Abstract
Purpose of review
The right ventricle (RV) is uniquely at risk in many patients with repaired or palliated congenital heart disease (CHD) such as tetralogy of Fallot, corrected transposition, single right ventricle, and in those with pulmonary hypertension. These patients live with abnormal cardiac loading conditions throughout their life, predisposing them to right heart failure.
Recent findings
Standard heart failure therapies, developed to treat left ventricular failure, have failed to improve function or survival in patients with RV failure, suggesting a divergence in the molecular mechanisms of right versus left ventricular failure. As surgical techniques for repair of the most complex forms of RV-affecting CHDs continue to improve, more children with CHD will survive into adulthood. Long-term survival and quality of life will ultimately depend on our ability to preserve RV function.
Summary
The purpose of this review is to highlight the differences between the right and left ventricular responses to stress, our current knowledge of how the RV adapts to the unique hemodynamic stressors experienced by patients with CHD, and the need to better understand the molecular mechanisms of RV failure, providing new targets for the development of RV-specific heart failure therapeutics.
Keywords: angiogenesis, congenital, heart defects, heart failure, hypertrophy, oxidative stress
INTRODUCTION
Congenital heart disease (CHD) is the single most common class of birth defects and one of the leading causes of infant mortality [1,2]. Most CHDs involve lesions in which loading conditions on the ventricles are abnormal. Despite major advances in surgical techniques, repair of these lesions is often imperfect, leading to lifelong chronic pressure and/or volume loading. For example, repair of tetralogy of Fallot, especially when requiring a transannular patch) will often result in moderate-to-severe pulmonary valve regurgitation; in patients with systemic right ventricles (RV), for example, in hypoplastic left heart syndrome (HLHS), the RV is exposed to abnormally high afterload throughout life. The molecular events that mark the transition from a stressed but compensated state, for example, stable hypertrophy, to overt heart failure are still not completely understood. Because the most common cause of heart failure in adults in the USA is ischemic left ventricular cardiomyopathy, there is an abundance of animal and human data which have advanced our understanding of the mechanisms underlying the progression from compensated left ventricular hypertrophy to a maladaptive state of left ventricular dysfunction and then to overt heart failure [3,4]. In contrast, there are few data on RV remodeling in response to hemodynamic stressors and the pathways leading to RV failure [5,6]. This is a critical issue for patients with CHD wherein the RV is uniquely at risk, for example, in patients with right-sided obstructive lesions (tetralogy of Fallot, pulmonary atresia), in patients with systemic right ventricles [l-transposition of the great arteries (TGA), HLHS, d-transposition after a Mustard or Senning (atrial switch)], and also in patients with pulmonary hypertension. As surgical techniques for repair of the most complex forms of RV-affecting congenital heart lesions continue to improve, long-term survival and quality of life will ultimately depend on our ability to preserve long-term RV function.
As more children with CHD survive into early and middle adulthood, RV failure secondary to residual hemodynamic stressors in the form of pressure and volume overload is fast becoming a major problem [7], Twenty five percent of patients with a systemic RV due to l-transposition of the great arteries (congenitally corrected transposition) develop congestive heart failure by 40 years of age, and having an associated lesion such as tricuspid insufficiency increases the incidence of heart failure to over 50% [8], Single ventricle patients with a systemic, single RV have worse survival compared with those with a systemic, single left ventricular [9], Myocardial fibrosis has emerged as an important feature predisposing to RV dysfunction, and using MRI delayed enhancement, has been demonstrated to be present in 99% of patients with tetralogy of Fallot, 28% of those with a systemic RV in the Fontan circulation, and in 61% of those with a systemic RV in TGA [10–12],
The purpose of this review is to highlight recent advances in understanding differences between the right and left ventricular responses to stress, our current knowledge of how the RV adapts to the unique hemodynamic stressors experienced by patients with CHD, and the critical need to better understand the molecular mechanisms of RV failure, providing new targets for the development of RV-specific heart failure therapeutics. Finally, the progression of the RV from a compensated to a decompensated state is often difficult to follow clinically, given the limitations of noninvasive imaging (echocardiogram, MRI) in assessing RV contractile function, Planning for surgical interventions, for example, pulmonary valve replacement would be greatly enhanced if serum biomarkers marking the earliest stages of RV failure could be developed.
HOW CLOSELY DOES THE RV MIRROR THE LEFT VENTRICULAR?
Embryological and structural differences between the right ventricle and left ventricular
In the past, differences in global structure and loading conditions were thought to represent the main differences between the right and left ventricles. We now recognize that these differences begin early in development) before afterload differences become operative (the fetal right and left ventricles are both coupled to the systemic circulation so both are exposed to similar – although not identical – afterloads). This cellular divergence begins with the primary and secondary heart fields, leading to the differentiation of left and right ventricular cardiomyocytes, respectively, during early development, and continues with chamber-specific differences in cell signaling and Ca2+ handling, all suggesting that there are some fundamental differences between the two ventricles at the cellular level [13]. During fetal life, the RV pumps blood to the pulmonary circulation, placenta, and to the lower body. With the transition from the fetal to the postnatal circulation and the fall in pulmonary vascular resistance, the RV becomes a thin-walled, heavily trabeculated chamber pumping a cardiac output equal to that of the left ventricular but at a much lower energy cost. The crescent-shaped RV has a trapezoidal pressure-volume loop with few isovolumic periods. In contrast, the ellipsoid-shaped left ventricular has a rectangular pressure-volume loop with well defined isovolumic contraction and relaxation phases. RV cardiomyocytes are oriented longitudinally and demonstrate faster twitch velocities than the radially oriented left ventricular cardiomyocytes. Thus, although the RV is a low-resistance and low-capacitance pump, the left ventricular is a high-resistance and high-pressure pump [14].
lood supply to the right ventricle
RV coronary blood flow is supplied mostly during systole but also during diastole, whereas left ventricular coronary blood flow is supplied almost exclusively during diastole. Therefore, left ventricular coronary flow is less susceptible to changes in left ventricular afterload (e.g., in aortic stenosis), whereas the RV is exquisitely sensitive to changes in RV afterload, such as occurs in RV outflow tract obstruction (pulmonary stenosis) and pulmonary hypertension, increasing the risk for subendocardial ischemia and subsequent fibrosis
Loading conditions
Under conditions of rest, the RV pumps against the low-resistance pulmonary circulation, whereas the left ventricular pumps against the high-resistance systemic circulation. Normal pulmonary vascular resistance is about 10% of that of the systemic circulation. Therefore, although the RV pumps the same cardiac output as the left ventricular) it does so at 20% of the energy cost. When the RV is subject to increased afterload in the form of pulmonary stenosis or pulmonary hypertension or when it functions as a systemic RV, it is more likely to progress to RV failure when compared with the left ventricular subjected to a similar afterload [15]. Patients with l-transposition of the great arteries, wherein the RV functions as the systemic ventricle, have an increased risk of RV failure as they age, even in the absence of atrioventricular valve regurgitation or other lesions. Similarly, the systemic RV is at risk in patients who have undergone an atrial switch operation for d-transposition of the great arteries. These systemic RVs develop hypertrophy at a very early age or never lose their fetal RV hypertrophy, however, increased wall stress alone cannot be the only factor predisposing these ventricles to failure.
Heart failure therapy in congenital heart disease
The fundamental differences in the mechanisms of right versus left ventricular failure are best demonstrated by the divergence in the response of the two ventricles to heart failure therapies. Multiple clinical trials have shown that standard heart failure drugs (β-blockers, angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor blockers), developed and tested in patients with left ventricular failure) do not improve function or survival in patients with CHD and RV failure [16–19], Randomized controlled studies of the use of ACE inhibitors in patients with tetralogy of Fallot and transposition with a systemic RV have not demonstrated a beneficial effect on oxygen consumption or ejection fraction [20–23]. In a landmark multicenter randomized trial by the Pediatric Heart Network investigators, the use of enalapril in infants with a single ventricle did not improve ventricular function, heart failure, or growth. Although both single RVs and left ventriculars were included, 65% of these patients had a systemic RV [24]. Van der Bom et al. [25] evaluated the effect of valsartan on systemic RV function and also found no difference in survival.
The results with β-blockers have been more equivocal with some improvement seen in small series of transposition patients. Carvedilol improved RV ejection fraction at 1 year in some studies whereas in others, although it improved New York Heart Association (NYHA) class, it did not improve ejection fraction or oxygen consumption [26,27], In the largest US study of β-blockers in children, Shaddy et al. [18] demonstrated no difference in death, hospitalization, NYHA class, or clinical status with the use of carvedilol in 161 pediatric heart failure patients. Importantly, this study suggested that β-blockers could possibly worsen outcomes in patients with a systemic RV, although the patient numbers were too small for any certain conclusions, Phosphodiesterase inhibitors such as sildenafil have been tested in 24 patients after the Fontan operation of whom 54% had a systemic RV, There was improvement in echocardiographic measures of myocardial performance index and velocity time integral but no change in diastolic indices [28].
In summary, heart failure therapies developed and tested in patients with left ventricular failure appear to be less effective or ineffective in patients with CHD and RV failure, In the case of ACE inhibitors, their lack of efficacy in the failing pulmonary RV could be explained by the fact that systemic vasodilation would not be expected to alter loading conditions of the pulmonary circulation, Differences in the cause of heart failure (ischemic in most left ventricular studies versus congenital in most RV studies) may be another reason for differences in drug efficacy, However, the broad failure of these agents in this patient population is also likely to be at least in part related to fundamental differences in the systemic RV versus left ventricular in its cellular and molecular response to stress.
Molecular response to stress
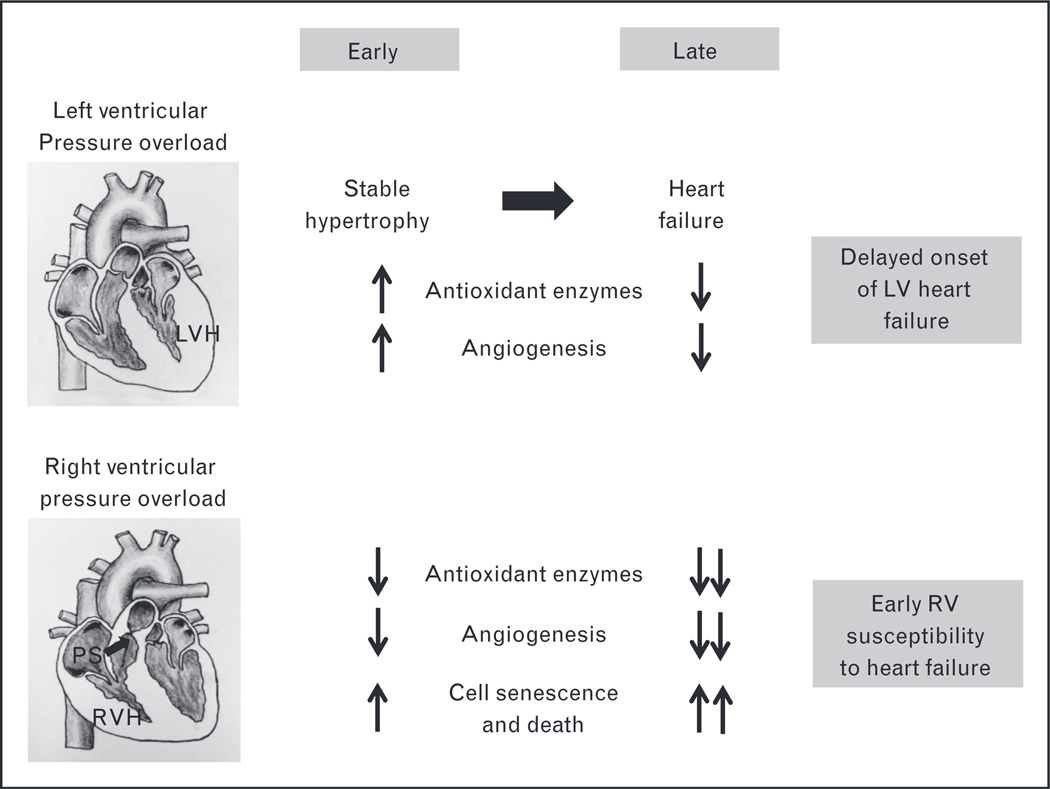
Although the majority of the cellular and molecular responses to stress are similar between the RV and the left ventricular, we and others have shown several key differences at the cellular and molecular levels in their responses to stress such as pressure overload, Although the two ventricles exhibit similar alterations in genes regulating extracellular matrix and cytoskeletal remodeling, there are important differences in genes regulating energy production, mitochondrial function, reactive oxygen species production, antioxidant protection, and angiogenesis (Fig, 1) [29,30], Importantly, when the left ventricular is exposed to afterload stress, it initially increases the production of new capillaries (angiogenesis), in order to keep up with the increased blood flow requirements of hypertrophied cardiomyocytes, This process is mediated by increased production of the proangiogenic factors hypoxia inducible factor-1α and vascular endothelial growth factor. Only when the stressed left ventricular begins to fail does capillary density begin to fall. In contrast, in the RV, even at the onset of pressure overload, capillary density decreases, rendering the stressed RV more susceptible to microischemic injury.
Figure 1.
Right ventricular susceptibility to heart failure. Stable LVH is characterized by an increase in antioxidant enzymes and angiogenesis and decreased ROS generation, which protects the LV from early progression to heart failure. However, in RVH, there is an early decrease in antioxidant enzymes and failure of angiogenesis along with increased ROS and cell death thereby making the RV more susceptible to early heart failure. LV, left ventricular; LVH, left ventricular hypertrophy; PS, pulmonary stenosis; ROS, reactive oxygen species; RV, right ventricular; RVH, right ventricular hypertrophy. Cardiac illustrations by Mingming Zhao.
As key regulators of entire gene networks, micro-RNAs (miRs) have emerged as important links for understanding basic cardiovascular processes-miRs are small, noncoding RNAs of, approximately, 22 nucleotides that regulate gene expression by degradation or translational suppression of mRNA. miRs also have the potential to serve as therapeutic targets, based on our ability to manipulate their expression with miR mimics and inhibitors. The role of miRs in left ventricular hypertrophy (LVH) and left ventricular failure (LVF) has received much recent attention, with miR-208, miR-1, miR-133, and miR-21 emerging as key regulators of LVH and fibrosis [31–36]. As miR expression is dynamic during a disease state, and miRs are released into the plasma, they may also be valuable as biomarkers for diagnosis and prognosis [37]. Despite a wealth of knowledge in the left ventricular, there has been less information on miR regulation in the afterload-stressed RV [7,38,39]. We characterized the murine RV miRome during both normal and stress conditions and described significant differences in the baseline expression of the most highly expressed miRs in the RV versus the left ventricular. We also found four unique RV-specific miRs: 28, 93, 148a, and 34a, which are upregulated during RV hypertrophy and failure but not during LVH/LVF [40]. The first three have not been previously evaluated in the heart but have been implicated in regulating oxidant stress, angiogenesis and cell death, and senescence in other cell types [40–43], the very pathways in which the RV and left ventricular appear to differ most.
iomarkers in heart failure
Classic left ventricular heart failure biomarkers B-type natriuretic peptide (BNP) and pro-N terminal BNP (pro-NTBNP) have changed the management and follow-up of heart failure patients. Additional biomarkers reflecting different pathophysiological processes are being developed to better understand myocardial stress, inflammation, and remodeling such as adrenomedullin, troponins, soluble ST2, growth differential factor-15, galectin-2, copectin, C-reactive protein, and microRNAs [44–46]. However, there is little consensus on which biomarkers provide the most useful information on diagnosis and prognosis of left ventricular heart failure. The lack of reliability in data stems from variability in timing of collections, different methods of measurements, contamination by other cells types, and influence of medications. Much less is known about biomarkers of RV failure. Di Salvo et al. [47] performed comprehensive profiling of the RV transcriptome and identified myocardial biomarkers unique to the RV involving abnormalities in structure and inflammation, six transmembrane epithelial antigen of prostate, secreted protein acidic and rich in cysteine-like 1, and V-Set And Immunoglobulin Domain Containing 4. Interestingly, established and or evolving left ventricular heart failure biomarkers showed substantial overlap between RV heart failure and normal RV donor hearts highlighting the difficulties in using left ventricular biomarkers for RV failure. In pulmonary hypertension with or without RV failure, pro-NTBNP is used in everyday clinical practice to guide management and prognosis [48]. We have identified plasma micro-RNAs mediating fibrosis and are developing these as biomarkers to use in conjunction with cardiac MRI to guide the timing of pulmonary valve replacement in patients with RV volume overload [49].
Finally, most animal models of left ventricular failure have used pressure overload (aortic banding) to induce cardiomyopathy. Many patients with CHD and vulnerable RVs (especially those with repaired tetralogy of Fallot) have minimal pressure overload and predominantly volume overload, due to pulmonary valve insufficiency. Yet, until recently there have been no animal models of this pathophysiology. To address this lapse, we developed a murine model of RV volume overload by suturing the pulmonary valve leaflets to the pulmonary artery wall, which recapitulates many of the clinical findings in these patients. We found upregulation in genes encoding the extracellular matrix, stress fibers, cell cycling, and Wnt signaling pathways, whereas α1-adrenergic receptors, transmembrane transport, and anion transport-related genes were downregulated [50]. These results confirm important differences at the cellular and molecular level in the mechanisms leading to heart failure between the right and left ventricles and also major differences depending on the form of stress (pressure overload versus volume overload).
CONCLUSION
The RV in children with CHD is uniquely at risk. With the growing adolescent and adult congenital heart disease population, RV failure is becoming a major long-term problem. Although there are considerable data on the mechanisms of left ventricular dysfunction and failure, the mechanisms of RV dysfunction and remodeling are only now beginning to be understood. New models of RV failure are beginning to uncover basic differences between the two ventricles in their cellular and molecular response to stress. Defining molecular mechanisms for the increased susceptibility of the RV in patients with CHD to progress from a compensated state to overt heart failure is the first step toward developing RV-specific heart failure therapies. Identifying and developing new biomarkers of the progression from RV pressure/volume overload to failure is another critical need, given the limitations of clinical assessment and current imaging modalities (echo, MRI) in determining the optimal timing for surgical intervention.
KEY POINTS.
Standard left ventricular failure therapies are less effective or ineffective in right ventricle (RV) failure secondary to CHD.
New models of RV failure are beginning to uncover basic differences between the two ventricles in their cellular and molecular response to stress.
Although the two ventricles exhibit similar alterations in genes regulating extracellular matrix and cytoskeletal remodeling, there are important differences in genes regulating energy production, mitochondrial function, reactive oxygen species production and antioxidant protection, and angiogenesis.
Acknowledgements
Mingming Zhao, Dong-Qing Hu, and Giovanni Fajardo.
Financial support and sponsorship
NIH/NHLBI grant HL061535 (D.B.); Children’s Heart Foundation grant (D.B. and S.R.); Packard Children’s Hospital Pediatric Research Fund, Heart Center Research Fund and Reddy Foundation grant (S.R); NIH/NHLBI 1K08HL127277 (S.R.).
Footnotes
Conflicts of interest
There are no conflicts of interest to declare.
REFERENCES
- 1.Jl Hoffman, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol 2002; 39:1890–1900. [DOI] [PubMed] [Google Scholar]
- 2.Belmont JW. Recent progress in the molecular genetics of congenital heart defects. Clin Genet 1998; 54:11–19. [DOI] [PubMed] [Google Scholar]
- 3.Lund O, Kristensen LH, Baandrup U, et al. Myocardial structure as a determinant of pre and postoperative ventricular function and long-term prognosis after valve replacement for aortic stenosis. Eur Heart J 1998; 19:1099–1108. [DOI] [PubMed] [Google Scholar]
- 4.Douglas PS, Reichek N, Hackney K, et al. Contribution of afterload, hypertrophy and geometry to left ventricular ejection fraction in aortic valve stenosis, pure aortic regurgitation and idiopathic dilated cardiomyopathy. Am J Cardiol 1987; 59:1398–1404. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman BD, Desai M, Reddy S, et al. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J Card Fail 2008; 14: 760–767. [DOI] [PubMed] [Google Scholar]
- 6.Buermans HP, Redout EM, Schiel AE, et al. Microarray analysis reveals pivotal divergent mrna expression profiles early in the development of either compensated ventricular hypertrophy or heart failure. Physiol Genomics 2005; 21:314–323. [DOI] [PubMed] [Google Scholar]
- 7.Wiliams RG, Pearson GD, Barst RJ, et al. Blood Institute Working Group on research in adult congenital heart disease. Report of the national heart, lung, and blood institute working group on research in adult congenital heart disease. J Am Coll Cardiol 2006; 47:701–707. [DOI] [PubMed] [Google Scholar]
- 8.Graham TP Jr, Bernard YD, Mellen BG, et al. Long-term outcome in congenitally corrected transposition of the great arteries: a multiinstitutional study. J Am Coll Cardiol 2000; 36:255–261. [DOI] [PubMed] [Google Scholar]
- 9.Julsrud PR, Weigel TJ, Van Son JA, et al. Influence of ventricular morphology on outcome after the fontan procedure. Am J Cardiol 2000; 86:319–323. [DOI] [PubMed] [Google Scholar]
- 10.Babu-Narayan SV, Goktekin O, Moon JC, et al. Late gadolinium enhancement cardiovascular magnetic resonance of the systemic right ventricle in adults with previous atrial redirection surgery for transposition of the great arteries. Circulation 2005; 111:2091–2098. [DOI] [PubMed] [Google Scholar]
- 11.Babu-Narayan SV, Kilner PJ, Li W, et al. Ventricular fibrosis suggested by cardiovascular magnetic resonance in adults with repaired tetralogy of fallot and its relationship to adverse markers of clinical outcome. Circulation 2006; 113:405–413. [DOI] [PubMed] [Google Scholar]
- 12.Rathod RH, Prakash A, Powell AJ, Geva T. Myocardial fibrosis identified by cardiac magnetic resonance late gadolinium enhancement is associated with adverse ventricular mechanics and ventricular tachycardia late after fontan operation. J Am Coll Cardiol 2010; 55:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo RP, Dederko DA, Teutsch C, et al. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol 2006;571:131–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friedberg MK, Redington AN. Right versus left ventricular failure: differences, similarities, and interactions. Circulation 2014; 129:1033–1044. [DOI] [PubMed] [Google Scholar]
- 15.Gentles TL, Mayer JE Jr, Gauvreau K, et al. Fontan operation in five hundred consecutive patients: factors influencing early and late outcome. J Thorac Cardiovasc Surg 1997; 114:376–391. [DOI] [PubMed] [Google Scholar]
- 16.Winter MM, Bouma BJ, Groenink M, et al. Latest insights in therapeutic options for systemic right ventricular failure: a comparison with left ventricular failure. Heart 2009; 95:960–963. [DOI] [PubMed] [Google Scholar]
- 17.Szymanski P, Klisiewicz A, Hoffman P. Therapeutic options for systemic right ventricular failure. Heart 2009; 95:1950–1951; author reply 1951. [DOI] [PubMed] [Google Scholar]
- 18.Shaddy RE, Boucek MM, Hsu DT, et al. Carvedilol for children and adolescents with heart failure: a randomized contro led trial. JAMA 2007; 298: 1171–1179. [DOI] [PubMed] [Google Scholar]
- 19.Hsu DT, Zak V, Mahony L, et al. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation 2010; 122:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hechter SJ, Fredriksen PM, Liu P, et al. Angiotensin-converting enzyme inhibitors in adults after the mustard procedure. Am J Cardiol 2001; 87: 660–663; A611. [DOI] [PubMed] [Google Scholar]
- 21.Robinson B, Heise CT, Moore JW, et al. Afterload reduction therapy in patients following intraatrial baffle operation for transposition of the great arteries. Pediatr Cardiol 2002; 23:618–623. [DOI] [PubMed] [Google Scholar]
- 22.Therrien J, Provost Y, Harrison J, et al. Effect of angiotensin receptor blockade on systemic right ventricular function and size: a small, randomized, placebocontrolled study. Int J Cardiol 2008; 129:187–192. [DOI] [PubMed] [Google Scholar]
- 23.Babu-Narayan SV, Uebing A, Davlouros PA, et al. Randomised trial of ramipril in repaired tetralogy of fa lot and pulmonary regurgitation: the appropriate study (ace inhibitors for potential prevention of the deleterious effects of pulmonary regurgitation in adults with repaired tetralogy of fallot). Int J Cardiol 2012; 154:299–305. [DOI] [PubMed] [Google Scholar]
- 24.Hsu DT, Zak V, Mahony L, et al. , Pediatric Heart Network I.. Enalapril in infants with single ventricle: results of a multicenter randomized trial. Circulation 2010; 122:333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van der Bom T, Winter MM, Bouma BJ, et al. Effect of valsartan on systemic right ventricular function: a double-blind, randomized, placebocontrolled pilot trial. Circulation 2013; 127:322–330. [DOI] [PubMed] [Google Scholar]
- 26.Giardini A, Lovato L, Donti A, et al. A pilot study on the effects of carvedilol on right ventricular remodelling and exercise tolerance in patients with systemic right ventricle. Int J Cardiol 2007; 114:241–246. [DOI] [PubMed] [Google Scholar]
- 27.Doughan AR, McConnell ME, Book WM. Effect of beta blockers (carvedilol or metoprolol xl) in patients with transposition of great arteries and dysfunction of the systemic right ventricle. Am J Cardiol 2007; 99:704–706. [DOI] [PubMed] [Google Scholar]
- 28.Goldberg DJ, French B, Szwast AL, et al. Impact of sildenafil on echocardiographic indices of myocardial performance after the fontan operation. Pediatr Cardiol 2012; 33:689–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piao L, Marsboom G, Archer SL. Mitochondrial metabolic adaptation in right ventricular hypertrophy and failure. J Mol Med; 88:1011–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urashima T, Zhao M, Wagner R, et al. Molecular and physiological characterization of rv remodeling in a murine model of pulmonary stenosis. Am J Physiol Heart Circ Physiol 2008; 295:H1351–H1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordes KR, Srivastava D. Microrna regulation of cardiovascular development Circ Res 2009; 104:724–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callis TE, Wang DZ. Taking micrornas to heart. Trends Mol Med 2008; 14:254–260. [DOI] [PubMed] [Google Scholar]
- 33.van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress-responsive micrornas that can evoke cardiac hypertrophy and heart failure. Proc Natl Acad Sci U S A 2006; 103:18255–18260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thum T, Galuppo P, Wolf C, et al. Micrornas in the human heart: a clue to fetal gene reprogramming in heart failure. Circulation 2007; 116:258–267. [DOI] [PubMed] [Google Scholar]
- 35.El-Armouche A, Schwoerer AP, Neuber C, et al. Common microrna signatures in cardiac hypertrophic and atrophic remodeling induced by changes in hemodynamic load. PLoS One; 5:e14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Care A, Catalucci D, Feliceti F, et al. Microrna-133 controls cardiac hypertrophy. Nat Med 2007; 13:613–618. [DOI] [PubMed] [Google Scholar]
- 37.Sheehy SP, Huang S, Parker KK. Time-warped comparison of gene expression in adaptive and maladaptive cardiac hypertrophy. Circ Cardiovasc Genet 2009; 2:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogaard HJ, Abe K, Vonk Noordegraaf A, Voelkel NF. The right ventricle under pressure: cellular and molecular mechanisms of right-heart failure in pulmonary hypertension. Chest 2009; 135:794–804. [DOI] [PubMed] [Google Scholar]
- 39.Kret M, Arora R. Pathophysiological basis of right ventricular remodeling. J Cardiovasc Pharmacol Ther 2007; 12:5–14. [DOI] [PubMed] [Google Scholar]
- 40.Reddy S, Zhao M, Hu DQ, et al. Dynamic microrna expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics 2012; 44:562–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang M, Yao Y, Eades G, et al. Mir-28 regulates nrf2 expression through a keap1-independent mechanism. Breast Cancer Res Treat 2011; 129:983–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith-Vikos T, Slack FJ. Micrornas and their roles in aging. J Cell Sci 2012; 125:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yu J, Li Q, Xu Q, et al. Mir-148a inhibits angiogenesis by targeting erbb3. J Biomed Res 2011; 25:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaggin HK, Januzzi JL Jr. Biomarkers and diagnostics in heart failure. Biochimica et biophysica acta 2013; 1832:2442–2450. [DOI] [PubMed] [Google Scholar]
- 45.Meluzin J, Tomandl J. Can biomarkers help to diagnose early heart failure with preserved ejection fraction? Dis Markers 2015; 2015:426045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong LL, Armugam A, Sepramaniam S, et al. Circulating micrornas in heart failure with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail 2015; 17:393–404. [DOI] [PubMed] [Google Scholar]
- 47.di Salvo TG, Yang KC, Brittain E, et al. Right ventricular myocardial biomarkers in human heart failure. J Card Fail 2015; 21:398–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fijalkowska A, Torbicki A. Role of cardiac biomarkers in assessment of rv function and prognosis in chronic pulmonary hypertension. Eur Heart J Suppl 2007; 9:H41–H47. [Google Scholar]
- 49.Bernstein SRD-QHMZEBGFD. Mir-21 is a biomarker for fibrosis and the progression to right ventricular systolic failure in a model of pulmonary volume and pressure overload. Circulation 2013; 128:A18046. [Google Scholar]
- 50.Reddy S, Zhao M, Hu DQ, et al. Physiologic and molecular characterization of a murine model of right ventricular volume overload. Am J Physiol Heart Circ Physiol 2013; 304:H1314–H1327. [DOI] [PMC free article] [PubMed] [Google Scholar]