Abstract
Utilization of functional ultrasound in cerebral vascular imaging is gaining popularity amongst neuroscientists. In this protocol, we describe a chronic surgical preparation method that allows longitudinal studies possible, therefore is applicable for a wide range of studies especially on aging, stroke and neurodegenerative diseases. This method can also be used with awake mice, hence the deleterious effects of anesthesia on neurovascular responses can be avoided. In addition to functional ultrasound imaging, this surgical preparation allows the researchers to take advantage of the common optical imaging methods to acquire complimentary data sets to help increase the technical rigor of the studies.
Keywords: Imaging, chronic, mouse, optical, functional ultrasound
INTRODUCTION
Functional ultrasound imaging (fUS) of the brain is a recently introduced imaging technique in the neuroscience field. Power Doppler-based fUS has shown promising results for imaging cerebral hemodynamics of the entire mouse brain with ~100 μm resolution. Microbubble tracking-based ultrasound localization microscopy has the ability to map the microvasculature of the entire brain with ~10 μm resolution. The whole rodent brain imaging capability makes fUS a great tool for obtaining macro scale information complementary to commonly used optical imaging techniques. The inclusion of macro-scale information can be critical in the study of functional changes due to aging, stroke, and neurodegenerative diseases. Chronic preparations make longitudinal studies possible and performing multimodal, repeated measurements within the same subject decreases the variability leading to higher scientific rigor. Chronic models also allow awake imaging that removes the confounds of anesthesia for functional imaging. Here we propose a chronic preparation method using polymethylpentene sealed cranial windows suitable for imaging with fUS and optical methods such as optical coherence tomography, 2-photon microscopy, intrinsic optical signal imaging and laser speckle contrast imaging for a span of three to six months.
This protocol describes the surgical preparation of chronic mouse cranial windows that is compatible with optical and fUS imaging. As opposed to most chronic window preparation protocols that employ the use of glass coverslips that are not compatible with imaging with fUS, this protocol uses a biocompatible polymer (polymethylpentene, PMP) that is suitable for both optical and fUS imaging. Since chronic preparations make longitudinal studies possible, performing multimodal and repeated measurements within the same subject is decreases the variability and can lead to higher scientific rigor. Chronic models also allow awake imaging that removes the confounds of anesthesia for functional imaging. This protocol can also be used for magnetic resonance imaging since it does not involve any metal implants. Given that all imaging methods have pros and cons, the ability to employ a diverse spectrum of them will enrich the data sets acquired from subjects. This method could be used for multimodal imaging to increase the understanding of physiological and pathological mechanisms, could allow longitudinal studies to test the effects of possible interventions or treatments, and could be coupled with behavioral testing paradigms. Since PMP is optically transparent, this method can also be used for optogenetic manipulation in a behaving animal whether or not coupled with imaging.
While this protocol emphasizes on the surgical preparation, brief information about the optical imaging and fUS imaging is also included in the descriptions.
BASIC PROTOCOL TITLE
Surgical Preparation of Mouse Chronic Cranial Windows Using PMP
Introductory paragraph
In this protocol, we describe how to prepare a mouse model for chronic imaging that is compatible with fUS imaging as well as optical imaging. This protocol includes the materials needed for preparation, surgical steps, post-operative care and training, imaging systems used by our group and example images acquired.
Materials
Subjects
Wild-type mice (C57Bl/6); (Jackson Laboratories, both sexes, 10 weeks old at the time of surgery)
Any experiments using animals must be conducted in accordance with institutional and national guidelines and regulations.
Solutions, Medicine and Ointments
Antiseptic solution (like Virex)
Isoflurane
Sterile eye ointment
Lubricant (for placement of the rectal temperature probe)
Water (to facilitate hair removal)
Hair removal cream
70% ethanol (Sterile alcohol wipes are recommended)
Betadine (Sterile betadine wipes are recommended)
Dexamethasone filled syringe (Please see the preoperative measures section for details).
Dextrose (5%) and buprenorphine filled syringe (Please see the preoperative measures section for details).
Cefazolin filled syringe (Please see the preoperative measures section for details).
Ketamine/xylazine and ketamine filled syringes (Optional. Please see the preoperative measures section for details).
Sterile saline (at 37°C and 4°C)
Surgical Supplies
Non-sterile cotton tipped applicators
Non-sterile gauze (for hair clean up)
Marker (e.g. Fine tip Sharpie)
Sterile cotton tipped applicators
Glue (Loctite 401)
Dental acrylic
Vetbond
Surgifoam (sterile) in saline
Kim wipes
Sterile glass pipettes
Bone wax
PMP sheet (50 μm thickness, Goodfellow, ME311050)
One sided razor blades
Pressurized air canister or supply.
Surgical Instruments
Sterile
One pair of Bonn scissors
Two blade handles (equipped with 11 and 15 blades)
One pair of craniotomy forceps (e.g. #2 Dumont)
One pair of soft tissue forceps (e.g. Adson)
Surgical drill bit (0.3 mm burr preferred)
Sterilize with autoclave or a hot bead sterilizer and place on a sterile metal tray.
Non-sterile
One pair of Mayo scissors
One pair of soft tissue forceps
Place these on the table, not on the sterile tray.
Miscellaneous
Oxygen tank
Isoflurane vaporizer
Homeothermic heating pad
Stereotaxic frame
Surgical drill
Bead sterilizer
Trash can
Biological waste container
20–50 ml beakers or vials (for water, saline)
Lab tape (to mark syringes)
Polyethyl ether ketone (PEEK) head plate
Protocol steps — Step annotations
Preoperative Measures
Planning and preparation decreases the time spent during surgery and risk of infection / inflammation and increase the success rate of surgery.
A. Preparation of the Surgical Room and Table
The preparation of the room and the table is important to decrease the risk of complications. Once the surgery starts the surgeon should not leave the room or the table. Otherwise, the sterility may be compromised and the monitoring of the subject would not be optimal.
Stainless surgical tables are recommended for easier and better cleaning.
Remove all the removable items from the table. If the microscope base is really heavy, that can be left in place.
Clean all the table surface with an antiseptic solution and let dry. Clean the outside of every item that will be placed on the table (stereotaxic frame, drill, bead sterilizer, etc.) with antiseptic solution.
Adjust the microscope so that height and focus is in middle of dynamic range.
Cover the heating pad with a thin, waterproof, disposable cover (plastic sheets or zipper bags can be used). This should be changed between cages.
Place a sterilized stainless steel tray on the left side of the surgeon. Place the other materials on the right side. This will prevent accidental mixing of sterile and non-sterile tools and supplies.
Turn on the bead sterilizer and the heating pad at least 30 minutes before the procedure to make sure they reach the operating temperature before the animal is anesthetized.
Check oxygen tanks and isoflurane levels. If levels are low, replace tank and replenish isoflurane in the vaporizer.
B. Anesthesia, antibiotics, anti-inflammatories
1. Dexamethasone injection
Dexamethasone is used to prevent cerebral edema (brain swelling) during and right after the surgery.
Inject dexamethasone intraperitoneally 4–6 hours before the craniotomy. Please see the table 1 for doses.
2. Ketamine/ Xylazine (K/X) anesthesia
In short surgeries / imaging sessions (up to 30 minutes) K/X anesthesia may be preferred. If the procedure takes longer than 30 minutes, a second dose may be needed. It is important to know that xylazine should be only used for the first injection, the other injections should only include ketamine.
Inject K/X as intraperitoneally. Please see the table 1 for doses.
3. Isoflurane anesthesia
Isoflurane anesthesia is recommended since the dosing is more controllable during the surgery and the recovery time is short from the anesthesia.
Induce isoflurane anesthesia induced by 3% (in 1L/min oxygen), followed by a maintenance dose of 1–1.5%.
4. Buprenorphine injection
Buprenorphine injection is recommended for pain management. Buprenorphine is diluted to have the final concentration of 0.03 mg/ml in saline.
Inject buprenorphine solution subcutaneously with the addition of 0.1 ml (per 25 g animal) 5% dextrose solution to help fight the dehydration during surgery. Please see the table 1 for doses.
5. Cefazolin injection
Since the surgical protocol includes implanting of foreign materials and dexamethasone injection, there is a slight chance that the infection risk would be increased. Cefazolin is injected as one dose before the start of the surgery to decrease this risk. Cefazolin is diluted to have the final concentration of 200 mg/ml in saline.
Inject cefazolin solution intraperitoneally. Please see the table 1 for doses.
6. Trimethioprim-Sulfametoxazole (TMP-SMX)/Ibuprofen solution
As a post-operative measure to decrease the risk of infection and inflammation, oral antibiotics and anti-inflammatories are administered. A final concentration of TMP 8mg/ml -SMX 40 mg/ml, ibuprofen 20 mg/ml is prepared in drinking water.
Administer TMP/SMX and ibuprofen solution in drinking water from 24 hours before through five days after the surgery.
C. Sterilization
All tools and supplies used, starting from the dissection of the skin, should be sterile.
Autoclave or bead sterilize the surgical tools.
Autoclave the tool tray.
Autoclave batches of Kimwipes and glass pipettes.
Autoclave saline in glass bottles (~10 ml) (Purchasing sterile 10 ml vials is recommended).
Purchase surgifoam, cotton tipped applicators and bone wax in sterile batches as heat sterilization is not recommended.
Intraoperative Measures
Induction of anesthesia
Let mouse rest in the cage rested for at least 15 minutes following the transport and weigh it before injection of dexamethasone.
4–6 hours after dexamethasone injection, induce the anesthesia with isoflurane at 3% in an induction chamber.
Place the mouse in the stereotaxic frame and decrease the anesthesia concentration to 1–1.5% for maintenance.
Note: K/X may be used for induction and can be supplemented with extra doses of ketamine through surgery or isoflurane.
Surgical procedure
- Cover the eyes of the mouse with sterile eye ointment.The application of this ointment can be repeated as often as necessary. Not only does this ointment protects the eyes from drying and keratitis, it also protects against accidental exposure to Betadine and alcohol.
- Weigh the whiskers down with some lubricant so that they are not accidentally damaged during the surgery.This is particularly critical because it can affect the somatosensory cortical organization significantly.
Place the temperature probe in the rectum using lubricant.
Inject cefazolin intraperitoneally (for doses, please check Table 1).
Inject dextrose and buprenorphine subcutaneously (for doses, please check Table 1, will be repeated after 12 hours in the presence of signs of pain).
Remove hair with water and cream leaving the cream up to 1 min on hair. Longer exposures could lead to chemical skin burns. Wipe skin with wet surgical sponges to remove the remaining hair or cream.
Clean skin with Betadine followed by alcohol swabs (this procedure is repeated three times with alternating wipes). Betadine should be completely rinsed off since it may cause the mouse to itch when dried up.
Mark the round incision borders with a sharpie. Make sure to mark the area a little bit smaller than the intended size since the skin will stretch after the cut making the incision bigger.
Cut skin with #11 blade and scissors following the markings.
Separate the sub-cutaneous tissue from the skin with scissors.
Push the remaining subcutaneous tissue aside with sterile cotton tip applicators starting from the middle and moving across the edges. Repeat this until the bone looks dry.
Scratch periosteum with #15 blade. Repeat this until there is no tissue left on the bone.
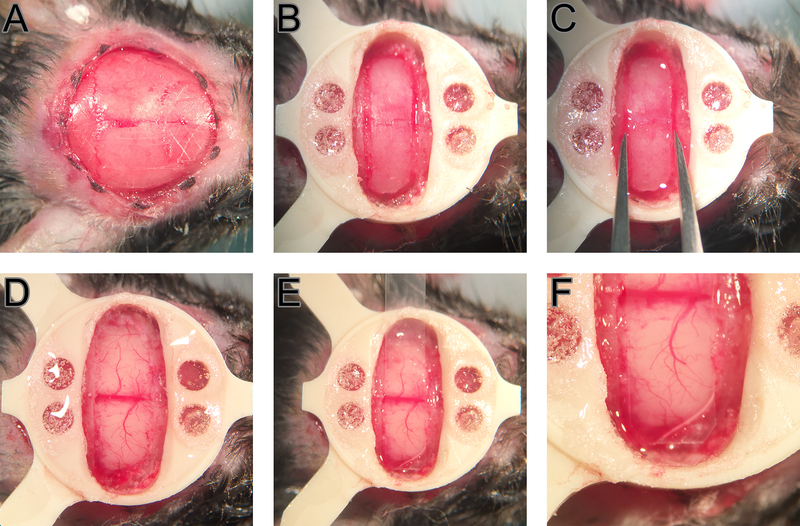
Carve a crosshatch pattern on the skull with #15 blade sparing the exposure and surroundings to improve adherence (Fig 1, A).
Dry the skull completely with pressurized air and cover with glue (Loctite 4014). To better control the glue, place a drop of glue in the middle of the skull and drag the drop to the edge of the skin. It is best to position the skin before the application of the glue. Drag the glue just until the skin edge to attach it in place. Make sure not to get glue in the eyes or on fur.
Mark the exposure using a semi-permanent marker (e.g. fine tip Sharpie). Marking the exposure on dried glue has an advantage since the marking can be cleaned with a sterile cotton tip applicator and a small amount of alcohol before it is drawn again if the surgeon wants to adjust the exposure position.
Attach the head plate with glue (Loctite 401) and dental acrylic. It will help to apply the acrylic before the glue completely dries since they will interact to yield a stronger bond.
- When the head plate is secure in its place, drill the bone on the exposure (Fig 1, B).Use pressurized air intermittently to remove bone dust It is important to drill slowly and softly not to heat the bone in which case the inflammatory processes could be activated. The drill bit can be dipped in chilled saline to avoid excessive heating. Bone can be wiped with chilled saline prior to removal.In case of bleeding from the bone, sterile sponges in saline and bone wax can be utilized. Stop drilling until the bleeding is completely under control. Periodically check the thickness of the craniotomy edges by pressing gently on the edges of the bone flap.
Prior to removal of the bone, a drop of sterile saline at 37°C is put on the exposure site to help rehydrate and “soften” the drilled bone.
- Using a pair of craniotomy forceps, hold the bone flap at two edges close to the midline. Gently pull anterior then posterior repeating the motion several times until the bone releases the dura and the underlying vessels (Fig 1, C).It is important to take the time to avoid bleeding. At this point a piece of saline soaked gel foam may be used to keep the dura moist and remove small residues of bleeding and clots.
- Cut a PMP strip using #11 blade.Use a clean razorblade on its side as a guide. The strips should be cut with at least a cm excess in length to facilitate the handling with forceps without touching the part that will end up on the craniotomy. You can prepare multiple strips of slightly different sizes ahead of time and choose the right one by comparing the width to the removed bone flap.
When all the bleeding is under control, place the PMP strip on the exposure. Start fixing the polymer with Vetbond at one end and move to the other step by step (Fig 1, E and F). Get small drops of glue on a beveled sterile wood applicator or a sterile blunt needle for this application. In order to minimize the contact of Vetbond with brain, apply the glue on the bone 0.5–1 mm away from the edge of the craniotomy line and slowly and controllably drag the glue to the adhesion site.
Push polymer strip down gently while attaching so that it would be inside the craniotomy lying against the brain with minimal gap.
After passing the midline, trim the excess part of the polymer and complete the attachment.
Reinforce edges with dental acrylic. Acrylic should be applied to cover the edges of the polymer and the attachment is extended until the inner edges of the headplate is also thinly covered.
Inject the animal 0.1 ml of 5% dextrose subcutaneously for every 2 hours if the surgery takes longer than 2 hours (optional).
Wean the mouse from anesthesia when glue and acrylic are completely dry.
Return the mouse to the cage and place the cage on a heating pad. Monitor the mouse for a few hours before returning to the vivarium. Mouse should be standing up in 10 minutes and eating / drinking / walking in 1 hour after a successful surgery. Mice should be housed singly since the PEEK head plate or the coverings can be damaged by a cage mate. If metal head plates and metal protective covers for PMP is used, mice could be housed with cage mates.
Figure 1.
Surgical steps. A crosshatch pattern is carved on the skull anterior to the bregma and posterior to the lambda (A). A PEEK head plate is attached and craniotomy borders are drilled (B). Bone flap is “loosened” by grabbing form the long edges and repetitively moving anterior and posteriorly (C). Minor dural bleedings are controlled using sterile saline and surgifoam (D). PMP is positioned to fit inside the craniotomy fully leaving minimum gap between the brain and the polymer (E and F).
Postoperative Measures (up to 14 days)
1. Monitoring
Supply TMP-SMX/Ibuprofen in drinking water for 5 days.
If there are signs of pain (1), give additional buprenorphine injections.
It is also helpful to give some softened pellets of food immersed in TMP-SMX/Ibuprofen drinking water, or gel diet to feed and hydrate the mouse.
2. Training
Detailed procedures are described in Desjardins M., Kılıç K. et al. Supplemental Methods.
Handle the mouse until calm and train to sit still in the cradle for up to 1 hour with increasing duration (e.g. 15 mins, 30 mins, 45 mins, 1 hour).
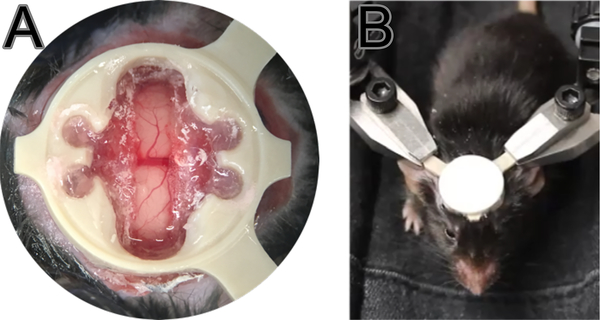
On imaging days, once the mouse is in the imaging cradle (Fig 2,B) fix the head quickly and leave the mouse to rest for 5 minutes before moving the cradle under the microscope or ultrasound.
Reward the mouse with a drop of sweetened condensed milk using a plastic pipette every 15 minutes (or however it is experimentally applicable and approved by local animal use committee).
End the session if the mouse shows signs of discomfort/anxiety since extreme, uncontrolled movement can lead to the mouse detaching its head plate.
Figure 2.
Monitoring and training. Successful surgeries should have a clear craniotomy (A) as blurry and opaque looking would suggest infection and/or inflammation. Position of the mouse in cradle (B). Mouse is trained to stay relaxed and still in the cradle with the help of sweetened condensed milk treat. Note that the imaging site is protected with a cap except for imaging sessions.
Imaging (After day 14)
Injection of intravenous contrast agents
Inject contrast agents (e.g. 0.05 ml FITC-dextran at 5% in PBS or 0.03 ml commercial microbubble suspension (5.0–8.0× 108 microbubbles per ml, Optison, GE Healthcare) retro-orbitally using a tuberculin syringe 31 G, 0.5” needle. Take care not to scratch the orbital fossa since this would be painful for mouse when it wakes up.
Awake Imaging
Allow the mouse to wake up and recover completely from anesthesia (15–60 minutes) if a retro-orbital injection is performed. Handle the mouse until calm and bring it under the imaging system five minutes after the head plate is fixed. Reward the mouse with a drop of sweetened condensed milk using a plastic pipette every 15 minutes (or however it is experimentally applicable and approved by local animal use committee). Session should not be over 60–90 minutes since it would stress the mouse. End the imaging session if the mouse if it is moving more than usual and does not accept condensed milk. Return the mouse its cage and the cage to vivarium after the imaging session is over.
Ultrasound Imaging
We use clear agarose phantom liquid (~38–40 oC) to fill the cranial window and it will be solidified after around 2–3 mins. A water container with an opening bottom but covered with a cling wrap membrane to prevent leakage is mounted on top of the solidified agarose phantom. A layer of ultrasound gel is applied between the food wrap membrane and the agarose phantom for acoustic transmission. The ultrasound probe is put inside in the container which has warm water (~36–37 oC) circulated through for ultrasound transmission to the transducer surface.
The ultrasound signal was acquired with a commercial ultrafast ultrasound imaging system (Vantage 256, Verasonics Inc. Kirkland, WA, USA) and a linear ultrasonic probe (L22–14v, Verasonics Inc. Kirkland, WA, USA). The Vantage 256 system has 256 parallelized emission and receiving channels, and can acquire planar images at a frame rate up to 30 kHz when the imaging depth is ~15 mm. The L22–14v ultrasonic probe has 128 transducer elements with a pitch of 0.1 mm and a center frequency of 18.5 MHz with a bandwidth of 12.4 MHz (67%, −6 dB). It has an elevation focus at z=6 mm. The data were acquired with 5 tilted ultrasound plane wave emission angles (–6, –3, 0, 3, 6) and coherently added to form a compounded ultrasound image at a frame rate of 5 KHz. With the multi-angle coherent compounding emission and acquisition, the contrast-to-noise ration of the compounded ultrasound image can be enhanced by 4–5 times while preserving very high frame rate.
The ultrasound localization microscopy (ULM) images and the ULM-based velocity maps (vULM) were obtained based on a microbubble tracking and accumulation method described Errico et al and Song et al. (2,3). Briefly, a frame-to-frame subtraction was applied to the data to get the dynamic microbubble signal. The images of the microbubble were rescaled to have a pixel size of 10 μm × 10 μm. The centroid position for each microbubble was then identified with 10 μm precision by deconvolving the system point spread function. By accumulating the centroid positions over time, a high resolution image of the cerebral vasculature image (ULM) is obtained. Further, by identifying and tracking the same microbubble’s position, the in-plane flow velocity of the microbubble can be calculated based on the travel distance and the imaging frame rate. The final velocity for coordinates consists of descending and ascending flows, and the speed for each direction was obtained by averaging the same directional flow speed at all time points when the absolute value was greater than 0, respectively.
Euthanasia
If the animal is distressed or the exposure is not imageable, perform euthanasia in accordance with animal use committee guidelines.
Sample Data
In this section, we present some data collected from animals that are prepared in accordance to the described surgical protocol. We have acquired images with optical imaging methods and ultrasound imaging. 2-photon angiography images were acquired after the intravenous injection of FITC-Dextran (2MDa, Sigma, 0.05 cc, 5% in PBS) with a resolution of 512×512 using a commercial microscope (Bruker Investigator, WI, USA). OCT images were acquired with a commercial system (Thorlabs Telesto III 1310 nm center wavelength, bandwidth 170 nm). Laser speckle imaging system (4) and intrinsic optical imaging system were custom built (5). The ultrasound signal was acquired with a commercial ultrafast ultrasound imaging system (Vantage 256, Verasonics Inc. Kirkland, WA, USA) and a linear ultrasonic probe (L22–14v, Verasonics Inc. Kirkland, WA, USA)(6).
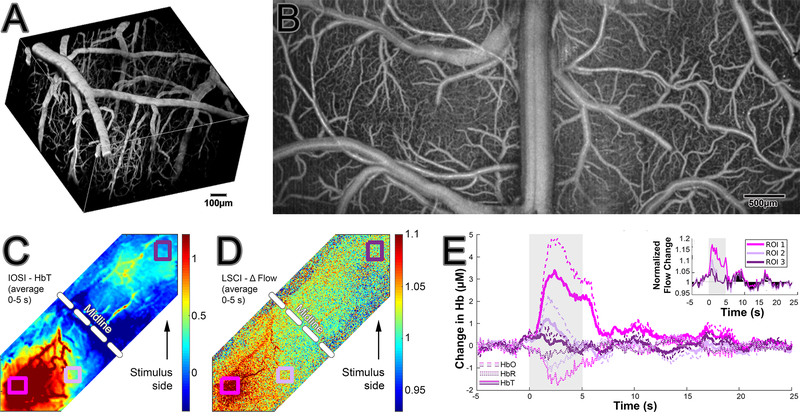
An example of 2-photon FITC vascular angiography images from cortex is presented (Fig 3, A). OCT angiography images are taken from a large field of view including bilateral hemispheres (Fig 3, B). Intrinsic optical signal imaging (Fig 3, C) and laser speckle imaging (Fig 3, D) are performed during a unilateral whisker stimulation. For details please see Desjardins M., Kılıç K. et al. (7). Graphs showing the change in hemoglobin concentration detected by IOSI and in flow detected by LSCI (inset) are presented (Fig 3, E) showing that blood flow, total and oxygenated hemoglobin concentration increases during stimulation while reduced hemoglobin decreases. These effects are localization specific as demonstrated by different region of interests displaying different results (ROI 1–3).
Figure 3.
Examples of optical imaging. 2-photon FITC vascular angiography (A). OCT angiography (B). Intrinsic optical signal imaging (C) and laser speckle imaging (D) during a unilateral whisker stimulation with changes demonstrated in the graphs (E).
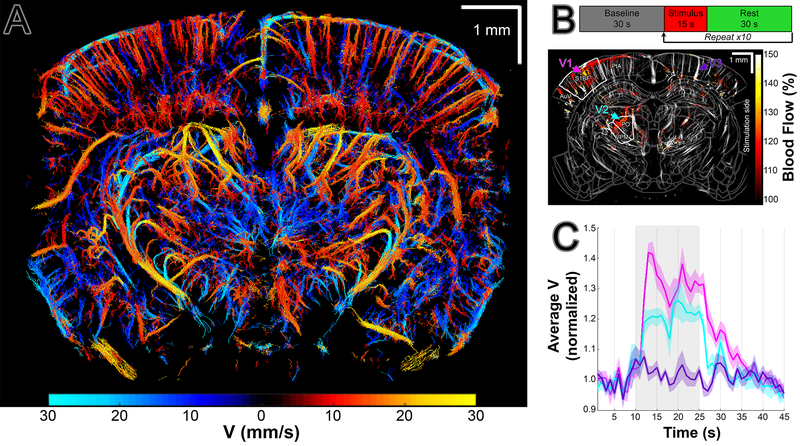
ULM can be used to view the vasculature structure. By tracking the flow of microbubbles, we can further get the flow velocity of the microbubbles in the blood vessels, i.e. the blood flow velocity. This method can be used in variety of applications including visualization the vascular response to sensory activation and vascular changes during stroke and following traumatic brain injury. It can also be employed chronically to track the vasculature and the blood flow velocity variations of the whole brain during disease progression. While ULM provides structural information concerning the vasculature, vUS gives blood flow velocity information as relative change to the baseline. An example of quantitative velocimetry by ultrasound localization microscopy (vULM) is shown in Fig 4, A. For details please see Tang et al. (6). For functional US application an experiment is conducted with application of whisker stimulus as described in Desjardins M., Kılıç K. et al. (7). Activation map (obtained with vUS on top of ULM masked brain vasculature) in response to the mouse’s left whisker stimulation is demonstrated in Fig 4, B. (BF: Primary somatosensory barrel field; PO: Posterior complex of the thalamus; VPM: Ventral posteromedial nucleus of the thalamus; AuD: Dorsal auditory area; PtA: Posterior parietal association.) Trial average of 10 stimuli is presented in a normalized velocity graph over time in Fig 4, C.
Figure 4.
Ultrasound imaging. vULM (A). Experimental paradigm and fUS imaging (B). Normalized velocity graph over time (C, trial average of 10 stimuli, stimulus duration is highlighted in grey).
COMMENTARY
BACKGROUND INFORMATION
The utilization of ultrasound imaging for imaging cerebrovascular hemodynamics is a recent advance in neuroscience (8) that provides unparalleled access to deeper brain regions in rodents. Therefore, the popularity of cerebral ultrasound imaging is thriving especially with its application to chronic awake subjects (9). However, employing other imaging methods besides ultrasound imaging to address related scientific questions has added benefits since all imaging methods have advantages and disadvantages. For example, while US could give us whole depth cerebrovascular imaging at once due to high penetration, it cannot provide submicron resolution. On the other hand, multiphoton microscopy can yield high planar resolution so much so that we can detect percentage changes in vascular diameters but this method is limited by shallow penetration (up to ~1–1.3 mm) and requires to image vessels individually or in small groups at once to acquire the aforementioned resolution. The versatility of the described surgery protocol will help researchers dealing with a broad range of applications.
Chronic preparations make longitudinal studies and therefore multimodal imaging and repeated measurements possible. Chronic models also allow awake imaging that removes the confounds of anesthesia for functional imaging. Most of the common described methods for chronic preparation of mice use glass for replacement of removed skull (7). Although, these methods yield quite favorable outcomes for optical and MRI imaging, they are unfortunately not suitable for imaging with ultrasound. Hence, we developed a surgical protocol that allows for imaging with ultrasound in addition to several optical methods such as optical coherence tomography, 2-photon microscopy, intrinsic optical signal imaging and laser speckle contrast imaging. Even though there are few publications that describe the use of polymers for chronic surgical preparation of mice, the region of interest (for example cerebellum or olfactory bulbs) and/or the diversity of imaging techniques are limited (10, 11).
Although we have not applied this preparation method for MRI imaging, the previous preparations using similar polymers (PEEK and PMP) makes us hopeful that these preparations are also MRI compatible (5, 11) since the preparation does not involve any metal implants. Due to high optical transparency of 50 μm thick PMP, it should also be possible to use these preparations for diverse optical manipulation methods like optogenetic stimulation and photothrombosis in addition to a wide spectrum of imaging techniques including calcium imaging and voltage sensitive sensor imaging.
CRITICAL PARAMETERS
Sterility and hygiene: Sterility of the surgical tools/supplies and general cleanliness of the surgical room is very important to achieve optimal results. The details are described in the following sections.
Anti-inflammatory medications: Anti-inflammatory treatments are important to get a minimal tissue response. Use and dosage is described in the following sections.
General wellbeing of the animal: Not only it is a violation of animal use protocols to let an animal recover suboptimally after surgery, it also affects the experimental results. General well-being of the animal should be assessed as well as the pain scale for 5 days after the surgery.
TROUBLESHOOTING
| Problem | Cause | Solution(s) |
|---|---|---|
| Folds appearing on PMP / uneven imaging surface | Most likely mouse hitting the head in the cage to something hard | • Make sure that the PMP is correctly sealed to the bone and a cap is provided to protect the PMP from external environment. |
| Imaging “gaps” (dark areas in images) | Having bubble or bone under the PMP | • Make sure that all the bubbles and bone dust are washed away before sealing the PMP • If there is any bone growth, PMP could be replaced after removal of the bone |
| Extensive growth of dural vessels or increased migration of inflammatory cells in the exposure | Extensive manipulation of dura or large gap between PMP and dura | • Inject dexamethasone before surgery • Minimize the manipulation of dura • Minimize the gap between PMP and dura |
UNDERSTANDING RESULTS
A good-quality preparation will allow a sufficient, continuous and rather homogeneous microcirculatory flow. OCT can serve as a good quality control measure. In poorly prepared mice OCT angiograms (Fig 3, B) show areas devoid of capillary flow and/or extremely high number of flow stalls in capillaries (12). Insufficient imaging penetration (especially with optical imaging) is another indicator of less-than optimal imaging. Tissue inflammation, dural growth, bone regrowth all contribute to a decreased penetration depth in imaging. The researchers should keep in mind that depending on the imaging system and the wavelength used for data acquisition, the imaging penetration depth may vary. Problems in acoustic imaging quality usually arise as areas that are devoid of signal. Although the dural growth would not significantly affect the US signal, bubbles under the PMP or bone regrowth will have a deleterious effect. Another measure of the quality of the preparation is the stability over time. A good preparation should give good quality of images for at least three months.
TIME CONSIDERATIONS
Preoperative Measures : ~1 hour
Intraoperative Measures: ~1–2 hours
Postoperative Measures: 10–14 days
Imaging: ~1 hour per session, up to 3–6 months
Supplementary Material
Supplementary File 3: Mouse Head Plate Holder Bottom.tif
Supplementary File 2: Mouse Head Plate Holder Top.tif
Supplementary File 1: Mouse Head Plate.tif
Table 1.
Medicine Doses
| Dexamethasone (4 mg/ml, 4.8 mg/kg) | Ketamine (40 mg/ml, 100 mg/kg) / Xylazine (4 mg/ml, 10 mg/kg) | ||||
| Weight (g) | Dose (mg) | Volume (ml) | Weight (g) | Dose (mg) | Volume (ml) |
| 20 | 0.096 | 0.03 | 20 | 2 / 0.2 | 0.05 |
| 25 | 0.120 | 0.03 | 25 | 2.5 / 0.25 | 0.06 |
| 30 | 0.144 | 0.04 | 30 | 3 / 0.3 | 0.08 |
| 35 | 0.168 | 0.04 | 35 | 3.5 / 0.35 | 0.09 |
| 40 | 0.192 | 0.05 | 40 | 4 / 0.4 | 0.10 |
| 45 | 0.216 | 0.05 | 45 | 4.5 / 0.45 | 0.11 |
| 50 | 0.240 | 0.06 | 50 | 5 / 0.5 | 0.13 |
| Cefazolin (200 mg/ml, 0.5 g/kg) | Buprenorphine (0.03 mg/ml, 0.05 mg/kg) | ||||
| Weight (g) | Dose (mg) | Volume (ml) | Weight (g) | Dose (mg) | Volume (ml) |
| 20 | 10 | 0.05 | 20 | 0.001 | 0.03 |
| 25 | 12.5 | 0.06 | 25 | 0.00125 | 0.04 |
| 30 | 15 | 0.08 | 30 | 0.0015 | 0.05 |
| 35 | 17.5 | 0.09 | 35 | 0.00175 | 0.06 |
| 40 | 20 | 0.10 | 40 | 0.002 | 0.07 |
| 45 | 22.5 | 0.11 | 45 | 0.00225 | 0.08 |
| 50 | 25 | 0.13 | 50 | 0.0025 | 0.08 |
SIGNIFICANCE STATEMENT.
Chronic preparations of experimental animals are widely used since they make longitudinal studies possible and allow awake imaging without the confounds of anesthesia. Most available chronic mouse preparations involving craniotomy employ a glass coverslip for the replacement of the skull. Although this approach allows the optical and MR imaging, it is not suitable for use with ultrasound imaging. This is important to allow better validation of these novel acoustic techniques and to enhance their biological applications in a multimodal setting. Here we describe a surgical protocol that optimizes the image quality with several optical imaging systems as well as with functional ultrasound imaging. Albeit functional ultrasound imaging of the brain is a recently introduced imaging technique in the neuroscience field, it is highly desirable due to increased penetration that cannot be achieved with optical methods. We believe our protocol for surgical preparation will serve many researchers working in the area.
ACKNOWLEDGEMENTS
Authors acknowledge funding from NIH R01-NS108472 and NIH R01-EB021018. S.E E ‘s work was additionally supported by Turkish Neurological Society.
Footnotes
APPENDIX
Mouse Head Plate and Holder Designs
Head plates are machined from PEEK. Head plate receivers are machined from stainless steel.
LITERATURE CITED
- 1.Langford D, Bailey A, Chanda M et al. 2010. Coding of facial expressions of pain in the laboratory mouse. Nat Methods 7, 447–449 [DOI] [PubMed] [Google Scholar]
- 2.Errico C, Pierre J, Pezet S, Desailly Y, Lenkei Z, Couture O, Tanter M. 2015. Ultrafast ultrasound localization microscopy for deep super- resolution vascular imaging. Nature 527, 499–502 [DOI] [PubMed] [Google Scholar]
- 3.Song P, Trzasko JD, Manduca A, Huang R, Kadirvel R, Kallmes DF, and Chen S. 2017. Improved Super-Resolution Ultrasound Microvessel Imaging with Spatiotemporal Nonlocal Means Filtering and Bipartite Graph-Based Microbubble Tracking. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 3010, 1–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Postnov DD, Erdener SE., Kılıç K. and Boas David A.. 2018. Cardiac pulsatility mapping and vessel type identification using laser speckle contrast imaging. Biomed. Opt. Express 9, 6388–6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sunil S, Erdener SE, Lee BS, Postnov DD, Tang J, Kura S, Cheng X, Chen IA, Boas DA, Kılıç K. 2020. Awake chronic mouse model of targeted pial vessel occlusion via photothrombosis. Neurophotonics. Jan;7(1):015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang J, Postnov DD, Kılıç K, Erdener SE, Lee BS, Szabo TL, Boas DA. 2019. Functional ultrasound speckle decorrelation-based velocimetry of the brain. bioRxiv 686774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desjardins M, Kılıç K, Thunemann M, Mateo C, Holland D, Ferri CGL, Cremonesi JA, Li B, Cheng Q, Weldy KL, Saisan PA, Kleinfeld D, Komiyama T, Liu TT, Bussell R, Wong EC, Scadeng M, Dunn AK, Boas DA, Sakadžić S, Mandeville JB, Buxton RB, Dale AM, Devor A. 2018. Awake mouse imaging: from 2-photon microscopy to BOLD fMRI. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Macé E, Montaldo G, Cohen I et al. 2011. Functional ultrasound imaging of the brain. Nat Methods 8, 662–664 [DOI] [PubMed] [Google Scholar]
- 9.Sieu L, Bergel A, Tiran E et al. 2015. EEG and functional ultrasound imaging in mobile rats. Nat Methods 12, 831–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Askoxylakis V, Badeaux M, Roberge S et al. 2017. A cerebellar window for intravital imaging of normal and disease states in mice. Nat Protoc 12, 2251–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boido D, Rungta RL, Osmanski B et al. Mesoscopic and microscopic imaging of sensory responses in the same animal. 2019. Nat Commun 10, 1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erdener ŞE, Tang J, Sajjadi A, Kılıç K, Kura S, Schaffer CB, & Boas DA 2019. Spatio-temporal dynamics of cerebral capillary segments with stalling red blood cells. Journal of Cerebral Blood Flow & Metabolism, 39(5), 886–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary File 3: Mouse Head Plate Holder Bottom.tif
Supplementary File 2: Mouse Head Plate Holder Top.tif
Supplementary File 1: Mouse Head Plate.tif