Abstract
Adipose tissues are present at multiple locations in the body. Most blood vessels are surrounded with adipose tissue which is referred to as perivascular adipose tissue (PVAT). Similarly to adipose tissues at other locations, PVAT harbors many types of cells which produce and secrete adipokines and other undetermined factors which locally modulate PVAT metabolism and vascular function. Uncoupling protein-1, which is considered as a brown fat marker, is also expressed in PVAT of rodents and humans. Thus, compared to other adipose tissues in the visceral area, PVAT displays brown-like characteristics. PVAT shows a distinct function in the cardiovascular system compared to adipose tissues in other depots which are not adjacent to the vascular tree. Growing and extensive studies have demonstrated that presence of normal PVAT is required to maintain the vasculature in a functional status. However, excessive accumulation of dysfunctional PVAT leads to vascular disorders, partially through alteration of its secretome which, in turn, affects vascular smooth muscle cells (VSMC) and endothelial cells (EC). In this review, we highlight the crosstalk between PVAT and VSMC and its roles in vascular remodeling and blood pressure regulation.
Keywords: Perivascular adipose tissue, vascular smooth muscle cells, hypertension
Graphical abstract

Adipose tissue develops naturally and is required for normal physiology in mammals. It is regarded as the largest endocrine organ which produces and secretes a large number of adipokines, cytokines and chemokines into the blood, affecting the functions of other organs from a distance1. Adipose tissues at different locations are distinct in morphology and composition. The visceral and omental adipose tissues are pure WAT (white adipose tissue), while the subcutaneous adipose tissue is BeAT (beige adipose tissue) with clusters of adipocytes showing similar characteristics to classical BAT (brown adipose tissue) among the white adipocytes2. PVAT (perivascular adipose tissue) shows characteristics of BeAT in human3 and BAT-like in mice4. However, PVAT is not always BAT in mice and humans. It depends on the anatomic location and environmental/metabolic context5, 6. Excessive WAT accumulation is associated with CVD (cardiovascular diseases), hypertension, type 2 diabetes, cancers and other diseases, while BeAT and BAT might be negatively associated with CVD7–10. The association between CVD and obesity was thought to operate through endocrine roles of adipose tissue. However, PVAT (perivascular adipose tissue) is tightly adherent to most blood vessels, including the aorta and arteries such as carotid, coronary, and mesenteric arteries. The paracrine effects of PVAT on blood vessels have not received much attention for a long time until 199111. Growing data from clinical and experimental animal models indicate that PVAT is engaged in paracrine cross-talk with blood vessels and is involved in the physiological homeostasis and pathological changes of the cardiovascular system12–15. Other reviews in this series discuss the crosstalk between PVAT and other vascular cells, such as EC (endothelial cells) and immune cells. This review specifically discusses the crosstalk between PVAT and VSMC (vascular smooth muscle cells) and its roles in vascular remodeling and BP (blood pressure) regulation.
1. Developmental origins of PVAT adipocytes
1.1. UCP-1 and PPARγ are key molecules of PVAT adipocytes
The precise process and mechanisms of PVAT development remain unclear. The discovery of unique cell markers provided powerful tools to study the development of adipose tissues, including PVAT. Thoracic PVAT harbors two types of adipose progenitor cells: CD105+/CD73+/CD45-/CD140A+/CD31-, and CD105-/CD73+/CD45-/CD44+/CD90+/CD29+/CD34-, both able to differentiate into UCP-1 (uncoupling protein-1, brown adipocyte marker) positive adipocytes in vitro16, 17. PPARγ (peroxisome proliferator-activated receptor γ) is a key transcription factor for differentiation of all types of adipocytes. Disruption of PPARγ expression in adipocyte precursors will block maturation of adipose tissues. Consistently, PPARγ deletion in adipocytes results in lipodystrophy in mice18.
1.2. Adipocytes might originate from precursors in neighboring tissues.
Adipocytes from different locations may have distinct precursors. Albeit limited, experimental evidence documented that adipocytes share the same precursors with neighboring organs. For example, brown adipocytes in the interscapular area may share common progenitors with myoblasts which can be differentiated into either brown adipocytes or skeletal muscle fibroblasts19. PRDM16 (PR domain containing 16) contributes to adipogenesis as a coregulator of PPARγ. Compared to WAT in other depots, PRDM16 is highly expressed in subcutaneous WAT and BAT. Overexpression of PRDM16 in precursors of myoblasts resulted in a switch of differentiation to brown adipocyte lineage19.
1.3. PVAT adipocytes and VSMC share common progenitors
In a similar fashion that brown adipocytes in BAT and myoblasts share common progenitors, adipocytes in PVAT may share common progenitors with VSMC in the artery. Primary VSMC with overexpression of PRDM16 cultured in adipocyte differentiation cocktails had increased accumulation of lipid droplets and expression of thermogenic marker genes, suggesting a trans-differentiation to thermogenic adipocytes20. Additionally, SM22α (smooth muscle protein 22 alpha) is first expressed within the dorsal aorta at embryonic day 9.5 and continues to be expressed in VSMC into adulthood21. We generated a smooth muscle-specific Cre mouse by knocking in the Cre into the SM22α promoter and deleted floxed PPARγ in VSMC22. We found that PVAT was completely missing throughout the thoracic and abdominal aorta and the mesenteric artery. However, the adipose tissues in other locations were not affected4. Together with a recent study that showed that PVAT in mouse starts to develop after birth23, this phenotype suggests that PVAT adipocytes potentially share the same SM22α+ precursors with VSMC.
1.4. NCC and MSC are other sources of PVAT adipocytes
A more precise and recent study identified that adipocytes in PVAT from the aortic arch area belong to distinct populations. Lineage tracing indicated that adipocytes in the anterior PVAT are primarily derived from SM22α+ progenitors, whereas lateral PVAT contains both SM22α+ and Myf5+ (myogenic factor 5) cells23. An additional study found that a subset of adipocytes in the PVAT at the aortic arch area originates from NCC (neural crest cells). The NCC-derived stromal vascular fraction (SVF) can be differentiated into both brown and white adipocytes24. These studies demonstrated that PVAT adipocytes originate from SM22α+ progenitors and NCC. On the other side, PVAT harbors multipotent MSC (mesenchymal stem cells). Single-cell RNA sequencing revealed that stromal cells in thoracic PVAT express genes related to vasculature development25. Furthermore, MSC in PVAT could differentiate to the VSMC lineage26.
In summary, these studies demonstrated that VSMC, perivascular MSCs and PVAT adipocytes share common precursors. Although the literature discussed above show the contributions of various lineages to PVAT and to VSMC, there are several interesting questions that are still left unaddressed. For example, it is unknown whether PVAT from different lineages behaves any differently from one another; it is unknown whether the plasticity of PVAT lineages is involved in development of CVD such as atherosclerosis, hypertension, aortic aneurysm, etc. It was reported that adipose lineage cells in general, participate in formation of the blood vessels and enhance neovascularization in ischemic muscle in mice27. It is likely that the progenitors in PVAT could be differentiated into VSMC which may contribute to vascular remodeling, as described below.
2. Contributions of signaling from PVAT to VSMC and vascular remodeling
Neointima formation is one of the major features of vascular remodeling, especially under vascular injury conditions such as atherosclerosis or mechanical damage. As described below, the adipocytes, stem cells, immune cells and other components in PVAT, such as EV (extracellular vesicles), can produce and secrete PVAT-derived factors such as adipokines, cytokines and growth factors which regulate vascular remodeling28.
2.1. Perivascular cells and vascular remodeling
The origins of cells in the neointima remains unclear. It was reported that MSC from bone marrow contribute to neointima formation29–35. Other literature points to contributions of local VSMC to neointima formation. Injurious stimuli may change VSMC from a contractile to a synthetic phenotype, which results in migration and proliferation36–42. Recently, the contribution of resident progenitors from the adventitia or PVAT to neointima formation has also been explored. Those progenitors could migrate to the injury site where they differentiate towards vascular lineages. A study using a vein graft model in the mouse carotid artery demonstrated this possibility. Transplantation of RFP (red fluorescent protein)-labeled progenitors isolated from PVAT into the adventitial side of the vein graft significantly increased neointima formation in the graft. Interestingly, the RFP signal was found in the neointima area, and the RFP-positive cells in the neointima expressed the VSMC marker ACTA2 (alpha smooth muscle actin), indicating that progenitors in PVAT contributed to neointima formation43.
The characteristics of the progenitors in PVAT change during the aging process. Matrigel implants with embedded periaortic stem cells from young mice placed in the perivascular area of carotid arteries after ligation injury, showed that those cells differentiated into EC or myofibroblasts in the neointima. These cells also differentiated into brown-like adipocytes in the perivascular region. Importantly, co-culture of VSMC with brown adipocytes differentiated from periaortic stem cells from young mice significantly inhibited VSMC proliferation. In contrast, brown adipocytes differentiated from periaortic stem cells of old mice promoted VSMC proliferation. These results suggest that periaortic stem cells from young mice differentiate into brown-like adipocytes and inhibit neointima formation, while aged stem cells lose this capability and promote neointima hyperplasia in the injured artery25.
Additionally, as described above, periaortic PVAT may differentiate from NCC which highly express Wnt1 (wingless-type MMTV integration site family, member 1). Knockout of PPARγ mediated by Wnt1-Cre resulted in delay and dysplasia of PVAT development. Infusion of Ang II (angiotensin II) to these mice did not significantly change the SBP (systolic blood pressure) when compared to that of wild-type mice. However, Ang II infusion markedly aggravated media thickness and collagen accumulation in common carotid arteries but not the aortic arch24.
Thus, in addition to MSC from bone marrow, local VSMC and progenitors from the adventitia, resident progenitors from the PVAT contribute to neointima formation during vascular remodeling. Mechanistically, TGF-β (transforming growth factor-beta) signaling promotes differentiation of PVAT resident progenitors into VSMC since they express Tgfbr2 (transforming growth factor beta receptor 2) and Anxa1 (annexin A1). Treatment of PVAT-derived progenitors with TGFβ promoted differentiation towards VSMC43. The characteristics of those progenitors in PVAT and other signaling pathways which contribute to vascular remodeling remain unexplored. Additionally, the mechanisms underlying the enhanced Ang II-induced vascular remodeling in mice lacking PVAT in the aortic arch region due to deletion of PPARγ in NCC remain unknown. Additionally, the contributions of PVAT to vascular remodeling in disease conditions could be mediated by increased macrophages and T-lymphocytes infiltrating into a proinflammatory PVAT, which is reviewed independently in this series.
2.2. EV in PVAT and vascular remodeling
Most cell types in the cardiovascular system including EC, VSMC, macrophage and cardiomyocytes produce and release EV into the extracellular space. EV enclose biological contents derived from the original cells, and can be classified into exosomes (ranging from 30 to 100 nm), microvesicles/microparticles (ranging from 200 nm to 1 μm) and apoptotic bodies (ranging from 1 to 4 μm) according to their biogenesis and sizes. Exosomes are formed within the endosomal network and released by fusion with the plasma membrane. Microvesicles/microparticles are directly shed from the plasma membrane. Apoptotic bodies are released as blebs from cells undergoing apoptosis. EV are crucial regulators of vascular homeostasis by transferring biological messages including mRNAs, proteins, and noncoding RNAs to neighboring cells as cargo44,45. For example, EC and VSMC can communicate through the release of EV, and laminar shear stress on EC induce production of EV containing miR-143/145, which inhibits proliferation and migration of VSMC46–48.
PVAT also secretes EV which may convey paracrine signaling from PVAT to VSMC through microRNAs in EV. Obese mice secrete abundant EV containing microRNAs, which evoke inflammatory responses in PVAT and VSMC phenotypic switch from a contractile to a synthetic phenotype in aorta. VSMC can uptake EV secreted from PVAT and the encapsulated microRNAs, such as miR-221–3p, promote VSMC proliferation and migration49. It is unclear which types of cells in PVAT generate EV. Mesenchymal stem cells in adipose tissue generate EV which were found to exert effects on angiogenesis, cell survival and apoptosis, inflammation, tissue regeneration, and reduction of disease pathology50. Further studies are needed to examine the origins, characteristics and function of EV in PVAT and their potential as mediators of crosstalk with VSMC and EC.
2.3. PVAT-derived adipokines and vascular remodeling
PVAT-derived mediators such as adipokines, cytokines, ROS (reactive oxygen species) and gaseous compounds might contribute to vascular remodeling. We focus on PVAT-derived adipokines and their roles in vascular remodeling in this review.
Adiponectin is one of the most abundant adipokines normally produced and released by PVAT under physiological or pathophysiological conditions51. Neointima formation upon intravascular injury was markedly enhanced when PVAT was removed. Interestingly, neointima formation was markedly reduced by local perivascular delivery of recombinant adiponectin or transplantation of subcutaneous adipose tissue to the artery injury area52. Accordingly, neointima after injury was increased in adiponectin-deficient mice, and the conditioned medium from subcutaneous fat attenuated VSMC proliferation in response to PDGF-BB (platelet-derived growth factor-BB)52.
Leptin is another abundant adipokine in adipose tissues, including PVAT. Recombinant leptin or conditioned medium from visceral adipose tissue stimulated VSMC proliferation in vitro53. Hydrosoluble protein growth factor(s) with a molecular mass >100 kDa released from PVAT adipocytes stimulated VSMC proliferation, which is enhanced in leptin receptor-deficient obese Zucker rats54. These studies suggested that PVAT-derived leptin influences VSMC and is involved in neointima formation. Indeed, overexpression of leptin in PVAT enhanced cell proliferation and neointima formation in wild-type mice, but not in leptin receptor-deficient mice53. Neointima formation was also enhanced by transplantation of visceral adipose tissue from obese wild-type mice on a high-fat diet to the carotid artery of immune-deficient mice. Interestingly, transplantation of visceral adipose tissue from ob/ob mice could not increase neointima formation, highlighting the importance of PVAT-derived leptin on vascular remodeling53.
It has been well established that obesity is positively correlated with vascular dysfunction, partially due to inflammation and oxidative stress in adipose tissues55. However, PVAT mass is markedly increased under the obese condition. Incubation of VSMC with conditioned medium from PVAT of obese rats promoted VSMC phenotypic switch56, which was defined as any change in the normal function or structure of the differentiated VSMC57. PVAT-derived adipokines might contribute to the VSMC phenotypic switch. Obesity is associated with increased leptin expression in visceral adipose tissue as well as in PVAT53. Pretreatment with a leptin receptor antagonist inhibited obese PVAT-induced VSMC phenotypic switch56. Mechanistically, p38 MAPK (p38 mitogen-activated protein kinases) signaling pathway is involved in VSMC phenotypic switch induced by obese PVAT. Leptin receptor antagonist upregulated p38 MAPK phosphorylation which was associated with inhibition of VSMC phenotypic switch56.
The roles of the cAMP/ERK/p38 MAPK signaling pathway on PVAT-mediated VSMC proliferation was further evidenced by studies of visfatin, an adipokine preferentially expressed in PVAT, compared with subcutaneous and visceral adipose tissues. Visfatin could act as a nicotinamide phosphoribosyltransferase and biosynthesize NMN (nicotinamide mononucleotide) which mediated a proliferative response in VSMC via ERK (extracellular signal-regulated kinase) and p38 MAPK signaling pathways58.
ERK signaling also contributes to VSMC phenotypic switch mediated by the adipokine CTRP 9 (C1q/TNF-related protein 9), which is secreted by adipose tissue. CTRP 9 functions as an adipokine that reduces serum glucose levels and is down-regulated in obese mice59. Interestingly, CTRP9 enhanced VSMC differentiation mediated by hypoxia60. Systemic delivery of an adenoviral vector expressing CTRP9 or CTRP9 recombinant protein significantly reduced neointima formation61. CTRP9 could attenuate VSMC proliferation induced by PDGF-BB which was associated with an increase in cAMP levels and a decrease of ERK phosphorylation in VSMCs61, suggesting that PVAT-derived CTRP9 might inhibit neointima formation via the cAMP-ERK signaling pathway. However, direct evidence is still needed. CTRP9 also significantly restrained VSMC proliferation through suppression of the TGF-β1/ERK1/2 pathway and promoted apoptosis in response to hypoxia62, suggesting that the TGF-β1/ERK pathway in VSMC inhibits proliferative signals from PVAT. Additionally, CTRP9 treatment significantly blocked the migratory potential of VSMC by decreasing the expression of the matrix metallopeptidases MMP-2/9. PVAT inflammatory responses significantly increased expression of activated MMP-2 in PVAT which leads to increased TGF-β1 in VSMCs. This, in turn, results in VSMC proliferation62.
PVAT-derived resistin might mediate OPN (Osteopontin) expression since a resistin-neutralizing antibody could attenuate obese PVAT-induced OPN expression63. Alternatively, physiologic levels of resistin could induce a shift of VSMC proliferation to apoptosis when VSMC are co-cultured with macrophages64. Since resistin in PVAT is mostly co-localized with macrophages, questions arise on the cellular source of local resistin in PVAT and the relative contribution of local and systemic resistin to vascular remodeling. Additionally, homocysteine is a known independent risk factor for CVD. Homocysteine can induce VSMC migration through adipocyte-derived resistin65, which was associated with increased PKCε-dependent expression of MMP66. Intimal hyperplasia and VSMC dysfunction associated with resistin were also related to PKCε- dependent Nox activation and ROS generation67. Co-culture with PVAT from obese mice significantly increased OPN expression via the AP-1 signaling pathway in VSMC.
There are more adipokines secreted from PVAT than those discussed above68. However, their role in VSMC growth and physiology and the underlying mechanisms remain to be systematically investigated. Adipokines such as leptin and chemerin also mediate formation of blood vessels through stimulating EC proliferation and migration, which is discussed in another review in this series. Furthermore, inflammatory signals from PVAT regulate the functions of cells in the vascular wall including VSMC, which is specifically discussed in another review in this series.
2.4. PVAT-derived growth factors and vascular remodeling
Growth factors, including thrombospondin-1, serpin-E1, TGF-β, PDGF-BB, VEGF (vascular endothelial growth factor), bFGF (basic fibroblast growth factor), PLGF (placental growth factor), HGF (hepatocyte growth factor) and ILGFBP-3 (insulin-like growth factor-binding protein-3), have well-known effects in stimulation of VSMC proliferation and migration and have been previously reviewed69–71. PVAT adipocytes express and secrete high amounts of all those growth factors72, 73. The significance of PVAT-derived growth factors on vascular remodeling is highlighted by a study on VEGF from PVAT of type 2 diabetic patients. Conditioned medium from PVAT adipocytes significantly increased expression of VEGF-R (VEGF-receptor) 1 and 2, and VEGF secretion from VSMC, which further induced VSMC proliferation74. Further studies are needed to investigate the effects of growth factors from PVAT on VSMC survival and growth.
In summary, these studies have demonstrated that PVAT-derived factors or multiple types of cells in PVAT are actively involved in adjacent vascular remodeling under disease conditions (Figure 1). This crosstalk is critical to normal vascular function as well. However, PVAT produces and secretes a multitude of factors including adipokines, cytokines, growth factors and other factors yet undetermined. The types and mechanisms of these PVAT-derived factors on vascular remodeling remain to be further investigated. Follow up extensive studies will be needed to further understand the contribution of PVAT to vascular remodeling and elucidate the underlying mechanisms.
Figure 1. Crosstalk between PVAT and VSMC in vascular remodeling.
Smooth muscle-like cells in the neointimal area of the vascular wall originate from four sources: 1) Wnt1 positive progenitors in PVAT; 2) PVAT-resident or bone marrow-derived mesenchymal stem cells; 3) local VSMC stimulated by signaling from PVAT adipocytes and mediated by adipokines, growth factors and extracellular vesicles; and 4) adventitia-derived cells. Additionally, Wnt1-positive progenitors in PVAT and VSMC in the vascular wall can be differentiated into brown-like adipocytes in PVAT.
3. PVAT regulates vascular tone by targeting VSMC
PVAT-derived bioactive factors not only contribute to vascular remodeling but are also involved in vascular homeostasis by modulating vascular tone. Next, we summarize the recent progress regarding PVAT-derived factors on vascular tone regulation.
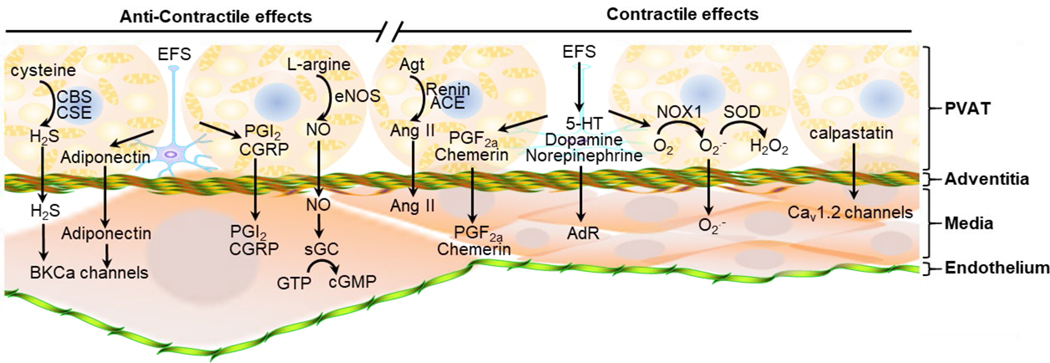
Using in vitro organ bath or wire myograph techniques, the vasoactivities of blood vessel rings from thoracic aorta, abdominal aorta, mesenteric, carotid or coronary arteries with or without PVAT (or by incubation of vessel rings with a conditioned buffer from an intact piece of PVAT or with PVAT extracts) was studied. The presence of PVAT on the vessel rings markedly attenuated the contractile response to norepinephrine (referred to as anti-contractile effect)11. PVAT’s anti-contractile effects were observed as well in response to stimulation with phenylephrine, 5-HT (serotonin) or Ang II. The anti-contractile property of PVAT was further confirmed by incubation of PVAT-free vessel rings with a PVAT-conditioned solution prepared in vitro, which produced a significant relaxation response75, 76.
Anti-contractile effects of PVAT might be mediated by uptake and/or metabolism of the vasoactive amines such as dopamine, norepinephrine, and serotonin in PVAT itself 77. PVAT adipocytes have MAO-A/B (monoamine oxidase A/B) and SSAO (semicarbazide-sensitive amine oxidase) which catalyze the metabolism of amines in PVAT77. MAO-A/B catalyzes the oxidative deamination of amines78, and SSAO is a multi-functional enzyme that promotes the generation of H2O2 and NH379. Indeed, inhibition of MAO and SSAO, or inhibition of NET (norepinephrine transporter) with nisoxetine increased vasocontraction induced by norepinephrine on vessel rings with PVAT77. Additionally, the PVAT’s anti-contractile effects are mediated by PVRF (PVAT-derived relaxing factors). Recent studies also demonstrated that PVAT secretes PVCF (PVAT-derived contracting factors), which induce blood vessel contraction4, 80–82. PVRF and PVCF influence local vascular tone through endothelium-dependent or endothelium-independent effects83, 84.
3.1. PVRF regulate anti-contractile effects through NO production by the endothelium
The anti-contractile effect of PVAT was lost in endothelium-denuded rings85, suggesting that intact-endothelium is required to induce relaxation of vessel rings. Endothelium-derived NO (nitric oxide), one of the most potent vascular dilators, stimulates guanylate cyclase in VSMCs, resulting in increase of cyclic GMP and activation of signaling cascades to induce blood vessel relaxation. PVAT expresses eNOS and produces NO83. The anti-contractile effects of PVAT on blood vessels might be mediated by PVAT-derived NO, since NOS (nitric oxide synthase) inhibition with L-NAME (Nω-nitro-L-arginine methyl ester) enhanced the phenylephrine-induced contraction in endothelial-denuded rings with PVAT83, indicating that NO might be one of the PVRF. Ang-(1–7) and leptin are PVRF which mediate anti-contractile effects in an endothelium-dependent manner since this effect was prevented by inhibition of NOS or NO scavenger85, 86. The details of endothelium-dependent anti-contractile effects of PVAT are discussed in another review in this series.
3.2. PVRF regulate anti-contractile effects through modulation of VSMC function
Inhibition of NOS attenuated the anti-contractile effects of PVAT in PVAT-intact vessel rings83, 84, 87, 88. Therefore, it is possible that PVRF, such as NO, directly target VSMC to induce vessel relaxation. Indeed, numerous studies documented that PVAT controls vascular tone through potassium channels in VSMC. Blockage of Ca2+-activated potassium channels by tetraethylammonium chloride, or inhibition of adenosine triphosphate (ATP)-dependent potassium channels by glibenclamide, or Kv7 (KCNQ) voltage-dependent potassium channels by XE991 in VSMC markedly reduced the anti-contractile effects of PVAT89–92.
H2S (hydrogen sulfide) gas is a vasodilator93, 94. Interestingly, expression of cystathionine β-synthase and cystathionine-γ-lyase, the enzymes required for H2S endogenous production in PVAT95 suggests that H2S is a PVRF. Pre-treatment with propargylglycine, an inhibitor of H2S production, significantly increased the noradrenaline-induced contraction of vessel rings with PVAT93, 96. ATP-sensitive K+ channels in VSMC might mediate the anti-contractile effects of PVAT-derived H2S93, 97, 98. Ang-(1–7) might act as a hyperpolarizing factor through K(Ca) channels as well to cause relaxation of the blood vessel86.
However, these results are based on pharmacological evidence in vitro. The specificity of these drugs in vivo is uncertain. Studies using K+ channel knockout mice suggest more complicated mechanisms behind the roles of K+ channels in PVAT’s anti-contractile effects. Kcna5−/− arteries and wild-type arteries incubated with DPO-1 (neomenthyl diphenylphosphine oxide), an inhibitor of the Kv1.5 potassium channel, showed normal vasoconstriction in response to phenylephrine in the presence and absence of PVAT. KV current density and response to inhibition by XE991, a KCNQ channel blocker, were normal in mesenteric artery VSMC isolated from Kcna5−/− mice91. Additionally, the anti-contractile effects of PVAT in Kcnq1−/− mesenteric arteries were comparable to those in wild-type mice. The anti-contractile effects of PVAT were not affected by KV 7.1 channel blockers such as chromanol 293B and HMR1556. VSMCs isolated from Kcnq1−/− mice exhibited normal peak KV currents. The KV 7.2–5 channel opener retigabine caused similar relaxation in Kcnq1−/− and wild-type vessels as well. Therefore, KV 7.1 channels were apparently not involved in the control of arterial tone by PVAT99.
It is possible that only part of them mediate PVAT’s anti-contractile effects. At least five classes of K+ channels including BKCa (large-conductance Ca2+-activated K+) channels, KCa3.1(intermediate-conductance Ca2+-activated K+) channels, KV (multiple isoforms of voltage-gated K+) channels, KATP channels, KIR (inward-rectifier K+) channels, and K2P (members of the two-pore K+) channels are expressed in VSMC100. Only inhibition of BKCa channel or knockout BKCa blocked PVAT-induced anti-contractile effects101. Additionally, PVAT from different locations (i.e., thoracic aorta, abdominal aorta or mesenteric artery) might mediate anti-contractile effects via distinct K+ channels.
Whether other mechanisms contribute to PVAT’s anti-contractile effects needs to be further investigated. Thus, for instance, it is unclear whether PVAT-derived adipokines mediate PVAT’s anti-contractile effects through modulation of VSMC function. PVAT-conditioned solution transfer studies documented that PKG (cGMP-dependent protein kinase) was necessary for PVAT’s paracrine effects on smooth muscle and endothelium of blood vessels. The anti-contractile effect of PVAT was not present in arterial rings isolated from PKG−/− mice, or arterial rings isolated from wild-type mice with inhibition of PKG signaling. Activation of PKG by ANP (atrial natriuretic peptide) rescued the loss of PVAT’s anti-contractile effects on wild-type vessel rings, but not on PKG−/− vessel rings upon hypoxia stimulation. Interestingly, adiponectin expression in PVAT adipocytes was reduced in PKG−/− mice, and ANP could not restore the reduced anti-contractile capacity of PVAT from adiponectin−/− mice. These data suggest that PVAT-derived adiponectin modulates vascular tone via PKG signaling in VSMC102.
3.3. PVCF induces contractile effects
Apart from anti-contractile effects, PVAT also induces contractile effects on VSMC. Perivascular nerve activation by EFS (electrical field stimulation) elicited a frequency-dependent contractile response in mesenteric artery rings both in the presence and absence of PVAT, but the amplitude of contraction was higher in arterial rings with PVAT than in those without PVAT103, suggesting that PVAT can release vasoconstrictors. Interestingly, Ang II treatment of mesenteric artery rings enhanced EFS-induced contraction, while arterial rings with intact PVAT treated with ACE (angiotensin-converting enzyme) inhibitor or AT1R (type 1 angiotensin II receptor) blockers showed reduced vessel contraction induced by EFS104.
PVAT’s contractile effects were further evidenced by incubation of PVAT-null vessel rings with extracts or conditioned buffer of PVAT. We found that incubation of vessel rings of aorta, mesenteric or carotid artery from mice lacking PVAT with extracts of thoracic or mesenteric PVAT, which were obtained by mincing PVAT into small pieces or homogenizing PVAT, markedly constricted the vessel rings4. Pretreatment of the blood vessel rings with AT1R blockers significantly attenuated contraction of the vessel rings induced by PVAT extracts80, demonstrating that PVAT-derived Ang II contributed to the contractile effects of PVAT. Additionally, the conditioned buffer of coronary PVAT increased the baseline tension of coronary arteries, and this effect was dependent on the amount of PVAT added to the bath81. The increase in the basal tone of the coronary artery was markedly augmented by the conditioned buffer from PVAT of obese swine compared to lean swine. Additional proof-of-principle studies demonstrated that PVAT-derived calpastatin constricts arterial rings via the Rho-kinase pathway and an increase in VSMC Ca2+ handling via Cav1.2 channels and H2O2-sensitive K+ channels81.
PVAT can release neurotransmitters to constrict blood vessels as well82. PVAT surrounding the rat thoracic aorta contains significant amounts of 5-HT. Fenfluramine increased the secretion of 5-HT and norepinephrine from PVAT. Consistently, the contraction of thoracic aortic rings with PVAT in response to fenfluramine was higher than rings without PVAT. This effect was diminished by prazosin (an α-adrenergic receptor antagonist) or nisoxetine (a norepinephrine transporter inhibitor)82. This phenotype was also observed in PVAT from the renal artery. The maximum contraction to the sympathomimetic tyramine was higher in the renal artery with PVAT versus without PVAT, and this effect was also reduced by prazosin and nisoxetine105.
In addition to 5-HT, the adipokine chemerin and its receptor ChemR23 co-localize with tyrosine hydroxylase, a sympathetic nerve marker, in mesenteric PVAT. Treatment of the vessel rings with intact PVAT with CCX832, an antagonist of ChemR23, or with prazosin blocked chemerin-9 or EFS induced vessel ring contraction106, 107.
These studies demonstrate that calpastatin, Ang II, 5-HT, chemerin and norepinephrine locally-produced in PVAT are potent PVCF which stimulate blood vessel constriction, most likely through their respective receptors on VSMC.
3.4. Potential mechanisms underlying PVAT-induced VSMC contractility
PVAT’s contractile effects might be regulated by oxidases including NADPH (nicotinamide adenine dinucleotide phosphate) oxidase, SOD (superoxide dismutase) and catalase. PVAT expresses the p67phox subunit of the NAD(P)H oxidase, Mn-SOD (superoxide dismutase), and CuZn-SOD84, 103. Stimulation of PVAT with norepinephrine increased Mn-SOD expression, decreased catalase expression, and induced O2.- generation in PVAT 108. Thus, it was hypothesized that mitochondria-derived ROS (reactive oxygen species) in PVAT modulates vascular reactivity108. Uncoupling mitochondria, as well as removal of H2O2, increased the contraction in vessel rings with PVAT present in response to norepinephrine108. EFS increased O2.- generation in isolated PVAT, and this effect was attenuated by NAD(P)H oxidase inhibition. Treatment with NAD(P)H oxidase inhibitors (apocynin and DPI) further attenuated the contraction in response to EFS in the vessel rings with PVAT than in those without PVAT, while exogenous O2.- augmented the contractile response to EFS and to phenylephrine in vessel rings without PVAT103. Therefore, PVCF mediated ROS generation in PVAT acts as a pivotal signaling molecule regulating VSMC contraction.
3.5. Bioactive factors in PVAT can act as either PVRF or PVCF
EFS elicits an anti-contractile effect on mesenteric arteries with PVAT in an endothelium-independent manner109, 110. Furthermore, sympathetic stimulation in PVAT triggers the release of adiponectin via β3-adrenoceptor activation110. PVAT also acts as a reservoir for noradrenaline, preventing it from reaching the vessel and causing contraction110. Additionally, perivascular sympathetic-sensory interactions have been shown to regulate calcitonin gene-related peptide (CGRP)-mediated vasodilation in rats via the release of methyl palmitate109. Therefore, in addition to the data discussed above showing that EFS induces VSMC contraction via ROS, stimulation of perivascular sympathetic nerves results in secretion of both PVCF and PVRF.
Actually, the same factor in PVAT can act as either PVRF or PVCF. PVAT-derived H2S acted as an anti-contractile factor in normotensive Wistar rats. However, in SHR (spontaneously hypertensive rat), the H2S in PVAT acted as a pro-contractile factor which was associated with the stimulation of perivascular nerves96 The pro-contractile effect of H2S in the arterial wall could represent a pathologic feature. Similar to H2S, PVAT-derived prostanoids such as PGE2 (prostaglandin E2) and PGI2 (prostacyclin) were responsible for anti-contractile effects of the normal PVAT84, 89, 103, 111. On the other side, PVAT-secreted thromboxane TXB2, PGE2 and PGF2α were responsible for the impairment of PVAT’s anti-contractile effects upon HFD (high fat diet) feeding112. Therefore, H2S and prostanoids in PVAT have anti-contractile effects under normal conditions, while they induce contractile effects under disease conditions, likely reflecting pathological changes in the target cells. Further studies are needed to distinguish the contributions of the cellular targets, VSMC and EC, on the contractile/anti-contractile effects of these PVAT-secreted bioactive factors.
In summary, the experimental data discussed demonstrate that PVAT produces PVRF such as H2S, Ang (1–7) and methyl palmitate, which could induce vasodilation by opening K+ channels on VSMC. PVAT also releases PVCF such as calpastatin, Ang II, 5-HT, chemerin and norepinephrine which could induce vasoconstriction by, in some cases, still underdetermined mechanisms, albeit most likely mediated by their receptors on VSMC. Thus, those PVAT-derived bioactive factors collectively and coordinately regulate vascular tone by targeting VSMC (Figure 2).
Figure 2. PVAT regulates VSMC dilation and contraction.
PVAT (perivascular adipose tissue) regulates vascular tone through relaxing factors and contractile factors. PVAT adipocytes express CBS (cystathionine β-synthase), CSE (cystathionine γ-lyase) and eNOS (endothelial nitric oxide synthase). CBS and CSE catalyze generation of H2S (hydrogen sulfide) from cysteine, which induces VSMC (vascular smooth muscle cells) dilation by opening BKCa (calcium activated K+-channels) on VSMC, and eNOS-dependent NO production from L-arginine which induces vasodilation mediated by the cGMP signaling pathway. Additionally, EFS (electrical field stimulation) induces PVAT adipocytes to release adiponectin, PGI2 and CGRP which induce vasodilation as well. On the other side, EFS induces PVAT release of 5-HT, dopamine and norepinephrine which cause VSMC contraction thorough adrenergic receptors (AdR) on VSMC. EFS also induces VSMC contraction by stimulating secretion of PGF2a, chemerin and O2.- from PVAT adipocytes. Additionally, PVAT adipocyte-derived calpastatin might induce VSMC contraction mediated by Cav1.2 channels on VSMC.
4. PVAT modulates vascular tone and BP in vivo
VSMC are the medium layer and an essential structural component of the blood vessels. VSMC play critical physiological functional roles in the regulation of vascular tone. As described above, in vitro experimental data documented that PVAT-derived factors regulate vascular tone in part by targeting VSMC. Therefore, it was hypothesized that PVAT modulates BP under physiological and/or pathophysiological conditions.
BP exhibits a robust circadian rhythm, which causes a dipping phenotype of BP between light-on and light-off periods in mice. It is unclear regarding the essential and physiological significance of the circadian rhythm and dipping pattern of BP in humans. However, in hypertension, sleep apnea, and even shift work, this balanced rhythm is perturbed113, 114. Changes in the dipping phenotype in particular, which may manifest from dippers to extreme dippers, non-dippers and reverse dippers, have been associated with adverse outocomes115–117. Lack of nocturnal BP fall (non-dipping) has been shown to be more closely associated with target organ damage and worsened cardiovascular outcome compared with essential hypertension in patients with dipping pattern118. Apart from the central circadian clock in the suprachiasmatic nucleus of the hypothalamus, a local biological clock in VSMC of the blood vessels contributes to regulation of BP circadian rhythm58, 119, 120. It was unknown if PVAT-derived factors target VSMC to regulate circadian rhythm and dipping patterns of BP.
We reported that the BP of mice lacking PVAT generated by deletion of PPARγ in VSMC was comparable with that in mice with intact PVAT during the night period (active phase in mice). However, the BP of mice lacking PVAT was markedly lower during day-time when the mice were in the resting phase, which resulted in an extreme-dipper phenotype4, 22. This phenotype might be caused by a lack of PVAT because the development of adipose tissue in other locations such as subcutaneous, gonadal, para-renal and interscapular was not affected4. Notably, another mouse model, in which PPARγ was deleted in VSMC by a transgenic SM22α Cre mouse tool, showed intact PVAT. This mouse model displayed a hypertensive phenotype121. A dominant negative PPARγ mutation (P467L) associated with hypertension in humans122, showed hypertension as well in a mouse model carrying the same mutation in VSMC120. Therefore, the hypotensive phenotype in mice lacking PVAT was most likely caused by a lack of PVAT, rather than by deficiency of PPARγ in VSMC.
PVAT regulation of the BP dipper pattern was further documented by another mouse model with Agt (angiotensinogen) deficiency in brown adipocytes80. As described above, Ang II is one of the PVCF. We found that UCP1-Cre-mediated deficiency of Agt in brown adipocytes reduced Ang II levels in thoracic PVAT, and decreased BP in the resting phase without affecting BP in the active period, which also caused an extreme-dipper BP80. However, the limitation of this mouse model is that Agt was also deleted in brown adipocytes in other locations which may affect the overall secretome from BAT. Currently, we do not have evidence that BAT-derived Ang II systemically affects BP because the plasma levels of Ang II were not reduced in brown adipocyte selective Agt deficient mice80. Additionally, we reported that PVAT has a peripheral clock. Knockout of Bmal1 in brown adipocytes of mice also reduced BP during the resting phase and we further documented that Bmal1 transcriptionally regulates Agt expression and Ang II generation in PVAT80.
Thus, while there are limitations intrinsic to each model, these results from mouse models demonstrated that paracrine effects of PVAT contribute to circadian BP regulation and may account for the yet not fully understood clinical observations associated with the dipper and non-dipper phenotypes in humans. The CVD outcomes associated with the regulation of BP dipping by PVAT, and the contributions of crosstalk between PVAT and VSMC to this phenotype should be further investigated.
5. Altered PVAT features from BAT-like to WAT-like contributes to obesity-associated hypertension
PVAT is not only involved in BP regulation under physiological conditions but also in hypertension. The results from animal studies indicated that HFD (high-fat diet) feeding significantly increased PVAT mass123, which results in white-like characteristics of PVAT. Notably, the anti-contractile effects of PVAT were attenuated under the obese condition87, 124, 125. The PVAT with changes in features from BAT-like to WAT-like might be associated with hypertension due to loss of PVAT’s anti-contractile effects. When the PVAT was removed from vascular rings, the relaxant responses to vasodilators were no different between rings isolated from obese and lean mice, suggesting that obesity did not impair the intrinsic vascular bed reactivity but rather the regulatory PVAT function126. Therefore, it is likely that PVAT’s dysfunction is related to the development of obesity-associated hypertension. Indeed, mesenteric arterial rings incubated with aortic PVAT from rats on a HFD for 6 months demonstrated lower endothelium-dependent relaxation. This effect was absent in mesenteric arterial rings incubated with aortic PVAT from rats on a standard chow diet123. The impaired anti-contractile effects of PVAT upon HFD feeding were not only dependent on the endothelium, but also consequence of reduced NO bioavailability due to L-arginine deficiency and eNOS uncoupling in white-like PVAT88, 127–129. Ex vivo L-arginine treatment and arginase inhibition could reverse obesity-induced vascular dysfunction128.
Additionally, obesity and aging cause inflammation in white-like PVAT characterized by infiltration of macrophage and dendritic cells with high expression of inflammatory adipokines and cytokines, including resistin, visfatin, leptin, MCP-1 (monocyte chemotactic protein-1), TNF-α and IL-663, 125, 130, 131, while the mRNA levels of the anti-inflammatory mediator adiponectin are decreased in obese PVAT. Depletion of dendritic cells improved the ability of PVAT to augment acetylcholine-induced vasorelaxation and anti-contractile activity132. On the other side, HFD reduction of the anti-contractile effect of PVAT was associated with reduced adiponectin secretion and AMPK (AMP-activated protein kinase) phosphorylation133. Chronic adiponectin treatment normalized NO-dependent vasorelaxation by increasing eNOS phosphorylation in mesenteric arteries of HFD-fed rats134. PVAT from AMPKα1 knockout mice had increased macrophage infiltration and significantly reduced adiponectin secretion133. Co-culture of VSMCs with PVAT adipocytes from rats on an HFD reduced the AMPK phosphorylation and increased mTOR (mammalian target of rapamycin) phosphorylation123. Aortic Rictor is an essential mTORC2 component. HFD feeding reduced Rictor gene expression in PVAT. Increased contraction and impaired dilation were found in thoracic aortic rings with PVAT from adipocyte-specific Rictor deficient mice, and inhibition of iNOS normalized vascular reactivity in aortic rings from Rictor−/− mice135. PVAT’s anti-contractile effect mimicking the obese phenotype was also lost in mice deficient in eosinophils. Eosinophil reconstitution restored PVAT’s anti-contractile effects accompanied by increased NO bioavailability and adiponectin in PVAT136.
Inflammation also stimulated generation of O2.- and H2O2 in PVAT from HFD-fed mice. Vessel rings with intact obese PVAT showed increased contraction to phenylephrine. Inactivation of O2.-, dismutation of mitochondrial-derived H2O2, or uncoupling of oxidative phosphorylation decreased phenylephrine-induced contraction in vessels with PVAT from HFD-fed mice124, 125. Consistently, SOD [Cu-Zn], peroxiredoxin-1 and adiponectin were reduced in obese humans compared to healthy subjects127. Incubation with SOD, catalase or TNF-α attenuated contractility in vessel rings presenting normal PVAT, while incubation of aortic rings containing obese PVAT with anti-TNF-α antibodies or free radical scavengers partially restored the anti-contractile effect of PVAT87, 127. Additional data from genetically modified mice further support the conclusion that inflammation and oxidative stress in PVAT altered the PVAT’s anti-contractile effects under obese conditions. PVAT from obese mice lacking TNF-α receptors in PVAT prevented H2O2 generation and vasocontraction124. Conversely, the anti-contractile function of PVAT was impaired in mice with PVAT-specific IL-18 deficiency. This was accompanied by decreased MnSOD expression in deformed mitochondria in PVAT and increased PVAT whitening 137.
Thus, brown-like PVAT could prevent inflammation and oxidative stress under physiological conditions, while white-like PVAT was accompanied by increases in inflammation and oxidative stress, and decrease of NO bioavailability under obese conditions. The alteration of brown-like PVAT to white-like PVAT might be associated with the development of hypertension under obese conditions.
6. Potential for prevention of hypertension by restoring brown-like PVAT
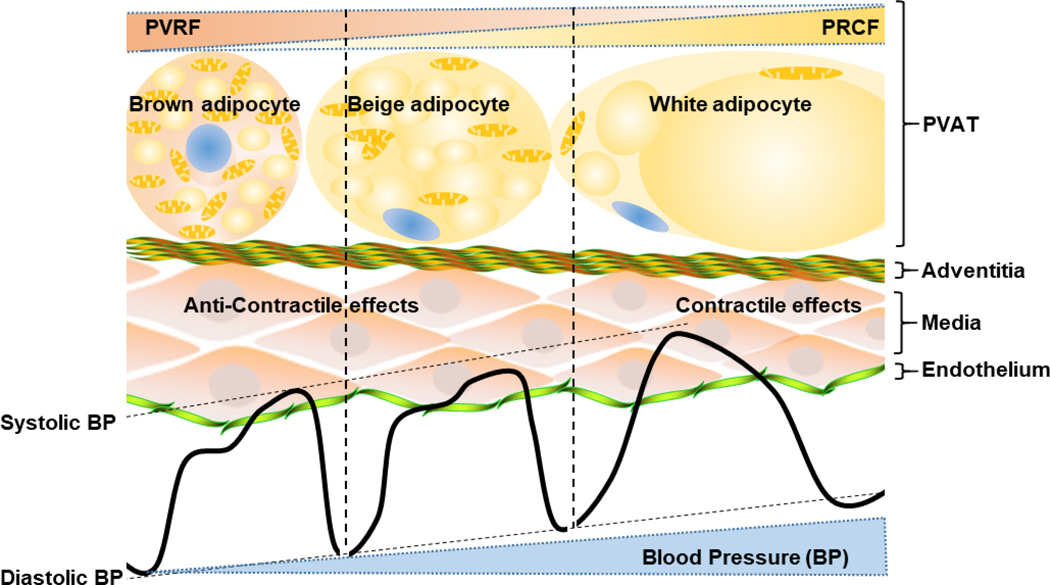
PVAT displays heterogeneity according to species and locations. The PVAT is brown-like at areas of larger size blood vessels, and is white-like at areas of the smaller size blood vessels. Compared to the same locations, the PVAT of rodents is more brown-like than that of humans. Nevertheless, there are clusters of brown-like adipocytes in PVAT, or BeAT, of both rodents and humans138. Obesity is one of the risk factors for primary hypertension. As described above, the PVAT gradually changes into white-like characteristics during development of obesity, which is associated with alterations of PVAT paracrine profiles, including those factors involved in VSMC growth, regulation of vascular tone and BP. Conservation of PVAT’s brown-like features might be a strategy to prevent development of hypertension by maintaining the homeostasis of blood vessels (Figure 3).
Figure 3. Strategy for hypertension prevention through PVAT browning.
Brown-like adipocytes in PVAT produce more PVAT-derived relaxing factors (PVRF), while white-like adipocytes produce more PVAT-derived contraction factors (PVCF). PVRF mediate anti-contractile effects of PVAT and reduce BP (blood pressure), while PVCF account for contractile effects of PVAT and increase blood pressure. Induction or maintenance of PVAT browning by drugs, but not cold stimuli, can be an efficient strategy to prevent hypertension development.
Multiple strategies including cold stimuli or growth factors such as FGF21 (fibroblast growth factor 21)139, ANP (atrial natriuretic peptide)140 and BMP (bone morphogenetic proteins) 141 are able to convert white-like WAT into BeAT through a “browning” process. It is hypothesized that browning is beneficial to prevent obesity and associated CVD. Therefore, restoring the normal beige features of human PVAT might reverse or prevent development of vascular disorders142. It is unknown whether the whitening-like process of PVAT under obese conditions results in elevation of BP. However, it is reasonable to hypothesize that restoring PVAT to brown-like characteristics could be a potential strategy for hypertension treatment.
Even though cold acclimation is the strongest stimuli to induce WAT browning process, unfortunately, cold significantly increases BP and heart rate and leads to more cardiac events143. Therefore, strategies other than cold stimuli are required to safely induce WAT or PVAT browning. Indeed, mitoNEET is a mitochondrial membrane protein that is regulated by thermogenic genes such as PGC1 (peroxisome proliferator-activated receptor-gamma coactivator 1). We reported that overexpression of mitoNEET in brown adipocytes, including PVAT adipocytes, significantly prevented arterial stiffness144 and atherosclerosis145. The Crataegus sp. extract WS® 1442 is an herbal compound that is beneficial in supporting cardiovascular function in heart failure patients146. Treatment of obese mice with WS® 1442 normalized vascular function without changing fat mass. The effect was associated with enhanced acetylation and phosphorylation levels of eNOS in PVAT147. Further studies are needed to test the effects of these factors on the PVAT browning process and the readouts on BP regulation, especially under hypertension associated with obesity. Further studies are needed to elucidate the underlying mechanisms for these observations and develop targeted therapeutics.
PERSPECTIVES AND CONCLUSIONS
There is no doubt that endocrine roles of adipose tissues, through adipokines, chemo/cytokines, hormones and other yet unknown factors, contribute systemically to many aspects of the physiology of the cardiovascular system. The dysfunction of adipose tissues, such as obesity, is one of the major risk factors for CVD. In that regard, PVAT is unique because it is adjacent and intimately integrated within the blood vessel wall and the significance of PVAT in development and prevention of CVD should not be ignored. PVAT is involved in all aspects of vascular physiology and pathophysiology. As a component of the vasculature, development and existence of PVAT is critical to maintenance of the vasculature in a normal functional status, partially through paracrine factors. Dysfunctional PVAT, such as obese PVAT or aging PVAT, secretes disease-promoting factors which cause abnormal changes towards various pathologies in the underlying layers of the blood vessels, the so-called “outside-to-inside” paradigm of PVAT effects on vascular pathologies. To comprehensively understand the vascular physiology and pathophysiology, in addition to understanding PVAT itself, more extensive and deeper research on the paracrine signaling from PVAT and its cross-talk with the underlying vascular cells, including EC, VSMC and fibroblasts under physiological and pathological conditions, is urgently needed. Adipocytes in PVAT are beige adipocytes which show mixed features of both brown and white adipocytes. Brown adipocytes are believed to promote energy expenditure and promote beneficial effects on the vascular system. There is evidence showing that PVAT is able to transform towards a fat tissue with increased white features during development of obesity and aging. Thus, reversing the white features of PVAT to brown features or maintaining PVAT beige features might be a crucial strategy to maintain a healthy vasculature. Additionally, PVAT secretes both PVCF and PVRF, especially in response to the same stimuli, suggesting that PVCF and PVRF precisely and concurrently control the blood vessel tone together with the systemic neurohumoral fluid system. The balance of PVCF and PVRF in the regulation of BP dipper pattern should be given more attention in order to develop novel strategies to maintain the physiological dipper pattern in order to prevent exacerbated adverse outcomes and CVD development. Finally, even though this review focuses on the crosstalk between PVAT and VSMC and its roles in vascular remodeling and BP regulation, the paracrine secretomes of PVAT and their roles in development of CVD should be systematically and extensively studied.
Highlights:
PVAT is a unique adipose tissue and is involved in all the aspects of vascular physiology and pathophysiology.
Existence of PVAT is critical to maintenance of the vasculature in a normal functional status, partially through paracrine factors. Dysfunctional PVAT secretes disease-promoting factors which cause abnormal changes towards various pathologies in the underlying layers of the blood vessels.
Reversing the white features of PVAT to brown characteristics or maintaining PVAT beige features might be a crucial strategy to maintain a healthy vasculature.
PVAT secretes both PVCF and PVRF, which precisely and concurrently control the blood vessel tone together with the systemic neurohumoral fluid system.
Extensive and deeper research on the paracrine signaling from PVAT and its cross talk with the underlying vascular cells, including EC, VSMC and fibroblasts under physiological and pathological conditions, is urgently needed.
Acknowledgments
a) Acknowledgments:
L.C and M.G wrote the manuscript. Y.E.C edited and approved the submission.
b) Sources of Funding: This work was supported by National Institutes of Health grants HL122664 (L.C.) and HL068878, HL134569 and HL134569 (Y.E.C,).
NONSTANDARD ABBREVIATIONS AND ACRONYMS:
- PVAT
(perivascular adipose tissue)
- WAT
(white adipose tissue)
- BeAT
(beige adipose tissue)
- BAT
(brown adipose tissue)
- CVD
(cardiovascular diseases)
- EC
(endothelial cells)
- VSMC
(vascular smooth muscle cells)
- BP
(blood pressure)
- UCP-1
(uncoupling protein-1)
- PPARγ
(peroxisome proliferator-activated receptor γ)
- PRDM16
(PR domain containing 16)
- SM22α
(smooth muscle protein 22 alpha)
- NCC
(neural crest cells)
- MSCs
(mesenchymal stem cells)
- EV
(extracellular vesicles)
- TGFβ
(transforming growth factor beta)
- Ang II
(angiotensin II)
- PDGF-BB
(platelet-derived growth factor-BB)
- p38 MAPK
(p38 mitogen-activated protein kinases)
- CTRP 9
(C1q/TNF-related protein 9)
- ERK
(extracellular signal–regulated kinase)
- PVRF
(PVAT-derived relaxing factors)
- PVCF
(PVAT-derived contracting factors)
- NO
(nitric oxide)
- NOS
(nitric oxide synthase)
- BKCa
(large-conductance Ca2+-activated K+)
- EFS
(electrical field stimulation)
- SOD
(superoxide dismutase)
- CBS
(cystathionine β-synthase)
- CSE
(cystathionine γ-lyase)
- H2S
(hydrogen sulfide)
- HFD
(high-fat diet)
Footnotes
c) Disclosures: None.
REFERENCES
- 1.Pond CM. Paracrine interactions of mammalian adipose tissue. J Exp Zool A Comp Exp Biol. 2003;295:99–110. [DOI] [PubMed] [Google Scholar]
- 2.Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerback S, Schrauwen P and Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Efremova A, Senzacqua M, Venema W, Isakov E, Di Vincenzo A, Zingaretti MC, Protasoni M, Thomski M, Giordano A and Cinti S. A large proportion of mediastinal and perirenal visceral fat of Siberian adult people is formed by UCP1 immunoreactive multilocular and paucilocular adipocytes. J Physiol Biochem. 2019; December 18[Online ahead of print]. [DOI] [PubMed] [Google Scholar]
- 4.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R and Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J and Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am J Physiol Heart Circ Physiol. 2011;301:H1425–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Padilla J, Jenkins NT, Vieira-Potter VJ and Laughlin MH. Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am J Physiol Regul Integr Comp Physiol. 2013;304:R543–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hildebrand S, Stumer J and Pfeifer A. PVAT and Its Relation to Brown, Beige, and White Adipose Tissue in Development and Function. Front Physiol. 2018;9:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thyagarajan B and Foster MT. Beiging of white adipose tissue as a therapeutic strategy for weight loss in humans. Horm Mol Biol Clin Investig. 2017;31:1–12. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez N, Moreno-Villegas Z, Gonzalez-Bris A, Egido J and Lorenzo O. Regulation of visceral and epicardial adipose tissue for preventing cardiovascular injuries associated to obesity and diabetes. Cardiovasc Diabetol. 2017;16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dam AD, Boon MR, Berbee JFP, Rensen PCN and van Harmelen V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol. 2017;816:82–92. [DOI] [PubMed] [Google Scholar]
- 11.Soltis EE and Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–296. [DOI] [PubMed] [Google Scholar]
- 12.Lian X and Gollasch M. A Clinical Perspective: Contribution of Dysfunctional Perivascular Adipose Tissue (PVAT) to Cardiovascular Risk. Curr Hypertens Rep. 2016;18:82. [DOI] [PubMed] [Google Scholar]
- 13.Gaggini M, Saponaro C and Gastaldelli A. Not all fats are created equal: adipose vs. ectopic fat, implication in cardiometabolic diseases. Horm Mol Biol Clin Investig. 2015;22:7–18. [DOI] [PubMed] [Google Scholar]
- 14.Villacorta L and Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig. 2015;21:137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE and Chang L. Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boucher JM, Robich M, Scott SS, Yang X, Ryzhova L, Turner JE, Pinz I and Liaw L. Rab27a Regulates Human Perivascular Adipose Progenitor Cell Differentiation. Cardiovasc Drugs Ther. 2018;32:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaviani M, Azarpira N, Aghdaie MH, Esfandiari E, Geramizadeh B, Nikeghbalian S and Dehghani M. Comparison of Human Mesenchymal Stem Cells Derived from Various Compartments of Human Adipose Tissue and Tunica Adventitia Layer of the Arteries Subsequent to Organ Donation. Int J Organ Transplant Med. 2019;10:65–73. [PMC free article] [PubMed] [Google Scholar]
- 18.Wang F, Mullican SE, DiSpirito JR, Peed LC and Lazar MA. Lipoatrophy and severe metabolic disturbance in mice with fat-specific deletion of PPARgamma. Proc Natl Acad Sci U S A. 2013;110:18656–18661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seale P, Bjork B, Yang W, Kajimura S, Chin S, Kuang S, Scime A, Devarakonda S, Conroe HM, Erdjument-Bromage H, Tempst P, Rudnicki MA, Beier DR and Spiegelman BM. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, Rao RR, Lou J, Lokurkar I, Baur W, Castellot JJ Jr., Rosen ED and Spiegelman BM. A smooth muscle-like origin for beige adipocytes. Cell Metab. 2014;19:810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li L, Miano JM, Cserjesi P and Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78:188–195. [DOI] [PubMed] [Google Scholar]
- 22.Chang L, Villacorta L, Zhang J, Garcia-Barrio MT, Yang K, Hamblin M, Whitesall SE, D’Alecy LG and Chen YE. Vascular smooth muscle cell-selective peroxisome proliferator-activated receptor-gamma deletion leads to hypotension. Circulation. 2009;119:2161–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye M, Ruan CC, Fu M, Xu L, Chen D, Zhu M, Zhu D and Gao P. Developmental and functional characteristics of the thoracic aorta perivascular adipocyte. Cell Mol Life Sci. 2019;76:777–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu M, Xu L, Chen X, Han W, Ruan C, Li J, Cai C, Ye M and Gao P. Neural Crest Cells Differentiate Into Brown Adipocytes and Contribute to Periaortic Arch Adipose Tissue Formation. Arterioscler Thromb Vasc Biol. 2019;39:1629–1644. [DOI] [PubMed] [Google Scholar]
- 25.Pan XX, Ruan CC, Liu XY, Kong LR, Ma Y, Wu QH, Li HQ, Sun YJ, Chen AQ, Zhao Q, Wu F, Wang XJ, Wang JG, Zhu DL and Gao PJ. Perivascular adipose tissue-derived stromal cells contribute to vascular remodeling during aging. Aging Cell. 2019;18:e12969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gu W, Ni Z, Tan YQ, Deng J, Zhang SJ, Lv ZC, Wang XJ, Chen T, Zhang Z, Hu Y, Jing ZC and Xu Q. Adventitial Cell Atlas of wt (Wild Type) and ApoE (Apolipoprotein E)-Deficient Mice Defined by Single-Cell RNA Sequencing. Arterioscler Thromb Vasc Biol. 2019;39:1055–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L and Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–663. [DOI] [PubMed] [Google Scholar]
- 28.Rajsheker S, Manka D, Blomkalns AL, Chatterjee TK, Stoll LL and Weintraub NL. Crosstalk between perivascular adipose tissue and blood vessels. Curr Opin Pharmacol. 2010;10:191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang Z, Wang A, Yuan F, Yan Z, Liu B, Chu JS, Helms JA and Li S. Differentiation of multipotent vascular stem cells contributes to vascular diseases. Nat Commun. 2012;3:875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng X, Chen M, Su W, Tao X, Sun M, Zou X, Ying R, Wei W and Wang B. The differentiation of mesenchymal stem cells to vascular cells regulated by the HMGB1/RAGE axis: its application in cell therapy for transplant arteriosclerosis. Stem Cell Res Ther. 2018;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang M, Guo Q, Huang F, Han G, Song K, Luo J, Cheng H, Hu H, Peden EK, Chen C, Mitch WE, Du J, Fu X, Truong L and Cheng J. Notch signaling in bone marrow-derived FSP-1 cells initiates neointima formation in arteriovenous fistulas. Kidney Int. 2019;95:1347–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen Q, Yang M, Wu H, Zhou J, Wang W, Zhang H, Zhao L, Zhu J, Zhou B, Xu Q and Zhang L. Genetic lineage tracing analysis of c-kit(+) stem/progenitor cells revealed a contribution to vascular injury-induced neointimal lesions. J Mol Cell Cardiol. 2018;121:277–286. [DOI] [PubMed] [Google Scholar]
- 33.Onwuka E, Best C, Sawyer A, Yi T, Heuer E, Sams M, Wiet M, Zheng H, Kyriakides T and Breuer C. The role of myeloid cell-derived PDGF-B in neotissue formation in a tissue-engineered vascular graft. Regen Med. 2017;12:249–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Wu D, Yang Y, Ji K and Gao P. Endotheliallike cells differentiated from mesenchymal stem cells attenuate neointimal hyperplasia after vascular injury. Mol Med Rep. 2016;14:4830–4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sahara M, Sata M, Morita T, Nakamura K, Hirata Y and Nagai R. Diverse contribution of bone marrow-derived cells to vascular remodeling associated with pulmonary arterial hypertension and arterial neointimal formation. Circulation. 2007;115:509–517. [DOI] [PubMed] [Google Scholar]
- 36.Mallawaarachchi CM, Weissberg PL and Siow RC. Antagonism of platelet-derived growth factor by perivascular gene transfer attenuates adventitial cell migration after vascular injury: new tricks for old dogs? FASEB J. 2006;20:1686–1688. [DOI] [PubMed] [Google Scholar]
- 37.Mallawaarachchi CM, Weissberg PL and Siow RC. Smad7 gene transfer attenuates adventitial cell migration and vascular remodeling after balloon injury. Arterioscler Thromb Vasc Biol. 2005;25:1383–1387. [DOI] [PubMed] [Google Scholar]
- 38.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S and Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;89:1111–1121. [DOI] [PubMed] [Google Scholar]
- 39.Shi Y, O’Brien JE, Fard A, Mannion JD, Wang D and Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. [DOI] [PubMed] [Google Scholar]
- 40.Yuan F, Wang D, Xu K, Wang J, Zhang Z, Yang L, Yang GY and Li S. Contribution of Vascular Cells to Neointimal Formation. PLoS One. 2017;12:e0168914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang M, Liang A, Wang Y, Jiang J and Cheng J. Smooth muscle cells from the anastomosed artery are the major precursors for neointima formation in both artery and vein grafts. Basic Res Cardiol. 2014;109:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganapathi SB, Wei SG, Zaremba A, Lamb FS and Shears SB. Functional regulation of ClC-3 in the migration of vascular smooth muscle cells. Hypertension. 2013;61:174–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu W, Nowak WN, Xie Y, Le Bras A, Hu Y, Deng J, Issa Bhaloo S, Lu Y, Yuan H, Fidanis E, Saxena A, Kanno T, Mason AJ, Dulak J, Cai J and Xu Q. Single-Cell RNA-Sequencing and Metabolomics Analyses Reveal the Contribution of Perivascular Adipose Tissue Stem Cells to Vascular Remodeling. Arterioscler Thromb Vasc Biol. 2019;39:2049–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jansen F, Nickenig G and Werner N. Extracellular Vesicles in Cardiovascular Disease: Potential Applications in Diagnosis, Prognosis, and Epidemiology. Circ Res. 2017;120:1649–1657. [DOI] [PubMed] [Google Scholar]
- 45.van der Vorst EPC, de Jong RJ and Donners M. Message in a Microbottle: Modulation of Vascular Inflammation and Atherosclerosis by Extracellular Vesicles. Front Cardiovasc Med. 2018;5:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J, Yan S, Tan H, Ma L, Feng H, Han H, Pan M, Yu L and Fang C. The miR-143/145 cluster reverses the regulation effect of KLF5 in smooth muscle cells with proliferation and contractility in intracranial aneurysm. Gene. 2018;679:266–273. [DOI] [PubMed] [Google Scholar]
- 47.Yue Y, Zhang Z, Zhang L, Chen S, Guo Y and Hong Y. miR-143 and miR-145 promote hypoxia-induced proliferation and migration of pulmonary arterial smooth muscle cells through regulating ABCA1 expression. Cardiovasc Pathol. 2018;37:15–25. [DOI] [PubMed] [Google Scholar]
- 48.Hergenreider E, Heydt S, Treguer K, Boettger T, Horrevoets AJ, Zeiher AM, Scheffer MP, Frangakis AS, Yin X, Mayr M, Braun T, Urbich C, Boon RA and Dimmeler S. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat Cell Biol. 2012;14:249–256. [DOI] [PubMed] [Google Scholar]
- 49.Li X, Ballantyne LL, Yu Y and Funk CD. Perivascular adipose tissue-derived extracellular vesicle miR-221–3p mediates vascular remodeling. FASEB J. 2019;33:12704–12722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong DE, Banyard DA, Santos PJF, Sayadi LR, Evans GRD and Widgerow AD. Adipose-derived stem cell extracellular vesicles: A systematic review(). J Plast Reconstr Aesthet Surg. 2019;72:1207–1218. [DOI] [PubMed] [Google Scholar]
- 51.Weston AH, Egner I, Dong Y, Porter EL, Heagerty AM and Edwards G. Stimulated release of a hyperpolarizing factor (ADHF) from mesenteric artery perivascular adipose tissue: involvement of myocyte BKCa channels and adiponectin. Br J Pharmacol. 2013;169:1500–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R and Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circ Res. 2009;105:906–911. [DOI] [PubMed] [Google Scholar]
- 53.Schroeter MR, Eschholz N, Herzberg S, Jerchel I, Leifheit-Nestler M, Czepluch FS, Chalikias G, Konstantinides S and Schafer K. Leptin-dependent and leptin-independent paracrine effects of perivascular adipose tissue on neointima formation. Arterioscler Thromb Vasc Biol. 2013;33:980–987. [DOI] [PubMed] [Google Scholar]
- 54.Barandier C, Montani JP and Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–1813. [DOI] [PubMed] [Google Scholar]
- 55.Stapleton PA, James ME, Goodwill AG and Frisbee JC. Obesity and vascular dysfunction. Pathophysiology. 2008;15:79–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li H, Wang YP, Zhang LN and Tian G. Perivascular adipose tissue-derived leptin promotes vascular smooth muscle cell phenotypic switching via p38 mitogen-activated protein kinase in metabolic syndrome rats. Exp Biol Med (Maywood). 2014;239:954–965. [DOI] [PubMed] [Google Scholar]
- 57.Owens GK, Kumar MS and Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. [DOI] [PubMed] [Google Scholar]
- 58.Wang P, Xu TY, Guan YF, Su DF, Fan GR and Miao CY. Perivascular adipose tissue-derived visfatin is a vascular smooth muscle cell growth factor: role of nicotinamide mononucleotide. Cardiovasc Res. 2009;81:370–380. [DOI] [PubMed] [Google Scholar]
- 59.Wong GW, Krawczyk SA, Kitidis-Mitrokostas C, Ge G, Spooner E, Hug C, Gimeno R and Lodish HF. Identification and characterization of CTRP9, a novel secreted glycoprotein, from adipose tissue that reduces serum glucose in mice and forms heterotrimers with adiponectin. FASEB J. 2009;23:241–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li YX, Run L, Shi T and Zhang YJ. CTRP9 regulates hypoxia-mediated human pulmonary artery smooth muscle cell proliferation, apoptosis and migration via TGF-beta1/ERK1/2 signaling pathway. Biochem Biophys Res Commun. 2017;490:1319–1325. [DOI] [PubMed] [Google Scholar]
- 61.Uemura Y, Shibata R, Ohashi K, Enomoto T, Kambara T, Yamamoto T, Ogura Y, Yuasa D, Joki Y, Matsuo K, Miyabe M, Kataoka Y, Murohara T and Ouchi N. Adipose-derived factor CTRP9 attenuates vascular smooth muscle cell proliferation and neointimal formation. FASEB J. 2013;27:25–33. [DOI] [PubMed] [Google Scholar]
- 62.Moe KT, Naylynn TM, Yin NO, Khairunnisa K, Allen JC, Wong MC, Chin-Dusting J and Wong P. Tumor necrosis factor-alpha induces aortic intima-media thickening via perivascular adipose tissue inflammation. J Vasc Res. 2013;50:228–237. [DOI] [PubMed] [Google Scholar]
- 63.Park SY, Kim KH, Seo KW, Bae JU, Kim YH, Lee SJ, Lee WS and Kim CD. Resistin derived from diabetic perivascular adipose tissue up-regulates vascular expression of osteopontin via the AP-1 signalling pathway. J Pathol. 2014;232:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zuniga MC, Raghuraman G and Zhou W. Physiologic levels of resistin induce a shift from proliferation to apoptosis in macrophage and VSMC co-culture. Surgery. 2018;163:906–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jiang C, Zhang H, Zhang W, Kong W, Zhu Y, Zhang H, Xu Q, Li Y and Wang X. Homocysteine promotes vascular smooth muscle cell migration by induction of the adipokine resistin. Am J Physiol Cell Physiol. 2009;297:C1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ding Q, Chai H, Mahmood N, Tsao J, Mochly-Rosen D and Zhou W. Matrix metalloproteinases modulated by protein kinase Cepsilon mediate resistin-induced migration of human coronary artery smooth muscle cells. J Vasc Surg. 2011;53:1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raghuraman G, Zuniga MC, Yuan H and Zhou W. PKCepsilon mediates resistin-induced NADPH oxidase activation and inflammation leading to smooth muscle cell dysfunction and intimal hyperplasia. Atherosclerosis. 2016;253:29–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fernandez-Alfonso MS, Somoza B, Tsvetkov D, Kuczmanski A, Dashwood M and Gil-Ortega M. Role of Perivascular Adipose Tissue in Health and Disease. Compr Physiol. 2017;8:23–59. [DOI] [PubMed] [Google Scholar]
- 69.Bowen-Pope DF, Ross R and Seifert RA. Locally acting growth factors for vascular smooth muscle cells: endogenous synthesis and release from platelets. Circulation. 1985;72:735–740. [DOI] [PubMed] [Google Scholar]
- 70.Reidy MA and Jackson CL. Factors controlling growth of arterial cells following injury. Toxicol Pathol. 1990;18:547–553. [PubMed] [Google Scholar]
- 71.Millette E, Rauch BH, Kenagy RD, Daum G and Clowes AW. Platelet-derived growth factor-BB transactivates the fibroblast growth factor receptor to induce proliferation in human smooth muscle cells. Trends Cardiovasc Med. 2006;16:25–28. [DOI] [PubMed] [Google Scholar]
- 72.Siegel-Axel DI, Ullrich S, Stefan N, Rittig K, Gerst F, Klingler C, Schmidt U, Schreiner B, Randrianarisoa E, Schaller HE, Stock UA, Weigert C, Konigsrainer A and Haring HU. Fetuin-A influences vascular cell growth and production of proinflammatory and angiogenic proteins by human perivascular fat cells. Diabetologia. 2014;57:1057–1066. [DOI] [PubMed] [Google Scholar]
- 73.Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, Kuper M, Stock UA, Staiger H, Machicao F, Schaller HE, Konigsrainer A, Haring HU and Siegel-Axel DI. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–1525. [DOI] [PubMed] [Google Scholar]
- 74.Schlich R, Willems M, Greulich S, Ruppe F, Knoefel WT, Ouwens DM, Maxhera B, Lichtenberg A, Eckel J and Sell H. VEGF in the crosstalk between human adipocytes and smooth muscle cells: depot-specific release from visceral and perivascular adipose tissue. Mediators Inflamm. 2013;2013:982458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Malinowski M, Deja MA, Janusiewicz P, Golba KS, Roleder T and Wos S. Mechanisms of vasodilatatory effect of perivascular tissue of human internal thoracic artery. J Physiol Pharmacol. 2013;64:309–316. [PubMed] [Google Scholar]
- 76.Lee MH, Chen SJ, Tsao CM and Wu CC. Perivascular adipose tissue inhibits endothelial function of rat aortas via caveolin-1. PLoS One. 2014;9:e99947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ayala-Lopez N, Thompson JM and Watts SW. Perivascular Adipose Tissue’s Impact on Norepinephrine-Induced Contraction of Mesenteric Resistance Arteries. Front Physiol. 2017;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tong JH, D’Iorio A and Kandaswami C. On the characteristics of mitochondrial monoamine oxidase in pancreas and adipose tissues from genetically obese mice. Can J Biochem. 1979;57:197–200. [DOI] [PubMed] [Google Scholar]
- 79.Boomsma F, Hut H, Bagghoe U, van der Houwen A and van den Meiracker A. Semicarbazide-sensitive amine oxidase (SSAO): from cell to circulation. Med Sci Monit. 2005;11:RA122–126. [PubMed] [Google Scholar]
- 80.Chang L, Xiong W, Zhao X, Fan Y, Guo Y, Garcia-Barrio M, Zhang J, Jiang Z, Lin JD and Chen YE. Bmal1 in Perivascular Adipose Tissue Regulates Resting-Phase Blood Pressure Through Transcriptional Regulation of Angiotensinogen. Circulation. 2018;138:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M and Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation. 2013;128:9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kumar RK, Darios ES, Burnett R, Thompson JM and Watts SW. Fenfluramine-induced PVAT-dependent contraction depends on norepinephrine and not serotonin. Pharmacol Res. 2019;140:43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Victorio JA, Fontes MT, Rossoni LV and Davel AP. Different Anti-Contractile Function and Nitric Oxide Production of Thoracic and Abdominal Perivascular Adipose Tissues. Front Physiol. 2016;7:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Awata WMC, Gonzaga NA, Borges VF, Silva CBP, Tanus-Santos JE, Cunha FQ and Tirapelli CR. Perivascular adipose tissue contributes to lethal sepsis-induced vasoplegia in rats. Eur J Pharmacol. 2019;863:172706. [DOI] [PubMed] [Google Scholar]
- 85.Galvez-Prieto B, Somoza B, Gil-Ortega M, Garcia-Prieto CF, de Las Heras AI, Gonzalez MC, Arribas S, Aranguez I, Bolbrinker J, Kreutz R, Ruiz-Gayo M and Fernandez-Alfonso MS. Anticontractile Effect of Perivascular Adipose Tissue and Leptin are Reduced in Hypertension. Front Pharmacol. 2012;3:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee RM, Lu C, Su LY and Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27:782–790. [DOI] [PubMed] [Google Scholar]
- 87.Sun X, Hou N, Han F, Guo Y, Hui Z, Du G and Zhang Y. Effect of high free fatty acids on the anti-contractile response of perivascular adipose tissue in rat aorta. J Mol Cell Cardiol. 2013;63:169–174. [DOI] [PubMed] [Google Scholar]
- 88.Zaborska KE, Wareing M, Edwards G and Austin C. Loss of anti-contractile effect of perivascular adipose tissue in offspring of obese rats. Int J Obes (Lond). 2016;40:1205–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zeng ZH, Zhang ZH, Luo BH, He WK, Liang LY, He CC and Su CJ. The functional changes of the perivascular adipose tissue in spontaneously hypertensive rats and the effects of atorvastatin therapy. Clin Exp Hypertens. 2009;31:355–363. [DOI] [PubMed] [Google Scholar]
- 90.Li R, Andersen I, Aleke J, Golubinskaya V, Gustafsson H and Nilsson H. Reduced anti-contractile effect of perivascular adipose tissue on mesenteric small arteries from spontaneously hypertensive rats: role of Kv7 channels. Eur J Pharmacol. 2013;698:310–315. [DOI] [PubMed] [Google Scholar]
- 91.Tsvetkov D, Tano JY, Kassmann M, Wang N, Schubert R and Gollasch M. The Role of DPO-1 and XE991-Sensitive Potassium Channels in Perivascular Adipose Tissue-Mediated Regulation of Vascular Tone. Front Physiol. 2016;7:335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang N, Kuczmanski A, Dubrovska G and Gollasch M. Palmitic Acid Methyl Ester and Its Relation to Control of Tone of Human Visceral Arteries and Rat Aortas by Perivascular Adipose Tissue. Front Physiol. 2018;9:583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kohn C, Schleifenbaum J, Szijarto IA, Marko L, Dubrovska G, Huang Y and Gollasch M. Differential effects of cystathionine-gamma-lyase-dependent vasodilatory H2S in periadventitial vasoregulation of rat and mouse aortas. PLoS One. 2012;7:e41951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beltowski J, Guranowski A, Jamroz-Wisniewska A, Wolski A and Halas K. Hydrogen-sulfide-mediated vasodilatory effect of nucleoside 5’-monophosphorothioates in perivascular adipose tissue. Can J Physiol Pharmacol. 2015;93:585–595. [DOI] [PubMed] [Google Scholar]
- 95.Donovan J, Wong PS, Garle MJ, Alexander SPH, Dunn WR and Ralevic V. Coronary artery hypoxic vasorelaxation is augmented by perivascular adipose tissue through a mechanism involving hydrogen sulphide and cystathionine-beta-synthase. Acta Physiol (Oxf). 2018;224:e13126. [DOI] [PubMed] [Google Scholar]
- 96.Cacanyiova S, Majzunova M, Golas S and Berenyiova A. The role of perivascular adipose tissue and endogenous hydrogen sulfide in vasoactive responses of isolated mesenteric arteries in normotensive and spontaneously hypertensive rats. J Physiol Pharmacol. 2019;70:295–306. [DOI] [PubMed] [Google Scholar]
- 97.Dongo E, Beliczai-Marosi G, Dybvig AS and Kiss L. The mechanism of action and role of hydrogen sulfide in the control of vascular tone. Nitric Oxide. 2018;81:75–87. [DOI] [PubMed] [Google Scholar]
- 98.Olson KR. Vascular actions of hydrogen sulfide in nonmammalian vertebrates. Antioxid Redox Signal. 2005;7:804–812. [DOI] [PubMed] [Google Scholar]
- 99.Tsvetkov D, Kassmann M, Tano JY, Chen L, Schleifenbaum J, Voelkl J, Lang F, Huang Y and Gollasch M. Do KV 7.1 channels contribute to control of arterial vascular tone? Br J Pharmacol. 2017;174:150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jackson WF. Potassium Channels in Regulation of Vascular Smooth Muscle Contraction and Growth. Adv Pharmacol. 2017;78:89–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH and Heagerty AM. Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am J Physiol Heart Circ Physiol. 2013;304:H786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Withers SB, Simpson L, Fattah S, Werner ME and Heagerty AM. cGMP-dependent protein kinase (PKG) mediates the anticontractile capacity of perivascular adipose tissue. Cardiovasc Res. 2014;101:130–137. [DOI] [PubMed] [Google Scholar]
- 103.Gao YJ, Takemori K, Su LY, An WS, Lu C, Sharma AM and Lee RM. Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc Res. 2006;71:363–373. [DOI] [PubMed] [Google Scholar]
- 104.Lu C, Su LY, Lee RM and Gao YJ. Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte-derived angiotensin II. Eur J Pharmacol. 2010;634:107–112. [DOI] [PubMed] [Google Scholar]
- 105.Restini CBA, Ismail A, Kumar RK, Burnett R, Garver H, Fink GD and Watts SW. Renal perivascular adipose tissue: Form and function. Vascul Pharmacol. 2018;106:37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Darios ES, Winner BM, Charvat T, Krasinksi A, Punna S and Watts SW. The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am J Physiol Heart Circ Physiol. 2016;311:H498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Watts SW, Dorrance AM, Penfold ME, Rourke JL, Sinal CJ, Seitz B, Sullivan TJ, Charvat TT, Thompson JM, Burnett R and Fink GD. Chemerin connects fat to arterial contraction. Arterioscler Thromb Vasc Biol. 2013;33:1320–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Costa RM, Filgueira FP, Tostes RC, Carvalho MH, Akamine EH and Lobato NS. H2O2 generated from mitochondrial electron transport chain in thoracic perivascular adipose tissue is crucial for modulation of vascular smooth muscle contraction. Vascul Pharmacol. 2016;84:28–37. [DOI] [PubMed] [Google Scholar]
- 109.Chang HH, Shei-Dei Yang S and Chang SJ. Perivascular adipose tissue modulation of neurogenic vasorelaxation of rat mesenteric arteries. J Cardiovasc Pharmacol. 2019;75:21–30. [DOI] [PubMed] [Google Scholar]
- 110.Saxton SN, Ryding KE, Aldous RG, Withers SB, Ohanian J and Heagerty AM. Role of Sympathetic Nerves and Adipocyte Catecholamine Uptake in the Vasorelaxant Function of Perivascular Adipose Tissue. Arterioscler Thromb Vasc Biol. 2018;38:880–891. [DOI] [PubMed] [Google Scholar]
- 111.Ozen G, Topal G, Gomez I, Ghorreshi A, Boukais K, Benyahia C, Kanyinda L, Longrois D, Teskin O, Uydes-Dogan BS and Norel X. Control of human vascular tone by prostanoids derived from perivascular adipose tissue. Prostaglandins Other Lipid Mediat. 2013;107:13–17. [DOI] [PubMed] [Google Scholar]
- 112.Lee HJ, Cantu SM, Donoso AS, Choi MR, Peredo HA and Puyo AM. Metformin prevents vascular prostanoid release alterations induced by a high-fat diet in rats. Auton Autacoid Pharmacol. 2017;37:37–43. [DOI] [PubMed] [Google Scholar]
- 113.Cugini P, Danese D, Battisti P, Di Palma L, Leone G and Kawasaki T. Usefulness of twenty-four-hour blood pressure patterns and response to short-term sodium restriction in normotensive subjects in detecting a predisposition to systemic arterial hypertension. Am J Cardiol. 1989;64:604–608. [DOI] [PubMed] [Google Scholar]
- 114.Pati P, Fulton DJ, Bagi Z, Chen F, Wang Y, Kitchens J, Cassis LA, Stepp DW and Rudic RD. Low-Salt Diet and Circadian Dysfunction Synergize to Induce Angiotensin II-Dependent Hypertension in Mice. Hypertension. 2016;67:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fagard RH. Dipping pattern of nocturnal blood pressure in patients with hypertension. Expert Rev Cardiovasc Ther. 2009;7:599–605. [DOI] [PubMed] [Google Scholar]
- 116.Najafi MT, Khaloo P, Alemi H, Jaafarinia A, Blaha MJ, Mirbolouk M, Mansournia MA, Afarideh M, Esteghamati S, Nakhjavani M and Esteghamati A. Ambulatory blood pressure monitoring and diabetes complications: Targeting morning blood pressure surge and nocturnal dipping. Medicine (Baltimore). 2018;97:e12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodrigues JCL, Amadu AM, Ghosh Dastidar A, Harries I, Burchell AE, Ratcliffe LEK, Hart EC, Hamilton MCK, Paton JFR, Nightingale AK and Manghat NE. Noctural dipping status and left ventricular hypertrophy: A cardiac magnetic resonance imaging study. J Clin Hypertens (Greenwich). 2018;20:784–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kanbay M, Turgut F, Uyar ME, Akcay A and Covic A. Causes and mechanisms of nondipping hypertension. Clin Exp Hypertens. 2008;30:585–597. [DOI] [PubMed] [Google Scholar]
- 119.Xie Z, Su W, Liu S, Zhao G, Esser K, Schroder EA, Lefta M, Stauss HM, Guo Z and Gong MC. Smooth-muscle BMAL1 participates in blood pressure circadian rhythm regulation. J Clin Invest. 2015;125:324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Halabi CM, Beyer AM, de Lange WJ, Keen HL, Baumbach GL, Faraci FM and Sigmund CD. Interference with PPAR gamma function in smooth muscle causes vascular dysfunction and hypertension. Cell Metab. 2008;7:215–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang N, Yang G, Jia Z, Zhang H, Aoyagi T, Soodvilai S, Symons JD, Schnermann JB, Gonzalez FJ, Litwin SE and Yang T. Vascular PPARgamma controls circadian variation in blood pressure and heart rate through Bmal1. Cell Metab. 2008;8:482–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK and O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. [DOI] [PubMed] [Google Scholar]
- 123.Ma L, Ma S, He H, Yang D, Chen X, Luo Z, Liu D and Zhu Z. Perivascular fat-mediated vascular dysfunction and remodeling through the AMPK/mTOR pathway in high-fat diet-induced obese rats. Hypertens Res. 2010;33:446–453. [DOI] [PubMed] [Google Scholar]
- 124.da Costa RM, Fais RS, Dechandt CRP, Louzada-Junior P, Alberici LC, Lobato NS and Tostes RC. Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice. Br J Pharmacol. 2017;174:3527–3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ketonen J, Shi J, Martonen E and Mervaala E. Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ J. 2010;74:1479–1487. [DOI] [PubMed] [Google Scholar]
- 126.Gil-Ortega M, Stucchi P, Guzman-Ruiz R, Cano V, Arribas S, Gonzalez MC, Ruiz-Gayo M, Fernandez-Alfonso MS and Somoza B. Adaptative nitric oxide overproduction in perivascular adipose tissue during early diet-induced obesity. Endocrinology. 2010;151:3299–3306. [DOI] [PubMed] [Google Scholar]
- 127.Aghamohammadzadeh R, Unwin RD, Greenstein AS and Heagerty AM. Effects of Obesity on Perivascular Adipose Tissue Vasorelaxant Function: Nitric Oxide, Inflammation and Elevated Systemic Blood Pressure. J Vasc Res. 2015;52:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, Mikhed Y, Munzel T, Daiber A, Forstermann U and Li H. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler Thromb Vasc Biol. 2016;36:78–85. [DOI] [PubMed] [Google Scholar]
- 129.Gil-Ortega M, Condezo-Hoyos L, Garcia-Prieto CF, Arribas SM, Gonzalez MC, Aranguez I, Ruiz-Gayo M, Somoza B and Fernandez-Alfonso MS. Imbalance between pro and anti-oxidant mechanisms in perivascular adipose tissue aggravates long-term high-fat diet-derived endothelial dysfunction. PLoS One. 2014;9:e95312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chen Y, Wang X, Mai J, Zhao X, Liang Y, Gu M, Chen Z, Nie R and Wang J. C-reactive protein promotes vascular endothelial dysfunction partly via activating adipose tissue inflammation in hyperlipidemic rabbits. Int J Cardiol. 2013;168:2397–2403. [DOI] [PubMed] [Google Scholar]
- 131.Bailey-Downs LC, Tucsek Z, Toth P, Sosnowska D, Gautam T, Sonntag WE, Csiszar A and Ungvari Z. Aging exacerbates obesity-induced oxidative stress and inflammation in perivascular adipose tissue in mice: a paracrine mechanism contributing to vascular redox dysregulation and inflammation. J Gerontol A Biol Sci Med Sci. 2013;68:780–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Qiu T, Li M, Tanner MA, Yang Y, Sowers JR, Korthuis RJ and Hill MA. Depletion of dendritic cells in perivascular adipose tissue improves arterial relaxation responses in type 2 diabetic mice. Metabolism. 2018;85:76–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Almabrouk TAM, White AD, Ugusman AB, Skiba DS, Katwan OJ, Alganga H, Guzik TJ, Touyz RM, Salt IP and Kennedy S. High Fat Diet Attenuates the Anticontractile Activity of Aortic PVAT via a Mechanism Involving AMPK and Reduced Adiponectin Secretion. Front Physiol. 2018;9:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sena CM, Pereira A, Fernandes R, Letra L and Seica RM. Adiponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet: role of perivascular adipose tissue. Br J Pharmacol. 2017;174:3514–3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bhattacharya I, Dragert K, Albert V, Contassot E, Damjanovic M, Hagiwara A, Zimmerli L, Humar R, Hall MN, Battegay EJ and Haas E. Rictor in perivascular adipose tissue controls vascular function by regulating inflammatory molecule expression. Arterioscler Thromb Vasc Biol. 2013;33:2105–2111. [DOI] [PubMed] [Google Scholar]
- 136.Withers SB, Forman R, Meza-Perez S, Sorobetea D, Sitnik K, Hopwood T, Lawrence CB, Agace WW, Else KJ, Heagerty AM, Svensson-Frej M and Cruickshank SM. Eosinophils are key regulators of perivascular adipose tissue and vascular functionality. Sci Rep. 2017;7:44571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li W, Jin D, Takai S, Hayakawa T, Ogata J, Yamanishi K, Yamanishi H and Okamura H. Impaired function of aorta and perivascular adipose tissue in IL-18-deficient mice. Am J Physiol Heart Circ Physiol. 2019;317:H1142–H1156. [DOI] [PubMed] [Google Scholar]
- 138.Chang L, Garcia-Barrio MT and Chen YE. Brown Adipose Tissue, Not Just a Heater. Arterioscler Thromb Vasc Biol. 2017;37:389–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E and Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessi-Fulgheri P, Zhang C, Takahashi N, Sarzani R and Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122:1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schulz TJ, Huang P, Huang TL, Xue R, McDougall LE, Townsend KL, Cypess AM, Mishina Y, Gussoni E and Tseng YH. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature. 2013;495:379–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aldiss P, Davies G, Woods R, Budge H, Sacks HS and Symonds ME. ‘Browning’ the cardiac and peri-vascular adipose tissues to modulate cardiovascular risk. Int J Cardiol. 2017;228:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bai L, Li Q, Wang J, Lavigne E, Gasparrini A, Copes R, Yagouti A, Burnett RT, Goldberg MS, Cakmak S and Chen H. Increased coronary heart disease and stroke hospitalisations from ambient temperatures in Ontario. Heart. 2018;104:673–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chang L, Zhao X, Garcia-Barrio M, Zhang J and Eugene Chen Y. MitoNEET in Perivascular Adipose Tissue Prevents Arterial Stiffness in Aging Mice. Cardiovasc Drugs Ther. 2018;32:531–539. [DOI] [PubMed] [Google Scholar]
- 145.Xiong W, Zhao X, Garcia-Barrio MT, Zhang J, Lin J, Chen YE, Jiang Z and Chang L. MitoNEET in Perivascular Adipose Tissue Blunts Atherosclerosis under Mild Cold Condition in Mice. Front Physiol. 2017;8:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Eggeling T, Regitz-Zagrosek V, Zimmermann A and Burkart M. Baseline severity but not gender modulates quantified Crataegus extract effects in early heart failure--a pooled analysis of clinical trials. Phytomedicine. 2011;18:1214–1219. [DOI] [PubMed] [Google Scholar]
- 147.Xia N, Weisenburger S, Koch E, Burkart M, Reifenberg G, Forstermann U and Li H. Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight. Br J Pharmacol. 2017;174:3443–3453. [DOI] [PMC free article] [PubMed] [Google Scholar]