized patients with COVID-19. This recommendation was formulated in light of Solidarity trial interim results showing that HCQ therapy is associated with little or no reduction in the mortality of hospitalized patients with COVID-19 when compared to standard of care. However, this decision applies only to the conduct of the Solidarity trial in hospitalized patients and does not affect the possible evaluation in other studies of HCQ in non-hospitalized patients or as prophylaxis for COVID-19 [59]. In this regard, some randomized controlled trials are actively recruiting participants to investigate the efficacy of early outpatient treatment with HCQ in symptomatic patients with mild to moderate COVID-19 [60]. However, a recent multicenter, open-label, randomized controlled trial conducted on 293 non-hospitalized adult patients with recently confirmed SARS-CoV-2 infection and less than 5 days of mild symptoms showed that the use of HCQ (at a dose of 800 mg on day 1, followed by 400 mg once daily for 6 days) did not significantly reduce the viral load at day 3 or at day 7, and did not reduce the risk of hospitalization nor shortened the time to complete resolution of symptoms up to 28 days following diagnosis, as compared to the control arm (no treatment aside from usual care) [61].
Recently, FDA also warned about a potential drug interaction of chloroquine and HCQ with remdesivir, a nucleotide analogue prodrug which also received an EUA for the treatment of hospitalized patients with severe COVID-19 based on preliminary findings of a large double-blind, randomized, placebo-controlled trial [62,63]. Therefore, co-administration of HCQ or chloroquine with remdesivir is not recommended [64]. In vitro data showed a dose-dependent antagonistic effect of chloroquine phosphate on the intracellular metabolic activation and antiviral activity of remdesivir when the two drugs were co-incubated at clinically relevant concentrations in human epithelial type 2 (HEp-2) cells infected with respiratory syncytial virus [64]. Of note, increasing concentrations of chloroquine phosphate have been shown to reduce formation of remdesivir triphosphate in normal human bronchial epithelial cells [64]. Accordingly, preliminary additional data from the randomized, open-label phase 3 trial evaluating the safety and antiviral activity of remdesivir in patients with severe COVID-19 (Phase 3 SIMPLE-Severe study; ClinicalTrials.gov Identifier: NCT04292899) recently showed that the concomitant use of HCQ in such individuals was associated with significantly 12% lower rates of clinical improvement or recovery compared to patients who did not receive HCQ. Also, the analysis showed that patients in the concomitant HCQ group experienced significantly higher rates of adverse events [65].
5. Hydroxychloroquine as a prophylactic agent against COVID-19
The use of HCQ has also been proposed as a potential pre-exposure or post-exposure prophylactic strategy against COVID-19 for individuals at high risk of being accidentally exposed to SARS-CoV-2 [6,48,66]. Some countries approved the empiric use of HCQ-based chemoprophylaxis in subjects at high risk for SARS-CoV-2 infection. For instance, the Indian Council of Medical Research (ICMR) has recommended the prophylactic use of HCQ in high-risk categories [67], namely:
asymptomatic household contacts of laboratory-confirmed cases: HCQ administered at a dose of 400 mg twice a day on day 1, followed by 400 mg once weekly for the next 3 weeks.
all asymptomatic healthcare workers involved in containment and treatment of COVID-19 and asymptomatic healthcare workers working in non-COVID hospitals/non-COVID areas of COVID hospitals/blocks, as well as asymptomatic frontline workers: HCQ administered at a dose of 400 mg twice a day on day 1, followed by 400 mg once weekly for the next 7 weeks.
Nevertheless, several concerns have been raised about the safety of HCQ use for prophylactic purposes, along with the inability of healthcare systems to screen such a large number of healthy contacts for risk factors of HCQ-related side effects (e.g., concomitant use of other QT-prolonging drugs, long QT syndrome, glucose-6-phosphate dehydrogenase deficiency) [68].
Some studies investigated the role of HCQ in post-exposure prophylaxis (PEP) for SARS-CoV-2 infection among high-risk individuals, such as healthcare workers or inpatients of COVID hospitals (Table 1). Lee et al. [69] showed that PEP based on HCQ administration (at a dose of 400 mg/day) was able to prevent SARS-CoV-2 infection among healthcare workers and inpatients after a large SARS-CoV-2 exposure event in a long-term care hospital. In particular, all 189 patients and 22 healthcare workers who received PEP did not develop COVID-19 (follow-up PCR tests were all negative at the end of 14 days of quarantine), nor did they report any serious adverse event. However, the interpretation of these results is strongly limited by the lack of a control group in the study [69]. Thereafter, Boulware et al. [70] conducted a randomized, double-blind, placebo-controlled trial across the United States and parts of Canada to investigate the use of HCQ as PEP for COVID-19 in 821 asymptomatic adult participants who reported household or occupational exposure to someone with confirmed disease. The primary outcome of the study was the incidence of either laboratory-confirmed COVID-19 or illness compatible with COVID-19 within 14 days. The participants were randomly assigned to receive either placebo or HCQ (800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg/day for 4 additional days) within 4 days after exposure. The authors found that the incidence of new illness compatible with COVID-19 did not differ significantly between HCQ and placebo groups [70]. Nonetheless, this trial has several limitations [71]. In particular, the study employed an Internet-based approach to recruit participants and almost all data were obtained by means of participant report. Moreover, the study methods did not allow certain proof of exposure to SARS-CoV-2 or consistent laboratory confirmation that reported symptoms represented a SARS-CoV-2 infection. The specificity of COVID-19 reported symptoms by participants was low and enrolled participants were generally younger (median age: 40 years) and had fewer coexisting conditions compared to those at risk for severe COVID-19. More important, the long delay between perceived exposure to SARS-CoV-2 and HCQ administration (≥3 days in most participants) cannot exclude that this trial primarily investigated the efficacy of HCQ in prevention of symptoms or disease progression in persons who may already have been infected (early treatment) rather than in prevention of SARS-CoV-2 infection itself [71]. Also, the trial was powered to detect a 50% relative reduction in new symptomatic infections, which is an overly optimistic and robust estimate [72,73].
Table 1.
Summary of the main published and ongoing protocols evaluating the use of hydroxychloroquine as pre- and post-exposure prophylaxis for COVID-19.
| Study | Study design | HCQ dose and schedule of administration |
Results |
|---|---|---|---|
| Pre-exposure prophylaxis (PrEP) | |||
| Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine (HERO-HCQ) study ClinicalTrials.gov Identifier: NCT04334148 |
Phase 3, double-blind, placebo-controlled trial involving 15,000 healthcare workers at risk of being exposed to COVID-19. Eligible participants will be randomly assigned (1:1) to either treatment group (HCQ) or placebo in a double-blind fashion. Course of treatment is 30 days. Baseline assessments include nasopharyngeal swab for COVID-19 and a blood sample to detect seroconversion to COVID-19. At the end of treatment participants will repeat nasopharyngeal swab for COVID-19 and a blood sample to detect seroconversion to COVID-19. The primary outcome is the number of participants with clinical COVID-19 infection. |
HCQ 600 mg bid (loading dose) on day 1, followed by 400 mg on days 2–30. | Ongoing study (results expected by September 2020) |
| WHIP COVID-19 (Will Hydroxychloroquine Impede or Prevent COVID-19) ClinicalTrials.gov Identifier: NCT04341441 |
Phase 3, triple-blind, randomized, placebo-controlled trial involving 3,000 healthcare workers, nursing home workers, first responders and bus drivers in Michigan (USA). Participants will be randomized in a 1:1:1 blinded comparison of daily HCQ, weekly HCQ, or placebo for 8 weeks. A fourth non-randomized comparator group of participants who are chronically on HCQ as part of their standard of care for autoimmune diseases will be recruited to provide information of chronic weight-based daily therapy of HCQ efficacy as a prophylactic/preventive strategy. Eligible participants who are asymptomatic for pre-specified signs and symptoms suggestive of COVID-19 infection will have a whole blood specimen obtained at study entry. Participants will receive monitoring at each study week visit to evaluate for the development of COVID-19 related symptoms, COVID-19 clinical disease and drug-related side effects. At week 8 or if diagnosed positive, participants will provide additional samples of whole blood. Primary outcome: To determine if the use of HCQ as preventive therapy decreases the rate of acquisition of SARS-CoV 2 infection and clinical COVID-19 disease compared to placebo. |
Arm 1 (daily dosing): HCQ at a dose of 200 mg/day administered orally following a loading dose of 400 mg administered on day 1. This dose represents approximately half the standard weight-based dosing recommended for the management of autoimmune diseases. Arm 2 (weekly dosing): HCQ is administered orally once weekly (6.5 mg/kg per dose; maximum of 400 mg per dose) on the same day of each week. This dosage is based on the recommended dose for prophylaxis of malaria. Arm 3 (placebo oral tablet): subjects randomized to this arm will be provided with daily dosing of oral placebo to have the participants take 2 pills a day. |
Ongoing study (estimated study completion date: 30 April 2021) |
| Advisory on the use of HCQ as prophylaxis for SARS-CoV-2 infection issued by the Indian Council of Medical Research (ICMR) [67] |
Prophylactic use of HCQ in high-risk categories | Asymptomatic household contacts of laboratory confirmed cases: HCQ administered at a dose of 400 mg twice a day on day 1, followed by 400 mg once weekly for the next 3 weeks. All asymptomatic healthcare workers involved in containment and treatment of COVID-19 and asymptomatic healthcare workers working in non-COVID hospitals/non-COVID areas of COVID hospitals/blocks, as well as asymptomatic frontline workers: HCQ administered at a dose of 400 mg twice a day on day 1, followed by 400 mg once weekly for the next 7 weeks. |
n/a |
| Post-exposure prophylaxis (PEP) | |||
| Lee et al. [69] | PEP using HCQ was initiated after a large COVID-19 exposure event in a long-term care hospital in Korea. The study was conducted on 211 participants (189 patients and 22 healthcare workers), whose baseline PCR tests for COVID-19 were negative. |
HCQ was administrated at a dose of 400 mg/day until the completion of 14 days of quarantine. PEP was initiated within a median 58 hours (range 48–106 hours) and a median 18 hours (range 6–66 hours) after detection of the index case and the second case, respectively. PEP was completed in 184 (97.4%) patients and 21 (95.5%) healthcare workers. Median duration of PEP was 10 days (range 2–15 days). |
At the end of 14 days of quarantine, follow-up PCR tests were negative in all 211 subjects who received PEP. Serious adverse events were not reported. |
| Boulware et al. [70] | Randomized, double-blind, placebo-controlled trial conducted across the United States and parts of Canada to investigate the use of HCQ as PEP for COVID-19 in 821 asymptomatic adult participants who reported household or occupational exposure to someone with confirmed disease. The primary outcome was the incidence of either laboratory-confirmed COVID-19 or illness compatible with COVID-19 within 14 days. |
The participants were randomly assigned to receive either placebo or HCQ (800 mg once, followed by 600 mg in 6 to 8 hours, then 600 mg/day for 4 additional days) within 4 days after SARS-CoV-2 exposure. | The incidence of new illness compatible with COVID-19 did not differ significantly between participants receiving HCQ (49 of 414 [11.8%]) and those receiving placebo (58 of 407 [14.3%]). The absolute difference was −2.4 percentage points (95% CI, −7.0 to 2.2; p-value = 0.35). Serious adverse events were not reported. |
Abbreviations: bid, twice a day; CI, confidence interval; HCQ, hydroxychloroquine; n/a, not applicable; PCR, polymerase chain reaction; PEP, post-exposure prophylaxis; PrEP, pre-exposure prophylaxis; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Altogether, the aforementioned findings do not allow for definitive conclusions regarding the efficacy of HCQ as PEP for COVID-19. On the other hand, whether HCQ use as pre-exposure prophylaxis (PrEP) for COVID-19 would be effective in high-risk individuals remains to be ascertained. Several clinical trials are currently ongoing to investigate the role of HCQ as PrEP and PEP for COVID-19 in high-risk individuals [74,75]. Amongst them, the Healthcare Worker Exposure Response and Outcomes of Hydroxychloroquine (HERO-HCQ; ClinicalTrials.gov Identifier: NCT04334148) study is a large phase 3, double-blind, placebo-controlled trial involving 15,000 healthcare workers at risk of being exposed to COVID-19. In this trial, HCQ is administered 600 mg bid (loading dose) on day 1, followed by 400 mg daily on days 2–30. The primary outcome is the number of participants with COVID-19 infection at the end of the study period. Another phase 3, triple-blind, randomized, placebo-controlled trial is actively recruiting 3,000 healthcare workers, nursing home workers, first responders and bus drivers to evaluate whether the use of daily or weekly HCQ therapy can prevent SARS-CoV-2 infection and COVID-19 viremia and clinical COVID-19 (WHIP COVID-19; ClinicalTrials.gov Identifier: NCT04341441). In particular, weekly dosing of HCQ is based on the recommended dose for prophylaxis of malaria (6.5 mg/kg per dose; maximum of 400 mg per dose) administered orally once weekly on the same day of each week (Table 1). Additionally, weekly dosing may also be superior in preventing HCQ-related toxicity in light of the large volume of distribution and long terminal half-life of this drug (Figure 1).
6. Conclusion
Over the last few months, HCQ has attained the status of one of the most disputed drugs in recent years due to its widespread off-label use for the treatment of COVID-19 on the basis of low-quality evidence. Clinical evidence suggests lack of benefit from HCQ use in hospitalized patients with COVID-19. In such patients, HCQ also appears to be associated with an increased risk of QT interval prolongation and potentially lethal ventricular arrhythmias. On June 15 2020, FDA definitively revoked the EUA for emergency use of HCQ and chloroquine to treat COVID-19. On July 4 2020, WHO discontinued the HCQ treatment arm for hospitalized patients with COVID-19. Therefore, the recent experience of antimalarial drug repositioning in the era of COVID-19 showed us that even in pandemic times the use of repurposed drugs should be cautiously investigated only in randomized controlled trials, mainly to avoid drug-related toxicity and potentially life-threatening adverse events. Moreover, it is worth outlining that healthcare decision-making should be based on key ethical concepts, such as evidence-based practice, sustainable allocation and meaningful consent [76].
However, various randomized studies are currently ongoing to investigate the safety and efficacy of HCQ as early treatment of non-hospitalized patients with mild to moderate COVID-19, as well as PrEP and PEP for COVID-19. In particular, a potential role of HCQ in the prevention of SARS-CoV-2 infection is still under debate and various studies are currently underway to evaluate the efficacy of HCQ as a prophylactic strategy against COVID-19. To date, more than 2,500 studies registered on ClinicalTrials.gov are addressing different aspects of COVID-19, including intervention studies and randomized clinical trials [77]. Yet, a series of challenging issues may limit the interpretation of many ongoing trials, such as lack of control groups, limited sample size, remarkable heterogeneity of treatment effects based on the timing of administration and cluster of disease manifestations, as well as co-administration of investigational agents with multiple other therapies given at different stages of the disease [78]. In this scenario, the safety and efficacy of HCQ in the setting of prophylaxis (both PrEP and PEP) of SARS-CoV-2 infection will necessarily be established by well-designed double-blind, randomized-controlled trials, which should still be regarded as critical research tools able to yield the highest level of scientific evidence, despite the pandemic era.
7. Expert opinion
Although there is a long-standing experience with the use of HCQ as a DMARD as well as a safe and effective intervention for malaria chemoprophylaxis in endemic areas [12,45], a careful risk-benefit assessment of HCQ is critical for a cautious use of this drug during the COVID-19 pandemic, particularly outside of hospital- or clinical trial settings. Some of the most serious HCQ-related side effects include retinal toxicity [12,79], neuromyotoxicity [80–85] and cardiotoxic effects leading to potentially lethal heart rhythm disorders, such as prolonged QT interval and ventricular arrhythmias [12,86–88]. Besides excluding subjects with known hypersensitivity to HCQ or 4-aminoquinoline compounds, upcoming trials investigating the use of HCQ should also consider to exclude the enrollment of patients with underlying coexisting conditions that increase the risk of HCQ-related side effects, such as: i) patients with preexisting retinopathy/maculopathy or history or risk for macular edema [89,90]; ii) patients with preexisting cardiomyopathy, heart failure, ventricular hypertrophy, left ventricular dysfunction, coronary artery disease and/or heart rhythm disorders such as long QT syndrome [27,91–93]; iii) diabetic patients with macrovascular and/or microvascular complications [42]; iv) electrolyte abnormalities such as hypokalemia and hypomagnesemia [93]; v) family history of premature sudden cardiac death or cardiac ion channelopathies [91–93]; vi) patients with preexisting myopathy and/or neuropathy; vii) patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency [94,95], although chloroquine- and HCQ-induced hemolysis in subjects with G6PD-deficiency has not been conclusively proven [96]; viii) patients using certain concomitant QT interval-prolonging medications such as azithromycin [22,91–93]. Thus, baseline and follow-up electrocardiography to evaluate for prolonged QT interval may be advisable prior to and following the initiation of HCQ, even in clinical trial settings. It is worth specifying that high-dose and long-term use (>5–10 years) are the most important risk factors for HCQ-induced retinal toxicity [12,79]. Also, the American Academy of Ophthalmology recommends a maximum HCQ dose of ≤5 mg/kg real body weight per day to mitigate the risk of retinopathy [89]. Short-term use of HCQ will likely have negligible risk, even with doses over three times higher than usual recommended maximum dosage [79]. Given these remarks and the current inability of healthcare systems to screen large subsets of individuals for risk factors of HCQ-related side effects, ophthalmic screening may not be necessary for patients using HCQ (or chloroquine) for short-term periods [79], including those enrolled in trials investigating the role of HCQ as prophylaxis for SARS-CoV-2 infection.
Clinical evidence suggests lack of benefit from HCQ in hospitalized patients with COVID-19. Also, HCQ appears to be associated with an increased risk for life-threatening side effects. In this regard, the risk of QT interval prolongation and subsequent cardiac arrhythmias related to HCQ may further increase in the context of systemic inflammation and severe manifestations of COVID-19 [93]. Severe cases of COVID-19 often develop the so-called ‘cytokine release syndrome’ (CRS), which is a systemic inflammatory response characterized by the excessive production of several pro-inflammatory cytokines [97,98]. In turn, CRS can ultimately lead to the development of acute respiratory distress syndrome (ARDS) and multiorgan failure [98–100]. Emerging evidence suggests interleukin-6 (IL-6) as one of the main drivers of the systemic pro-inflammatory responses accompanying the CRS in COVID-19 [98]. Importantly, it has been shown that the basis for the observed clinical QT interval prolongation in the context of systemic inflammation may rely on IL-6 ability to inhibit the rapid component of the delayed rectifier K current (IKr), which is encoded by the human-ether-á-go-go-related gene (hERG) and plays a crucial role in cardiac repolarization [101]. IL-6 receptor (IL-6R) blockade is able to reverse the inhibitory effects of IL-6 on IKr [101]. Notably, antimalarial drugs, such as chloroquine, can also cause a QT interval prolongation via inhibition of IKr [102]. Altogether, these findings suggest that COVID-19-related CRS represents an independent risk factor for QT interval prolongation and consequent life-threatening arrhythmias. This risk may be significantly exacerbated by the use of antimalarial drugs such as chloroquine and HCQ, particularly when these drugs are administered in combination with other QT interval-prolonging drugs (e.g., azithromycin) and/or in presence of COVID-19-related myocardial injury and hypoxia [103,104]. These hypotheses well align with the preliminary observational findings regarding the HCQ-related risk of QT interval prolongation among hospitalized patients with COVID-19 [53–55].
On the other hand, whether HCQ use represents an effective prophylactic strategy (PrEP or PEP) against COVID-19 is a separate question that still remains to be answered through well-designed randomized-controlled trials. As previously mentioned, the postulated mechanisms by which HCQ may play a role in the prevention of SARS-CoV-2 infection include the potential ability of this drug to exert an antiviral activity either directly or indirectly by: i) interfering with the viral binding to ACE2 receptor and subsequent host cell entry, ii) inhibiting the lysosomal activity and autophagy in host cells, and iii) reducing the pro-inflammatory state which is commonly associated with several chronic conditions that negatively affect the outcomes of patients with COVID-19. However, chloroquine and HCQ may also alter the differentiation of T-helper 2 (Th2) cells by reducing interleukin-2 (IL-2) production and responsiveness, resulting in reduced Th2 cell ability to suppress inflammation in SARS-CoV-2 infection [105–107]. Hence, it cannot be ruled out that HCQ may negatively impact the immune response to SARS-CoV-2 by delaying the host antiviral response, potentially leading to an increase in the initial viral load [108]. Thus, it may be worth investigating the use of HCQ in combination with micronutrients potentially able to promote innate immune responses, such as zinc [109,110], vitamin C [111,112] and vitamin D [113–115]. In this regard, a prospective case-control study is currently evaluating the efficacy of HCQ in combination with vitamin C, vitamin D and zinc in preventing SARS-CoV-2 infection among healthcare professionals (ClinicalTrials.gov Identifier: NCT04326725). Interestingly, vitamin D deficiency has been suggested as a potential risk factor for COVID-19 infection and subsequent development of CRS [116]. Moreover, patients with SLE and RA on HCQ appear to exhibit a significantly higher risk for vitamin D deficiency compared to those who do not use HCQ [117,118].
HCQ has a large volume of distribution and a long half-life, which account for its slow onset of action and prolonged effects after drug discontinuation [12]. The use of HCQ for malaria chemoprophylaxis is recommended to start 2 weeks before travel to an endemic area. On the contrary, prevention of SARS-CoV-2 infection would require a more rapid attainment of HCQ therapeutic concentrations, supporting the rationale for the use of a loading dose in the setting of prophylaxis [21]. Notably, the window for PEP against SARS-CoV-2 infection is narrow [73,119]. In addition, conventional HCQ dosing regimens used for malaria prevention and treatment, which have been approved in some countries as empiric regimens for prophylaxis of COVID-19 in subjects at high risk for infection, may not be sufficient to reach plasma concentrations that would be expected to suppress or inhibit the replication of SARS-CoV-2.
As different studies keep on recruiting patients to investigate the use of HCQ for prophylaxis of COVID-19, a number of critical questions still need to be addressed. For example: Which are the subsets of individuals for whom benefits of HCQ use may outweigh the risks, and vice versa? What are the optimal doses (both loading and maintenance doses), timing and schedule of administration, as well as duration of treatment with HCQ that are worth to be tested, without posing serious health risks? Could the combination with other anti-inflammatory or immunomodulatory drugs be associated with a better safety and efficacy profile of HCQ?
Additional major challenges also remain to be addressed. For instance, inadequate drug stockpiling [120] may pose serious health risks to patients with rheumatic diseases for whom HCQ has demonstrated benefits, and may lead to preventable morbidity and mortality in malaria-endemic areas [68,121]. Furthermore, the shortage of medical personnel, equipment and funds caused by the current pandemic scenario in different countries may prevent the ability of healthcare systems to screen large subsets of healthy contacts for risk factors of HCQ-related side effects, such as preexisting heart rhythm disorders and G6PD deficiency.
Acknowledgments
We would like to thank Mr. Enzo Luchetti (Studio Cyan, Via Beccari Odoardo 32, 00154 Rome, Italy) for his thorough work on creating the digital figure drawing.
Funding Statement
This study was not funded.
Article highlights
Over the last few months, drug repositioning has allowed to investigate the off-label use of several drugs for prophylaxis and treatment of COVID-19, including the antimalarial drug hydroxychloroquine (HCQ).
The postulated mechanisms of action by which HCQ may play an antiviral activity against SARS-CoV-2 include the potential HCQ ability to: i) inhibit viral binding to ACE2 receptor, membrane fusion and subsequent host cell entry by interfering with the terminal glycosylation of ACE2 and viral spike S protein, ii) inhibit the fusion between the viral envelope and lysosomal or endosomal membrane by increasing the pH within cytoplasmic acidic organelles, and iii) exert anti-inflammatory and immunomodulatory effects.
Although preliminary studies showed that HCQ exerts an anti-SARS-CoV-2 activity in vitro, in vivo evidence for the efficacy of this drug as either prophylaxis or treatment of COVID-19 is lacking.
To date, clinical evidence suggests lack of benefit from HCQ use in hospitalized patients with COVID-19. In such patients, HCQ also appears to be associated with an increased risk of QT interval prolongation and potentially lethal ventricular arrhythmias.
On the other hand, the safety and efficacy of HCQ use as pre-exposure prophylaxis (PrEP) or post-exposure prophylaxis (PEP) for SARS-CoV-2 infection still remain to be established.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauchner H, Fontanarosa P.. Thinking of risk in the era of COVID-19. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 3.Leiva Sisnieguez CE, Espeche WG, Salazar MR. Arterial hypertension and the risk of severity and mortality of COVID-19. Eur Respir J. 2020;55:2001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drucker DJ. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocr Rev. 2020;41:457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sattar N, McInnes IB, McMurray JJV. Obesity a risk factor for severe COVID-19. Infection: multiple potential mechanisms. Circulation. 2020;142(1):4–6. [DOI] [PubMed] [Google Scholar]
- 6.Agrawal S, Goel AD, Gupta N. Emerging prophylaxis strategies against COVID-19. Monaldi Arch Chest Dis. 2020;90:1. [DOI] [PubMed] [Google Scholar]
- 7.Rogosnitzky M, Berkowitz E, Jadad AR. Delivering benefits at speed through real-world repurposing of off-patent drugs: the COVID-19 pandemic as a case in point. JMIR Public Health Surveill. 2020;6(2):e19199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.SS Jean, PR Hsueh. Old and re-purposed drugs for the treatment of COVID-19. Expert Rev Anti Infect Ther. 2020:1–5 . [DOI] [PMC free article] [PubMed] [Google Scholar]; • These publications provide relevant information on the latest development on pharmacologic treatments for COVID-19
- 9.Zhou D, Dai SM, Tong Q. COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]; • These publications provide relevant information on pharmacokinetics, pharmacodynamics and potential antiviral properties of hydroxychloroquine
- 10.Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. [DOI] [PubMed] [Google Scholar]; •• These publications provide relevant information on pharmacokinetics, pharmacodynamics and potential antiviral properties of hydroxychloroquine.
- 13.SE T, DJ C, RO D, et al. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989;27(6):771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler DJ, MacIntyre AC, Tett SE. Pharmacokinetics and cellular uptake of 4-aminoquinoline antimalarials. Agents Actions Suppl. 1988;24:142–157. [DOI] [PubMed] [Google Scholar]
- 15.Furst DE. Pharmacokinetics of hydroxychloroquine and chloroquine during treatment of rheumatic diseases. Lupus. 1996;5(Suppl 1):S11–5. [PubMed] [Google Scholar]
- 16.Pereira BB. Challenges and cares to promote rational use of chloroquine and hydroxychloroquine in the management of coronavirus disease 2019 (COVID-19) pandemic: a timely review. J Toxicol Environ Health B Crit Rev. 2020;23(4):177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]; • These publications provide relevant information on pharmacokinetics, pharmacodynamics and potential antiviral properties of hydroxychloroquine
- 17.Ponticelli C, Moroni G. Hydroxychloroquine in systemic lupus erythematosus (SLE). Expert Opin Drug Saf. 2017;16(3):411–419. [DOI] [PubMed] [Google Scholar]
- 18.Wahie S, Daly AK, Cordell HJ, et al. Clinical and pharmacogenetic influences on response to hydroxychloroquine in discoid lupus erythematosus: a retrospective cohort study. J Invest Dermatol. 2011;131(10):1981–1986. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naghipour S, Ghodousi M, Rahsepar S, et al. Repurposing of well-known medications as antivirals: hydroxychloroquine and chloroquine - from HIV-1 infection to COVID-19. Expert Rev Anti Infect Ther. 2020. 1–15. [DOI] [PubMed] [Google Scholar]
- 21.Al-Kofahi M, Jacobson P, Boulware DR, et al. Finding the dose for hydroxychloroquine prophylaxis for COVID-19: the desperate search for effectiveness. Clin Pharmacol Ther. 2020. DOI: 10.1002/cpt.1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanders JM, Monogue ML, Jodlowski TZ, et al. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020. DOI: 10.1001/jama.2020.6019 [DOI] [PubMed] [Google Scholar]; •• These publications provide relevant information on the latest development on pharmacologic treatments for COVID-19
- 23.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–80.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glinsky GV. Tripartite combination of candidate pandemic mitigation agents: vitamin D, quercetin, and estradiol manifest properties of medicinal agents for targeted mitigation of the COVID-19 pandemic defined by genomics-guided tracing of SARS-CoV-2 targets in human cells. Biomedicines. 2020;8:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmann M, Kleine-Weber H, Pöhlmann S. A multibasic cleavage site in the spike protein of SARS-CoV-2 is essential for infection of human lung cells. Mol Cell. 2020;78(4):779–84.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lukassen S, Chua RL, Trefzer T, et al. SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. Embo J. 2020;39(10):e105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalra RS, Tomar D, Meena AS, et al. SARS-CoV-2, ACE2, and hydroxychloroquine: cardiovascular complications, therapeutics, and clinical readouts in the current settings. Pathogens. 2020;9:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181(2):281–92.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brufsky A. Hyperglycemia, hydroxychloroquine, and the COVID-19 epidemic. J Med Virol. 2020;92:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savarino A, Boelaert JR, Cassone A, et al. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3(11):722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• These publications provide relevant information on pharmacokinetics, pharmacodynamics and potential antiviral properties of hydroxychloroquine.
- 31.Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Millet JK, Whittaker GR. Host cell proteases: critical determinants of coronavirus tropism and pathogenesis. Virus Res. 2015;202:120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perricone C, Triggianese P, Bartoloni E, et al. The anti-viral facet of anti-rheumatic drugs: lessons from COVID-19. J Autoimmun. 2020;111:102468. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• These publications provide relevant information on pharmacokinetics, pharmacodynamics and potential antiviral properties of hydroxychloroquine
- 34.Al-Bari MAA. Targeting endosomal acidification by chloroquine analogs as a promising strategy for the treatment of emerging viral diseases. Pharmacol Res Perspect. 2017;5(1):e00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotteau V, Teyton L, Peleraux A, et al. Intracellular transport of class II MHC molecules directed by invariant chain. Nature. 1990;348(6302):600–605. [DOI] [PubMed] [Google Scholar]
- 36.Kuznik A, Bencina M, Svajger U, et al. Mechanism of endosomal TLR inhibition by antimalarial drugs and imidazoquinolines. J Immunol. 2011;186(8):4794–4804. [DOI] [PubMed] [Google Scholar]
- 37.van den Borne BE, Dijkmans BA, de Rooij HH, et al. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24(1):55–60. [PubMed] [Google Scholar]
- 38.Andersson U, Ottestad W, Tracey KJ. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med. 2020;26(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang R, Chen R, Zhang Q, et al. HMGB1 in health and disease. Mol Aspects Med. 2014;40:1–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chiappetta S, Sharma AM, Bottino V, et al. COVID-19 and the role of chronic inflammation in patients with obesity. Int J Obes (Lond). 2020;44(8):1790–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Infante M, Ricordi C, Fabbri A. Antihyperglycemic properties of hydroxychloroquine in patients with diabetes: risks and benefits at the time of COVID-19 pandemic. J Diabetes. 2020. DOI: 10.1111/1753-0407.13053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pablos JL, Abasolo L, Alvaro-Gracia JM, et al. Prevalence of hospital PCR-confirmed COVID-19 cases in patients with chronic inflammatory and autoimmune rheumatic diseases. Ann Rheum Dis. 2020. DOI: 10.1136/annrheumdis-2020-217763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosenke K, Jarvis MA, Feldmann F, et al. Hydroxychloroquine proves ineffective in hamsters and macaques infected with SARS-CoV-2. bioRxiv. 2020. [Google Scholar]
- 45.Spinelli FR, Ceccarelli F, Di Franco M, et al. To consider or not antimalarials as a prophylactic intervention in the SARS-CoV-2 (Covid-19) pandemic. Ann Rheum Dis. 2020;79:666–667. [DOI] [PubMed] [Google Scholar]
- 46.Colson P, Rolain JM, Lagier JC, et al. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55(4):105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim AHJ, Sparks JA, Liew JW, et al. A rush to judgment? Rapid reporting and dissemination of results and its consequences regarding the use of hydroxychloroquine for COVID-19. Ann Intern Med. 2020;172:819–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Principi N, Esposito S. Chloroquine or hydroxychloroquine for prophylaxis of COVID-19. Lancet Infect Dis. 2020. DOI: 10.1016/S1473-3099(20)30296-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Risch HA. Early outpatient treatment of symptomatic, high-risk covid-19 patients that should be ramped-up immediately as key to the pandemic crisis. Am J Epidemiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gautret P, Lagier JC, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020. 105949. DOI: 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;382:2411–2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients with COVID-19 in New York state. JAMA. 2020;323(24):2493–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mercuro NJ, Yen CF, Shim DJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bessière F, Roccia H, Delinière A, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonow RO, Hernandez AF, Hydroxychloroquine TM, et al. Coronavirus disease 2019, and QT prolongation. JAMA Cardiol. 2020. [DOI] [PubMed] [Google Scholar]
- 56.FDA Drug Safety Communication . Safety announcement. [cited 2020 April24]. Available from: https://www.fda.gov/media/137250/download
- 57.Horby P, Landray M. Statement from the chief investigators of the randomised evaluation of COVID-19 therapy (RECOVERY) trial on hydroxychloroquine: no clinical benefit from use of hydroxychloroquine in hospitalised patients with COVID-19. [updated 2020 June5; cited 2020 July19]. Available from: https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf
- 58.FDA . Hydroxycholoquine and chloroquine letter [updated 2020 June15; cited 2020 July19]. Available from: https://www.fda.gov/media/138945/download
- 59.WHO discontinues hydroxychloroquine and lopinavir/ritonavir treatment arms for COVID-19 . [updated 2020 July4; cited 2020 July19]. Available from: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19
- 60.Göpel S, Bethge W, Martus P, et al. Test and treat COVID 65 plus - Hydroxychloroquine versus placebo in early ambulatory diagnosis and treatment of older patients with COVID19: A structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitjà O, Corbacho-Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild Covid-19: a randomized-controlled trial. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Remdesivir EUA letter of authorization. [updated 2020 May1; cited 2020 July19]. Available from: https://www.fda.gov/media/137564/download
- 63.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 - preliminary report. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2007764 [DOI] [PubMed] [Google Scholar]
- 64.Remdesivir EUA fact sheet for healthcare providers. [updated 2020 June15; cited 2020 July19]. Available form: https://www.fda.gov/media/137566/download
- 65.Diaz G, Cattelan AM, Balani B, et al. Association between concomitant hydroxychloroquine use and safety and efficacy of remdesivir in severe COVID-19 patients. Presented at Virtual COVID-19 Conference, 23rd International AIDS Conference (2020). [upated 2020 July10–11; cited 2020 July19]. Available from: https://cattendee.abstractsonline.com/meeting/9307/Presentation/3945 [Google Scholar]
- 66.Pagliano P, Piazza O, De Caro F, et al. Is hydroxychloroquine a possible post-exposure prophylaxis drug to limit the transmission to health care workers exposed to COVID19? Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.ICMR , Revised advisory on the use of hydroxychloroquine (HCQ) as prophylaxis for SARS-CoV-2 infection (in supersession of previous advisory dated 23rd March. 2020. [cited 2020 July19]. Available from: https://www.icmr.gov.in/pdf/covid/techdoc/V5_Revised_advisory_on_the_use_of_HCQ_SARS_CoV2_infection.pdf
- 68.Rathi S, Ish P, Kalantri A, et al. Hydroxychloroquine prophylaxis for COVID-19 contacts in India. Lancet Infect Dis. 2020. DOI: 10.1016/S1473-3099(20)30313-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lee SH, Son H, Peck KR. Can post-exposure prophylaxis for COVID-19 be considered as an outbreak response strategy in long-term care hospitals? Int J Antimicrob Agents. 2020;55(6):105988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020. DOI: 10.1056/NEJMoa2016638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cohen MS. Hydroxychloroquine for the prevention of Covid-19 - searching for evidence. N Engl J Med. 2020. DOI: 10.1056/NEJMe2020388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khan MS, Butler J. Hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383. [DOI] [PubMed] [Google Scholar]
- 73.Avidan MS, Dehbi HM, Delany-Moretlwe S. Hydroxychloroquine as postexposure prophylaxis for Covid-19. N Engl J Med. 2020;383. [DOI] [PubMed] [Google Scholar]
- 74.[cited 2020 July19] Available from: https://clinicaltrials.gov/(“condition or disease”: COVID; “other terms”: hydroxychloroquine, prophylaxis)
- 75.Galvis V, Spinelli FR, Tello A, et al. Hydroxychloroquine as prophylaxis for coronavirus SARS-CoV-2 infection: review of the ongoing clinical trials. Arch Bronconeumol. 2020. DOI: 10.1016/j.arbres.2020.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Aquino YSJ, Cabrera N. Hydroxychloroquine and COVID-19: critiquing the impact of disease public profile on policy and clinical decision-making. J Med Ethics. 2020. DOI: 10.1136/medethics-2020-106306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.[cited 2020 July19]. Available from: https://clinicaltrials.gov/(“condition or disease”: COVID-19)
- 78.Bauchner H, Fontanarosa PB. Randomized clinical trials and COVID-19: managing expectations. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 79.Marmor MF. COVID-19 and chloroquine/hydroxychloroquine: is there ophthalmological concern? Am J Ophthalmol. 2020. DOI: 10.1016/j.ajo.2020.03.029 [DOI] [Google Scholar]
- 80.Kwon JB, Kleiner A, Ishida K, et al. Hydroxychloroquine-induced myopathy. J Clin Rheumatol. 2010;16(1):28–31. [DOI] [PubMed] [Google Scholar]
- 81.Rothenberg RJ, Sufit RL. Drug-induced peripheral neuropathy in a patient with psoriatic arthritis. Arthritis Rheum. 1987;30(2):221–224. [DOI] [PubMed] [Google Scholar]
- 82.Stein M, Bell MJ, Ang LC. Hydroxychloroquine neuromyotoxicity. J Rheumatol. 2000;27(12):2927–2931. [PubMed] [Google Scholar]
- 83.Bolaños-Meade J, Zhou L, Hoke A, et al. Hydroxychloroquine causes severe vacuolar myopathy in a patient with chronic graft-versus-host disease. Am J Hematol. 2005;78(4):306–309. [DOI] [PubMed] [Google Scholar]
- 84.Casado E, Gratacós J, Tolosa C, et al. Antimalarial myopathy: an underdiagnosed complication? Prospective longitudinal study of 119 patients. Ann Rheum Dis. 2006;65(3):385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shukla S, Gultekin SH, Pearls SM. Oy-sters: hydroxychloroquine-induced toxic myopathy mimics Pompe disease: critical role of genetic test. Neurology. 2019;92(7):e742–e5. [DOI] [PubMed] [Google Scholar]
- 86.Chen CY, Wang FL, Lin CC. Chronic hydroxychloroquine use associated with QT prolongation and refractory ventricular arrhythmia. Clin Toxicol (Phila). 2006;44(2):173–175. [DOI] [PubMed] [Google Scholar]
- 87.Morgan ND, Patel SV, Dvorkina O. Suspected hydroxychloroquine-associated QT-interval prolongation in a patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19(5):286–288. [DOI] [PubMed] [Google Scholar]
- 88.O’Laughlin JP, Mehta PH, Wong BC. Life threatening severe QTc prolongation in patient with systemic lupus erythematosus due to hydroxychloroquine. Case Rep Cardiol. 2016;2016:4626279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Marmor MF, Kellner U, Lai TY, et al. American academy of ophthalmology. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision). Ophthalmology. 2016;123(6):1386–1394. [DOI] [PubMed] [Google Scholar]
- 90.Yusuf IH, Sharma S, Luqmani R, et al. Hydroxychloroquine retinopathy. Eye (Lond). 2017;31(6):828–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sapp JL, Alqarawi W, MacIntyre CJ, et al. Guidance on minimizing risk of drug-induced ventricular arrhythmia during treatment of COVID-19: A statement from the canadian heart rhythm society. Can J Cardiol. 2020;36:948–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kapoor A, Pandurangi U, Arora V, et al. Cardiovascular risks of hydroxychloroquine in treatment and prophylaxis of COVID-19. Patients: a scientific statement from the Indian heart rhythm society. Indian Pacing Electrophysiol J. 2020;20(3):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Roden DM, Harrington RA, Poppas A, et al. Considerations for drug interactions on QTc in exploratory COVID-19 (coronavirus disease 2019) treatment. Circulation. 2020;141(24):e906–e907. [DOI] [PubMed] [Google Scholar]
- 94.Beauverd Y, Adam Y, Assouline B, et al. COVID-19 infection and treatment with hydroxychloroquine cause severe haemolysis crisis in a patient with glucose-6-phosphate dehydrogenase deficiency. Eur J Haematol. 2020. DOI: 10.1111/ejh.13432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Capoluongo ED, Amato F, Castaldo G. The friendly use of chloroquine in the COVID-19 disease: a warning for the G6PD-deficient males and for the unaware carriers of pathogenic alterations of the G6PD gene. Clin Chem Lab Med. 2020;58:1162–1164. [DOI] [PubMed] [Google Scholar]
- 96.Afra TP, Nampoothiri RV, T M R, et al. Linking hydroxychloroquine to hemolysis in a ‘suspected’ glucose-6-phosphate dehydrogenase deficient patient with COVID-19 infection - a critical appraisal. Eur J Intern Med. 2020. DOI: 10.1016/j.ejim.2020.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science. 2020;368(6490):473–474. [DOI] [PubMed] [Google Scholar]
- 99.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zaim S, Chong JH, Sankaranarayanan V, et al. COVID-19 and multiorgan response. Curr Probl Cardiol. 2020;45:100618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aromolaran AS, Srivastava U, Alí A, et al. Interleukin-6 inhibition of hERG underlies risk for acquired long QT in cardiac and systemic inflammation. PLoS One. 2018;13(12):e0208321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Traebert M, Dumotier B, Meister L, et al. Inhibition of hERG K+ currents by antimalarial drugs in stably transfected HEK293 cells. Eur J Pharmacol. 2004;484(1):41–48. [DOI] [PubMed] [Google Scholar]
- 103.Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5(7):802–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Guastalegname M, Vallone A. Could chloroquine/hydroxychloroquine be harmful in coronavirus disease 2019 (COVID-19) treatment? Clin Infect Dis. 2020. DOI: 10.1093/cid/ciaa321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Landewé RB, Miltenburg AM, Verdonk MJ, et al. Chloroquine inhibits T cell proliferation by interfering with IL-2 production and responsiveness. Clin Exp Immunol. 1995;102(1):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liao W, Schones DE, Oh J, et al. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9(11):1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fung KL, Chan PL. Comment on: COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75(7):2016–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Knoell DL, Liu MJ. Impact of zinc metabolism on innate immune function in the setting of sepsis. Int J Vitam Nutr Res. 2010;80(4–5):271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Derwand R, Scholz M. Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine to win today’s battle against COVID-19? Med Hypotheses. 2020;142:109815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Carr AC, Maggini S. Vitamin C and Immune Function. Nutrients. 2017;9:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.RML CB, Berrill M, Marik PE. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18(2):99–101. [DOI] [PubMed] [Google Scholar]
- 113.Caprio M, Infante M, Calanchini M, et al. Vitamin D: not just the bone. Evidence for beneficial pleiotropic extraskeletal effects. Eat Weight Disord. 2017;22(1):27–41. [DOI] [PubMed] [Google Scholar]
- 114.Fabbri A, Infante M, Editorial RC. - Vitamin D status: a key modulator of innate immunity and natural defense from acute viral respiratory infections. Eur Rev Med Pharmacol Sci. 2020;24(7):4048–4052. [DOI] [PubMed] [Google Scholar]
- 115.Infante M, Ricordi C, Padilla N, et al. The role of vitamin D and Omega-3 PUFAs in Islet transplantation. Nutrients. 2019;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grant WB, Lahore H, McDonnell SL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Islam MA, Khandker SS, Alam SS, et al. Vitamin D status in patients with systemic lupus erythematosus (SLE): A systematic review and meta-analysis. Autoimmun Rev. 2019;18(11):102392. [DOI] [PubMed] [Google Scholar]
- 118.Gheita TA, Sayed S, Gheita HA, et al. Vitamin D status in rheumatoid arthritis patients: relation to clinical manifestations, disease activity, quality of life and fibromyalgia syndrome. Int J Rheum Dis. 2016;19(3):294–299. [DOI] [PubMed] [Google Scholar]
- 119.Linton NM, Kobayashi T, Yang Y, et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: a statistical analysis of publicly available case data. J Clin Med. 2020;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McElhiney LF. Pharmacies on the frontline: responding to the COVID-19 pandemic. Int J Pharm Compd. 2020;24(4):287–295. [PubMed] [Google Scholar]
- 121.Mvumbi DM. Mass intake of hydroxychloroquine or chloroquine in the present context of the Covid-19 outbreak: possible consequences in endemic malaria settings. Med Hypotheses. 2020;143:109912. [DOI] [PMC free article] [PubMed] [Google Scholar]


